Abstract
Significance: Alterations in endothelial function contribute to a variety of vascular diseases. In pathological conditions, the endothelium shows a reduced ability to regulate vasodilation (endothelial dysfunction) and a conversion toward a proinflammatory and leaky phenotype (endothelial activation). At the interface between the vessel wall and blood, the endothelium exists in a complex microenvironment and must translate changes in these environmental signals to alterations in vessel function. Mechanical stimulation and endothelial cell interactions with the vascular matrix, as well as a host of soluble factors, coordinately contribute to this dynamic regulation. Recent Advances: Blood hemodynamics play an established role in the regulation of endothelial function. However, a growing body of work suggests that subendothelial matrix composition similarly and coordinately regulates endothelial cell phenotype such that blood flow affects matrix remodeling, which affects the endothelial response to flow. Critical Issues: Hemodynamics and soluble factors likely affect endothelial matrix remodeling through multiple mechanisms, including transforming growth factor β signaling and alterations in cell–matrix receptors, such as the integrins. Likewise, differential integrin signaling following matrix remodeling appears to regulate several key flow-induced responses, including nitric oxide production, regulation of oxidant stress, and activation of proinflammatory signaling and gene expression. Microvascular remodeling responses, such as angiogenesis and arteriogenesis, may also show coordinated regulation by flow and matrix. Future Directions: Identifying the mechanisms regulating the dynamic interplay between hemodynamics and matrix remodeling and their contribution to the pathogenesis of cardiovascular disease remains an important research area with therapeutic implications across a variety of conditions. Antioxid. Redox Signal. 25, 415–434.
Introduction
Blood flow has a multifaceted role on vascular structure and function (64, 72). The vascular endothelium senses the frictional force generated by blood flow, termed shear stress, and alters vessel function accordingly. Rapid changes in flow activate endothelial paracrine signaling to the medial smooth muscle to regulate vessel tone and counteract the transient change in flow volume. Chronic elevations in flow promote outward vessel remodeling, whereas chronically reduced flow stimulates inward vessel remodeling to normalize shear stress in the vessel. The gradual or sudden cessation of blood flow in diseased arteries shunts blood flow through collateral vessels stimulating arteriogenesis, the maturation of vessel structure typically due to enhanced mechanical load, and acute changes in shear stress play an important role in this response (158). Therefore, shear stress acting on the endothelial layer profoundly affects multiple aspects of physiological and pathological vascular remodeling.
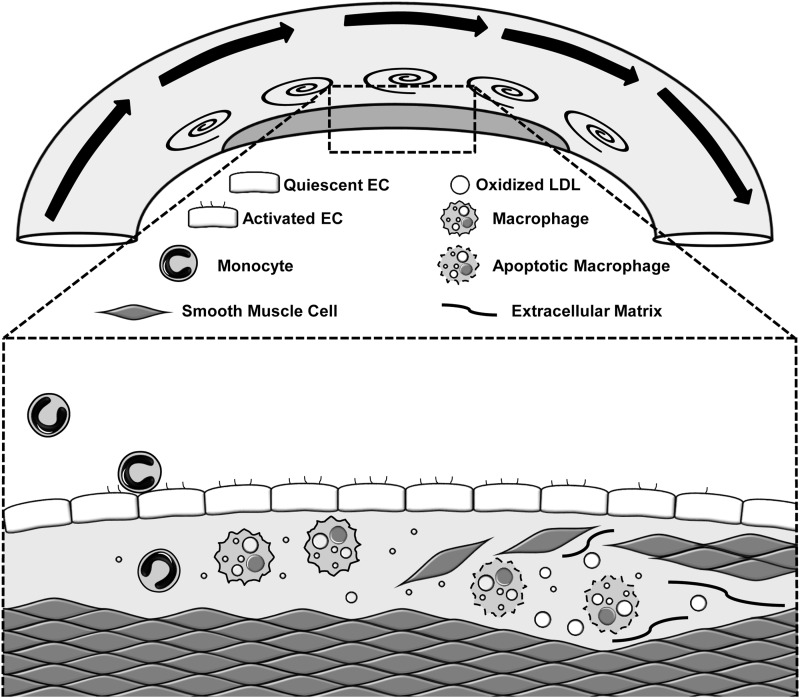
Specific flow patterns differentially regulate multiple aspects of endothelial cell phenotype and atherosclerotic plaque formation (36, 64). Atherosclerotic plaques form in medium to large vessels through the progressive deposition of cholesterol, primarily carried by low-density lipoproteins (LDL), and accumulation of dysfunctional macrophages (Fig. 1). These early inflammatory plaques result in a fibroproliferative smooth muscle response forming a collagen-rich fibrous cap, which separates the thrombotic plaque material from the blood and prevents plaque rupture and thrombosis (103). Despite the systemic nature of most atherosclerotic risk factors (elevated cholesterol, smoking, hypertension), vascular regions exposed to unidirectional laminar blood flow show protection from inflammation and atherosclerotic plaque formation (36, 64). As such, atherosclerotic plaques form preferentially at sites of low and turbulent blood flow, such as vessel curvatures, branch points, and bifurcations (Fig. 1). The vascular endothelium senses the frictional force generated by blood flow, termed shear stress, and alters vessel function accordingly. Over the past 30 years, endothelial cell culture and animal models have shown that high unidirectional flow drives an adaptive endothelial cell response, resulting in endothelial cell alignment parallel to flow direction, reduced endothelial turnover, and alterations in the endothelial cell gene expression pattern to reduce inflammation and enhance antioxidant responses (36, 64). In contrast, endothelial cells exposed to low or oscillatory flow (model of turbulent flow) do not align, show enhanced turnover, demonstrate elevated oxidant stress, and show an enhanced inflammatory response, both basally and in response to stimulus. Therefore, local components of the vessel microenvironment, such as local hemodynamics, profoundly affect the endothelial cell phenotype and their response to systemic atherogenic factors.
FIG. 1.
Model of atherosclerotic plaque formation at sites of disturbed flow. While endothelial cells in areas of high flow show a quiescent and anti-inflammatory phenotype, the endothelium in regions of turbulent flow exhibits an activated phenotype, characterized by high levels of proinflammatory gene expression that facilitates monocyte recruitment. Monocyte-derived macrophages avidly engulf lipoprotein deposits and potentiate local inflammation. Inflammation in the vessel wall stimulates smooth muscle recruitment, enhancing fibrous cap formation and preventing plaque rupture and thrombogenesis.
Subendothelial Matrix Remodeling and Cell–Matrix Interactions
The extracellular matrix (ECM) consists of more than 300 genes (including collagens, proteoglycans, and glycoproteins) that dynamically assemble to form the tissue's structural scaffold (78). Since the ECM consumes nearly 50% of blood vessel weight, it is unsurprising that the ECM profoundly influences vascular functions, with ECM remodeling that interrupts normal vascular function being a hallmark of cardiovascular disease (181). Endothelial cells natively reside on a basement membrane, an elaborate thin layer of collagen IV, laminin, nidogen, and heparan sulfate proteoglycans (86). Basement membranes are evolutionarily archaic structures, likely appearing when animal cells first started organizing into multilayered structures (77, 86). Normal vascular function relies on the supramolecular architecture of the basement membrane, with alterations in both the quantity and composition of basement membrane components underlying several vascular diseases (77, 181).
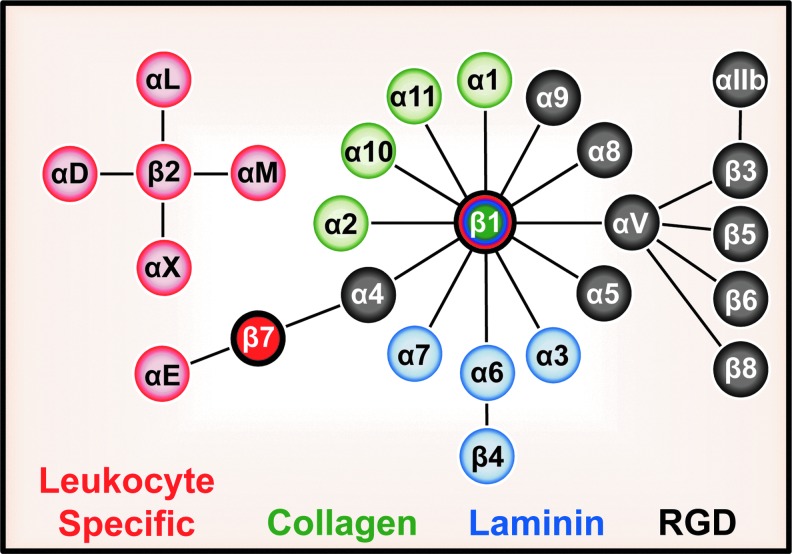
As cells and their matrix exist in a state of dynamic reciprocity (9), endothelial cells both remodel their basement membrane upon pathological stimulation and modulate cell function in response to altered basement membrane composition. The remodeling-associated provisional matrix proteins, largely absent from the vasculature under quiescent conditions, become rapidly deposited upon vessel activation or injury. Several provisional matrix proteins, including fibrinogen, vitronectin, and fibronectin, circulate in high amounts in blood and contribute to clot formation, establishing a permissive matrix for proliferation and migration during tissue wounding (109). Enhanced endothelial permeability in disease states allows these proteins to leak into the vessel wall and deposit into the endothelial matrix. Although typically absent in quiescent endothelium, fibronectin and thrombospondin production by endothelial cells in culture similarly enhances proliferation (151, 155). Cell–matrix receptors differentially interact with basement membrane and provisional matrix components to translate changes in matrix composition into altered endothelial cell function. The most prominent matrix receptors belong to the integrin family, encompassing 18 α subunits and 8 β subunits that heterodimerize into 24 different integrins, each with distinct ligand-binding specificities as well as disparate and often nonredundant functions (76). Endothelial cells express multiple integrins, including the collagen-binding integrins, α1β1 and α2β1, the laminin-binding integrins, α3β1, α6β1, and α6β4, and the provisional matrix-binding (RGD-binding), integrins α4β1, α5β1, αvβ1, αvβ3, and αvβ5 (Fig. 2) (163). However, some integrins show restricted expression based on the vascular bed, with α1β1 and α4β1 predominantly expressed in the microvasculature (38, 67, 163). While these integrins do not contain enzymatic activity, they associate with potentially hundreds of adaptors and kinases that mediate signal transduction (153).
FIG. 2.
Depiction of the 24 mammalian integrin heterodimers. Integrins are divided based on ligand specificity for provisional matrix (gray), collagen (green), and laminin (blue) or by their restricted expression in leukocytes (red).
The sticky side of fibronectin
During pathological vascular remodeling, such as angiogenesis, arteriogenesis, and atherosclerosis, deposition of provisional matrix proteins (e.g., fibronectin) into the endothelial matrix is greatly enhanced (15, 46, 136, 148). Integrin activation, a conversion from a low-affinity bent conformation to a high-affinity extended confirmation, plays a major role in fibronectin deposition. As such, stimulating endothelial β1 integrin activation drives fibronectin deposition, while preventing integrin activation hinders fibronectin deposition (132, 187). Most of the fibronectin in the body circulates in the blood in a soluble globular form. Provisional matrix-binding integrins, particularly α5β1, mechanically unravel globular fibronectin, revealing cryptic nucleation sites that promote its assembly into the fibrillar form (53).
Fibronectin expression is classically regulated by members of the transforming growth factor β (TGFβ) superfamily (e.g., TGFβ, bone morphogenic proteins [BMPs]), potent regulators of ECM remodeling, and tissue fibrosis (107). TGFβ drives matrix gene expression by activating the Smad transcription factors, Smad2 and Smad3. In addition, TGFβ stimulates an endothelial phenotypic conversion, termed EndoMT, resulting in the loss of endothelial markers (platelet–endothelial cell adhesion molecule-1 [PECAM-1], vascular endothelial-cadherin [VE-cadherin]), expression of smooth muscle markers, and induction of fibronectin deposition (28). In addition to enhancing fibronectin expression, TGFβ promotes α5β1 activation, suggesting that TGFβ can induce fibronectin deposition independent of its effects on fibronectin expression (170). Reciprocally, ECM content and mechanical properties critically regulate TGFβ signaling. TGFβ is secreted in an inactive form due to the latency-associated peptide (LAP) blocking the TGFβ active sites and unmasking active TGFβ requires LAP cleavage or specific protein–protein (111). Direct thrombospondin-TGFβ interactions activate latent TGFβ (154), and thrombospondin deposition during wound healing accounts for a significant amount of active TGFβ in the tissue (32, 60). In addition, latent TGFβ-binding protein (LTBP) anchors TGFβ to the endothelial matrix through interactions with fibronectin (70, 203). Interactions between αv integrins (e.g., αvβ3) and an RGD motif in the LAP allows for force-dependent activation of latent TGFβ (70, 126). Fibronectin-dependent α5β1 ligation also potentiates TGFβ signaling by targeting its receptors (e.g., endoglin, activin receptor-like kinase 1 [Alk1]) to the cell surface (170).
Fibronectin in atherosclerosis
Reducing fibronectin deposition in atherosclerosis, both genetically or with a peptide inhibitor, limits endothelial cell proinflammatory gene expression and reduces atherosclerosis (23, 148). Despite this atheroprotective benefit, lowering fibronectin levels also reduces smooth muscle recruitment and fibrous cap size (148), suggesting that fibronectin plays a dual role in promoting both plaque inflammation and plaque stability. In contrast to plasma fibronectin, cell-derived fibronectin contains the alternative splice domains (extradomain A [EDA], extradomain B), and interfering with normal fibronectin splicing, both positively and negatively, limits atherosclerosis (1, 168). Levels of EDA-containing fibronectin correlate with plaque stability, and loss of EDA-fibronectin mimics the reduction in fibrous cap size associated with loss of total fibronectin (143, 178) and increases the propensity for focal hemorrhage (128). However, inhibiting the fibronectin receptor, α5β1, using a small peptide reduces proinflammatory gene expression and plaque burden without affecting fibrous cap size, suggesting that these disparate functions of fibronectin can be separated by targeting specific integrin receptors (195).
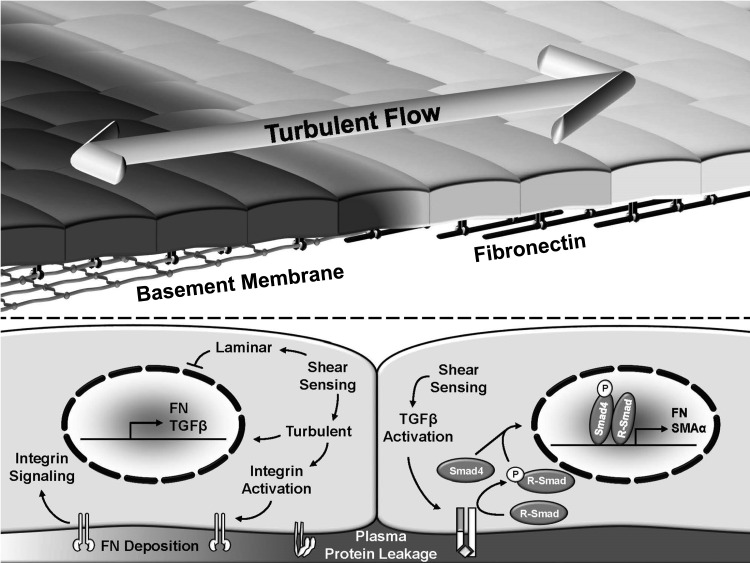
Shear stress patterns significantly affect fibronectin matrix deposition during early atherosclerosis (Fig. 3). In cell culture models, exposure to oscillatory flow (model of turbulent flow) is sufficient to promote endothelial fibronectin expression and deposition (45). However, atherosclerosis-prone regions exposed to turbulent flow show very little fibronectin in the absence of circulating risk factors (50, 135). Other studies, including a model of diabetes, show a more prominent reduction in fibronectin expression and deposition by protective laminar flow than stimulation by oscillatory flow (54, 60). Shear stress stimulates TGFβ mRNA expression as early as 2 h and generates active TGFβ shortly thereafter (131). However, shear stress also activates Smad2/3 phosphorylation within minutes of shear exposure, presumably through transphosphorylation (157), suggesting that shear stress may activate this pathway through both ligand-dependent and ligand-independent mechanisms. However, the role of TGFβ/Smad signaling in shear-induced fibronectin deposition remains undefined.
FIG. 3.
Flow patterns regulate subendothelial matrix remodeling from a native basement membrane (collagen IV, laminin) to a fibronectin-rich provisional matrix. While fibronectin deposition is inhibited by laminar flow, disturbed flow promotes fibronectin deposition, potentially through effects on endothelial integrin activation, transforming growth factor-β (TGFβ) signaling, and fibronectin expression.
Pathologic insults induce rapid and robust changes in both quantity and composition of the vascular matrix, including the provisional matrix proteins, fibronectin, fibrinogen, and thrombospondin. Endothelial cells display an amazing ability to remodel their microenvironment through changes in gene expression, promoting matrix deposition or degradation, and through alterations in the cell's ability to remodel the matrix via changes in matrix receptor activity of cellular contractility. Given its role in early endothelial activation, gaining further insight into early matrix remodeling may unravel novel therapeutic strategies to curb atherosclerosis. However, limiting incorporation of provisional matrix proteins into the vessel wall affects multiple aspects of plaque formation and vascular remodeling. Therefore, while the importance of matrix remodeling during the early stages of atherosclerosis may provide therapeutic benefit, more studies are necessary to understand the role of matrix remodeling in plaque progression or plaque regression.
Shear Stress Sensing and Signaling
The remarkable extent of mechanical stimuli that affect cellular function makes it highly unlikely that a limited repertoire of mechanosensors exists to account for all of these events (134). While organisms have evolved cell-type-specific mechanosensors for certain mechanical stimuli (e.g., hair cells in the inner ear), other mechanical responses, such as sensitivity to tissue stiffness and strain, appear to affect nearly every cell of the body. Endothelial cells express a variety of mechanosensors that account for their unique ability to respond to flow. PECAM-1, VE-cadherin, and the vascular endothelial growth factor receptor 2 (VEGFR2) form a mechanosensory complex that accounts for a significant portion of shear stress-induced responses (122, 175). While endothelial cells are the only cell type to align parallel to flow, expression of PECAM-1, VE-Cadherin, and VEGFR2 allows flow-induced alignment in normally shear-insensitive cells (175). Since shear stress also acts directly on the apical surface, apical proteins and structures are proposed as shear stress mechanosensors, including ion channels, G-protein-coupled receptors (GPCRs), primary cilia, and the endothelial glycocalyx. While the mechanosensory complex and apical mechanosensors endow endothelial cells the capacity to respond to shear stress, mechanosensing through cell–matrix interactions allows the cells to tune these responses according to the surrounding microenvironment.
Force transmission to cell–matrix adhesions
Integrins couple the ECM to the contractile actin cytoskeleton and, as such, serve as mechanosensors for forces born by the matrix, including tissue stiffness and strain (149). This mechanical coupling occurs at large clusters of integrins, termed focal complexes or focal adhesions, and force-sensitive actin-binding proteins such as talin and vinculin allow for rapid reinforcement of the focal adhesion sites in response to changes in either extracellular or intracellular tension (149). Shear stress promotes directional remodeling of focal adhesions (37), and cells under laminar flow show larger tractional forces than cells under disturbed flow (139, 171). However, the forces applied to the endothelial cell surface by physiological shear stress remain orders of magnitude less than the contractile forces exerted by the cell's cytoskeleton, suggesting that shear forces do not likely contribute directly to mechanical tension at focal adhesion sites (89). Rather, several groups have shown that shear stress results in the activation of a pool of inactive low-affinity integrins on the endothelial cell surface (Fig. 4) (80, 174, 190). This activation response, driven by the PECAM-1 mechanosensor, stimulates new integrin–matrix interactions and activates ligation-dependent signaling (132, 175). Therefore, in this system, cell–matrix adhesions serve as mechanosensitive signaling proteins rather than as mechanosensors. Preventing new integrin engagement to the matrix using chemical inhibitors and function-blocking antibodies reduces many shear stress responses (64). However, integrin signaling may affect shear stress mechanosensation indirectly by altering the mechanical stiffness of the cell. Direct application of force to PECAM-1 results in endothelial cell stiffening in cells on fibronectin (29). However, collagen prevents this cytoskeletal stiffening response through a protein kinase A (PKA)-dependent pathway (29, 50). Taken together, the current literature supports a role for integrins as mechanosensitive components of shear stress-induced signaling responses, but not as direct mechanosensors of shear stress.
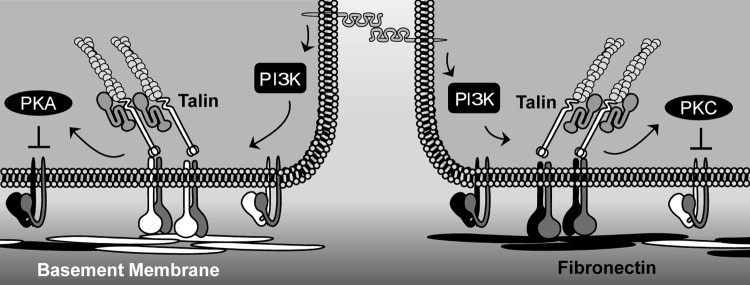
FIG. 4.
Tuning flow-induced integrin activation through transdominant suppression. Shear stress promotes platelet–endothelial cell adhesion molecule-1 (PECAM-1)-dependent integrin activation through phosphoinositide 3-kinase (PI3K) signaling. Ligation of collagen-binding integrins (white) promotes protein kinase A (PKA)-dependent suppression of fibronectin-binding integrins. In contrast, integrin ligation on fibronectin (black) promotes protein kinase Cα (PKCα)-dependent suppression of collagen-binding integrins.
Endothelial integrin activation
Despite the growing literature on integrin activation in the endothelial response to flow, the mechanisms regulating this response remain virtually unknown. Studies on integrin activation mechanisms, primarily performed in leukocytes and platelets, have shown critical roles for the integrin β tail-binding proteins, talin1 and kindlin (kindlin-1, −2, and −3), although the latter may play a larger role in integrin clustering than activation (191, 192). Binding of talin1 to integrin β tails is sufficient to stimulate integrin activation (192), and knockout of the talin1 gene severely impairs integrin activation (121). While the closely related protein, talin2, can compensate for loss of talin1 in some tissues (196), endothelial cells do not express talin2 and loss of talin1 in the endothelium results in rapid embryonic lethality with severe defects in blood vessel formation (120). While the talin1 activation mechanisms are only partially understood, the best model of talin activation involves interaction with the Rap1 effector RIAM (66). Rap1 mediates integrin activation in a variety of conditions and cell types, including endothelial cells (96), and shear stress stimulates Rap1 activity (97). However, the role of Rap1 in shear stress-induced integrin activation has yet to be determined. Induced PECAM-1 clustering stimulates phosphoinositide 3-kinase (PI3K)-dependent integrin activation (24, 199), and PI3K inhibitors blunt shear stress-induced activation of α2β1, α5β1, and αvβ3 (Fig. 4) (132, 175). However, the mechanisms mediating PI3K-dependent integrin activation remain unknown.
Ligation-dependent integrin signaling can also affect inside-out integrin activation, either perpetuating integrin activation or suppressing activation. Integrin signaling in response to mechanical strain promotes PI3K-dependent activation of β1 integrins (88, 169). In contrast, shear-induced ligation of the collagen-binding integrin α2β1 prevents α5β1 and αvβ3 activation, whereas ligation of fibronectin-binding integrins prevents α2β1 activation (132). This transdominant suppression model may amplify signaling by the dominant matrix protein in the context of a mixed matrix by actively blunting the endothelial cell response to the minor matrix components. Shear stress promotes PKA activation in endothelial cells on collagen (132), and inhibiting PKA signaling completely blunts the suppressive effects of the collagen matrix (Fig. 4) (132). In contrast, enhanced flow-induced protein kinase Cα (PKCα) activation on fibronectin prevents α2β1 activation (Fig. 4) (132). Talin1 overexpression relieves this transdominant suppression, suggesting that PKA and PKC affect flow-induced integrin activation at the level of talin1 activation or talin1 interactions with the integrin β subunit tail. Phosphoproteomic analysis of talin1 identified multiple PKA and PKC phosphorylation sites (144), but the relevance of these phosphorylation sites for talin1 function remains unknown. In addition to talin1, PKA also stimulates serine phosphorylation of the β3 tail (58).
Taken together, these data suggest that activation of PECAM-1 mechanosensors in the endothelial cell–cell junction drives integrin activation through a PI3K-dependent pathway. However, the mechanisms mediating PI3K-dependent integrin activation remain unknown. Much of the work on integrin activation mechanisms involves cells typically in suspension, such as leukocytes and platelets. While these models can provide useful information concerning the mechanisms of integrin activation, it is unclear whether other cell types with different protein expression patterns and baseline adhesive states utilize the same pathways to affect integrin activity. Furthermore, the role of integrin activation in the endothelial cell response to flow has yet to be definitively verified in animal models.
Cell–Matrix Interactions in Shear Stress-Induced Endothelial Quiescence
In regions of unidirectional, laminar, or pulsatile flow, endothelial cells exhibit a quiescent phenotype characterized by endothelial alignment with flow, low endothelial turnover, elevated nitric oxide (NO) production, reduced oxidant stress, and limited proinflammatory signaling (64). These regions resist atherosclerotic plaque formation and show reduced responsiveness to systemic proinflammatory stimuli. Laminar flow reduces endothelial cell proliferation and apoptosis in cell culture models, and regions exposed to unidirectional laminar flow in vivo show reduced turnover compared with turbulent flow regions (93). While matrix composition and integrin-specific signaling profoundly affect proliferation and apoptosis in other systems (155), no studies to date have examined the role of integrin-specific signaling on endothelial cell turnover under flow. Therefore, we will focus our discussion on shear-induced protective signaling, gene expression, and alignment.
Protective signaling and gene expression
Prolonged laminar shear stress activates a pattern of endothelial gene expression that promotes endothelial quiescence and limits the endothelial proinflammatory response. Even in the presence of the proinflammatory cytokine, tumor necrosis factor α (TNFα), prolonged laminar flow reduces monocyte adhesion to the endothelium associated with diminished expression of cell adhesion molecules (E-selectin, vascular cell adhesion molecule-1 [VCAM-1] expression) (25) and reduced endothelial apoptosis (42). Several signaling pathways mediate this potent protective response to flow, including activation of endothelial NO synthase (eNOS), elevated kruppel-like factor 2 (KLF2) expression, and reduced oxidant stress.
Shear stress and eNOS regulation
Endothelial cells respond to increased blood flow through the shear stress-dependent production of vasodilators, including NO, prostacyclin I2 (PGI2), and endothelial-derived hyperpolarization factor (EDHF) (162). While PGI2 and EDHF play a role in the flow-mediated dilation responses (162), the production of NO has proven to be a major contributor of shear-dependent vasodilation as well as shear-induced endothelial quiescence. Endothelial dysfunction, a reduced capacity for NO production, in either the coronary or peripheral circulation serves as a strong predictor of clinical events (68). This effect is likely multifaceted as NO has been shown to limit proinflammatory gene expression, prevent platelet aggregation, and suppress vascular smooth muscle cell proliferation and migration (6).
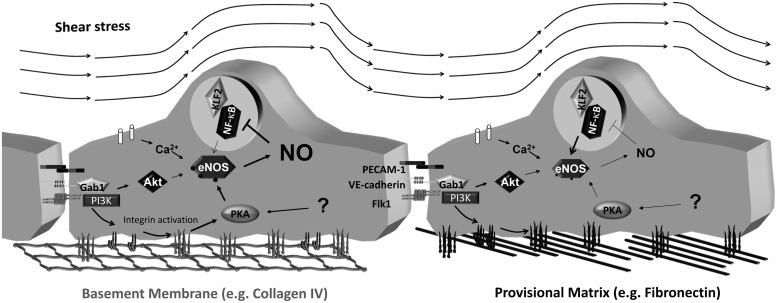
Shear stress activates eNOS in cultured endothelial cells transiently through calcium–calmodulin signaling and chronically through a sustained increase in eNOS phosphorylation (Ser1177, Ser633) and expression (Fig. 5) (48). Multiple pathways regulate flow-induced eNOS phosphorylation, including Akt, PKA, and AMPK (48). VEGF and shear stress-induced eNOS phosphorylation on Ser1177 was originally shown to occur through the Akt pathway (41, 49). While Akt still appears to be the dominant pathway in VEGF-induced eNOS phosphorylation, the role of the Akt pathway in shear stress-induced phosphorylation has been called into question. PKA inhibitors blunt shear stress-induced eNOS phosphorylation on Ser1177, but do not affect eNOS phosphorylation by active Akt (10, 43). The adaptor protein, Gab1, originally shown to mediate shear-induced eNOS phosphorylation through Akt (82), can also stimulate eNOS through PKA (43). Additionally, mutant Gab1 constructs suggest that the ability of Gab1 to mediate Akt activation and eNOS phosphorylation are functionally separate pathways (43).
FIG. 5.
Matrix-specific regulation of endothelial nitric oxide synthase (eNOS) by shear stress. Shear stress results in calcium-dependent eNOS activation and eNOS phosphorylation through PKA and potentially Akt. Enhanced PKA signaling in cells on basement membrane proteins results in a large increase in NO production, whereas the lack of PKA signaling in cells on fibronectin hinders shear-induced NO production. Shear-induced eNOS expression is enhanced on fibronectin, potentially due to reduced negative feedback between NO and the transcription factor, nuclear factor-κB (NF-κB).
A considerable amount of data link cell–matrix interactions with alterations in flow-mediated vasodilation and NO production. Early studies show conflicting results, with the addition of αvβ3 inhibitors stimulating vasodilation in vivo, but blunting flow-mediated dilation in isolated arterioles (116, 125). Blocking antibodies against focal adhesion kinase (FAK), a classic integrin signaling partner, prevents flow-mediated dilation in isolated arterioles, but does not affect vasodilation induced by other agonists (95). Endothelial cells bound to basement membrane proteins show enhanced shear-induced PKA activation and PKA-mediated eNOS phosphorylation compared with cells on fibronectin (Fig. 5) (194). Shear-induced PKA signaling is specifically enhanced in cells on Matrigel (basement membrane-rich matrix), collagen I, and collagen IV compared with cells on laminin, fibronectin, or fibrinogen, suggesting that collagen receptors facilitate flow-induced PKA activation (29, 50). Even in the absence of flow, endothelial cells on collagen IV produce more NO through an integrin-dependent mechanism (59), and collagen glycation, which is known to reduce integrin binding sites, prevents flow-induced eNOS Ser1177 phosphorylation and NO production (90). The mechanisms by which collagen mediates enhanced PKA activation by flow remain unknown. However, signaling through collagen-binding integrins (α2β1, α3β1) enhances PKA activation in other systems through a mechanism involving the phosphatase SHP-1 (92, 130).
In addition to eNOS phosphorylation, matrix composition also affects eNOS expression. Under static conditions, fibronectin reduces endothelial eNOS expression through an α5β1 integrin-dependent pathway (180). However, fibronectin enhances eNOS expression in response to either laminar or oscillatory flow compared with cells on collagen (194), potentially due to a negative feedback between eNOS-dependent NO production and the transcription factor, nuclear factor-κB (NF-κB) (Fig. 5). Shear-induced eNOS expression requires NF-κB activation (62), and collagen promotes shear-induced activation of PKA and eNOS/NO/protein kinase G signaling, which limits NF-κB activation (50, 194).
KLF2 in laminar flow-induced quiescence
One of the most highly upregulated genes in response to protective laminar flow (39), KLF2 expression is sufficient to drive a gene expression pattern, promoting vasodilation (increased eNOS), limiting endothelial migration (decreased VEGFR2, p21-activated kinase-1 [PAK1]), and preventing the endothelial inflammatory responses (decreased intercellular adhesion molecule [ICAM-1], VCAM-1) (39, 94, 156). KLF2's multifaceted anti-inflammatory properties involve reduction of NF-κB-dependent proinflammatory gene expression (156) and suppression of AP1 and TGFβ signaling activation (11, 12, 47). Shear stress stimulates KLF2 expression through extracellular signal-regulated kinase 5 (ERK5)-dependent activation of the transcription factor, myocyte enhancer factor 2 (MEF2), which activates the KLF2 promoter (137). However, the mechanisms by which shear stress activates ERK5 and its upstream kinase MEK5 are not completely clear.
While the data linking cell–matrix interactions to KLF2 expression are limited, several lines of evidence suggest a functional association. KLF2 overexpression enhances RhoA signaling and endothelial actin stress fiber formation (12), suggesting that KLF2 may promote integrin signaling through enhanced clustering and cytoskeletal tension. Matrix composition does not affect laminar flow-induced KLF2 expression or inhibition of TNFα-induced inflammation (106). However, enhanced β1 integrin signaling in zebrafish and endothelial cell culture models promotes KLF2 expression (145). Depletion of the proteoglycan syndecan 4, which contributes to both cell–matrix interactions and the glycocalyx, reduces both laminar flow-induced alignment and KLF2 expression associated with enhanced NF-κB activation and atypical atherosclerotic plaque formation in protected vascular regions (2). However, this study did not address the mechanisms by which syndecan 4 mediates these protective effects or the pool of syndecan 4 involved.
Laminar flow and NF-E2-related factor 2-driven antioxidant gene expression
Oxidant stress affects multiple aspects of endothelial function, stimulating endothelial turnover, permeability, and proinflammatory gene expression, and promoting endothelial dysfunction through NO scavenging (51). Atheroprotected regions show reduced oxidant stress and reduced susceptibility to oxidant stress in response to systemic proinflammatory stimuli (147). Laminar shear stress alters the endothelial redox state through NF-E2-related factor 2 (Nrf2) signaling (35). Laminar flow upregulates Nrf2-dependent antioxidant gene expression (e.g., heme oxygenase-1, peroxiredoxin) (35, 183), which is further enhanced by the interaction between Nrf2 and KLF2 (47). Laminar flow-induced alterations in the intracellular redox state require Nrf2 signaling (35), and Nrf2 depletion enhances shear-induced proinflammatory gene expression (167).
The inhibitory protein, Keap1, which maintains Nrf2 in the cytosol, contains several reactive cysteines sensitive to oxidant stress or electrophiles (61). Shear-induced Nrf2 activation involves oxidant stress (183) and cyclooxygenase-2 (COX-2) signaling, which drives the production of electrophilic oxoderivatives from arachidonic acid (61). Laminar flow induces COX-2 expression and activity, and inhibiting COX-2 blunts Nrf2 target gene expression (40). Fibronectin deposition into the matrix promotes endothelial COX-2 expression (179), and ligation of the fibronectin-binding integrin αvβ3 stimulates both COX-2 expression and activity in endothelial cells in culture (127). This association between fibronectin and anti-inflammatory COX-2 expression appears paradoxical. However, like eNOS, NF-κB promotes COX-2 expression (198), suggesting that inflammation contributes to this antioxidant response as a negative feedback pathway. Consistent with this idea, regions of the porcine aorta exposed to turbulent flow show both proinflammatory and antioxidant gene expression (138). Since oxidant stress promotes endothelial α5 and αv integrin expression (98), the Nrf2-driven antioxidant response may provide negative feedback for provisional matrix-binding integrin signaling at atheroprone sites and limit expression of these integrins in protected regions.
Endothelial cell alignment
Exposure to unidirectional flow stimulates endothelial elongation parallel with flow direction and cytoskeletal alignment in the direction of flow occurring gradually over several hours to days (30). These cytoskeletal alterations have several beneficial effects, most notably limiting further mechanical stress due to the shear forces (3). Mimicking shear-induced alignment with micropatterned substrates also shows a mild but significant reduction in the expression of proinflammatory adhesion molecules (ICAM-1, VCAM-1) and chemokines (monocyte chemotactic protein-1 [MCP-1]) and a reduction in monocyte adhesion (74). However, this effect is much weaker than that seen with prolonged unidirectional flow.
Cell–matrix interactions regulate endothelial cell alignment to flow through effects on the Rho family of small GTPases and actin cytoskeletal dynamics. Shear stress stimulates the polarized activation of Rac1 and cdc42 preferentially in the downstream side of the cell (173, 176), whereas shear induces a transient inactivation of RhoA resulting in the disassembly of actin stress fibers (174). All of these signaling responses require new integrin–matrix interaction and all are required for proper endothelial cell alignment with flow (173, 174, 176). RhoA inactivation involves integrin-dependent p190RhoGAP signaling in response to shear (189). While the guanine nucleotide exchange factors (GEFs) coupling shear-induced integrin activation to cdc42 are unknown, shear stress-induced Rac activity requires the GEF Vav2 (104). Both cell–cell interactions and cell–matrix interactions can activate Vav2, and the relative roles they play in shear-induced Vav2 activation are unclear (8, 110). Interestingly, Vav2 is only required for shear-induced Rac GTP loading, whereas another Rac GEF, Tiam1, mediates the spatial localization of active Rac to the downstream side of the cell (104). This localization requires the interaction of Tiam1 with VE-cadherin, but did not require Tiam1 GEF activity, suggesting that Tiam1 plays predominantly a scaffolding function in this regard.
While laminar flow provides many protective effects to endothelial cells that drive a quiescent phenotype, the role of integrin signaling in this protective response is not well characterized. Endothelial cells play a clear role in the endothelial alignment response to flow, but the relevance of this pathway in the chronic response to flow in the adult is unclear. A majority of the protective properties of laminar flow are attributed to its ability to increase KLF2 expression, and several studies suggest that integrin signaling can affect KLF2 expression. However, integrins and cell–matrix interactions do not appear to play a major role in flow-induced KLF2 expression. Laminar flow stimulates KLF2-dependent eNOS expression and NO production, and NO maintains endothelial quiescence, in part, by reducing NF-κB activation and proinflammatory gene expression. Cell–matrix interactions appear to affect endothelial eNOS expression; however, the mechanisms underlying this regulation remain unclear. Furthermore, cell–matrix interactions affect flow-induced eNOS phosphorylation and NO production in cell culture models. However, the role in NO regulation in vivo remains virtually unexplored.
Integrin Signaling in Shear Stress-Induced Endothelial Activation
In early atherosclerosis, endothelial cells transition from a quiescent state to a state of endothelial activation, characterized by enhanced proinflammatory gene expression and reduced barrier function. Unlike the protective effects of laminar flow, turbulent or oscillatory flow promotes the expression of leukocyte-binding cell adhesion molecules (E-selectin, ICAM-1, VCAM-1) (18). Consistent with in vitro studies, ICAM-1 and VCAM-1 expression preferentially localizes to atheroprone sites exposed to turbulent flow in both mice and humans (34). In addition, vascular regions experiencing turbulent flow show enhanced endothelial permeability compared with protected, laminar flow regions (69), and exposing endothelial cells to atheroprone flow patterns shows a similar enhancement in permeability compared with atheroprotective flow patterns (136). These deleterious effects of turbulent shear stress involve several integrin-dependent signaling pathways, including the induction of oxidant stress, enhanced endothelial permeability, and activation of proinflammatory signaling pathways, including NF-κB.
Oxidant stress
Oxidant stress promotes several of the deleterious responses associated with shear-induced endothelial activation. Cellular redox status governs both proinflammatory gene expression (123) and endothelial barrier function (13). Furthermore, oxidative stress at sites of turbulent flow actively represses endothelial-protective pathways. Oxidant stress stimulates the addition of a small ubiquitin-like modifier (SUMO) on ERK5, inhibiting its transactivator function and reducing KLF2 expression (186). Both protein kinase C ζ (PKCζ) and the redox-sensitive kinase, p90 ribosomal S6 kinase (p90RSK), show enhanced activation in regions of turbulent flow (99, 108) and both phosphorylate ERK5 inducing its SUMOylation (99, 129).
Shear stress increases endothelial reactive oxygen species (ROS) production, including superoxide (O2•−), hydrogen peroxide (H2O2), and peroxynitrite (ONOO•−) in vitro (83), through the small GTPase Rac and the NADPH oxidase (NOX) complex (83, 193). While new integrin–matrix interactions critically regulate Rac activation by flow (173), shear stress promotes similar Rac activation and ROS production regardless of matrix composition, suggesting that this pathway is conserved among the different endothelial cell integrins (50). Rac activates NOX1/2/3 by binding to an NOX subunit (p67phox for NOX2), and dominant-negative Rac expression prevents flow-induced oxidant stress (173, 193). However, scaffold proteins appear to regulate the formation of this complex since shear-induced binding between active Rac and NOX2 requires the scaffolding functions of Tiam1 (104). Oscillatory shear stress promotes NOX2 and NOX4 expression, as well as NOX-dependent O2− production (75), while prolonged laminar flow downregulates the NOX genes (75). In addition to integrins, oscillatory flow-induced NOX activation and monocyte binding also involve enhanced BMP4 expression (159). However, current data are conflicting as to the role of ligand-dependent or ligand-independent activation of BMP signaling by oscillatory flow (17, 202). Oscillatory flow-induced associations between the BMPRII receptor and αvβ3 integrins suggest one potential mechanism of ligand-independent signaling (201). However, oscillatory flow downregulates the BMPRII receptor, and depletion of BMPRII enhances oxidant stress in a ligand-independent manner (91), calling into question the utility of this ligand-independent mechanism. Since BMP4 has not been shown to activate Rac, BMP4 and integrin signaling may coordinately regulate NOX activation in oscillatory flow by upregulating NOX and activating NOX, respectively.
Enhanced permeability
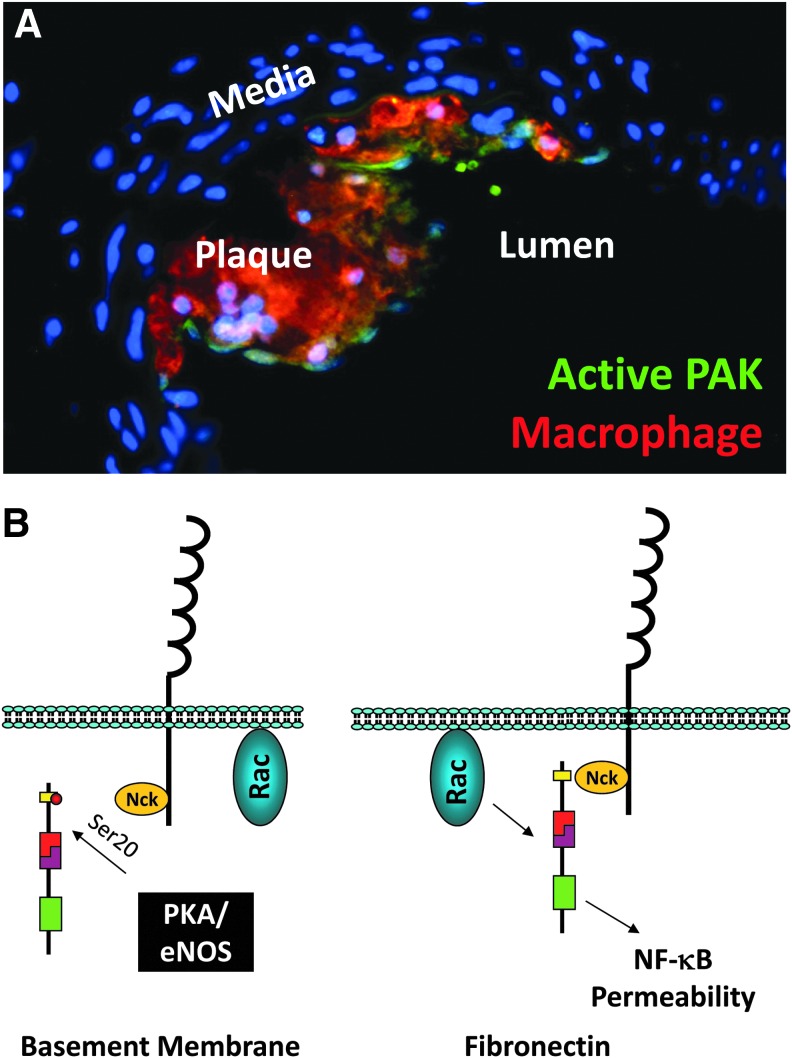
Endothelial cell–cell junctions, including adherens junctions and particularly tight junctions, establish a semipermeable barrier preventing the contents of the bloodstream from leaking into the interstitial tissue. In atherosclerosis, endothelial permeability is thought to contribute to the local accumulation of LDL cholesterol at sites of turbulent flow (152). Acute shear stress and chronic oscillatory shear stress promote endothelial permeability, whereas prolonged laminar shear stress reduces endothelial permeability associated with enhanced expression of junctional components, VE-cadherin, occludin, and ZO-1 (27, 113, 136, 184). Fibronectin in the subendothelial matrix enhances shear stress-induced permeability, compared with cells on Matrigel or collagen IV alone, associated with the induction of paracellular pores (136). Like oxidant stress, shear stress promotes integrin-dependent activation of the Rac effector PAK, a family of Ser/Thr kinases (136). Regions of turbulent flow in vivo show enhanced PAK activation associated with early plaque formation, whereas PAK activation is absent in vessel regions negative for atherosclerosis (Fig. 6A). Active PAK localizes to the cell–cell junctions through interactions with the adapter protein Nck, and blocking the PAK-Nck interaction prevents shear stress-induced paracellular pore formation, oscillatory flow-induced endothelial permeability, and endothelial permeability at sites of turbulent flow in vivo (136). While all matrices support shear stress-induced Rac activation, basement membrane proteins inhibit PAK activation through a PKA/eNOS-dependent pathway (Fig. 6B) (50, 194). However, it should be noted that PAK signaling promotes microvascular endothelial cell permeability at multiple sites, including the lung, suggesting that these pathways may not be universally employed in all vascular beds (7).
FIG. 6.
Regulation of p21 activated kinase (PAK) activation in atherosclerosis. (A) Immunohistochemical staining for active p21-activated kinase (PAK) (phospho-Ser141; green) in an early atherosclerotic plaque isolated from ApoE knockout atherosclerosis-prone mice. Macrophage staining (Mac2, red) shows the focal nature of PAK activation to early plaque sites. (B) Schematic of PAK regulation by Nck-mediated membrane targeting and its inhibition by PKA/eNOS-dependent PAK phosphorylation at an inhibitory (Ser20) site.
NF-κB activation
The NF-κB family of hetero- and homodimeric transcription factors drives proinflammatory gene expression in response to multiple stimuli (31). Shear stress has long been known to promote NF-κB signaling, with NF-κB binding to the original shear stress-responsive element identified in platelet-derived growth factorβ, ICAM-1, and VCAM-1 (118, 146), and sites of disturbed flow in vivo show both enhanced expression and activation of NF-κB (65). NF-κB is a redox-sensitive transcription factor, and shear stress-induced ROS production critically regulates NF-κB-driven proinflammatory gene expression (26). Inhibitory inhibitor of κB (IκB) proteins typically sequester NF-κB dimers in the cytosol until their phosphorylation by IκB kinases (IKKα/β) stimulates their ubiquitination and degradation (57). Shear stress-induced activation of IKKβ, the primary upstream mediator, requires both integrins and ROS production (5, 117), but the mechanisms linking these pathways to IKKβ signaling remain largely unexplored.
Cell–matrix interactions play an important role in shear stress-induced NF-κB activation. While endothelial cells on provisional matrix proteins show strong NF-κB activation, cells on basement membrane proteins do not, suggesting that cell–matrix interactions with the provisional matrix mediate this pathway (135). Endothelial cells interact with the provisional matrix through the integrins, α5β1, αvβ3, and αvβ5. However, only deletion of αv or β3 reduces shear stress-induced NF-κB activation, suggesting that specific signaling through this integrin activates this pathway (20). Mice deficient for αv in the endothelium show reduced oscillatory flow-associated proinflammatory gene expression and reduced atherosclerotic plaque formation (20). Similarly, treatment with an inhibitor of αv integrins, the RGD-mimetic S247, prevents both oscillatory flow-induced inflammation and early plaque formation, whereas the α5 signaling inhibitor, ATN-161, does not (20). Several integrin-binding proteins function to mediate shear stress-induced NF-κB activation, including FAK and Shc (105, 140). Inhibition of either FAK or Shc blunts shear stress-dependent proinflammatory gene expression (ICAM-1, VCAM-1); however, the mechanisms employed appear to differ. FAK mediates the shear stress-induced NF-κB phosphorylation in its transactivation domain required for maximal transcriptional activity, but does not affect shear stress-induced NF-κB nuclear translocation (140). FAK deletion does not affect shear stress-induced Rac activation, ROS production, or IκB degradation, consistent with a lack of FAK-dependent IKK regulation in this system. In contrast, Shc mediates NF-κB nuclear translocation, but does not appear to significantly affect shear-induced NF-κB phosphorylation (105), suggesting that integrins may utilize multiple parallel pathways to stimulate maximal NF-κB activity.
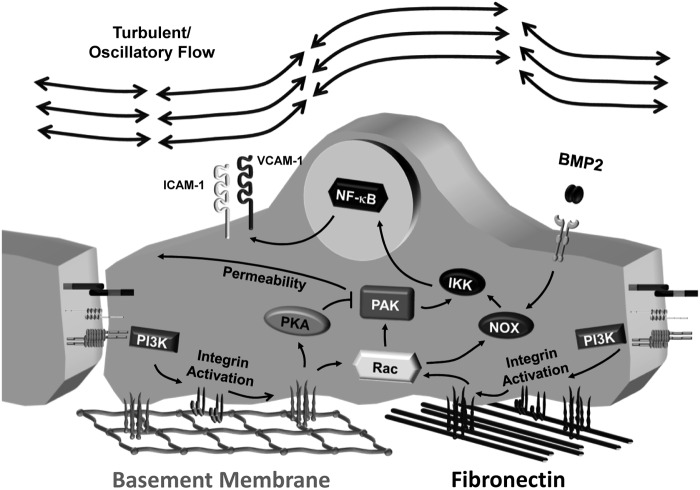
Matrix-specific PAK signaling also regulates shear stress-dependent proinflammatory signaling through NF-κB and c-jun N-terminal kinase (JNK) in conjunction with ROS (21, 133). In cells on basement membrane proteins, shear stress promotes Rac activation and ROS production without subsequent NF-κB activation or proinflammatory gene expression, suggesting that ROS production is insufficient for NF-κB activation in this system (Fig. 7) (50). Rather, ROS-dependent NF-κB activation requires PAK signaling as PAK inhibitors blunt NF-κB activation by shear stress, exogenous H2O2, and endogenous oxidant stress (21, 133). Oxidant stress promotes PAK activation by stimulating PAK-Nck interactions and enhancing Nck-dependent PAK membrane targeting (21). Preventing PAK activation reduces NF-κB activation and proinflammatory gene expression at sites of turbulent flow in vivo (81, 133). Basement membrane proteins uncouple this pathway by inhibiting PAK-Nck interactions through a PKA/eNOS-dependent mechanism (Fig. 6B) (50, 194). As such, inhibiting PKA or eNOS enhances shear stress-induced PAK activation, NF-κB activation, and proinflammatory gene expression in response to flow in cells on basement membrane proteins (50, 194).
FIG. 7.
Matrix-specific endothelial activation by oscillatory flow. Endothelial cells on the fibronectin-rich provisional matrix show enhanced PAK-dependent NF-κB activation, proinflammatory gene expression, and permeability in response to oscillatory flow. Oscillatory flow activates PKA in endothelial cells attached to basement membrane proteins, leading to PAK suppression. Both matrices potentiate shear-induced Rac activation and NADPH oxidase (NOX)-dependent reactive oxygen species (ROS) production, potentially in concert with secreted bone morphogenic protein 2 (BMP2).
Cell–matrix interactions regulate multiple aspects of shear stress-induced endothelial cell activation, which may explain the difficulty in correlating disturbed flow conditions observed in vivo with the endothelial activation response to models of disturbed flow in vitro. Endothelial cells in culture rapidly deposit a fibronectin matrix and are exposed to the provisional matrix proteins abundant in serum. Therefore, most models of disturbed flow utilize cell culture conditions that mimic the matrix observed during early endothelial activation. However, regions of disturbed flow in vivo do not show strong endothelial activation in the absence of systemic atherogenic risk factors such as elevated cholesterol. This may be due to protective signaling produced by the endogenous endothelial basement membrane to limit shear-induced endothelial activation. Shear stress stimulates signaling through the fibronectin-binding integrins, αvβ3 and α5β1 (132, 174), and signaling through αvβ3 critically regulates shear stress-induced activation of PAK and NF-κB (20), critical pathways regulating endothelial permeability and proinflammatory gene expression. While the role of α5β1 in the endothelial response to flow remains unknown, its ability to mediate fibronectin fibrillogenesis and the endothelial response to oxidized LDL suggest that α5β1 may play a unique but equally important role in early atherosclerotic remodeling (53, 195).
JNK activation
Signaling through the JNK pathway also contributes to flow-induced endothelial cell activation. Transient activation of JNK in response to laminar flow promotes endothelial cell alignment in the direction of flow (63, 112), whereas chronic activation of JNK in response to disturbed flow contributes to endothelial cell inflammation, apoptosis, and autophagy. JNK activation at atherosclerosis-prone sites mediates enhanced NF-κB expression, VCAM-1 expression, and monocyte recruitment (33, 182). Inhibiting JNK signaling limits expression of the proapoptotic proteins, RIP1 and caspase 3, in endothelial cells in culture, and JNK1 deletion reduces apoptosis at atherosclerosis-prone sites associated with reduced caspase 3 expression (19). Oscillatory flow-induced JNK signaling promotes mitochondrial superoxide production and initiation of autophagy (102). Mitochondrial targeting of active JNK through the scaffolding protein, Sab, significantly enhances complex I-induced superoxide production, reduces mitochondrial respiration, and enhances mitochondrial DNA damage (16). Whereas autophagy limits mitochondrial oxidative stress by depleting damaged mitochondria, oscillatory flow results in impaired autophagic flux by preventing the formation of the mature autophagosome (102).
Like NF-κB, shear stress-induced JNK signaling requires new integrin–matrix interactions and displays matrix-specific activation, with flow-induced JNK activation enhanced on fibronectin compared with basement membrane proteins (63, 80). Activation of the upstream kinase, MKK4, shows a similar matrix-specific activation profile, and inhibiting MKK4 completely suppresses flow-induced JNK activation (63). Both the NAPDH oxidase inhibitor, apocyanin, and the nonspecific antioxidant, N-acetylcysteine, inhibit JNK activation by oscillatory flow, suggesting a role for oxidative stress (166). However, oxidant stress classically promotes JNK signaling through the redox-sensitive kinase, apoptosis signal-related kinase 1 (ASK1), and only inhibition of ASK1 by laminar flow has been described to date (188). Instead, matrix-specific PAK signaling drives flow-induced JNK activation both in endothelial cells exposed to transient laminar flow and to chronic oscillatory flow. Consistent with PAK-dependent JNK signaling, inhibiting PAK signaling with the PAK-Nck blocking peptide blunts JNK activation at atherosclerosis-prone sites in vivo (63). Therefore, oxidant stress and matrix-specific PAK activation drive both JNK and NF-κB activation in response to oscillatory flow.
Cell–Matrix Interactions in Shear-Mediated Regulation of Angiogenesis and Arteriogenesis
Cardiovascular disease often results in poor perfusion of target tissue, and vascular remodeling to regain adequate perfusion tissue perfusion requires both angiogenesis, the growth of new vessels from preexisting vessels, and arteriogenesis, an increase in vessel diameter associated with hemodynamic stress (158). Angiogenesis can occur through sprouting of a new vessel branch from a preexisting vessel or by intussusception, the splitting of preexisting vessels. Endothelial cell sprouting occurs in a subpopulation of endothelial cells, termed tip cells, which produce matrix-degrading enzymes and show filopodia-like extensions into the matrix, while the stalk cells behind them proliferate to drive sprout extension (142). Local VEGF gradients drive tip cell formation through alterations in the Notch pathway. VEGF promotes the expression of the Notch ligand delta-like ligand 4 (Dll4) in tip cells, activating Notch in the adjacent cells, resulting in cleavage of the Notch intracellular domain (142). The Notch intracellular domain translocates into the nucleus to affect target gene expression, including the downregulation of VEGFRs and Dll4 (164). The Dll4-positive tip cells invade the matrix, while Notch signaling in the adjacent stalk cells prevents filopodia formation and promotes proliferation. In contrast to sprouting, intussusceptive angiogenesis occurs through the formation of tissue pillars in the vessel lumen, splitting the vessel into two distinct vessels in response to increased blood flow (177). Arteriogenesis plays a major role in tissue reperfusion responses following vessel blockage as increased blood flow through collateral vessels stimulates their remodeling (158). Both shear stress and cell–matrix interactions play well-described roles in angiogenesis that have been reviewed extensively elsewhere (72, 185). In this section, we discuss how these environmental signals function together to regulate vascular remodeling responses.
Sprouting angiogenesis
While the direct role of integrin signaling in shear stress regulation of angiogenesis has not been addressed, shear stress and integrin signaling both regulate similar pathways in angiogenic endothelium. Sprouting primarily occurs in postcapillary venues, with shear ranging from 1 to 6 dynes/cm2. During capillary plexus remodeling, where shear stress forces are similar (84), onset of flow drives Notch1-dependent expression of arterial marker genes, such as ephrinB2 and Dll4 (79). Activation of Notch may affect vascular cell integrin function as well. Constitutively active Notch4 stimulates β1 integrin activation, enhances adhesion to collagen, and reduces angiogenesis (101), while the cleaved Notch-1 cytoplasmic tail is sufficient to stimulate α5β1 integrin activation (71). Like other shear stress responses, the composition of the subendothelial matrix significantly affects Notch pathway activation. Collagen I and IV inhibit Notch signaling by binding to Notch and Jagged (197). In contrast, laminin-binding integrins, particularly those engaged by laminin-411, promote Dll4 expression and Notch signaling in endothelial cells (44). Consistent with this, laminin-411 knockout mice show enhanced tip formation and a hypersprouting phenotype (160).
Moderate levels of shear stress (∼5 dynes/cm2) regulate matrix proteolysis during sprouting angiogenesis, enhancing the expression of matrix metalloproteinases (MMPs), including MT1-MMP and MMP2 (114, 150). The effect of shear on MMP expression and endothelial invasion occurs maximally in the presence of soluble sphingosine-1-phosphate (S1P) (87). Interestingly, deletion of the S1P receptor 1 (S1PR1) causes defects in vascularization with hypersprouting, vessel dilation, and increased branching, whereas overexpressing S1PR1 shows reduced tip cell formation (52, 85). Furthermore, S1PR1 knockout endothelial cells show diminished shear stress-induced signaling and alignment; however, it is unclear whether S1PR1 is activated by shear stress or if S1PR1 deletion causes significant changes in the shear stress sensors in the adherens junctions (85).
Intussusception angiogenesis
The mechanisms regulating intussusceptive angiogenesis remain largely unknown, and the role of cell–matrix interactions in intussusception has yet to be explored. However, shear stress profoundly affects intussusceptive angiogenesis. High flow-induced angiogenesis in muscle appears to occur primarily through intussusception (14). Enhanced capillary shear stress following vasodilation (VEGF, Prazosin) stimulates intussusceptive angiogenesis in skeletal muscle (56, 200), and both eNOS deletion and treatment with the eNOS inhibitor, L-NAME, reduce angiogenesis (4). Interestingly, while increased blood flow promotes intussusceptive angiogenesis, analysis of capillary plexus remodeling suggests that intussusceptive angiogenesis occurs in regions exposed to turbulent flow induced by elevated blood flow through the vascular network (177).
Arteriogenesis and collateralization
Increased blood flow through collateral arterioles following vessel occlusion drives arteriogenesis through proliferative outward vessel remodeling. While this enhanced flow increases vessel strain immediately, multiple lines of evidence suggest an important role for shear stress in arteriogenic remodeling. Creating a sustained increase in shear stress in the collateral circulation by anastomosis of the femoral artery with the femoral vein significantly enhances collateralization while preventing the increase in postocclusive pressure (141). Mice deficient for PECAM-1, a vital shear stress sensor, show reduced arteriogenesis (22), whereas PECAM-1 does not affect endothelial activation in response to stretch. In addition, despite the rapid increase in vessel strain, smooth muscle proliferation does not occur for several days, potentially due to the antiproliferative effects of the endogenous matrix (119).
Endothelial cells show enhanced activation (ICAM-1, VCAM-1 expression) during arteriogenesis (100, 141), and preventing this activation by inhibiting NF-κB or ICAM-1 significantly reduces arteriogenic remodeling (73, 172). Fibronectin deposition and endothelial cell integrins contribute to flow-induced inflammation in models of early atherogenesis (135, 148, 195), but their role in shear stress-induced inflammation during arteriogenesis remains unknown. While the endothelium in normal vessels shows low levels of fibronectin, α5β1, and αvβ3, all three proteins show elevated expression in developing collaterals, both in the endothelium and smooth muscle cells (15, 46). Developing collaterals similarly show enhanced activation of the integrin signaling partners, FAK (15, 46) and Shc (165). Shear stress induces Shc recruitment to integrin β subunit tails (80), and mice lacking Shc specifically in the endothelium undergo diminished collateral luminal expansion associated with reduced endothelial proliferation, NF-κB activation, and VCAM-1 expression (165).
Clinical Perspectives
Although targeting cell–matrix interactions may be useful to modulate vascular remodeling responses, integrin-based therapeutics have a long and often disappointing history. These inhibitors typically take the form of cyclic peptides, inhibitory antibodies, or small-molecule inhibitors (115). While several αIIbβ3 and α4β1-based therapeutics have made their way into the clinic, therapeutics targeting the provisional matrix-binding integrins in cardiovascular disease have had significantly less success. Both αvβ3 and α5β1 inhibitors have shown considerable promise in mouse models of angiogenesis, but these inhibitors have almost universally failed to show a therapeutic benefit in patients. Integrin inhibition may prove difficult to maintain over the time scales studied in human disease compared with those observed in mice. Acute deletion of endothelial β3 integrins blunts tumor-associated angiogenesis, whereas chronic deletion does not (161), suggesting that prolonged inhibition caused compensatory alterations in the mechanisms employed. Additionally, acute β3 deletion only reduced angiogenesis and tumorigenesis in a preventative manner, whereas deletion in already established tumors proved ineffective (161). Another potential reason integrin ligation inhibitors have failed could be the activation state of the integrin being targeted. Platelets and leukocytes generally maintain αIIbβ3 and α4β1 in an inactive state (76), suggesting that the inhibitor may bind the ligand binding site before relevant stimulation of integrin function. Adherent cells maintain both an active and inactive pool of integrins, and some integrin activation may occur intracellularly before surface presentation, making targeting the ligand binding site in these integrins difficult. Alternative methods to prevent integrin signaling could also be explored, such as allosteric integrin inhibitors, which reduce preformed integrin–ligand complexes in contrast to inhibitors that target the ligand binding site (124). In addition, targeting the molecular mechanisms mediating integrin-dependent effects may functionally separate integrin effects on endothelial dysfunction and proinflammatory activation from those regulating cytoskeletal remodeling and angiogenesis. While several recent proteomic and phosphoproteomic analyses of integrin signaling components may provide new directions in this regard, we will have much to learn concerning how integrins initiate and regulate local signaling responses and how these converge with other environmental signals.
Conclusions
The current data suggest a complex and dynamic reciprocal relationship between shear stress and the endothelial cell matrix. Shear stress patterns regulate the local susceptibility to atherosclerosis through effects on endothelial activation. However, the remodeling of the local vascular matrix, a function of both systemic atherosclerotic risk factors and local hemodynamics, plays a similar role in tuning the endothelial cell response to shear stress. The role of matrix remodeling in the endothelial response to laminar flow is not entirely clear, although the current data suggest against a large role for matrix remodeling in the regulation of laminar flow's protective properties (106). This finding is consistent with data suggesting that laminar flow itself prevents provisional matrix deposition, which would limit the impact of provisional matrix deposition on the subsequent endothelial response to laminar flow (54, 60). However, the endothelial cell response to acute changes in flow and to models of chronic oscillatory flow suggests that matrix composition plays a vital role in regulating this response. In particular, signaling through the endothelial integrin αvβ3 appears to play a critical role in shear stress-induced NF-κB activation as well as activation of the PAK pathway critical for shear stress-induced endothelial permeability (20). While the provisional matrix-binding integrins appear to promote endothelial activation in models of early atherosclerosis, their role in shear stress-associated angiogenesis and arteriogenesis responses remains unknown.
Genome-wide association studies underscore the importance of the vascular matrix to cardiovascular disease, with both the ECM and cell–matrix interactions associating strongly with human coronary artery disease (55). While we have an emerging understanding of how cell–matrix interactions modulate endothelial phenotype, beyond their classic roles in proliferation and migration, it remains unclear whether these mechanisms represent feasible therapeutic targets for cardiovascular disease. Additionally, pathological situations such as atherosclerosis may employ cell–matrix interactions for contrasting roles, promoting both plaque inflammation and plaque stability through integrin-specific pathways while enhancing angiogenesis and arteriogenesis to limit the effects of atherosclerotic disease on target tissue. Future work elucidating the molecular mechanisms underlying these conflicting properties of the matrix may provide novel targets to alleviate chronic proinflammatory signaling responses while allowing tissue remodeling to deal with changes in mechanical and metabolic stress.
Abbreviations Used
- Alk1
activin receptor-like kinase 1
- ASK1
apoptosis signal-related kinase 1
- BMP
bone morphogenic protein
- COX-2
cyclooxygenase-2
- Dll1
delta-like ligand 1
- Dll4
delta-like ligand 4
- ECM
extracellular matrix
- EDA
extradomain A
- EDHF
endothelial-derived hyperpolarization factor
- EndoHT
endothelial-to-hematopoietic transition
- EndoMT
endothelial-to-mesenchymal transition
- eNOS
endothelial nitric oxide synthase
- ERK5
extracellular signal-regulated kinase 5
- FAK
focal adhesion kinase
- GEF
guanine nucleotide exchange factor
- GPCR
G-protein-coupled receptor
- H2O2
hydrogen peroxide
- ICAM-1
intercellular adhesion molecule-1
- IKK
IκB kinase
- IκB
inhibitor of κB
- JNK
c-jun N-terminal kinase
- KLF2
kruppel-like factor 2
- LAP
latency-associated peptide
- LDL
low-density lipoproteins
- LTBP
latent TGFβ-binding protein
- MCP-1
monocyte chemotactic protein-1
- MEF2
myocyte enhancer factor 2
- MMP
matrix metalloproteinase
- NF-κB
nuclear factor-κB
- NO
nitric oxide
- NOX
NADPH oxidase
- Nrf2
NF-E2-related factor 2
- O2•−
superoxide
- ONOO•−
peroxynitrite
- p90RSK
p90 ribosomal S6 kinase
- PAK
p21-activated kinase
- PECAM-1
platelet–endothelial cell adhesion molecule-1
- PGI2
prostacyclin I2
- PI3K
phosphoinositide 3-kinase
- PKA
protein kinase A
- PKCα
protein kinase Cα
- PKCζ
protein kinase C ζ
- ROS
reactive oxygen species
- S1P
sphingosine-1-phosphate
- S1PR1
S1P receptor 1
- SUMO
small ubiquitin-like modifier
- TGFβ
transforming growth factor-β
- TNFα
tumor necrosis factor α
- VCAM-1
vascular cell adhesion molecule-1
- VE-cadherin
vascular endothelial-cadherin
- VEGF
vascular endothelial growth factor
- VEGFR
VEGF receptor
References
- 1.Babaev VR, Porro F, Linton MF, Fazio S, Baralle FE, and Muro AF. Absence of regulated splicing of fibronectin EDA exon reduces atherosclerosis in mice. Atherosclerosis 197: 534–540, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baeyens N, Mulligan-Kehoe MJ, Corti F, Simon DD, Ross TD, Rhodes JM, Wang TZ, Mejean CO, Simons M, Humphrey J, and Schwartz MA. Syndecan 4 is required for endothelial alignment in flow and atheroprotective signaling. Proc Natl Acad Sci U S A 111: 17308–17313, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbee KA, Davies PF, and Lal R. Shear stress-induced reorganization of the surface topography of living endothelial cells imaged by atomic force microscopy. Circ Res 74: 163–171, 1994 [DOI] [PubMed] [Google Scholar]
- 4.Baum O, Da Silva-Azevedo L, Willerding G, Wockel A, Planitzer G, Gossrau R, Pries AR, and Zakrzewicz A. Endothelial NOS is main mediator for shear stress-dependent angiogenesis in skeletal muscle after prazosin administration. Am J Physiol Heart Circ Physiol 287: H2300–H2308, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Bhullar IS, Li YS, Miao H, Zandi E, Kim M, Shyy JY, and Chien S. Fluid shear stress activation of IkappaB kinase is integrin-dependent. J Biol Chem 273: 30544–30549, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Bian K, Doursout MF, and Murad F. Vascular system: role of nitric oxide in cardiovascular diseases. J Clin Hypertens (Greenwich) 10: 304–310, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birukova AA, Alekseeva E, Cokic I, Turner CE, and Birukov KG. Cross talk between paxillin and Rac is critical for mediation of barrier-protective effects by oxidized phospholipids. Am J Physiol Lung Cell Mol Physiol 295: L593–L602, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birukova AA, Tian Y, Dubrovskyi O, Zebda N, Sarich N, Tian X, Wang Y, and Birukov KG. VE-cadherin trans-interactions modulate Rac activation and enhancement of lung endothelial barrier by iloprost. J Cell Physiol 227: 3405–3416, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bissell MJ. and Aggeler J. Dynamic reciprocity: how do extracellular matrix and hormones direct gene expression? Prog Clin Biol Res 249: 251–262, 1987 [PubMed] [Google Scholar]
- 10.Boo YC, Sorescu G, Boyd N, Shiojima I, Walsh K, Du J, and Jo H. Shear stress stimulates phosphorylation of endothelial nitric-oxide synthase at Ser1179 by Akt-independent mechanisms: role of protein kinase A. J Biol Chem 277: 3388–3396, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Boon RA, Fledderus JO, Volger OL, van Wanrooij EJ, Pardali E, Weesie F, Kuiper J, Pannekoek H, ten Dijke P, and Horrevoets AJ. KLF2 suppresses TGF-beta signaling in endothelium through induction of Smad7 and inhibition of AP-1. Arterioscler Thromb Vasc Biol 27: 532–539, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Boon RA, Leyen TA, Fontijn RD, Fledderus JO, Baggen JM, Volger OL, van Nieuw Amerongen GP, and Horrevoets AJ. KLF2-induced actin shear fibers control both alignment to flow and JNK signaling in vascular endothelium. Blood 115: 2533–2542, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Boueiz A. and Hassoun PM. Regulation of endothelial barrier function by reactive oxygen and nitrogen species. Microvasc Res 77: 26–34, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Brown MD. and Hudlicka O. Modulation of physiological angiogenesis in skeletal muscle by mechanical forces: involvement of VEGF and metalloproteinases. Angiogenesis 6: 1–14, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Cai WJ, Li MB, Wu X, Wu S, Zhu W, Chen D, Luo M, Eitenmuller I, Kampmann A, Schaper J, and Schaper W. Activation of the integrins alpha 5beta 1 and alpha v beta 3 and focal adhesion kinase (FAK) during arteriogenesis. Mol Cell Biochem 322: 161–169, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chambers JW. and LoGrasso PV. Mitochondrial c-Jun N-terminal kinase (JNK) signaling initiates physiological changes resulting in amplification of reactive oxygen species generation. J Biol Chem 286: 16052–16062, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang K, Weiss D, Suo J, Vega JD, Giddens D, Taylor WR, and Jo H. Bone morphogenic protein antagonists are coexpressed with bone morphogenic protein 4 in endothelial cells exposed to unstable flow in vitro in mouse aortas and in human coronary arteries: role of bone morphogenic protein antagonists in inflammation and atherosclerosis. Circulation 116: 1258–1266, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Chappell DC, Varner SE, Nerem RM, Medford RM, and Alexander RW. Oscillatory shear stress stimulates adhesion molecule expression in cultured human endothelium. Circ Res 82: 532–539, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Chaudhury H, Zakkar M, Boyle J, Cuhlmann S, van der Heiden K, Luong le A, Davis J, Platt A, Mason JC, Krams R, Haskard DO, Clark AR, and Evans PC. c-Jun N-terminal kinase primes endothelial cells at atheroprone sites for apoptosis. Arterioscler Thromb Vasc Biol 30: 546–553, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Chen J, Green J, Yurdagul A, Jr., Albert P, McInnis MC, and Orr AW. alphavbeta3 integrins mediate flow-induced NF-kappaB activation, proinflammatory gene expression, and early atherogenic inflammation. Am J Pathol 185: 2575–2589, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J, Leskov IL, Yurdagul A, Jr., Thiel B, Kevil CG, Stokes KY, and Orr AW. Recruitment of the adaptor protein Nck to PECAM-1 couples oxidative stress to canonical NF-kappaB signaling and inflammation. Sci Signal 8: ra20, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Z, Rubin J, and Tzima E. Role of PECAM-1 in arteriogenesis and specification of preexisting collaterals. Circ Res 107: 1355–1363, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiang HY, Korshunov VA, Serour A, Shi F, and Sottile J. Fibronectin is an important regulator of flow-induced vascular remodeling. Arterioscler Thromb Vasc Biol 29: 1074–1079, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiba R, Nakagawa N, Kurasawa K, Tanaka Y, Saito Y, and Iwamoto I. Ligation of CD31 (PECAM-1) on endothelial cells increases adhesive function of alphavbeta3 integrin and enhances beta1 integrin-mediated adhesion of eosinophils to endothelial cells. Blood 94: 1319–1329, 1999 [PubMed] [Google Scholar]
- 25.Chiu JJ, Lee PL, Chen CN, Lee CI, Chang SF, Chen LJ, Lien SC, Ko YC, Usami S, and Chien S. Shear stress increases ICAM-1 and decreases VCAM-1 and E-selectin expressions induced by tumor necrosis factor-[alpha] in endothelial cells. Arterioscler Thromb Vasc Biol 24: 73–79, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Chiu JJ, Wung BS, Shyy JY, Hsieh HJ, and Wang DL. Reactive oxygen species are involved in shear stress-induced intercellular adhesion molecule-1 expression in endothelial cells. Arterioscler Thromb Vasc Biol 17: 3570–3577, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Colgan OC, Ferguson G, Collins NT, Murphy RP, Meade G, Cahill PA, and Cummins PM. Regulation of bovine brain microvascular endothelial tight junction assembly and barrier function by laminar shear stress. Am J Physiol Heart Circ Physiol 292: H3190–H3197, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Coll-Bonfill N, Musri MM, Ivo V, Barbera JA, and Tura-Ceide O. Transdifferentiation of endothelial cells to smooth muscle cells play an important role in vascular remodelling. Am J Stem Cells 4: 13–21, 2015 [PMC free article] [PubMed] [Google Scholar]
- 29.Collins C, Osborne LD, Guilluy C, Chen Z, O'Brien ET, 3rd, Reader JS, Burridge K, Superfine R, and Tzima E. Haemodynamic and extracellular matrix cues regulate the mechanical phenotype and stiffness of aortic endothelial cells. Nat Commun 5: 3984, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collins C. and Tzima E. Rac[e] to the pole: setting up polarity in endothelial cells. Small GTPases 5: e28650, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collins T. and Cybulsky MI. NF-kappaB: pivotal mediator or innocent bystander in atherogenesis? J Clin Invest 107: 255–264, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP, and Bouck N. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell 93: 1159–1170, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Cuhlmann S, Van der Heiden K, Saliba D, Tremoleda JL, Khalil M, Zakkar M, Chaudhury H, Luong le A, Mason JC, Udalova I, Gsell W, Jones H, Haskard DO, Krams R, and Evans PC. Disturbed blood flow induces RelA expression via c-Jun N-terminal kinase 1: a novel mode of NF-kappaB regulation that promotes arterial inflammation. Circ Res 108: 950–959, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Cybulsky MI, and Gimbrone MA., Jr. Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science 251: 788–791, 1991 [DOI] [PubMed] [Google Scholar]
- 35.Dai G, Vaughn S, Zhang Y, Wang ET, Garcia-Cardena G, and Gimbrone MA., Jr. Biomechanical forces in atherosclerosis-resistant vascular regions regulate endothelial redox balance via phosphoinositol 3-kinase/Akt-dependent activation of Nrf2. Circ Res 101: 723–733, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Davies PF, Civelek M, Fang Y, and Fleming I. The atherosusceptible endothelium: endothelial phenotypes in complex haemodynamic shear stress regions in vivo. Cardiovasc Res 99: 315–327, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davies PF, Robotewskyj A, and Griem ML. Quantitative studies of endothelial cell adhesion. Directional remodeling of focal adhesion sites in response to flow forces. J Clin Invest 93: 2031–2038, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Defilippi P, van Hinsbergh V, Bertolotto A, Rossino P, Silengo L, and Tarone G. Differential distribution and modulation of expression of alpha 1/beta 1 integrin on human endothelial cells. J Cell Biol 114: 855–863, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dekker RJ, van Soest S, Fontijn RD, Salamanca S, de Groot PG, VanBavel E, Pannekoek H, and Horrevoets AJ. Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Kruppel-like factor (KLF2). Blood 100: 1689–1698, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Di Francesco L, Totani L, Dovizio M, Piccoli A, Di Francesco A, Salvatore T, Pandolfi A, Evangelista V, Dercho RA, Seta F, and Patrignani P. Induction of prostacyclin by steady laminar shear stress suppresses tumor necrosis factor-alpha biosynthesis via heme oxygenase-1 in human endothelial cells. Circ Res 104: 506–513, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, and Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399: 601–605, 1999 [DOI] [PubMed] [Google Scholar]
- 42.Dimmeler S, Haendeler J, Rippmann V, Nehls M, and Zeiher AM. Shear stress inhibits apoptosis of human endothelial cells. FEBS Lett 399: 71–74, 1996 [DOI] [PubMed] [Google Scholar]
- 43.Dixit M, Loot AE, Mohamed A, Fisslthaler B, Boulanger CM, Ceacareanu B, Hassid A, Busse R, and Fleming I. Gab1, SHP2, and protein kinase A are crucial for the activation of the endothelial NO synthase by fluid shear stress. Circ Res 97: 1236–1244, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Estrach S, Cailleteau L, Franco CA, Gerhardt H, Stefani C, Lemichez E, Gagnoux-Palacios L, Meneguzzi G, and Mettouchi A. Laminin-binding integrins induce Dll4 expression and Notch signaling in endothelial cells. Circ Res 109: 172–182, 2011 [DOI] [PubMed] [Google Scholar]
- 45.Feaver RE, Gelfand BD, Wang C, Schwartz MA, and Blackman BR. Atheroprone hemodynamics regulate fibronectin deposition to create positive feedback that sustains endothelial inflammation. Circ Res 106: 1703–1711, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fernandez B. and Broich K. Cell-cell and cell-matrix interactions. In: Arteriogenesis, edited by Schaper W. and Schaper J. Boston: Kluwer Academic Publishers, 2004, pp. 173–190 [Google Scholar]
- 47.Fledderus JO, van Thienen JV, Boon RA, Dekker RJ, Rohlena J, Volger OL, Bijnens AP, Daemen MJ, Kuiper J, van Berkel TJ, Pannekoek H, and Horrevoets AJ. Prolonged shear stress and KLF2 suppress constitutive proinflammatory transcription through inhibition of ATF2. Blood 109: 4249–4257, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Fleming I. Molecular mechanisms underlying the activation of eNOS. Pflugers Arch 459: 793–806, 2010 [DOI] [PubMed] [Google Scholar]
- 49.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, and Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 399: 597–601, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Funk SD, Yurdagul A, Jr., Green JM, Jhaveri KA, Schwartz MA, and Orr AW. Matrix-specific protein kinase A signaling regulates p21-activated kinase activation by flow in endothelial cells. Circ Res 106: 1394–1403, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Funk SD, Yurdagul A, Jr., and Orr AW. Hyperglycemia and endothelial dysfunction in atherosclerosis: lessons from type 1 diabetes. Int J Vasc Med 2012: 569654, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gaengel K, Niaudet C, Hagikura K, Lavina B, Muhl L, Hofmann JJ, Ebarasi L, Nystrom S, Rymo S, Chen LL, Pang MF, Jin Y, Raschperger E, Roswall P, Schulte D, Benedito R, Larsson J, Hellstrom M, Fuxe J, Uhlen P, Adams R, Jakobsson L, Majumdar A, Vestweber D, Uv A, and Betsholtz C. The sphingosine-1-phosphate receptor S1PR1 restricts sprouting angiogenesis by regulating the interplay between VE-cadherin and VEGFR2. Dev Cell 23: 587–599, 2012 [DOI] [PubMed] [Google Scholar]
- 53.Gao M, Craig D, Lequin O, Campbell ID, Vogel V, and Schulten K. Structure and functional significance of mechanically unfolded fibronectin type III1 intermediates. Proc Natl Acad Sci U S A 100: 14784–14789, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gelfand BD, Meller J, Pryor AW, Kahn M, Bortz PD, Wamhoff BR, and Blackman BR. Hemodynamic activation of beta-catenin and T-cell-specific transcription factor signaling in vascular endothelium regulates fibronectin expression. Arterioscler Thromb Vasc Biol 31: 1625–1633, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ghosh S, Vivar J, Nelson CP, Willenborg C, Segre AV, Makinen VP, Nikpay M, Erdmann J, Blankenberg S, O'Donnell C, Marz W, Laaksonen R, Stewart AF, Epstein SE, Shah SH, Granger CB, Hazen SL, Kathiresan S, Reilly MP, Yang X, Quertermous T, Samani NJ, Schunkert H, Assimes TL, and McPherson R. Systems genetics analysis of genome-wide association study reveals novel associations between key biological processes and coronary artery disease. Arterioscler Thromb Vasc Biol, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gianni-Barrera R, Trani M, Fontanellaz C, Heberer M, Djonov V, Hlushchuk R, and Banfi A. VEGF over-expression in skeletal muscle induces angiogenesis by intussusception rather than sprouting. Angiogenesis 16: 123–136, 2012 [DOI] [PubMed] [Google Scholar]
- 57.Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene 25: 6680–6684, 2006 [DOI] [PubMed] [Google Scholar]
- 58.Gonzalez AM, Claiborne J, and Jones JC. Integrin cross-talk in endothelial cells is regulated by protein kinase A and protein phosphatase 1. J Biol Chem 283: 31849–31860, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gonzalez-Santiago L, Lopez-Ongil S, Rodriguez-Puyol M, and Rodriguez-Puyol D. Decreased nitric oxide synthesis in human endothelial cells cultured on type I collagen. Circ Res 90: 539–545, 2002 [DOI] [PubMed] [Google Scholar]
- 60.Green J, Yurdagul A, Jr., McInnis MC, Albert P, and Orr AW. Flow patterns regulate hyperglycemia-induced subendothelial matrix remodeling during early atherogenesis. Atherosclerosis 232: 277–284, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Groeger AL, Cipollina C, Cole MP, Woodcock SR, Bonacci G, Rudolph TK, Rudolph V, Freeman BA, and Schopfer FJ. Cyclooxygenase-2 generates anti-inflammatory mediators from omega-3 fatty acids. Nat Chem Biol 6: 433–441, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grumbach IM, Chen W, Mertens SA, and Harrison DG. A negative feedback mechanism involving nitric oxide and nuclear factor kappa-B modulates endothelial nitric oxide synthase transcription. J Mol Cell Cardiol 39: 595–603, 2005 [DOI] [PubMed] [Google Scholar]
- 63.Hahn C, Orr AW, Sanders JM, Jhaveri KA, and Schwartz MA. The subendothelial extracellular matrix modulates JNK activation by flow. Circ Res 104: 995–1003, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hahn C. and Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol 10: 53–62, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hajra L, Evans AI, Chen M, Hyduk SJ, Collins T, and Cybulsky MI. The NF-kappa B signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc Natl Acad Sci U S A 97: 9052–9057, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Han J, Lim CJ, Watanabe N, Soriani A, Ratnikov B, Calderwood DA, Puzon-McLaughlin W, Lafuente EM, Boussiotis VA, Shattil SJ, and Ginsberg MH. Reconstructing and deconstructing agonist-induced activation of integrin alphaIIbbeta3. Curr Biol 16: 1796–1806, 2006 [DOI] [PubMed] [Google Scholar]
- 67.Heino J. The collagen receptor integrins have distinct ligand recognition and signaling functions. Matrix Biol 19: 319–323, 2000 [DOI] [PubMed] [Google Scholar]
- 68.Heitzer T, Baldus S, von Kodolitsch Y, Rudolph V, and Meinertz T. Systemic endothelial dysfunction as an early predictor of adverse outcome in heart failure. Arterioscler Thromb Vasc Biol 25: 1174–1179, 2005 [DOI] [PubMed] [Google Scholar]
- 69.Himburg HA, Grzybowski DM, Hazel AL, LaMack JA, Li XM, and Friedman MH. Spatial comparison between wall shear stress measures and porcine arterial endothelial permeability. Am J Physiol Heart Circ Physiol 286: H1916–H1922, 2004 [DOI] [PubMed] [Google Scholar]
- 70.Hinz B. The extracellular matrix and transforming growth factor-beta1: tale of a strained relationship. Matrix Biol 47: 54–65, 2015 [DOI] [PubMed] [Google Scholar]
- 71.Hodkinson PS, Elliott PA, Lad Y, McHugh BJ, MacKinnon AC, Haslett C, and Sethi T. Mammalian NOTCH-1 activates beta1 integrins via the small GTPase R-Ras. J Biol Chem 282: 28991–29001, 2007 [DOI] [PubMed] [Google Scholar]
- 72.Hoefer IE, den Adel B, and Daemen MJ. Biomechanical factors as triggers of vascular growth. Cardiovasc Res 99: 276–283, 2013 [DOI] [PubMed] [Google Scholar]
- 73.Hoefer IE, van Royen N, Rectenwald JE, Deindl E, Hua J, Jost M, Grundmann S, Voskuil M, Ozaki CK, Piek JJ, and Buschmann IR. Arteriogenesis proceeds via ICAM-1/Mac-1- mediated mechanisms. Circ Res 94: 1179–1185, 2004 [DOI] [PubMed] [Google Scholar]
- 74.Huang NF, Lai ES, Ribeiro AJ, Pan S, Pruitt BL, Fuller GG, and Cooke JP. Spatial patterning of endothelium modulates cell morphology, adhesiveness and transcriptional signature. Biomaterials 34: 2928–2937, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hwang J, Saha A, Boo YC, Sorescu GP, McNally JS, Holland SM, Dikalov S, Giddens DP, Griendling KK, Harrison DG, and Jo H. Oscillatory shear stress stimulates endothelial production of O2- from p47phox-dependent NAD(P)H oxidases, leading to monocyte adhesion. J Biol Chem 278: 47291–47298, 2003 [DOI] [PubMed] [Google Scholar]
- 76.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell 110: 673–687, 2002 [DOI] [PubMed] [Google Scholar]
- 77.Hynes RO. The evolution of metazoan extracellular matrix. J Cell Biol 196: 671–679, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hynes RO. and Naba A. Overview of the matrisome—an inventory of extracellular matrix constituents and functions. Cold Spring Harb Perspect Biol 4: a004903, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jahnsen ED, Trindade A, Zaun HC, Lehoux S, Duarte A, and Jones EA. Notch1 is pan-endothelial at the onset of flow and regulated by flow. PLoS One 10: e0122622, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jalali S, del Pozo MA, Chen K, Miao H, Li Y, Schwartz MA, Shyy JY, and Chien S. Integrin-mediated mechanotransduction requires its dynamic interaction with specific extracellular matrix (ECM) ligands. Proc Natl Acad Sci U S A 98: 1042–1046, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jhaveri KA, Debnath P, Chernoff J, Sanders J, and Schwartz MA. The role of p21-activated kinase in the initiation of atherosclerosis. BMC Cardiovasc Disord 12: 55, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jin ZG, Wong C, Wu J, and Berk BC. Flow shear stress stimulates Gab1 tyrosine phosphorylation to mediate protein kinase B and endothelial nitric-oxide synthase activation in endothelial cells. J Biol Chem 280: 12305–12309, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jo H, Song H, and Mowbray A. Role of NADPH oxidases in disturbed flow- and BMP4- induced inflammation and atherosclerosis. Antioxid Redox Signal 8: 1609–1619, 2006 [DOI] [PubMed] [Google Scholar]
- 84.Jones EA, Baron MH, Fraser SE, and Dickinson ME. Measuring hemodynamic changes during mammalian development. Am J Physiol Heart Circ Physiol 287: H1561–H1569, 2004 [DOI] [PubMed] [Google Scholar]
- 85.Jung B, Obinata H, Galvani S, Mendelson K, Ding BS, Skoura A, Kinzel B, Brinkmann V, Rafii S, Evans T, and Hla T. Flow-regulated endothelial S1P receptor-1 signaling sustains vascular development. Dev Cell 23: 600–610, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer 3: 422–433, 2003 [DOI] [PubMed] [Google Scholar]
- 87.Kang H, Kwak HI, Kaunas R, and Bayless KJ. Fluid shear stress and sphingosine 1-phosphate activate calpain to promote membrane type 1 matrix metalloproteinase (MT1-MMP) membrane translocation and endothelial invasion into three-dimensional collagen matrices. J Biol Chem 286: 42017–42026, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Katsumi A, Naoe T, Matsushita T, Kaibuchi K, and Schwartz MA. Integrin activation and matrix binding mediate cellular responses to mechanical stretch. J Biol Chem 280: 16546–16549, 2005 [DOI] [PubMed] [Google Scholar]
- 89.Katsumi A, Orr AW, Tzima E, and Schwartz MA. Integrins in mechanotransduction. J Biol Chem 279: 12001–12004, 2004 [DOI] [PubMed] [Google Scholar]
- 90.Kemeny SF, Figueroa DS, Andrews AM, Barbee KA, and Clyne AM. Glycated collagen alters endothelial cell actin alignment and nitric oxide release in response to fluid shear stress. J Biomech 44: 1927–1935, 2011 [DOI] [PubMed] [Google Scholar]
- 91.Kim CW, Song H, Kumar S, Nam D, Kwon HS, Chang KH, Son DJ, Kang DW, Brodie SA, Weiss D, Vega JD, Alberts-Grill N, Griendling K, Taylor WR, and Jo H. Anti-inflammatory and antiatherogenic role of BMP receptor II in endothelial cells. Arterioscler Thromb Vasc Biol 33: 1350–1359, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim SH, Cho YR, Kim HJ, Oh JS, Ahn EK, Ko HJ, Hwang BJ, Lee SJ, Cho Y, Kim YK, Stetler-Stevenson WG, and Seo DW. Antagonism of VEGF-A-induced increase in vascular permeability by an integrin alpha3beta1-Shp-1-cAMP/PKA pathway. Blood 120: 4892–4902, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kliche K, Jeggle P, Pavenstadt H, and Oberleithner H. Role of cellular mechanics in the function and life span of vascular endothelium. Pflugers Arch 462: 209–217, 2011 [DOI] [PubMed] [Google Scholar]
- 94.Komaravolu RK, Adam C, Moonen JR, Harmsen MC, Goebeler M, and Schmidt M. Erk5 inhibits endothelial migration via KLF2-dependent down-regulation of PAK1. Cardiovasc Res 105: 86–95, 2015 [DOI] [PubMed] [Google Scholar]
- 95.Koshida R, Rocic P, Saito S, Kiyooka T, Zhang C, and Chilian WM. Role of focal adhesion kinase in flow-induced dilation of coronary arterioles. Arterioscler Thromb Vasc Biol 25: 2548–2553, 2005 [DOI] [PubMed] [Google Scholar]
- 96.Lakshmikanthan S, Sobczak M, Chun C, Henschel A, Dargatz J, Ramchandran R, and Chrzanowska-Wodnicka M. Rap1 promotes VEGFR2 activation and angiogenesis by a mechanism involving integrin alphavbeta(3). Blood 118: 2015–2026, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lakshmikanthan S, Zheng X, Nishijima Y, Sobczak M, Szabo A, Vasquez-Vivar J, Zhang DX, and Chrzanowska-Wodnicka M. Rap1 promotes endothelial mechanosensing complex formation, NO release and normal endothelial function. EMBO Rep 16: 628–637, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lamari F, Braut-Boucher F, Pongnimitprasert N, Bernard M, Foglietti MJ, Derappe C, and Aubery M. Cell adhesion and integrin expression are modulated by oxidative stress in EA.hy 926 cells. Free Radic Res 41: 812–822, 2007 [DOI] [PubMed] [Google Scholar]
- 99.Le NT, Heo KS, Takei Y, Lee H, Woo CH, Chang E, McClain C, Hurley C, Wang X, Li F, Xu H, Morrell C, Sullivan MA, Cohen MS, Serafimova IM, Taunton J, Fujiwara K, and Abe J. A crucial role for p90RSK-mediated reduction of ERK5 transcriptional activity in endothelial dysfunction and atherosclerosis. Circulation 127: 486–499, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee CW, Stabile E, Kinnaird T, Shou M, Devaney JM, Epstein SE, and Burnett MS. Temporal patterns of gene expression after acute hindlimb ischemia in mice: insights into the genomic program for collateral vessel development. J Am Coll Cardiol 43: 474–482, 2004 [DOI] [PubMed] [Google Scholar]
- 101.Leong KG, Hu X, Li L, Noseda M, Larrivee B, Hull C, Hood L, Wong F, and Karsan A. Activated Notch4 inhibits angiogenesis: role of beta 1-integrin activation. Mol Cell Biol 22: 2830–2841, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li R, Jen N, Wu L, Lee J, Fang K, Quigley K, Lee K, Wang S, Zhou B, Vergnes L, Chen YR, Li Z, Reue K, Ann DK, and Hsiai TK. Disturbed flow induces autophagy, but impairs autophagic flux to perturb mitochondrial homeostasis. Antioxid Redox Signal 23: 1207–1219, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med 368: 2004–2013, 2013 [DOI] [PubMed] [Google Scholar]
- 104.Liu Y, Collins C, Kiosses WB, Murray AM, Joshi M, Shepherd TR, Fuentes EJ, and Tzima E. A novel pathway spatiotemporally activates Rac1 and redox signaling in response to fluid shear stress. J Cell Biol 201: 863–873, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu Y, Sweet DT, Irani-Tehrani M, Maeda N, and Tzima E. Shc coordinates signals from intercellular junctions and integrins to regulate flow-induced inflammation. J Cell Biol 182: 185–196, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Luu NT, Glen KE, Egginton S, Rainger GE, and Nash GB. Integrin-substrate interactions underlying shear-induced inhibition of the inflammatory response of endothelial cells. Thromb Haemost 109: 298–308, 2013 [DOI] [PubMed] [Google Scholar]
- 107.MacDonald EM. and Cohn RD. TGFbeta signaling: its role in fibrosis formation and myopathies. Curr Opin Rheumatol 24: 628–634, 2012 [DOI] [PubMed] [Google Scholar]
- 108.Magid R. and Davies PF. Endothelial protein kinase C isoform identity and differential activity of PKCzeta in an athero-susceptible region of porcine aorta. Circ Res 97: 443–449, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Magnusson MK. and Mosher DF. Fibronectin: structure, assembly, and cardiovascular implications. Arterioscler Thromb Vasc Biol 18: 1363–1370, 1998 [DOI] [PubMed] [Google Scholar]
- 110.Marignani PA. and Carpenter CL. Vav2 is required for cell spreading. J Cell Biol 154: 177–186, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Massague J. TGFbeta signalling in context. Nat Rev Mol Cell Biol 13: 616–630, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mengistu M, Brotzman H, Ghadiali S, and Lowe-Krentz L. Fluid shear stress-induced JNK activity leads to actin remodeling for cell alignment. J Cell Physiol 226: 110–121, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Miao H, Hu YL, Shiu YT, Yuan S, Zhao Y, Kaunas R, Wang Y, Jin G, Usami S, and Chien S. Effects of flow patterns on the localization and expression of VE-cadherin at vascular endothelial cell junctions: in vivo and in vitro investigations. J Vasc Res 42: 77–89, 2005 [DOI] [PubMed] [Google Scholar]
- 114.Milkiewicz M, Kelland C, Colgan S, and Haas TL. Nitric oxide and p38 MAP kinase mediate shear stress-dependent inhibition of MMP-2 production in microvascular endothelial cells. J Cell Physiol 208: 229–237, 2006 [DOI] [PubMed] [Google Scholar]
- 115.Millard M, Odde S, and Neamati N. Integrin targeted therapeutics. Theranostics 1: 154–188, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mogford JE, Davis GE, Platts SH, and Meininger GA. Vascular smooth muscle alpha v beta 3 integrin mediates arteriolar vasodilation in response to RGD peptides. Circ Res 79: 821–826, 1996 [DOI] [PubMed] [Google Scholar]
- 117.Mohan S, Koyoma K, Thangasamy A, Nakano H, Glickman RD, and Mohan N. Low shear stress preferentially enhances IKK activity through selective sources of ROS for persistent activation of NF-kappaB in endothelial cells. Am J Physiol Cell Physiol 292: C362–C371, 2007 [DOI] [PubMed] [Google Scholar]
- 118.Mohan S, Mohan N, Valente AJ, and Sprague EA. Regulation of low shear flow-induced HAEC VCAM-1 expression and monocyte adhesion. Am J Physiol 276: C1100–C1107, 1999 [DOI] [PubMed] [Google Scholar]
- 119.Moiseeva EP. Adhesion receptors of vascular smooth muscle cells and their functions. Cardiovasc Res 52: 372–386, 2001 [DOI] [PubMed] [Google Scholar]
- 120.Monkley SJ, Kostourou V, Spence L, Petrich B, Coleman S, Ginsberg MH, Pritchard CA, and Critchley DR. Endothelial cell talin1 is essential for embryonic angiogenesis. Dev Biol 349: 494–502, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Monkley SJ, Zhou XH, Kinston SJ, Giblett SM, Hemmings L, Priddle H, Brown JE, Pritchard CA, Critchley DR, and Fassler R. Disruption of the talin gene arrests mouse development at the gastrulation stage. Dev Dyn 219: 560–574, 2000 [DOI] [PubMed] [Google Scholar]
- 122.Monvoisin A, Alva JA, Hofmann JJ, Zovein AC, Lane TF, and Iruela-Arispe ML. VE-cadherin-CreERT2 transgenic mouse: a model for inducible recombination in the endothelium. Dev Dyn 235: 3413–3422, 2006 [DOI] [PubMed] [Google Scholar]
- 123.Morgan MJ. and Liu ZG. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res 21: 103–115, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mould AP, Craig SE, Byron SK, Humphries MJ, and Jowitt TA. Disruption of integrin-fibronectin complexes by allosteric but not ligand-mimetic inhibitors. Biochem J 464: 301–313, 2014 [DOI] [PubMed] [Google Scholar]
- 125.Muller JM, Chilian WM, and Davis MJ. Integrin signaling transduces shear stress—dependent vasodilation of coronary arterioles. Circ Res 80: 320–326, 1997 [DOI] [PubMed] [Google Scholar]
- 126.Munger JS. and Sheppard D. Cross talk among TGF-beta signaling pathways, integrins, and the extracellular matrix. Cold Spring Harb Perspect Biol 3: a005017, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Murphy JF, Steele C, Belton O, and Fitzgerald DJ. Induction of cyclooxygenase-1 and -2 modulates angiogenic responses to engagement of alphavbeta3. Br J Haematol 121: 157–164, 2003 [DOI] [PubMed] [Google Scholar]
- 128.Murphy PA. and Hynes RO. Alternative splicing of endothelial fibronectin is induced by disturbed hemodynamics and protects against hemorrhage of the vessel wall. Arterioscler Thromb Vasc Biol 34: 2042–2050, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nigro P, Abe J, Woo CH, Satoh K, McClain C, O'Dell MR, Lee H, Lim JH, Li JD, Heo KS, Fujiwara K, and Berk BC. PKCzeta decreases eNOS protein stability via inhibitory phosphorylation of ERK5. Blood 116: 1971–1979, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nystrom A, Shaik ZP, Gullberg D, Krieg T, Eckes B, Zent R, Pozzi A, and Iozzo RV. Role of tyrosine phosphatase SHP-1 in the mechanism of endorepellin angiostatic activity. Blood 114: 4897–4906, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ohno M, Cooke JP, Dzau VJ, and Gibbons GH. Fluid shear stress induces endothelial transforming growth factor beta-1 transcription and production. Modulation by potassium channel blockade. J Clin Invest 95: 1363–1369, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Orr AW, Ginsberg MH, Shattil SJ, Deckmyn H, and Schwartz MA. Matrix-specific suppression of integrin activation in shear stress signaling. Mol Biol Cell 17: 4686–4697, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Orr AW, Hahn C, Blackman BR, and Schwartz MA. p21-activated kinase signaling regulates oxidant-dependent NF-kappa B activation by flow. Circ Res 103: 671–679, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Orr AW, Helmke BP, Blackman BR, and Schwartz MA. Mechanisms of mechanotransduction. Dev Cell 10: 11–20, 2006 [DOI] [PubMed] [Google Scholar]
- 135.Orr AW, Sanders JM, Bevard M, Coleman E, Sarembock IJ, and Schwartz MA. The subendothelial extracellular matrix modulates NF-kappaB activation by flow: a potential role in atherosclerosis. J Cell Biol 169: 191–202, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Orr AW, Stockton R, Simmers MB, Sanders JM, Sarembock IJ, Blackman BR, and Schwartz MA. Matrix-specific p21-activated kinase activation regulates vascular permeability in atherogenesis. J Cell Biol 176: 719–727, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Parmar KM, Larman HB, Dai G, Zhang Y, Wang ET, Moorthy SN, Kratz JR, Lin Z, Jain MK, Gimbrone MA, Jr., and Garcia-Cardena G. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J Clin Invest 116: 49–58, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Passerini AG, Polacek DC, Shi C, Francesco NM, Manduchi E, Grant GR, Pritchard WF, Powell S, Chang GY, Stoeckert CJ, Jr., and Davies PF. Coexisting proinflammatory and antioxidative endothelial transcription profiles in a disturbed flow region of the adult porcine aorta. Proc Natl Acad Sci U S A 101: 2482–2487, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Perrault CM, Brugues A, Bazellieres E, Ricco P, Lacroix D, and Trepat X. Traction forces of endothelial cells under slow shear flow. Biophys J 109: 1533–1536, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Petzold T, Orr AW, Hahn C, Jhaveri KA, Parsons JT, and Schwartz MA. Focal adhesion kinase modulates activation of NF-kappaB by flow in endothelial cells. Am J Physiol Cell Physiol 297: C814–C822, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pipp F, Boehm S, Cai WJ, Adili F, Ziegler B, Karanovic G, Ritter R, Balzer J, Scheler C, Schaper W, and Schmitz-Rixen T. Elevated fluid shear stress enhances postocclusive collateral artery growth and gene expression in the pig hind limb. Arterioscler Thromb Vasc Biol 24: 1664–1668, 2004 [DOI] [PubMed] [Google Scholar]
- 142.Potente M, Gerhardt H, and Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell 146: 873–887, 2011 [DOI] [PubMed] [Google Scholar]
- 143.Pulakazhi Venu VK, Uboldi P, Dhyani A, Patrini A, Baetta R, Ferri N, Corsini A, Muro AF, Catapano AL, and Norata GD. Fibronectin extra domain A stabilises atherosclerotic plaques in apolipoprotein E and in LDL-receptor-deficient mice. Thromb Haemost 114: 186–197, 2015 [DOI] [PubMed] [Google Scholar]
- 144.Ratnikov B, Ptak C, Han J, Shabanowitz J, Hunt DF, and Ginsberg MH. Talin phosphorylation sites mapped by mass spectrometry. J Cell Sci 118: 4921–4923, 2005 [DOI] [PubMed] [Google Scholar]
- 145.Renz M, Otten C, Faurobert E, Rudolph F, Zhu Y, Boulday G, Duchene J, Mickoleit M, Dietrich AC, Ramspacher C, Steed E, Manet-Dupe S, Benz A, Hassel D, Vermot J, Huisken J, Tournier-Lasserve E, Felbor U, Sure U, Albiges-Rizo C, and Abdelilah-Seyfried S. Regulation of beta1 integrin-Klf2-mediated angiogenesis by CCM proteins. Dev Cell 32: 181–190, 2015 [DOI] [PubMed] [Google Scholar]
- 146.Resnick N, Collins T, Atkinson W, Bonthron DT, Dewey CF, Jr., and Gimbrone MA., Jr. Platelet-derived growth factor B chain promoter contains a cis-acting fluid shear-stress-responsive element [erratum appears in Proc Natl Acad Sci U S A 90: 7908, 1993]. Proc Natl Acad Sci U S A 90: 4591–4595, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rochfort KD, Collins LE, McLoughlin A, and Cummins PM. Shear-dependent attenuation of cellular ROS levels can suppress proinflammatory cytokine injury to human brain microvascular endothelial barrier properties. J Cereb Blood Flow Metab 35: 1648–1656, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rohwedder I, Montanez E, Beckmann K, Bengtsson E, Duner P, Nilsson J, Soehnlein O, and Fassler R. Plasma fibronectin deficiency impedes atherosclerosis progression and fibrous cap formation. EMBO Mol Med 4: 564–576, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ross TD, Coon BG, Yun S, Baeyens N, Tanaka K, Ouyang M, and Schwartz MA. Integrins in mechanotransduction. Curr Opin Cell Biol 25: 613–618, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sacharidou A, Koh W, Stratman AN, Mayo AM, Fisher KE, and Davis GE. Endothelial lumen signaling complexes control 3D matrix-specific tubulogenesis through interdependent Cdc42- and MT1-MMP-mediated events. Blood 115: 5259–5269, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sage H, Pritzl P, and Bornstein P. Secretory phenotypes of endothelial cells in culture: comparison of aortic, venous, capillary, and corneal endothelium. Arteriosclerosis 1: 427–442, 1981 [DOI] [PubMed] [Google Scholar]
- 152.Sakellarios AI, Papafaklis MI, Siogkas P, Athanasiou LS, Exarchos TP, Stefanou K, Bourantas CV, Naka KK, Michalis LK, Parodi O, and Fotiadis DI. Patient-specific computational modeling of subendothelial LDL accumulation in a stenosed right coronary artery: effect of hemodynamic and biological factors. Am J Physiol Heart Circ Physiol 304: H1455–H1470, 2013 [DOI] [PubMed] [Google Scholar]
- 153.Schiller HB, Hermann MR, Polleux J, Vignaud T, Zanivan S, Friedel CC, Sun Z, Raducanu A, Gottschalk KE, Thery M, Mann M, and Fassler R. beta1- and alphav-class integrins cooperate to regulate myosin II during rigidity sensing of fibronectin-based microenvironments. Nat Cell Biol 15: 625–636, 2013 [DOI] [PubMed] [Google Scholar]
- 154.Schultz-Cherry S, Lawler J, and Murphy-Ullrich JE. The type 1 repeats of thrombospondin 1 activate latent transforming growth factor-beta. J Biol Chem 269: 26783–26788, 1994 [PubMed] [Google Scholar]
- 155.Schwartz MA. and Assoian RK. Integrins and cell proliferation: regulation of cyclin-dependent kinases via cytoplasmic signaling pathways. J Cell Sci 114: 2553–2560, 2001 [DOI] [PubMed] [Google Scholar]
- 156.SenBanerjee S, Lin Z, Atkins GB, Greif DM, Rao RM, Kumar A, Feinberg MW, Chen Z, Simon DI, Luscinskas FW, Michel TM, Gimbrone MA, Jr., Garcia-Cardena G, and Jain MK. KLF2 Is a novel transcriptional regulator of endothelial proinflammatory activation. J Exp Med 199: 1305–1315, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Shepherd RD, Kos SM, and Rinker KD. Flow-dependent Smad2 phosphorylation and TGIF nuclear localization in human aortic endothelial cells. Am J Physiol Heart Circ Physiol 301: H98–H107, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Simons M. and Eichmann A. Molecular controls of arterial morphogenesis. Circ Res 116: 1712–1724, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sorescu GP, Song H, Tressel SL, Hwang J, Dikalov S, Smith DA, Boyd NL, Platt MO, Lassegue B, Griendling KK, and Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based NADPH oxidase. Circ Res 95: 773–779, 2004 [DOI] [PubMed] [Google Scholar]
- 160.Stenzel D, Franco CA, Estrach S, Mettouchi A, Sauvaget D, Rosewell I, Schertel A, Armer H, Domogatskaya A, Rodin S, Tryggvason K, Collinson L, Sorokin L, and Gerhardt H. Endothelial basement membrane limits tip cell formation by inducing Dll4/Notch signalling in vivo. EMBO Rep 12: 1135–1143, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Steri V, Ellison TS, Gontarczyk AM, Weilbaecher K, Schneider JG, Edwards D, Fruttiger M, Hodivala-Dilke KM, and Robinson SD. Acute depletion of endothelial beta3-integrin transiently inhibits tumor growth and angiogenesis in mice. Circ Res 114: 79–91, 2013 [DOI] [PubMed] [Google Scholar]
- 162.Stoner L, Erickson ML, Young JM, Fryer S, Sabatier MJ, Faulkner J, Lambrick DM, and McCully KK. There's more to flow-mediated dilation than nitric oxide. J Atheroscler Thromb 19: 589–600, 2012 [DOI] [PubMed] [Google Scholar]
- 163.Stupack DG. and Cheresh DA. ECM remodeling regulates angiogenesis: endothelial integrins look for new ligands. Sci STKE 2002: PE7, 2002 [DOI] [PubMed] [Google Scholar]
- 164.Suchting S, Freitas C, le Noble F, Benedito R, Breant C, Duarte A, and Eichmann A. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc Natl Acad Sci U S A 104: 3225–3230, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sweet DT, Chen Z, Givens CS, Owens AP, 3rd, Rojas M, and Tzima E. Endothelial Shc regulates arteriogenesis through dual control of arterial specification and inflammation via the notch and nuclear factor-kappa-light-chain-enhancer of activated B-cell pathways. Circ Res 113: 32–39, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Takabe W, Jen N, Ai L, Hamilton R, Wang S, Holmes K, Dharbandi F, Khalsa B, Bressler S, Barr ML, Li R, and Hsiai TK. Oscillatory shear stress induces mitochondrial superoxide production: implication of NADPH oxidase and c-Jun NH2-terminal kinase signaling. Antioxid Redox Signal 15: 1379–1388, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Takabe W, Warabi E, and Noguchi N. Anti-atherogenic effect of laminar shear stress via Nrf2 activation. Antioxid Redox Signal 15: 1415–1426, 2011 [DOI] [PubMed] [Google Scholar]
- 168.Tan MH, Sun Z, Opitz SL, Schmidt TE, Peters JH, and George EL. Deletion of the alternatively spliced fibronectin EIIIA domain in mice reduces atherosclerosis. Blood 104: 11–18, 2004 [DOI] [PubMed] [Google Scholar]
- 169.Thodeti CK, Matthews B, Ravi A, Mammoto A, Ghosh K, Bracha AL, and Ingber DE. TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling. Circ Res 104: 1123–1130, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tian H, Mythreye K, Golzio C, Katsanis N, and Blobe GC. Endoglin mediates fibronectin/alpha5beta1 integrin and TGF-beta pathway crosstalk in endothelial cells. EMBO J 31: 3885–3900, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ting LH, Jahn JR, Jung JI, Shuman BR, Feghhi S, Han SJ, Rodriguez ML, and Sniadecki NJ. Flow mechanotransduction regulates traction forces, intercellular forces, and adherens junctions. Am J Physiol Heart Circ Physiol 302: H2220–H2229, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tirziu D, Jaba IM, Yu P, Larrivee B, Coon BG, Cristofaro B, Zhuang ZW, Lanahan AA, Schwartz MA, Eichmann A, and Simons M. Endothelial nuclear factor-kappaB-dependent regulation of arteriogenesis and branching. Circulation 126: 2589–2600, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tzima E, Del Pozo MA, Kiosses WB, Mohamed SA, Li S, Chien S, and Schwartz MA. Activation of Rac1 by shear stress in endothelial cells mediates both cytoskeletal reorganization and effects on gene expression. EMBO J 21: 6791–6800, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tzima E, del Pozo MA, Shattil SJ, Chien S, and Schwartz MA. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO J 20: 4639–4647, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, and Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437: 426–431, 2005 [DOI] [PubMed] [Google Scholar]
- 176.Tzima E, Kiosses WB, del Pozo MA, and Schwartz MA. Localized cdc42 activation, detected using a novel assay, mediates microtubule organizing center positioning in endothelial cells in response to fluid shear stress. J Biol Chem 278: 31020–31023, 2003 [DOI] [PubMed] [Google Scholar]
- 177.Udan RS, Vadakkan TJ, and Dickinson ME. Dynamic responses of endothelial cells to changes in blood flow during vascular remodeling of the mouse yolk sac. Development 140: 4041–4050, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.van Keulen JK, de Kleijn DP, Nijhuis MM, Busser E, Velema E, Fijnheer R, van der Graaf Y, Moll FL, de Vries JP, and Pasterkamp G. Levels of extra domain A containing fibronectin in human atherosclerotic plaques are associated with a stable plaque phenotype. Atherosclerosis 195: e83–e91, 2007 [DOI] [PubMed] [Google Scholar]
- 179.Viji RI, Kumar VB, Kiran MS, and Sudhakaran PR. Modulation of cyclooxygenase in endothelial cells by fibronectin: relevance to angiogenesis. J Cell Biochem 105: 158–166, 2008 [DOI] [PubMed] [Google Scholar]
- 180.Viji RI, Sameer Kumar VB, Kiran MS, and Sudhakaran PR. Modulation of endothelial nitric oxide synthase by fibronectin. Mol Cell Biochem 323: 91–100, 2008 [DOI] [PubMed] [Google Scholar]
- 181.Wagenseil JE. and Mecham RP. Vascular extracellular matrix and arterial mechanics. Physiol Rev 89: 957–989, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wang J, An FS, Zhang W, Gong L, Wei SJ, Qin WD, Wang XP, Zhao YX, Zhang Y, Zhang C, and Zhang MX. Inhibition of c-Jun N-terminal kinase attenuates low shear stress-induced atherogenesis in apolipoprotein E-deficient mice. Mol Med 17: 990–999, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Warabi E, Takabe W, Minami T, Inoue K, Itoh K, Yamamoto M, Ishii T, Kodama T, and Noguchi N. Shear stress stabilizes NF-E2-related factor 2 and induces antioxidant genes in endothelial cells: role of reactive oxygen/nitrogen species. Free Radic Biol Med 42: 260–269, 2007 [DOI] [PubMed] [Google Scholar]
- 184.Warboys CM, Eric Berson R, Mann GE, Pearson JD, and Weinberg PD. Acute and chronic exposure to shear stress have opposite effects on endothelial permeability to macromolecules. Am J Physiol Heart Circ Physiol 298: H1850–H1856, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Weis SM. and Cheresh DA. alphaV integrins in angiogenesis and cancer. Cold Spring Harb Perspect Med 1: a006478, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Woo CH, Shishido T, McClain C, Lim JH, Li JD, Yang J, Yan C, and Abe J. Extracellular signal-regulated kinase 5 SUMOylation antagonizes shear stress-induced antiinflammatory response and endothelial nitric oxide synthase expression in endothelial cells. Circ Res 102: 538–545, 2008 [DOI] [PubMed] [Google Scholar]
- 187.Wu C, Keivens VM, O'Toole TE, McDonald JA, and Ginsberg MH. Integrin activation and cytoskeletal interaction are essential for the assembly of a fibronectin matrix. Cell 83: 715–724, 1995 [DOI] [PubMed] [Google Scholar]
- 188.Yamawaki H, Pan S, Lee RT, and Berk BC. Fluid shear stress inhibits vascular inflammation by decreasing thioredoxin-interacting protein in endothelial cells. J Clin Invest 115: 733–738, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yang B, Radel C, Hughes D, Kelemen S, and Rizzo V. p190 RhoGTPase-activating protein links the beta1 integrin/caveolin-1 mechanosignaling complex to RhoA and actin remodeling. Arterioscler Thromb Vasc Biol 31: 376–383, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yang B. and Rizzo V. Shear stress activates eNOS at the endothelial apical surface through 1 containing integrins and caveolae. Cell Mol Bioeng 6: 346–354, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ye F, Petrich BG, Anekal P, Lefort CT, Kasirer-Friede A, Shattil SJ, Ruppert R, Moser M, Fassler R, and Ginsberg MH. The mechanism of kindlin-mediated activation of integrin alphaIIbbeta3. Curr Biol 23: 2288–2295, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ye F, Snider AK, and Ginsberg MH. Talin and kindlin: the one-two punch in integrin activation. Front Med 8: 6–16, 2014 [DOI] [PubMed] [Google Scholar]
- 193.Yeh LH, Park YJ, Hansalia RJ, Ahmed IS, Deshpande SS, Goldschmidt-Clermont PJ, Irani K, and Alevriadou BR. Shear-induced tyrosine phosphorylation in endothelial cells requires Rac1-dependent production of ROS. Am J Physiol 276: C838–C847, 1999 [DOI] [PubMed] [Google Scholar]
- 194.Yurdagul A, Jr., Chen J, Funk SD, Albert P, Kevil CG, and Orr AW. Altered nitric oxide production mediates matrix-specific PAK2 and NF-kappaB activation by flow. Mol Biol Cell 24: 398–408, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yurdagul A, Jr., Green J, Albert P, McInnis MC, Mazar AP, and Orr AW. alpha5beta1 integrin signaling mediates oxidized low-density lipoprotein-induced inflammation and early atherosclerosis. Arterioscler Thromb Vasc Biol 34: 1362–1373, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhang X, Jiang G, Cai Y, Monkley SJ, Critchley DR, and Sheetz MP. Talin depletion reveals independence of initial cell spreading from integrin activation and traction. Nat Cell Biol 10: 1062–1068, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhang X, Meng H, and Wang MM. Collagen represses canonical Notch signaling and binds to Notch ectodomain. Int J Biochem Cell Biol 45: 1274–1280, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhao H, Hiroi T, Hansen BS, and Rade JJ. Cyclic stretch induces cyclooxygenase-2 gene expression in vascular endothelial cells via activation of nuclear factor kappa-beta. Biochem Biophys Res Commun 389: 599–601, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zhao T. and Newman PJ. Integrin activation by regulated dimerization and oligomerization of platelet endothelial cell adhesion molecule (PECAM)-1 from within the cell. J Cell Biol 152: 65–73, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhou A, Egginton S, Hudlicka O, and Brown MD. Internal division of capillaries in rat skeletal muscle in response to chronic vasodilator treatment with alpha1-antagonist prazosin. Cell Tissue Res 293: 293–303, 1998 [DOI] [PubMed] [Google Scholar]
- 201.Zhou J, Lee PL, Lee CI, Wei SY, Lim SH, Lin TE, Chien S, and Chiu JJ. BMP receptor-integrin interaction mediates responses of vascular endothelial Smad1/5 and proliferation to disturbed flow. J Thromb Haemost 11: 741–755, 2013 [DOI] [PubMed] [Google Scholar]
- 202.Zhou J, Lee PL, Tsai CS, Lee CI, Yang TL, Chuang HS, Lin WW, Lin TE, Lim SH, Wei SY, Chen YL, Chien S, and Chiu JJ. Force-specific activation of Smad1/5 regulates vascular endothelial cell cycle progression in response to disturbed flow. Proc Natl Acad Sci U S A 109: 7770–7775, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zilberberg L, Todorovic V, Dabovic B, Horiguchi M, Courousse T, Sakai LY, and Rifkin DB. Specificity of latent TGF-beta binding protein (LTBP) incorporation into matrix: role of fibrillins and fibronectin. J Cell Physiol 227: 3828–3836, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]