Abstract
Heparanase (HPSE) is the only endo-β-D-glucuronidase that is correlated with the progression of neuroblastoma (NB), the most common extracranial malignancy in childhood. However, the mechanisms underlying HPSE expression in NB still remain largely unknown. Herein, through analyzing cis-regulatory elements and mining public microarray datasets, we identified SMAD family member 4 (Smad4) as a crucial transcription regulator of HPSE in NB. We demonstrated that Smad4 repressed the HPSE expression at the transcriptional levels in NB cells. Mechanistically, Smad4 suppressed the HPSE expression through directly binding to its promoter and repressing the lymphoid enhancer binding factor 1 (LEF1)-facilitated transcription of HPSE via physical interaction. Gain- and loss-of-function studies demonstrated that Smad4 inhibited the growth, invasion, metastasis, and angiogenesis of NB cells in vitro and in vivo. Restoration of HPSE expression prevented the NB cells from changes in these biological features induced by Smad4. In clinical NB specimens, Smad4 was under-expressed and inversely correlated with HPSE levels, while LEF1 was highly expressed and positively correlated with HPSE expression. Patients with high Smad4 expression, low LEF1 or HPSE levels had greater survival probability. These results demonstrate that Smad4 suppresses the tumorigenesis and aggressiveness of NB through repressing the HPSE expression.
Neuroblastoma (NB), a malignancy derived from neural crest cells of sympathetic nervous system, accounts for approximately 15% of all cancer-related mortality in childhood1. The clinical features of NB are heterogeneous, ranging from spontaneous regression to rapid progression and resistance to multimodal therapy1. For high-risk NB patients, tumor invasion and metastasis are the main causes of death, suggesting the urgency to investigate the underlying mechanisms for improving the outcome of NB patients1. It has been established that extracellular matrix (ECM) is an important structure surrounding the cells and vessels, and provides a physical barrier for the migration of tumor cells2. During the process of tumor invasion and metastasis, degradation of ECM and basement membrane is an initial and essential step3, which is also linked with tumor angiogenesis3. Thus, identification of crucial proteolytic enzymes that drive ECM degrading and remodeling will provide novel insights to improve the therapeutic efficiency of NB.
Heparanase (HPSE) is the only mammalian endo-β-D-glucuronidase that degrades the heparan sulphate glycosaminoglycan within the ECM and basement membrane4. In normal tissues, HPSE is detectable in platelets, neutrophils, and activated T lymphocytes 5. Meanwhile, up-regulation of HPSE has been demonstrated in a number of primary human malignancies, such as bladder cancer, prostate cancer, melanoma, pancreas cancer, colorectal cancer, and gallbladder cancer6, and is correlated with increased metastatic potentials and decreased survival rates6. It has also been documented that HPSE is functionally related to the invasion and metastasis of tumor cells4. In addition, HPSE is tightly involved in tumor angiogenesis by releasing angiogenic factors stored in the ECM such as basic fibroblast growth factor7, and promoting vascular endothelial growth factor (VEGF) expression via activation of the Src pathway8. Our previous studies have shown that HPSE is highly expressed in NB tissues, and is a prognostic factor for poor outcome of NB patients9. However, the mechanisms underlying the HPSE expression in NB still remain largely unknown.
In the current study, through analyzing the cis-regulatory elements and mining public microarray datasets, we identified SMAD family member 4 (Smad4) as a crucial transcription regulator of HPSE expression in NB. We demonstrate, for the first time, that Smad4 is under-expressed and inversely correlated with HPSE expression in clinical NB specimens. In addition, Smad4 represses the expression of HPSE through directly binding to its promoter and attenuating lymphoid enhancer binding factor 1 (LEF1)-facilitated transcription of HPSE via physical interaction, thus suppressing the growth, invasion, metastasis and angiogenesis of NB cells in vitro and in vivo, suggesting the tumor suppressive roles of Smad4 in the progression of NB.
Results
Smad4 represses the expression of HPSE in cultured NB cell lines
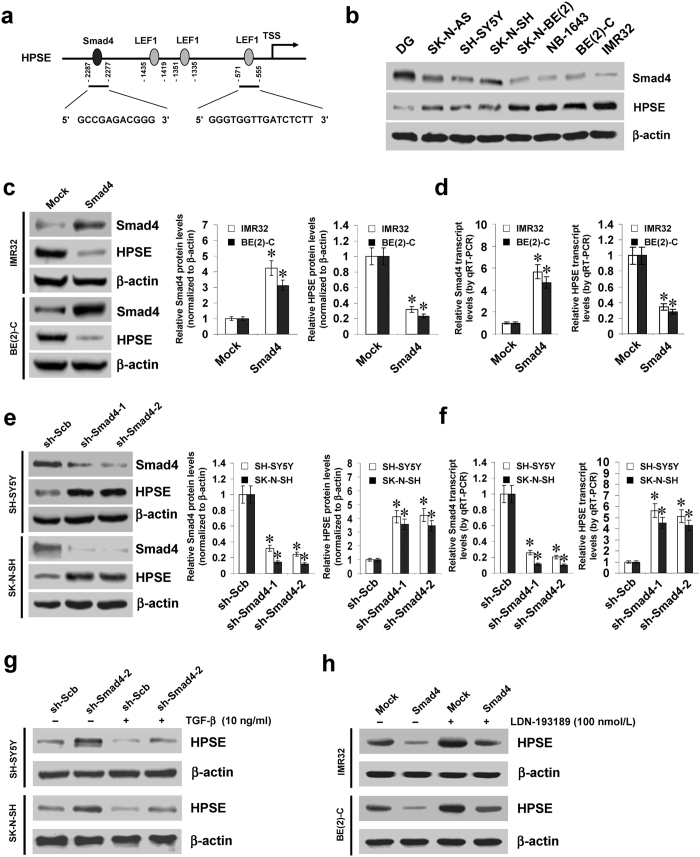
Mining the publicly available databases Genomatix10 and R2: microarray analysis and visualization platform (http://r2.amc.nl) revealed three potential transcription factors correlated with HPSE expression in NB tissues, including Smad4, LEF1, and peroxisome proliferator-activated receptor gamma (PPARG) (Supplementary Fig. S1a). However, transfection of short hairpin RNA (shRNA) specific for PPARG did not affect the HPSE promoter activity in NB cell lines (Supplementary Fig. S1b). Meanwhile, one Smad4 binding site and three LEF1 binding sites were noted within the HPSE promoter, locating at bases −2287/−2277, −1435/−1419, −1351/−1335, and −571/−555 relative to the transcription start site (TSS), respectively (Fig. 1a). Notably, negative or positive correlation between HPSE and Smad4 or LEF1 expression was observed in different NB and neuroblastic tumor cohorts (Supplementary Fig. S1c). Lower Smad4 and higher HPSE levels were observed in NB cell lines than those in normal dorsal ganglia (DG; Fig. 1b).
Figure 1. Smad4 represses the expression of HPSE in cultured NB cell lines.
(a) scheme of the potential binding sites of Smad4 and LEF1 within HPSE promoter, locating at bases −2287/−2277, −1435/−1419, −1351/−1335, and −571/−555 upstream the transcription start site (TSS). (b) western blot showing the expression levels of Smad4 and HPSE in normal dorsal ganglia (DG) and NB cell lines. (c,d) western blot and real-time quantitative RT-PCR indicating the protein and transcript levels of Smad4 and HPSE in IMR32 and BE(2)-C cells stably transfected with empty vector (mock) or Smad4. (e,f) western blot and real-time quantitative RT-PCR showing the protein and transcript levels of Smad4 and HPSE in SH-SY5Y and SK-N-SH cells stably transfected with scramble shRNA (sh-Scb) or shRNA specific for Smad4 (sh-Smad4). (g,h) western blot indicating the expression levels of HPSE in NB cells stably transfected with sh-Scb, sh-Smad4, mock or Smad4, and those treated with TGF-β or LDN-193189. *P < 0.01 vs. mock or sh-Scb.
To investigate the effects of Smad4 on HPSE expression in NB cells, we performed the Smad4 over-expression and knockdown experiments. Western blot and real-time quantitative RT-PCR assays indicated that stable transfection of Smad4 into IMR32 and BE(2)-C cells obviously increased the expression of Smad4, and decreased the HPSE levels, than those of empty vector (mock)-transfected cells (Fig. 1c,d). In contrast, transfection of shRNAs targeting Smad4 (sh-Smad4) into SH-SY5Y and SK-N-SH cells resulted in decreased protein and transcript levels of Smad4 and increased HPSE expression in NB cells, when compared to those stably transfected with scramble short hairpin RNA (sh-Scb) (Fig. 1e,f). Nuclear run-on assay indicated that stable over-expression or knockdown of Smad4 decreased or increased the nascent transcript levels of HPSE in NB cells, respectively (Supplementary Fig. S2a). In SH-SY5Y and SK-N-SH cells, administration of transforming growth factor beta (TGF-β) resulted in increased activity of Smad4 response element reporter (Supplementary Fig. S2b) and decreased HPSE levels (Fig. 1g and Supplementary Fig. S2c), which were abolished by knockdown of Smad4 (Fig. 1g, Supplementary Fig. S2b,c). However, treatment of IMR32 and BE(2)-C cells with either TGF-β or inhibitor of its receptors (LY364947)11, did not affect the nuclear translocation of Smad4 and inhibition of HPSE expression induced by stable transfection of Smad4 (Supplementary Fig. S3a,b). Co-immunoprecipitation (Co-IP) and western blot assays indicated that transfection of D351H and R361H, two Smad4 constructs with mutation of the loop-helix region12, abolished the interaction of Smad4 with R-Smads, phosphorylated Smad1 and Smad2 (p-Smad1 and p-Smad2), in these NB cells (Supplementary Fig. S3c). In addition, transfection of D351H or R361H did not influence the expression levels and promoter activity of HPSE (Supplementary Fig. S3d–f). Treatment with activin A-neutralizing antibody did not diminish the activation of Smad4 response element reporter in IMR32 and BE(2)-C cells stably transfected with Smad4 (Supplementary Fig. S3g). Instead, administration of LDN-193189, the inhibitor of bone morphogenetic protein (BMP) type I receptors13, abolished the increased activity of BMP/Smad transcriptional reporter induced by BMP-2 (Supplementary Fig. S2h). Moreover, LDN-193189 treatment also prevented the IMR32 and BE(2)-C cells from decrease in the expression of HPSE induced by stable transfection of Smad4 (Fig. 1h and Supplementary Fig. S3i). These results demonstrated that Smad4 considerably repressed the HPSE expression at the transcriptional levels in NB cells.
Smad4 represses the transcription of HPSE through direct binding to its promoter
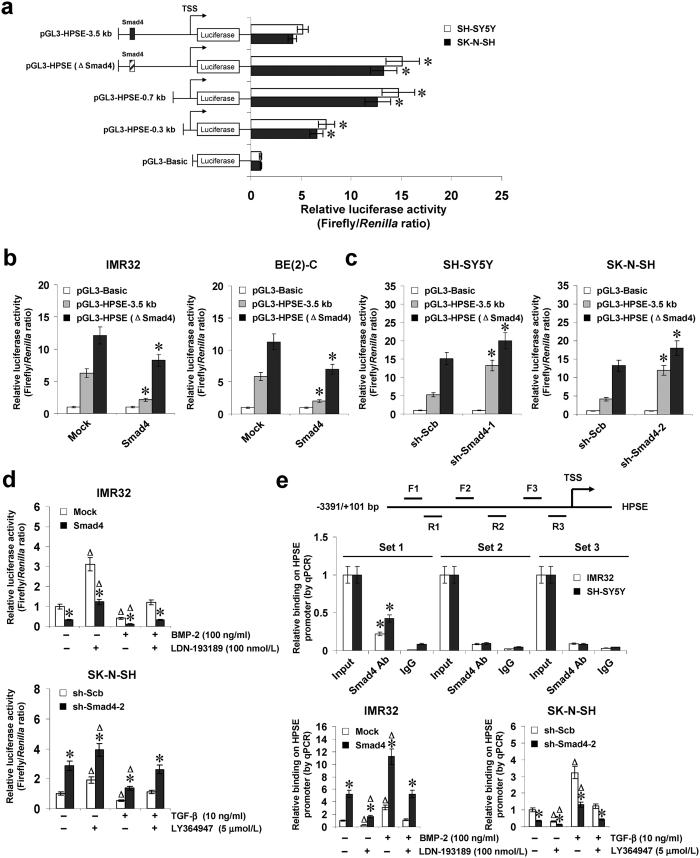
To determine whether Smad4 could directly target its binding site, the HPSE promoter luciferase reporter vector and its truncates were transfected into NB cells stably transfected with empty vector (mock) or Smad4. Dual-luciferase assay indicated that −3.5 to −0.7 kb relative to TSS was essential for the negative control of HPSE promoter activity, and mutation of Smad4 binding site within this region resulted in increased HPSE promoter activity in cultured SH-SY5Y and SK-N-SH cells (Fig. 2a). Ectopic expression or knockdown of Smad4 attenuated and enhanced the promoter activity of HPSE in NB cells, respectively (Fig. 2b,c), and mutation of Smad4 binding site partially abolished these effects (Fig. 2b,c). In addition, BMP-2 treatment facilitated the decreased HPSE promoter activity in IMR32 cells stably transfected with Smad4, which was abolished by administration of LDN-193189 (Fig. 2d). Meanwhile, administration of TGF-β attenuated the increase in HPSE promoter activity induced by stable knockdown of Smad4 in SK-N-SH cells, and these effects were abolished by LY364947 treatment (Fig. 2d). Chromatin immunoprecipitation (ChIP) and quantitative PCR (qPCR) assays were applied to measure the enrichment of Smad4 on HPSE promoter with three tiled primer sets. In cultured NB cells, enrichment of Smad4 was observed at the region (−2347/−2148) around its binding site (Fig. 2e). As controls, no HPSE promoter regions were immunoprecipitated with unspecific antibody (isotype IgG) (Fig. 2e). Stable transfection of Smad4 or sh-Smad4 into IMR32 and SK-N-SH cells resulted in increased and decreased binding of Smad4 to HPSE promoter, respectively (Fig. 2e), which were abolished by treatment with LDN-193189 or TGF-β (Fig. 2e). These results indicated that Smad4 directly interacted with the binding site within HPSE promoter to repress its transcription.
Figure 2. Smad4 represses the transcription of HPSE through direct binding to its promoter.
(a) dual-luciferase assay showing the activity of HPSE promoter reporter and its truncates and mutant in SH-SY5Y and SK-N-SH cells. (b,c) dual-luciferase assay indicating the activity of HPSE promoter and its mutant in NB cells stably transfected with empty vector (mock), Smad4, scramble shRNA (sh-Scb), or shRNA specific for Smad4 (sh-Smad4). (d) dual-luciferase assay showing the activity of pGL3-HPSE-3.5 kb in IMR32 and SK-N-SH cells stably transfected with mock, Smad4, sh-Scb, or sh-Smad4, and those treated with BMP-2, LDN-193189, TGF-β protein, or LY364947. (e) ChIP and qPCR assay indicating the enrichment of Smad4 on HPSE promoter in IMR32 and SK-N-SH cells stably transfected with mock, Smad4, sh-Scb, or sh-Smad4, and those treated with BMP-2, LDN-193189, TGF-β protein, or LY364947. *P < 0.01 vs. pGL3-HPSE-3.5 kb, mock, sh-Scb, or IgG; ΔP < 0.01 vs. control untreated with BMP-2, LDN-193189, TGF-β protein, or LY364947.
Smad4 represses the LEF1-facilitated transcription of HPSE in NB cells
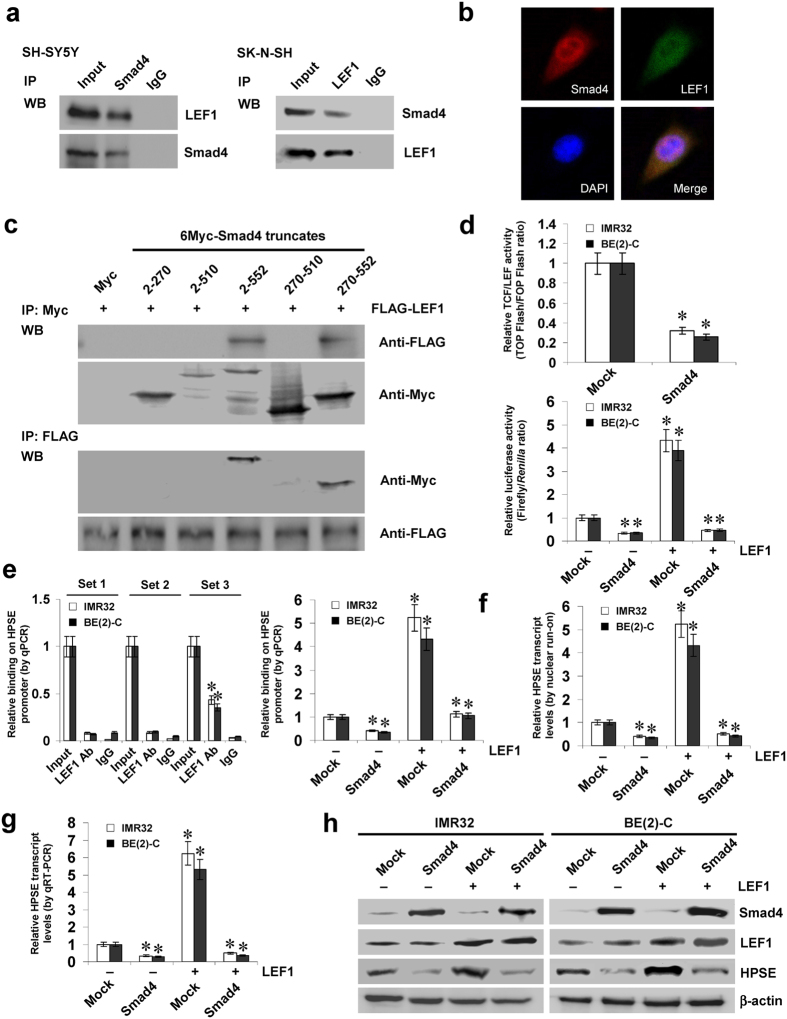
Since previous studies indicate the interaction between Smad4 and LEF114,15, and combining above evidence showing the potential roles of LEF1 in HPSE expression, we further investigated the effects of Smad4 on LEF1-mediated HPSE expression. As shown in Fig. 3a,b, Co-IP and immunofluorescence assays revealed the endogenous protein interaction between Smad4 and LEF1 in cultured NB cells. To determine the domain essential for this interaction, a series of Myc-tagged Smad4 truncates were co-transfected with FLAG-tagged LEF1 construct into NB cells, and the results indicated that the 511–552 amino acids of Smad4 was crucial for the interaction with LEF1 (Fig. 3c). Dual-luciferase assay indicated that stable transfection of Smad4 attenuated the activity of T-cell factor (TCF)/LEF-responsive construct, and inhibited the LEF1-facilitated activity of HPSE promoter in IMR32 and BE(2)-C cells (Fig. 3d). ChIP and qPCR assays revealed the endogenous enrichment of LEF1 on the −673/−476 bp region, but not the −2347/−2148 or −1505/−1268 bp region, of HPSE promoter (Fig. 3e). Transfection of LEF1 increased the HPSE promoter activity in NB cells, while mutation of LEF1 binding site within −673/−476 bp region abolished these effects (Supplementary Fig. S4a). In addition, transfection of Smad4 prevented the increased enrichment of LEF1 on HPSE promoter induced by ectopic expression of LEF1 (Fig. 3e). Dual-luciferase, nuclear run-on, real-time quantitative RT-PCR, and western blot assays further indicated that stable transfection of Smad4 prevented the NB cells from increased promoter activity and expression levels of HPSE induced by LEF1 over-expression (Supplementary Fig. S4a, Fig. 3f–h). These results indicated that Smad4 repressed the LEF1-facilitated HPSE transcription in NB cells.
Figure 3. Smad4 represses the LEF1-facilitated transcription of HPSE in NB cells.
(a,b) Co-IP and immunofluorescence assays revealing the endogenous interaction between Smad4 and LEF1 in NB cells. (c) IP and western blot assays showing the interaction between Smad4 and LEF1 in IMR32 cells transfected with different Myc-tagged Smad4 truncates and FLAG-tagged LEF1 construct, running under the same experimental conditions (full-length blots are presented in Supplementary Figure S7). (d) dual-luciferase assay indicating the activity of TCF/LEF-responsive constructs TOP-FLASH and FOP-FLASH and the HPSE promoter reporter in NB cells stably transfected with empty vector (mock) or Smad4, and those co-transfected with LEF1. (e) ChIP and qPCR assay showing the enrichment of LEF1 on HPSE promoter in NB cells stably transfected with mock or Smad4, and those co-transfected with LEF1. (f,g) nuclear run-on and real-time quantitative RT-PCR assays indicating the HPSE transcript levels in NB cells stably transfected with mock or Smad4, and those co-transfected with LEF1. (h) western blot assay showing the expression of Smad4, LEF1, and HPSE in NB stably transfected with mock or Smad4, and those co-transfected with LEF1. *P < 0.01 vs. mock or IgG.
Smad4 suppresses the growth, invasion, and angiogenesis of NB cells in vitro
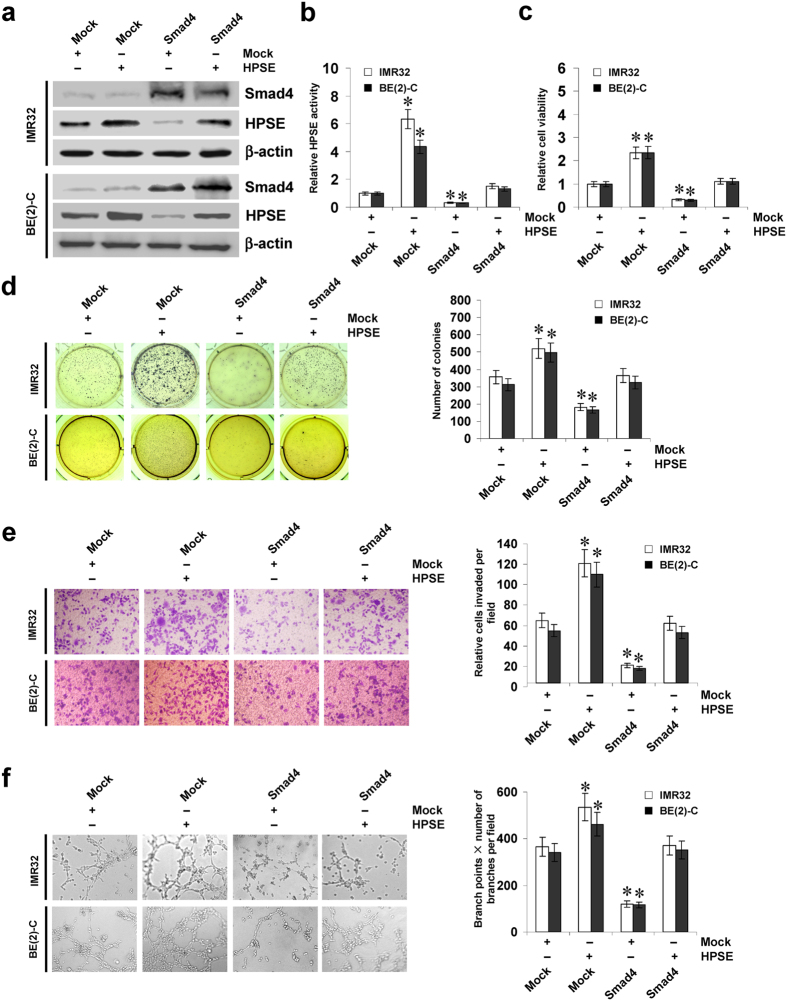
Since previous studies indicate that HPSE participates in the growth, invasion, and angiogenesis of cancer cells16,17, and combining the evidence that Smad4 directly regulated the expression of HPSE, we investigated the effects of Smad4 over-expression and HPSE restoration on cultured NB cells. As shown in Fig. 4a,b, transfection of HPSE increased the expression and activity of HPSE, and restored the decrease in HPSE expression and activity induced by Smad4 in IMR32 and BE(2)-C cells. In MTT colorimetric and soft agar assays, stable transfection of Smad4 suppressed the viability and anchorage-independent growth of NB cells, when compared to those stably transfected with empty vector (mock) (Fig. 4c,d). In matrigel invasion assay, Smad4 over-expression decreased the invasion capability of IMR32 and BE(2)-C cells (Fig. 4e). The tube formation of endothelial cells was decreased by treatment with the medium preconditioned by stable transfection of NB cells with Smad4 (Fig. 4f). In addition, restoration of HPSE expression via transfection of HPSE vector prevented the NB cells from their decrease in growth, invasion, and angiogenesis induced by stable over-expression of Smad4 (Fig. 4c–f).
Figure 4. Smad4 suppresses the growth, invasion and angiogenesis of NB cells in vitro.
(a) western blot indicating the expression of Smad4 and HPSE in IMR32 and BE(2)-C cells stably transfected with empty vector (mock) or Smad4, and those co-transfected with HPSE. (b) colorimetric assay for the HPSE activity in NB cells stably transfected with mock or Smad4, and those co-transfected with HPSE. (c,d) MTT colorimetric and soft agar assays [representation (left) and quantification (right)] showing the viability and anchorage-independent growth capability of NB cells stably transfected with mock or Smad4, and those co-transfected with HPSE. (e) representation (left) and quantification (right) of matrigel invasion assay indicating the invasion capability of NB cells transfected with mock or Smad4, and those co-transfected with HPSE. (f) representation (left) and quantification (right) of tube formation assay showing the angiogenic capability of NB cells transfected with mock or Smad4, and those co-transfected with HPSE. *P < 0.01 vs. mock.
On the other hand, we investigated the effects of Smad4 on LEF1-mediated HPSE activity, growth, invasion, and angiogenesis of NB cells. As shown in Supplementary Fig. S4b, increased HPSE activity was observed in NB cells stably transfected with LEF1. In MTT colorimetric and soft agar assays, LEF1 over-expression promoted the viability and anchorage-independent growth of IMR32 and BE(2)-C cells, when compared to those stably transfected with mock (Supplementary Fig. S4c,d). Matrigel invasion assay showed that NB cells stably transfected with LEF1 presented an increased invasion capacity than mock-transfected cells (Supplementary Fig. S4e). The tube formation of endothelial cells was enhanced by treatment with the medium preconditioned by stable transfection of NB cells with LEF1 (Supplementary Fig. S4f). In addition, transfection of Smad4 rescued the IMR32 and BE(2)-C cells from their changes in HPSE activity, growth, invasion, and angiogenesis induced by ectopic expression of LEF1 (Supplementary Fig. S4b–f). Collectively, these results revealed the tumor suppressive roles of Smad4 in regulating the growth, invasion, and angiogenesis of NB cells.
Smad4 suppresses the growth, metastasis, and angiogenesis of NB cells in vivo
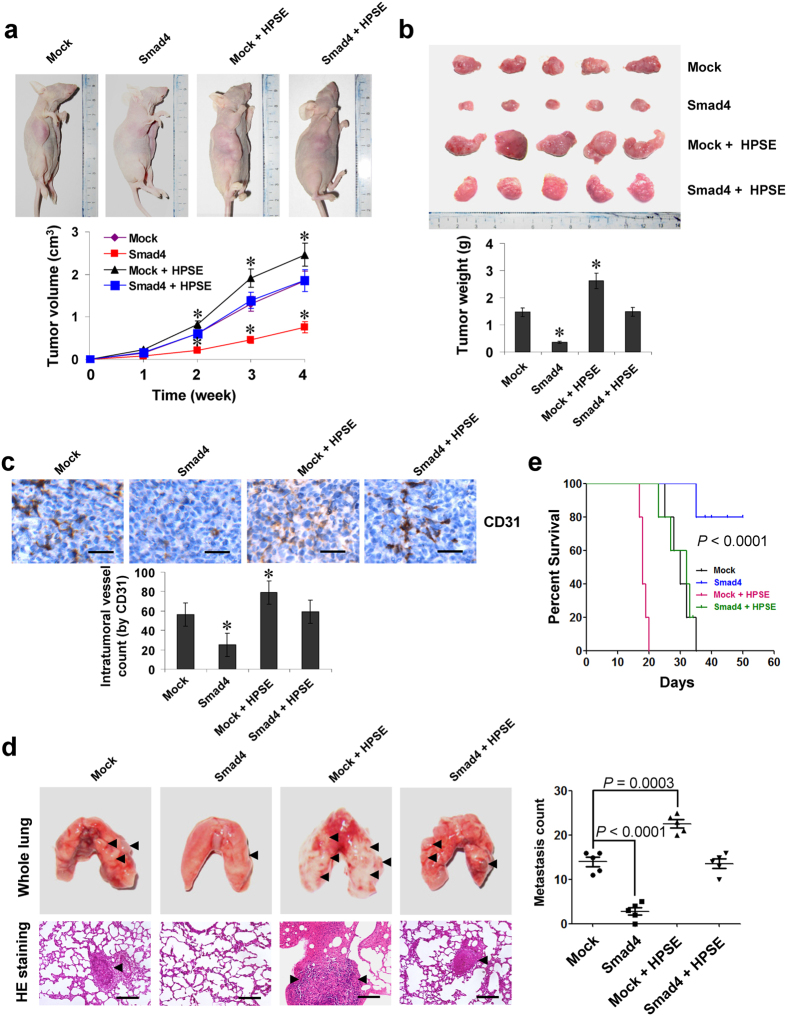
We next investigated the efficacy of Smad4 over-expression against tumor growth, metastasis, and angiogenesis in vivo. Stable transfection of Smad4 into BE(2)-C cells resulted in decreased growth and tumor weight of subcutaneous xenograft tumors in athymic nude mice, when compared to those stably transfected with empty vector (mock; Fig. 5a,b). In addition, stable transfection of Smad4 resulted in a decrease in CD31-positive mean vessel density within tumors (Fig. 5c). In the experimental metastasis studies, BE(2)-C cells stably transfected with Smad4 established statistically fewer lung metastatic colonies and higher survival probability than mock group (Fig. 5d,e). Restoration of HPSE expression abolished these changes induced by stable over-expression of Smad4 (Fig. 5a–e). These results were consistent with the findings that Smad4 suppressed the growth, invasion, and angiogenesis of NB cells in vitro.
Figure 5. Smad4 suppresses the growth, metastasis and angiogenesis of NB cells in vivo.
(a) tumor growth curve of BE(2)-C (1 × 106) stably transfected with empty vector (mock) or Smad4, and those co-transfected with HPSE in athymic nude mice (n = 5 for each group), after hypodermic injection for 4 weeks. (b) representation (top) and quantification (bottom) of xenograft tumors formed by hypodermic injection of BE(2)-C cells stably transfected with mock or Smad4, and those co-transfected with HPSE. (c) immunohistochemical staining (top) and quantification (bottom) of CD31 expression within tumors formed by hypodermic injection of BE(2)-C cells stably transfected with mock or Smad4, and those co-transfected with HPSE. Scale bars: 100 μm. (d) representation (left, arrowhead) and quantification (right) of lung metastasis in nude mice with injection of BE(2)-C cells (0.4 × 106) stably transfected with mock or Smad4, and those co-transfected with HPSE via the tail vein (n = 5 for each group). Scale bars: 100 μm. (e) Kaplan–Meier survival plots of athymic nude mice with injection of BE(2)-C cells (0.4 × 106) stably transfected with mock or Smad4, and those co-transfected with HPSE via the tail vein (n = 5 for each group). *P < 0.01 vs. mock.
Smad4 and LEF1 are inversely or positively correlated with HPSE expression in NB tissues
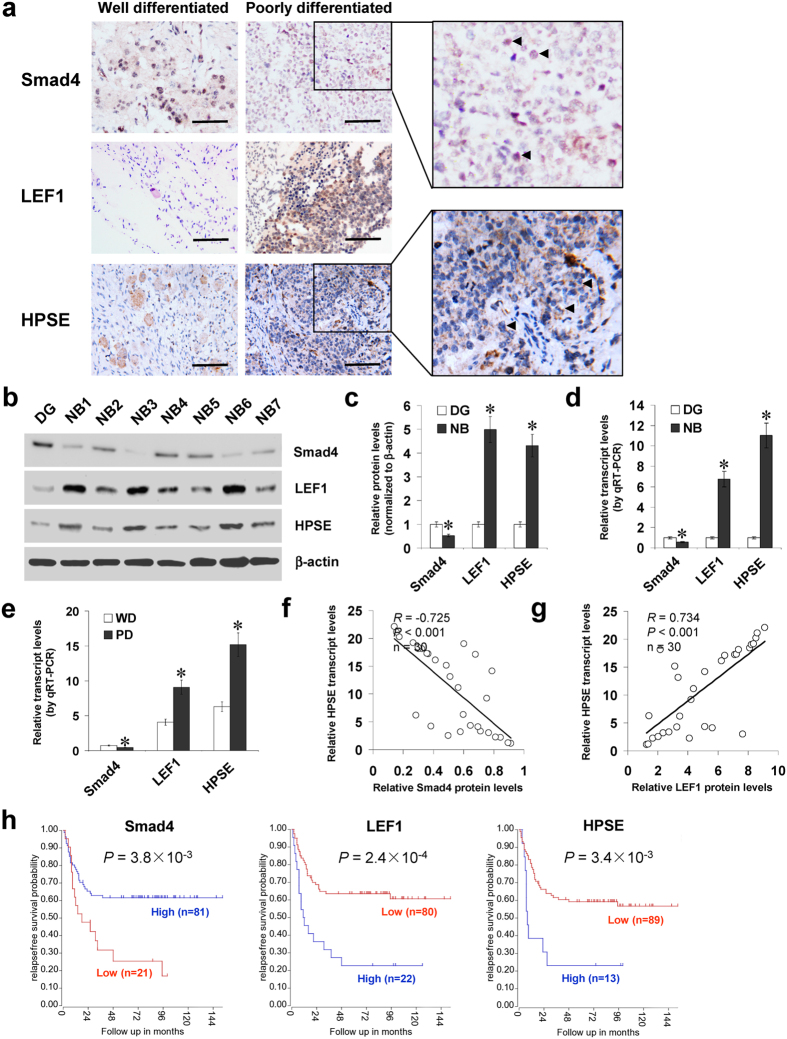
Mining the publicly available data derived from Oncogenomics (https://pob.abcc.ncifcrf. gov/cgi-bin/JK) and TARGET (https://target.nci.nih.gov/dataMatrix/) indicated no copy number loss and low mutation frequency of Smad4 gene in NB (Supplementary Fig. S5a,b). To investigate the expression of Smad4 and LEF1 in NB tissues, paraffin-embedded sections from 42 well-established primary cases were collected. Immunohistochemical staining revealed that Smad4 and LEF1 were expressed in the nuclei of tumor cells (Fig. 6a). Smad4 expression was detected in 16/42 (38.1%) cases and the staining was weak in 8, moderate in 5, and intense in 3 (Supplementary Table S1). The Smad4 immunoreactivity was significantly lower in NB cases with poor differentiation (P < 0.001), higher mitosis karyorrhexis index (MKI) (P = 0.002), and advanced international neuroblastoma staging system (INSS) stages (P = 0.016) (Supplementary Table S1). Notably, the immunostaining of HPSE was inversely or positively correlated with that of Smad4 (correlation coefficient R = −0.583, P < 0.001) and LEF1 (correlation coefficient R = 0.553, P < 0.001) in NB cases, respectively (Fig. 6a and Supplementary Table S2). Western blot and real-time quantitative RT-PCR were applied to measure the expression levels of Smad4, LEF1, and HPSE in 30 NB specimens and DG. As shown in Fig. 6b–d, lower Smad4 expression and higher LEF1 or HPSE levels were observed in NB tissues than those in DG, which was in line with the data derived from R2: microarray analysis and visualization platform (Supplementary Fig. S5c). In addition, lower Smad4 levels and higher expression of LEF1 or HPSE were observed in NB specimens with poorer differentiation (Fig. 6e), advanced INSS stages (Supplementary Fig. S5d), or relapse (Supplementary Fig. S5d). There was an inverse correlation between Smad4 protein and HPSE transcript levels in NB tissues (correlation coefficient R = −0.725, P < 0.001, Fig. 6f), while LEF1 protein levels were positively correlated with HPSE transcript levels (correlation coefficient R = 0.734, P < 0.001, Fig. 6g). Kaplan–Meier survival plots of 102 well-defined NB cases derived from R2 microarray analysis and visualization platform revealed that patients with high Smad4 expression (P = 3.8 × 10−3), low LEF1 levels (P = 2.4 × 10−4), or low HPSE expression (P = 3.4 × 10−3) expression had greater survival probability (Fig. 6h), which was further validated by different NB cohorts derived from R2 database (Supplementary Fig. S6). These results indicated that Smad4 and LEF1 were inversely or positively correlated with HPSE expression in NB tissues.
Figure 6. Smad4 and LEF1 are inversely or positively correlated with HPSE expression in NB tissues.
(a) immunohistochemical staining revealing the expression of Smad4, LEF1, and HPSE in NB specimens (arrowheads, brown). Scale bars: 100 μm. (b–d) western blot and real-time quantitative RT-PCR assays showing the protein and transcript levels of Smad4, LEF1, and HPSE in NB tissues (n = 30) and normal dorsal ganglia (DG). (e) real-time quantitative RT-PCR indicating the transcript levels of Smad4, LEF1, and HPSE in NB tissues with well (WD) or poor differentiation (PD). (f–g) the correlation between Smad4 protein, LEF1 protein, and HPSE transcript levels in NB tissues (n = 30). (h) Kaplan–Meier survival plots of 102 well-defined NB cases derived from R2 microarray analysis and visualization platform (http://r2.amc.nl) showing the survival probability of patients with high or low expression of Smad4, LEF1 or HPSE. *P < 0.01 vs. DG or WD.
Discussion
Smad4, locating at chromosome 18q21, was first identified as a tumor suppressor gene in pancreatic cancer18. Subsequent studies show that Smad4 is involved in the TGF-β family signaling pathways19. The TGF-β family contains about 40 structurally related factors, including TGF-β, activin, inhibins, and BMPs, which exert signaling through binding to their receptors; upon receptor activation, the receptor-regulated Smads (R-Smads), including Smad1, Smad2, Smad3, Smad5 and Smad8, are phosphorylated to associate with the common Smad, Smad4, and mediate the nuclear translocation of heteromeric complex that regulates gene transcription19,20. It has been indicated that Smad4 is down-regulated in many human cancers, as a result of mutation, homozygous deletion, and heterozygous loss21. Mutation of Smad4 gene occurs frequently in pancreatic22 and colorectal cancer23. Transfection of Smad4 into pancreatic cancer cells reduces the anchorage-independent growth by more than 50%, and inhibits xenograft tumor growth24. In addition, stable over-expression of Smad4 in colon cancer cells suppresses their growth in vivo25. However, few mutation of Smad4 gene is seen in the rest of human cancers26. In the current study, we searched the publicly available databases Oncogenomics and TARGET, and found no copy number loss and low mutation frequency of Smad4 in NB. We demonstrated the down-regulation of Smad4 in NB tissues and cell lines. Low Smad4 expression was associated with poor prognosis of NB patients, suggesting that Smad4 may be a potential outcome predictor for NB. In addition, we found that ectopic expression of Smad4 suppressed the growth, invasion, metastasis, and angiogenesis of NB cells, suggesting the tumor suppressive roles of Smad4 in the progression of NB.
The heteromeric Smad complexes regulate target gene expression through direct binding to DNA, interaction with DNA-binding proteins, or recruitment of transcriptional co-activators or co-repressors19. Smad4 binds to the Smad-binding element, an 8-bp palindromic sequence (5′-GTCTAGAC-3′), for transcriptional regulation of TGF-β target genes27. Through targeting the promoter regions, Smad4 induces the expression of p15ink4b to inhibit the growth of pancreatic cancer cells24. Enhanced Smad4 binding to the promoter of snail family zinc finger (Snail) contributes to its expression and epithelial-mesenchymal transition in skin carcinogenesis28. Over-expression of Smad4 induces the expression of p21waf1 in breast cancer cells in the presence or absence of TGF-β stimulation, and Smad4 can bypass the TGF-β receptor activation to increase p21waf1 levels in pancreatic cancer cells29. In addition, Smad4 positively regulates the c-Myc expression in a TGF-β-independent manner12. In this study, we demonstrated that HPSE was a novel transcriptional target gene of Smad4. Previous studies have shown the existence of TGF-β/Smad signaling in some NB cell lines. TGF-β treatment facilitates the nuclear localization of Smad2 in SK-N-SH cells30. Ectopic over-expression of TGF-β type II receptor suppresses the tumor-forming ability and induces differentiation in LAN5 cells31. However, TGF-β and its receptors are usually down-regulated in aggressive or advanced stage NB32,33. Our findings indicated that TGF-β/Smad4 signaling was able to repress the HPSE expression in two non-MYCN amplified NB cells (SK-N-SH and SH-SY5Y), but not in MYCN amplified ones. In IMR32 and BE(2)-C cells, the Smad4-repressed HPSE expression was abolished by disruption of its interaction with p-Smad1 or p-Smad2, two R-Smads activated by BMP and activin receptors19,20. Since administration of activin A-neutralizing antibody did not affect the activity of Smad4 response element reporter in these cells, we ruled out the possible involvement of activin A signaling. Instead, as a member of BMP family expressing mainly in the nervous system, BMP-2 activated the BMP/Smad transcriptional reporter and facilitated the down-regulation of HPSE induced by Smad4 in IMR32 and BE(2)-C cells, which were abolished by the inhibitor of BMP type I receptors. It has been indicated that BMP-2 induces the phosphorylation of Smad1/5/834, and is capable of facilitating growth arrest and neuronal differentiation in NB cells35. We believe that both TGF-β-dependent and -independent R-Smad activation occurs in NB cells to regulate the Smad4-mediated repression of HPSE expression in a context-dependent manner.
Human HPSE gene is located at chromosome 4q21.3, and its expression is regulated at the transcriptional levels36. The HPSE promoter contains GC-rich sequences and lacks a typical TATA or CCAAT box36. Previous studies indicate that transcription factors specific protein 1 and ETS are associated with basal HPSE promoter activity in thyroid tumor cells37, while early growth response 1 is implicated in inducible transcription of HPSE gene in prostate cancer cells38. In glioblastoma, glioma-associated oncogene homolog 1 facilitates the transcription of HPSE to promote tumor angiogenesis and aggressive growth39. Nuclear factor κB mediates the hypoxia-induced up-regulation of HPSE in pancreatic caner cells40. In addition, HPSE is induced in tumor cells by hepatocyte growth factor41, basic fibroblast growth factor41, and platelet-derived growth factor41. On the other hand, tumor suppressor p53 negatively regulates HPSE gene expression, suggesting that p53 gene mutation may contribute to increased HPSE expression in tumors42. Despite extensive studies on the regulation of HPSE expression during tumorigenesis, little is known about HPSE gene transcription in NB. In this study, we demonstrated that LEF1 facilitated the HPSE transcription via directly binding to its promoter. Previous studies indicate that LEF1 forms a complex with Smad4 to activate the promoter of msh homeobox 2 in murine embryonic stem cells15. However, in palate medial-edge epithelial cells, LEF1 is activated by forming a complex with Smad4 to bind with the E-cadherin promoter to repress its transcription14. Our evidence shows that Smad4 is able to interact with LEF1 to abolish its binding to proximal HPSE promoter region. Over-expression of Smad4 rescued the NB cells from LEF1-facilitated growth, invasion and angiogenesis, suggesting that Smad4 may exert its tumor suppressive functions, at least in part, through interacting and repressing the LEF1 activity in regulation of HPSE expression in NB.
In summary, we have shown that Smad4 is down-regulated in NB tissues, and inhibits the growth, invasion, metastasis, and angiogenesis of NB cells in vitro and in vivo. Furthermore, Smad4 suppresses the expression of HPSE via targeting its binding site within the promoter and attenuating LEF1-faclitated transcription in NB cell lines. This study extends our knowledge about the regulation of HPSE at the transcriptional level by transcription factors, and suggests that Smad4 and LEF1 may be of potential values as novel therapeutic targets for human NB. Meanwhile, the roles and underlying mechanisms of down-regulation of Smad4 in TGF-β family signaling pathways during the tumorigenesis and aggressiveness of NB warrant further investigation.
Methods
Cell culture
Human NB cell lines SK-N-AS (CRL-2137), SH-SY5Y (CRL-2266), SK-N-SH (HTB-11), SK-N-BE(2) (CRL-2271), NB-1643, BE(2)-C (CRL-2268), and IMR32 (CCL-127), and human endothelial cell line HUVEC (CRL-1730) were purchased from American Type Culture Collection (Rockville, MD) and Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). Cell lines were authenticated by the provider, used within 6 months after resuscitation of frozen aliquots, and grown in RPMI1640 medium (Life Technologies, Inc., Gaithersburg, MD) supplemented with 10% fetal bovine serum (Life Technologies, Inc.), penicillin (100 U/ml) and streptomycin (100 μg/ml). Cells were maintained at 37 °C in a humidified atmosphere of 5% CO2, and applied for transfection or treatment with TGF-β protein, LY364947, BMP-2 protein, LDN-193189 (Sigma, St. Louis, MO), or activin A-neutralizing antibody (R&D Systems Inc., Minneapolis, MN) as indicated.
Gene over-expression and knockdown
Human Smad4 cDNA (1659 bp) was provided by Dr. Anna Coppa43 and subcloned into pcDNA3.1 (Invitrogen, Carlsbad, CA) with primer indicated in Supplementary Table S3. The 6Myc-tagged truncates and FLAG-tagged construct of Smad4 were provided by Dr. Ralf Janknecht44 and Dr. Caroline S. Hill45, respectively. The Smad4 mutant constructs (D351H and R361H) were generated with GeneTailorTM Site-Directed Mutagenesis System (Invitrogen) and PCR primers (Supplementary Table S3). Human HPSE cDNA (1632 bp) and LEF1 cDNA (1200 bp) were amplified from NB tissue (Supplementary Table S3), and subcloned into pcDNA3.1 (Invitrogen) and pCMV-3Tag-1A (Addgene, Cambridge, MA), respectively. The empty vector (mock) and Smad4 or HPSE constructs were transfected into tumor cells, and stable cell lines were screened by administration of neomycin or puromycin (Invitrogen). The mock-transfected cells were applied as controls. To restore the Smad4-induced down-regulation of HPSE, stable cell lines were transfected with the recombinant vector pcDNA3.1-HPSE or pCMV-3Tag-1A-LEF1. The oligonucleotides encoding shRNA specific for Smad4 or PPARG, and their scramble sequences were subcloned into GV102 (Genechem Co., Ltd, Shanghai, China; Supplementary Table S3), and transfected into tumor cells with Genesilencer Transfection Reagent (Genlantis, San Diego, CA).
Luciferase reporter assay
Human HPSE promoter luciferase reporter constructs were kindly provided by Dr. Xiulong Xu (Rush University Medical Center)16,17,46,47. The TCF/LEF-responsive reporter (TOP-FLASH and FOP-FLASH), Smad4 response element reporter (pSBE4-Luc), and BMP/Smad transcriptional reporter (pGL3-BRE-Luc) plasmids were obtained from Millipore (Billerica, MA) and Addgene, respectively. Mutation of Smad4 or LEF1 binding site was performed with GeneTailorTM Site-Directed Mutagenesis System (Invitrogen) and PCR primers (Supplementary Table S3). Dual-luciferase assay was performed as previously described16,17,46,47.
Nuclear run-on assay
Nuclear run-on assay was performed based on the incorporation of biotin-16-uridine- 5’-triphosphate into nascent transcripts as previously described16,17,48,49. Total RNA was extracted using Trizol (Invitrogen), and biotinylated nascent RNA was purified using agarose-conjugated streptavidin beads (Invitrogen). Beads were then eluted, and biotinylated RNA was isolated for real-time quantitative RT-PCR assay with primers (Supplementary Table S4).
Western blot
Tissue or cellular protein was extracted with 1× cell lysis buffer (Promega, Madison, WI). Western blot was performed as previously described47,50,51,52,53, with antibodies specific for Smad4, p-Smad1, p-Smad2, LEF1, HPSE, H3 histone, and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA). ECL substrate kit (Amersham, Piscataway, NJ) was used for the chemiluminscent detection of signals with autoradiography film (Amersham).
Real-time quantitative RT-PCR
Total RNA was isolated with RNeasy Mini Kit (Qiagen Inc., Valencia, CA). The reverse transcription reactions were conducted with Transcriptor First Strand cDNA Synthesis Kit (Roche, Indianapolis, IN). Real-time PCR was performed with SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA) and primers indicated in Supplementary Table S4. The transcript levels were analyzed by 2−△△Ct method.
Co-immunoprecipitation
Co-IP was performed as previously described16, with antibodies specific for Smad4, LEF1, Myc, and FLAG (Upstate Biotechnology, Temacula, CA). The bead-bound proteins were released by boiling the protein A-Sepharose beads (Santa Cruz Biotechnology) in 1 × SDS-PAGE loading buffer and analyzed by western blot.
Chromatin immunoprecipitation
ChIP assay was performed according to the manufacture’s instructions of EZ-ChIP kit (Upstate Biotechnology)16,54,55. DNA was sonicated into fragments of an average size of 200 bp. Different sets of PCR primers were designed, targeting the Smad4 and LEF1 binding site within HPSE promoter (Supplementary Table S4). Real-time qPCR with SYBR Green PCR Master Mix was performed using ABI Prism 7700 Sequence Detector.
HPSE activity assay
The HPSE activity of tumor cells was measured according to the manufacture’s instructions of HepActivTM Heparanase Activity Assay Kit (InSight Biopharmaceuticals Ltd., Rehovot, Israel)56,57.
Cell viability assay
Tumor cells were cultured in 96-well plates at 5 × 103 cells per well. Cell viability was monitored by the 2-(4,5-dimethyltriazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT, Sigma) colorimetric assay16,55. All experiments were done with 6–8 wells per experiment and repeated at least three times.
Soft agar assay
Tumor cells at 5 × 103 were mixed with 0.05% Nobel agar (Fisher Scientific, Pittsburgh, PA) in growth medium and plated onto 6-well plates containing a solidified bottom layer (0.1% Noble agar in growth medium). After the incubation of cells for 21 days, the number of cell colonies was counted under the microscope, and the cells were fixed with 100% methanol and stained with 0.5% crystal violet dye50,54.
Cell invasion assay
Matrigel invasion assay was performed using membranes coated with Matrigel matrix (BD Science, Sparks, MD). Homogeneous single cell suspensions (1 × 105 cells/well) were added to the upper chambers and allowed to invade for 24 hrs at 37 °C in a CO2 incubator. Invaded cells were stained with 0.1% crystal violet for 10 min at room temperature and examined by light microscopy. Quantification of invaded cells was performed according to published criteria47,50,51,52,55,58.
Tube formation assay
Fifty microliters of growth factor-reduced matrigel were polymerized on 96-well plates. HUVECs were serum starved in RPMI1640 medium for 24 hrs, suspended in RPMI1640 medium preconditioned with tumor cells, added to the matrigel-coated wells at the density of 5 × 104 cells/well, and incubated at 37 °C for 18 hrs. Quantification of angiogenic activity was calculated by measuring the length of tube walls formed between discrete endothelial cells in each well relative to the control17,48,49,51,55.
Immunofluorescence assay
Tumor cells were plated on coverslips, permeabilized with 0.3% Triton X-100, and blocked with 5% milk for 1 hr. Cells were incubated at 4 °C overnight with antibodies specific for Smad4 or LEF1 (Santa Cruz Biotechnology; 1:200 dilutions). Then, cells were incubated with Alexa Fluor 594 goat anti-rabbit IgG (1:1000 dilution), stained with 4′,6-diamidino-2-phenylindole (DAPI, 300 nmol/L) to visualize nuclei, and photographed under a microscope.
In vivo growth, metastasis and angiogenesis assay
All animal experiments were carried out in accordance with NIH Guidelines for the Care and Use of Laboratory Animals, and approved by the Animal Care Committee of Tongji Medical College (approval number: Y20080290). For the in vivo tumor growth studies, 2-month-old male nude mice (n = 5 per group) were injected subcutaneously in the upper back with 1 × 106 tumor cells stably transfected with empty vector, Smad4 or HPSE. One month later, mice were sacrificed and examined for tumor weight and angiogenesis. The experimental metastasis (0.4 × 106 tumor cells per mouse, n = 5 per group) studies were performed with 2-month-old male nude mice as previously described17,47,48,49,50,54,58.
Patient tissue samples
Approval to conduct this study was obtained from the Institutional Review Board of Tongji Medical College (approval number: 2011-S085). All procedures were carried out in accordance with the approved guidelines. Paraffin-embedded specimens from 42 well-established primary NB cases were obtained from the Department of Pediatric Surgery, Union Hospital, Tongji Medical College9,47,50,54,58. Informed consent was obtained from all of the patients. The pathological diagnosis of NB was confirmed by at least two pathologists. Based on Shimada classification system, including the MKI, degree of neuroblastic differentiation and stromal maturation, and patient’s age, 19 patients were classified as favorable histology and 23 as unfavorable histology. According to the INSS, 7 patients were classified as stage 1, 7 as stage 2, 9 as stage 3, 11 as stage 4 and 8 as stage 4S. In subtotal 30 NB patients, fresh tumor specimens were collected at surgery and stored at −80 °C until use. Protein and RNAs of normal human dorsal ganglia were obtained from Clontech (Mountain View, CA).
Immunohistochemistry
Immunohistochemical staining was performed as previously described47,50,54,58, with antibodies specific for Smad4, LEF1, HPSE (Abcam Inc, Cambridge, MA; Santa Cruz Biotechnology; 1:200 dilutions) and CD31 (Santa Cruz Biotechnology; 1:200 dilution). The negative controls included parallel sections treated with omission of the primary antibody, in addition to an adjacent section of the same block in which the primary antibody was replaced by rabbit polyclonal IgG (Abcam Inc.) as an isotype control. The immunoreactivity in each tissue section was assessed by at least two pathologists without knowledge of the clinicopathological features of tumors or patients’ survival. The degree of positivity was initially classified according to the percentage of positive tumor cells as the following: (−) <5% cells positive, (1+) 6–25% cells positive, (2+) 26–50% cells positive, and (3+) >50% cells positive.
Statistical analysis
Unless otherwise stated, all data were shown as mean ± standard error of the mean (SEM). The χ2 analysis and Fisher exact probability analysis were applied for comparison among the gene expression and individual clinicopathological features. Pearson’s coefficient correlation was applied for analyzing the relationship among gene expression. The Kaplan-Meier method was used to estimate survival rates. Difference of tumor cells was determined by t test or analysis of variance (ANOVA).
Additional Information
How to cite this article: Qu, H. et al. Smad4 suppresses the tumorigenesis and aggressiveness of neuroblastoma through repressing the expression of heparanase. Sci. Rep. 6, 32628; doi: 10.1038/srep32628 (2016).
Supplementary Material
Acknowledgments
We are grateful for Drs Anna Coppa, Ralf Janknecht, Caroline S. Hill, and Xiulong Xu for providing vectors. This work was supported by the National Natural Science Foundation of China (81272779, 81372667, 81372401, 81472363, 81402301, 81402408, 81572423, 81672500), Fundamental Research Funds for the Central Universities (2012QN224, 2013ZHYX003, 01-18-530112, 01-18-530115), and Natural Science Foundation of Hubei Province (2014CFA012).
Footnotes
Author Contributions H.Q. and L.Z. conceived and performed most of the experiments; W.J., H.M., D.L. and H.S. performed some of the in vitro studies; E.F and X.W. performed the in vivo experiments; S.L. performed the analysis of publicly available clinical tumor datasets; K.H. critically reviewed the manuscript; L.Z. and Q.T. wrote the manuscript.
References
- Westermann F. & Schwab M. Genetic parameters of neuroblastomas. Cancer Lett 184, 127–147 (2002). [DOI] [PubMed] [Google Scholar]
- Yanagishita M. & Hascall V. C. Cell surface heparan sulfate proteoglycans. J Biol Chem 267, 9451–9454 (1992). [PubMed] [Google Scholar]
- Liotta L. A., Steeg P. S. & Stetler-Stevenson W. G. Cancer metastasis and angiogenesis: An imbalance of positive and negative regulation. Cell 64, 327–336 (1991). [DOI] [PubMed] [Google Scholar]
- Hulett M. D. et al. Cloning of mammalian heparanase, an important enzyme in tumor invasion and metastasis. Nat Med 5, 803–809 (1999). [DOI] [PubMed] [Google Scholar]
- Nasser N. J. Heparanase involvement in physiology and disease. Cell Mol Life Sci 65, 1706–1715 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentina M., Maria Francesca S., Giovanni G. & Maurizio O. Heparanase as a target in cancer therapy. Curr Cancer Drug Targets 14, 286–293 (2014). [DOI] [PubMed] [Google Scholar]
- Elkin M. et al. Heparanase as mediator of angiogenesis: mode of action. FASEB J 15, 1661–1663 (2001). [DOI] [PubMed] [Google Scholar]
- Zetser A. et al. Heparanase induces vascular endothelial growth factor expression: correlation with p38 phosphorylation levels and Src activation. Cancer Res 66, 1455–1463 (2006). [DOI] [PubMed] [Google Scholar]
- Zheng L. D. et al. Expression and clinical significance of heparanase in neuroblastoma. World J Pediatr 5, 206–210 (2009). [DOI] [PubMed] [Google Scholar]
- Cartharius K. et al. MatInspector and beyond: promoter analysis based on transcription factor binding sites. Bioinformatics 21, 2933–2942 (2005). [DOI] [PubMed] [Google Scholar]
- Shiou S. R. et al. Smad4 regulates claudin-1 expression in a transforming growth factor-beta-independent manner in colon cancer cells. Cancer Res 67, 1571–1579 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S. K. & Hoffmann F. M. Smad4 cooperates with lymphoid enhancer-binding factor 1/T cell-specific factor to increase c-myc expression in the absence of TGF-β signaling. Proc Natl Acad Sci USA 103, 18580–18585 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P. B. et al. BMP type I receptor inhibition reduces heterotopic ossification. Nat Med 14, 1363–1369 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawshad A., Medici D., Liu C. C. & Hay E. D. TGFβ3 inhibits E-cadherin gene expression in palate medial-edge epithelial cells through a Smad2-Smad4-LEF1 transcription complex. J Cell Sci 120, 1646–1653 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein S. M., Duff E. K. & Sirard C. Smad4 and beta-catenin co-activators functionally interact with lymphoid-enhancing factor to regulate graded expression of Msx2. J Biol Chem 278, 48805–48814 (2003). [DOI] [PubMed] [Google Scholar]
- Jiang G. et al. Small RNAs targeting transcription start site induce heparanase silencing through interference with transcription initiation in human cancer cells. PLoS One 7, e31379 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu H. et al. miRNA-558 promotes tumorigenesis and aggressiveness of neuroblastoma cells through activating the transcription of heparanase. Hum Mol Genet 24, 2539–2551 (2015). [DOI] [PubMed] [Google Scholar]
- Hahn S. A. et al. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science 271, 350–353 (1996). [DOI] [PubMed] [Google Scholar]
- Massagué J. TGF-beta signal transduction. Annu Rev Biochem 67, 753–791 (1998). [DOI] [PubMed] [Google Scholar]
- Katz L. H. et al. Targeting TGF-β signaling in cancer. Expert Opin Ther Targets 17, 743–760 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G. & Yang X. Smad4-mediated TGF-β signaling in tumorigenesis. Int J Biol Sci 6, 1–8 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilentz R. E. et al. Loss of expression of Dpc4 in pancreatic intraepithelial neoplasia: evidence that DPC4 inactivation occurs late in neoplastic progression. Cancer Res 60, 2002–2006 (2000). [PubMed] [Google Scholar]
- Maitra A., Molberg K., Albores-Saavedra J. & Lindberg G. Loss of Dpc4 expression in colonic adenocarcinomas correlates with the presence of metastatic disease. Am J Pathol 157, 1105–1111 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng B. et al. Suppression of tumorigenesis and induction of p15(ink4b) by Smad4/DPC4 in human pancreatic cancer cells. Clin Cancer Res 8, 3628–3638 (2002). [PubMed] [Google Scholar]
- Schwarte-Waldhoff I. & Schmiegel W. Smad4 transcriptional pathways and angiogenesis. Int J Gastrointest Cancer 31, 47–59 (2002). [DOI] [PubMed] [Google Scholar]
- Miyaki M. & Kuroki T. Role of Smad4 (DPC4) inactivation in human cancer. Biochem Biophys Res Commun 306, 799–804 (2003). [DOI] [PubMed] [Google Scholar]
- Zawel L. et al. Human Smad3 and Smad4 are sequence-specific transcription activators. Mol Cell 1, 611–617 (1998). [DOI] [PubMed] [Google Scholar]
- Hoot K. E. et al. Keratinocyte-specific Smad2 ablation results in increased epithelial- mesenchymal transition during skin cancer formation and progression. J Clin Invest 118, 2722–2732 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt K. K. et al. Overexpression of the tumor suppressor gene Smad4/DPC4 induces p21waf1 expression and growth inhibition in human carcinoma cells. Cancer Res 58, 5656–5661 (1998). [PubMed] [Google Scholar]
- Qiao J., Kang J., Ko T. C., Evers B. M. & Chung D. H. Inhibition of transforming growth factor-beta/Smad signaling by phosphatidylinositol 3-kinase pathway. Cancer Lett 242, 207–214 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco A. et al. Increased TGFbeta type II receptor expression suppresses the malignant phenotype and induces differentiation of human neuroblastoma cells. Exp Cell Res 255, 77–85 (2000). [DOI] [PubMed] [Google Scholar]
- McCune B. K. et al. Expression of transforming growth factor-beta isoforms in small round cell tumors of childhood. An immunohistochemical study. Am J Pathol 142, 49–58 (1993). [PMC free article] [PubMed] [Google Scholar]
- Iolascon A. et al. Reduced expression of transforming growth factor-beta receptor type III in high stage neuroblastomas. Br J Cancer 82, 1171–1176 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y. & Yip H. Effects of bone morphogenetic protein 2 on Id expression and neuroblastoma cell differentiation. Differentiation 79, 84–92 (2010). [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Ozaki T., Koseki H., Nakagawara A. & Sakiyama S. Accumulation of p27KIP1 is associated with BMP2-induced growth arrest and neuronal differentiation of human neuroblastoma-derived cell lines. Biochem Biophys Res Commun 307, 206–213 (2003). [DOI] [PubMed] [Google Scholar]
- Dong J., Kukula A. K., Toyoshima M. & Nakajima M. Genomic organization and chromosome localization of the newly identified human heparanase gene. Gene 253, 171–178 (2000). [DOI] [PubMed] [Google Scholar]
- Lu W. C., Liu Y. N., Kang B. B. & Chen J. H. Trans-activation of heparanase promoter by ETS transcription factors. Oncogene 22, 919–923 (2003). [DOI] [PubMed] [Google Scholar]
- Ogishima T. et al. Increased heparanase expression is caused by promoter hypomethylation and up-regulation of transcriptional factor early growth response-1 in human prostate cancer. Clin Cancer Res 11, 1028–1036 (2005). [PubMed] [Google Scholar]
- Zhu H., Carpenter R. L., Han W. & Lo H. W. The GLI1 splice variant TGLI1 promotes glioblastoma angiogenesis and growth. Cancer Lett 343, 51–61 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W. et al. Hypoxia activates heparanase expression in an NF-kappaB dependent manner. Oncol Rep 23, 255–261 (2010). [PubMed] [Google Scholar]
- Sasaki M. et al. Erythromycin and clarithromycin modulation of growth factor-induced expression of heparanase mRNA on human lung cancer cells in vitro. Mediators Inflamm 10, 259–267 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraz L., Haupt Y., Elkin M., Peretz T. & Vlodavsky I. Tumor suppressor p53 regulates heparanase gene expression. Oncogene 25, 3939–3947 (2006). [DOI] [PubMed] [Google Scholar]
- D’Inzeo S. et al. A novel human Smad4 mutation is involved in papillary thyroid carcinoma progression. Endocr Relat Cancer 19, 39–55 (2012). [DOI] [PubMed] [Google Scholar]
- Janknecht R., Wells N. J. & Hunter T. TGF-β-stimulated cooperation of Smad proteins with the coactivators CBP/p300. Genes Dev 12, 2114–2119 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierreux C. E., Nicolás F. J. & Hill C. S. Transforming growth factor beta-independent shuttling of Smad4 between the cytoplasm and nucleus. Mol Cell Biol 20, 9041–9054 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D. et al. Intelectin 1 suppresses the growth, invasion and metastasis of neuroblastoma cells through up-regulation of N-myc downstream regulated gene 2. Mol Cancer 14, 47 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X. et al. CTCF cooperates with noncoding RNA MYCNOS to promote neuroblastoma progression through facilitating MYCN expression. Oncogene 35, 3565–3576 (2016). [DOI] [PubMed] [Google Scholar]
- Xiang X. et al. miRNA-584-5p exerts tumor suppressive functions in human neuroblastoma through repressing transcription of matrix metalloproteinase 14. Biochim Biophys Acta 1852, 1743–1754 (2015). [DOI] [PubMed] [Google Scholar]
- Xiang X. et al. miRNA-337-3p suppresses neuroblastoma progression by repressing the transcription of matrix metalloproteinase 14. Oncotarget 6, 22452–22466 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H. et al. MicroRNA-145 inhibits the growth, invasion, metastasis and angiogenesis of neuroblastoma cells through targeting hypoxia-inducible factor 2 alpha. Oncogene 33, 387–397 (2014). [DOI] [PubMed] [Google Scholar]
- Zheng L. et al. miRNA-145 targets v-ets erythroblastosis virus E26 oncogene homolog 1 to suppress the invasion, metastasis, and angiogenesis of gastric cancer cells. Mol Cancer Res 11, 182–193 (2013). [DOI] [PubMed] [Google Scholar]
- Zheng L. et al. microRNA-9 suppresses the proliferation, invasion and metastasis of gastric cancer cells through targeting cyclin D1 and Ets1. PLoS One 8, e55719 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang X. et al. Hepatocyte nuclear factor 4 alpha promotes the invasion, metastasis and angiogenesis of neuroblastoma cells via targeting matrix metalloproteinase 14. Cancer Lett 359, 187–197 (2015). [DOI] [PubMed] [Google Scholar]
- Li D. et al. FOXD3 is a novel tumor suppressor that affects growth, invasion, metastasis and angiogenesis of neuroblastoma. Oncotarget 4, 2021–2044 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L. et al. Methyl jasmonate abolishes the migration, invasion and angiogenesis of gastric cancer cells through down-regulation of matrix metalloproteinase 14. BMC Cancer 13, 74 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond E., Li C. P. & Ferro V. Development of a colorimetric assay for heparanase activity suitable for kinetic analysis and inhibitor screening. Anal Biochem 396, 112–116 (2010). [DOI] [PubMed] [Google Scholar]
- Nadanaka S., Purunomo E., Takeda N., Tamura J. & Kitagawa H. Heparan sulfate containing unsubstituted glucosamine residues: biosynthesis and heparanase-inhibitory activity. J Biol Chem 289, 15231–15243 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H. et al. microRNA-9 targets matrix metalloproteinase 14 to inhibit invasion, metastasis, and angiogenesis of neuroblastoma cells. Mol Cancer Ther 11, 1454–1466 (2012). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.