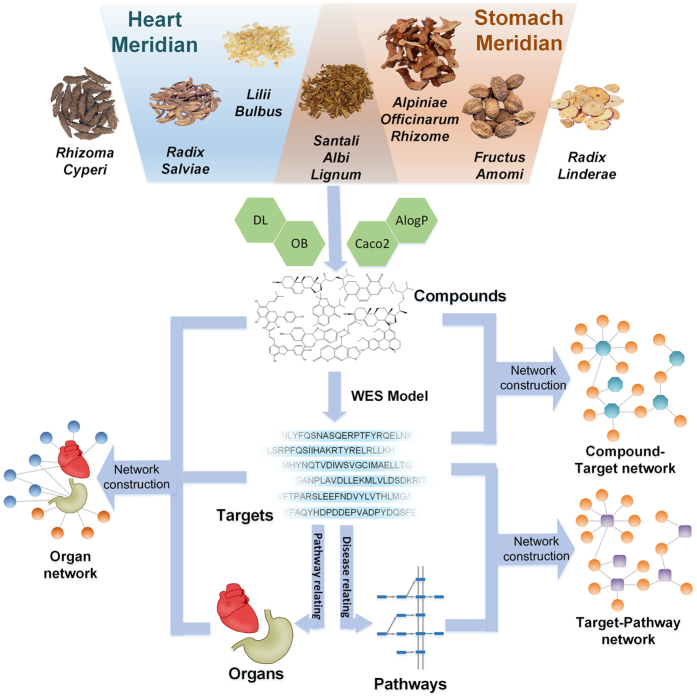
Abstract
Though cardiovascular diseases (CVDs) and gastrointestinal disorders (GIDs) are different diseases associated with different organs, they are highly correlated clinically. Importantly, in Traditional Chinese Medicine (TCM), similar treatment strategies have been applied in both diseases. However, the etiological mechanisms underlying them remain unclear. Here, an integrated systems pharmacology approach is presented for illustrating the molecular correlations between CVDs and GIDs. Firstly, we identified pairs of genes that are associated with CVDs and GIDs and found that these genes are functionally related. Then, the association between 115 heart meridian (HM) herbs and 163 stomach meridian (SM) herbs and their combination application in Chinese patent medicine was investigated, implying that both CVDs and GIDs can be treated by the same strategy. Exemplified by a classical formula Sanhe Decoration (SHD) treating chronic gastritis, we applied systems-based analysis to introduce a drug-target-pathway-organ network that clarifies mechanisms of different diseases being treated by the same strategy. The results indicate that SHD regulated several pathological processes involved in both CVDs and GIDs. We experimentally confirmed the predictions implied by the effect of SHD for myocardial ischemia. The systems pharmacology suggests a novel integrated strategy for rational drug development for complex associated diseases.
Over the past decade, there has been a marked increase in our understanding that there are higher prevalence rates of gastrointestinal disease (GIDs) in patients with cardiovascular disease (CVDs)1,2 with similar dysfunctional phenotypes, such as rib pain, stomach pain, nausea and vomiting. However, the underlying co-occurrence mechanisms of CVDs and GIDs are unclear, thereby hampering development of drugs for both diseases in humans3. In modern Western medicine, usually, it has been observed that cardiovascular diseases have an etiological relationship with gastrointestinal disorders. Several studies have reported that the risk of cardiovascular disease in patients with gastrointestinal disease appears to be far greater than in the general population4. Moreover, some gastrointestinal disorders may increase patients’ risk of cardiovascular disease as well. For example, patients with chronic gastrointestinal ischemia have an increased CVDs’ risk and excess mortality5. Furthermore, the pathophysiological mechanisms between the two organs would supply realistic treatment for CVDs and GIDs6. For example, Iranian traditional physicians have introduced several remedies for heart-stomach association ailments in a previous study7. In addition, novel studies demonstrated the close relationship between gastroesophageal reflux disease (GERD) and the development of atrial fibrillation (AF), notably, acid-suppressive therapy is an effective strategy for the management of AF and may help to minimize the use of anti-arrhythmic agents8,9. However, the pathophysiology or mediators underlying these comorbidities were not clearly elucidated due to the concept of one-gene/one-enzyme/one-function, which viewed these disorders are independent from each other.
Differently, holistic medicine is understood herein including alternative, complementary, and traditional medical practices6, sharing consequences in relation to overall health, which may lead to the advancement of therapeutic strategies for complex disorders. In traditional Chinese Medicine, human body is considered as a holistic being in which each organ or each specific physiological part is interrelated. Based on Huangdi Neijing (The Yellow Emperor’s Inner Canon)10, traditional Chinese medical practitioners diagnose and treat patients beneath the guidance of meridian theory, declaring that the heart and stomach linked through some specific Jingluo (meridians)11 and they have an interaction effect in terms of both physiological function and pathological basis. Under the Jingluo theory, both heart and stomach are located in the epigastrium, and they are separated only by the diaphragm. Furthermore, the relationship between heart and stomach is inter-promoting. As for physiological function, the heart is able to control the blood and vessels and govern the mind, while the stomach transforms food into usable nutrition to ensure the production and storage of blood.
Traditional Chinese Medicine (TCM), which is an integral knowledge system of holistic medicine for disease prevention and treatment based on practitioner’s dynamic observation and practice12. TCM is becoming more popular because it is widely available and relatively effective, and has fewer side effects13. More importantly, TCM does not focus merely on diseases that are defined by specific pathological changes, but concentrates on the overall functional states of the patients in a holistic fashion14. As for synchronic treatment of CVDs and GIDs, the earliest depiction has been documented in Jingui Yaolue (Synopsis of Golden Chamber), a classic clinical book written by Zhang Zhongjing, which considered that adjusting the function of spleen and stomach is of great significance for the treatment of heart disease and designed a series of herbal formulae to treat XiongBi (thoracic obstruction) and XinTong syndrome (cardiodynia). For example, the Zhishi Xiebai Guizhi decoction, which consists of Zhishi (Fructus Aurantii Immaturus), Houpu (Cortex Magnoliae Officinalis), Xiebai (Bulbus Allii Macrostemonis), Guizhi (Ramulus Cinnamomi), Gualoushi (Fructus Trichosanthis), is used to strengthen the function of stomach, moreover, it also can be used to treat coronary heart diseases15. Besides, some heart Meridian herbs, such as Baihe (Lilium brownii var.viridulum), Hehuanpi (bark of Albizia julibrissin Durazz), which can nourish the heart, are often added into the formulae to enhance the therapeutic effect for stomach diseases.
Despite the promising effects of TCM in the treatment of CVDs and GIDs, how formulae of heart meridian herbs and stomach meridian herbs work together and what their common targets are is still ambiguous. To clarify molecular mechanisms of herbal drugs for complex related diseases from a system level, the following numerous issues need to be solved urgently: 1) what the relationship is between heart meridian (HM) herbs and stomach meridian (SM) herbs? And how the HM herbs and SM herbs are combined in the treatment of CVDs and GIDs clinically? 2) Which active compounds are involved in the regulatory processes of formulae in CVDs and GIDs treatment? 3) Which targets are modulated by the active ingredients to achieve the therapeutic effect? 4) Which pathologic processes are regulated by the active compounds and herbal medicine to treat CVDs and GIDs simultaneously? 5) Whether the formulae for the GIDs is effective in treating CVDs experimentally? Nowadays, systems pharmacology is an emerging field implicated in the application of network pharmacology and pharmacokinetics evaluation16, which clarifies the therapeutic effects and underlying mechanisms of multi-component and multi-target agents17,18,19. In our previous work, we have successfully built an integrated platform of systems pharmacology combined the discovery of bioactive ingredients, prediction of drug targets, exploration of therapeutic mechanisms and revelation of TCM combination rule, etc20,21,22,23. For example, under the paradigm, we have investigated the mechanisms for the well-known herbal recipe Compound Danshen Formula (CDF) and the botanical drug licorice24,25.
In this study, we proposed an integrated systems pharmacology approach to illustrate the mechanisms of action in the systematic combination of different herbs for treating relevant diseases simultaneously. Firstly, we attempted to dissect underlying mechanisms of CVDs and GIDs. Then we explored the association between heart meridian (HM) herbs and stomach meridian (SM) herbs, and evaluate their combination application in Chinese Patent Medicine (CPM). TCM has achieved notable success in treating related complex diseases, here, a classical formula Sanhe Decoction (SHD) was selected as a case study26. SHD is a complex system with 7 herbs, including Lilli Bulbus, Salvia Miltiorrhiza, Alpinia Officinarum Rhizoma, Amomum Villosum, Santail Albi Lignum, Lindera Aggregata and Cyperus Rotundus. Among these herbs, Radix Salvia and Lilli Bulbus belong to heart meridian; Alpiniae Officinarum Rhizome and Fructus Amomi belong to stomach meridian; Santail Albi Lignum belongs to both HM and SM; Rhizoma Cyperi and Radix Linderae are assigned to other meridians. At first, Sanhe Decoration (SHD) has been applied to treat GIDs such as chronic gastritis, epigastric pain and peptic ulcer, and the stomach meridian herbs in the formula have been reported to treat stomach diseases in previous study. For example, Alpiniae Officinarum Rhizome and Fructus Amomi have been validated to strengthen the stomach and relieve stomach ache27,28. In addition, modern pharmacological studies have proven that Radix Salvia could treat CVDs by enhancing myocardial anti-hypoxia capacity, increasing myocardial contraction and improving cardiac function29, thus we inferred that Sanhe Decoction may be applied for the treatment of both CVDs and GIDs.
An integrated systems pharmacology approach was used to depict the holistic healing of SHD for cardiovascular-gastrointestinal diseases from a molecular to holistic level. Briefly, as shown in Fig. 1, we screened the biochemical compounds by in silico ADME system and predicted the potential related targets of these compounds by weighted ensemble similarity (WES) method30,31. The obtained targets were mapped onto relevant databases to find out their corresponding pathways of CVDs and GIDs. Furthermore, network construction, pathway enrichment analysis and tissue location analysis were performed to illustrate the molecular mechanisms of SHD on CVDs and GIDs holistically. Finally, we administered SHD to LAD occlusion model rats and investigated the effect of SHD on apomorphosis and necrosis of cardiac muscle cells and the activity change of superoxide dismutase (SOD), creatine kinase (CK), cyclic adenosine monophosphate (cAMP) and cardiac troponin I (cTnI). These results further supplied the in vivo experimental evidence to validate the therapeutic effect of SHD for CVDs that were predicted in the systems pharmacology. In summary, our systems pharmacology approach would provide guidance for complicated related disease treatment and new drug development.
Figure 1. Systems pharmacology approach framework.
Results
The closeness analysis between genes of CVDs and GIDs
We collected 288 and 353 genes associated with CVDs (31 types) and GIDs (13 types), respectively. The detailed information of these genes were listed in supplementary Tables S1 and S2, respectively. As shown in Table 1, 47 shared genes of CVDs and GIDs were identified. If two diseases share a large number of disease genes, the disease pairs become more comorbid and they are closely associated32. Therefore, shared genes of CVDs and GIDs could provide novel target potential for the treatment of CVDs and GIDs. Among these 38 genes, many targets have been proved effective in the clinical practices, for example, the regulation of gene angiotensin I converting enzyme (ACE) has therapeutic effect on chronic heart failure and gastric cancer33,34. ACE blockers are now first-line treatments for hypertensive target organ damage. In addition, ACE is located at the upstream of renin-angiotensin system pathway. Renin-angiotensin system blockade exerts potent anti-atherosclerotic effects, which are mediated by their antihypertensive, anti-inflammatory, anti-proliferative, and oxidative stress lowering properties35. Moreover, the renin-angiotensin system pathway plays a pivotal role in regulating the blood volume and systemic vascular resistance, which together influence cardiac output and arterial pressure. ACE inhibitors are often used to prevent angiotensin I and angiotensin II from binding to blood vessels and causing vasoconstriction, since angiotensin II is a strong hormone in renin-angiotensin system pathway, and can act directly on blood vessels to cause blood pressure increases. Gene Nitric oxide synthase 2 (NOS2) is associated with both heart failure and gastric ulcer, which is involved in inflammatory response and its expression could prevent harmful processes to human36,37. Specially, the transcription regulation of NOS2 depends on the mediation of specific transcription factors, such as CREB, NF-κB and C/EBP8138, thus playing the therapeutic effects.
Table 1. The shared genes of CVDs and GIDs.
| GeneSymbol | Gene name | Types of CVDs | Types of GIDs |
|---|---|---|---|
| ABCB1 | ATP-binding cassette, sub-family B (MDR/TAP), member 1 | Heart Defects, Congenital | Gastroesophageal Reflux |
| ACE | angiotensin I converting enzyme | Heart Failure, Cardiovascular Diseases | Stomach Neoplasms |
| ADRB1 | adrenoceptor beta 1 | Heart Failure, Cardiovascular Diseases | Stomach Neoplasms |
| ADRB2 | adrenoceptor beta 2, surface | Heart Failure | Stomach Neoplasms |
| AHR | aryl hydrocarbon receptor | Heart Defects, Congenital; Heart diseases | Stomach Neoplasms |
| ALB | albumin | Heart Diseases; Heart Failure; Cardiovascular Diseases | Stomach Neoplasms |
| APEX1 | APEX nuclease (multifunctional DNA repair enzyme) 1 | Heart Diseases | Stomach Neoplasms |
| AVP | arginine vasopressin | Heart Failure | Gastrointestinal Hemorrhage |
| CAT | catalase | Heart Failure | Stomach Ulcer |
| CSF2 | colony stimulating factor 2 (granulocyte-macrophage) | Heart Failure | Gastrointestinal Hemorrhage |
| CSF3 | colony stimulating factor 3 (granulocyte) | Heart Failure; Heart Diseases | Gastrointestinal Diseases |
| CYP2C19 | cytochrome P450, family 2, subfamily C, polypeptide 19 | Cardiovascular Diseases | Gastroesophageal Reflux |
| EDN1 | endothelin 1 | Heart Failure; Cardiovascular Diseases; Heart Defects, Congenital; Cardiovascular Abnormalities; Cardiovascular disease, unspecified; Ischemic heart disease | Gastrointestinal Diseases |
| F2R | coagulation factor II (thrombin) receptor | Cardiovascular Disorders | Stomach Neoplasms |
| GCG | glucagon | Heart Failure; Heart Diseases | Stomach Diseases |
| GDF15 | growth differentiation factor 15 | Heart Failure; Cardiovascular disease, unspecified | Gastrointestinal Neoplasms |
| GHRL | ghrelin/obestatin prepropeptide | Heart Failure | Stomach Ulcer |
| GHSR | Cardiovascular disease, unspecified | Gastrointestinal Diseases and Disorders, miscellaneous | |
| HMOX1 | heme oxygenase (decycling) 1 | Heart Failure; Cardiovascular disease, unspecified | Stomach Neoplasms; Gastroparesis |
| HSPB1 | heat shock 27 kDa protein 1 | Heart Failure | Stomach Neoplasms |
| IL1B | interleukin 1, beta | Heart Failure; Heart Valve Diseases | Stomach Ulcer; Stomach Neoplasms; Gastritis, Atrophic |
| IL6 | interleukin 6 | Heart Failure | Stomach Neoplasms |
| MT2A | metallothionein 2A | Heart Diseases | Stomach Neoplasms |
| MTHFR | methylenetetrahydrofolate reductase (NAD(P)H) | Cardiovascular Diseases; Heart Defects, Congenital | Stomach Neoplasms; |
| NOS2 | nitric oxide synthase 2, inducible | Heart Failure, Cardiovascular Abnormalities | Stomach Ulcer |
| NOS3 | nitric oxide synthase 3 (endothelial cell) | Cardiovascular Diseases; Heart Failure | Stomach Ulcer, Stomach Neoplasms |
| NRG1 | neuregulin 1 | Heart Failure | Stomach Ulcer |
| PLAU | plasminogen activator, urokinase | Heart Rupture, Post-Infarction | Stomach Neoplasms |
| POMC | proopiomelanocortin | Heart Failure | Gastrointestinal Diseases |
| PPARG | peroxisome proliferator-activated receptor gamma | Ischemic heart disease | Stomach Neoplasms |
| PTGS1 | prostaglandin-endoperoxide synthase 1 (prostaglandin G/H synthase and cyclooxygenase) | Heart Failure | Stomach Ulcer |
| PTGS2 | prostaglandin-endoperoxide synthase 2 (prostaglandin G/H synthase and cyclooxygenase) | Cardiovascular Diseases; Heart Failure | Stomach Ulcer; Stomach Neoplasms |
| PYCARD | PYD and CARD domain containing | Heart Valve Diseases | Stomach Neoplasms |
| RELA | Cardiovascular disease, unspecified | Gastric cancer | |
| SERPINE1 | serpin peptidase inhibitor, clade E (nexin, plasminogen activator inhibitor type 1), member 1 | Heart Failure | Stomach Neoplasms |
| SOD2 | superoxide dismutase 2, mitochondrial | Heart Failure | Stomach Neoplasms |
| TNF | tumor necrosis factor | Congestive heart failure | Stomach Ulcer; Stomach Neoplasms |
| VEGFA | vascular endothelial growth factor A | Heart Diseases; Heart Failure | Stomach Ulcer |
| ACHE | Acetylcholinesterase | Heart Failure | Stomach Tumor |
| AR | Androgen Receptor | Atherosclerosis | Gastric Cancer |
| CYP2A6 | Cytochrome P450 2A6 | Cardiovascular Disease | Gastric Carcinoma |
| ESR1 | Estrogen Receptor | Ischemia Reperfusion Injury | Gastric Cancer |
| GLO1 | Glyoxalase I | Diabetic Cardiomyopathy | Gastric Cancer |
| MMP9 | Matrix metalloproteinase 9 | Heart Failure | Gastric Carcinoma |
| NR3C1 | Glucocorticoid receptor | Coronary Heart Disease | Gastric Cancer |
| PLA2G2A | Phospholipase A2 group IIA | Atherosclerosis | Gastric Cancer |
| PLK2 | Polo-Like Kinase 2 | Myocardial Infarction | Stomach Tumor |
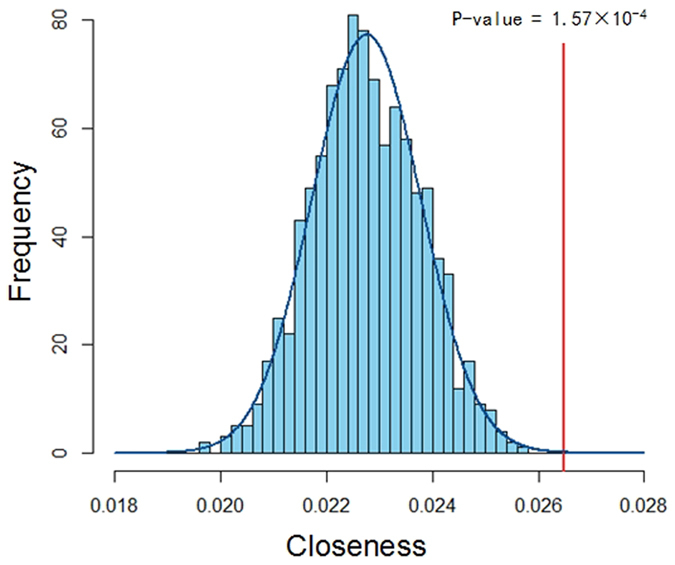
Though shared genes of CVDs and GIDs are not so abundant, previous study found that if pairs of genes are closely related in PPI network, these pairs of genes are significantly correlated39. To assess the correlation of pairs of genes between CVDs and GIDs, we illustrate their closeness in PPI network. According to the formula (1), the obtained closeness between genes of CVDs and GIDs is 0.0264 by the 10,000 times of randomization (Fig. 2). To evaluate the statistical significance between the actual distance and those of random counterpart, Z test is carried out and the significant difference is defined as P < 0.01. Compared with the random-selected two proteins from PPI network, two group genes associated with CVDs and GIDs show significantly close linkage relevance (ultimate nearness = 1.57 × 10−4 (p < 0.01)). These results above indicated that genes associated with CVDs and GIDs are close related.
Figure 2. The closeness correlation of gene pair between CVDs and GIDs.
Correlation of the heart meridian (HM) and stomach meridian (SM) herbs
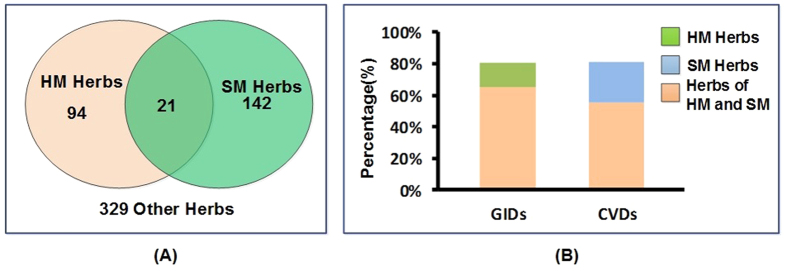
115 and 163 herbs that belonged to heart meridian and stomach meridian were collected from Chinese Pharmacopeia, respectively (The herbs were presented in Supplementary Tables S3 and S4, respectively). The Venn diagram illustrated that 21 herbs belonged to both HM and SM (Fig. 3(A), the detailed information of common herbs was listed in Table 2). Specifically, common herbs belonged to both HM and SM meridians includes licorice, Santalum Albi Lignum, and dried Ginger and so on. In traditional medicine, licorice has been widely used for treating cardiovascular diseases40. In fact, licorice is a common herb with desirable pharmacological effects on inhibiting inflammatory processes of blood vessels, preventing atherosclerosis, reducing plasma lipid levels, and decreasing systolic blood pressure40,41, thus exerting cardio-protective activities42,43,44. Also, licorice has been used as an anti-peptic ulcer agent45, and its antiulcer and mucosal protective actions have been confirmed by numerous clinical trials and animal experiments46,47,48. Santalum Albi Lignum showed good curative effect in the treatment of anginal attacks49 and ulceration in vivo50. The dried Ginger Rhizome has been used as an herbal therapy for treating cardiovascular diseases such as atherosclerosis, hyperglycemia and hyperinsulinemia51,52. Moreover, the active constituents of Ginger Rhizome are effective in alleviating the symptoms of gastrointestinal illnesses53. In addition, the Fisher’s test exhibited the significant correlation of the HM and SM herbs (P < 0.01).
Figure 3. Correlation of the HM and SM herbs.
(A) The relationship of HM and SM Herbs. (B) Distribution of these herbs in CPM for CVDs and GIDs.
Table 2. The shared herbs that belong to heart and stomach meridians.
| Herb names | Pinyin | Meridians | |
|---|---|---|---|
| Acori Tatarinowii Rhizoma | Shichangpu | heart, stomach | pungent, bitter, temperature |
| Allii Macrostemonis Bulbus | Xiebai | heart, lung, stomach, large intestine | pungent, bitter, temperature |
| Ampelopsis Radix | Bailian | heart, stomach | bitter, little cold |
| Bambusae Caulis in Taenias | Zhuru | lung, stomach, heart, gallbladder | sweet, little cold |
| Baphicacanthis Cusiae Rhizoma et Radix | Nanbanlangen | heart, stomach | bitter, cold |
| Coptidis Rhizoma | Huanglian | heart, spleen, stomach, liver, gallbladder, large intestine | bitter, cold |
| Glycyrrhizae Radix et Rhizoma Praeparata Cum Melle | Zhigancao | heart, lung, spleen, stomach | sweet, natured |
| Hippophae Fructus | Shaji | spleen, stomach, lung, heart | sour, astringent, temperature |
| Hyoscyami Semen | Tianxianzi | heart, stomach, liver | bitter, pungent, temperature, large toxic |
| Isatidis Folium | Daqingye | heart, stomach | bitter, cold |
| Isatidis Radix | Banlangen | heart, stomach | bitter, cold |
| Liriopes Radix | Shanmaidong | heart, lung, stomach | sweet, little bitter, little cold |
| Lonicerae Flos | Shanyinhua | lung, heart, stomach | sweet, cold |
| Lonicerae Japonicae Flos | Jinyinhua | lung, heart, stomach | sweet, cold |
| Lophatheri Herba | Danzhuye | heart, stomach, small intestine | sweet, bland, cold |
| Ophiopogonis Radix | Maidong | heart, lung, stomach | sweet, little bitter, little cold |
| Polygoni Tinctorii Folium | Liaodaqingye | heart, stomach | bitter, cold |
| Santali Albi Lignum | Tanxiang | spleen, stomach, heart, lung | pungent, temperature |
| Sophorae Flavescentis Radix | Kushen | heart, liver, stomach, large intestine, bladder | bitter, cold |
| Tamaricis Cacumen | Xiheliu | heart, lung, stomach | sweet, pungent, natured |
| Zingiberis Rhizoma | Ganjiang | spleen, stomach, kidney, heart, lung | pungent, hot |
Furthermore, to explore the clinical application of the herbs of HM and SM, we analyzed the distribution of these herbs which are used in Chinese Patent Medicine (CPM) for CVDs and GIDs (The list of these CPM are displayed in Tables S5 and S6, respectively). As shown in Fig. 3(B), 81.01% (128/158) herbs of heart meridian appeared in the CPM which are used to treat CVDs, and 68.75% (88/128) herbs of stomach meridian are also found in these CPM. Interestingly, in terms of the CPM for stomach diseases, there was 80.72% (67/83) botanic drugs of stomach meridian, among these TPM, 80.60% (54/67) herbs of heart meridian appeared simultaneously. We concluded that herbs belonging to heart and stomach meridians were highly overlapped in CPMs for the treatment of heart and stomach diseases, indicating that the combination of heart and stomach herbs may be a successful strategy to treat CVDs and GIDs.
Screening active ingredients of Sanhe Decoction
Sanhe Decoction consists of 7 herbs, Radix Salvia and Lilli Bulbus belong to heart meridian; Alpiniae Officinarum Rhizome and Fructus Amomi belong to stomach meridian; and Santail Albi Lignum belongs to both HM and SM; Rhizoma Cyperi and Radix Linderae are assigned to other meridians. By in silico ADME model, 59 compounds were predicted as the potential active compounds with appropriate pharmaceutical properties, which accounted for 8.9% of the total compounds, including 32 phenanthrenequinone compounds, 10 terpenoid compounds, 8 flavonoid compounds, 4 ligand compounds and 2 coumarin compounds. The ADME parameters and structural information of these 59 compounds are shown in Table 3. Among 59 predicted ingredients, 6 compounds converted by intestinal microbes. M1 (Luteolin) is converted into baicalein 6-methylether54, M2 (Quercetin) is metabolized to myricetin, 8-hydroxy quercetin and 3′-O-methylquercetin by biotransformation55, M8 (Galangin) is metabolized to kaempferol. M31 (Cryptotanshinone) is able to be biotransformed to three new products, (3R,15R)-3-hydroxycryptotanshinone, (3S,15R)-3-hydroxycryptotanshinone, and (4S,15R)-18-hydroxycryptotanshinone56. M45 (Neocryptotanshinone) could be convert into CT, TIIA, TIIB and TI, and probably DH-TI stepwisely57. The metabolites of M52 (Tanshinone IIA) are formed through hydroxylation and dehydrogenation, including tanshinone IIA, hydroxytanshinone IIA, przewaquinone A and dehydrotanshinone IIA58.
Table 3. Active constituents of herbs in Sanhe Decoration and their corresponding ADME parameters.
There are 41 ingredients in Radix Salviae that exhibit valuable therapeutic effects for CVDs and GIDs. For instance, M1 (Luteolin) is a representative compound of SHD that shows cardio-protective effects against ischemia/reperfusion (I/R) injury by reducing necrosis and apoptosis in rat cardiomyocytes59. Additionally, Luteolin is also a potential alternative in the treatment of GIDs60. M52 (Tanshinone IIA) is one of the key active ingredients of Radix Salviae and it protects cardiac myocytes against oxidative stress-induced apoptosis in vivo61. Tanshinone IIA plays important roles in ischemic heart diseases, especially in reduction of myocardial infarct size and decrease of myocardial consumption of oxygen62.
Bioactive ingredients in Alpinia Officinarum Rhizoma are M2 (Quercetin), M3 (Isorhamnetin), M04 (Kampferol), M08 (Galangin), M09 (Medicarpin) and so forth. Quercetin is a typical flavonol-type flavonoid with strong antioxidative and cardioprotective properties, thus preventing cardiovascular diseases63. M4 (Kaempferol) was reported to protect against coronary heart disease, and a person with higher kaempferol intakes would have the lower incidence of cardiovascular diseases64,65. M8 (Galangin) has promising effect in the treatment of cardiovascular by reducing lipid peroxidation66. In addition, M3 (Isorhamnetin) may be a potential candidate in the treatment of gastric cancer67.
6 potential active compounds are identified in Rhizoma Cyperi, among them, M16 (Sugeonyl acetate) is the potential effective ingredient for treating CVDs and GIDs pharmacological and biological activities68. In Lilli Bulbus, 3-Demethylcolchicine is predicted to reduce the risk of cardiovascular diseases (CVDs). High intake of M54 (Vitamin E, in Fructus Amomi) through diet or supplements decrease occurrence of cardiovascular diseases and stomach cancer69,70. M59 (Boldine, Radix Linderae) is a major alkaloid with antioxidant activity and anti-inflammatory effect71, therefore it is the therapeutic agent for CVDs.
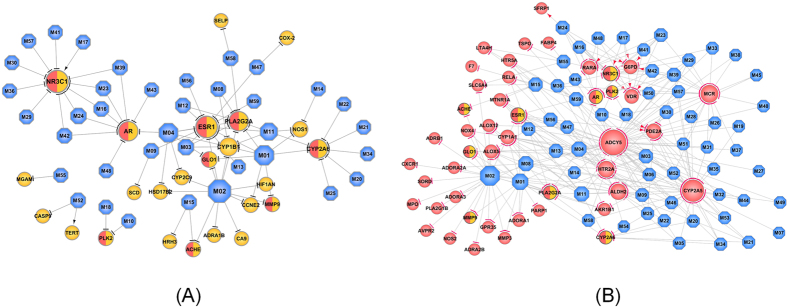
Compound-Target network for GIDs (C-T1)
We identified proteins that could be potential therapeutic targets of heart and stomach disorders and elucidated the mechanisms of SHD from a systematic level. A compound-target network was constructed to interpret the therapeutic mechanisms of the Sanhe Decoction for GIDs (Fig. 4 (A)). 80 interactions are generated by modulating 24 stomach related targets through 37 compounds. In a network, the node degree (the number of connections or edges the node has to other nodes) is one of the most basic quantitative properties and nodes with highly connections (high degree) are referred to as hubs72. Of the 24 targets, 7 proteins possess degree larger than 4 under an average value of 3.3, therefore, these candidate targets participating in more interactions than other proteins are the hubs in this C-T1 Network.
Figure 4. Therapeutic compound-target network.
(A) Therapeutic compound-target network of GIDs. (B) Therapeutic compound-target network of CVDs. The hexagon nodes represent compounds, and circles are targets. Node size is proportional to its degree. Arrows indicate activation and T-arrows represent inhibition of the action mode of compound and target interaction.
In the network, ESR1 (Estrogen Receptor) was the target with the highest degree (DD = 11), followed by NR3C1 (Glucocorticoid receptor, DD = 10), PLA2G2A (Phospholipase A2 group IIA, DD = 8), AR (Androgen Receptor, DD = 8), CYP2A6 (Cytochrome P450 2A6, DD = 7), CYP1B1 (Cytochrome P450 1B1, DD = 6) and etc. Evidence suggests that the potential active compounds in SHD can act on these targets, thus contributing to therapeutic effect on stomach diseases. For example (as shown in Tables 4), (1) Estrogen receptor (ER) mediates estrogenic activity in several organs, including those in the stomach. The estrogen receptor-α could be expressed in cardiomyocytes and plays an acute cardioprotective role in ischemia reperfusion injury73,74. Besides, the clinical significance and prognostic effect of ER-β was evaluated, ER-β has a protective effect against gastric cancer, which indicating that hormone therapy may be a useful new strategy for the treatment of gastric cancer75. (2) The activity of PLA2G2A (by M1 (Luteolin), M2 (Quercetin), M3 (Isorhamnetin), M4 (Kaempferol), M8 (Galangin), M11 (Chryseriol), M12 (8-Isopentenyl-kaempferol), M58 (Nubigenol)) suppresses progression or metastasis of human gastric cancer76. Elevated expression of PLA2G2A might inhibit progression of gastric cancer through increased release of arachidonic acid, thus preventing bacteria invading during inflammation process77. (3) Androgen receptor (AR) plays an important role in gastric cancers and is involved in tumor progression54. Especially, AR promotes esophageal cancer cell migration and proliferation via matrix metalloproteinase 278. In Sanhe Decoction, M4 (Kaempferol), M16 (Sugeonyl acetate), M23 (Przewalskin A), M24 (Przewalskin B), M39 (Microstegiol), M43 (Miltirone II) and M48 (Salvilenone I) were identified to block AR, highlighting therapeutic effects of on the stomach diseases.
Table 4. The GIDs target information.
| Gene name | Protein name | Degree |
|---|---|---|
| ESR1 | Estrogen Receptor | 18 |
| NR3C1 | Glucocorticoid receptor | 12 |
| AR | Androgen Receptor | 8 |
| PlA2G2A | Phospholipase A2 group IIA | 8 |
| CYP2A6 | Cytochrome P450 2A6 | 7 |
| CYP1B1 | Cytochrome P450 1B1 | 6 |
| GLO1 | Glyoxalase I | 4 |
| ACHE | Acetylcholinesterase | 3 |
| NOS1 | Nitric Oxide Synthase, brain | 3 |
| HSD17B2 | Estradiol 17-beta-dehydrogenase 2 | 2 |
| PLK2 | Polo-Like Kinase 2 | 2 |
| CCNE2 | Cyclin-dependent kinase 2 | 2 |
| MMP9 | Matrix metalloproteinase 9 | 2 |
| CYP2C9 | Cytochrome P450 2C9 | 2 |
| SCD | Acyl-CoA desaturase | 1 |
| COX-2 | Cyclooxygenase-2 | 1 |
| HRH3 | Histamine H3 Receptor | 1 |
| MGAM | Maltase-glucoamylase | 1 |
| SELP | P-selectin | 1 |
| HIF1AN | Hypoxia-inducible factor 1-alpha inhibitor | 1 |
| CA9 | Carbonic anhydrase 9 | 1 |
| CASP9 | Caspase-9 | 1 |
| ADRA1B | Alpha-1b adrenergic receptor | 1 |
| TERT | Telomerase reverse transcriptase | 1 |
Interestingly, though proteins such as Glyoxalase I (GLO1, DD = 3), Nitric Oxide Synthase (NOS1, DD = 3), Matrix metalloproteinase-9 (MMP9, DD = 2) and Acetylcholinesterase (ACHE, DD = 2) do not have high topological properties, they are all the therapeutic targets of stomach diseases. For example, detoxifying enzyme GLO1 is a potential therapeutic target of human gastric cancer79, it was inhibited by M1 (Luteolin), M2 (Quercetin) and M4 (Kaempferol) (in herbs Salvia miltiorrhiza; Alpinia Officinarum Rhizoma; Rhizoma Galangae and Lindera Aggregate), therefore enhancing the therapeutic effect for GIDs by the synergistic effect in Sanhe Decoction. In addition, the nitric oxide synthase (NOS) family of enzymes synthesize NO in endothelial cells80, which augments the generation of reactive oxygen species by mitochondria, and thereby triggering mechanisms of cell survival or death81. NO is a mediator of vasodilatation, platelet aggregation, and regulates various cellular functions in immune and inflammatory processes82. Besides, endogenous NO derived from NOS contributes to mucosal defense against gastric damage83. MMP-9 has been shown to be one of the important enzymes in the invasion and metastatic cascade of gastric cancer8,84,85, thus the MMP9-inhibitor agent is the effective treatment for GIDs. In summary, the action mechanism for SHD is most probably due to that the potential active compounds target at multiple proteins in the biological network, and these targets interacted with each other so that play therapeutic effect as a whole for GIDs.
Compound-Target network for CVDs (C-T2)
The network of compounds and targets for CVDs is shown in Fig. 4(B). And the interaction modes of the compounds and targets are depicted as in the materials and methods of ‘Predicting the mode of action of drugs’. As shown in Fig. 4(B), there are 294 compound-target interactions in the network, and 98% of the targets were inhibited by the potential compounds in SHD. The targets with high degree (DD > 3) were listed in Table 5. Among these targets, Adenylate cyclase type V (ADCY5, DD = 47) is the protein with highest degree, that is to say that, ADCY5 could interact with the most nodes in the network, which plays a key role in regulating the network as the hub target. Moreover, followed by Cytochrome P450 2A5 (CYP2A5, DD = 35), Mineralocorticoid receptor (NR3C2, DD = 20), 5-hydroxytryptamine receptor 2A (HTR2A, DD = 18) and so forth, which are potential therapeutic targets for CVDs. Among these targets, Adenylate cyclase (AC) is the keystone of sympathetic transmission in β-AR signaling in myocardium, and β-AR plays a role in the development of aging cardiomyopathy86. The inhibition of ADCY5 prevents myocardial apoptosis potentially by enhancing resistance to oxidative stress and apoptosis87. NR3C2 (Mineralocorticoid Receptor) antagonists improve outcomes in patients with chronic heart failure caused by LV systolic dysfunction and hypertension by minimizing the cardiovascular damages88,89. In Sanhe Decoction, 20 molecules such as M18 (3α-hydroxytanshinoneIIa), M23 (Przewalskin a), M26 (Przewaquinone c) and M28 (Tanshinaldehyde) can inhibit NR3C2 activity, highlighting their therapeutic effect for CVDs. Most drugs used clinically are metabolized by cytochromes P450, specifically, CYP2A5 plays an important role in the regulation of oxidative stress in mice90. Intriguingly, the therapeutic target CYP2A5 regulates platelet activation in blood to reduce coronary heart disease in animal models91. Notably, CYP2A5 was predicted to be inhibited by 18 molecules in herbs Salvia Miltiorrhiza and Alpinia Officinarum Rhizoma, further highlighting the synergistic effect in treatment of CVDs. All these results above indicate that the CVDs treats the cardiovascular disease based on the synergistic interactions of different components.
Table 5. The CVDs target information.
| Gene_Name | Protein Name | Degree |
|---|---|---|
| ADCY5 | Adenylate cyclase type V | 47 |
| CYP2A5 | Cytochrome P450 2A5 | 35 |
| NR3C2 | Glucocorticoid receptor | 20 |
| HTR2A | 5-hydroxytryptamine receptor 2A | 18 |
| ESR1 | Estrogen Receptor | 18 |
| ALDH2 | Aldehyde dehydrogenase | 15 |
| CYP1A1 | Cytochrome P450 1A1 | 13 |
| NR3C1 | Glucocorticoid receptor | 12 |
| PDE2A | Phosphodiesterase 2A | 12 |
| VDR | Vitamin D receptor | 9 |
| AKR1B1 | Aldose reductase | 9 |
| AR | Androgen Receptor | 8 |
| ALOX5 | 5-Lipoxygenase | 8 |
| G6PD | glucose-6-phosphate 1-dehydrogenase isoform b | 8 |
| PLA2G2A | Phospholipase A2 group IIA | 8 |
| RARA | Retinoic acid receptor alpha | 8 |
| CYP2A6 | Cytochrome P450 2A6 | 7 |
| MTNR1A | Melatonin receptor 1A | 5 |
| ALOX12 | Arachidonate 12-lipoxygenase | 4 |
| GLO1 | Glyoxalase I | 4 |
| ACHE | Acetylcholinesterase | 3 |
| HTR5A | Serotonin 5a receptor | 3 |
| LTA4H | Leukotriene A4 Hydrolase | 3 |
| SLC6A4 | Serotonin Transporter | 3 |
| RELA | v-rel reticuloendotheliosis viral oncogene homolog A isoform 1 | 3 |
| ADORA1 | Adenosine A1 receptor | 3 |
| ALOX5 | 5-Lipoxygenase | 2 |
| PLK2 | Polo-Like Kinase 2 | 2 |
| TSPO | Translocator protein | 2 |
| NOX4 | NADPH oxidase 4 | 2 |
| GPR35 | G protein-coupled receptor 35 | 2 |
| ADORA2A | Adenosine Receptor A2A | 2 |
| MMP9 | Matrix metalloproteinase 9 | 2 |
| ADORA3 | Adenosine receptor A3 | 2 |
| ADRA2B | Alpha-2b adrenergic receptor | 2 |
| MMP3 | Matrix metalloproteinase-3 | 2 |
| FABP4 | Fatty acid binding protein adipocyte | 1 |
| SFRP1 | Secreted frizzled-related protein 1 | 1 |
| PLA2G1B | Phospholipase A2 | 1 |
| AVPR2 | Vasopressin V2 Receptor | 1 |
| CXCR1 | Interleukin-8 receptor A | 1 |
| MPO | Myeloperoxidase | 1 |
| SORD | Sorbitol dehydrogenase | 1 |
| F7 | Coagulation factor III/VII | 1 |
| PARP1 | Poly [ADP-ribose] polymerase-1 | 1 |
| ADRB1 | Beta-1 adrenergic receptor | 1 |
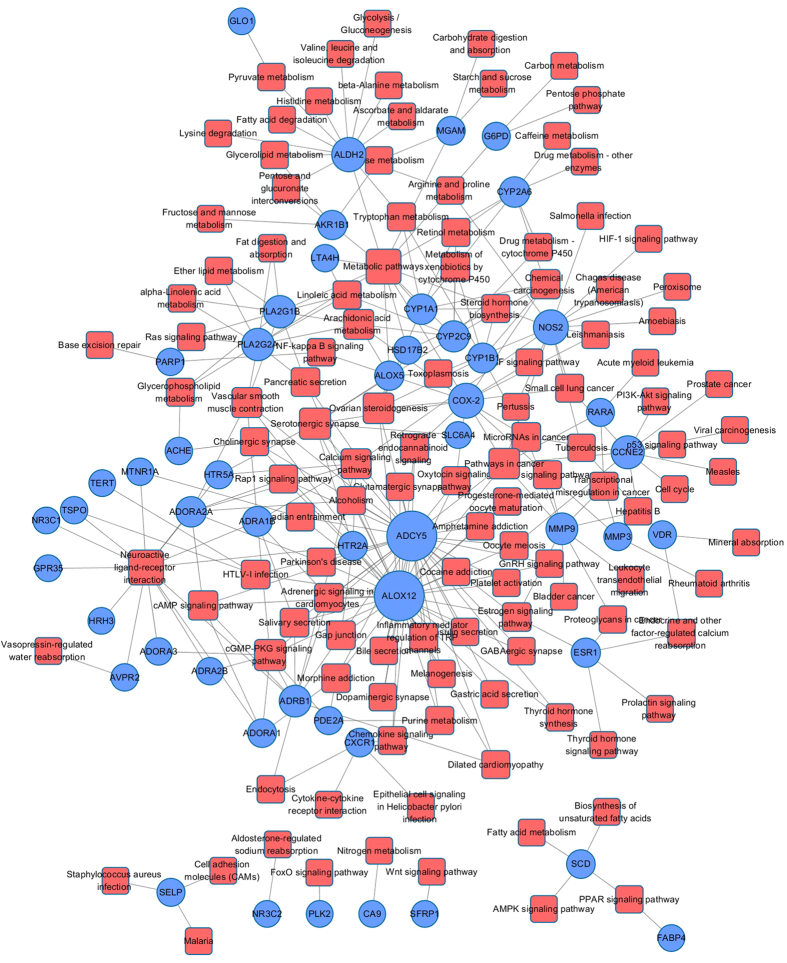
Target-Pathway network
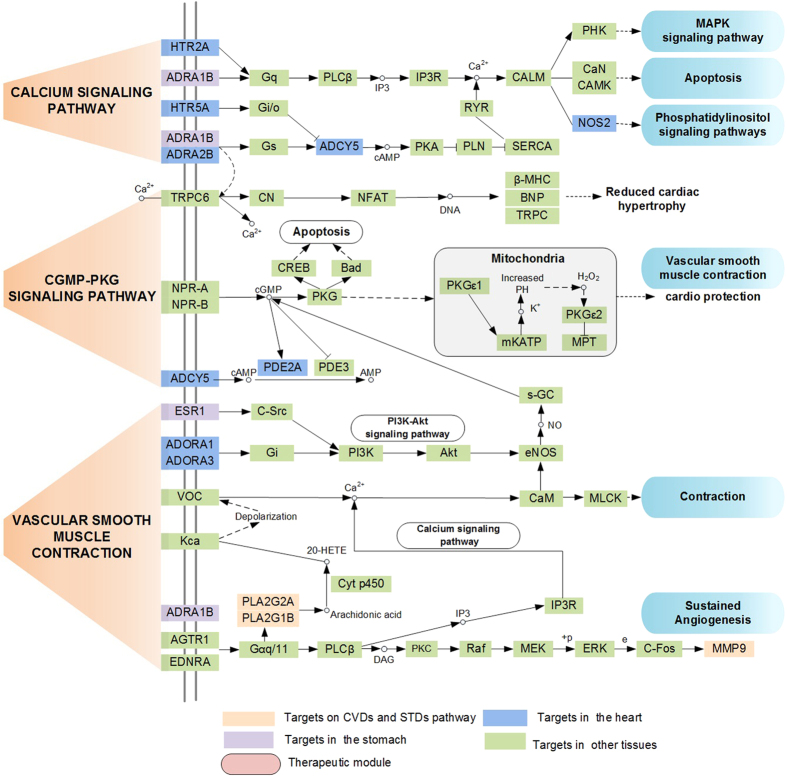
Different network regions may underlie different biological pathways, processes or cellular localizations. Drugs not only regulate their related targets, but also affect various metabolic enzymes and downstream proteins, as well as pathways related to the specific disease24. To understand the therapeutic mechanisms of SHD, we mapped the predicted targets onto their related pathways extracted from KEGG database (www.genome.jp/kegg)92 and generated a bipartite graph of T-P Network (Target-Pathway Network, Fig. 5), in which a compound and a signal pathway were linked if the compound targets on the proteins appeared in the signal pathways. Target-Pathway network consists of 171 nodes (51 targets and 120 pathways) and 294 edges after discarding 8 target proteins not participating in any pathways. Clearly, these pathways are linked with target proteins intensively, nearly 80% target proteins (40/51) are mapped in multiple pathways, indicating that these targets may regulate the interactions and cross-talk between multiple pathways. Similarly, major pathways (72/120) are also modulated through more than one target protein, and many of them have been proved as suitable therapeutic pathways for CVDs and GIDs, such as cGMP-PKG signaling pathway (hsa04022), calcium signaling pathway (hsa04020), cAMP signaling pathway (hsa04024), vascular smooth muscle contraction (hsa04270) and Arachidonic acid metabolism (hsa00590). The cGMP-PKG signaling pathway plays a significant role in cardioprotection by monitoring cell death and maintaining intracellular acidosis93. Calcium signaling pathway could mediate autophagy and apoptosis, and Calcium (Ca2+) ions regulate muscle contraction, electrical signals which determine the cardiac rhythm and cell growth, exerting pleiotropic effects on cardiovascular cells94,95. Vascular smooth muscle contraction also plays an important role in the regulation of CVDs, there is due to that proliferation of intimal vascular smooth muscle cells could induce the development of atherosclerosis. Therefore, inhibiting the proliferation of vascular smooth muscle cells is an effective way to control the process of CVDs24.
Figure 5. Target-Pathway network of active ingredients in Sanhe Decoction for CVDs and GIDs.
Circles are targets and squares are pathways.
Subsequently, based on the current knowledge of cardiovascular-gastrointestinal disease pathology, we mapped these targets into the DAVID database (https://david.ncifcrf.gov/)96 to identify the significantly overrepresented incorporated “cardiovascular-gastrointestinal disease pathway”. 51 targets were involved in the most highly related pathways associated with CVD and GIDs in three biological processes, which may provide basis for CVDs and GIDs treatment strategies as well. The functional annotation clustering analysis further illustrated that, as shown in Fig. 6, the enriched pathways under different biological processes are mainly enriched in several modules, including inflammation, contraction, cardiac hypertrophy, cardio protection and apoptosis.
Figure 6. The representative cardiovascular-gastrointestinal disease pathway and therapeutic modules.
Cardiac contraction process
Calcium signaling pathway and vascular smooth contraction pathway play important roles in the contraction function, which are disturbed by the active ingredients in Sanhe Decoction. As shown in Fig. 6, M2 (Quercetin) could reduce ADRA1B protein levels in the upstream pathways-vascular smooth muscle contraction pathway and calcium signaling pathway. Specially, ADRA1B participates in the control of vascular tone and plays an important role in regulating systolic blood pressure levels97, thus treating CVDs. In addition, PLA2G1B protein is the key gene in the control of contractions98, and it serves as a distinct target in the regulation of active lipid metabolites that promote inflammatory metabolic diseases including CVDs, such as atherosclerosis and hyperlipidemia99. Moreover, eight molecules (M1 (Luteolin), M2 (Quercetin), M3 (Isorhamnetin), M4 (Kaempferol), M5 (1,2,5,6-tetrahydrotanshinone), M6 (Isoimperatorin), M8 (Galangin), M9 (Medicarpin)) in SHD serve as activators of ADCY5, which is involved in vascular smooth muscle contraction100. In addition, calcium channel blockers are promising interventions for preventing cardiovascular events by lowering blood pressure101. The cyclic nucleotides signaling regulates cardiac function, therefore, it may provide novel treatments to improve heart function for hypertrophy and heart failure102. The central role of cyclic nucleotide phosphodiesterase (PDE) regulates the activities of the cardiovascular system103.
Inflammation process
NOS2 plays a central role in the inflammatory reactions and expresses protective effects against detrimental damage104. Regulation of NOS2 gene transcription appears to be the primary mechanism of action of cAMP, and whether it is stimulatory or inhibitory depends on the regulation of transcription factors including CREB, NF-κB and C/EBP105. Taken together, herbal ingredients in SHD mainly target on proteins ADORA1 and ADORA3, thereby regulating pathways such as PI3K-AKT pathway106 and MAPK pathway to reduce stomach inflammation. Besides, controlling of chronic infection in stomach is appropriate for its local effects, at the same time, proving the efficacy in the prevention of CVDs107. Therefore, Sanhe Decoction might provide an efficient system for preventing/treating both CVDs and GIDs by the anti-inflammation effect.
Apoptosis Process
Analogously, the activation of cGMP-PKG signaling pathway is essential for inhibiting the apoptosis and protecting the heart from ischemia/reperfusion injury93. And 13 herbal ingredients like M2 (Quercetin), M8 (Galangin) and M10 (Dehydrotanshinone II A) have been identified to regulate the cGMP-PKG signaling pathway in cardiac ischemia, thereby exhibiting protective effects against heart injury93. In addition, the majority of active ingredients in Sanhe Decoction targeted on proteins such as PLA2G1B, ADCY5 and ADORA1, and thereby exhibiting anti-apoptosis effects by regulating the cGMP-PKG signaling pathway108,109.
cAMP is a unique second messenger, which plays an anti-apoptotic role in protecting rat cardiac myocytes110, the changes in the phosphorylation of individual substrates of cAMP-dependent protein kinase is beneficial for the CVDs111. Additionally, the alterations of cAMP-mediated signaling pathway have different roles in the pathophysiology of the dilated cardiomyopathy111. Therefore, we speculate that herbal medicines of Sanhe Decoction probably mediates these pathways to exhibit the anti-apoptosis, thereby providing an effective strategy to treat CVDs and GIDs systematically. Adenylyl cyclase activity regulates heart failure due to myocardial infarction (MI) in rats112, especially, the soluble adenylyl cyclase plays an important role in apoptosis in coronary endothelial cells and cardiomyocytes113. Moreover, and adenylyl cyclase also regulates the function of gastrointestinal tract114. Therefore, the inhibition of adenylyl cyclase activity is an effective strategy to treat cardiovascular disease and gastrointestinal disorders.
In summary, all the pathways were interdependent with each other through the potential active compounds, which further indicates that SHD can exert synergistic influences on different pathways. In addition, a compound may target multiple proteins involved in multiple pathways, which indicates that the multiple targets of SHD could act on multiple biological processes to treat CVDs and GIDs effectively. We conclude that SHD probably mediates multiple pathways to promote the cardiac contractility properties, display anti-inflammatory and anti-apoptosis properties, and thereby exhibiting synergistic effect for CVDs and GIDs.
Target tissue location
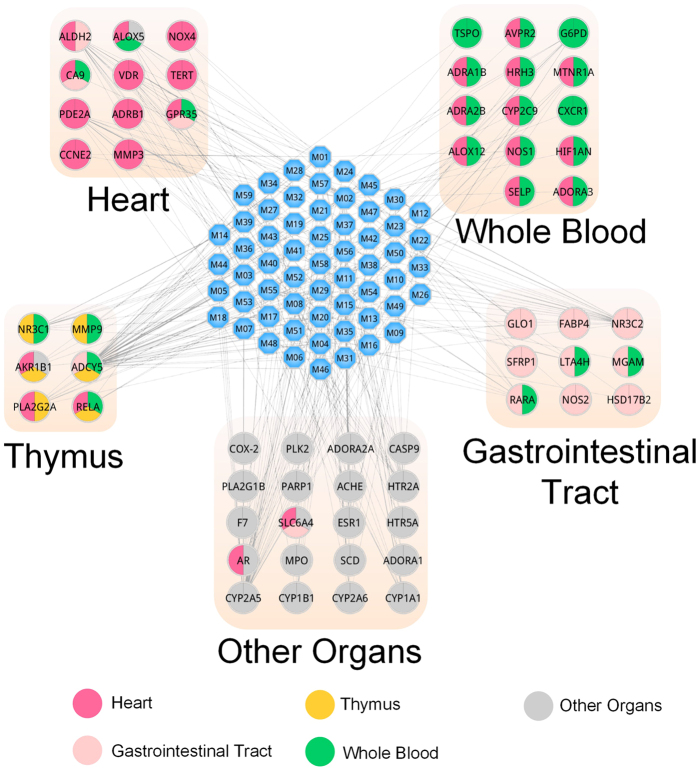
Understanding, on a system level, how the multi-organs respond to indications may facilitate the development of enhanced detection and treatment modalities for complex disease. By microarray analyses of mRNA expression, we found that 70 targets were mapped on 84 organs at different levels according to the BioGPS database (accessible at http://biogps.org)115. More importantly, we compared the expression patterns across different tissues, the tissue distribution network of the 70 targets are shown in Fig. 7. Based on the target expression pattern, the network is divided into several tissue modules, such as heart, stomach, whole blood and so on. Specifically, 24 targets contain higher mRNA expression in stomach than the average value of 84 tissues for each target. Therefore, these 24 stomach high-abundant targets are considered as therapeutic targets for GIDs, accounting for 34% of all the targets. There are 26 targets (accounting for 37% of all the targets) located in the heart, they are potential effective targets for the treatment of CVDs. Besides, most targets acted on two or more tissues, which suggests that these tissues are closely correlated. Consistent with this, patients with thymus hypoplasia have a high incidence of congenital heart disease116. Notably, the targets in whole blood are linked with tissues in almost all the forms. These results indicated that whole blood acts as the bridge and these tissues are closely related to the cardiovascular-gastrointestinal diseases.
Figure 7. Target organ location map.
The blue node represents the molecule and colored circles represents the target nodes along with the organs in which the target is located.
Experimental Validation
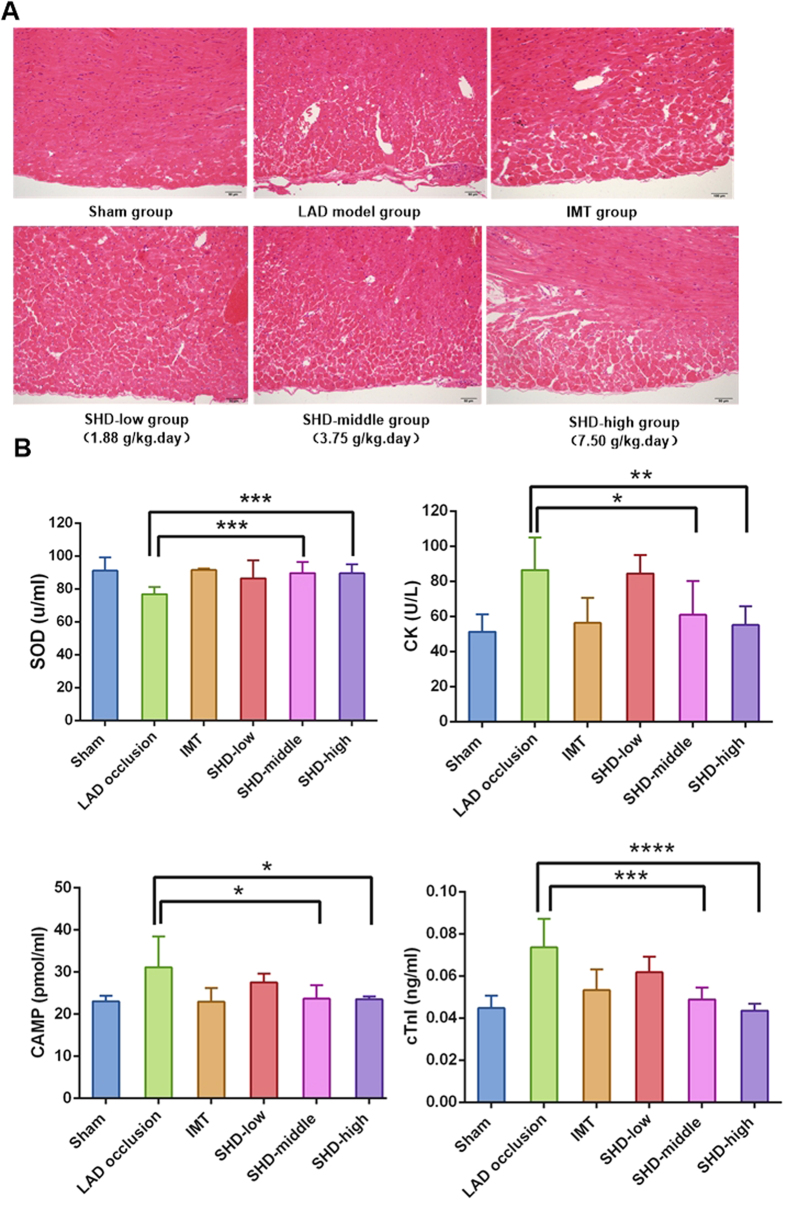
To investigate whether SHD has a pathological effect on CVDs, we evaluated the effects of SHD on a rat model of left anterior descending coronary artery (LAD) occlusion. The light microscopy results showed that unimpaired integrity and intact layers of cells in hematoxylin-eosin (HE)-stained heart of non-LAD occlusion rats. In addition, there was no fibrous hyperplasia or edema in intercellular substance (Fig. 8(A)). In contrast, the LAD occlusion model group rats showed varying degrees of apomorphosis and necrosis of cardiac muscle cells. In addition, intercellular substance was slightly edema, fibrous hyperplasia and slight inflammatory cell invasion. Compared with LAD occlusion model group, pathological changes decreased significantly with less edema, fibrous hyperplasia and slight inflammatory cell invasion in rats pre-treated with SHD (SHD-low 1.88 g/(kg/day), SHD-middle 3.75 g/(kg/day) and SHD-high 7.5 g/(kg/day)) and isosorbide mononitrate. Notably, SHD-middle (3.75 g/(kg/day) group achieved the best myocardial protection effect, which significantly attenuated pathological changes (i.e., slight apomorphosis and necrosis, intercellular substance showed slight edema, and there was slight inflammatory cell invasion), which was similar as the positive group with isosorbide mononitrate (IMT). The results indicated that the myocardial damage was reduced by SHD from the morphological change.
Figure 8.
Effect of Sanhe Decoction (SHD) on myocardial ischemia pathological changes (A) and biochemical changes of SOD, CK, cAMP and cTnI serum levels (B) in left anterior descending coronary artery (LAD) occlusion rats. LAD occlusion rats were intragastricly treated with SHD-low 1.88 g/(kg.day), SHD-middle 3.75 g/(kg.day), SHD-high 7.5 g/(kg.day) and isosorbide mononitrate (IMT, 4 mg/kg) for one week. The myocardial pathological changes were analyzed by optical microscope analysis on the eighth day. The muscular fibers (A) (magnification, × 30,000) of myocardial ultrastructure images were taken. Values represent means ± SEM, n = 8. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 vs. model.
Because SOD, CK, cAMP and serum cardiac troponin I (cTnI) concentrations are widely used as a biomarker for the detection of myocardial infarction, therefore, we measured SOD, CK, cAMP and cTnI in the serum to qualitatively assess the effect of SHD on the cardiac protection. As shown in Fig. 8(B), compared with model rats, SHD significantly increased the activity of antioxidant enzymes SOD and decreased levels of serum CK, cAMP and cTnI. These findings demonstrated that SHD treatment could effectively inhibit the production of free radicals of heart, and the results are consistent with those obtained by target protein function analysis.
Discussion and Conclusion
It has been observed that CVDs have an etiologic relationship with GIDs1,2, and currently there is no effective treatment strategy for treating CVD and GID simultaneously in western medicine. Compared with western medicine, TCM has been used in the synchronic treatment of heart and stomach for a long time in China. However, the complex action mechanisms of TCM have hindered the development of effective therapy for such kind of systematic diseases. How to understand the TCM as the whole and identification of the shared potential active compounds, targets and biological processes become the bottleneck restrictions for modern TCM study.
(1) In this study, we first proposed a novel strategy integrating gene co-expression, meridian theory and systems pharmacology approach to explore the mechanisms of TCM in the synchronic treatment of CVDs and GIDs. Our main findings are as follows:
(2) A novel system is constructed to investigate the closeness analysis between genes of CVDs and GIDs. The shared genes of CVDs and GIDs were identified and the association was evaluated.
(3) Based on the meridian theory, herbs belonged to the heart meridian and stomach meridian were collected. These two sets of herbs were found to exhibit significant correlations through the Fisher’s test. More importantly, the combinations of HM and SM drugs were widely applied in clinical use of CPM for the treatment of CVDs and GIDs simultaneously.
The systems pharmacology approach, which integrates ADME evaluation, multi-target/pathway regulations, and multi-organ cooperation, was then applied to SHD to clarify the mechanisms involved in the co-treatment of heart and stomach diseases. In addition, an experiment was carried out to validate the therapeutic effects of SHD in CVDs.
In summary, this study provided an integrative analysis of related complex diseases by the systems pharmacology approach to find potential active compounds and understand the action mechanisms of TCM. Despite these potentially interesting findings above, further interpretation such as the drug-drug interaction and the herb dose-effect relationship is necessary to consider relied on experimental data analysis. Moreover, further experimental testing of these compound-target binding actions and molecular mechanism of active compounds in vivo will be required to support further assessments of potential clinical application.
Materials and Methods
To understand the integrated treatment for co-occurring cardiovascular and gastrointestinal disorders, we firstly collected and analyzed the correlation of genes associated with CVDs and GIDs. Then, herbs of heart meridian (HM) and stomach meridian (SM) were extracted and their correlation was evaluated by Fisher’s test. In addition, these herbs were mapped into Chinese Patent Medicine (CPM) to investigate their clinical application and explore the principles of drug combination of heart and stomach meridians. Based on these systematic analyses of Chinese medicine, we selected Sanhe Decoction as a typical example to elaborate the molecular mechanisms of the co-treatment for cardiovascular and gastrointestinal disorders.
The gene analysis of CVDs and GIDs
The genes associated with CVDs and GIDs were obtained from literature mining and several disease-gene databases: Therapeutic Target Database (TTD, http://bidd.nus.edu.sg/group/ttd/)117, DrugBank (http://www.drugbank.ca/)118, HIT (Herbal Ingredients,Targets Database, http://lifecenter.sgst.cn/hit/)119, PharmGKB (http://www.pharmgkb.org)120 and Comparative Toxicogenomics Database (CTD, http://ctdbase.org/)121.
To evaluate the correlation between genes associated with CVDs and GIDs, we mapped these genes to Human Protein-Protein Interaction (PPI) Network (from Hint Database: http://hint.yulab.org/)122. In the PPI network, the correlation between target ai associated with CVDs and bj associated with GIDs was calculated by the following formula:
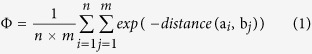 |
where n and m represent numbers of genes associated with CVDs and GIDs in the PPI network, respectively. Distance (ai, bj) represents the shortest path between gene ai and bj in the PPI network. If distance (ai, bj) is considered be infinite, it means that there is no association between CVDs’ and GIDs’ genes. The parameter exp (−distance (ai, bj)) could transfer the distance of two genes of CVDs and GIDs into the closeness between them. To further compare the associations between genes of CVDs and GIDs with that in the random two genes, we randomly selected two genes from PPI network and calculate the association between them, the process was repeated 1000 times.
Analysis of HM and SM herbs and their application in Chinese Patent Medicine
In this work, the available information of the heart meridian (HM) and stomach meridian (SM) herbs were extracted from pharmacopoeia of the People’s republic of China (2010)123, which consists of 586 different species of drugs in text, such as herbs, plant oils and extracts. The Fisher’s test was applied to assess the relationship between HM and SM drugs.
To further investigate the clinical application of these HM and SM drugs, we restricted these drugs into a comprehensive encyclopedia of CPM, the National Chinese patent medicine124. CPM used in current clinical practice for CVDs (including angina, cardiodynia and angina pectoris,) and GIDs (such as gastrointestinal ulcers, chronic atrophic gastritis and chronic gastritis) were extracted for further study. Considering the fact that herbs related to heart and stomach meridians occurred in CPM for the treatment of CVDs and GIDs, t-test was used to evaluate the combination of HM and SM in the integrated treatment for co-occurring disorders.
Systematic analysis of Sanhe Decoction
Here, Sanhe Decoction (consists of Lilli Bulbus, Salvia Miltiorrhiza, Alpinia Officinarum Rhizoma, Amomum Villosum, Santail Albi Lignum, Lindera Aggregata and Cyperus Rotundus) was selected as a case study, which is a famous Chinese medicine prescription in the treatment of epigastric pain including chronic atrophic gastritis, gastric ulcer and gastroesophageal reflux disease125 designed by TCM expert Jiao Shude. Besides, several previous studies have reported that SHD could be used to treat heart diseases as well126,127, but there was no study on the dissection of SHD for CVDs and GIDs. Therefore, we investigated the mechanisms of Sanhe Decoction in the treatment of CVDs and GIDs from a molecular to system level.
(1) ADME Screening
To screen for potentpharmaceutical compounds from Sanhe Decoction, an in silico ADME-systems evaluation model, which integrated drug-likeness (DL), oral bioavailability (OB), aqueous solubility (logS, the logarithm of aqueous solubility), lipophilicity (logP, logarithm of octanol-water partition coefficient) and Caco-2 permeability was proposed.
Lipophilicity: The lipophilicity was expressed as the partition coefficient P (log P), which is calculated by ALOGPS 2.1 software128. The value of log P less than 5 was selected for further analysis.
Aqueous solubility: Log S, a measure of aqueous solubility, which has been considered as an important factor in drug absorption and distribution. The value of Log S is also calculated by ALOGPS 2.1 software128 and the threshold value is range from −5 to −1.
Drug-likeness: To filter out the drug-like molecules, we have developed a database-dependent model to discriminate between drug-like and nondrug-like chemicals using the Tanimoto coefficient129. This model is constructed based on the molecular descriptors and Tanimoto coefficient (as displayed in Equation2).
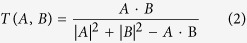 |
where A is the molecular properties of herbal ingredients, and B represents the average molecular properties of molecules in DrugBank database (http://www.drugbank.ca/) based on Dragon soft descriptors130. In this work, the molecules with satisfying drug-likeness index (DL ≥ 0.18) (average value for Drugbank) were selected as candidate compounds.
Oral bioavailability: OB, represents the rate and extent to which the active ingredient or active moiety is absorbed from a drug product and becomes available at the site of action131. Here the OB screening was performed by an in-house system OBioavail1.131 and the compounds with OB ≥ 30% were kept in the database.
Caco-2 permeability: For an orally administered drug, the majority of drug absorption occurs in the small intestine where the presence of villi and microvilli greatly increases the surface available for absorption132. Here, we employed a robust in silico Caco-2 permeability prediction model PreCaco2133 to predict the drug absorption. We set the threshold of Caco-2 permeability to −0.4, because the compounds with Caco-2 value less than −0.4 are considered to be not permeable.
(2) Drug targeting
The identification of drug targets is a critical step for both the pharmaceutical industry and academic biomedical research. In this work, a newly developed weighted ensemble similarity (WES) method based on over 900,000 drug-target relations was proposed to predict the direct targets of drugs. The proposed method consists of two steps: (1) evaluating the significance of ligand structure parameters for each target and (2) calculating the potential unity of a molecule for a specific target based on the top-important chemical features that are strongly related to pharmacological properties, then adopting a statistical model to control for random similarity. The method integrates chemical, genomic and pharmacological data to build a high level model for acquiring higher prediction ability. More importantly, the reliability of the theoretical model is validated by a rat experiment. The WES model shows impressive performance for both internal and external validation data30.
(3) Predicting the mode of action of drugs
To characterize the interactions of drugs and target proteins, an in silico PreAM (Prediction of the Action Mode) model based on random forests (RF) algorithm was built. Firstly, drug structures and protein sequences were converted into numerical descriptors. Secondly, the multiple Compound-Target Interactions (CTIs) were represented by concatenating these chemical and protein descriptors, and minimal-redundancy–maximal-relevance (mRMR) was applied as a variable selected strategy to identify the best combination of descriptors to ensure the model with the highest predictive power. Thirdly, a random forests (RF) algorithm was trained to generate a nonlinear classifier tailored to CTIs with known action modes. The PreAM model shows impressive performance of prediction for drug-target interactions, with an overall accuracy of 97.3%, an activated prediction accuracy of 87.7%, an inhibited prediction accuracy of 99.8%134.
(4) Network construction
To further explore the multi-scale action mechanisms of herbal medicines in the prevention and treatment of CVDs and GIDs, we constructed two networks: Compound-Target network (C-T network) and Target-Pathway network (T-P network). In the network, the nodes represent compounds/targets/pathways, and edges represent they are linked with each other. The canonical pathways were extracted from KEGG database (http://www.genome.jp/kegg/)92. The enriched KEGG pathways of targets with a false discovery rate less than 0.05 by Fisher’s Exact test in DAVID database (https://david.ncifcrf.gov/)96 were analyzed96. Finally, the pathways were divided into several modules after the enrichment analysis. In these networks, degree (DD)135 is used to characterize the connectedness of a node. The degree of a node is the number of edges associated with it. The topological properties of these networks were analyzed using the Network Analysis plugin and CentiScaPe 1.2 of Cytoscape136.
(5) Compound organ location
To further explore the underlying mechanisms of Sanhe Decoction that provides therapeutic effects in CVDs and GIDs, it is essential to validate the functional and tissue expression profile of the protein targets at the organ level. We enriched the overrepresented gene ontology (GO) terms and checked the tissue distribution of the obtained targets. For GO analysis, the biological process of GO vocabulary (GOBP) was identified through GOBP terms by DAVID database (https://david.ncifcrf.gov/)96 and GOBP terms with adjusted P-values < 0.005 were observed. The target tissue distribution was determined based on the microarray analyses data of different tissue types lodged in the BioGPS bank (accessible at http://biogps.org)115.
Animal experimental validation: A total of 72 Sprague Dawley male rats (150–200 g) were obtained from Shanghai Super-B&K laboratory animal Co.Ltd. (Shanghai, China). All rats were housed in a controlled environment (temperature 23° ± 2 °C, relative humidity 50% ± 10%, 12 h light/dark cycle). SD rats were randomly divided into 6 groups with the equal number (n = 10): Sham-operated control group (Sham), LAD occlusion model control group(Vehicle), LAD occlusion rats treated with 1.88 g/(kg.day) SHD (SHD-low), 3.75 g/(kg.day) SHD (SHD-middle), 7.5 g/(kg.day) SHD (SHD-high), and 5.4 mg/kg isosorbide mononitrate (IMT). IMT is used as the positive control, which inhibits angiogenesis and mediates vasoconstriction to treat myocardial ischemia137.
The components of SHD were as follows: Radix Salvia 30 g, Lilli Bulbus 30 g, Radix Linderae 12 g, Alpiniae Officinarum Rhizome 9 g, Santail Albi Lignum 9 g, Rhizoma Cyperi 9 g and Fructus Amomi 6 g. All these herbal drugs were identified and prepared in fluid extract for use. For in vivo experimental validation, the route of SHD and IMT delivery were oral administration when the weight of rats was 300–350 g. Pre-treatment was given daily for a period of 7 days. Then, LAD occlusion rat model was used to assess the cardiac protection function of SHD and IMT. Following a procedure established in related reports138,139. Sham group underwent a thoracotomy without infarct induction. At 4 hours after LAD occlusion, rats were exposed to anesthesia. Prior to histopathologic examination, we harvested blood from the abdominal aorta and stored them in −20 °C temperature.
Animal experiments were conducted in accordance with the Guiding Principles for the Care and Use of Laboratory Animals. Our experimental protocol was approved by the Ethics Committee for Animal Experiments at State Key Laboratory of New-tech for Chinese Medicine Pharmaceutical Process of Jiangsu Kanion Parmaceutical Co.LtD. All efforts were made to minimize the number of the animals used and their suffering.
Histology lesion analysis: After taking blood samples from the abdominal aorta, we quickly removed the hearts of rats, storing them in 4% paraformaldehyde for histological analysis. Next, we cut consecutive cross-sections (4 μm thick) of the hearts, and stained with hematoxylin-eosin (HE). Morphology were determined under an Olympus BH20 microscope (Olympus; Tokyo, Japan).
Biochemical analysis: We collected blood samples and measured serum levels of superoxide dismutase (SOD), creatine kinase (CK), cyclic adenosine monophosphate (cAMP), and cardiac troponin I (cTnI) using chemical colorimetry assay kits (Nanjing Jiancheng Bioengineering Institute) according to manufacturer instructions.
Statistical analysis: Mean values ± S.E. was calculated from independent experiments. Data were analyzed by Student’s t test and one-way analysis of variance. P value less than 0.05 was considered significant.
Additional Information
How to cite this article: Zhang, W. et al. Systems Pharmacology Dissection of the Integrated Treatment for Cardiovascular and Gastrointestinal Disorders by Traditional Chinese Medicine. Sci. Rep. 6, 32400; doi: 10.1038/srep32400 (2016).
Supplementary Material
Acknowledgments
This work was supported by the Fund of Northwest A & F University and was financially supported by the National Natural Science Foundation of China [Grant number 31170796, 81373892] and New Century Excellent Talents in University of Ministry of Education of China.
Footnotes
Author Contributions Y.W. and W.X. formulated the idea of the paper and supervised the research. W.Z. and Z.G. performed the research. Q.T. draw the Figures 1, 2, 3, 4, 5 and 6, W.Z. and Y.B. drew the Figure 7 originally. Xuetong Chen collected data. J.Z., J.X., Y.B. and Z.W. performed the experiments. W.Z. wrote the paper. Y.F., M.S., Z.W. and P.A.S. revised the paper. All authors reviewed the manuscript.
References
- Budzyński J. et al. The effect of double dose of omeprazole on the course of angina pectoris and treadmill stress test in patients with coronary artery disease-a randomized, double-blind, placebo controlled, crossover trial. International journal of cardiology 127, 233–239 (2008). [DOI] [PubMed] [Google Scholar]
- Dobrzycki S. et al. Does gastro-esophageal reflux provoke the myocardial ischemia in patients with CAD? International journal of cardiology 104, 67–72 (2005). [DOI] [PubMed] [Google Scholar]
- Xie C., Wang Z. C., Liu X. F. & Yang M. S. The common biological basis for common complex diseases: evidence from lipoprotein lipase gene. European Journal of Human Genetics 18, 3–7 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicano R., Broutet N., Ponzetto A. & Mégraud F. Helicobacter pylori: from the stomach to the heart. European journal of gastroenterology & hepatology 11, 1335–1338 (1999). [PubMed] [Google Scholar]
- Sana A. et al. Patients with chronic gastrointestinal ischemia have a higher cardiovascular disease risk and mortality. Atherosclerosis 224, 235–241 (2012). [DOI] [PubMed] [Google Scholar]
- Shirzad M., Mosaddegh M., Minaii B., Nikbakht Nasrabadi A. & Ahmadian-Attari M. M. The relationship between heart and stomach in Iranian traditional medicine: a new concept in cardiovascular disease management. Int J Cardiol 165, 556–557 (2013). [DOI] [PubMed] [Google Scholar]
- Chen K.-j., Hui K. K., Lee M. S. & Xu H. The potential benefit of complementary/alternative medicine in cardiovascular diseases. Evidence-Based Complementary and Alternative Medicine 2012, 125029 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman C., Bruley des Varannes S., Muresan L., Picos A. & Dumitrascu D. L. Atrial fibrillation in patients with gastroesophageal reflux disease: a comprehensive review. World J Gastroenterol 20, 9592–9599 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velagapudi P., Turagam M. K., Leal M. A. & Kocheril A. G. Atrial fibrillation and acid reflux disease. Clinical cardiology 35, 180–186 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unschuld P. U. Huang Di nei jing su wen. University of California Press I, 7–57 (2003). [Google Scholar]
- Li-Ling J. Human phenome based on Traditional Chinese Medicine-a solution to congenital syndromology. The American journal of Chinese medicine 31, 991–1000 (2003). [DOI] [PubMed] [Google Scholar]
- Qiu J. When the East meets the West: the future of traditional Chinese medicine in the 21st century. National Science Review 2, 377–380 (2015). [Google Scholar]
- Steinmann D. & Ganzera M. Recent advances on HPLC/MS in medicinal plant analysis. J. Pharm. Biomed. Anal. 55, 744–757 (2011). [DOI] [PubMed] [Google Scholar]
- Patwardhan B., Warude D., Pushpangadan P. & Bhatt N. Ayurveda and traditional Chinese medicine: a comparative overview. Evidence-Based Complementary and Alternative Medicine 2, 465–473 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can E., Cheng M. Z., Xiong Bi & Xin Tong Syndrome in Jin Kui Yao Lue. The Journal of Chinese Medicine and Acupuncture 20, 2–6 (2013). [Google Scholar]
- Berger S. I. & Iyengar R. Network analyses in systems pharmacology. Bioinformatics 25, 2466–2472 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins A. L. Network pharmacology: the next paradigm in drug discovery. Nat. Chem. Biol. 4, 682–690 (2008). [DOI] [PubMed] [Google Scholar]
- Janga S. C. & Tzakos A. Structure and organization of drug-target networks: insights from genomic approaches for drug discovery. Mol. Biosyst. 5, 1536–1548 (2009). [DOI] [PubMed] [Google Scholar]
- Hao D. C. & Xiao P. G. Network pharmacology: a Rosetta Stone for traditional Chinese medicine. Drug Dev. Res. 75, 299–312 (2014). [DOI] [PubMed] [Google Scholar]
- Li P. et al. Systems pharmacology strategies for drug discovery and combination with applications to cardiovascular diseases. J. Ethnopharmacol. 151, 93–107 (2014). [DOI] [PubMed] [Google Scholar]
- Huang C. et al. Systems pharmacology in drug discovery and therapeutic insight for herbal medicines. Briefings in bioinformatics bbt035 (2013). [DOI] [PubMed] [Google Scholar]
- Tao W. et al. Network pharmacology-based prediction of the active ingredients and potential targets of Chinese herbal Radix Curcumae formula for application to cardiovascular disease. J. Ethnopharmacol. 145, 1–10 (2013). [DOI] [PubMed] [Google Scholar]
- Yu H. et al. A systematic prediction of multiple drug-target interactions from chemical, genomic, and pharmacological data. PLoS One 7, e37608 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. et al. A system-level investigation into the mechanisms of Chinese Traditional Medicine: Compound Danshen Formula for cardiovascular disease treatment. PLoS One 7, e43918 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Wang J., Zhou W., Wang Y. & Yang L. Systems approaches and polypharmacology for drug discovery from herbal medicines: an example using licorice. J. Ethnopharmacol. 146, 773–793 (2013). [DOI] [PubMed] [Google Scholar]
- Shude. J., Sanhe Decoction in the treatment of epigastric pain. Practical internal medicine of traditional Chinese medicine 1, 49–51 (1987). [Google Scholar]
- An N., Xu L.-Z., Zou Z.-M. & Yang S.-L. Diarylheptanoids from Alpinia officinarum. J. Asian Nat. Prod. Res. 8, 637–641 (2006). [DOI] [PubMed] [Google Scholar]
- Tian J.-X., Li M., Liao J.-Q., Liu W.-K. & Tong X.-L. Xiangshaliujunzi Decoction for the treatment of diabetic gastroparesis: a systematic review. World journal of gastroenterology: WJG 20, 561–568 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- DU G.-h. & Zhang J.-t. The General Situation and Progress of the Modern Research of Red Sage Root (Radix Salviae Miltiorrhizae). Herald of Medicine 6, 000 (2004). [Google Scholar]
- Zheng C. et al. Large-scale Direct Targeting for Drug Repositioning and Discovery. Scientific reports 5, 11970 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X. et al. A novel chemometric method for the prediction of human oral bioavailability. Int. J. Mol. Sci. 13, 6964–6982 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Lee D. S., Christakis N. A. & Barabási A. L. The impact of cellular networks on disease comorbidity. Molecular systems biology 5, 7 APR (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röcken C. et al. The number of lymph node metastases in gastric cancer correlates with the angiotensin I–converting enzyme gene insertion/deletion polymorphism. Clinical Cancer Research 11, 2526–2530 (2005). [DOI] [PubMed] [Google Scholar]
- Granger C. B. et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. The Lancet 362, 772–776 (2003). [DOI] [PubMed] [Google Scholar]
- Schmieder R. E., Hilgers K. F., Schlaich M. P. & Schmidt B. M. Renin-angiotensin system and cardiovascular risk. Lancet 369, 1208–1219 (2007). [DOI] [PubMed] [Google Scholar]
- Nishijima Y. et al. Tetrahydrobiopterin depletion and NOS2 uncoupling contribute to heart failure-induced alterations in atrial electrophysiology. Cardiovascular research 91, 71–79 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jainu M. & Devi C. S. S. Gastroprotective action of Cissus quadrangularis extract against NSAID induced gastric ulcer: role of proinflammatory cytokines and oxidative damage. Chemico-biological interactions 161, 262–270 (2006). [DOI] [PubMed] [Google Scholar]
- Galea E. & Feinstein D. L. Regulation of the expression of the inflammatory nitric oxide synthase (NOS2) by cyclic AMP. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 13, 2125–2137 (1999). [DOI] [PubMed] [Google Scholar]
- Li M., Wu X., Wang J. & Pan Y. Towards the identification of protein complexes and functional modules by integrating PPI network and gene expression data. BMC Bioinf. 13, 109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee N. P. Pharmacopoeia of the People’s Republic of China. China Medical Science Press, Beijing, Part 1, 376–377 (2010). [Google Scholar]
- Fuhrman B. et al. Antiatherosclerotic effects of licorice extract supplementation on hypercholesterolemic patients: increased resistance of LDL to atherogenic modifications, reduced plasma lipid levels, and decreased systolic blood pressure. Nutrition 18, 268–273 (2002). [DOI] [PubMed] [Google Scholar]
- Xie S. et al. Antiarrhythmic effect of glycyrrhetinic acid. Herald Med 23, 140–143 (2004). [Google Scholar]
- Zhan C. & Yang J. Protective effects of isoliquiritigenin in transient middle cerebral artery occlusion-induced focal cerebral ischemia in rats. Pharmacol. Res. 53, 303–309 (2006). [DOI] [PubMed] [Google Scholar]
- Zhang M. & Shen Y. Advances in studies on Glycyrrhizae Radix et Rhizoma and its active components in anti-inflammation and mechanism. Drugs & Clinic 4, 261–268 (2011). [Google Scholar]
- Revers F. Licorice juice in therapy of ventricular and duodenal ulcers. Ned Tijdschr Geneeskd 92, 2968–2973 (1948).18894329 [Google Scholar]
- Van Marle J., Aarsen P. N., Lind A. & Van Weeren-Kramer J. Deglycyrrhizinised liquorice (DGL) and the renewal of rat stomach epithelium. Eur. J. Pharmacol. 72, 219–225 (1981). [DOI] [PubMed] [Google Scholar]
- Kassir Z. Endoscopic controlled trial of four drug regimens in the treatment of chronic duodenal ulceration. Irish medical journal 78, 153–156 (1985). [PubMed] [Google Scholar]
- Aly A. M., Al-Alousi L. & Salem H. A. Licorice: a possible anti-inflammatory and anti-ulcer drug. AAPS PharmSciTech 6, E74–E82 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagetia G. C. & Baliga M. S. The evaluation of nitric oxide scavenging activity of certain Indian medicinal plants in vitro: a preliminary study. Journal of Medicinal Food 7, 343–348 (2004). [DOI] [PubMed] [Google Scholar]
- Sivaramakrishnan V. & Shankaranarayana K. Investigation on the insecticidal properties of plant extractives-I testing of new medicinal oils, HESP from spent sandalwood powder on insects. Science & culture 56, 124–127 (1990). [Google Scholar]
- Verma S., Singh M., Jain P. & Bordia A. Protective effect of ginger, Zingiber officinale Rose on experimental atherosclerosis in rabbits. Indian journal of experimental biology 42, 736–738 (2004). [PubMed] [Google Scholar]
- Ali B. H., Blunden G., Tanira M. O. & Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food and chemical Toxicology 46, 409–420 (2008). [DOI] [PubMed] [Google Scholar]
- Afzal Μ., Al-Hadidi D., Menon M., Pesek J. & Dhami M. Ginger: an ethnomedical, chemical and pharmacological review. Drug metabolism and drug interactions 18, 159–190 (2001). [DOI] [PubMed] [Google Scholar]
- Wang Y.-y., Liu J.-h. & Yu B.-y. Biotransformation of Flavonoids by Streptomyces griseus ATCC 13273. Pharmaceutical biotechnology-Beijing 12, 308 (2005). [Google Scholar]
- Cao H., Chen X., Jassbi A. R. & Xiao J. Microbial biotransformation of bioactive flavonoids. Biotechnol. Adv. 33, 214–223 (2015). [DOI] [PubMed] [Google Scholar]
- Walle T. Absorption and metabolism of flavonoids. Free Radical Biol. Med. 36, 829–837 (2004). [DOI] [PubMed] [Google Scholar]
- Zhang Y. et al. Tanshinones: sources, pharmacokinetics and anti-cancer activities. Int. J. Mol. Sci. 13, 13621–13666 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Wang G. J., Li J., Hao H. P. & Zheng C. N. Characterization of metabolites of tanshinone IIA in rats by liquid chromatography/tandem mass spectrometry. J. Mass Spectrom. 41, 670–684 (2006). [DOI] [PubMed] [Google Scholar]
- Qi L. et al. Luteolin improves contractile function and attenuates apoptosis following ischemia–reperfusion in adult rat cardiomyocytes. Eur. J. Pharmacol. 668, 201–207 (2011). [DOI] [PubMed] [Google Scholar]
- Lagiou P. et al. Flavonoids, vitamin C and adenocarcinoma of the stomach. Cancer Causes & Control 15, 67–72 (2004). [DOI] [PubMed] [Google Scholar]
- Fu J. et al. Tanshinone IIA protects cardiac myocytes against oxidative stress-triggered damage and apoptosis. Eur. J. Pharmacol. 568, 213–221 (2007). [DOI] [PubMed] [Google Scholar]
- Sun J., Tan B. K., Huang S.-H., Whiteman M. & Zhu Y.-Z. Effects of natural products on ischemic heart diseases and cardiovascular system. Acta Pharmacol. Sin. 23, 1142–1151 (2002). [PubMed] [Google Scholar]
- Hrelia S., Angeloni C., Watson R. & Preedy V. Quercetin and its metabolites in heart health. Bioactive Food as Dietary Interventions for Cardiovascular Disease Chap 13, 217–228 (2012). [Google Scholar]
- Hollman P., Hertog M. & Katan M. Role of dietary flavonoids in protection against cancer and coronary heart disease. Biochem. Soc. Trans. 24, 785–789 (1996). [DOI] [PubMed] [Google Scholar]
- M Calderon-Montano J., Burgos-Morón E., Pérez-Guerrero C. & López-Lázaro M. A review on the dietary flavonoid kaempferol. Mini reviews in medicinal chemistry 11, 298–344 (2011). [DOI] [PubMed] [Google Scholar]
- Khalil M. & Sulaiman S. The potential role of honey and its polyphenols in preventing heart disease: a review. African Journal of Traditional, Complementary and Alternative Medicines 7, 315–321 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran L. & Sethi G. Abstract C43: Potential role of isorhamnetin to overcome chemoresistance in gastric cancer. Cancer research 71, C43–C43 (2011). [Google Scholar]
- Sivapalan S. R. Medicinal uses and pharmacological activities of Cyperus rotundus Linn-A Review. Int J Sci Res Pub 3, 1–7 (2013). [Google Scholar]
- Lee I.-M. et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women’s Health Study: a randomized controlled trial. Jama 294, 56–65 (2005). [DOI] [PubMed] [Google Scholar]
- Jacobs E. J. et al. Vitamin C, vitamin E, and multivitamin supplement use and stomach cancer mortality in the Cancer Prevention Study II cohort. Cancer Epidemiology Biomarkers & Prevention 11, 35–41 (2002). [PubMed] [Google Scholar]
- Lau Y. S. et al. Boldine protects endothelial function in hyperglycemia-induced oxidative stress through an antioxidant mechanism. Biochemical pharmacology 85, 367–375 (2013). [DOI] [PubMed] [Google Scholar]
- Smoak K. A. & Cidlowski J. A. Mechanisms of glucocorticoid receptor signaling during inflammation. Mechanisms of ageing and development 125, 697–706 (2004). [DOI] [PubMed] [Google Scholar]
- Deschamps A. M., Murphy E. & Sun J. Estrogen receptor activation and cardioprotection in ischemia reperfusion injury. Trends in cardiovascular medicine 20, 73–78 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth E. A., Obeid N. R. & Lucchesi B. R. Activation of estrogen receptor-α protects the in vivo rabbit heart from ischemia-reperfusion injury. American Journal of Physiology-Heart and Circulatory Physiology 289, H2039–H2047 (2005). [DOI] [PubMed] [Google Scholar]
- Ryu W. S. et al. Expression of estrogen receptors in gastric cancer and their clinical significance. Journal of surgical oncology 106, 456–461 (2012). [DOI] [PubMed] [Google Scholar]
- Leung S. Y. et al. Phospholipase A2 group IIA expression in gastric adenocarcinoma is associated with prolonged survival and less frequent metastasis. Proceedings of the National Academy of Sciences 99, 16203–16208 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan T. A., Morin P. J., Vogelstein B. & Kinzler K. W. Mechanisms underlying nonsteroidal antiinflammatory drug-mediated apoptosis. Proceedings of the National Academy of Sciences 95, 681–686 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B.-q.Y. et al. Androgen receptor promotes gastric cancer cell migration and invasion via AKT-phosphorylation dependent upregulation of matrix metalloproteinase 9. Oncotarget 5, 10584–10595 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoda F. et al. Integrated genomic and functional analyses reveal glyoxalase I as a novel metabolic oncogene in human gastric cancer. Oncogene 34, 1196–1206 (2015). [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. & Higgs E. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacological reviews 43, 109–142 (1991). [PubMed] [Google Scholar]
- Moncada S. & Erusalimsky J. D. Does nitric oxide modulate mitochondrial energy generation and apoptosis? Nat. Rev. Mol. Cell Biol. 3, 214–220 (2002). [DOI] [PubMed] [Google Scholar]
- Coleman J. W. Nitric oxide in immunity and inflammation. International immunopharmacology 1, 1397–1406 (2001). [DOI] [PubMed] [Google Scholar]
- Nishio H., Hayashi Y., Terashima S. & Takeuchi K. Role of endogenous nitric oxide in mucosal defense of inflamed rat stomach following iodoacetamide treatment. Life Sci. 79, 1523–1530 (2006). [DOI] [PubMed] [Google Scholar]
- St-Pierre Y., Themsche C. V. & Esteve P.-O. Emerging features in the regulation of MMP-9 gene expression for the development of novel molecular targets and therapeutic strategies. Current Drug Targets-Inflammation & Allergy 2, 206–215 (2003). [DOI] [PubMed] [Google Scholar]
- Vandooren J., Van den Steen P. E. & Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): the next decade. Crit. Rev. Biochem. Mol. Biol. 48, 222–272 (2013). [DOI] [PubMed] [Google Scholar]
- Swynghedauw B. et al. Molecular and cellular biology of the senescent hypertrophied and failing heart. The American journal of cardiology 76, 2D–7D (1995). [DOI] [PubMed] [Google Scholar]
- Yan L., Vatner S. F. & Vatner D. E. Disruption of type 5 adenylyl cyclase prevents β-adrenergic receptor cardiomyopathy: a novel approach to β-adrenergic receptor blockade. Am J Physiol Heart Circ Physiol. 307, H1521–H1528 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youcef G. et al. 0298: The early mineralocorticoid receptor antagonism mitigates the metabolic syndrome symptoms and transition to heart failure in the SHHF rat model. Archives of Cardiovascular Diseases Supplements 6, 49 (2014). [Google Scholar]
- Iqbal J. et al. Selection of a mineralocorticoid receptor antagonist for patients with hypertension or heart failure. European Journal of Heart Failure 16, 143–150 (2014). [DOI] [PubMed] [Google Scholar]
- Renton K. W. Cytochrome P450 regulation and drug biotransformation during inflammation and infection. Current drug metabolism 5, 235–243 (2004). [DOI] [PubMed] [Google Scholar]
- Golimbet V., Dolzhikov A., Korovaitseva G. & Isaeva M. Association of 5-HTR2A and 5-HTR2C serotonin receptor gene polymorphisms with depression risk in patients with coronary heart disease. Bull. Exp. Biol. Med. 156, 680–683 (2014). [DOI] [PubMed] [Google Scholar]
- Kanehisa M. & Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic acids research 28, 27–30 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbe A. et al. Role of cGMP-PKG signaling in the protection of neonatal rat cardiac myocytes subjected to simulated ischemia/reoxygenation. Basic research in cardiology 105, 643–650 (2010). [DOI] [PubMed] [Google Scholar]
- Marks A. R. Ryanodine receptors/calcium release channels in heart failure and sudden cardiac death. Journal of molecular and cellular cardiology 33, 615–624 (2001). [DOI] [PubMed] [Google Scholar]
- Wang S., Shih Y., Ko W., Wei Y.-H. & Shih C.-M. Cadmium-induced autophagy and apoptosis are mediated by a calcium signaling pathway. Cell. Mol. Life Sci. 65, 3640–3652 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang da W., Sherman B. T. & Lempicki R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature protocols 4, 44–57 (2009). [DOI] [PubMed] [Google Scholar]
- Krushkal J. et al. Linkage and association of adrenergic and dopamine receptor genes in the distal portion of the long arm of chromosome 5 with systolic blood pressure variation. Human molecular genetics 7, 1379–1383 (1998). [DOI] [PubMed] [Google Scholar]
- Torkamani A., Topol E. J. & Schork N. J. Pathway analysis of seven common diseases assessed by genome-wide association. Genomics 92, 265–272 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui D. Y. Phospholipase A2 enzymes in metabolic and cardiovascular diseases. Current opinion in lipidology 23, 235 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T. et al. Milk-Derived Peptides, Val-Pro-Pro and Ile-Pro-Pro, Attenuate Atherosclerosis Development in Apolipoprotein E–Deficient Mice: A Preliminary Study. Journal of medicinal food 16, 396–403 (2013). [DOI] [PubMed] [Google Scholar]
- Dahlöf B. et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. The Lancet 366, 895–906 (2005). [DOI] [PubMed] [Google Scholar]
- Fischmeister R. et al. Compartmentation of cyclic nucleotide signaling in the heart the role of cyclic nucleotide phosphodiesterases. Circulation research 99, 816–828 (2006). [DOI] [PubMed] [Google Scholar]
- Movsesian M. A. PDE3 cyclic nucleotide phosphodiesterases and the compartmentation of cyclic nucleotide-mediated signalling in cardiac myocytes. Basic research in cardiology 97, I83–I90 (2002). [DOI] [PubMed] [Google Scholar]
- Tripathi P., Tripathi P., Kashyap L. & Singh V. The role of nitric oxide in inflammatory reactions. FEMS Immunology & Medical Microbiology 51, 443–452 (2007). [DOI] [PubMed] [Google Scholar]
- Galea E. & FEINSTEIN D. L. Regulation of the expression of the inflammatory nitric oxide synthase (NOS2) by cyclic AMP. The FASEB Journal 13, 2125–2137 (1999). [DOI] [PubMed] [Google Scholar]
- Schabbauer G., Tencati M., Pedersen B., Pawlinski R. & Mackman N. PI3K-Akt pathway suppresses coagulation and inflammation in endotoxemic mice. Arteriosclerosis, Thrombosis, and Vascular Biology 24, 1963–1969 (2004). [DOI] [PubMed] [Google Scholar]
- Lowe G. D. The relationship between infection, inflammation, and cardiovascular disease: an overview. Annals of periodontology 6, 1–8 (2001). [DOI] [PubMed] [Google Scholar]
- Wang L., Huang J., Jiang M. & Lin H. Tissue-specific transplantation antigen P35B (TSTA3) immune response–mediated metabolism coupling cell cycle to postreplication repair network in no-tumor hepatitis/cirrhotic tissues (HBV or HCV infection) by biocomputation. Immunologic research 52, 258–268 (2012). [DOI] [PubMed] [Google Scholar]
- Sheng H. et al. Omega-3 PUFAs induce apoptosis of gastric cancer cells via ADORA1. Frontiers in bioscience (Landmark edition) 19, 854–861 (2013). [DOI] [PubMed] [Google Scholar]
- Iwai-Kanai E. et al. α-and β-Adrenergic pathways differentially regulate cell type–specific apoptosis in rat cardiac myocytes. Circulation 100, 305–311 (1999). [DOI] [PubMed] [Google Scholar]
- Movsesian M. A. & Bristow M. R. Alterations in cAMP‐Mediated Signaling and Their Role in the Pathophysiology of Dilated Cardiomyopathy. Current topics in developmental biology 68, 25–48 (2005). [DOI] [PubMed] [Google Scholar]
- Sethi R., Dhalla K., Beamish R. E. & Dhalla N. S. Differential changes in left and right ventricular adenylyl cyclase activities in congestive heart failure. American Journal of Physiology-Heart and Circulatory Physiology 272, H884–H893 (1997). [DOI] [PubMed] [Google Scholar]
- Chen J., Levin L. R. & Buck J. Role of soluble adenylyl cyclase in the heart. American Journal of Physiology-Heart and Circulatory Physiology 302, H538–H543 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbatini M. E., Gorelick F. & Glaser S. Adenylyl cyclases in the digestive system. Cell. Signalling 26, 1173–1181 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. et al. BioGPS: an extensible and customizable portal for querying and organizing gene annotation resources. Genome biology 10, R130 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedom R. M., Rosen F. S. & Nadas A. S. Congenital cardiovascular disease and anomalies of the third and fourth pharyngeal pouch. Circulation 46, 165–172 (1972). [DOI] [PubMed] [Google Scholar]
- Zhu F. et al. Therapeutic target database update 2012: a resource for facilitating target-oriented drug discovery. Nucleic acids research 40, D1128–D1136 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart D. S. et al. DrugBank: a comprehensive resource for in silico drug discovery and exploration. Nucleic acids research 34, D668–D672 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye H. et al. HIT: linking herbal active ingredients to targets. Nucleic acids research 39, D1055–D1059 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong L., Owen R. P., Gor W., Altman R. B. & Klein T. E. PharmGKB: an integrated resource of pharmacogenomic data and knowledge. Current protocols in bioinformatics/editoral board, Andreas D. Baxevanis Chapter 14, Unit14–17 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A. P. et al. The Comparative Toxicogenomics Database: update 2013. Nucleic acids research 41, D1104–D1114 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das J. & Yu H. HINT: High-quality protein interactomes and their applications in understanding human disease. BMC systems biology 6, 92 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee N. P. Pharmacopoeia of the People’s Republic of China. China Medical Science Press, Beijing, Part 1, 181–182 (2010). [Google Scholar]
- Minxian S. & New edition fo National chinese patent medicine (second edition). People’s Medical Publishing House April, 1307–1311, 1319–1322 (2011). [Google Scholar]
- Peng B. Chinese and western medicine clinical digestive epidemiology. Chinese press of traditional Chinese medicine, Beijing, 180–187 (1997). [Google Scholar]
- Fan G. & L. Y. Sanhe decoction in the treatment of 36 angina patients. National physician BBS 4, 36 (1996). [Google Scholar]
- Jianlan L. The clinical application of Sanhe Decoction. Zhejiang Journal of Integrated Traditional Chinese and Western Medicine 17, 596 (2007). [Google Scholar]
- Tetko I. V., Tanchuk V. Y. & Villa A. E. Prediction of n-octanol/water partition coefficients from PHYSPROP database using artificial neural networks and E-state indices. Journal of chemical information and computer sciences 41, 1407–1421 (2001). [DOI] [PubMed] [Google Scholar]
- Willett P., Barnard J. M. & Downs G. M. Chemical similarity searching. Journal of chemical information and computer sciences 38, 983–996 (1998). [Google Scholar]
- Mauri A., Consonni V., Pavan M. & Todeschini R. Dragon software: An easy approach to molecular descriptor calculations. Match 56, 237–248 (2006). [Google Scholar]
- Chen M.-L. et al. Bioavailability and bioequivalence: an FDA regulatory overview. Pharm. Res. 18, 1645–1650 (2001). [DOI] [PubMed] [Google Scholar]
- Pang K. S. Modeling of intestinal drug absorption: roles of transporters and metabolic enzymes (for the Gillette Review Series). Drug metabolism and disposition 31, 1507–1519 (2003). [DOI] [PubMed] [Google Scholar]
- Li L., Li Y., Wang Y., Zhang S. & Yang L. Prediction of human intestinal absorption based on molecular indices. J Mol Sci 23, 286–291 (2007). [Google Scholar]
- Wang Y. et al. Systems Pharmacology Dissecting Holistic Medicine for Treatment of Complex Diseases: An Example Using Cardiocerebrovascular Diseases Treated by TCM. Evidence-Based Complementary and Alternative Medicine 2015, 980190 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuaje F. J., Zhang L., Devaux Y. & Wagner D. R. Drug-target network in myocardial infarction reveals multiple side effects of unrelated drugs. Scientific reports 1, 3216539 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon P. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome research 13, 2498–2504 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes P., Westaby D. & Williams R. Effect and mechanism of action of isosorbide-5-mononitrate. Gut 29, 752–755 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki K. G. et al. Effects of (−)-epicatechin on myocardial infarct size and left ventricular remodeling after permanent coronary occlusion. Journal of the American College of Cardiology 55, 2869–2876 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.-Y. et al. Gadd45β is a novel mediator of cardiomyocyte apoptosis induced by ischaemia/hypoxia. Cardiovascular research 87, 119–126 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.