Abstract
Campylobacter jejuni (C. jejuni), a Gram-negative microaerophilic bacterium, is a predominant cause of bacterial foodborne gastroenteritis in humans worldwide. Despite its importance as a major foodborne pathogen, our understanding of the molecular mechanisms underlying C. jejuni stress survival and pathogenesis is limited. Inorganic polyphosphate (poly P) has been shown to play significant roles in bacterial resistance to stress and virulence in many pathogenic bacteria. C. jejuni contains the complete repertoire of enzymes required for poly P metabolism. Recent work in our laboratory and others have demonstrated that poly P controls a plethora of C. jejuni properties that impact its ability to survive in the environment as well as to colonize/infect mammalian hosts. This review article summarizes the current literature on the role of poly P in C. jejuni stress survival and virulence and discusses on how poly P-related enzymes can be exploited for therapeutic/prevention purposes. Additionally, the review article identifies potential areas for future investigation that would enhance our understanding of the role of poly P in C. jejuni and other bacteria, which ultimately would facilitate design of effective therapeutic/preventive strategies to reduce not only the burden of C. jejuni-caused foodborne infections but also of other bacterial infections in humans.
Keywords: Campylobacter jejuni, Stress tolerance, Inorganic polyphosphate, Virulence, Colonization/infection
Core tip: Recent studies show that inorganic polyphosphate (poly P) plays several important roles in the biology of Campylobacter jejuni (C. jejuni), a major cause of bacterial foodborne gastroenteritis in humans. This review summarizes the latest findings on the role of poly P in C. jejuni stress survival and virulence, provides directions for future investigation, and discusses the potential of polyphosphate kinase enzymes as drug/vaccine targets to control C. jejuni infections in humans.
INTRODUCTION
Campylobacter (Greek word “Kampylos” means curved) species are curved to spiral-shaped, non-spore forming, Gram-negative bacteria that contain a single flagellum at one or both ends. Most of the Campylobacter species are microaerophilic and use a respiratory type of metabolism; however, some species prefer to grow anaerobically. Campylobacter species are chemoorganotrophs - they primarily depend on amino acids or Kreb’s cycle intermediates for energy. Based on their optimal growth temperature, Campylobacter species are classified into thermophilic and non-thermophilic species. The thermophilic Campylobacter species grow optimally at 42 °C [Campylobacter jejuni (C. jejuni) and Campylobacter coli (C. coli)], while the non-thermophilic species grow optimally at the range between 30-37 °C (Campylobacter fetus). The genus Campylobacter consists of 34 species and 14 subspecies, which are human and animal pathogens (http://www.bacterio.net/campylobacter.html, last accessed 17 March 2016). Several of these species cause a plethora of clinical manifestations in humans (Table 1); however, C. jejuni and C. coli are the predominant species associated with human disease[1].
Table 1.
Comprehensive summary of Campylobacter infections in humans
| Type | Clinical form/presentation | Risk factors | Target site | Symptoms | Species associated |
| (A) Intestinal | Periodontal diseases | Oral bleeding, increased vascular permeability[70], and pregnancy[71]? | Oral cavity | Bleeding, tenderness, and tooth loss | C. rectus[70], C. gracilis, C. showae[72] and C. concisus[73] |
| Esophageal diseases | Mucosal damage due to stomach contents regurgitation[1] | Esophagus | Heart burn, regurgitation, bloating, bad breath, nausea, and abdominal pain | C. concisus[74] | |
| (gastroesophageal reflux disease, Barrett’s esophagus, and esophageal adenocarcinoma) | |||||
| Self-limited gastroenteritis, most common form | All factors as described in review | Jejunum and ileum | Diarrhea, fever, and abdominal pain | C. jejuni and C. coli[1,75] | |
| Post-infectious functional gastrointestinal disorder, irritable bowel syndrome, and functional dyspepsia | Infection with C. jejuni and other species[1] | Diarrhea, constipation or both, and abdominal pain | C. jejuni, C. coli and C. consisus[76] | ||
| Inflammatory bowel disease, Crohn’s diseases (CD), and ulcerative colitis (UC) | Gut dysbiosis[77] | CD-any part of intestine UC- colon | Diarrhea, fever and fatigue, abdominal pain, weight loss, and reduced appetite | C. concisus, C. showae, C. hominis, C. rectus, and C. ureolyticus[78] | |
| Colorectal cancer | Gut dysbiosis[79] | Colon | Diarrhea, constipation, abdominal pain, weight loss, and rectal bleeding | C. showae[80] | |
| Cholecystitis | Gall stones[1] | Gall bladder | Pain and tenderness in right abdomen, nausea, vomiting, and fever | C. jejuni[81] | |
| (B) Extra-intestinal | Guillain-Barre syndrome | C. jejuni infection[82] | Nervous system | Progressive symmetric weakness in limbs, below or lack of reflex (hyporeflexia) | C. jejuni[1,82] |
| Miller Fisher syndrome | C. jejuni[83] | Nervous system | Oculo-motor weakness | C. jejuni[83] | |
| Reactive arthritis | Infection with enteric bacterial pathogens, including Campylobacter[84] | Joints, eyes, and genitourinary tract | Pain and stiffness of joints, swollen toes, eye inflammation, and urinary problems | C. jejuni and C. coli[1,84] | |
| Cardiovascular complications | Immuno-compromised condition, and bacteremia[1] | Cardiovascular system | Chest pain leading to arrhythmia, dilated cardiomyopathy, and sudden death due to congestive heart failure | C. jejuni and C. fetus[85,86] | |
| Meningitis | Immuno-compromised condition[1,87] | Meninges of brain and spinal cord | Headache with nausea or vomiting, seizures, sensitive to light, and loss of appetite | C. jejuni and C. fetus[88,89] | |
| Abscesses of breast, brain, vertebra, and liver | Secondary bacterial infection | Breast, brain, vertebra, and liver | Varies with target organ | C. rectus[90], C. curvus, C. gracilis and C. showae[91] | |
| Reproductive complication | Bowel infection, and periodontal disease[92] | Uterus, and placenta | Preterm birth, low birth weight, and intra-uterine growth restriction | C. jejuni, C. coli, C. fetus and C. upsaliensis[92] |
Campylobacter infection types, clinical presentations, risk factors, target organ or tissues involved, symptoms and associated Campylobacter species are described.
C. jejuni is one of the major causes of bacterial foodborne diarrheal disease in the United States and worldwide[2]. According to Foodborne Diseases Active Surveillance Network, Campylobacter spp. account for 9% of total foodborne illnesses, 15% of hospitalizations and 0.1% of deaths from foodborne illnesses each year[3]. In immunocompetent patients, C. jejuni infection typically manifests as a self-limiting acute gastroenteritis characterized by severe watery and sometimes bloody diarrhea, fever, nausea, and vomiting. However, in immunocompromised patients, C. jejuni can cause a severe, life-threatening disease, often requiring hospitalization and antibiotic treatment. In addition to causing gastroenteritis, C. jejuni is also associated with post-infection complications such as Guillain-Barre syndrome, which is a rare neuromuscular disease that is thought to occur in 1 in 1000 individuals infected with C. jejuni[4]. Other sequelae such as Reiter’s syndrome, inflammatory bowel syndrome, and immunoproliferative small intestinal disease also significantly add to the burden of C. jejuni infection[5-7].
Unlike in humans, C. jejuni lives as a commensal in the gut of a variety of domestic and wild animals and birds[4]. Epidemiological studies show that the majority (50%-80%) of human infections are acquired by consumption of contaminated poultry and poultry products and that nearly 90% of the poultry flocks in the United States are colonized with C. jejuni, suggesting that controlling C. jejuni colonization in poultry is an effective strategy to control human infections[4]. Rarely, contaminated raw milk, water, and vegetables also serve as sources of human infection[1,4]. Although transmission through ingestion of contaminated food and water is the major route, person-to-person contact, as well as contact with pets or their feces is not uncommon[1,4]. Although C. jejuni is a major public health concern worldwide, the genetic determinants that contribute to its ability to survive in different host and non-host environments, to colonize poultry and other domestic animals, and to cause disease in humans are relatively poorly understood.
C. jejuni is unique among the enteric bacterial pathogens in that it lacks many classical stress response and virulence mechanisms. More specifically, C. jejuni lacks the stationary phase sigma factor RpoS, the heat shock sigma factor RpoH, the cold shock protein CspA, the oxidative stress response genes SoxRS, OxyR, SodA and KatG, the osmoprotectants ProU, OtsAB and BetAB, and the leucine-responsive global regulator Lrp, which are all essential for stress tolerance in other enteric pathogens[8]. These findings are consistent with the unusual sensitivity of C. jejuni to various environmental stresses i.e., C. jejuni is unable to grow in the presence of oxygen, has a narrow growth temperature range, is normally incapable of multiplication outside the host, does not survive well on dry surfaces, cannot withstand high temperaturature, and is more sensitive to osmotic and low pH stresses[8]. C. jejuni also lacks many classical virulence mechanisms, including a type III secretion system and exotoxins, which play important roles in the pathogenesis of other enteric bacteria[9]. Several C. jejuni strains contain type IV and type VI secretion systems but the absence of these systems in many pathogenic C. jejuni strains questions their requirement for virulence[10,11]. Nevertheless, the increasing incidence of C. jejuni infections in humans suggests that this organism may have evolved alternative mechanisms for stress survival and virulence. One such mechanism that has been relatively well characterized by several recent studies and plays important roles in C. jejuni survival and virulence involves inorganic polyphosphate (poly P). In this review, we summarize recent data on the role of poly P and related enzymes in C. jejuni biology, with particular focus on stress survival and virulence.
INORGANIC POLYPHOSPHATE
Even after the discovery of the subatomic particle (the God particle), the origin of the universe is still a debated topic. However, there is almost a universal agreement that phosphate has played a key role in the origin of life on earth. Poly P is a linear polymer of ten to hundreds of phosphate residues linked by high-energy phosphoanhydride bonds. Poly P granules were first seen in bacteria as “Volutin granules” or “Metachromatic granules”, named after their tendency to stain pink with basic blue dyes[12]. Indeed, the presence of metachromatic granules was used as a diagnostic feature for pathogenic bacteria such as Corynebacterium diptheriae. Later with the advent of electron microscopy, these granules were identified in nonpathogenic bacteria, which refuted the idea of using poly P granules as a marker for pathogenic bacteria[12]. Recent studies have shown that poly P is essential for numerous cellular functions in bacteria, such as energy source, phosphate reservoir, cation sequestration, buffering role against alkali, participation in membrane transport, cell envelope formation and function, regulator of enzyme activities, gene activity control and development, chromatin destabilization, DNA replication and phage production, sporulation and germination, bacterial virulence/pathogenesis, and regulator of stress and survival[13]. Additionally, bacteria capable of storing large amounts of intracellular poly P are used in the biological treatment of wastewater[14].
GENERAL ASPECTS OF POLY P METABOLISM IN BACTERIA
Polyphosphate kinase 1 (PPK1) is the principal enzyme responsible for poly P synthesis in many bacteria[15,16]. PPK1 is highly conserved; homologs of this enzyme have been found in over 100 bacterial species, including 20 major human and animal pathogens. PPK1 is perhaps the most known of all poly P-related enzymes for its role in bacterial survival under conditions of stress, virulence, and host colonization[13]. Many bacterial species contain another enzyme, PPK2, which preferentially mediates poly P-driven generation of guanosine triphosphate (GTP)[17,18], a molecule known to have important roles in cell signaling and DNA, RNA, protein, and polysaccharide synthesis[19,20]. Similar to PPK1, PPK2 is also widely conserved in bacteria, including major human pathogens such as Mycobacterium tuberculosis, Pseudomonas aeruginosa, and Vibrio cholerae among others[17]. Although relatively less studied compared to PPK1, few recent studies have shown a role for PPK2 in bacterial survival and virulence[18]. The third family of enzymes involved in poly P metabolism includes exopolyphosphatases, which degrade poly P to inorganic phosphate[13,21]. Many bacteria contain 2 types of exopolyphosphatases[22], one that only hydrolyzes poly P (hereafter referred to as PPX) and the other that, in addition to hydrolyzing poly P, also hydrolyzes guanosine pentaphosphate (pppGpp) to guanosine tetraphosphate (ppGpp) (hereafter referred to as PPX/GPPA)[13,23]. ppGpp is a signaling molecule that plays an important role in bacterial stringent response induced by starvation[13,23]. Of all the poly P-metabolizing enzymes, PPX enzymes are the least understood with regards to their role in bacterial survival and virulence.
POLY P METABOLISM IN C. JEJUNI
C. jejuni contains all the enzymes involved in poly P metabolism- PPK1, PPK2, and 2 PPX/GPPA enzymes (Figure 1). Two independent studies have evaluated the contribution of PPK1 to poly P metabolism in C. jejuni[24,25]. Using a toluidine blue O assay, both studies demonstrated that the C. jejuni wildtype strain accumulated more poly P in stationary phase than in mid-log and transition phases and that deletion of ppk1 significantly reduced poly P accumulation (Figure 1)[24,25]. The C. jejuni ppk1 mutant retained a modest ability to synthesize poly P[24,25]; thus, the alternative enzyme that contributes to residual poly P levels in the ppk1 mutant remains to be identified. Using electron microscopy, the authors further demonstrated that the C. jejuni wildtype strain contained several poly P-like granules and that the ppk1 mutant contained fewer of these granules[25]. More recently, a detailed ultrastructure analysis of C. jejuni at 5 nm resolutions also showed the presence of orange, poly P storage granules in C. jejuni[26]. Although the two studies suggested that the observed granules are poly P granules, additional work is required to confirm that these granules are indeed poly P granules.
Figure 1.
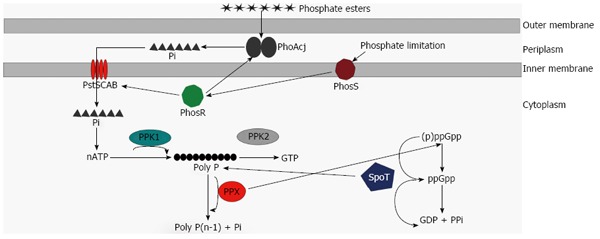
Model of poly P metabolism in Campylobacter jejuni. Phosphate esters are hydrolyzed to inorganic phosphate (Pi) by alkaline phosphatase (PhoAcj) in the periplasm. Phosphate uptake proteins and alkaline phosphatase are directly regulated by the PhosS/PhosR two-component system. Pi is transported across the inner membrane via the high affinity phosphate transport system PstSCAB. ATP generated from Pi is utilized for poly P synthesis by PPK1. PPK2 utilizes poly P to generate GTP, while PPX hydrolyzes poly P back to Pi. PPX also affects conversion of (p)ppGpp to ppGpp. SpoT is a bifunctional enzyme involved in both ppGpp synthesis as well as ppGpp hydrolysis. SpoT is also linked to poly P metabolism and a spoT mutant has reduced ability to accumulate poly P.
In another study, the role of PPK2 in poly P metabolism was investigated[27]. The authors of this study demonstrated that a ppk2 mutant was deficient in poly P-dependent GTP synthesis. The authors also showed that the ratio of ATP:GTP was altered in the ppk2 mutant, thus suggesting that PPK2 is required for poly P-dependent GTP synthesis as well as for maintenance of nucleotide balance in the cell (Figure 1). GTP is a signaling molecule that modulates many physiological functions in bacteria[19,20]. Unlike studies in other bacteria, which showed that PPK2 possesses poly P-synthetic activity[28], the authors demonstrated that poly P levels were unaltered in the C. jejuni ppk2 mutant when compared to wildtype, suggesting that PPK2 does not appear to contribute to poly P synthesis in C. jejuni[27].
Malde et al[29] evaluated the contributions of PPX/GPPA enzymes to poly P metabolism in C. jejuni. They demonstrated that both ppx1/gppa and ppx2/gppa mutants accumulated more poly P than the wildtype strain and that the ppx1/gppa mutant accumulated relatively more poly P than the ppx2/gppa mutant when compared to wildtype. Based on these data, the authors suggested that PPX1/GPPA and PPX2/GPPA are involved in poly P degradation and that PPX1/GPPA is probably the primary enzyme that contributes to poly P degradation in C. jejuni (Figure 1)[29]. However, whether PPX/GPPA enzymes possess PPX activity needs to be further experimentally confirmed.
PPKS: ROLE IN C. JEJUNI SURVIVAL AND VIRULENCE
Mutations in ppk1 and ppk2 genes have resulted in a variety of phenotypic changes in C. jejuni. Although the mechanisms underlying these phenotypes are poorly understood, the phenotypes as such are significant and relevant to C. jejuni pathogenesis. In the following sections, we will review our latest understanding of the role of PPKs in these phenotypes, which are summarized in Table 2.
Table 2.
Phenotypes associated with enzymes of poly P and ppGpp metabolism
| Phenotype | PPK1 | PPK2 | PPX1 | PPX2 | PPX1-PPX2 | PhoAcj | SpoT |
| Poly P metabolism | |||||||
| Poly P synthesis/accumulation | ↑ | - | - | - | - | ↑ | ↑ |
| Poly P-dependent GTP synthesis | - | ↑ | NT | NT | NT | NT | NT |
| Poly P degradation | - | - | ↑ | ↑ | ↑ | NT | NT |
| Maintenance of ATP:GTP ratio | NT | ↑ | NT | NT | NT | NT | NT |
| ppGpp metabolism | |||||||
| ppGpp synthesis | NT | - | ↑ | - | ↑ | NT | ↑ |
| Stress survival | |||||||
| Stationary phase survival and growth | - | - | - | - | - | - | ↑ |
| Survival under low CO2 | NT | NT | NT | NT | NT | NT | ↑ |
| Osmotic shock survival | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | NT |
| Low nutrient stress survival | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | NT |
| Aerobic stress survival | - | ↑ | NT | NT | NT | NT | ↑ |
| VBNC formation | ↑ | ↑ | NT | NT | NT | NT | NT |
| Natural transformation | ↑ | NT | NT | NT | NT | NT | NT |
| Antimicrobial resistance | ↑ | ↑ | NT | NT | NT | ↓ | R |
| Virulence-related | |||||||
| Motility | - | - | ↑ | ↑ | ↑ | NT | NT |
| Biofilm formation | ↓ | ↓ | ↑ | ↑ | ↑ | ↓ | NT |
| Resistance to complement-mediated killing | NT | NT | ↑ | ↑ | ↑ | NT | NT |
| Adherence | NT | - | NT | NT | NT | NT | ↑ |
| Invasion | - | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ |
| Intraepithelial survival | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ |
| Chicken colonization | ↑ | ↑ | NT | NT | NT | ↑ | NT |
NT: Not tested; R: Tested only for Rifampicin resistance; -: Phenotype absent; ↑: Phenotype is positively regulated i.e., deletion of the gene reduces the phenotype when compared to wildtype; ↓: Phenotype is negatively regulated i.e., deletion of the gene enhances the phenotype when compared to wildtype.
PPKs: Role in growth and stationary phase survival
Several studies evaluated the effect of ppk1 and ppk2 mutations on C. jejuni growth and demonstrated that these mutations do not affect C. jejuni growth[24,25,27]. In other organisms, ppk1 mutants are defective in stationary phase growth and survival[30,31]. The C. jejuni wildtype strain accumulated more poly P in stationary phase than in mid-log and transition phases and more genes were differentially expressed in the ppk1 mutant in stationary phase when compared to mid-log phase[24,25,32]. However, the C. jejuni ppk1 mutant grew and survived similar to wildtype in stationary phase. A possible explanation for this difference is that C. jejuni lacks RNA polymerase, sigma S (RpoS), which regulates stationary phase survival and itself is modulated by poly P in other organisms[8,33].
PPKs: Role in stress survival and adaptation
C. jejuni encounters a number of unfavorable environments both in the external environment during transmission as well as in the host during colonization/infection[8]. Poly P is known for its role in modulating stress tolerance in bacteria[13]. Two independent studies by Candon et al[24] and Gangaiah et al[25] demonstrated that the ppk1 mutant had a reduced ability to survive under low nutrient stress and osmotic shock. Similarly, in another study, Gangaiah et al[27] showed that the ppk2 mutant, in addition to displaying a reduced ability to survive under low nutrient stress and osmotic shock, also had reduced ability to survive under aerobic stress. Bacteria generally respond to nutrient stress by eliciting a stringent response regulated by ppGpp and/or a general stress response regulated by RpoS. A C. jejuni bifunctional (p)ppGpp synthase/hydrolase (spoT) mutant, which is defective in eliciting a stringent response, was compromised in its ability to survive under low nutrient stress[34]. Thus, to rule out the possible role of ppGpp in PPK1-mediated nutrient stress survival phenotype, it would be interesting to determine if ppGpp levels are altered in the ppk1 mutant. Transcriptome analysis revealed that, despite C. jejuni lacking RpoS, homologs of several genes involved in general stress response were downregulated in ppk1 and ppk2 mutants; this might partly explain the stress survival deficiencies in the ppk mutants[32]. In E. coli, poly P also affects stress tolerance by several other mechanisms- poly P is thought to be involved in sensing minor changes at the cell surface, serves as a source of energy during times of stress, and regulates Lon protease, which degrades nonessential proteins providing free amino acids for survival during stress[13,35-38]. Whether such mechanisms also play a role in poly P-mediated stress resistance in C. jejuni is worth investigating.
PPKs: Role in viable but nonculturable cell formation
C. jejuni is also capable of forming viable but nonculturable cell (VBNC) under stressful conditions; this phenotype is thought to provide a survival advantage to C. jejuni during times of stress[39,40]. Two recent studies showed that both ppk1 and ppk2 mutants were compromised in their ability to form VBNCs after formic acid treatment[25,27]. The reduced ability of the ppk mutants to form VBNCs could be due to the fact that these mutants were more sensitive to other stresses such as low nutrient stress and osmotic shock. Poly P can serve as a source of energy during times of stress[13]; this could also contribute to the reduced ability of the ppk1 mutant to form VBNCs. A ppk2 mutant had similar poly P levels but was compromised in its ability to regulate nucleotide balance; thus, whether altered nucleotide balance is the cause of the ppk2 mutant’s reduced ability to form VBNCs needs to be further studied. One might also speculate that PPK enzymes, as global regulators, might modulate VBNC formation by affecting the expression of other genes involved in VBNC formation; however, transcriptome analysis of ppk mutants did not appear to provide any additional insights into this mechanism. For more information on the role of poly P in VBNC formation in C. jejuni, refer to the review by Kassem et al[39].
PPKs: Role in antimicrobial resistance and natural transformation
C. jejuni is increasingly becoming resistant to several clinically-relevant antimicrobials[41-43]. Two recent studies demonstrated that both ppk1 and ppk2 mutants were more susceptible to several antimicrobials (erythromycin, cefotaxime, ciprofloxacin, rifampin, polymyxin, and tetracycline) compared to their respective wildtypes[25,27]. The stringent-response mediator ppGpp affects bacterial resistance to antimicrobials[44]; studies looking at ppGpp levels in the ppk1 mutant would provide some insights on this mechanism. Both ppk1 and ppk2 mutants had altered outer membrane profiles, suggesting that membrane permeability of these mutants may be compromised[45]; where altered membrane permeability could be the cause of increased susceptibility of ppk mutants to antimicrobials. Stress resistance is an emerging mechanism of antimicrobial resistance in bacteria[44]; thus, the susceptibility of ppk mutants to antimicrobials may also be explained by their general sensitivity to other stresses. In E. coli, poly P is known to regulate adaptive evolution and ribosome fidelity, both of which modulate resistance to antimicrobials[46]. Whether these mechanisms play a role in PPK1-mediated resistance to antimicrobials in C. jejuni also need to be investigated.
C. jejuni is naturally competent (i.e, ability to take up DNA from its surroundings) and this feature impacts the organism’s ability to adapt to different environments as well as to acquire antimicrobial resistance genes. Gangaiah et al[25] showed that a ppk1 mutant was compromised in its ability to acquire foreign DNA for natural transformation. Poly P is a component of the membrane channels that mediate DNA uptake[47,48]; the potential of such a mechanism for PPK1 to mediate natural competence in C. jejuni should be explored. Evidence suggests that outer membrane composition of the ppk1 mutant is altered[45]; whether this influences the ability of C. jejuni to take up DNA is also unknown.
PPKs: Role in motility and biofilm formation
Several recent studies have evaluated the role of PPKs in virulence-associated phenotypes such as motility, biofilm formation, adherence, invasion, and intracellular survival[24,25,27,45]. Motility is an essential C. jejuni virulence mechanism[49]. Three independent studies demonstrated that both PPK1 and PPK2 were not required for C. jejuni motility[24,25,27]. This is in contrast to other organisms, where PPK1 is required for motility. Surprisingly, Chandrashekhar et al[32] demonstrated that several flagella-associated genes and flagellar glycosylation genes were downregulated in the ppk mutants; the authors hypothesized that the degree of downregulation in the ppk mutants may not have been enough to impair motility. Although motility was not affected by PPKs in C. jejuni, the potential role of PPKs on other phenotypes associated with flagella such as autoagglutination, secretion, and/or invasion needs further investigation.
Biofilms play an important role in C. jejuni tolerance to environmental stresses, disinfectants, and antimicrobials, as well as facilitating colonization of animal and human hosts[50-52]. Two independent groups demonstrated that the ppk1 mutant formed higher amount of biofilms than the wildtype[24,25]. This finding is in contrast to other organisms, where PPK1 is required for biofilm formation[13]. In another study, Gangaiah et al[27] demonstrated that ppk2 mutant also formed higher amount of biofilms than its parent. Drozd et al[53] went onto characterize how PPK enzymes modulate biofilm formation in C. jejuni and demonstrated that ppk1 and ppk2 mutants formed higher adherent colonies on day 1 and 2 compared to wildtype. This study suggested that, compared to mutant strains, the wildtype might spend more time in planktonic phase, delaying biofilm formation[53]. The authors further demonstrated that ppk1 and ppk2 mutants had reduced calcofluor white reactivity, suggesting that polysaccharide structures might be altered in the mutant strains[53]. The quorum sensing molecule Autoinducer-2, which is required for biofilm formation, was increased in both mutants[53]. Finally, the authors evaluated the mutants for altered expression of genes involved in biofilm formation and found that several genes (pglH, kpsM, cj0688, neuB1, neuB1, fliS, and maf5) involved in biofilm formation were altered in ppk1 and ppk2 mutants[53].
PPKs: Role in adherence, invasion and intracellular survival
Adherence, invasion, and intracellular survival are also essential virulence mechanisms for C. jejuni[54]. Recent studies demonstrated that both PPK1 and PPK2 are important for invasion and intracellular survival within INT-407 human intestinal epithelial cells[24,25,27]. Pina-Mimbela et al[45] showed that ppk1 and ppk2 mutants had qualitative and quantitative differences in outer membrane composition compared to wildtype. They also demonstrated that the differences in outer membrane composition in the mutants were directly related to the ability of C. jejuni to invade and survive within INT-407 cells[45]. The authors of this study went one step further to evaluate which fractions were associated with invasion and intracellular survival[45]. They found that outer membrane proteins were uniquely associated with invasion, whereas outer membrane proteins, lipids, and lipoglycans all were associated with intracellular survival[45].
PPKs: Role in chicken colonization
Two independent studies evaluated the role of PPK1 in C. jejuni colonization in day-old chicks (Table 3)[24,25]. Candon et al[24] demonstrated that, after 7 days of oral inoculation, the wildtype colonized the ceca of chicks at a minimum average of 1.79 × 108 CFU/g of cecal contents at all inoculation doses examined. The ppk1 mutant colonized the ceca of all chicks at a rate similar to that of wildtype at a dosage of 1.5 × 107 CFU/chick, of 8 out of 10 chicks at a dosage of 1.5 × 106 CFU/chick, and of 0 out of 10 chicks at a dosage of 1.5 × 105 CFU/chick[24]. In another study, Gangaiah et al[25] evaluated the role of PPK1 in colonization of ceca and bursa as well as C. jejuni load in feces using low inoculation doses i.e., 103, 104, and 105 CFU/chick. They demonstrated that, after 8 d of oral inoculation, the ppk1 mutant colonized the ceca and bursa and showed a C. jejuni load in feces of all chicks but at a significantly lower rate than the wildtype at a dosage of 105 CFU/chick[25]. While the wildtype colonized to an average of about 1.0 × 108 CFU/g of ceca and about 1.0 × 105 CFU/g of bursa, and showed a C. jejuni load of about 1.0 × 107 CFU/g of feces, none of the chicks were colonized with the mutant strain at a dosage of 104 CFU/chick[25]. Similarly, while the wildtype colonized to an average of about 1.0 × 106 CFU/g of ceca and about 1.0 × 104 CFU/g of bursa, and showed a C. jejuni load of about 1.0 × 105 CFU/g of feces, none of the chicks were colonized with the mutant strain at a dosage of 103 CFU/chick[25]. Except for 105 CFU/chick, the two studies used different dosages for inoculation. Both studies were done in the same strain background; thus, the reason for the difference in the results between the two studies is unclear.
Table 3.
Summary of chicken colonization phenotypes of ppk1 and ppk2 mutants
| Study | Organ/feces | Inoculation dose (CFU/chick) | Wildtype (CFU/g) | ppk1 mutant (No. of chicks colonized) | ppk2 mutant (No. of chicks colonized) |
| Candon et al[24] 2007 | Ceca | 1.5 × 105 | 1.8 × 108 | 0/10 chicks | NS |
| 1.5 × 106 | 1.8 × 108 | 8/10 chicks | NS | ||
| 1.5 × 107 | 1.8 × 108 | 10/10 chicks at an average of 1.8 × 108 CFU/g | NS | ||
| Gangaiah et al[25] 2009 and 2010 | Ceca | 1.0 × 103 | 1.0 × 106 | 0/5 chicks | 0/5 chicks |
| 1.0 × 104 | 1.0 × 108 | 0/5 chicks | 0/5 chicks | ||
| 1.0 × 105 | 8.0 × 108 | 5/5 chicks at an average of 2.0 × 104 CFU/g | 5/5 chicks at an average of 9.0 × 103 CFU/g | ||
| Bursa | 1.0 × 103 | 1.0 × 104 | 0/5 chicks | 0/5 chicks | |
| 1.0 × 104 | 1.0 × 105 | 0/5 chicks | 0/5 chicks | ||
| 1.0 × 105 | 1.5 × 105 | 5/5 chicks at an average of 1.0 × 102 CFU/g | 5/5 chicks at an average of 8.0 × 102 CFU/g | ||
| Feces | 1.0 × 103 | 1.0 × 105 | 0/5 chicks | 0/5 chicks | |
| 1.0 × 104 | 1.0 × 107 | 0/5 chicks | 0/5 chicks | ||
| 1.0 × 105 | 3.0 × 107 | 5/5 chicks at an average of 1.5 × 103 CFU/g | 5/5 chicks at an average of 8.0 × 103 CFU/g |
NS: Not studied.
Furthermore, Gangaiah et al[27] evaluated the role of PPK2 in colonization of ceca and bursa as well in C. jejuni load in feces using 103, 104, and 105 CFU/chick inoculation doses (Table 3). They demonstrated that, at 105 CFU/chick inoculation dose, the ppk2 mutant colonized the ceca and bursa as well as showed a C. jejuni load in feces of all chicks but at a significantly lower rate than the wildtype[27]. While the wildtype colonized to an average of about 1.0 × 108 CFU/g of ceca and about 1.0 × 105 CFU/g of bursa, and showed a C. jejuni load of about 1.0 × 107 CFU/g of feces, none of the chicks were colonized with the mutant strain at a dosage of 104 CFU/chick, except for 1 chick[27]. Similarly, while the wildtype colonized to an average of about 4.0 × 106 CFU/g of ceca and about 1.0 × 104 CFU/g of bursa, and showed a C. jejuni load of about 1.0 × 105 CFU/g of feces, none of the chicks were colonized with the mutant strain at a dosage of 103 CFU/chick[27].
It is intriguing that both ppk1 and ppk2 mutants behave similar with respect to chicken colonization. Several C. jejuni genes have been identified to be important for chicken colonization[55]; transcriptome and outer membrane proteome analyses showed that these genes did not appear to be regulated by PPK1 and PPK2[32,45]. Thus, the mechanisms underlying the contribution of poly P to chicken colonization are unclear. Both ppk1 and ppk2 mutants showed dose-dependent colonization defects in day-old chicks[24,25,27]. The authors hypothesized that the dose-dependency may be related to the hyperbiofilm phenotype of ppk1 and ppk2 mutants[24,25,27]. According to this hypothesis, at lower inoculation doses, the ppk mutants are more sensitive to in vivo stresses and thus, display no or reduced colonization. At higher doses, the ppk mutants form hyperbiofilms, conferring resistance to in vivo stresses and thus, similar colonization of the mutants as that of wildtype. Additional in vivo studies complementing the mutants to rule out the effect of secondary mutations on colonization are warranted.
PPX/GPPA ENZYMES: ROLE IN C. JEJUNI SURVIVAL AND VIRULENCE
Malde et al[29] demonstrated that mutations in ppx/gppa genes were associated with a variety of phenotypes in C. jejuni. In this study, it was shown that ppx/gppa mutants were deficient in survival under nutrient limitation and osmotic stress. The authors further showed that the nutrient survival defect in the mutants could be complemented by amino acid supplementation. Based on these findings, it was hypothesized that the nutrient survival phenotype in the mutants is likely due to reduced ppGpp or increased poly P levels. Both ppx/gppa mutants had increased poly P levels; however, only ppx1/gppa mutant had reduced ppGpp levels when compared to wildtype but not ppx2/gppa mutant, which indeed accumulated more ppGpp than the wildtype[29]. This suggests that the nutrient survival defect in the mutants is more likely due to increased poly P levels rather than due to reduced ppGpp levels.
Further, Malde et al[29] also demonstrated that ppx/gppa mutants were compromised in several virulence-associated phenotypes such as motility, biofilm formation, and invasion and intracellular survival within human intestinal epithelial cells. Unlike ppk1 and ppk2 mutants, which had similar motility as the wildtype, the ppx/gppa mutants were defective in motility compared to wildtype. The ppx/gppa mutants also had decreased ability to form biofilms. Poly P is essential for chelation of cations[13], which are required for biofilm formation[56]; whether such a mechanism is the cause of reduced biofilms in ppx/gppa mutants is an interesting question. The group further went on to test the contributions of PPX/GPPA enzymes to serum resistance and demonstrated that ppx/gppa mutants were resistant to human complement but not to chicken complement.
Although the contributions of PPX/GPPA enzymes to C. jejuni biology is well characterized at the phenotypic level using in vitro assays, it remains to be understood if these contributions impact colonization of C. jejuni in day-old chicks and infection in humans. The mechanisms underlying the PPX/GPPA-associated phenotypes are also largely unknown; a transcriptome analysis of the ppx mutants would provide some insights on this aspect. In other bacteria, it has been shown that excess poly P in the ppx mutants restricts growth and downregulates metabolism, which is thought to have caused the underlying phenotypes[57]. The C. jejuni ppx/gppa mutants grew very similar to the wildtype strain, yet were compromised in several phenotypes (i.e., motility, biofilm formation, nutrient stress survival, invasion and intracellular survival, and resistance to human complement-mediated killing), suggesting that growth restriction and metabolic downshift are less likely the reasons for PPX-dependent phenotypes in C. jejuni. Phenotypes associated with excess of poly P were modest compared to those associated with poly P deficiency, suggesting that lack of poly P impacts C. jejuni biology more than excess of poly P. It is also intriguing to note that the phenotypes of the double mutant lacking both ppx1/gppa and ppx2/gppa genes were more severe compared to those of the individual mutants, suggesting some degree of functional redundancy in these enzymes; the reason behind this functional redundancy is unclear. Overall, findings from the analysis of ppx/gppa mutants suggest that poly P levels are tightly regulated and that dysregulation of poly P levels as seen in the ppx/gppa mutants compromises C. jejuni’s survival and virulence properties.
LINK BETWEEN POLY P AND (P)PPGPP METABOLISM IN C. JEJUNI
Malde et al[29] assessed the role of PPX/GPPA enzymes in ppGpp synthesis and demonstrated that PPX1/GPPA but not PPX2/GPPA is important for ppGpp synthesis. In another study, Candon et al[24] demonstrated that a spoT mutant, which is deficient in ppGpp synthesis, had significantly reduced poly P levels than its parent. These findings suggest that (p)ppGpp and poly P metabolism are linked in C. jejuni (Figure 1). Both, (p)ppGpp and poly P metabolism are known to be linked in E. coli; in this organism, ppGpp is known to modulate poly P levels by inhibiting PPX enzymes[58,59]. Analogous to E. coli, the authors speculated that (p)ppGpp might modulate poly P levels in C. jejuni by inhibiting PPX enzymes, which would not only affect the dynamic balance between poly P degradation by PPX enzymes but also affects poly P synthesis by PPK1. However, this hypothesis remains to be experimentally confirmed. In Pseudomonas aeruginosa, PPK2 affects (p)ppGpp accumulation; this role of PPK2 is attributed to its ability to serve as a source of GTP, which is a precursor for (p)ppGpp synthesis[60]. However, a C. jejuni ppk2 mutant accumulated similar (p)ppGpp levels to that of wildtype, suggesting that PPK2 is less likely the link between poly P and (p)ppGpp metabolism in C. jejuni. Although PPK1 does not appear to have ppGpp synthetic/hydrolytic activity, it remains to be understood if (p)ppGpp levels are altered in the ppk1 mutant.
POLY P AND PHOSPHATE METABOLISM
Inorganic phosphate (Pi) is an essential nutrient for many bacterial species. As most natural environments are limiting in Pi, phosphate esters are the preferred source of Pi for bacteria[61]. Phosphate esters are broken down into Pi in the periplasm by alkaline phosphatase. PhoAcj is the only alkaline phosphatase in C. jejuni[62]. Drozd et al[53] recently demonstrated that a phoAcj mutant had significantly reduced intracellular poly P levels compared to its parent, suggesting that PhoAcj is likely the primary source of Pi for poly P synthesis[53,63]. The authors also demonstrated that the phoAcj mutant had a significantly reduced ability to survive under nutrient stress, to invade and survive within INT407 cells and to colonize day-old chicks[63]. The phenotypes in the phoAcj mutant were in general less severe when compared to those in the ppk1 mutant. This is likely due to the fact that phoAcj mutant only had a modest defect in poly P accumulation compared to the ppk1 mutant, which had a severe deficiency in poly P accumulation[63]. Unlike ppk1 mutant, the phoAcj mutant had enhanced resistance to antimicrobials, suggesting that this phenotype is less likely due to poly P deficiency[63].
PHENOTYPIC OVERLAP BETWEEN POLY P AND RELATED ENZYMES
Several phenotypes of PPK1, PPK2, PPX/GPPA, SpoT, and PhoAcj enzymes overlap between each other as shown in Table 2[24,25,27,29,34,63,64]. For example, osmotic shock survival and low nutrient stress survival phenotypes were common to PPK1, PPK2, PPX/GPPA, and PhoAcj enzymes. Similarly, intraepithelial survival phenotype was common to all enzymes and, except for PPK1, invasion was also common to all the enzymes. There are two possible explanations for this overlap; the enzymes likely affect the shared phenotypes independently, or the enzymes might impact the phenotypes through a common protein or molecule. As PPK2 is required for poly P-dependent GTP synthesis, it is conceivable that poly P deficiency in the ppk1 mutant likely also affects GTP levels[25,27]. Thus, the phenotypic overlap between PPK1 and PPK2 may be the consequence of low GTP levels in these mutants. Both spoT and phoAcj mutants had low poly P levels[34,63]; thus, the phenotypic overlap between PPK1, PhoAcj, and SpoT may be due to low poly P in these mutants. The ability to form biofilms was upregulated in the ppk1 mutant[24,25], which has low poly P levels, and downregulated in ppx/gppa mutants[29], which have high poly P levels; this suggests that the biofilm phenotype is directly associated with poly P. This is further supported by the fact that spoT mutant, which has low poly P, also formed more biofilms than its parent[34]. However, the ppk2 mutant, which has similar poly P levels as its parent, also formed more biofilms compared to its parent[27]; suggesting that the effect of PPK2 on biofilm formation is independent of poly P. Further studies are required to precisely define phenotypic overlap between poly P and related enzymes. For example, if the biofilm phenotype of the spoT mutant is due to low poly P levels, supplementation with poly P might rescue the biofilm phenotype in this mutant. Similarly, if the phenotypic overlap between ppk1 and ppk2 mutants is due to altered GTP levels, supplementation with GTP might rescue the associated phenotypes in the ppk1 mutant. Strains containing mutations in multiple of these enzymes accompanied by complementation may also provide insights as to whether these enzymes have a synergistic or antagonistic effect on the overlapping phenotypes.
PPKS AS PROMISING DRUG AND VACCINE TARGETS
PPK enzymes have the potential to be ideal drug targets for controlling C. jejuni and other bacterial infections. First, these enzymes are highly conserved across a broad array of bacterial species[13]; thus, the identified drugs will be effective against many bacterial species. Second, homologs of PPKs are absent in higher eukaryotes[13]; thus, it is less likely that the drugs will be toxic to host cells. Third, a recent study by Pina-Mimbela et al[45] showed that PPKs modulate outer membrane composition of C. jejuni; thus, anti-PPK drugs could expose surface antigens, which are otherwise hidden, and make C. jejuni vulnerable to host defense mechanisms. Fourth, deletion of ppk1 and ppk2 does not affect growth of bacteria in vitro[24,25,27]; thus, there is less selection pressure on the bacteria to develop resistance to anti-PPK drugs. Lastly, ppk mutants are more sensitive to several conventional antibiotics[24,25,27]; thus, anti-PPK drugs could be used in conjunction with existing antibiotics for drug-resistant strains.
Mutants of poly P enzymes could also be sought as potential live attenuated vaccine candidates. Strains with deleted virulence genes have been successfully used for controlling Salmonella colonization in poultry[65,66]. To have a synergistic effect on virulence/colonization attenuation, mutants containing deletions in ppk genes and other established virulence determinants could be used. As discussed before, using ppk mutants as live attenuated vaccine candidates not only has the advantage of the strain being attenuated for colonization but also could expose previously hidden antigens, likely inducing a strong protective immunity.
Epidemiological studies have shown that nearly 50%-80% of the human infections originate from ingestion of poultry and poultry products[4]. Both PPK1 and PPK2 are necessary for C. jejuni colonization in chickens[4,24,25,27]. These data suggest that PPKs could at least be targeted for controlling C. jejuni colonization in chickens, which likely would aid in reducing human infections. A human challenge model is available to study campylobacteriosis using the C. jejuni strain CG8421, which lacks ganglioside mimicry, a mechanism known to cause Guillain Barre Syndrome[67-69]. The potential of using PPKs as drug and vaccine targets for human infections warrants that the contributions of these enzymes to human infection be studied using the human challenge model of campylobacteriosis.
CLOSING REMARKS AND PERSPECTIVES
Recent studies have yielded several important insights into the role of poly P in C. jejuni biology. Poly P is associated with a plethora of C. jejuni phenotypes, which not only impact how C. jejuni survives in the environment under different stress conditions but also impact how this organism colonizes poultry and other domestic animals and causes disease in humans. Transcriptome and outer membrane proteomics analyses of the ppk1 and ppk2 mutants have provided some valuable mechanistic insights with regards to poly P-associated phenotypes. Nevertheless, our understanding of poly P in C. jejuni is still in its first steps. For example, the signals that activate poly P-mediated response are largely unknown. Most bacteria, including C. jejuni, contain two PPX enzymes; the reason behind this redundancy is an open question. Although transcriptome and outer membrane proteomic studies have yielded valuable insights into poly P functions, the few genes or proteins differentially expressed in the ppk mutants compared to wildtype do not seem to explain the plethora of phenotypes arising from mutants of poly P-associated enzymes. This suggests that poly P may also mediate its functions by affecting its targets posttranscriptionally; global proteomic analyses of the ppk1 and ppk2 mutants will likely shed some light on this aspect. It is also not known how poly P is regulated; in other words, what are the upstream components that feed into poly P-mediated response? How poly P feeds into the global regulatory network also remains to be understood. Poly P and ppGpp are linked in C. jejuni and other bacteria but the precise mechanisms underlying their interaction are poorly understood.
Given that poly P-related enzymes affect numerous aspects of C. jejuni life, these enzymes have the potential to be promising drug and vaccine targets. Therefore, it would be worthwhile to determine the contributions of poly P-related enzymes in a human model of Campylobacter infection. Gaynor et al[34] demonstrated that ppk1 was upregulated during C. jejuni infection of human intestinal epithelial cells. Thus, studies defining at which stage of human infection and chicken colonization the poly P-mediated response is activated would provide additional insights into its role in C. jejuni pathogenesis/colonization. The recent finding that Poly P enzymes modulate IL-8 production in INT-407 cells suggests that host immune response to the mutant strains may be critical to study[45]. Such studies could facilitate development of ppk mutants alone or in combination with mutations in other established virulence genes as live attenuated vaccines for reducing C. jejuni colonization in poultry and one day for controlling human infections.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: No potential conflicts of interest.
Peer-review started: March 27, 2016
First decision: May 27, 2016
Article in press: July 20, 2016
P- Reviewer: Diefenbach R, Pogreba-Brown K, Zhang L S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
References
- 1.Kaakoush NO, Castaño-Rodríguez N, Mitchell HM, Man SM. Global Epidemiology of Campylobacter Infection. Clin Microbiol Rev. 2015;28:687–720. doi: 10.1128/CMR.00006-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization FaAOotUN, World Organisation for Animal Health (WHO) The global view of campylobacteriosis: report of an expert consultation. Utrecht, Netherlands: World Health Organization; 2013. [Google Scholar]
- 3.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. Foodborne illness acquired in the United States--major pathogens. Emerg Infect Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Louwen R, van Baarlen P, van Vliet AH, van Belkum A, Hays JP, Endtz HP. Campylobacter bacteremia: a rare and under-reported event? Eur J Microbiol Immunol (Bp) 2012;2:76–87. doi: 10.1556/EuJMI.2.2012.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalischuk LD, Buret AG. A role for Campylobacter jejuni-induced enteritis in inflammatory bowel disease? Am J Physiol Gastrointest Liver Physiol. 2010;298:G1–G9. doi: 10.1152/ajpgi.00193.2009. [DOI] [PubMed] [Google Scholar]
- 6.Lecuit M, Suarez F, Lortholary O. [Immunoproliferative small intestinal disease associated with Campylobacter jejuni] Med Sci (Paris) 2004;20:638–640. doi: 10.1051/medsci/2004206-7638. [DOI] [PubMed] [Google Scholar]
- 7.Pönkä A, Martio J, Kosunen TU. Reiter’s syndrome in association with enteritis due to Campylobacter fetus ssp. jejuni. Ann Rheum Dis. 1981;40:414–415. doi: 10.1136/ard.40.4.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park SF. The physiology of Campylobacter species and its relevance to their role as foodborne pathogens. Int J Food Microbiol. 2002;74:177–188. doi: 10.1016/s0168-1605(01)00678-x. [DOI] [PubMed] [Google Scholar]
- 9.Parkhill J, Wren BW, Mungall K, Ketley JM, Churcher C, Basham D, Chillingworth T, Davies RM, Feltwell T, Holroyd S, et al. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature. 2000;403:665–668. doi: 10.1038/35001088. [DOI] [PubMed] [Google Scholar]
- 10.Bacon DJ, Alm RA, Hu L, Hickey TE, Ewing CP, Batchelor RA, Trust TJ, Guerry P. DNA sequence and mutational analyses of the pVir plasmid of Campylobacter jejuni 81-176. Infect Immun. 2002;70:6242–6250. doi: 10.1128/IAI.70.11.6242-6250.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siddiqui F, Champion O, Akram M, Studholme D, Eqani SA, Wren BW, Titball R, Bokhari H. Molecular detection identified a type six secretion system in Campylobacter jejuni from various sources but not from human cases. J Appl Microbiol. 2015;118:1191–1198. doi: 10.1111/jam.12748. [DOI] [PubMed] [Google Scholar]
- 12.Pallerla SR, Knebel S, Polen T, Klauth P, Hollender J, Wendisch VF, Schoberth SM. Formation of volutin granules in Corynebacterium glutamicum. FEMS Microbiol Lett. 2005;243:133–140. doi: 10.1016/j.femsle.2004.11.047. [DOI] [PubMed] [Google Scholar]
- 13.Rao NN, Gómez-García MR, Kornberg A. Inorganic polyphosphate: essential for growth and survival. Annu Rev Biochem. 2009;78:605–647. doi: 10.1146/annurev.biochem.77.083007.093039. [DOI] [PubMed] [Google Scholar]
- 14.Seviour RJ, Mino T, Onuki M. The microbiology of biological phosphorus removal in activated sludge systems. FEMS Microbiol Rev. 2003;27:99–127. doi: 10.1016/S0168-6445(03)00021-4. [DOI] [PubMed] [Google Scholar]
- 15.Ahn K, Kornberg A. Polyphosphate kinase from Escherichia coli. Purification and demonstration of a phosphoenzyme intermediate. J Biol Chem. 1990;265:11734–11739. [PubMed] [Google Scholar]
- 16.Akiyama M, Crooke E, Kornberg A. The polyphosphate kinase gene of Escherichia coli. Isolation and sequence of the ppk gene and membrane location of the protein. J Biol Chem. 1992;267:22556–22561. [PubMed] [Google Scholar]
- 17.Ishige K, Zhang H, Kornberg A. Polyphosphate kinase (PPK2), a potent, polyphosphate-driven generator of GTP. Proc Natl Acad Sci USA. 2002;99:16684–16688. doi: 10.1073/pnas.262655299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sureka K, Sanyal S, Basu J, Kundu M. Polyphosphate kinase 2: a modulator of nucleoside diphosphate kinase activity in mycobacteria. Mol Microbiol. 2009;74:1187–1197. doi: 10.1111/j.1365-2958.2009.06925.x. [DOI] [PubMed] [Google Scholar]
- 19.Sundin GW, Shankar S, Chakrabarty AM. Mutational analysis of nucleoside diphosphate kinase from Pseudomonas aeruginosa: characterization of critical amino acid residues involved in exopolysaccharide alginate synthesis. J Bacteriol. 1996;178:7120–7128. doi: 10.1128/jb.178.24.7120-7128.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chakrabarty AM. Nucleoside diphosphate kinase: role in bacterial growth, virulence, cell signalling and polysaccharide synthesis. Mol Microbiol. 1998;28:875–882. doi: 10.1046/j.1365-2958.1998.00846.x. [DOI] [PubMed] [Google Scholar]
- 21.Kumble KD, Kornberg A. Endopolyphosphatases for long chain inorganic polyphosphate in yeast and mammals. J Biol Chem. 1996;271:27146–27151. doi: 10.1074/jbc.271.43.27146. [DOI] [PubMed] [Google Scholar]
- 22.Rangarajan ES, Nadeau G, Li Y, Wagner J, Hung MN, Schrag JD, Cygler M, Matte A. The structure of the exopolyphosphatase (PPX) from Escherichia coli O157: H7 suggests a binding mode for long polyphosphate chains. J Mol Biol. 2006;359:1249–1260. doi: 10.1016/j.jmb.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 23.Kristensen O, Laurberg M, Liljas A, Kastrup JS, Gajhede M. Structural characterization of the stringent response related exopolyphosphatase/guanosine pentaphosphate phosphohydrolase protein family. Biochemistry. 2004;43:8894–8900. doi: 10.1021/bi049083c. [DOI] [PubMed] [Google Scholar]
- 24.Candon HL, Allan BJ, Fraley CD, Gaynor EC. Polyphosphate kinase 1 is a pathogenesis determinant in Campylobacter jejuni. J Bacteriol. 2007;189:8099–8108. doi: 10.1128/JB.01037-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gangaiah D, Kassem II, Liu Z, Rajashekara G. Importance of polyphosphate kinase 1 for Campylobacter jejuni viable-but-nonculturable cell formation, natural transformation, and antimicrobial resistance. Appl Environ Microbiol. 2009;75:7838–7849. doi: 10.1128/AEM.01603-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Müller A, Beeby M, McDowall AW, Chow J, Jensen GJ, Clemons WM. Ultrastructure and complex polar architecture of the human pathogen Campylobacter jejuni. Microbiologyopen. 2014;3:702–710. doi: 10.1002/mbo3.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gangaiah D, Liu Z, Arcos J, Kassem II, Sanad Y, Torrelles JB, Rajashekara G. Polyphosphate kinase 2: a novel determinant of stress responses and pathogenesis in Campylobacter jejuni. PLoS One. 2010;5:e12142. doi: 10.1371/journal.pone.0012142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindner SN, Vidaurre D, Willbold S, Schoberth SM, Wendisch VF. NCgl2620 encodes a class II polyphosphate kinase in Corynebacterium glutamicum. Appl Environ Microbiol. 2007;73:5026–5033. doi: 10.1128/AEM.00600-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malde A, Gangaiah D, Chandrashekhar K, Pina-Mimbela R, Torrelles JB, Rajashekara G. Functional characterization of exopolyphosphatase/guanosine pentaphosphate phosphohydrolase (PPX/GPPA) of Campylobacter jejuni. Virulence. 2014;5:521–533. doi: 10.4161/viru.28311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crooke E, Akiyama M, Rao NN, Kornberg A. Genetically altered levels of inorganic polyphosphate in Escherichia coli. J Biol Chem. 1994;269:6290–6295. [PubMed] [Google Scholar]
- 31.Rao NN, Kornberg A. Inorganic polyphosphate regulates responses of Escherichia coli to nutritional stringencies, environmental stresses and survival in the stationary phase. Prog Mol Subcell Biol. 1999;23:183–195. doi: 10.1007/978-3-642-58444-2_9. [DOI] [PubMed] [Google Scholar]
- 32.Chandrashekhar K, Kassem II, Nislow C, Gangaiah D, Candelero-Rueda RA, Rajashekara G. Transcriptome analysis of Campylobacter jejuni polyphosphate kinase (ppk1 and ppk2) mutants. Virulence. 2015;6:814–818. doi: 10.1080/21505594.2015.1104449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shiba T, Tsutsumi K, Yano H, Ihara Y, Kameda A, Tanaka K, Takahashi H, Munekata M, Rao NN, Kornberg A. Inorganic polyphosphate and the induction of rpoS expression. Proc Natl Acad Sci USA. 1997;94:11210–11215. doi: 10.1073/pnas.94.21.11210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gaynor EC, Wells DH, MacKichan JK, Falkow S. The Campylobacter jejuni stringent response controls specific stress survival and virulence-associated phenotypes. Mol Microbiol. 2005;56:8–27. doi: 10.1111/j.1365-2958.2005.04525.x. [DOI] [PubMed] [Google Scholar]
- 35.Brown MR, Kornberg A. The long and short of it - polyphosphate, PPK and bacterial survival. Trends Biochem Sci. 2008;33:284–290. doi: 10.1016/j.tibs.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Kuroda A. A polyphosphate-lon protease complex in the adaptation of Escherichia coli to amino acid starvation. Biosci Biotechnol Biochem. 2006;70:325–331. doi: 10.1271/bbb.70.325. [DOI] [PubMed] [Google Scholar]
- 37.Kuroda A, Nomura K, Takiguchi N, Kato J, Ohtake H. Inorganic polyphosphate stimulates lon-mediated proteolysis of nucleoid proteins in Escherichia coli. Cell Mol Biol (Noisy-le-grand) 2006;52:23–29. [PubMed] [Google Scholar]
- 38.Kuroda A, Tanaka S, Ikeda T, Kato J, Takiguchi N, Ohtake H. Inorganic polyphosphate kinase is required to stimulate protein degradation and for adaptation to amino acid starvation in Escherichia coli. Proc Natl Acad Sci USA. 1999;96:14264–14269. doi: 10.1073/pnas.96.25.14264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kassem II, Chandrashekhar K, Rajashekara G. Of energy and survival incognito: a relationship between viable but non-culturable cells formation and inorganic polyphosphate and formate metabolism in Campylobacter jejuni. Front Microbiol. 2013;4:183. doi: 10.3389/fmicb.2013.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tholozan JL, Cappelier JM, Tissier JP, Delattre G, Federighi M. Physiological characterization of viable-but-nonculturable Campylobacter jejuni cells. Appl Environ Microbiol. 1999;65:1110–1116. doi: 10.1128/aem.65.3.1110-1116.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kashoma IP, Kassem II, John J, Kessy BM, Gebreyes W, Kazwala RR, Rajashekara G. Prevalence and Antimicrobial Resistance of Campylobacter Isolated from Dressed Beef Carcasses and Raw Milk in Tanzania. Microb Drug Resist. 2016;22:40–52. doi: 10.1089/mdr.2015.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kashoma IP, Kassem II, Kumar A, Kessy BM, Gebreyes W, Kazwala RR, Rajashekara G. Antimicrobial Resistance and Genotypic Diversity of Campylobacter Isolated from Pigs, Dairy, and Beef Cattle in Tanzania. Front Microbiol. 2015;6:1240. doi: 10.3389/fmicb.2015.01240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kashoma IP, Kumar A, Sanad YM, Gebreyes W, Kazwala RR, Garabed R, Rajashekara G. Phenotypic and genotypic diversity of thermophilic Campylobacter spp. in commercial turkey flocks: a longitudinal study. Foodborne Pathog Dis. 2014;11:850–860. doi: 10.1089/fpd.2014.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poole K. Bacterial stress responses as determinants of antimicrobial resistance. J Antimicrob Chemother. 2012;67:2069–2089. doi: 10.1093/jac/dks196. [DOI] [PubMed] [Google Scholar]
- 45.Pina-Mimbela R, Madrid JA, Kumar A, Torrelles JB, Rajashekara G. Polyphosphate kinases modulate Campylobacter jejuni outer membrane constituents and alter its capacity to invade and survive in intestinal epithelial cells in vitro. Emerg Microbes Infect. 2015;4:e77. doi: 10.1038/emi.2015.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stumpf JD, Foster PL. Polyphosphate kinase regulates error-prone replication by DNA polymerase IV in Escherichia coli. Mol Microbiol. 2005;57:751–761. doi: 10.1111/j.1365-2958.2005.04724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Castuma CE, Huang R, Kornberg A, Reusch RN. Inorganic polyphosphates in the acquisition of competence in Escherichia coli. J Biol Chem. 1995;270:12980–12983. doi: 10.1074/jbc.270.22.12980. [DOI] [PubMed] [Google Scholar]
- 48.Reusch RN, Sadoff HL. Putative structure and functions of a poly-beta-hydroxybutyrate/calcium polyphosphate channel in bacterial plasma membranes. Proc Natl Acad Sci USA. 1988;85:4176–4180. doi: 10.1073/pnas.85.12.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guerry P, Ewing CP, Schirm M, Lorenzo M, Kelly J, Pattarini D, Majam G, Thibault P, Logan S. Changes in flagellin glycosylation affect Campylobacter autoagglutination and virulence. Mol Microbiol. 2006;60:299–311. doi: 10.1111/j.1365-2958.2006.05100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haddock G, Mullin M, MacCallum A, Sherry A, Tetley L, Watson E, Dagleish M, Smith DG, Everest P. Campylobacter jejuni 81-176 forms distinct microcolonies on in vitro-infected human small intestinal tissue prior to biofilm formation. Microbiology. 2010;156:3079–3084. doi: 10.1099/mic.0.039867-0. [DOI] [PubMed] [Google Scholar]
- 51.Hanning I, Donoghue DJ, Jarquin R, Kumar GS, Aguiar VF, Metcalf JH, Reyes-Herrera I, Slavik M. Campylobacter biofilm phenotype exhibits reduced colonization potential in young chickens and altered in vitro virulence. Poult Sci. 2009;88:1102–1107. doi: 10.3382/ps.2008-00307. [DOI] [PubMed] [Google Scholar]
- 52.Reuter M, Mallett A, Pearson BM, van Vliet AH. Biofilm formation by Campylobacter jejuni is increased under aerobic conditions. Appl Environ Microbiol. 2010;76:2122–2128. doi: 10.1128/AEM.01878-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Drozd M, Chandrashekhar K, Rajashekara G. Polyphosphate-mediated modulation of Campylobacter jejuni biofilm growth and stability. Virulence. 2014;5:680–690. doi: 10.4161/viru.34348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu L, Kopecko DJ. Campylobacter jejuni 81-176 associates with microtubules and dynein during invasion of human intestinal cells. Infect Immun. 1999;67:4171–4182. doi: 10.1128/iai.67.8.4171-4182.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Young KT, Davis LM, Dirita VJ. Campylobacter jejuni: molecular biology and pathogenesis. Nat Rev Microbiol. 2007;5:665–679. doi: 10.1038/nrmicro1718. [DOI] [PubMed] [Google Scholar]
- 56.Grillo-Puertas M, Villegas JM, Rintoul MR, Rapisarda VA. Polyphosphate degradation in stationary phase triggers biofilm formation via LuxS quorum sensing system in Escherichia coli. PLoS One. 2012;7:e50368. doi: 10.1371/journal.pone.0050368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chuang YM, Bandyopadhyay N, Rifat D, Rubin H, Bader JS, Karakousis PC. Deficiency of the novel exopolyphosphatase Rv1026/PPX2 leads to metabolic downshift and altered cell wall permeability in Mycobacterium tuberculosis. MBio. 2015;6:e02428. doi: 10.1128/mBio.02428-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuroda A, Murphy H, Cashel M, Kornberg A. Guanosine tetra- and pentaphosphate promote accumulation of inorganic polyphosphate in Escherichia coli. J Biol Chem. 1997;272:21240–21243. doi: 10.1074/jbc.272.34.21240. [DOI] [PubMed] [Google Scholar]
- 59.Kuroda A, Ohtake H. Molecular analysis of polyphosphate accumulation in bacteria. Biochemistry (Mosc) 2000;65:304–308. [PubMed] [Google Scholar]
- 60.Kim HY, Schlictman D, Shankar S, Xie Z, Chakrabarty AM, Kornberg A. Alginate, inorganic polyphosphate, GTP and ppGpp synthesis co-regulated in Pseudomonas aeruginosa: implications for stationary phase survival and synthesis of RNA/DNA precursors. Mol Microbiol. 1998;27:717–725. doi: 10.1046/j.1365-2958.1998.00702.x. [DOI] [PubMed] [Google Scholar]
- 61.Siuda W, Chrost R. Utilization of selected dissolved organic phosphorus compounds by bacteria in lake water under non-limiting orthophosphate conditions. Polish J Environ Stud. 2001;10:475–484. [Google Scholar]
- 62.van Mourik A, Bleumink-Pluym NM, van Dijk L, van Putten JP, Wösten MM. Functional analysis of a Campylobacter jejuni alkaline phosphatase secreted via the Tat export machinery. Microbiology. 2008;154:584–592. doi: 10.1099/mic.0.2007/012120-0. [DOI] [PubMed] [Google Scholar]
- 63.Drozd M, Gangaiah D, Liu Z, Rajashekara G. Contribution of TAT system translocated PhoX to Campylobacter jejuni phosphate metabolism and resilience to environmental stresses. PLoS One. 2011;6:e26336. doi: 10.1371/journal.pone.0026336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jeon B. A tangle of poly-phosphate in Campylobacter. Virulence. 2014;5:449–450. doi: 10.4161/viru.28690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matsuda K, Chaudhari AA, Lee JH. Evaluation of safety and protection efficacy on cpxR and lon deleted mutant of Salmonella Gallinarum as a live vaccine candidate for fowl typhoid. Vaccine. 2011;29:668–674. doi: 10.1016/j.vaccine.2010.11.039. [DOI] [PubMed] [Google Scholar]
- 66.Si W, Wang X, Liu H, Yu S, Li Z, Chen L, Zhang W, Liu S. Physiology, pathogenicity and immunogenicity of live, attenuated Salmonella enterica serovar Enteritidis mutants in chicks. Microb Pathog. 2015;83-84:6–11. doi: 10.1016/j.micpath.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 67.Baqar S, Tribble DR, Carmolli M, Sadigh K, Poly F, Porter C, Larsson CJ, Pierce KK, Guerry P, Darsley M, et al. Recrudescent Campylobacter jejuni infection in an immunocompetent adult following experimental infection with a well-characterized organism. Clin Vaccine Immunol. 2010;17:80–86. doi: 10.1128/CVI.00252-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tribble DR, Baqar S, Carmolli MP, Porter C, Pierce KK, Sadigh K, Guerry P, Larsson CJ, Rockabrand D, Ventone CH, et al. Campylobacter jejuni strain CG8421: a refined model for the study of Campylobacteriosis and evaluation of Campylobacter vaccines in human subjects. Clin Infect Dis. 2009;49:1512–1519. doi: 10.1086/644622. [DOI] [PubMed] [Google Scholar]
- 69.Tribble DR, Baqar S, Scott DA, Oplinger ML, Trespalacios F, Rollins D, Walker RI, Clements JD, Walz S, Gibbs P, et al. Assessment of the duration of protection in Campylobacter jejuni experimental infection in humans. Infect Immun. 2010;78:1750–1759. doi: 10.1128/IAI.01021-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Macuch PJ, Tanner AC. Campylobacter species in health, gingivitis, and periodontitis. J Dent Res. 2000;79:785–792. doi: 10.1177/00220345000790021301. [DOI] [PubMed] [Google Scholar]
- 71.Ercan E, Eratalay K, Deren O, Gur D, Ozyuncu O, Altun B, Kanli C, Ozdemir P, Akincibay H. Evaluation of periodontal pathogens in amniotic fluid and the role of periodontal disease in pre-term birth and low birth weight. Acta Odontol Scand. 2013;71:553–559. doi: 10.3109/00016357.2012.697576. [DOI] [PubMed] [Google Scholar]
- 72.Etoh Y, Dewhirst FE, Paster BJ, Yamamoto A, Goto N. Campylobacter showae sp. nov., isolated from the human oral cavity. Int J Syst Bacteriol. 1993;43:631–639. doi: 10.1099/00207713-43-4-631. [DOI] [PubMed] [Google Scholar]
- 73.Kaakoush NO, Mitchell HM. Campylobacter concisus - A new player in intestinal disease. Front Cell Infect Microbiol. 2012;2:4. doi: 10.3389/fcimb.2012.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Macfarlane S, Furrie E, Macfarlane GT, Dillon JF. Microbial colonization of the upper gastrointestinal tract in patients with Barrett’s esophagus. Clin Infect Dis. 2007;45:29–38. doi: 10.1086/518578. [DOI] [PubMed] [Google Scholar]
- 75.Kumar A, Drozd M, Pina-Mimbela R, Xu X, Helmy YA, Antwi J, Fuchs JR, Nislow C, Templeton J, Blackall PJ, et al. Novel Anti-Campylobacter Compounds Identified Using High Throughput Screening of a Pre-selected Enriched Small Molecules Library. Front Microbiol. 2016;7:405. doi: 10.3389/fmicb.2016.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ericsson CD, Hatz C, DuPont AW. Postinfectious irritable bowel syndrome. Clin Infect Dis. 2008;46:594–599. doi: 10.1086/526774. [DOI] [PubMed] [Google Scholar]
- 77.Sartor RB, Mazmanian SK. Intestinal Microbes in Inflammatory Bowel Diseases. Am J Gastroenterol Suppl. 2012;1:15–21. [Google Scholar]
- 78.Mahendran V, Riordan SM, Grimm MC, Tran TA, Major J, Kaakoush NO, Mitchell H, Zhang L. Prevalence of Campylobacter species in adult Crohn’s disease and the preferential colonization sites of Campylobacter species in the human intestine. PLoS One. 2011;6:e25417. doi: 10.1371/journal.pone.0025417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gao Z, Guo B, Gao R, Zhu Q, Qin H. Microbiota disbiosis is associated with colorectal cancer. Front Microbiol. 2015;6:20. doi: 10.3389/fmicb.2015.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Warren RL, Freeman DJ, Pleasance S, Watson P, Moore RA, Cochrane K, Allen-Vercoe E, Holt RA. Co-occurrence of anaerobic bacteria in colorectal carcinomas. Microbiome. 2013;1:16. doi: 10.1186/2049-2618-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vaughan-Shaw PG, Rees JR, White D, Burgess P. Campylobacterjejuni cholecystitis: a rare but significant clinical entity. BMJ Case Rep. 2010;2010:bcr1020092365. doi: 10.1136/bcr.10.2009.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nachamkin I, Allos BM, Ho T. Campylobacter species and Guillain-Barré syndrome. Clin Microbiol Rev. 1998;11:555–567. doi: 10.1128/cmr.11.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Koga M, Gilbert M, Li J, Koike S, Takahashi M, Furukawa K, Hirata K, Yuki N. Antecedent infections in Fisher syndrome: a common pathogenesis of molecular mimicry. Neurology. 2005;64:1605–1611. doi: 10.1212/01.WNL.0000160399.08456.7C. [DOI] [PubMed] [Google Scholar]
- 84.Pope JE, Krizova A, Garg AX, Thiessen-Philbrook H, Ouimet JM. Campylobacter reactive arthritis: a systematic review. Semin Arthritis Rheum. 2007;37:48–55. doi: 10.1016/j.semarthrit.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morrison VA, Lloyd BK, Chia JK, Tuazon CU. Cardiovascular and bacteremic manifestations of Campylobacter fetus infection: case report and review. Rev Infect Dis. 1990;12:387–392. doi: 10.1093/clinids/12.3.387. [DOI] [PubMed] [Google Scholar]
- 86.Hannu T, Mattila L, Rautelin H, Siitonen A, Leirisalo-Repo M. Three cases of cardiac complications associated with Campylobacter jejuni infection and review of the literature. Eur J Clin Microbiol Infect Dis. 2005;24:619–622. doi: 10.1007/s10096-005-0001-2. [DOI] [PubMed] [Google Scholar]
- 87.Kogawa S, Furukawa K. [Campylobacter jejuni meningitis in an immunocompetent adult male] Rinsho Shinkeigaku. 2010;50:262–264. doi: 10.5692/clinicalneurol.50.262. [DOI] [PubMed] [Google Scholar]
- 88.Goossens H, Henocque G, Kremp L, Rocque J, Boury R, Alanio G, Vlaes L, Hemelhof W, Van den Borre C, Macart M. Nosocomial outbreak of Campylobacter jejuni meningitis in newborn infants. Lancet. 1986;2:146–149. doi: 10.1016/s0140-6736(86)91956-2. [DOI] [PubMed] [Google Scholar]
- 89.Suy F, Le Dû D, Roux AL, Hanachi M, Dinh A, Crémieux AC. Meningitis and endocarditis caused by Campylobacter fetus after raw-liver ingestion. J Clin Microbiol. 2013;51:3147–3150. doi: 10.1128/JCM.00631-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Han XY, Tarrand JJ, Rice DC. Oral Campylobacter species involved in extraoral abscess: a report of three cases. J Clin Microbiol. 2005;43:2513–2515. doi: 10.1128/JCM.43.5.2513-2515.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.de Vries JJ, Arents NL, Manson WL. Campylobacter species isolated from extra-oro-intestinal abscesses: a report of four cases and literature review. Eur J Clin Microbiol Infect Dis. 2008;27:1119–1123. doi: 10.1007/s10096-008-0550-2. [DOI] [PubMed] [Google Scholar]
- 92.Simor AE, Karmali MA, Jadavji T, Roscoe M. Abortion and perinatal sepsis associated with campylobacter infection. Rev Infect Dis. 1986;8:397–402. doi: 10.1093/clinids/8.3.397. [DOI] [PubMed] [Google Scholar]


