Abstract
AIM
To assess the practice of caring for acute liver failure (ALF) patients in varying geographic locations and medical centers.
METHODS
Members of the European Acute Liver Failure Consortium completed an 88-item questionnaire detailing management of ALF. Responses from 22 transplantation centers in 11 countries were analyzed, treating between 300 and 500 ALF cases and performing over 100 liver transplants (LT) for ALF annually. The questions pertained to details of the institution and their clinical activity, standards of care, referral and admission, ward- based care versus intensive care unit (ICU) as well as questions regarding liver transplantation - including criteria, limitations, and perceived performance. Clinical data was also collected from 13 centres over a 3 mo period.
RESULTS
The interval between referral and admission of ALF patients to specialized units was usually less than 24 h and once admitted, treatment was provided by a multidisciplinary team. Principles of care of patients with ALF were similar among centers, particularly in relation to recognition of severity and care of the more critically ill. Centers exhibited similarities in thresholds for ICU admission and management of severe hepatic encephalopathy. Over 80% of centers administered n-acetyl-cysteine to ICU patients for non-paracetamol-related ALF. There was significant divergence in the use of prophylactic antibiotics and anti-fungals, lactulose, nutritional support and imaging investigations in admitted patients and in the monitoring and treatment of intra-cranial pressure (ICP). ICP monitoring was employed in 12 centers, with the most common indications being papilledema and renal failure. Most patients listed for transplantation underwent surgery within an average waiting time of 1-2 d. Over a period of 3 mo clinical data from 85 ALF patients was collected. Overall patient survival at 90-d was 76%. Thirty six percent of patients underwent emergency LT, with a 90% post transplant survival to hospital discharge, 42% survived with medical management alone.
CONCLUSION
Alongside similarities in principles of care of ALF patients, major areas of divergence were present in key areas of diagnosis, monitoring, treatment and decision to transplant.
Keywords: Acute liver failure, Liver transplantation, Intra-cranial pressure, Hepatic encephalopathy
Core tip: Acute liver failure is rare, but carries high mortality and resource use. Standard of care and clinical practice varies between centers. In a survey conducted among members of the European-Acute-Liver-Failure consortium we have identified similarities in principles of care, including basic clinical management, recognition of severity and care of critically ill patients. Major areas of divergence were pre-intensive care unit (ICU) care and elements of ICU care. Further research is required regarding intra-cranial pressure monitoring and therapy, prophylactic antibiotics and anti-fungals, and liver support systems; we also identified a great need for improving prognostic evaluation for liver transplantation and refinement of transplantation criteria.
INTRODUCTION
Acute liver failure (ALF) is a rare clinical syndrome resulting in rapid loss of hepatocyte function in a patient without preexisting liver disease[1]. ALF accounts for 5%-7% of liver transplantations annually and its incidence rate is < 10 cases per million population[2]. ALF carries high morbidity and mortality, which often exceeded 90% in the pre-transplant era[3]. The introduction of liver transplantation (LT), along with changing patterns of etiology, has markedly increased short-term survival[4], but the treatment of ALF remains challenging. The rarity of ALF and its unpredictable course make it a difficult entity to study and treat. Only a few small, randomized controlled trials dealing with standard treatment have been performed in patients with ALF. As a result, many interventions continue to be administered on an intuitive basis or are adopted from other critical care settings. Familiarity with current real-life clinical practice is necessary to establish an outline for future therapeutic studies. We performed a survey among the European Acute Liver Failure (EUROALF) consortium members relating to medical ward and intensive care unit (ICU) management of ALF patients, assessing similarities and differences in management of these patients. Our findings show large variations in management among centers and call for urgent standardization of care. Furthermore, these findings identify opportunities for future interventional clinical trials.
MATERIALS AND METHODS
The EUROALF study group is an international consortium of 22 medical centers in 11 countries established in 2010 (For details of the consortium see www.medscinet.net/euroalf). A shared registry of ALF cases was developed, containing over 400 cases to date. ALF cases entered in to the registry are defined as marked hepatic synthetic dysfunction in patients with no previously known liver disease.
In order to survey clinical practices in ALF, an 88-item questionnaire was sent to the EUROALF consortium members (Appendix A). The first set of questions pertained to details of the institution and extent of clinical activity. The following sections referred to standards of care, including issues of referral and admission and ward versus ICU based care (triggers for admission, drug therapy, imaging studies, coagulation studies, renal replacement therapy, nutritional support, utilization of blood products etc.). The last section referred to liver transplantation (criteria and limitations, and perceived performance). Requested answers were oriented (different items to choose from), quantitative (a 0-100% scale) or open-ended (choice of words by the participant) (Appendix B).
Data regarding the “real-life” experience of 13 centers in 7 countries treating patients with ALF was collected over a 3 mo period (January- March 2009). All centres have an established programme of LT and ALF management. ALF was classified as being present in patients without clinical, histological or radiological evidence of chronic liver disease, an international normalized ratio (INR) of > 1.5 and any level of hepatic encephalopathy (HE). Data was collected concerning clinical presentation, management and outcome.
Statistical analysis
All data was summarized and reported as the number and percent of centers for each question. Comparison of variables between high- and low-volume centers was done using the Fischer exact test, while the paired comparison between the behaviors on the ward compared to the ICU in each center was done using the non-parametric McNemar analysis. All analyses were two-sided and P < 0.05 was considered statistically significant. The SPSS statistical package version 19.0 was used to perform all statistical analyses (SSPS Inc., Chicago, IL, United States).
RESULTS
The responses of 22 centers in 11 countries were analyzed (Appendix A). Nineteen centers (86.4%) had > 500 inpatients beds, with 10 (45.5%) having > 1000 beds (Figure 1A). Eleven centers performed between 50-100 elective and emergency LT annually, and 3 performed > 100 (Figure 1B).
Figure 1.
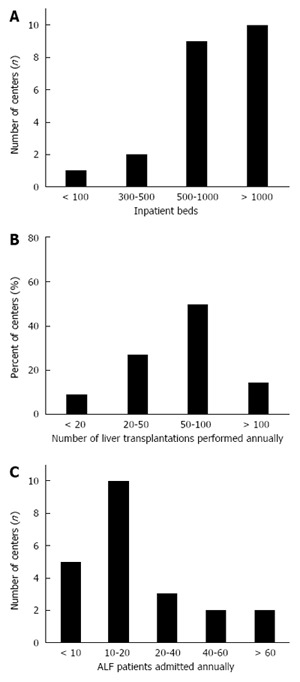
Demographic data. A: Number of inpatients beds per center; B: Number of liver transplantations performed annually per center; C: Number of ALF patients admitted annually per center.
Collectively, an estimated number of 300-500 ALF cases were treated annually. Ten centers (45%) admitted 10-20 patients with ALF annually, while 7 “high volume” centers (32%) admitted 20 to > 60 ALF patients annually. The remaining 23% centers treated < 10 ALF patients annually (with those admitting ≤ 20 cases annually classed as “low volume” centers) (Figure 1C). On average, > 100 LT of ALF patients are performed annually among consortium centers. However, most centers (77%, 17/22) perform < 10 LT, 4 perform 20-40 and only one center performed > 40 LT for ALF annually.
Practices involving referral, hospital placement and jurisdictions
Many patients were referred to the participating centers from other hospitals. The average time from referral to admission was less than one day in 16/22 (73%) of the centers, 1-2 d in 4 centers (18%) and 2-5 d in 2 (9%). In 96% (21/22) and 100% (22/22) of the participating centers, ALF patients in the medical ward or the ICU respectively, were interviewed and examined by a hepatology consultant or senior physician at least daily. Once in the ICU, or high dependency unit (HDU), patients were treated by a multidisciplinary team (consisting of a hepatologist, intensive care specialist and transplant surgeon) in 50% of the centers. In other centers, the ICU patients were managed by an intensive care specialist (32%), a hepatologist (9%) or co-treated by the two (9%).
The level of HE that prompted transfer to an ICU or HDU was grade 1 HE in 33%, grade 2 in most centers (43%), or grade 3 in 10%.
Practices of diagnostic studies
Imaging: Abdominal ultrasound (US) was used as the initial imaging modality in the majority of centers (95% and 91% on the ward or ICU respectively). Utilization of computed tomography (CT) imaging was more variable.
Liver biopsy: LBX was performed in < 25% of patients by the majority of centers. There was no difference in performing LBX between high and low volume centers or centers with high transplantation rates.
Practices involving patient monitoring
Intra-cranial pressure (ICP) monitoring: ICP monitoring was used in 55% of centers. The rate was higher in high volume centers (6/7, 86%) than low volume centers (6/15, 40%) (P = 0.074). The most common indications for ICP monitoring were papilledema (all centers) and renal failure (58%). Elevated ammonia (42%), cardiovascular instability (42%), young age (42%) and severity of coagulopathy (33%) were less commonly reported indications for ICP monitoring (Figure 2A).
Figure 2.
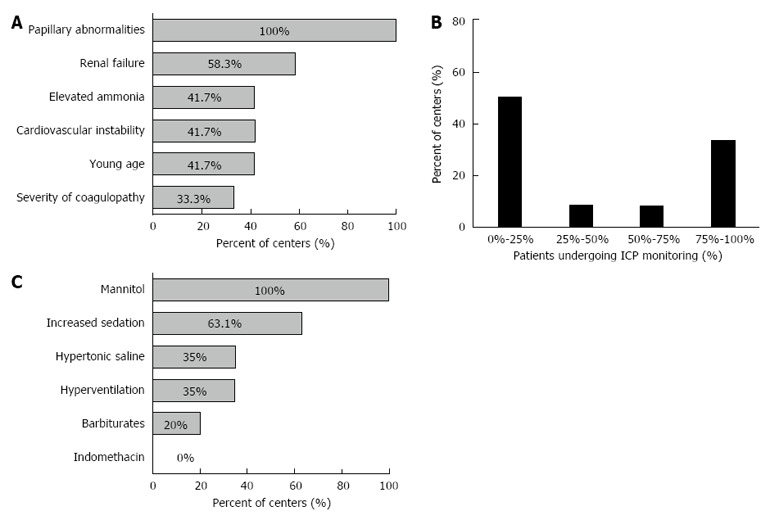
Practice involving intra-cranial pressure monitoring. A: Indications for intra-cranial pressure (ICP) monitoring in ALF patients; B: Percent of patients with HE ≥ grade 3 undergoing ICP monitoring (among the centers using ICP monitoring); C: First line treatment interventions for raised ICP.
Among the 12 centers reporting the use of ICP monitoring, 50% reported using it in < 25% of the patients with high grade HE, with no difference between high and low volume centers. However, 4 of the 12 centers performed ICP monitoring in > 75% of the patients with high grade HE (Figure 2B).
The ICP pressure that triggered treatment was 20-25 mmHg and 25-30 in 58% (7/12) and 33% (4/12) of centers respectively. Over 90% (11/12) of the centers targeted a specific cerebral perfusion pressure (CPP). The majority (54.5%, 6/11) used a CPP value of 50-60 mmHg, while 27% (3/11) used a CPP of 60-70 mmHg as their target.
Practices involving medical treatment
Antibiotics and Antifungals: Use of routine antibiotics prophylaxis was reported in < 50% of patients by 63% (12/19) of the centers. However, wide discrepancy existed as demonstrated in Figure 3A. High volume centers administered routine antibiotics on the ward significantly less than low volume centers (P = 0.001). Antibiotics were used in < 50% of the patients by 5 of the 7 high volume centers (71%) compared to 58% (7/12) of the low volume centers. Antibiotic use was more common in the ICU; 73% (16/22) of the centers reported that > 75% of the patients received antibiotics. There was no statistically significant difference between high and low volume centers regarding ICU antibiotic use (Figure 3B).
Figure 3.
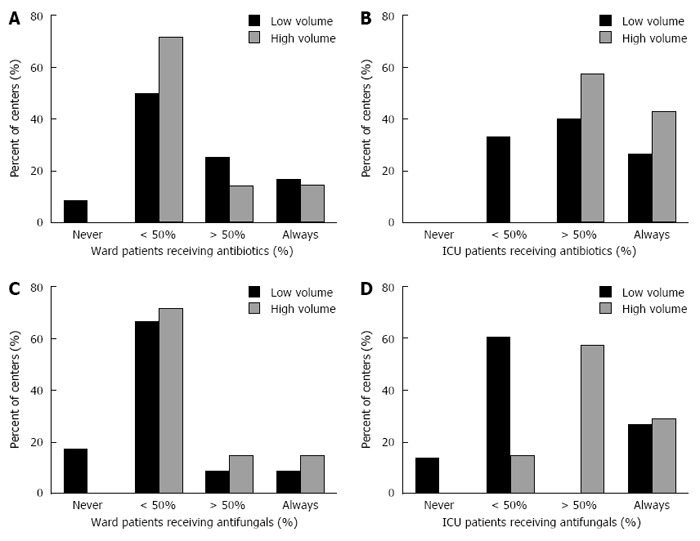
Use of prophylactic antibiotics and antifungals. A: Prophylactic antibiotics in ward based care, low versus high volume centers; B: Prophylactic antibiotics in ICU based care, low versus high volume centers; C: Prophylactic antifungals in ward based care, low versus high volume centers; D: Prophylactic antifungals in ICU based care, low versus high volume centers.
Systemic anti-fungals were given to < 25% of patients in 13/19 centers (68%) while on the ward. In the ICU a slightly higher rate was evident. Twelve centers (55%) administered anti-fungals to < 50% of patients and 10 (45%) treated > 50% of patients. We also observed a greater tendency to treat patients in the ICU with anti-fungals in the high volume centers, 85% (6/7) administering anti-fungals to the majority of their patients while only 27% (4/15) of low volume centers did so (P = 0.016) (Figure 3C and D).
N-acetylcysteine (NAC): Most centers administered NAC to the majority of their patients either on the ward (74%, 14/19) or the ICU (86%, 19/22), with 21% (4/19) and 36% (8/22) of the centers always doing so on the ward and ICU respectively. Seventy four percent (14/19) and 81% (18/22) of the centers administered NAC to patients with non-paracetamol ALF in the ward and ICU respectively.
ICP lowering medications: Mannitol (100%) and increased sedation (63%) were used as first-line treatment interventions. Other options less commonly used were hypertonic saline (35%), hyperventilation (35%) and barbiturates (20%) (Figure 2C). High volume centers reported greater use of hypertonic saline (86%) compared to low volume centers (31%) (P = 0.057).
Blood products: Fresh frozen plasma (FFP) or platelets were not routinely used in the vast majority of centers (95% 18/19 and 84%, 16/19 respectively). The threshold for platelets administration was < 20 × 109/L in 80% (15/19) of the centers. In the centers performing ICP monitoring, 92% (11/12) administered FFP prior to the procedure, 75% (9/12) administered platelets and 42% (5/12) used cryoprecipitate. Use of recombinant activated factor VII was rare (8%, 1/12).
Nutrition support: Feeding modalities differed markedly in the ward and ICU. While patients in the ICU where either mostly (> 50% of patients) or always fed via nasogastric tube (NGT) (73%, 16/22 and 23%, 5/22 respectively), only a minority of ward patients were fed by NGT (79%, 15/19 used NGT feeding in < 50% of the patients and 10%, 2/19 never used it). Total parenteral nutrition was used in < 50% of the patients in 77%-79% centers. Branched chain amino acids were rarely used.
Utilization of other treatment modalities: Ventilation and sedation: The level of HE that precipitated mechanical ventilation was grade ≥ 3 in 54% of the centers and ≥ 2 in 36%. Propofol was the sedative agent of choice in almost all centers, 68% also administered opiates. Only 1 center reported using benzodiazepines. Muscle relaxants were rarely used.
Renal replacement therapy (RRT): Continuous hemofiltration or hemodialysis was the most utilized primary form of RRT (86%, 19/22), as opposed to Intermittent hemodialysis (9%, 2/22). Indications for use of RRT are shown in Figure 4.
Figure 4.
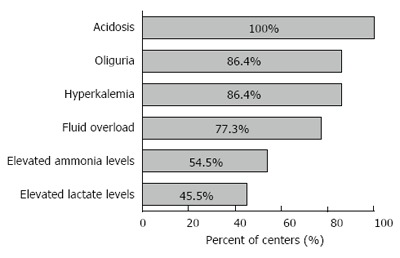
Indications for renal replacement therapy.
Liver transplantation: The King’s College criteria (KCC) (86%, 18/21) and Clichy criteria (33%, 7/21) were the most commonly used prognostic models to select patients for LT. The KCC and Clichy criteria were used as a single criteria system by 46% (10/22) and 4.5% (1/22) of the centers respectively. Thirty-eight percent (8/21) of the centers used a combination of the two criteria systems. The KCC were used in all the high volume centers.
There was no age limit for LT in 55% (12/22) centers, with 60 years of age being the limit in the remainder. The average waiting time for transplantation was 1-2 days in 55% (12/22) of the centers, 2-3 days in 27% (6/12) of the centers and > 3 d in 18% (4/22). None of the centers reported surgery occurring within 24 h of patient listing. The predicted survival rate of patients who fulfilled transplantation criteria but were not transplanted was < 50% in all participating centers, with 80% (18/21) reporting non-transplanted survival of < 25%.
Clinical presentation and outcome of ALF patients: A total of 85 patients were treated over a 3 mo period in 13 the centres that participated in our “real-life” data collection. Median age was 38 (29-52) and 54 (64%) were female. The etiologic mix varied by location with paracetamol-induced disease predominating in Denmark, Belgium and the United Kingdom. Overall 43 patients (51%) were diagnosed with paracetamol-induced disease. Other causes included: auto-immune (2 cases), hypoxic hepatitis (6), non-paracetamol drugs (7), viral disease (4), pregnancy-related (2), herbal remedies (1), malignant infiltration (1), Budd-Chiari syndrome (1) and unknown (18).
Severity of Illness: Coagulopathy: Median INR was 3.8 (2.1-6.5) at presentation and peak was 4.7 (2.9-7.3).
Encephalopathy: At presentation HE was mild [median grade 1 (0-2)], however high grade HE (grade 3-4) developed later in the clinical course in 51 (60%) of patients. Of these cases, 12 (24%) developed clinical evidence of ICH. Medical management: Seventy-six patients (89%) were admited to a critical care unit . During the course of illness 59 (69%) patients required intubation and mechanical ventilation, 54 (64%) vasopressor support and 48 (56%) RRT. Of the 30 patients who required one or fewer systemic support, 21 (70%) survived with medical management alone. However 40 of the 55 patients who required more than one organ system support either died or underwent LT and 15 (27%) survived with medical management alone.
Outcome: Overall 90-d survival was 65/85 (76%). Forty-two patients (49%) fulfilled transplantation selection criteria and of these 31 (74%) underwent emergency LT. Post transplant survival to hospital discharge was 28/31 (90%). Of the remaining eleven patients who fulfilled criteria but were not transplanted 7 died and 4 survived. Thirty-two (74%) of the 43 patients who did not fulfil transplant criteria survived with medical management alone. Eight (73%) of the 11 patients who died had hypoxic hepatitis as a cause of ALF and median age was 56 (49-79) years.
DISCUSSION
The results of our survey highlight important aspects regarding patterns of practice of ALF patients across various geographic locations. Whilst there were many similarities in the principles of care, particularly in relation to utilizing prognostic models and basic clinical management of the severely ill, major areas of divergence remain, including many aspects of ward and ICU care. These variations in care stem from a lack of high quality evidence-based data to guide the decision-making process, and uncertainty as to what constitutes best practice.
The data indicates that referral, hospitalization and jurisdiction practices were homogenous among EUROALF members. Almost 75% of the centers reported an average time from referral to admission of < 1 d. Given transportation logistics, space allocation and other bureaucratic barriers, it is unlikely that this time frame can be significantly shortened.
Indications for LBX in the management of patients with ALF are not well defined. Although it has been suggested to be of diagnostic and prognostic value, assisting clinical decision-making and timing of LT[5,6], in our survey it was not generally considered a requirement in clinical practice and was performed only in a small minority of patients.
While diagnostic procedures were relatively uniform in the consortium, management protocols varied more widely. NAC is routinely used in paracetamol induced hepatotoxicity[7,8]. Most centers administered NAC to the majority of patients early in the course of illness as part of ward and especially ICU based care. This is inspite of recent data suggesting that NAC is ineffective in critically ill patients[9], over 80% of the responders also routinely administered NAC to non-paracetamol ALF, in concordance with the findings of a recent RCT[10].
Patients with ALF are highly susceptible to infections. Bacterial infections have been documented in up to 80% of patients and fungal infections occur in a third[11]. Though early studies showed that prophylactic antimicrobial therapy decreased infections, a survival benefit was not demonstrated[12]. Currently, the use of prophylactic antibiotics and anti-fungals in patients with ALF is not generally recommended and instead, periodic surveillance for infection is advocated[13,14]. Whilst most centers did not routinely administer antibiotics to ALF patients in the wards, almost 75% of centers treated the majority of patients in the ICU (Figure 3B). Administration of anti-fungals in ward based care of ALF patients was limited, but marked variation was found regarding their use in the ICU (Figure 3D). This divergence in care suggests that the prophylactic anti-infectious treatment is an area of uncertainty for treating clinicians.
Coagulopathy and thrombocytopenia are frequently seen with ALF[15]. The most frequently used coagulation parameter was the INR test followed by PT and fibrinogen levels. Interestingly, only 52% of the centers measured Factor V levels, as a means of assessing liver function and prediction of patient outcome[16]. Finally, the thromboelastogram (TEG) test, which assesses overall homeostasis[17], was rarely used.
In the absence of bleeding, correction of INR or thrombocytopenia is not justified and may obscure the use of INR as a prognostic marker[13]. Our results show that most centers did not routinely administer coagulation factors or platelets.
The issue of bleeding subsequent to invasive procedures constitutes a more significant dilemma. In clinical practice, the risk of bleeding following routine procedures such as insertion of central venous catheters or paracentesis and even for more invasive procedures such as trans-jugular LBX is considered small[5,18]. In contrast, the risk of intracranial hemorrhage following ICP monitor insertion is a major cause of concern in ALF patients with an incidence of fatal hemorrhage ranging from 1%-5%[19,20]. No guidelines exist regarding administration of coagulation factors prior to specific procedures in the presence of coagulopathy[13,14]. In our questionnaire, we addressed utilization of coagulation factors in general and specifically with regards to ICP monitor insertion. Platelets and FFP were the most common factors given in the majority of centers prior to performing LBX and inserting ICP monitors. Cryoprecipitate was used by < 50% of the centers while the use of recombinant activated factor VII was rare.
The role of HE as a prognostic marker was reflected in the decision to transfer patients from the medical ward to the ICU. Grade 2 HE served as the most commonly accepted indication for ICU admission. However, over a third of the participants admitted to the ICU patients with HE grade 1 or did so regardless of the patients’ cognitive state. Most centers intubated patients with grade 3 HE and over a third reported intubating at grade 2 HE. These practices indicate that in clinical practice physicians prefer to treat patients earlier, perhaps recognizing the potential for abrupt deterioration. This approach is supported by our “real-life” results showing that although most patients presented with minor HE, 60% developed high grade HE later in the clinical course.
ICP monitoring use varied by center with only approximately 50% of the participating centers reported using it. The overall proportion of patients with HE of grade 3 or above that underwent ICP monitoring was low, even in centers that performed this procedure often. However, there was marked divergence, with a few centers reporting extensive use of this modality (Figure 2B). These centers were all considered high volume, admitting > 20 ALF patients annually and performing LT in > 50% of their patients. Our results reflect uncertainty regarding the specific indications and benefit of ICP monitoring (Figure 2A).
Mannitol is widely accepted as first-line therapy to decrease intracranial hypertension, followed by hypertonic saline and moderate hypothermia, but their use is supported only by limited evidence and doesn't appear to improve survival[13,14,21-24]. Barbiturates, Indomethacin and hyperventilation are considered short-term salvage therapies in refractory cases[13,14,25]. All centers reported using all these modalities (Figure 2C) without significant difference in treatment choices between centers that did or did not perform ICP monitoring.
Prognostic models to assess allocation to LT in ALF include the KCC[26], Clichy-Villejuif criteria[27,28], model for end stage liver disease, and the new Acute Liver Failure Study Group index[29]. All of which show good specificity but more limited sensitivity and accuracy[29-33]. We found that KCC criteria were the most commonly used, followed by the Clichy criteria, and were occasionally used in combination. The need for better prognostic models and biomarkers to accurately define indications for LT, were raised by many survey participants.
Most patients listed for LT in our survey underwent surgery. These transplantation rates appear to coincide with the current literature as 85% of the centers reported performing transplantation in > 50% of the patients; and 43% reported that over 75% of the patients underwent LT. The predicted survival rate of patients who fulfilled transplantation criteria but did not undergo transplantation was < 50% in all participating centers, with the majority of centers predicting a survival rate of < 25%.
“Real-life” data from our survey, showed 76% overall 90-day survival (65/85 patients). Although limited by a relatively small number of patients this figure demonstrates the improved survival of ALF patients over the years, as this number is higher than that previously reported in the United States and England[4,34]. Almost half of the patients fulfilled the criteria for emergency LT, 74% of them underwent LT. LT in this high risk group was associated with 90% survival. The death rate among the patients who fulfilled LT criteria but were not transplanted (63%) was considerably higher compared with those who were not LT candidates (25%) treated with medical management alone.
Although we did not directly address the role of liver-assist devices in our survey, many centers raised this issue as one deserving further investigation and definition, both as a potential bridge for LT and as a means of providing vital support in hope of functional recovery.
Our study is a survey addressing the clinical practice and management of ALF patients. One of the limitations of our study is the number of participating centers. Although treating a large group of patients, standards of treatment may not be representative of all treatment centers in Europe. As a survey, the results represent the perceived views of the participants and are not backed by evidence. However, all the participants are senior hepatology consultants, and we believe that their answers portray current practices in their respective centers. Furthermore, this is an inherent premise of any survey. Furthermore, 13 centers reported “real-life” data, which provides validation to the questionnaire.
Over the past decades the outcomes of patients with ALF have improved considerably. However, it still remains a disease with high mortality. Management of ALF is challenging not only because of its severity and rapid progression but also due to the many uncertainties accompanying current clinical practice. Whilst many similarities were found in principles of care of patients with ALF across the centers participating in our study, there are still major areas of divergence, representing a need for further studies. Areas for potential research include use of ICP monitors and ICP therapy, prophylactic use of antibiotics and anti-fungals, as well as further investigation into the role of liver support systems and establishing an ALF care bundle. There is also a great need for improving prognostic evaluation for LT and for the refinement of transplantation criteria.
COMMENTS
Background
Acute liver failure (ALF) is a rare condition with high mortality and resource use, but limited evidence to support clinical practice. Little is known as to what constitutes standard of care and how practice varies between centers.
Research frontiers
The rarity of ALF and its unpredictable course make it a difficult entity to study and treat. Only a few studies dealing with standard treatment have been performed and therefore many interventions continue to be administered on an intuitive basis or are adopted from other clinical settings. Familiarity with current real-life clinical practice is necessary to establish an outline for clinical management and future therapeutic studies.
Innovations and breakthroughs
This study examined the practice of caring for ALF patients in varying geographic locations and medical centers, pointing out the similarities alongside major variations in clinical management.
Applications
The authors findings identify major areas of disagreement and opportunities for future interventional clinical trials.
Terminology
ALF is the sudden onset of severe liver cell dysfunction, in a patient without previously known liver disease, leading to coagulopathy and hepatic encephalopathy.
Peer-review
This study provides data regarding clinical practice of caring for ALF patients in various centres. It is of interest for other clinicians treating liver patients as well as researchers in this field.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Israel
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: Non-identifiable data was gathered as part of the EuroALF registry. The study was performed with the approval of the local research ethics committee.
Informed consent statement: Survey - the participants are EUROALF members listed in appendix A. Informed consent waiver was obtained.
Conflict-of-interest statement: The authors declare no conflict of interest related to this publication.
Data sharing statement: Technical appendix, statistical code, and dataset available from the corresponding author at william.bernal@kcl.ac.uk.
Peer-review started: January 6, 2016
First decision: January 28, 2016
Article in press: March 30, 2016
P- Reviewer: Higuera-de la Tijera MF, Marzuillo P, Silva LD, Wang K S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
References
- 1.Trey C, Davidson CS. The management of fulminant hepatic failure. Prog Liver Dis. 1970;3:282–298. [PubMed] [Google Scholar]
- 2.Bower WA, Johns M, Margolis HS, Williams IT, Bell BP. Population-based surveillance for acute liver failure. Am J Gastroenterol. 2007;102:2459–2463. doi: 10.1111/j.1572-0241.2007.01388.x. [DOI] [PubMed] [Google Scholar]
- 3.Rakela J, Mosley JW, Edwards VM, Govindarajan S, Alpert E. A double-blinded, randomized trial of hydrocortisone in acute hepatic failure. The Acute Hepatic Failure Study Group. Dig Dis Sci. 1991;36:1223–1228. doi: 10.1007/BF01307513. [DOI] [PubMed] [Google Scholar]
- 4.Bernal W, Hyyrylainen A, Gera A, Audimoolam VK, McPhail MJ, Auzinger G, Rela M, Heaton N, O’Grady JG, Wendon J, et al. Lessons from look-back in acute liver failure? A single centre experience of 3300 patients. J Hepatol. 2013;59:74–80. doi: 10.1016/j.jhep.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Donaldson BW, Gopinath R, Wanless IR, Phillips MJ, Cameron R, Roberts EA, Greig PD, Levy G, Blendis LM. The role of transjugular liver biopsy in fulminant liver failure: relation to other prognostic indicators. Hepatology. 1993;18:1370–1376. [PubMed] [Google Scholar]
- 6.Miraglia R, Luca A, Gruttadauria S, Minervini MI, Vizzini G, Arcadipane A, Gridelli B. Contribution of transjugular liver biopsy in patients with the clinical presentation of acute liver failure. Cardiovasc Intervent Radiol. 2006;29:1008–1010. doi: 10.1007/s00270-006-0052-5. [DOI] [PubMed] [Google Scholar]
- 7.Prescott LF, Illingworth RN, Critchley JA, Stewart MJ, Adam RD, Proudfoot AT. Intravenous N-acetylcystine: the treatment of choice for paracetamol poisoning. Br Med J. 1979;2:1097–1100. doi: 10.1136/bmj.2.6198.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brok J, Buckley N, Gluud C. Interventions for paracetamol (acetaminophen) overdose. Cochrane Database Syst Rev. 2006;(2):CD003328. doi: 10.1002/14651858.CD003328.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Szakmany T, Hauser B, Radermacher P. N-acetylcysteine for sepsis and systemic inflammatory response in adults. Cochrane Database Syst Rev. 2012;(9):CD006616. doi: 10.1002/14651858.CD006616.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee WM, Hynan LS, Rossaro L, Fontana RJ, Stravitz RT, Larson AM, Davern TJ, Murray NG, McCashland T, Reisch JS, et al. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137:856–864, 864.e1. doi: 10.1053/j.gastro.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rolando N, Philpott-Howard J, Williams R. Bacterial and fungal infection in acute liver failure. Semin Liver Dis. 1996;16:389–402. doi: 10.1055/s-2007-1007252. [DOI] [PubMed] [Google Scholar]
- 12.Rolando N, Gimson A, Wade J, Philpott-Howard J, Casewell M, Williams R. Prospective controlled trial of selective parenteral and enteral antimicrobial regimen in fulminant liver failure. Hepatology. 1993;17:196–201. [PubMed] [Google Scholar]
- 13.Lee WM, Stravitz RT, Larson AM. Introduction to the revised American Association for the Study of Liver Diseases Position Paper on acute liver failure 2011. Hepatology. 2012;55:965–967. doi: 10.1002/hep.25551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stravitz RT, Kramer AH, Davern T, Shaikh AO, Caldwell SH, Mehta RL, Blei AT, Fontana RJ, McGuire BM, Rossaro L, et al. Intensive care of patients with acute liver failure: recommendations of the U.S. Acute Liver Failure Study Group. Crit Care Med. 2007;35:2498–2508. doi: 10.1097/01.CCM.0000287592.94554.5F. [DOI] [PubMed] [Google Scholar]
- 15.Munoz SJ, Rajender Reddy K, Lee W. The coagulopathy of acute liver failure and implications for intracranial pressure monitoring. Neurocrit Care. 2008;9:103–107. doi: 10.1007/s12028-008-9087-6. [DOI] [PubMed] [Google Scholar]
- 16.Elinav E, Ben-Dov I, Hai-Am E, Ackerman Z, Ofran Y. The predictive value of admission and follow up factor V and VII levels in patients with acute hepatitis and coagulopathy. J Hepatol. 2005;42:82–86. doi: 10.1016/j.jhep.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Stravitz RT, Lisman T, Luketic VA, Sterling RK, Puri P, Fuchs M, Ibrahim A, Lee WM, Sanyal AJ. Minimal effects of acute liver injury/acute liver failure on hemostasis as assessed by thromboelastography. J Hepatol. 2012;56:129–136. doi: 10.1016/j.jhep.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalambokis G, Manousou P, Vibhakorn S, Marelli L, Cholongitas E, Senzolo M, Patch D, Burroughs AK. Transjugular liver biopsy--indications, adequacy, quality of specimens, and complications--a systematic review. J Hepatol. 2007;47:284–294. doi: 10.1016/j.jhep.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Vaquero J, Fontana RJ, Larson AM, Bass NM, Davern TJ, Shakil AO, Han S, Harrison ME, Stravitz TR, Muñoz S, et al. Complications and use of intracranial pressure monitoring in patients with acute liver failure and severe encephalopathy. Liver Transpl. 2005;11:1581–1589. doi: 10.1002/lt.20625. [DOI] [PubMed] [Google Scholar]
- 20.Blei AT, Olafsson S, Webster S, Levy R. Complications of intracranial pressure monitoring in fulminant hepatic failure. Lancet. 1993;341:157–158. doi: 10.1016/0140-6736(93)90016-a. [DOI] [PubMed] [Google Scholar]
- 21.Canalese J, Gimson AE, Davis C, Mellon PJ, Davis M, Williams R. Controlled trial of dexamethasone and mannitol for the cerebral oedema of fulminant hepatic failure. Gut. 1982;23:625–629. doi: 10.1136/gut.23.7.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy N, Auzinger G, Bernel W, Wendon J. The effect of hypertonic sodium chloride on intracranial pressure in patients with acute liver failure. Hepatology. 2004;39:464–470. doi: 10.1002/hep.20056. [DOI] [PubMed] [Google Scholar]
- 23.Jalan R, Olde Damink SW, Deutz NE, Hayes PC, Lee A. Moderate hypothermia in patients with acute liver failure and uncontrolled intracranial hypertension. Gastroenterology. 2004;127:1338–1346. doi: 10.1053/j.gastro.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Larsen F, Murphy N, Bernal W, Bjerring P, Hauerberg A, Wendon J. The prophylactic effect of mild hypothermia to prevent brain oedema in patients with acute liver failure: results of a multicentre randomised controlled trial [Abstract] J Hepatol. 2011;54(Supplement 1):S26. [Google Scholar]
- 25.Ede RJ, Gimson AE, Bihari D, Williams R. Controlled hyperventilation in the prevention of cerebral oedema in fulminant hepatic failure. J Hepatol. 1986;2:43–51. doi: 10.1016/s0168-8278(86)80007-1. [DOI] [PubMed] [Google Scholar]
- 26.O’Grady JG, Alexander GJ, Hayllar KM, Williams R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989;97:439–445. doi: 10.1016/0016-5085(89)90081-4. [DOI] [PubMed] [Google Scholar]
- 27.Bernuau J, Goudeau A, Poynard T, Dubois F, Lesage G, Yvonnet B, Degott C, Bezeaud A, Rueff B, Benhamou JP. Multivariate analysis of prognostic factors in fulminant hepatitis B. Hepatology. 1986;6:648–651. doi: 10.1002/hep.1840060417. [DOI] [PubMed] [Google Scholar]
- 28.Bismuth H, Samuel D, Castaing D, Adam R, Saliba F, Johann M, Azoulay D, Ducot B, Chiche L. Orthotopic liver transplantation in fulminant and subfulminant hepatitis. The Paul Brousse experience. Ann Surg. 1995;222:109–119. doi: 10.1097/00000658-199508000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rutherford A, King LY, Hynan LS, Vedvyas C, Lin W, Lee WM, Chung RT. Development of an accurate index for predicting outcomes of patients with acute liver failure. Gastroenterology. 2012;143:1237–1243. doi: 10.1053/j.gastro.2012.07.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bailey B, Amre DK, Gaudreault P. Fulminant hepatic failure secondary to acetaminophen poisoning: a systematic review and meta-analysis of prognostic criteria determining the need for liver transplantation. Crit Care Med. 2003;31:299–305. doi: 10.1097/00003246-200301000-00048. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt LE, Larsen FS. MELD score as a predictor of liver failure and death in patients with acetaminophen-induced liver injury. Hepatology. 2007;45:789–796. doi: 10.1002/hep.21503. [DOI] [PubMed] [Google Scholar]
- 32.Yantorno SE, Kremers WK, Ruf AE, Trentadue JJ, Podestá LG, Villamil FG. MELD is superior to King’s college and Clichy’s criteria to assess prognosis in fulminant hepatic failure. Liver Transpl. 2007;13:822–828. doi: 10.1002/lt.21104. [DOI] [PubMed] [Google Scholar]
- 33.McPhail MJ, Wendon JA, Bernal W. Meta-analysis of performance of Kings’s College Hospital Criteria in prediction of outcome in non-paracetamol-induced acute liver failure. J Hepatol. 2010;53:492–499. doi: 10.1016/j.jhep.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 34.Ostapowicz G, Fontana RJ, Schiødt FV, Larson A, Davern TJ, Han SH, McCashland TM, Shakil AO, Hay JE, Hynan L, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]


