Summary
Conversion of arginine into citrulline is a post‐translational modification that is observed in normal physiological processes. However, abnormal citrullination can provoke autoimmunity by generating altered self‐epitopes that are specifically targeted by autoantibodies and T cells. In this review we discuss the recognition of citrullinated antigens in human autoimmune diseases and the role that this modification plays in increasing antigenic diversity and circumventing tolerance mechanisms. Early published work demonstrated that citrullinated proteins are specifically targeted by autoantibodies in rheumatoid arthritis and that citrullinated peptides are more readily presented to T cells by arthritis‐susceptible HLA class II ‘shared epitope’ proteins. Emerging data support the relevance of citrullinated epitopes in other autoimmune diseases, including type 1 diabetes and multiple sclerosis, whose susceptible HLA haplotypes also preferentially present citrullinated peptides. In these settings, autoimmune patients have been shown to have elevated responses to citrullinated epitopes derived from tissue‐specific antigens. Contrasting evidence implicates autophagy or perforin and complement‐mediated membrane attack as inducers of ectopic citrullination. In either case, the peptidyl deiminases responsible for citrullination are activated in response to inflammation or insult, providing a mechanistic link between this post‐translational modification and interactions with the environment and infection. As such, it is likely that immune recognition of citrullinated epitopes also plays a role in pathogen clearance. Indeed, our recent data suggest that responses to citrullinated peptides facilitate recognition of novel influenza strains. Therefore, increased understanding of responses to citrullinated epitopes may provide important insights about the initiation of autoimmunity and recognition of heterologous viruses.
Keywords: antigens/peptides/epitopes, arthritis (including rheumatoid arthritis), autoimmunity, diabetes, T cells
Introduction
Post‐translational modifications (PTMs) are alterations of specific amino acids that influence the structural and functional properties of proteins. Some PTMs (such as isoaspartylation) occur spontaneously whereas others (such as deamidation and citrullination) are enzymatically catalysed processes. In general, all PTMs are an important part of normal physiology and, accordingly, inappropriate or dysregulated modifications can have deleterious effects. Among the various PTMs that are known to occur within the human proteome, many (such as phosphorylation, acetylation, glycosylation and ubiquitination) have functional consequences with respect to signalling pathways, chromatin structure, protein–protein interactions, or protein turnover.1 However, specific PTMs have also been shown to directly modulate the antigenicity of self‐proteins, so are increasingly implicated in the aetiology of autoimmune diseases.2 A key example of this phenomenon is the conversion of arginine into its polar analogue citrulline by peptidyl arginine deiminase (PAD) enzymes (Fig. 1). This PTM has been shown to greatly enhance immune recognition of joint‐associated proteins, which are selectively targeted by autoreactive T and B cells in patients with rheumatoid arthritis (RA). However, analogous studies support the relevance and importance of this modification in other disease settings, including type 1 diabetes (T1D) and multiple sclerosis (MS). In this review we discuss the recognition of citrullinated antigens in human immune responses and the role that this modification plays in increasing antigenic diversity. We will detail the structural effects of citrullination on epitope presentation by autoimmune associated HLA‐DR proteins, the influence of environmental insults and inflammation on the process and resulting levels of citrullination, and the role that this may have in circumventing tolerance mechanisms. Finally, we will consider possible roles for citrullinated epitopes in the generation of broadly protective responses to viruses, address important unanswered questions in this area of research, and discuss the possibility that these pathways could be directly targeted for therapeutic intervention.
Figure 1.

Conversion of arginine to citrulline by peptidyl arginine deiminase. The conventional amino acid arginine is enzymatically converted into its polar analogue citrulline in a calcium‐dependent manner. This reaction consumes one water molecule and yields one ammonia, converting the positively charged ketamine group into a neutral ketone group.
Citrullination as a means for increasing antigenic diversity
The conversion of arginine to citrulline is an enzyme‐catalysed deimination that results in a modest (<1 Da) change in molecular mass.3 However, this conversion of the ketamine side chain to a neutral ketone group alters the overall charge and hydrogen bond capabilities of this amino acid, which can fundamentally alter its structural interactions with other proteins and, in some cases, the structure of the protein domain in which it resides. As such, at the level of intact proteins, citrullination can increase protein antigenicity by eliciting changes in the primary, secondary and tertiary structures of proteins which, in turn, can alter antigen processing, antigen presentation and immune recognition.2 Because citrullination alters the size and charge of amino acid side chains, this modification can alter signalling pathways and specific interactions with immune cells. Mounting evidence indicates that these biophysical changes elicit adaptive responses that preferentially target citrullated self‐proteins. As we will discuss further in the next section, such responses are prominently demonstrated by the selective recognition of citrullinated synovial proteins by autoantibodies in patients with RA. In particular, conversion of arginine to citrulline fundamentally alters the presentation of self‐peptides to CD4+ T cells. This alteration stems largely from the poor anchoring of arginine within some of the binding pockets of many HLA‐DR proteins. Arginine is essentially incapable of anchoring within the first binding pocket of all HLA DR proteins but this residue is also poorly accommodated elsewhere because of its charge and relatively large size.4 In contrast, as indicated in recent crystal structures of citrullinated self‐peptides within the binding cleft of HLA‐DR0401 (all of which adopt similar conformations), citrulline is more flexible and so is better accommodated than the unmodified arginine residue.5 In addition, the polar characteristics of citrulline provide a wider range of favourable electrostatic interactions between the peptide and amino acid side chains within certain HLA class II binding pockets, thereby increasing overall peptide‐binding affinity.6, 7
As summarized in Table 1, we have investigated the preferential binding of citrullinated peptides to various HLA‐DR proteins, including those that are strongly associated with genetic risk for developing autoimmune diseases such as RA, T1D and MS. Our results demonstrate that HLA‐DR proteins that are associated with RA risk (such as DRB1*04:01 and DRB1*15:01) prefer citrulline at key positions of their binding motifs.8 In contrast, HLA‐DR that are neutral or associated with reduced risk of RA show less of a preference for citrulline binding. We have also investigated citrulline binding for a limited number of HLA‐DQ proteins (Table 2) but for these we generally saw a preference for arginine over citrulline, suggesting that citrullination may have limited relevance for HLA‐DQ. The dichotomy between the capacity of citrulline and arginine to serve as an anchor residue for various binding pockets of certain HLA‐DR proteins creates a scenario in which peptides that are incapable of being presented in their unmodified form can be bound with appreciable affinity and presented in their citrullinated form. In addition, arginine residues at T‐cell receptor contact residues can modulate T‐cell recognition when modified. In cases where they are immunogenic to T cells, such peptides are termed ‘neo‐epitopes’ and are thought to be less subject to conventional tolerance mechanisms. Given these phenomena and the relative abundance of arginine residues present on many proteins, citrullination can play a large role in the generation of a more diverse array of self‐peptides that can be presented to CD4+ T cells.
Table 1.
Modulated binding of citrulline residues to HLA‐DR proteins
| HLA allele | Modification | P1 | P4 | P6 | P7 | P9 |
|---|---|---|---|---|---|---|
| DRB1*01:01 | Arg →Cit | ND | + | − | − | + |
| DRB1*04:01 | Arg →Cit | ND | + | ND | + | + |
| DRB1*04:02 | Arg →Cit | ND | ND | ND | ND | + |
| DRB1*04:04 | Arg →Cit | ND | + | ND | + | + |
| DRB1*07:01 | Arg →Cit | ND | ND | + | + | + |
| DRB1*10:01 | Arg →Cit | ND | + | ND | + | + |
| DRB1*15:01 | Arg →Cit | ND | + | ND | + | + |
| DRB5*01:01 | Arg →Cit | ND | + | ND | + | − |
+ indicates a significant increase in binding affinity.
− indicates a significant decrease in binding affinity.
ND indicates no difference in binding affinity.
Table 2.
Modulated binding of citrulline residues to HLA‐DQ proteins
| HLA allele | Modification | P1 | P4 | P6 | P7 | P9 |
|---|---|---|---|---|---|---|
| DQB1*02:01 | Arg →Cit | − | − | − | − | ND |
| DQB1*03:02 | Arg →Cit | ND | ND | − | − | ND |
− indicates a significant decrease in binding affinity.
ND indicates no difference in binding affinity.
Recognition of citrullinated epitopes in RA
The role of citrullination in autoimmune disease is perhaps best understood and most extensively studied in the context of RA.9, 10, 11 Anti‐citrullinated protein antibodies (ACPAs) are a constellation of partially cross‐reactive autoantibodies that recognize various citrullinated proteins including fillagrin (also known as anti‐perinuclear factor or anti‐keratin),12, 13, 14 vimentin,15 α‐enolase,16 collagen17 and fibrinogen.18 Notably, the anti‐cyclic citrullinated peptide assay, which provides a standardized means of measuring ACPA titres, provides the most relevant diagnostic biomarker for RA in the clinic. ACPAs are detected in 50–70% of RA patients before onset,19 and the fragments of citrullinated proteins that they recognize are reported to be present at elevated levels in inflamed synovial fluid.20 ACPAs are disease specific, but can be detected years before disease progression, implicating an early role for the recognition of citrullinated proteins and peptides in disease aetiology.21, 22, 23
It is established that the occurrence of ACPA in RA patients is further enriched in patients with HLA haplotypes that include a specific pattern of residues (either QKRAA or QRRAA) at positions 70–74 of their HLA‐DR β‐chain.24 A more recent study affirms that five distinct amino acids among the polymorphic residues that form the peptide‐binding grooves of HLA class II proteins (including β71 and β74) explain most of the HLA‐associated risk for RA.25 Because of its placement in the peptide binding cleft, this so‐called ‘shared epitope’ sequence dictates the preferences of the P4 binding pocket, conferring a positively charged region that excludes arginine but accommodates citrulline.6, 7 This in turn leads to a greater ability to bind the citrullinated versions of self‐peptides.5 The increased likelihood of ACPAs in RA patients with shared epitope haplotypes and the propensity for these HLA‐DR proteins to present citrullinated peptides suggests that CD4+ T‐cell responses to such epitopes actively contribute to disease. Indeed various studies have identified citrullinated vimentin,26, 27 aggrecan,28, 29 α‐enolase,30 and collagen31 peptides that elicit functional T‐cell responses in RA patients. Recognition of these citrullinated proteins by T cells has also been documented in HLA transgenic mice.26, 32 Recently, we directly characterized CD4+ T cells from RA patients that recognize citrullinated epitopes derived from four distinct antigens and showed that these citrulline‐specific T cells had an increased T helper type 1 memory polarization in RA patients and that their frequency was influenced by disease duration and biological therapy.33 As we will discuss later in this review, the PAD enzymes that affect protein citrullination are up‐regulated in response to stress and other environmental factors. Therefore, it is clear that the generation and recognition of citrullinated epitopes is an early event in the disease process that plays a prominent role in the loss of self‐tolerance in RA.
Recognition of citrullinated epitopes in T1D
Like RA, T1D is a T‐cell mediated autoimmune disease for which the appearance of autoantibodies is a relevant indicator for the risk of onset.34 Furthermore, there is significant overlap in genetic risk between these autoimmune diseases, including overlapping high‐risk HLA haplotypes.35 Mounting evidence supports the relevance of modified antigens in T1D. For example, it has been known for decades that an arginine residue of the insulin B chain can be citrullinated36 and a diversity of published evidence implies a possible role for modified epitopes in the development of T1D.2, 37, 38, 39 However, it was only recently demonstrated that citrullinated β cell antigens are selectively targeted by autoreactive T cells and autoantibodies in autoimmune diabetes. Our group recently reported that a citrullinated peptide derived from glutamic acid decarboxylase (GAD65) has enhanced recognition and elicits functional T‐cell responses in patients with T1D.40 Direct ex vivo analysis with the corresponding HLA class II tetramer indicated that citrullinated GAD65‐specific T cells have elevated frequencies in patients with T1D and an antigen‐experienced cell surface phenotype. Another recent study demonstrated the recognition of citrullinated glucose‐regulated protein 78 (GRP78), an endoplasmic reticulum chaperone protein that is also a putative antigen in RA, by autoantibodies in the NOD mice that have developed diabetes.41 Splenocytes isolated from these NOD mice produced interferon‐γ in response to citrullinated but not native GRP78, indicating selective recognition of the citrullinated protein by T cells. Furthermore, that study went on to demonstrate an up‐regulation of PAD2 in pancreatic islets, suggesting a mechanism through which inflammation and stress results in protein citrullination and loss of tolerance to β cell antigens.
Recognition of citrullinated epitopes in other settings
Both RA and T1D provide clear examples of HLA‐associated autoimmune diseases for which the preferential recognition of citrullinated antigens appears to play an important role in the loss of tolerance. MS provides another example of an HLA‐associated disease whose aetiology involves protein citrullination. The pathogenesis of MS is thought to occur by inflammation and immune mediated demyelination that progresses to cause dysfunction of the central nervous system and accompanying neurological symptoms.42 In inflamed neural tissue, the myelin sheath becomes compromised when myelin basic protein (MBP) is hyper‐citrullinated,43 leading to sheath instability. This hyper‐citrullination is thought to be caused by overexpression of PAD2 and possibly PAD444 that is caused at least in part by hypo‐methylation of the PAD2 promoter, which leads to dysregulation of the enzyme.45 In addition to these biophysical effects of citrullination, published evidence also suggests an immunological role for protein citrullination in MS. Dating back to the 1990s, it was shown that some T‐cell lines selectively respond to citrullinated MBP.46 A subsequent study demonstrated that stimulation with citrullinated MBP generated a higher number of T‐cell lines from MS patients and that these lines generally responded with greater sensitivity to citrullinated MBP than to the unmodified protein.47 An analogous study of antibody recognition of citrullinated MBP suggested that citrullinated MBP epitopes may be targeted by antibodies from the cerebrospinal fluid and sera of MS patients.48 As shown in Table 1, we have shown that DRB1*15:01 and DRB5*01:01, which comprise the DR2 haplotype that confers the greatest genetic risk for MS, preferentially present peptides that have been citrullinated at key HLA‐binding residues. Therefore, although definitive evidence remains lacking, it is highly probable that citrullinated neuronal antigens are preferentially presented by DRB1*15:01 and DRB5*01:01 and recognized by T cells and antibodies in MS patients.
Systemic lupus erythematosus (SLE) represents another setting in which immune recognition of citrullinated proteins may be relevant to disease. PAD4‐mediated citrullination of histones is a normal process that occurs in neutrophils upon bacterial invasion. Histone citrullination contributes to chromatin condensation and the formation of neutrophil extracellular traps (NETs), which are an integral part of the innate immune response to pathogens.49 In SLE, NET‐associated histones and other citrullinated proteins are often present at sites of inflammation.50 Therefore, it has been speculated that the citrullinated antigens, generated through NET formation and decorated by bacterial components that can act as adjuvants, play an important role in the induction of autoimmunity in SLE patients.51 However, studies of autoantibodies specific for citrullinated histones so far have not been conclusive. In one recent study, autoantibodies that bind to citrullinated histones were observed in the majority of patients with Felty syndrome but only in a subset of SLE patients.52 Another study observed that modest levels of reactivity to citrullinated histones were present in SLE patients but saw no significant disease association.53 Given that responses to citrullinated histone H4 peptides and to proteins extracted from NETs have been reported in RA54 it is plausible that such peptides are also immunogenic in SLE, but the role for these antigens in the disease has yet to be clearly elucidated.
Although there is an obvious role for responses to citrullinated self‐antigens in multiple autoimmune diseases, such responses may also contribute to protective immunity by expanding the repertoire of virus‐specific T cells. For example, through pioneering work using the hen egg‐white lysozyme model, it was shown that immunization of mice with this highly immunogenic protein elicits T cells that selectively respond to citrullinated peptides.55 In our own work, we have identified specific peptides derived from the haemagglutinin protein of an important influenza A strain that are selectively recognized by T cells. As shown in Fig. 2 we identified three distinct peptides (derived from the haemagglutinin protein of the pandemic influenza A/California/04/2009 strain) that elicited preferential responses in their citrullinated form in the context of DRB1*04:01 compared with the corresponding unmodified peptides (evidenced by the higher percentage of tetramer‐positive cells and brighter staining mean fluorescence intensity). As summarized in Table 3, each of the identified sequences contains a highly conserved positively charged residue that is predicted to reside within a binding pocket that favours the binding of citrulline. Interestingly, analysis of T‐cell responses following influenza virus infection, which should reflect a more inflammatory environment than vaccination, also confirmed the presence of influenza‐specific T cells that preferentially respond to these citrullinated peptides. Hence, it is possible that through the activity of PAD enzymes, tissue‐resident antigen‐presenting cells are able to unmask highly conserved T‐cell epitopes within viral proteins that facilitate adaptive immune responses against heterologous virus strains that have not been previously encountered.
Figure 2.
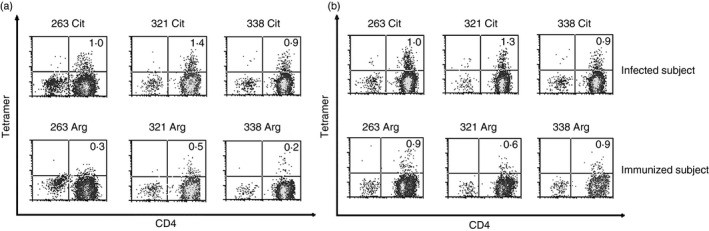
Selective T‐cell responses to citrullinated influenza peptides. (a) Representative T‐cell responses to citrullinated (upper panels) or unmodified (lower panels) influenza peptides, visualized using HLA class II tetramers loaded with the corresponding peptides using T‐cell lines that were expanded in vitro from the peripheral blood of immunized patients with DRB1*04:01 haplotypes. For analysis, cells were co‐stained using anti‐CD4 allophycocyanin. (b) Representative T‐cell responses to citrullinated influenza peptides for an influenza virus‐infected patient (upper panels) or immunized patient (lower panels).
Table 3.
Sequence conservation of influenza virus peptides that are recognized when citrullinated
| Influenza virus strain | HA 263 regiona | HA 321 regiona | HA 338 regiona |
|---|---|---|---|
| A/Texas/50/2012 (H3N2) | GNLIAPRGYFKIRSG | PRYVKQSTLKLATGM | MRNVPEKQTRGIFGA |
| A/Victoria/361/2011 (H3N2) | GNLIAPRGYFKIRSG | PRYVKQSTLKLATGM | MRNVPEKQTRGIFGA |
| A/Perth/16/2009 (H3N2) | GNLIAPRGYFKIRSG | PRYVKQNTLKLATGM | MRNVPEKQTRGIFGA |
| A/California/04/2009 (H1N1) | GNLVVPRYAFAMERN | PKYVKSTKLRLATGL | LRNIPSIQSRGLFGA |
| A/Brisbane/59/2007 (H1N1) | GNLIAPRYAFALSRG | PKYVRSAKLRMVTGL | LRNIPSIQSRGLFGA |
| A/Brisbane/10/2007 (H3N2) | GNLIAPRGYFKIRSG | PRYVKQNTLKLATGM | MRNVPEKQTRGIFGA |
| A/Solomon Islands/3/2006 (H1N1) | GNLIAPRYAFALSRG | PKYVRSAKLRMVTGL | LRNIPSIQSRGLFGA |
| A/Wisconsin/67/2005 (H3N2) | GNLIAPRGYFKIRSG | PRYVKQNTLKLATGM | MRNVPEKQTRGIFGA |
| Citrullianted 2009 H1N1b | GNLVVPXYAFAMERN | PKYVKSTKLXLATGL | LRNIPSIQSXGLFGA |
X denotes citrulline.
Each predicted citrullinated binding register is underlined.
Mechanistic importance of responses or citrullinated proteins
The PAD family consists of five distinct isoforms with varying distribution and function throughout the human body.56, 57 The calcium ion concentration that is required for optimal PAD activity has been shown to be in the micromolar range,57, 58 which would exceed typical intracellular levels. This would seem to indicate that an appropriate physiological stimulus must be provided to elicit protein citrullination. It is known that some degree of protein citrullination is carried out as an essential part of normal physiological processes. For example, the citrullination of proteins such as trichohyalin is essential for the formation of hair follicles in the skin.59 However, abnormally elevated levels of protein citrullination (termed hypercitrullination) can occur in response to various environmental stresses. Triggering events such as viral infections60 and reactive oxygen species61 can disrupt calcium gradients, potentially leading to increased PAD enzyme activity and hypercitrullination of endogenous proteins. In RA, several environmental factors that are associated with disease are thought to increase protein citrullination include smoking,11, 62 coal particle exposure63 and periodontal disease.64, 65 In T1D pancreatic β cells become overworked and experience heightened endoplasmic reticulum stress66, 67 as they are confronted with up to 50‐fold51 dynamic increases in demand for insulin production to maintain blood glucose levels.68, 69 Therefore, both diseases involve known environmental and physiological events that would be expected to elevate PAD activity. Speaking more generally, PAD dysregulation represents an intriguing link between environmental stresses, inflammation and the breakdown of peripheral tolerance through the generation of a unique class of epitopes that may not be subject to conventional central tolerance mechanisms.
One key unanswered question is the timing of responses in the overall autoimmune process. It could be argued that responses to modified self‐antigens could be the earliest initiating event in the loss of tolerance because neo‐epitopes may not be appreciably present in the thymus. However, it is equally plausible that tolerance may first be lost to an initiating ‘primary’ self‐antigen, after which ongoing responses contribute to the up‐regulation of PAD enzymes, peptide citrullination, and additional waves of autoimmune attack, epitope spreading and disease progression. Differentiating between these two models will require longitudinal study of at‐risk individuals carrying disease‐associated HLA alleles.
Another key unanswered question about this process is the precise pathway through which citrullinlated antigens are generated. There is contrasting evidence to support that either PAD activity in immune cells or in target cells may play the primary role. Data from the hen egg‐white lysozyme model supports the importance of intracellular citrullination by antigen‐presenting cells such as macrophages through their processing of proteins in autophagy vesicles, in that interruption of this pathway through treatment with 3‐methyladenine was shown to disrupt presentation of citrullinated but not unmodified hen egg‐white lysozyme peptides.70 This harmonizes with earlier studies showing PAD expression in infiltrating immune cells but not synovial tissue.71 In contrast, a recent study demonstrated that hypercitrullination in RA patients occurs as a result of immune‐mediated membranolytic pathways through which cytotoxic cells elicit hypercitrullination in their target cells mediated by perforin or membrane attack complex formation.72 In both scenarios, self‐antigens are citrullintated by PADs to generate epitopes and responses that break tolerance, but each pathway has different implications about modalities that should be pursued for effective treatment. In either scenario, PAD inhibitors that are specific for PAD2 or PAD4 (such as those that are currently under development) may be an effective means for halting this process.73
Conclusions and future prospects
Given what we have summarized here, accumulating evidence suggests that protein citrullination in response to biochemical stresses and inflammation initiates an immunological process that can unmask modified‐self epitopes, leading to a loss of self‐tolerance. More generally, citrullination expands epitope diversity by creating neo‐epitopes that elicit enhanced recognition by T cells and antibodies in a variety of contexts, including autoimmune diseases such as RA, T1D and MS as well as protective responses against heterologous virus strains. Autoimmune patients have been shown to have antibodies against citrullinated antigens and elevated frequencies of T cells that respond to citrullinated epitopes derived from tissue‐specific antigens, which can be monitored to draw inferences about disease risk and progression. Therefore, characterization of T‐cell and antibody responses to citrullinated self‐antigens in additional autoimmune settings such as SLE may be informative. As immune recognition of citrullinated epitopes reflects an important and distinct disease pathway, pursuit of treatment options, such as specific PAD inhibitors, may be an important avenue for therapeutic intervention.
Disclosures
The authors declare no competing interests.
References
- 1. Karve TM, Cheema AK. Small changes huge impact: the role of protein posttranslational modifications in cellular homeostasis and disease. J Amino Acids 2011; 2011:207691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Doyle HA, Mamula MJ. Autoantigenesis: the evolution of protein modifications in autoimmune disease. Curr Opin Immunol 2012; 24:112–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gyorgy B, Toth E, Tarcsa E, Falus A, Buzas EI. Citrullination: a posttranslational modification in health and disease. Int J Biochem Cell Biol 2006; 38:1662–77. [DOI] [PubMed] [Google Scholar]
- 4. James EA, Moustakas AK, Berger D, Huston L, Papadopoulos GK, Kwok WW. Definition of the peptide binding motif within DRB1*1401 restricted epitopes by peptide competition and structural modeling. Mol Immunol 2008; 45:2651–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scally SW, Petersen J, Law SC, Dudek NL, Nel HJ, Loh KL et al A molecular basis for the association of the HLA‐DRB1 locus, citrullination, and rheumatoid arthritis. J Exp Med 2013; 210:2569–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hill JA, Southwood S, Sette A, Jevnikar AM, Bell DA, Cairns E. Cutting edge: the conversion of arginine to citrulline allows for a high‐affinity peptide interaction with the rheumatoid arthritis‐associated HLA‐DRB1*0401 MHC class II molecule. J Immunol 2003; 171:538–41. [DOI] [PubMed] [Google Scholar]
- 7. Hill JA, Bell DA, Brintnell W, Yue D, Wehrli B, Jevnikar AM et al Arthritis induced by posttranslationally modified (citrullinated) fibrinogen in DR4‐IE transgenic mice. J Exp Med 2008; 205:967–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. James EA, Moustakas AK, Bui J, Papadopoulos GK, Bondinas G, Buckner JH et al HLA‐DR1001 presents “altered‐self” peptides derived from joint‐associated proteins by accepting citrulline in three of its binding pockets. Arthritis Rheum 2010; 62:2909–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Venrooij WJ, Pruijn GJM. Citrullination: a small change for a protein with great consequences for rheumatoid arthritis. Arthritis Res 2000; 2:249–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Imboden JB. The immunopathogenesis of rheumatoid arthritis. Annu Rev Pathol‐Mech Dis 2009; 4:417–34. [DOI] [PubMed] [Google Scholar]
- 11. Klareskog L, Malmstrom V, Lundberg K, Padyukov L, Alfredsson L. Smoking, citrullination and genetic variability in the immunopathogenesis of rheumatoid arthritis. Semin Immunol 2011; 23:92–8. [DOI] [PubMed] [Google Scholar]
- 12. Nienhuis RL, Mandema E. New serum factor in patients with rheumatoid arthritis – antiperinuclear factor. Ann Rheum Dis 1964; 23:302–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Young BJJ, Mallya RK, Leslie RDG, Clark CJM, Hamblin TJ. Anti‐keratin antibodies in rheumatoid‐arthritis. Br Med J 1979; 2:97–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vincent C, Nogueira L, Sebbag M, Chapuy‐Regaud S, Arnaud M, Letourneur O et al Detection of antibodies to deiminated recombinant rat. filaggrin by enzyme‐linked immunosorbent assay – a highly effective test for the diagnosis of rheumatoid arthritis. Arthritis Rheum 2002; 46:2051–8. [DOI] [PubMed] [Google Scholar]
- 15. Szekanecz Z, Soós L, Szabó Z, Fekete A, Kapitány A, Végvári A et al Anti‐citrullinated protein antibodies in rheumatoid arthritis: as good as it gets? Clin Rev Allergy Immunol 2008; 34:26–31. [DOI] [PubMed] [Google Scholar]
- 16. Lundberg K, Kinloch A, Fisher BA, Wegner N, Wait R, Charles P et al Antibodies to citrullinated α‐enolase peptide 1 are specific for rheumatoid arthritis and cross‐react with bacterial enolase. Arthritis Rheum 2008; 58:3009–19. [DOI] [PubMed] [Google Scholar]
- 17. Burkhardt H, Sehnert B, Bockermann R, Engström A, Kalden JR, Holmdahl R. Humoral immune response to citrullinated collagen type II determinants in early rheumatoid arthritis. Eur J Immunol 2005; 35:1643–52. [DOI] [PubMed] [Google Scholar]
- 18. Hill JA, Al‐Bishri J, Gladman DD, Cairns E, Bell DA. Serum autoantibodies that bind citrullinated fibrinogen are frequently found in patients with rheumatoid arthritis. J Rheumatol 2006; 33:2115–9. [PubMed] [Google Scholar]
- 19. van de Stadt LA, de Koning M, van de Stadt RJ, Wolbink G, Dijkmans BAC, Hamann D et al Development of the anti‐citrullinated protein antibody repertoire prior to the onset of rheumatoid arthritis. Arthritis Rheum 2011; 63:3226–33. [DOI] [PubMed] [Google Scholar]
- 20. Raijmakers R, van Beers JJ, El‐Azzouny M, Visser NF, Božič B, Pruijn GJ et al Elevated levels of fibrinogen‐derived endogenous citrullinated peptides in synovial fluid of rheumatoid arthritis patients. Arthritis Res Ther 2012; 14:R114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nielen MMJ, van Schaardenburg D, Reesink HW, van de Stadt RJ, van der Horst‐Bruinsma IE, de Koning M et al Specific autoantibodies precede the symptoms of rheumatoid arthritis – a study of serial measurements in blood donors. Arthritis Rheum 2004; 50:380–6. [DOI] [PubMed] [Google Scholar]
- 22. van Venrooij WJ, Hazes JM, Visser H. Anticitrullinated protein/peptide antibody and its role in the diagnosis and prognosis of early rheumatoid arthritis. Neth J Med 2002; 60:383–8. [PubMed] [Google Scholar]
- 23. Rantapaa‐Dahlqvist S, de Jong BAW, Berglin E, Hallmans G, Wadell G, Stenlund H et al Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum 2003; 48:2741–9. [DOI] [PubMed] [Google Scholar]
- 24. Gregersen PK, Silver J, Winchester RJ. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum 1987; 30:1205–13. [DOI] [PubMed] [Google Scholar]
- 25. Eyre S, Bowes J, Diogo D, Lee A, Barton A, Martin P et al High‐density genetic mapping identifies new susceptibility loci for rheumatoid arthritis. Nat Genet 2012; 44:1336–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Feitsma AL, van der Voort EIH, Franken K, el Bannoudi H, Elferink BG, Drijfhout JW et al Identification of citrullinated vimentin peptides as T cell epitopes in HLA‐DR4‐positive patients with rheumatoid arthritis. Arthritis Rheum 2010; 62:117–25. [DOI] [PubMed] [Google Scholar]
- 27. Snir O, Rieck M, Gebe JA, Yue BB, Rawlings CA, Nepom G et al Identification and functional characterization of T cells reactive to citrullinated vimentin in HLA‐DRB1*0401‐positive humanized mice and rheumatoid arthritis patients. Arthritis Rheum 2011; 63:2873–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. von Delwig A, Locke J, Robinson JH, Ng W‐F. Response of Th17 cells to a citrullinated arthritogenic aggrecan peptide in patients with rheumatoid arthritis. Arthritis Rheum 2010; 62:143–9. [DOI] [PubMed] [Google Scholar]
- 29. Law SC, Street S, Yu CH, Capini C, Ramnoruth S, Nel HJ et al T‐cell autoreactivity to citrullinated autoantigenic peptides in rheumatoid arthritis patients carrying HLA‐DRB1 shared epitope alleles. Arthritis Res Ther 2012; 14:R118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pieper J, Rieck M, James EA, Sandin C, Klareskog L, Buckner J et al α‐Enolase specific T cells in rheumatoid arthritis – a MHC class II tetramer approach. Ann Rheum Dis 2012; 71:A33–4. [Google Scholar]
- 31. Chemin K, Pollastro S, James E, Ge C, Albrecht I, Herrath J et al A novel HLA‐DRB1*10:01‐restricted T cell epitope from citrullinated type II collagen relevant to rheumatoid arthritis. Arthritis Rheumatol 2016; 68:1124–35. [DOI] [PubMed] [Google Scholar]
- 32. Cordova KN, Willis VC, Haskins K, Holers VM. A citrullinated fibrinogen‐specific T cell line enhances autoimmune arthritis in a mouse model of rheumatoid arthritis. J Immunol 2013; 190:1457–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. James EA, Rieck M, Pieper J, Gebe JA, Yue BB, Tatum M et al Citrulline‐specific Th1 cells are increased in rheumatoid arthritis and their frequency is influenced by disease duration and therapy. Arthritis Rheumatol 2014; 66:1712–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sabbah E, Savola K, Kulmala P, Veijola R, Vähäsalo P, Karjalainen J et al Diabetes‐associated autoantibodies in relation to clinical characteristics and natural course in children with newly diagnosed type 1 diabetes. The Childhood Diabetes In Finland Study Group. J Clin Endocrinol Metab 1999; 84:1534–9. [DOI] [PubMed] [Google Scholar]
- 35. The Wellcome Trust Case Control Consortium . Genome‐wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 2007; 447:661–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hayashi H, Morioka M, Ichimiya S, Yamato K, Hinode D, Nagata A et al Participation of an arginyl residue of insulin chain B in the inhibition of hemagglutination by Porphyromonas gingivalis . Oral Microbiol Immunol 1993; 8:386–9. [DOI] [PubMed] [Google Scholar]
- 37. Mannering SI, Harrison LC, Williamson NA, Morris JS, Thearle DJ, Jensen KP et al The insulin A‐chain epitope recognized by human T cells is posttranslationally modified. J Exp Med 2005; 202:1191–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Delong T, Baker RL, He J, Barbour G, Bradley B, Haskins K. Diabetogenic T‐cell clones recognize an altered peptide of chromogranin A. Diabetes 2012; 61:3239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van Lummel M, Duinkerken G, van Veelen PA, de Ru A, Cordfunke R, Zaldumbide A et al Posttranslational modification of HLA‐DQ binding islet autoantigens in type 1 diabetes. Diabetes 2014; 63:237–47. [DOI] [PubMed] [Google Scholar]
- 40. McGinty JW, Chow IT, Greenbaum C, Odegard J, Kwok WW, James EA. Recognition of posttranslationally modified GAD65 epitopes in subjects with type 1 diabetes. Diabetes 2014; 63:3033–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rondas D, Crèvecoeur I, D'Hertog W, Ferreira GB, Staes A, Garg AD et al Citrullinated glucose‐regulated protein 78 is an autoantigen in type 1 diabetes. Diabetes 2015; 64:573–86. [DOI] [PubMed] [Google Scholar]
- 42. Moscarello MA, Wood DD, Ackerley C, Boulias C. Myelin in multiple sclerosis is developmentally immature. J Clin Invest 1994; 94:146–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wood DD, Bilbao JM, Oconnors P, Moscarello MA. Acute multiple sclerosis (Marburg type) is associated with developmentally immature myelin basic protein. Ann Neurol 1996; 40:18–24. [DOI] [PubMed] [Google Scholar]
- 44. Mastronardi FG, Wood DD, Mei J, Raijmakers R, Tseveleki V, Dosch HM et al Increased citrullination of histone H3 in multiple sclerosis brain and animal models of demyelination: a role for tumor necrosis factor‐induced peptidylarginine deiminase 4 translocation. J Neurosci 2006; 26:11387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mastronardi FG, Noor A, Wood DD, Paton T, Moscarello MA. Peptidyl argininedeiminase 2 CpG island in multiple sclerosis white matter is hypomethylated. J Neurosci Res 2007; 85:2006–16. [DOI] [PubMed] [Google Scholar]
- 46. Martin R, Whitaker JN, Rhame L, Goodin RR, McFarland HF. Citrulline‐containing myelin basic protein is recognized by T‐cell lines derived from multiple sclerosis patients and healthy individuals. Neurology 1994; 44:123–9. [DOI] [PubMed] [Google Scholar]
- 47. Tranquill LR, Cao L, Ling NC, Kalbacher H, Martin RM, Whitaker JN. Enhanced T cell responsiveness to citrulline‐containing myelin basic protein in multiple sclerosis patients. Mult Scler 2000; 6:220–5. [DOI] [PubMed] [Google Scholar]
- 48. de Seze J, Dubucquoi S, Lefranc D, Virecoulon F, Nuez I, Dutoit V et al IgG reactivity against citrullinated myelin basic protein in multiple sclerosis. J Neuroimmunol 2001; 117:149–55. [DOI] [PubMed] [Google Scholar]
- 49. Wang Y, Li M, Stadler S, Correll S, Li P, Wang D et al Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol 2009; 184:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dwivedi N, Radic M. Citrullination of autoantigens implicates NETosis in the induction of autoimmunity. Ann Rheum Dis 2014; 73:483–91. [DOI] [PubMed] [Google Scholar]
- 51. Dwivedi N, Neeli I, Schall N, Wan H, Desiderio DM, Csernok E et al Deimination of linker histones links neutrophil extracellular trap release with autoantibodies in systemic autoimmunity. FASEB J 2014. Jul; 28(7):2840–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dwivedi N, Upadhyay J, Neeli I, Khan S, Pattanaik D, Myers L et al Felty's syndrome autoantibodies bind to deiminated histones and neutrophil extracellular chromatin traps. Arthritis Rheum 2012; 64:982–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Liu CL, Tangsombatvisit S, Rosenberg JM, Mandelbaum G, Gillespie EC, Gozani OP et al Specific post‐translational histone modifications of neutrophil extracellular traps as immunogens and potential targets of lupus autoantibodies. Arthritis Res Ther 2012; 14:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pratesi F, Dioni I, Tommasi C, Alcaro MC, Paolini I, Barbetti F et al Antibodies from patients with rheumatoid arthritis target citrullinated histone 4 contained in neutrophils extracellular traps. Ann Rheum Dis 2014; 73:1414–22. [DOI] [PubMed] [Google Scholar]
- 55. Ireland J, Herzog J, Unanue ER. Cutting edge: unique T cells that recognize citrullinated peptides are a feature of protein immunization. J Immunol 2006; 177:1421–5. [DOI] [PubMed] [Google Scholar]
- 56. Baka Z, Gyorgy B, Geher P, Buzas EI, Falus A, Nagy G. Citrullination under physiological and pathological conditions. Joint Bone Spine 2012; 79:431–6. [DOI] [PubMed] [Google Scholar]
- 57. Vossenaar ER, Zendman AJW, van Venrooij WJ, Pruijn GJM. PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. BioEssays 2003; 25:1106–18. [DOI] [PubMed] [Google Scholar]
- 58. Takahara H, Okamoto H, Sugawara K. Calcium‐dependent properties of peptidylarginine deiminase from rabbit skeletal muscle. Agric Biol Chem 1986; 50:2899–904. [Google Scholar]
- 59. Tarcsa E, Marekov LN, Andreoli J, Idler WW, Candi E, Chung SI et al The fate of trichohyalin. Sequential post‐translational modifications by peptidyl‐arginine deiminase and transglutaminases. J Biol Chem 1997; 272:27893–901. [DOI] [PubMed] [Google Scholar]
- 60. van Kuppeveld FJ, de Jong AS, Melchers WJ, Willems PH. Enterovirus protein 2B po(u)res out the calcium: a viral strategy to survive? Trends Microbiol 2005; 13:41–4. [DOI] [PubMed] [Google Scholar]
- 61. Gorlach A, Klappa P, Kietzmann T. The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxid Redox Signal 2006; 8:1391–418. [DOI] [PubMed] [Google Scholar]
- 62. Makrygiannakis D, Hermansson M, Ulfgren AK, Nicholas AP, Zendman AJ, Eklund A et al Smoking increases peptidylarginine deiminase 2 enzyme expression in human lungs and increases citrullination in BAL cells. Ann Rheum Dis 2008; 67:1488–92. [DOI] [PubMed] [Google Scholar]
- 63. Miall WE, Caplan A, Cochrane AL, Kilpatrick GS, Oldham PD. An epidemiological study of rheumatoid arthritis associated with characteristic chest x‐ray appearances in coal‐workers. Br Med J 1953; 2:1231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Arkema EV, Karlson EW, Costenbader KH. A prospective study of periodontal disease and risk of rheumatoid arthritis. J Rheumatol 2010; 37:1800–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bingham CO 3rd, Moni M. Periodontal disease and rheumatoid arthritis: the evidence accumulates for complex pathobiologic interactions. Curr Opin Rheumatol 2013; 25:345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Araki E, Oyadomari S, Mori M. Endoplasmic reticulum stress and diabetes mellitus. Intern Med 2003; 42:7–14. [DOI] [PubMed] [Google Scholar]
- 67. Volchuk A, Ron D. The endoplasmic reticulum stress response in the pancreatic β‐cell. Diabetes Obes Metab 2010; 12:48–57. [DOI] [PubMed] [Google Scholar]
- 68. Scheuner D, Kaufman RJ. The unfolded protein response: a pathway that links insulin demand with β‐cell failure and diabetes. Endocr Rev 2008; 29:317–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lipson KL, Fonseca SG, Urano F. Endoplasmic reticulum stress‐induced apoptosis and autoimmunity in diabetes. Curr Mol Med 2006; 2006(6):71–7. [DOI] [PubMed] [Google Scholar]
- 70. Ireland JM, Unanue ER. Autophagy in antigen‐presenting cells results in presentation of citrullinated peptides to CD4 T cells. J Exp Med 2011; 208:2625–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Vossenaar ER, Nijenhuis S, Helsen MM, van der Heijden A, Senshu T, van den Berg WB et al Citrullination of synovial proteins in murine models of rheumatoid arthritis. Arthritis Rheum 2003; 48:2489–500. [DOI] [PubMed] [Google Scholar]
- 72. Romero V, Fert‐Bober J, Nigrovic PA, Darrah E, Haque UJ, Lee DM et al Immune‐mediated pore‐forming pathways induce cellular hypercitrullination and generate citrullinated autoantigens in rheumatoid arthritis. Sci Transl Med 2013; 5:209ra150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bicker KL, Anguish L, Chumanevich AA, Cameron MD, Cui X, Witalison E et al D‐amino acid based protein arginine deiminase inhibitors: synthesis, pharmacokinetics, and in cellulo efficacy. ACS Med Chem Lett 2012; 3:1081–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


