Abstract
Vegetative but not reproductive stage of Saposhnikovia divaricate (Turxz.) schischk possesses pharmacological activities. However, our recent study showed that reproductive S. divaricate supplemented with polysaccharide showed evidently elevated pharmacological activities and increased cimifugin content in rat serum. The aims of present study were to assess the influence of polysaccharides on the chromones pharmacological activities in Radix Saposhnikoviae (RS), the dried root of vegetative stage of S. divaricate, and to explore the underlying mechanisms. Only cimifugin was detected in the plasma of chromone treated animals and RS polysaccharide significantly increased the plasma content of cimifugin. It was shown that neither cimifugin absorption nor glycoside components transformation in simulated digestive fluid was affected by RS polysaccharide. However, a significant promotion of transformation of cimifugin to more stable prime-O-glucosylcimifugin (PGCN) by RS polysaccharide, and a protective effect of polysaccharide on chromone components were observed in small intestine solutions. Meanwhile, RS polysaccharide produced a significant elevation of cimifugin and PGCN concentration in vivo. Based on these findings, we concluded that RS polysaccharide could greatly increase the content of cimifugin, which might be related to its degradation-proof effect on cimifugin, via transforming cimifugin to comparatively more stable PGCN and spatial structure protection.
Radix Saposhnikoviae (RS) is the dried root of vegetative stage of perennial herb Saposhnikovia divaricate (Turxz.) schischk in family Umbelliferae, and is commonly used for treating febrility, rheumatism, headache, vertigo, generalized aching, convulsion, arthralgia and inflammatory symptoms for thousands of years in China, Japan, and Korea1,2,3,4,5. Modern scientific experiments have also confirmed the beneficial effects of RS using its extracts1,5,6,7. RS contains various bioactive substances, such as chromones, coumarins, polyacetylenes, and polysaccharides. Among them, chromones and coumarins are the main components of RS4,8,9. Furthermore, chromones have also been identified as the main bioactive constituents with most-evident pharmacological activities as they possess potent analgesic, antifebric, anti-inflammatory and immune-regulatory effects1,5.
Currently, three major types of chromones were found in RS, including prime-O-glucosylcimifugin (PGCN), 4′-O-β-D-glucosyl-5-O-methylvisamminol (GML), and cimifugin (Fig. 1). In Chinese pharmacopoeia, PGCN and GML are designated as marker compounds for RS quality control due to their relatively high content and GML is also used for the identification of RS in Japanese Pharmacopoeia3,4,10,11. Cimifugin is aglycone of PGCN and has much lower content than that of PGCN and GML. Huge amount of cimifugin and trace amount of PGCN and GML were detected in the plasma sample of overdose chromone4,12. Generally, cimifugin is regarded as the pharmacologically active form of inactive chromone derivatives such as PGCN and GML, which are transformed into bioavailable cimifugin in the gastrointestinal tract4,7,11,12.
In addition to chromones, several other types of components were also identified in RS, including polysaccharides, coumarins, volatile oils, polyacetylenes, organic acids and so on4,8,9. Among these constituents, polysaccharides represent as immune-regulatory and antineoplastic components in RS13,14. Apart from its direct pharmacological activities, mounting researches showed that polysaccharides might indirectly effected on other active ingredients in medicinal herbs via influencing their pharmacokinetic process. For example, previous report showed that gastrodin, an effective constituent in Chinese traditional medical herb Rhizoma gastrodiae, exhibited greatly decreased retention time and serum content when polysaccharides were removed from this plant15. A significant increase in absorption and decrease in elimination of the effective components in Schisandra chinensis was also observed when this herb was administered in combination with polysaccharide-rich Radix ophiopogonis16. In our present study, we are focusing on RS (traditionally the officinal part is the dried root of its vegetative stage). Once entering the stage of reproductive growth, its medicinal value becomes nil. Intriguingly, relevant research revealed that it was the content of polysaccharide rather than active chromone substances that greatly decreased in reproductive stage of RS17. Furthermore, our recent study showed that when supplemented with polysaccharides, reproductive stage of RS showed enhanced antipyretic, analgesic, anti-inflammatory activities with evidently elevated content of cimifugin in rat serum18. Based on these facts, we reckoned that polysaccharide component might play an essential role when RS exerting its pharmacological activity, perhaps via improving the pharmacokinetic process of chromone ingredients. Therefore, the aims of present study were to determine the beneficial effect of RS polysaccharides on chromone pharmacokinetics and to clarify the underlying mechanisms.
Figure 1. Chemical structures of 4′-O-β-D-glucosyl-5-O-methylvisamminol (GML), cimifugin, and prime-O-glucosylcimifugin (PGCN).
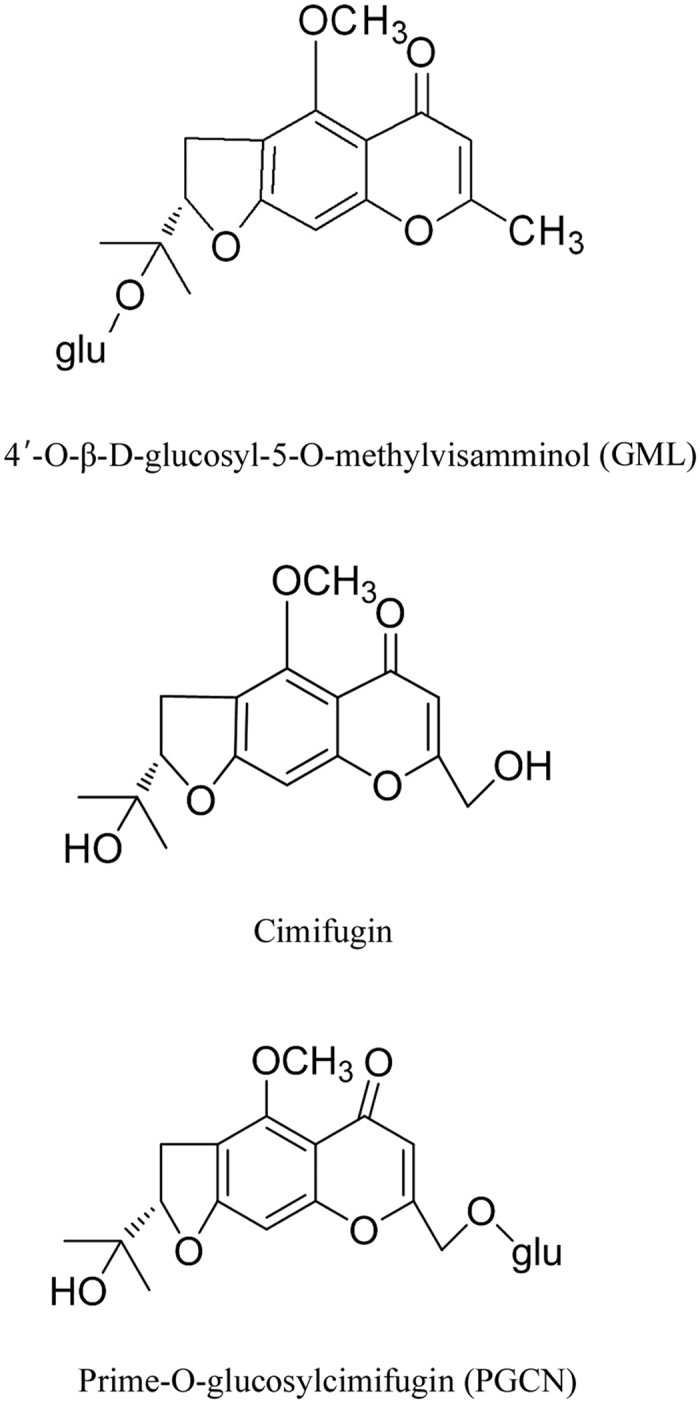
Figure 2. The concentration-time curve of cimifugin in rat plasma after oral administration of chromone extracts in the presence of glucuronic acid or Radix Saposhnikoviae (RS) polysaccharides.
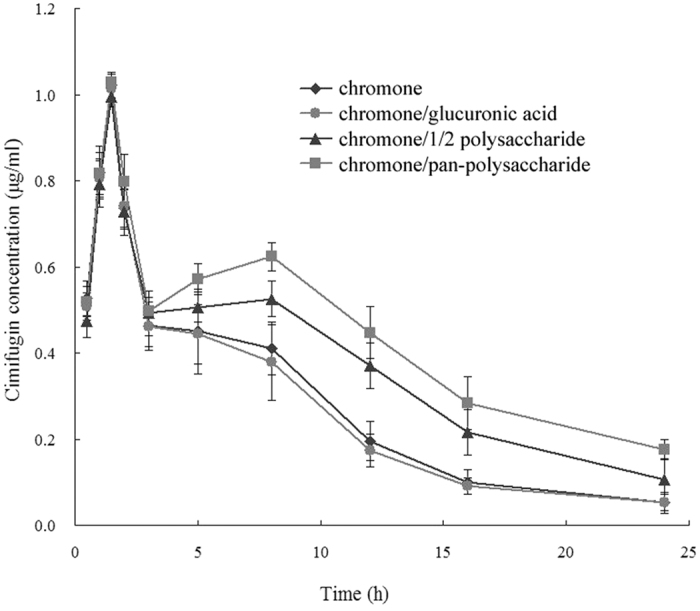
The rats were orally administered with chromone, chromone/glucuronic acid (CG), chromone/1/2 polysaccharie (CHP), and chromone/pan-polysaccharide (CP), respectively. Blood samples from rats were then collected 0.5, 1, 1.5, 2, 3, 5, 8, 12, 16, and 24 h following the drug delivery for high performance liquid chromatography (HPLC) analysis.
Materials and Methods
Reagents
Cimifugin, prime-O-glucosylcimifugin (PGCN), and 4′-O-β-D-glucosyl-5-O-methylvisamminol (GML), with all ≥ 98% purity, were provided by Shanghai Jinsui Bio-Technology Co., Ltd (Shanghai, China). LY335979 (Zosuquidar Trihydrochloride) was provided by Shanghai Mingrong Bio Co., Ltd (Shanghai, China). Troleandomycin was bought from Hubei Jusheng Technology Co. Ltd. Glucose was obtained from Shanghai Jingke Chemical Technology Co., Ltd (Shanghai, China). Glucuronic acid was obtained from Shenyang Jiuye Chemical Material Co., Ltd (Shenyang, China). HPLC-grade methanol was purchased from Tianjin Guangfu Fine Chemical Research Institute (Tianjin, China). Pepsin was obtained from Sigma-Aldrich (St. Louis, MO, USA). Other chemicals and solvents were analytical purity grade. Distilled water prepared from demineralized water was used throughout the experiment.
Animals
Animal experiments were conducted in accordance with the guidelines of the National Institutes of Health (NIH guidelines) and approved by the Ethical Committee of Heilongjiang University of Chinese Medicine (approval number: HUCM2014-00348). Sprague-Dawley rats weighing 180~220 g were maintained under a 12/12h light/dark cycle and kept at controlled temperature (25 ± 1 °C) and humidity (70 ± 10%) conditions. The rats were provided free access to standard laboratory diet and water. All efforts were made to guarantee minimally used animal number and suffering. Not any adverse health effects on animals were found with the test compounds.
Sample preparation
RS, the roots of S.divaricata (Turcz) schischk, were bought from Beijing Tong Ren Tang Group Co. Ltd and identified by Professor Xiang-Cai Meng, College of Pharmacy, Heilongjiang University of Chinese Medicine. The chopped dried roots were extracted thrice with distilled water at the reflux temperature and the solutions were concentrated into viscous state. Subsequently, about seven fold of 95% ethanol was added, this ethanol solution was set aside overnight and then subjected to decompress filtration followed by separate collection of filtrated liquid and filter residue. The filtrated liquid, which were just the chromone components in RS, were divided into four equal aliquots for follow-up experiments. Then the filtrate residues were washed with 95% ethanol, acetone and petroleum ether successively and underwent deproteinization and decoloration. The decolorated residues were eluted with distilled water with the effluent collected when Molish reaction was positive. This collection was stopped when Molish reaction exhibited negative. After decompressed filtration and freeze-drying, a total of 8.44 g RS polysaccharide was obtained.
Pharmacokinetic measurement of chromone
To explore the influence of RS polysaccharide on the pharmacokinetics of chromones, 2.11 g, 1.05 g RS polysaccharide, and 2.11 g glucuronic acid were added to the three aliquots of chromone components, with one aliquot left as control. Thus, totally four test compounds were obtained, including chromone/pan-polysaccharide (CP), chromone/1/2polysaccharide (CHP), chromone/glucuronic acid (CG), and chromone, which served as control. Forty male Sprague-Dawley rats were used in this experiment. They were randomly divided into 4 groups, each consisting of 10 rats. The rats in these four groups were orally administered with CP, CHP, CG and chromones at the dose of 4 g crude drug (equivalent to 10 ml RS extracts) per kg body weight, respectively for five consecutive days. After the final drug delivery of day 5 (at 0.5, 1, 1.5, 2, 3, 5, 8, 12, 16, and 24 h/day), rats were anesthetized by ether inhalation and rat blood samples were obtained from the orbit vein and centrifuged immediately at 3,000 rpm for 10 min to yield plasma. Each plasma sample (100 μl) was mixed with 20 μl of 70% perchloric acid and vortexed and centrifuged at 3,000 rpm for 10 min. The supernatant were subjected to 0.45 μm microporous membrane and the filtrate was collected for subsequent high performance liquid chromatography (HPLC) analysis. Chromatographic separation and analysis using an L-2000 Elite HPLC system has been previously reported7. Briefly, the separation of the analytes were performed on a Kromasil C18 column (200 mm × 4.6 mm i.d., particle size 5 μm), equipped with a Shim-pack security guard column at a column temperature of 25 °C. The binary mobile phase consisted of methanol and water. A flow rate of 1.0 ml/min with a gradient elution program as follows: 40–45% methanol at 0–5 min; 45–60% methanol at 5–10 min; 60–80% methanol at 10–15 min; 80–95% methanol at 15–20 min; 95–40% methanol at 20–30 min. The injection volume was 20 μl and detection wavelength was kept at 254 nm.
Caco-2 cell culture and bidirectional transport assay
Caco-2 cells were obtained from the American Type Culture Collection (ATCC). Cells were maintained in high glucose DMEM media supplemented with L-glutamine, 10% fetal bovine serum, 1% nonessential amino acids and 1% penicillin/streptomycin in a humidified atmosphere of 5% CO2 at 37 °C. For the permeability test, cells were seeded in transwell supports with polycarbonate membrane (0.4 μm, 1.12 cm2, Corning Costar) at a density of 6 × 104 cells/cm2. The integrity of the monolayers was tested by measuring the transepithelial electrical resistance (TEER) before and after every experiment. Only monolayers with TEER >200 Ω/cm2 were used. Test compounds in transport buffer were added to the donor compartments of either apical (A → B) or basolateral (B → A) wells of the Insert System and incubated at 37 °C. Samples were collected at 0, 30, 60, 90, 120, and 150 min time points and analyzed for HPLC analysis of content of cimifugin as described above.
Metabolism studies of PGCN and GML in SGF and SIF
Simulated gastric fluid (SGF, pH 1.2) and simulated intestinal fluid (SIF, pH 6.8) were prepared based on Chinese Pharmacopeia (2010) and have been depicted previously7,9. 100 μg PGCN or GML was added to 4 ml of either SGF or SIF to yield 4 reaction systems, i.e., PGCN/SGF (213.47 nM), PGCN/SIF (213.47 nM), GML/SGF (220.99 nM), GML/SIF (220.99 nM), respectively. Polysaccharides extracted in RS were added to these 4 reaction systems in accordance with the actual chromone-polysaccharide proportion, with each reaction further became three sub-systems, i.e., 0 polysaccharide/chrome, 1/2 polysaccharide/chromone, and pan-polysaccharide/chromone. Meanwhile, these 12 systems were allowed to react at 37 °C. Following 2 h, the reaction was stopped by 100 °C boiling for 10 min. Subsequently, these systems were evaporated to dryness and the residue was reconstituted in 1 ml methanol. After centrifugation at 3,000 rpm for 10 min, the supernatant was collected and used for HPLC analysis.
Preparation of enzyme solution from small intestine and grouping
Small intestine segment was taken from ten 12 h-fasting male Sprague-Dawley rats. The intestine tissue was first frozen at −80 °C for 12 h, and then ground and centrifuged at 10,000 rpm for 10 min. The supernatant constituted the enzyme solution used in the experiment. To explore the influence of RS polysaccharide on the metabolism of cimifugin, three groups were designed: control group containing 40 μg cimifugin in 1.2 ml enzyme solution; RS polysaccharide/cimifugin group containing 40 μg cimifugin and 8.7 mg RS polysaccharide in 1.2 ml enzyme solution; glucuronic acid/cimifugin group containing 40 μg cimifugin and 8.7 mg glucuronic acid in 1.2 ml enzyme solution. These groups were put in 38 °C water bath for reaction, while samples were taken at 15 and 30 min. After deproteinization by methanol, these samples were centrifuged at 10,000 rpm for 10 min. Subsequently, the supernatant was collected and analyzed for HPLC analysis as described above.
Transport study of chromones across semi-permeable membrane in the absence or presence of RS polysaccharide
Dialysis bags made by semi-permeable membrane materials were used in this analysis. ~3 cm long dialysis bags were first processed as the following procedures in succession: 1) boiled for 5 min in distilled water; 2) rinsed by 60 °C water for 2 min; 3) rinsed by small intestine buffer for 2 min; 4) rinsed by small intestine buffer for 2 min; 5) immersed in buffer at 4 °C. After 12 h, these processed dialysis bags were tied tightly at one end to form a dialysis bag. 1 ml small intestine buffer (pH 7.8) containing cimifugin, PGCN, cimifugin + polysaccharide, or PGCN + polysaccharide was added into this bag and a glass bead was put in to keep this bag vertical. When the other end was tied (an air bubble was retained), this dialysis bag was suspended in 15 ml small intestine buffer. This system was left to stand for 6 h in 37 °C water bath and chromone content inside and outside of this semi-permeable membrane was measured by HPLC analysis.
Results
Pharmacokinetic study of chromone in rat plasma
In this study, HPLC qualitative analysis showed that only cimifugin was detected in the plasma of chromone treated rats in the whole process of the experiment (data not shown). Based on the qualitative results, cimifugin concentrations in rat plasma were further quantitatively measured. As seen in Fig. 2, the concentration-time relationship exhibited as a double-peak curve, with Tmax at 1.5 and 8 h, respectively. In the first three hours, not any difference of AUC value was found among the four groups as indicated in the figure, however, a significant distinction of AUC was seen among them at 3 h and later. Specifically, glucuronic acid did not affect the absorption process of chromone, but polysaccharide could significantly increase the absorption of chromone in the form of cimifugin. Moreover, pan-polysaccharide displayed comparatively greater effect than 1/2 polysaccharide.
Influence of RS polysaccharide on transport of cimifugin after apical or basolateral application
As seen in Table 1, a time-dependent flux of cimifugin from the apical-to-basolateral (A → B) direction was observed after apical application of cimifugin. This flux was significantly enhanced by pretreatment of troleandomycin, a specific inhibitor of CYP3A, but not affected by LY335979, a specific inhibitor of P-glycoprotein inhibitor. Similar results was observed when cimifugin was applied in basolateral chamber (Table 2, B → A transport), thereby suggesting a CYP3A4 activity-dependent process of cimifugin transport. RS polysaccharide produced no effect on cimifugin transport either in the presence or absence of inhibitors. As such, it was concluded that RS polysaccharide was unable to affect the flux across the intestine tract.
Table 1. Time-dependent transport of cimifugin in Caco-2 cell monolayers after apical application of different test compounds.
| Test Compound | Basolateral content of cimifugin (ng) |
||||
|---|---|---|---|---|---|
| 30 min | 60 min | 90 min | 120 min | 150 min | |
| A | 15.21 ± 2.34 | 30.85 ± 3.64 | 143.92 ± 12.32 | 236.01 ± 22.86 | 201.33 ± 15.32 |
| A/B | 13.53 ± 4.22 | 31.35 ± 4.74 | 137.11 ± 9.46 | 222.66 ± 18.43 | 212.51 ± 20.47 |
| A/C | 16.32 ± 1.97 | 31.37 ± 4.64 | 142.82 ± 11.63 | 231.42 ± 24.55 | 204.35 ± 24.52 |
| A/B/C | 16.53 ± 2.01 | 32.67 ± 4.4 | 144.76 ± 12.23 | 237.12 ± 21.85 | 200.43 ± 10.11 |
| A/D | 48.54 ± 3.57* | 110.59 ± 11.50* | 247.57 ± 17.00* | 316.65 ± 31.53* | 252.83 ± 20.35* |
| A/B/D | 46.36 ± 6.46* | 104.63 ± 14.89* | 258.54 ± 18.58* | 325.34 ± 25.61* | 249.25 ± 17.54* |
A: cimifugin; B: polysaccharide; C: LY335979; D: troleandomycin.
Data were presented as mean ± SD (n = 10). *P < 0.05 versus A group.
Table 2. Time-dependent transport of cimifugin in Caco-2 cell monolayers after basolateral application of different test compounds.
| Test compounds | Apical content of cimifugin (ng) |
||||
|---|---|---|---|---|---|
| 30 min | 60 min | 90 min | 120 min | 150 min | |
| A | 26.87 ± 1.54 | 50.45 ± 2.27 | 234.35 ± 11.36 | 354.24 ± 15.47 | 289.46 ± 15.45 |
| A/B | 23.42 ± 1.65 | 52.23 ± 3.62 | 246.53 ± 13.34 | 348.84 ± 21.34 | 294.33 ± 13.67 |
| A/C | 25.25 ± 2.23 | 52.32 ± 4.54 | 263.33 ± 20.42 | 344.44 ± 21.34 | 278.35 ± 23.34 |
| A/B/C | 24.35 ± 1.87 | 49.24 ± 3.64 | 246.53 ± 18.32 | 351.26 ± 20.11 | 282.42 ± 21.18 |
| A/D | 20.85 ± 1.68* | 44.24 ± 3.24* | 225.44 ± 17.66* | 332.64 ± 22.85* | 256.66 ± 18.47* |
| A/B/D | 21.99 ± 2.26* | 46.82 ± 3.26* | 233.85 ± 16.90* | 326.5 ± 16.87* | 253.65 ± 20.79* |
A: cimifugin; B: polysaccharide; C: LY335979; D: troleandomycin.
Data were presented as mean ± SD (n = 10). *P < 0.05 versus A group at the same time point.
Metabolism studies of PGCN and GML in SGF and SIF
The metabolic profiles of PGCN and GML in the absence and presence of RS polysaccharide in simulated gastrointestinal fluid were investigated. In SGF, a small amount of PCGN was converted into cimifugin, and a little GML was converted into PGCN (Table 3). In SIF, an overwhelmingly large amount of PGCN was transformed into cimifugin, and GML underwent no change (Table 4). Neither in SGF (Table 3) nor in SIF (Table 4), there was significant influence of polysaccharide on the transformation of these two glycosides in RS.
Table 3. Metabolic study of PGCN and GML in SGF in the absence and presence of RS polysaccharide.
| Test compounds | HPLC peak area |
||
|---|---|---|---|
| Cimifugin | PGCN | GML | |
| PGCN | 33145 ± 2233 | 966390 ± 35555 | N.A. |
| PGCN/1/2 Polysaccharide | 26204 ± 1425 | 931227 ± 23420 | N.A. |
| PGCN/Pan-polysaccharide | 36062 ± 2151 | 936065 ± 42384 | N.A. |
| GML | N.A. | 22814 ± 4238 | 1369608 ± 83550 |
| GML/1/2 Polysaccharide | N.A. | 23657 ± 2427 | 1389523 ± 93428 |
| GML/Pan-polysaccharide | N.A. | 22253 ± 2154 | 1218659 ± 42338 |
Data were presented as mean ± SD (n = 10).
Table 4. Metabolic study of PGCN and GML in SIF in the absence and presence of RS polysaccharide.
| Test compounds | HPLC peak area |
||
|---|---|---|---|
| Cimifugin | PGCN | GML | |
| PGCN | 1101759 ± 81002 | 5159 ± 1238 | N.A. |
| PGCN/1/2 Polysaccharide | 1131570 ± 103451 | 5698 ± 2214 | N.A. |
| PGCN/Pan-polysaccharide | 1291256 ± 42563 | 5488 ± 2137 | N.A. |
| GML | N.A. | N.A. | 1369720 ± 112480 |
| GML/1/2 Polysaccharide | N.A. | N.A. | 1303171 ± 98550 |
| GML/Pan-polysaccharide | N.A. | N.A. | 1651263 ± 132537 |
Data were presented as mean ± SD (n = 10).
Effect of RS polysaccharide on the transformation of cimifugin in small intestine enzyme solutions
As shown in Table 5, cimifugin underwent degradation to a great extent in small intestine enzyme solutions, with small amount of which transformed into PGCN. Intriguingly, although RS polysaccharide produced no effect on cimifugin degradation, it promoted quite amount of cimifugin transformed into PGCN. After removing or denaturing the small intestine enzymes, RS polysaccharide still evoked this transformation to a similar extent. In contrast, as shown in Table 6, glucuronic acid and glucose did not produce any effect on cimifugin transformation. All these findings indicated that RS polysaccharide promoted the transformation of cimifugin to PGCN in a direct manner, rather than via affecting the bioactivity of the small intestine enzymes.
Table 5. Transformational study of cimifugin to PGCN and GML in the absence and presence of RS polysaccharide.
| Reaction time | Test compounds | Chromone content (μg/mL) | PGCN | GML |
|---|---|---|---|---|
| Cimifugin | ||||
| 15 min | A/B | 1.52 ± 0.16 | 39.88 ± 2.57 | 0.00 ± 0.00 |
| A/B/C | 13.65 ± 1.11* | 38.27 ± 2.45 | 1.09 ± 0.12* | |
| A/C | 16.58 ± 1.26* | 65.74 ± 3.68* | 2.74 ± 0.31* | |
| A/C/D | 16.92 ± 1.06* | 68.60 ± 4.62* | 2.92 ± 0.50* | |
| 30 min | A/B | 2.82 ± 0.14 | 32.26 ± 2.01 | 0.00 ± 0.00 |
| A/B/C | 13.79 ± 0.99* | 34.13 ± 2.46 | 1.31 ± 0.12* | |
| A/C | 20.71 ± 1.88* | 56.36 ± 3.56* | 2.95 ± 0.14* | |
| A/C/D | 22.68 ± 1.66* | 58.43 ± 5.37* | 2.94 ± 0.52* |
A: cimifugin; B: enzyme; C: polysaccharide; D: denatured enzyme.
Data were presented as mean ± SD (n = 10). *P < 0.05 vs “A/B” group at the same time point.
Table 6. Transformational study of cimifugin to PGCN and GML in the absence and presence of glucuronic acid and glucose.
| Reaction time | Chromone content (μg/mL) |
|||
|---|---|---|---|---|
| Test compounds | Cimifugin | PGCN | GML | |
| 15 min | A/B | 1.52 ± 0.16 | 39.88 ± 2.57 | 0.00 ± 0.00 |
| A/B/C | 4.35 ± 0.32 | 38.25 ± 2.23 | 0.00 ± 0.00 | |
| A/B/D | 6.70 ± 0.67 | 21.95 ± 2.01 | 0.00 ± 0.00 | |
| 30 min | A/B | 2.82 ± 0.14 | 32.26 ± 2.01 | 0.00 ± 0.00 |
| A/B/C | 4.73 ± 0.41 | 33.66 ± 2.58 | 0.00 ± 0.00 | |
| A/B/D | 8.89 ± 1.05 | 22.88 ± 1.40 | 0.00 ± 0.00 | |
A: cimifugin; B: enzyme; C: glucuronic acid; D: glucose.
Data were presented as mean ± SD (n = 10).
Effect of RS polysaccharide on the trans-membrane transport of chromones
As shown in Table 7, RS polysaccharide produced an 81.12% elevation of cimifugin concentration inside the semi-permeable membrane. Likewise, RS polysaccharide also caused a 32.24% elevation of PGCN concentration inside the semi-permeable membrane. These findings indicated that polysaccharide might have a remarkable “protective” effect on chromone components in intestine tracts.
Table 7. Protective effect of polysaccharide on cimifugin and PGCN.
| Groups | Fluid outside the semi-permeable membrane | Fluid inside the semi-permeable membrane | total chromone (nmol) | ||||
|---|---|---|---|---|---|---|---|
| PGCN (nmol) | cimifugin (nmol) | chromone (nmol) | PGCN (nmol) | cimifugin (nmol) | chromone (nmol) | ||
| Cimifugin | N.A. | 143.6 ± 0.1 | 143.6 ± 0.1 | N.A. | 18.1 ± 0.6 | 18.1 ± 0.6 | 161.7 ± 0.5 |
| Cimifugin/polysaccharide | 30.3 ± 1.5** | 98.1 ± 0.3** | 128.4 ± 1.4* | 6.3 ± 0.1** | 26.4 ± 1.2 | 32.7 ± 1.2** | 161.1 ± 0.4 |
| PGCN | 89.8 ± 0.4 | N.A. | 89.8 ± 0.4 | 15.2 ± 0.5 | N.A. | 15.2 ± 0.5 | 105.0 ± 0.1 |
| PGCN/polysaccharide | 85.7 ± 0.6# | N.A. | 85.7 ± 0.6# | 20.1 ± 0.2** | N.A. | 20.1 ± 0.2## | 105.8 ± 0.7 |
Data were presented as mean ± SD (n = 10). *P < 0.05, **P < 0.01 vs. “cimifugin” group; #P < 0.05, ##P < 0.01 vs. “PGCN” group.
Discussion
PGCN, GML and cimifugin are the three major chromones in RS with clear chemical structures. Although the content of cimifugin is the lowest among these three components in RS, it represented as the main absorbed and active component in plasma after oral administration of RS extract. In contrast, the other two chromones possess feeble pharmacological activities and are hard to be detected by HPLC when used at regular doses. It was generally speculated that these two glycosides might function after firstly biotransformed into cimifugin in the gastrointestinal tract, then the latter was absorbed into the blood4,11. As such, we used cimifugin here as the indicative component. Our present study showed that RS polysaccharide significantly promoted the absorption of cimifugin, with an obvious dose-response relationship. In the presence of polysaccharide, AUC0–24 of cimifugin showed a 0.56-fold increase, compared with sole administration of chromones. The polysaccharide structure might be essential for this absorption promoting effect, as this effect was not found with monosaccharides, such as glucose.
Subsequently, the underlying mechanism of RS polysaccharide increasing cimifugin content in plasma was also explored. CYP3A4 accelerates the decomposition of exogenous foreign bodies, hindering their absorption while, P-glycoprotein promotes the exocytosis of exogenous foreign bodies. Both of these proteins are the key to limit the absorption of exogenous agents through gastrointestinal tract. In order to figure out whether CYP3A4 and P-glycoprotein were involved in the cimifugin absorption promoting process of RS polysaccharide, two pharmacological inhibitors, LY33597 and troleandomycin targeting CYP3A4 and P-glycoprotein, were introduced, respectively. Our results revealed that LY353597 failed to increase the absorption of cimifugin, suggesting that cimifugin was not likely to be destroyed by intestinal CYP3A4. In contrast, troleandomycin has a significant influence on cimifugin content, indicative of an exocytosis promoting effect of P-glycoprotein. Besides, polysaccharide did not affect the transport of cimifugin between side A and B, and not increase the inhibitory effect of the two above-mentioned pharmacological inhibitors. Based on these, it was concluded that it was not through inhibiting P-glycoprotein and CYP3A that polysaccharide promoted the absorption of cimifugin.
PGCN is transformed into cimifugin by enzymes in gastrointestinal fluid. RS extract contained a huge amount of PGCN and traces of cimifugin. This had a significant bearing on the large quantities of transformation of PGCN into cimifugin at the very beginning. PGCN did not easily transport across the cell membrane for absorption due to its high polarity, whereas cimifugin was readily absorbed into blood4. The production of cimifugin was dominated at the beginning, when the amount of absorbed cimifugin was dependent on its generation. As such, it is understandable that a similar level of plasma content of cimifugin was found among groups within the first three hours. There are two active –OH in cimifugin molecule, which leads to its rapid degradation before absorption: the higher the cimifugin concentration, the more amount of degradation of this substance. Considerable breakdown of cimifugin in intestinal tract would result in the decrease of cimifugin in the blood. The amount of PGCN in intestinal tract was gradually decreased, when the reaction of cimifugin proceeded towards PGCN. On the other hand, the content of cimifugin steeply enhanced. As such, polysaccharide preventing cimfugin from degrading matters.
Above all, polysaccharide can convert cimifugin into chemically more stable PGCN. In view of the facts that: 1) the glycoside group of PGCN and GML is of glucose; 2) glucuronyl transferase exists in the small intestine of human and rats and is essential for glycoside synthesis, we initially reckoned that the polysaccharide might exert its protective effect depending on glucose and glucuronyl transferase. However, our results did not support this hypothesis, as these substances failed to increase the production of PGCN and GML. That is to say, polysaccharide itself was the main material basis for cimifugin transformation into PGCN. This experiment exploring the effect of polysaccharide on cimifugin transformation revealed that the relatively lower content of cimifugin was due to the presence of the transformation process from cimifugin to PGCN. RS polysaccharides are of macromolecular active substance with complex spatial structure and active groups, such as -OH and -COOH. It is still not clear how cimifugin and polysaccharide react. It is speculated that structurally complex polysaccharide catalyze the transformation from cimifugin to more stable PGCN and GML, thereby averting its breakdown. In addition, the complex spatial structure of polysaccharide might also provide physical protection to cimifugin and PGCN19. Cimifugin and PGCN might bind to polysaccharide through a certain manner after entry into this complex spatial structure, thus protecting them against being destroyed by gastrointestinal enzymes.
Transformation from PGCN to cimifugin increases the absorption of chromone, whereas the opposite process decreases the breakdown of chromones before their absorption. Polysaccharide was able to prevent chromones from being destroyed in intestinal tract. There exists a dynamic balance of the inter-transformation between RS chromone glycosides and its aglycone. The protective effect of polysaccharide on chromone transformation would increase the therapeutic effect of chromones. The present finding afforded us salutary lessons with regard to the quality control and clinical application of RS and other medicinal materials.
Additional Information
How to cite this article: Yang, J.-M. et al. Polysaccharide enhances Radix Saposhnikoviae efficacy through inhibiting chromones decomposition in intestinal tract. Sci. Rep. 6, 32698; doi: 10.1038/srep32698 (2016).
Acknowledgments
This work was supported by National Natural Science Foundation of China (81541079) and Research Project of Science and Technology of Liaoning Province (L2013330).
Footnotes
Author Contributions J.-M.Y. and H.J. were responsible for data acquisition, data analysis and figure/table preparation. H.-L.D. contributed to the study design, provided the technical support, and wrote the main manuscript text. Z.-W.W. and G.-Z.J. carried out the statistical analysis and interpretation of data. X.-C.M. participated in the conceptualization and design of the experiment, interpretation of data, and revision and approval of the final version of the manuscript. All authors reviewed and approved the final manuscript.
References
- Kim M. K. et al. Simultaneous determination of chromones and coumarins in Radix Saposhnikoviae by high performance liquid chromatography with diode array and tandem mass detectors. Journal of chromatography. A 1218, 6319–6330, doi: 10.1016/j.chroma.2011.06.103 (2011). [DOI] [PubMed] [Google Scholar]
- Okuyama E. et al. Analgesic components of saposhnikovia root (Saposhnikovia divaricata). Chemical & pharmaceutical bulletin 49, 154–160 (2001). [DOI] [PubMed] [Google Scholar]
- Kang J. et al. Three new compounds from the roots of Saposhnikovia divaricata. Journal of Asian natural products research 10, 971–976, doi: 10.1080/10286020802217556 (2008). [DOI] [PubMed] [Google Scholar]
- Li Y. et al. Comparative pharmacokinetics of prim-O-glucosylcimifugin and cimifugin by liquid chromatography-mass spectrometry after oral administration of Radix Saposhnikoviae extract, cimifugin monomer solution and prim-O-glucosylcimifugin monomer solution to rats. Biomedical chromatography: BMC 26, 1234–1240, doi: 10.1002/bmc.2684 (2012). [DOI] [PubMed] [Google Scholar]
- Dai J. et al. A sensitive liquid chromatography-mass spectrometry method for simultaneous determination of two active chromones from Saposhnikovia root in rat plasma and urine. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences 868, 13–19, doi: 10.1016/j.jchromb.2008.03.031 (2008). [DOI] [PubMed] [Google Scholar]
- Tai J. & Cheung S. Anti-proliferative and antioxidant activities of Saposhnikovia divaricata. Oncology reports 18, 227–234 (2007). [PubMed] [Google Scholar]
- Yang J. et al. Feeble antipyretic, analgesic and anti-inflammatory activities were found with regular dose 4′-O-β-D-glucosyl-5-O-methylvisamminol, one of the conventional marker compounds for quality evaluation of Radix Saposhnikoviae. Pharmacognosy magazine 12, xx-xx. Under preparation for issue (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z. Q., Tian Y. J. & Zhang J. [Studies on the antioxidative activity of polysaccharides from radix Saposhnikoviae]. Zhong yao cai = Zhongyaocai = Journal of Chinese medicinal materials 31, 268–272 (2008). [PubMed]
- Kang J. et al. Characterization of compounds from the roots of Saposhnikovia divaricata by high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry. Rapid communications in mass spectrometry: RCM 22, 1899–1911, doi: 10.1002/rcm.3559 (2008). [DOI] [PubMed] [Google Scholar]
- Committe C. P. Pharmacopoeia of the People’s Republic of China. Vol. 1 (China Chemical Industry Press, 2010). [Google Scholar]
- Zhao B., Yang X. B., Yang X. W. & Liu J. X. Biotransformation of prim-O-glucosylcimifugin by human intestinal flora and its inhibition on NO production and DPPH free radical. Journal of Asian natural products research 14, 886–896, doi: 10.1080/10286020.2012.702756 (2012). [DOI] [PubMed] [Google Scholar]
- Li Y. Y. et al. RRLC -TOF/MS in identification of constituents and metabolites of Radix Saposhnikoviae in rat plasma and urin. Academic Journal of Second Military Medical University 31, 760–763 (2010). [Google Scholar]
- Xuan Chen Y. Z. Jianbo Zhang. Advances in research on the plant polysaccharides. Chinese Journal of New Drugs 16, 1000–1005 (2007). [Google Scholar]
- Gengyuan Tian Y. F. Ying Lin. Advances in research on plant polysaccharide. China Journal of Chinese Materia Medica 20, 441–444 (1995). [PubMed] [Google Scholar]
- Xiangdong Wu, X. W. Juan Yang. The influence of polysaccharides of rhizoma gastrodiae on the absorption of gastrodin. Journal of Chengdu Meical College 7, 551–553 (2012). [Google Scholar]
- Xu M. et al. Pharmacokinetic comparisons of schizandrin after oral administration of schizandrin monomer, Fructus Schisandrae aqueous extract and Sheng-Mai-San to rats. Journal of ethnopharmacology 115, 483–488, doi: 10.1016/j.jep.2007.10.016 (2008). [DOI] [PubMed] [Google Scholar]
- Hui Sun X. S. Xiangcai Meng. Bolt effect on quality and yield of saposhnikovia divaricata roots. World Science and Technology-Modernization of Traditional Chinese Medicine and Materia Medica 10, 101–104,108 (2008). [Google Scholar]
- Ziwei Wang J. Y., Jiang Hua & Chen Yannan Hongyu Li. Influence of polysaccharides on pharmacodynamics and pharmacokinetics of bolting saposhnikoviae radix. Chinese Traditional Patent Medicine 37, 2392–2397 (2015). [Google Scholar]
- Jingjing Dai J. Z., Sun Runguang, Jiao Ziming, Zhang Peng & Zhang Huapeng Ajuan Liu. Analyses on properties for physicochemistry, morphology, and structure of Saposhnikovia divaricata polysaccharides. Chinese Traditional and Herbal Drugs 44, 391–396 (2013). [Google Scholar]


