Abstract
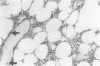
AIMS: To determine the distribution of tenascin in normal and pathological bone marrow. METHODS: 48 different bone marrow lesions were studied immunohistochemically using a monoclonal antibody to tenascin. RESULTS: Tenascin immunoreactivity was found in lesions with increased fibrosis and high numbers of reticular fibres. The strongest immunoreactivity was found in myelofibrosis. Bone marrow from acute and chronic myeloid and lymphatic leukaemias showed weak or moderate immunoreactivity. In hyperplasias inconsistent reticular tenascin immunoreactivity was found; in normal bone marrow, only a few scattered positive fibres were occasionally seen. CONCLUSIONS: Tenascin was generally observed in conditions in which megakaryocytic hyperplasia was a feature. This is in line with the notion that tenascin synthesis in bone marrow fibroblasts is stimulated by TGF-beta which is synthesised by the megakaryocytic lineage. Tenascin also contains EGF-like repeats. It might therefore function as a growth promoter and in this way could also stimulate synthesis of other matrix components. On the other hand, tenascin could function as an adhesive molecule to some cells of the bone marrow. The presence of tenascin in many pathological states of the bone marrow suggests that it may have a role in their pathogenesis and that it also could be a potential marker of disease.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apaja-Sarkkinen M., Autio-Harmainen H., Alavaikko M., Risteli J., Risteli L. Immunohistochemical study of basement membrane proteins and type III procollagen in myelofibrosis. Br J Haematol. 1986 Jul;63(3):571–580. doi: 10.1111/j.1365-2141.1986.tb07535.x. [DOI] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R. What distinguishes tenascin from fibronectin? FASEB J. 1990 Jun;4(9):2598–2604. doi: 10.1096/fasebj.4.9.1693347. [DOI] [PubMed] [Google Scholar]
- Ellingsworth L. R., Brennan J. E., Fok K., Rosen D. M., Bentz H., Piez K. A., Seyedin S. M. Antibodies to the N-terminal portion of cartilage-inducing factor A and transforming growth factor beta. Immunohistochemical localization and association with differentiating cells. J Biol Chem. 1986 Sep 15;261(26):12362–12367. [PubMed] [Google Scholar]
- Engel J. EGF-like domains in extracellular matrix proteins: localized signals for growth and differentiation? FEBS Lett. 1989 Jul 17;251(1-2):1–7. doi: 10.1016/0014-5793(89)81417-6. [DOI] [PubMed] [Google Scholar]
- Erickson H. P., Bourdon M. A. Tenascin: an extracellular matrix protein prominent in specialized embryonic tissues and tumors. Annu Rev Cell Biol. 1989;5:71–92. doi: 10.1146/annurev.cb.05.110189.000443. [DOI] [PubMed] [Google Scholar]
- Howeedy A. A., Virtanen I., Laitinen L., Gould N. S., Koukoulis G. K., Gould V. E. Differential distribution of tenascin in the normal, hyperplastic, and neoplastic breast. Lab Invest. 1990 Dec;63(6):798–806. [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Jones F. S., Burgoon M. P., Hoffman S., Crossin K. L., Cunningham B. A., Edelman G. M. A cDNA clone for cytotactin contains sequences similar to epidermal growth factor-like repeats and segments of fibronectin and fibrinogen. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2186–2190. doi: 10.1073/pnas.85.7.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A., Katoh O., Hyodo H., Kuramoto A. Transforming growth factor-beta regulates growth as well as collagen and fibronectin synthesis of human marrow fibroblasts. Br J Haematol. 1989 Aug;72(4):486–491. doi: 10.1111/j.1365-2141.1989.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Koch M., Wehrle-Haller B., Baumgartner S., Spring J., Brubacher D., Chiquet M. Epithelial synthesis of tenascin at tips of growing bronchi and graded accumulation in basement membrane and mesenchyme. Exp Cell Res. 1991 Jun;194(2):297–300. doi: 10.1016/0014-4827(91)90368-5. [DOI] [PubMed] [Google Scholar]
- Liakka K. A., Autio-Harmainen H. I. Distribution of the extracellular matrix proteins tenascin, fibronectin, and vitronectin in fetal, infant, and adult human spleens. J Histochem Cytochem. 1992 Aug;40(8):1203–1210. doi: 10.1177/40.8.1377736. [DOI] [PubMed] [Google Scholar]
- Natali P. G., Nicotra M. R., Bartolazzi A., Mottolese M., Coscia N., Bigotti A., Zardi L. Expression and production of tenascin in benign and malignant lesions of melanocyte lineage. Int J Cancer. 1990 Oct 15;46(4):586–590. doi: 10.1002/ijc.2910460406. [DOI] [PubMed] [Google Scholar]
- Natali P. G., Nicotra M. R., Bigotti A., Botti C., Castellani P., Risso A. M., Zardi L. Comparative analysis of the expression of the extracellular matrix protein tenascin in normal human fetal, adult and tumor tissues. Int J Cancer. 1991 Apr 1;47(6):811–816. doi: 10.1002/ijc.2910470603. [DOI] [PubMed] [Google Scholar]
- Pearson C. A., Pearson D., Shibahara S., Hofsteenge J., Chiquet-Ehrismann R. Tenascin: cDNA cloning and induction by TGF-beta. EMBO J. 1988 Oct;7(10):2977–2982. doi: 10.1002/j.1460-2075.1988.tb03160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto A. L., Jones F. S., Cunningham B. A., Crossin K. L., Edelman G. M. Localization during development of alternatively spliced forms of cytotactin mRNA by in situ hybridization. J Cell Biol. 1990 Aug;111(2):685–698. doi: 10.1083/jcb.111.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly J. T. Pathogenesis of idiopathic myelofibrosis: role of growth factors. J Clin Pathol. 1992 Jun;45(6):461–464. doi: 10.1136/jcp.45.6.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soini Y., Päkkö P., Virtanen I., Lehto V. P. Tenascin in salivary gland tumours. Virchows Arch A Pathol Anat Histopathol. 1992;421(3):217–222. doi: 10.1007/BF01611178. [DOI] [PubMed] [Google Scholar]
- Vollmer G., Siegal G. P., Chiquet-Ehrismann R., Lightner V. A., Arnholdt H., Knuppen R. Tenascin expression in the human endometrium and in endometrial adenocarcinomas. Lab Invest. 1990 Jun;62(6):725–730. [PubMed] [Google Scholar]
- Wang Q. R., Yan Z. J., Wolf N. S. Dissecting the hematopoietic microenvironment. VI. The effects of several growth factors on the in vitro growth of murine bone marrow CFU-F. Exp Hematol. 1990 May;18(4):341–347. [PubMed] [Google Scholar]