Abstract
Soil alkalization severely affects crop growth and agricultural productivity. Alkali salts impose ionic, osmotic, and high pH stresses on plants. The alkali tolerance molecular mechanism in roots from halophyte Puccinellia tenuiflora is still unclear. Here, the changes associated with Na2CO3 tolerance in P. tenuiflora roots were assessed using physiological and iTRAQ-based quantitative proteomic analyses. We set up the first protein dataset in P. tenuiflora roots containing 2,671 non-redundant proteins. Our results showed that Na2CO3 slightly inhibited root growth, caused ROS accumulation, cell membrane damage, and ion imbalance, as well as reduction of transport and protein synthesis/turnover. The Na2CO3-responsive patterns of 72 proteins highlighted specific signaling and metabolic pathways in roots. Ca2+ signaling was activated to transmit alkali stress signals as inferred by the accumulation of calcium-binding proteins. Additionally, the activities of peroxidase and glutathione peroxidase, and the peroxiredoxin abundance were increased for ROS scavenging. Furthermore, ion toxicity was relieved through Na+ influx restriction and compartmentalization, and osmotic homeostasis reestablishment due to glycine betaine accumulation. Importantly, two transcription factors were increased for regulating specific alkali-responsive gene expression. Carbohydrate metabolism-related enzymes were increased for providing energy and carbon skeletons for cellular metabolism. All these provide new insights into alkali-tolerant mechanisms in roots.
Soil alkalization is a major abiotic stress that severely affects crop growth and agricultural productivity worldwide. The alkaline soil contains high levels of Na2CO3 and NaHCO3, which leads to a high soil pH (>9.0)1. Relative to neutral salts, alkali salts impose more severe damage to plants due to the combination of ion toxicity, osmotic stress, and high pH stress. Especially, high pH environment surrounding the plant roots has great influence on nutrient uptake, organic acid balance, ion homeostasis, and especially pH stability at cell, tissue, and organ levels2,3,4.
Plant roots act as the primary site for perceiving the alkali stress. Alkaline soil always contains mixed saline-alkali, including NaCl, Na2CO3, NaHCO3, Na2SO4, and NaOH, which generally retards the root growth and even kills the plants5. A mixed saline-alkali (70 mM NaCl and 50 mM NaHCO3) stress activated a series of signaling and metabolic pathways in roots of glycophyte soybean (Glycine max), including hormone signaling, transcriptional regulation, ion homeostasis, antioxidant responses, transportation, protein synthesis and destination, cell rescue and defense6. Importantly, single alkali stress also has obvious effect on root growth. In the Na2CO3-stressed roots of glycophyte sunflower (Helianthus annuus), cellular homeostasis was disrupted due to the increased Na+ content, decreased K+ content and protein concentration7. While the increased content of free amino acid and the enhanced activities of ATPase and proteases in roots suggest that the osmotic homeostasis, energy supply, and protein turnover play crucial roles in alkali tolerance7.
The neutral salt-responsive physiological and molecular mechanisms in roots have been extensively studied8,9,10, but the specific molecular mechanism underlying alkali tolerance in roots is lacking. A number of genes in roots were affected by alkali stress. It was reported that 8,319 genes, representing over a quarter of the total number in the maize (Zea mays) genome, were significantly altered in roots under 50 mM Na2CO3 treatment for 5 h11. The expression patterns of these alkali-responsive genes reveal that maize roots possess unique biological pathways for adapting to Na2CO3 stress. They include Na2CO3-induced brassinosteroid biosynthesis, and Na2CO3-reduced ascorbate (AsA) and aldarate metabolism, protein processing in endoplasmic reticulum (ER), the biosynthesis of N-glycan and fatty acid, and circadian rhythm11.
The early and delayed alkali-responsive functional inclines are different in roots revealed from high-throughput transcriptomic analysis. The patterns of 7,088 differentially expressed genes in wild soybean (Glycine soja) under 50 mM NaHCO3 stress showed that at early stage of stress (3–6 h), a cascade of processes were initiated, including the induced signal transduction, secondary metabolism, and transcription regulation, but these molecular processes were reduced after 12 h of stress, following the subsequent induction of protein synthesis and energy metabolism after 24 h of stress12. In addition, the NaHCO3-responsive genes in roots of woody halophyte Tamarix hispida under 300 mM NaHCO3 for 12 h, 24 h, and 48 h imply that various specific strategies are employed for surviving from alkaline stress, such as induced biosynthesis of proline and trehalose, enhancement of protein folding and osmotic homeostasis, and diverse transcription regulations13.
Although a large amount of candidate alkali-responsive genes were found using transcriptomic approaches, only several of them have been cloned and characterized. It was reported that three genes, including rHsp90 (encoding a 90 kDa heat shock protein (Hsp))14, RMtATP6 (encoding a mitochondrial ATP synthase 6 kDa subunit)1, and NADP-ME2 (encoding an NADP-malic enzyme)15, were isolated from a cDNA library constructed from rice (Oryza sativa) roots under NaHCO3 stress. The expression levels of these genes and the activity of NADP-malic enzyme were increased in rice roots under NaHCO3 and Na2CO3 treatments1,14,15. Moreover, yeast or transgenic plants over-expressing these genes exhibited greater tolerance to alkali/salt stress, indicating their important roles in alkali/salt tolerance1,14,15. However, these results are not adequate for unraveling the molecular basis and dynamic networks underlying alkaline tolerance in plant roots.
Puccinellia tenuiflora is a monocotyledonous halophyte widely distributed in the Songnen Plain in Northeastern China. P. tenuiflora belongs to the genus Gramineae, and has close genetic relationships with rice and barley (Hordeum vulgare)16,17. Unlike these two relatives, P. tenuiflora has a strong ability of salt and alkali tolerance to grow normally under maximum stress up to 600 mM NaCl and 150 mM Na2CO3 (pH 11.0) for 6 days17. Therefore, P. tenuiflora is considered as an outstanding pasture for soil improvement, as well as a good plant model among monocotyledonous plants for understanding alkali tolerance mechanisms.
The salt/alkali tolerance of P. tenuiflora was due to its high selectivity for K+ over Na+ 2,18. The low net Na+ uptake was mainly resulted from the restriction of unidirectional Na+ influx2. In addition, the Casparian band in the root endodermis can also block the apoplastic path of Na+ entrance18. Genes encoding several plasma membrane (PM) located proteins have been characterized to be involved in transmembrane ion transport, such as PutPMP3-1 and PutPMP3-2 encoding PM protein 3 family proteins function to prevent the accumulation of excess Na+ 19, PutHKT2; 1 encoding a high-affinity K+ transporter which plays a role in K+ uptake to maintain a high ratio of K+/Na+ in the cells20, PtNHA1 encoding a Na+/H+ antiporter for the maintenance of low cytosolic Na+ 21, and PutAKT1 encoding a PM-localized K+ channel family protein that can interact with KPutB1 to alter K+ and Na+ homeostasis22. Besides of restriction of Na+ entrance, Na+ can also be secreted onto leaf surface through stomata or together with wax secretion under salt/alkali stress23,24,25. However, the Na+ secretion accounted for only a small portion of the whole plant Na+ content and was very small compared with other salt-secreting halophytes2.
To maintain intracellular ionic and osmotic balance under saline or alkaline stress, P. tenuiflora can accumulate organic acids and inorganic anions to balance the massive influx of cations4,24. Additionally, P. tenuiflora is able to accumulate Na+, K+, and organic acids in vacuoles, as well as proline, betaine, and soluble sugar in the protoplasm to maintain osmotic homeostasis4,24. Importantly, some genes were found to be involved in ion compartmentalization in stressed P. tenuiflora. PutCAX1 encoding a Ca2+/H+ antiporter in the vacuolar membrane was proposed to play a role in Ca2+, Ba2+, and Zn2+ transportation26. Besides, PutNHX encoding a vacuolar Na+/H+ antiporter was found to be responsible for Na+ sequestration into the vacuole27. Moreover, the vacuolar Na+/H+ antiporter might be involved in pH regulation under alkaline salt conditions due to higher NaHCO3-induced expression level of PutNHX in P. tenuiflora roots compared with that under NaCl condition27. To cope with the high pH of micro-environment around roots under alkali stress, P. tenuiflora can pump out H+ through the ATPase system on the cell membrane or secrete acidic metabolites such as organic acids4. Moreover, P. tenuiflora can also accumulate organic acids or other acidic metabolites in cells to adjust to the internal pH4. In addition, some antioxidant pathways were found to be activated in P. tenuiflora to scavenge excessive reactive oxygen species (ROS) caused by salt/alkali stress24,25,28. Two genes, PutAPX encoding ascorbate peroxidase (APX) and PutMT2 encoding a type-2 metallothionein-like protein, which are all involved in ROS scavenging, were identified in P. tenuiflora. Over-expressing PutAPX and PutMT2 in transgenic Arabidopsis thaliana and yeast increased the tolerance to H2O2, NaCl, and NaHCO329,30.
Besides the aforementioned genes, more candidate genes and/or proteins involved in alkali tolerance have been found in P. tenuiflora using high-throughput transcriptomic and proteomic approaches17,24,25,31,32. The genes participated in ion transport, Fe acquisition, metabolism, and defense were up-regulated in seedlings under 20 mM NaHCO331. Besides, the genes involved in metabolism, cell growth and photosynthesis were strongly affected in leaves stressed by 450 mM NaHCO332. Similarly, Na2CO3-responsive genes involved in metabolism, signal transduction, transcription, and cell rescue and defense were overrepresented in seedlings17. The expression patterns of Na2CO3-responsive genes imply that dynamic regulation of photosynthesis, cytoskeleton, kinase-mediated signaling, and transcription, maintenance of redox and osmotic homeostasis, and the increased ability to regulate intracellular pH homeostasis and synthesize citric acid are important in P. tenuiflora to cope with alkali stress17. In addition, our previous comparative proteomic studies based on two-dimensional gel electrophoresis have identified 93 NaCl-responsive and 43 Na2CO3-responsive proteins in P. tenuiflora leaves, respectively. These results reveal that NaCl- and Na2CO3-responsive regulatory mechanisms in P. tenuiflora leaves share some common pathways, including declined photosynthesis, activation of antioxidant systems, ion exclusion and compartmentalization, as well as enhanced energy supply24,25. Despite the progress, the precise molecular mechanisms and regulatory networks of alkali tolerance in P. tenuiflora roots are still unknown.
In the present study, we investigated the Na2CO3-responsive characteristics in P. tenuiflora roots using physiological approaches combined with isobaric tags for relative and absolute quantification (iTRAQ)-based quantitative proteomic approach. By integrating the physiological and proteomic results, some unique Na2CO3-responsive pathways including alkali signal transduction, ROS scavenging, ionic and osmotic homeostasis, and protein synthesis and turnover were observed to play vital roles in Na2CO3 tolerance in P. tenuiflora roots. These results provide novel insights into the alkali tolerance mechanisms in P. tenuiflora, which is required for further studies on improving the crop alkali tolerance.
Results
Root growth and biomass of P. tenuiflora under Na2CO3
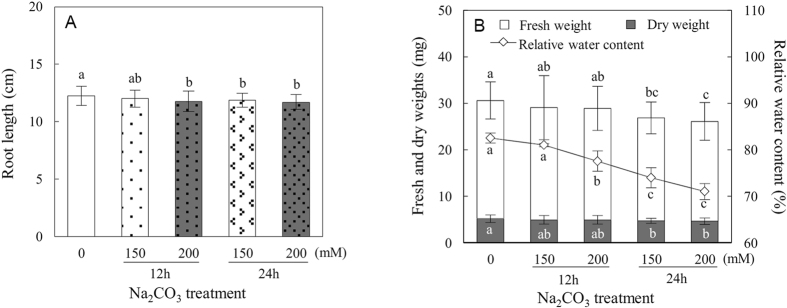
P. tenuiflora is a monocotyledonous halophyte with a high alkali tolerance. It can tolerate up to 150 mM Na2CO3 (pH 11.0) but not 200 mM or more Na2CO3 (pH 11.0) for 6 days17. In this study, 50-day-old seedlings were exposed to the tolerant level (150 mM) and lethal level (200 mM) of Na2CO3 for 12 h and 24 h, respectively. Root biomass was measured after Na2CO3 treatments. The root length was not significantly affected by 150 mM Na2CO3 for 12 h, but was decreased by 4%, 3.3% and 4.6% under 200 mM Na2CO3 for 12 h, 150 mM for 24 h, and 200 mM for 24 h, respectively (Fig. 1A). In addition, the fresh weight (Fw) and dry weight (Dw) of roots did not change significantly under Na2CO3 for 12 h (Fig. 1B). However, the root Fw was decreased by 12.9% and 15.6% under 150 mM and 200 mM Na2CO3 for 24 h, respectively. Similarly, the Dw of roots was also decreased by 9.1% and 10.6% under these two conditions (Fig. 1B). Besides, the relative water content (RWC) in roots was decreased by 6%, 10.3%, and 13.9% under 200 mM Na2CO3 for 12 h, 150 mM for 24 h, and 200 mM for 24 h, respectively (Fig. 1B). The data show that the root biomass and growth are apparently inhibited under Na2CO3 stress.
Figure 1. Root biomass of Puccinellia tenuiflora seedlings grown under Na2CO3 conditions.
(A) Root length (n = 35) and (B) fresh weight (white columns) (n = 35), dry weight (gray columns) (n = 35), and relative water content (diamonds) (n = 3). The values were determined under control, 150 mM Na2CO3 for 12 h, 200 mM Na2CO3 for 12 h, 150 mM Na2CO3 for 24 h, and 200 mM Na2CO3 for 24 h. The values are presented as means ± standard deviation. Different letters indicate significant differences among different treatments (p < 0.05).
Na+, K+, Ca2+, and Mg2+ contents in Na2CO3-stressed roots
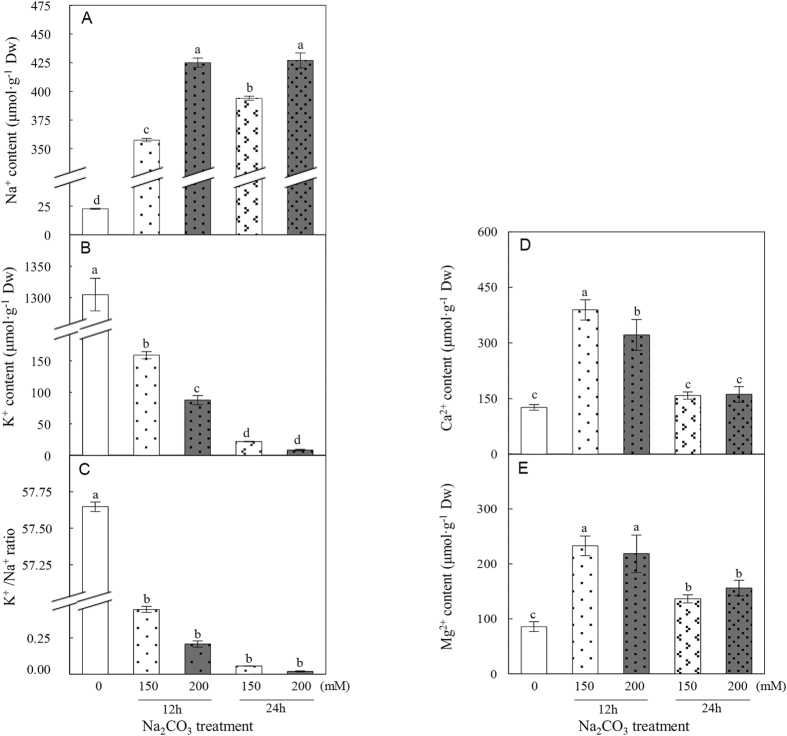
To monitor the ion homeostasis affected by Na2CO3 stress, the contents of Na+, K+, Ca2+, and Mg2+ in P. tenuiflora roots were measured. The Na+ content in Na2CO3-stressed roots was significantly increased by 15.8, 18.8, 17.4, and 18.9-fold under the four Na2CO3 conditions, respectively (Fig. 2A). This was accompanied by a decline in the root K+ content by about 8.2-, 14.9-, 59.0-, and 152.1-fold, respectively (Fig. 2B). The K+/Na+ ratio was also dramatically decreased under Na2CO3 stress (Fig. 2C). This implies that the uptake of K+ into roots is inhibited by the increasing Na+. In addition, the Ca2+ content was significantly increased after 12 h of Na2CO3 stress (Fig. 2D), and the Mg2+ content was increased under all of the four Na2CO3 conditions (Fig. 2E).
Figure 2. Effect of Na2CO3 on ion contents in Puccinellia tenuiflora roots.
(A) Na+ content; (B) K+ content; (C) K+/Na+ ratio; (D) Ca2+ content; and (E) Mg2+ content. The values were determined under control, 150 mM Na2CO3 for 12 h, 200 mM Na2CO3 for 12 h, 150 mM Na2CO3 for 24 h, and 200 mM Na2CO3 for 24 h. The values are presented as means ± standard deviation (n = 4). Different letters indicate significant differences among different treatments (p < 0.05).
Osmolyte contents in roots under Na2CO3
The accumulation of osmolytes within plant cells is involved in alleviating alkali-induced osmotic stress. The contents of two important osmolytes, soluble sugar and glycine betaine, were measured in P. tenuiflora roots under Na2CO3 stress. The soluble sugar content was dramatically decreased in the Na2CO3-stressed roots (Fig. 3A). In contrast, the glycine betaine content was significantly increased in roots under Na2CO3 stress (Fig. 3A). This suggests that accumulating glycine betaine in P. tenuiflora roots may contribute to reestablish the osmotic balance under Na2CO3.
Figure 3. Effects of Na2CO3 on contents of (A) soluble sugar (white diamonds), glycine betaine (black diamonds), (B) malondialdehyde, and (C) relative electrolyte leakage in Puccinellia tenuiflora roots.
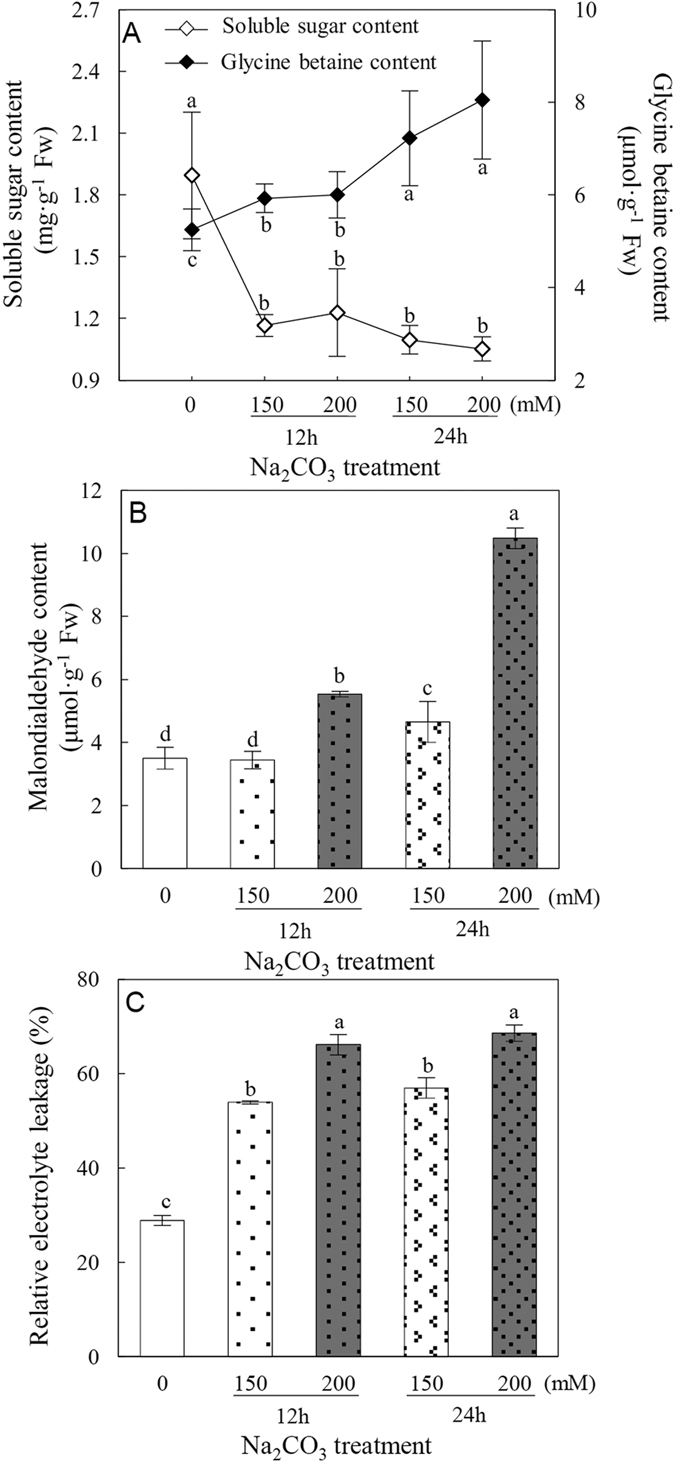
The values were determined under control, 150 mM Na2CO3 for 12 h, 200 mM Na2CO3 for 12 h, 150 mM Na2CO3 for 24 h, and 200 mM Na2CO3 for 24 h. The values are presented as means ± standard deviation (n = 3). Different letters indicate significant differences among different treatments (p < 0.05).
Effect of Na2CO3 on root membrane integrity
To assess the impact of Na2CO3 stress on membrane integrity of P. tenuiflora roots, the malondialdehyde (MDA) content and relative electrolyte leakage (REL), two reliable indicators for alkali salt-induced membrane damage, were measured. Our data showed that MDA content did not change significantly after 150 mM Na2CO3 treatment for 12 h, but was significantly increased under 200 mM Na2CO3 for 12 h, 150 mM for 24 h, and 200 mM for 24 h (Fig. 3B). As to REL, a substantial increase was observed in P. tenuiflora roots under all the four Na2CO3 treatments (Fig. 3C). These results indicate that the membrane integrity of P. tenuiflora roots is damaged by Na2CO3 stress.
Antioxidant enzyme activities in roots in response to Na2CO3
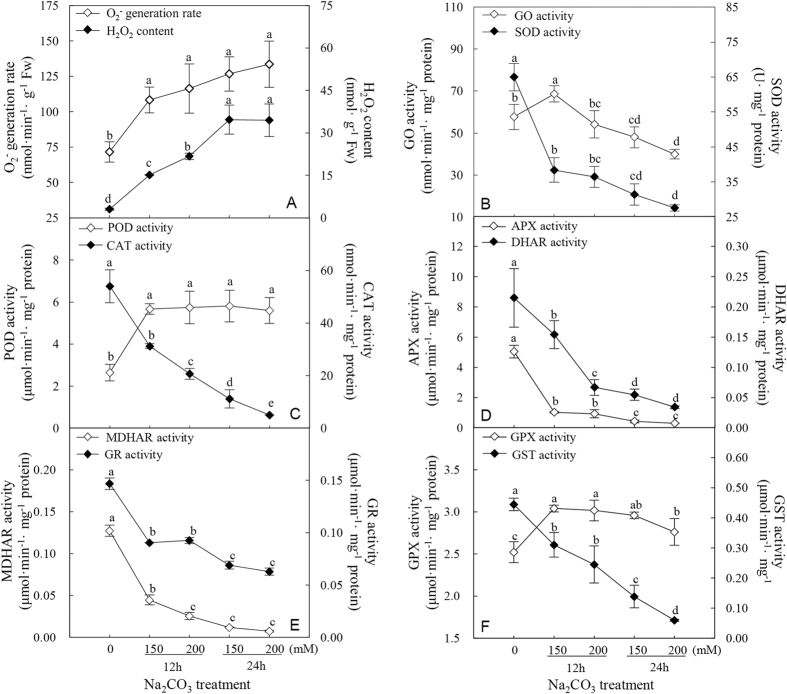
Since alkali salt imposes oxidative stress on plants by inducing the formation of ROS33, the O2− generation rate and H2O2 content were measured in P. tenuiflora roots. The data showed that Na2CO3 caused dramatic increases in O2− and H2O2 production in roots under all the four treatments (Fig. 4A), indicating that Na2CO3 resulted in oxidative damage to P. tenuiflora roots.
Figure 4. Effects of Na2CO3 on ROS production and antioxidant enzyme activities in Puccinellia tenuiflora roots.
(A) O2− generation rate (white diamonds) and H2O2 content (black diamonds); (B) glycolate oxidase (GO) (white diamonds) and superoxide dismutase (SOD) (black diamonds) activities; (C) peroxidase (POD) (white diamonds) and catalase (CAT) (black diamonds) activities; (D) ascorbate peroxidase (APX) (white diamonds) and dehydroascorbate reductase (DHAR) (black diamonds) activities; (E) monodehydroascorbate reductase (MDHAR) (white diamonds) and glutathione reductase (GR) (black diamonds) activities; and (F) glutathione peroxidase (GPX) (white diamonds) and glutathione S-transferase (GST) (black diamonds) activities. The values were determined under control, 150 mM Na2CO3 for 12 h, 200 mM Na2CO3 for 12 h, 150 mM Na2CO3 for 24 h, and 200 mM Na2CO3 for 24 h. The values are presented as means ± standard deviation (n = 3). Different letters indicate significant differences among different treatments (p < 0.05).
To determine the response of ROS scavenging system to oxidative stress induced by Na2CO3, the activities of various antioxidant enzymes including glycolate oxidase (GO), superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), AsA-glutathione (GSH) cycle-related enzymes, glutathione peroxidase (GPX), and glutathione S-transferase (GST) were measured. The activity of GO was initially enhanced under 150 mM Na2CO3 for 12 h, but decreased after 24 h of Na2CO3 treatment (Fig. 4B). A substantial decrease in SOD activity was observed under Na2CO3 treatment (Fig. 4B). In contrast, POD activity displayed a significant increase in Na2CO3-stressed roots, while CAT activity was decreased after Na2CO3 treatment (Fig. 4C). In addition, the activities of APX, monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), and glutathione reductase (GR) involved in AsA-GSH cycle all showed dramatic decreases in roots (Fig. 4D,E). The GPX activity was significantly increased, but GST activity was severely inhibited in roots under Na2CO3 stress (Fig. 4F). These results indicate that POD and GPX pathways have been initiated, while GO, SOD, CAT, AsA-GSH cycle, and GST pathways are inhibited in P. tenuiflora roots in response to Na2CO3 stress.
Identification of Na2CO3-responsive proteins using iTRAQ -based liquid chromatography-tandem mass spectrometry (LC-MS/MS)
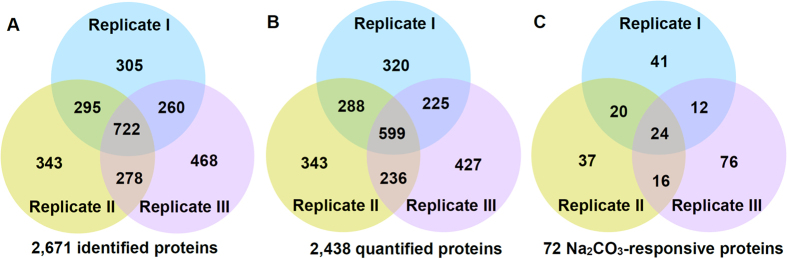
To explore the proteomic changes in P. tenuiflora roots in response to Na2CO3 stress, we employed the iTRAQ-based proteomics approach (Supplementary Fig. S1). Protein abundance profiles in roots under control, 150 mM and 200 mM Na2CO3 treated for 12 h and 24 h were analyzed in three independent biological replicates. The ProteinPilot cut-off score for proteins identified was set at 1.3, which corresponded to a confidence level of 95%. The proteins with similar protein family name and/or amino acid sequence but identified from only one replicate were taken as one unique protein to avoid the redundancy from combination of replicates. Finally, 2,671 non-redundant proteins were included in the dataset of P. tenuiflora roots (Fig. 5A; Supplementary Table S1). In this dataset, a total of 1,594 function unknown proteins were function annotated by searching against the NCBI non-redundant protein database using PSI and PHI-BLAST programs, and then all the 2,671 non-redundant proteins were classified into 17 functional categories based on BLAST alignment, information searching from KEGG pathway database, UniProt database, Gene Ontology, as well as literature (Supplementary Table S1).
Figure 5. Venn diagram analysis of protein identification and quantification in three biological replicates.
(A) The number of identified proteins with at least 95% confidence in three independent biological replicates. (B) The number of quantified proteins with at least 95% confidence in three independent biological replicates. (C) The number of Na2CO3-responsive proteins in three independent biological replicates.
Of the proteins identified, a total of 2,438 proteins were quantified in at least one of the replicates, and 1,348 proteins were quantified in at least two of the three independent replicates (Fig. 5B; Supplementary Table S1). A total of 226 Na2CO3-responsive proteins were identified and quantified in at least one of the three independent replicates under Na2CO3 stress based on the ratio fold change ≥1.5 and p < 0.05. Among them, 72 Na2CO3-responsive proteins were reproducibly identified in at least two replicates (Fig. 5C; Table 1 and Supplementary Table S2).
Table 1. Na2CO3-responsive proteins in roots of Puccinellia tenuiflora identified by iTRAQ-based proteomic analysis.
| Accession No.a | Protein nameb | Speciesc | Mw (Da)d | pIe | Relative protein abundancef | |||
|---|---|---|---|---|---|---|---|---|
| 150 mM, 12 h | 200 mM, 12 h | 150 mM, 24 h | 200 mM, 24 h | |||||
| Signaling (6) | ||||||||
| D2EDB7 | Salt-stress root protein, containing pfam05558 developmentally regulated plasma membrane polypeptide domains* (DREPP) | Lolium perenne | 22,106 | 4.97 | 8.015 ± 3.453* | 8.810 ± 2.054* | 12.880 ± 1.474* | 9.304 ± 1.366* |
| Q5MCL9 | Calreticulin-like protein (CRT) | Triticum aestivum | 47,204 | 4.49 | 5.203 ± 2.886* | 3.900 ± 2.185* | 7.697 ± 5.203* | 6.204 ± 3.544* |
| B4FAK8 | Putative uncharacterized protein, containing pfam00262 calreticulin domain* (CRT) | Zea mays | 60,246 | 4.70 | 0.748 ± 0.097 | 0.526 ± 0.149* | 2.110 ± 0.110 | 0.518 ± 0.037 |
| F2E2L5 | Predicted protein, containing cd00051 calcium binding motif, calmodulin* (CaM) | Hordeum vulgare var. distichum | 16,670 | 4.89 | 0.997 ± 0.071 | 1.043 ± 0.061 | 0.517 ± 0.303 | 0.458 ± 0.286* |
| Q6KCK6 | Calcium-dependent protein kinase (CDPK) | T. aestivum | 58,408 | 5.79 | 2.555 ± 1.201 | 2.764 ± 1.087 | 3.321 ± 1.145* | 3.351 ± 1.378* |
| F2CQQ4 | Serine/threonine-protein phosphatase, containing cd07414 protein phosphate type 1 and keltch like domain* (STPP) | H. vulgare var. distichum | 36,177 | 5.19 | 1.316 ± 0.339 | 1.888 ± 0.569 | 2.331 ± 0.527* | 2.506 ± 0.970 |
| ROS scavenging (2) | ||||||||
| F2DTT4 | Predicted protein, 2-Cys peroxiredoxin* (PrxR) | H. vulgare var. distichum | 28,230 | 6.33 | 2.128 ± 0.575* | 2.043 ± 0.729 | 3.282 ± 0.887* | 2.680 ± 0.724* |
| Q6UQ06 | Cytosolic glutathione reductase (GR) | Triticum. monococcum | 53,015 | 5.93 | 2.065 ± 0.440 | 2.118 ± 0.492 | 2.580 ± 0.318* | 3.445 ± 0.358* |
| Transportation (5) | ||||||||
| Q5PSM6 | Plasma membrane H+-ATPase (P-ATPase) | T. aestivum | 104,661 | 6.58 | 0.251 ± 0.024* | 0.163 ± 0.018* | 0.185 ± 0.026* | 0.289 ± 0.043* |
| F2CRB3 | Predicted protein, containing cd01869 Rab1 domain* | H. vulgare var. distichum | 22,503 | 5.14 | 2.100 ± 0.440 | 1.893 ± 0.588 | 2.712 ± 0.686* | 2.827 ± 1.043* |
| C5XQM5 | Putative uncharacterized protein Sb03g040890, homologue of vesicle-associated membrane protein family protein* (VAMP) | Sorghum bicolor | 39,780 | 9.86 | 0.438 ± 0.057* | 0.654 ± 0.119 | 0.318 ± 0.068 | 0.327 ± 0.023 |
| Q0DG31 | Os05g0556100 protein, dynamin-related protein* (DRP) | Oryza sativa subsp. japonica | 68,683 | 7.65 | 0.728 ± 0.005 | 0.662 ± 0.052 | 0.345 ± 0.040* | 0.525 ± 0.000 |
| A8TU59 | Mitochondrial phosphate transporter (MPT) | Paeonia suffruticosa | 39,853 | 9.31 | 0.194 ± 0.005 | 0.213 ± 0.000 | 0.105 ± 0.005* | 0.106 ± 0.005* |
| Chromosome assembly and transcription (4) | ||||||||
| F2E328 | Histone H2B | H. vulgare var. distichum | 16,236 | 10.02 | 0.215 ± 0.009* | 0.471 ± 0.035* | 0.239 ± 0.018* | 0.194 ± 0.043* |
| F2DVK7 | Predicted protein, nucleosome assembly protein* (NAP) | H. vulgare var. distichum | 42,009 | 4.32 | 3.057 ± 2.082 | 2.606 ± 1.402 | 4.897 ± 4.205* | 2.949 ± 1.780 |
| B4FYX0 | Putative uncharacterized protein, transcription factor purine-rich alpha 1* (PURα1) | Z. mays | 33,488 | 5.72 | 2.100 ± 0.749 | 2.189 ± 0.728 | 3.530 ± 1.472* | 2.249 ± 0.898 |
| F2D3D5 | Predicted protein, containing cd00590 RNA recognition motif* (RRM) | H. vulgare var. distichum | 41,295 | 5.82 | 1.319 ± 0.069 | 1.441 ± 0.224 | 2.177 ± 0.506* | 1.528 ± 0.000 |
| Protein synthesis (19) | ||||||||
| F2CQY1 | Predicted protein, 40S ribosomal protein S3* (RPS3) | H. vulgare var. distichum | 25,373 | 9.55 | 0.551 ± 0.100* | 0.413 ± 0.000* | 0.312 ± 0.085* | 0.307 ± 0.055* |
| F2DIR3 | Predicted protein, 40S ribosomal protein S4* (RPS4) | H. vulgare var. distichum | 29,949 | 10.15 | 0.164 ± 0.025* | 0.255 ± 0.111* | 0.073 ± 0.024* | 0.143 ± 0.003* |
| F2D448 | Predicted protein, containing pfam00333 ribosomal protein S5 domain* (RPS5) | H. vulgare var. distichum | 30,341 | 10.18 | 0.240 ± 0.083* | 0.297 ± 0.108* | 0.110 ± 0.115* | 0.175 ± 0.109* |
| B4FKA4 | Putative uncharacterized protein, 40S ribosomal protein S14* (RPS14) | Z. mays | 16,363 | 10.56 | 0.306 ± 0.115 | 0.324 ± 0.110* | 0.256 ± 0.114 | 0.307 ± 0.155 |
| D7KHV6 | 40S ribosomal protein S15a (RPS15a) | Arabidopsis lyrata subsp. lyrata | 14,804 | 9.89 | 0.714 ± 0.134 | 0.708 ± 0.092 | 0.395 ± 0.107* | 0.307 ± 0.231* |
| F2E598 | Predicted protein, containing pfam01090 ribosomal protein S19 domain* (RPS19) | H. vulgare var. distichum | 17,084 | 9.89 | 0.438 ± 0.062* | 0.599 ± 0.099 | 0.313 ± 0.047* | 0.277 ± 0.072* |
| Q6V959 | Ribosomal protein L3 (RPL3) | T. aestivum | 44,592 | 10.07 | 0.139 ± 0.016* | 0.277 ± 0.080 | 0.141 ± 0.063* | 0.255 ± 0.099 |
| Q0D868 | Os07g0180900 protein, containing PRK04042 ribosomal protein L4* (RPL4) | O. sativa subsp. japonica | 46,694 | 10.64 | 0.145 ± 0.017* | 0.287 ± 0.034* | 0.071 ± 0.032* | 0.162 ± 0.053* |
| F2DAK3 | Predicted protein, 60S ribosomal protein L6* (RPL6) | H. vulgare var. distichum | 24,372 | 10.10 | 0.462 ± 0.042* | 0.494 ± 0.108* | 0.321 ± 0.130* | 0.174 ± 0.044* |
| F2E0C0 | Predicted protein, 60S ribosomal protein L7* (RPL7) | H. vulgare var. distichum | 28,287 | 10.03 | 0.220 ± 0.061* | 0.350 ± 0.131* | 0.100 ± 0.026* | 0.157 ± 0.040* |
| F2DE13 | Predicted protein, 60S ribosomal protein L7a* (RPL7a) | H. vulgare var. distichum | 29,409 | 10.34 | 0.111 ± 0.005 | 0.208 ± 0.039* | 0.106 ± 0.021* | 0.164 ± 0.030* |
| F2CT73 | Predicted protein, 60S ribosomal protein L8* (RPL8) | H. vulgare var. distichum | 28,191 | 11.08 | 0.306 ± 0.105* | 0.444 ± 0.224* | 0.184 ± 0.152* | 0.181 ± 0.016* |
| F2DVU2 | 60S ribosomal protein L13 (RPL13) | H. vulgare var. distichum | 24,129 | 10.91 | 0.171 ± 0.051* | 0.217 ± 0.027* | 0.090 ± 0.004* | 0.176 ± 0.060* |
| Q5I7L1 | Ribosomal protein L13a (RPL13a) | T. aestivum | 23,530 | 10.39 | 0.290 ± 0.206* | 0.369 ± 0.205* | 0.197 ± 0.222* | 0.254 ± 0.207* |
| Q9AXS0 | Ribosomal protein L17-1 (RPL17-1) | Poa secunda | 19,564 | 10.25 | 0.338 ± 0.034 | 0.410 ± 0.100 | 0.128 ± 0.021* | 0.252 ± 0.037 |
| F2EAX5 | Predicted protein, 60S ribosomal protein L22* (RPL22) | H. vulgare var. distichum | 14,375 | 9.56 | 0.270 ± 0.037* | 0.243 ± 0.116* | 0.257 ± 0.040* | 0.156 ± 0.097* |
| Q07760 | 60S ribosomal protein L23 (RPL23) | Nicotiana tabacum | 14,988 | 10.48 | 0.275 ± 0.038 | 0.337 ± 0.042 | 0.200 ± 0.066* | 0.224 ± 0.073* |
| F2CTT6 | Predicted protein, containing COG0182 translation initiation factor 2b subunit domain* (eIF2b) | H. vulgare var. distichum | 38,573 | 5.56 | 4.924 ± 0.256* | 2.238 ± 1.266 | 5.062 ± 0.264* | 5.961 ± 1.536* |
| F2CS01 | Predicted protein, eukaryotic initiation factor 3 subunit* (eIF3) | H. vulgare var. distichum | 83,367 | 5.03 | 1.854 ± 0.384 | 1.517 ± 0.187 | 2.846 ± 0.449* | 2.466 ± 0.032* |
| Protein processing (4) | ||||||||
| A9U4U1 | Predicted protein, nascent polypeptide-associated complex subunit alpha-like protein-like* (NAC) | Physcomitrella patens subsp. patens | 21,604 | 4.35 | 0.275 ± 0.264* | 0.247 ± 0.274* | 0.766 ± 0.149 | 0.327 ± 0.267* |
| F2EE28 | Predicted protein, chaperonin 60* (CPN60) | H. vulgare var. distichum | 61,033 | 5.45 | 0.491 ± 0.107* | 0.446 ± 0.121* | 0.696 ± 0.221 | 0.423 ± 0.125* |
| F2DRC5 | Predicted protein, T-complex protein 1 subunit beta* (TCP1) | H. vulgare var. distichum | 57,337 | 5.63 | 0.570 ± 0.228 | 0.679 ± 0.132 | 0.327 ± 0.055* | 0.515 ± 0.020 |
| Q7XJ80 | Cytosolic heat shock protein 90 (Hsp90) | H. vulgare | 80,419 | 4.95 | 0.160 ± 0.056* | 0.077 ± 0.009* | 0.342 ± 0.122* | 0.147 ± 0.096* |
| Protein degradation (7) | ||||||||
| F2E7G1 | Predicted protein, 26S protease regulatory subunit 4* (P26S4) | H. vulgare var. distichum | 49,686 | 5.90 | 0.586 ± 0.015* | 0.714 ± 0.017 | 0.357 ± 0.117* | 0.556 ± 0.121 |
| F2D121 | Predicted protein, 26S protease regulatory subunit 6B* (P26S6B) | H. vulgare var. distichum | 45,685 | 5.74 | 0.639 ± 0.189 | 0.587 ± 0.053 | 0.237 ± 0.147* | 0.426 ± 0.112* |
| D3G8A3 | 26S protease regulatory subunit-like protein (P26SLP) | L. perenne | 48,018 | 4.84 | 0.649 ± 0.017 | 0.617 ± 0.004* | 0.451 ± 0.059* | 0.460 ± 0.092* |
| F2DQ10 | Predicted protein, 26S proteasome regulatory subunit S2* (P26S2) | H. vulgare var. distichum | 98,121 | 5.05 | 1.652 ± 0.255 | 1.797 ± 0.236 | 2.013 ± 0.175 | 2.517 ± 0.202* |
| Q6H852 | Proteasome subunit alpha type (PSA) | O. sativa subsp. japonica | 25,844 | 5.38 | 2.885 ± 0.996* | 2.288 ± 0.761* | 3.472 ± 1.378* | 2.515 ± 0.819* |
| Q941B7 | At2g39730/T5I7.3, containing pfam00004 ATPase family associated with various cellular activities domain* (AAA+ ATPase) | Arabidopsis thaliana | 52,039 | 5.69 | 1.538 ± 1.312 | 2.900 ± 1.528* | 8.982 ± 5.673* | 11.663 ± 6.928* |
| E0A9F0 | Methionine aminopeptidase (MAP) | H. vulgare | 43,373 | 6.58 | 0.805 ± 0.050 | 0.440 ± 0.100* | 0.501 ± 0.000* | 0.657 ± 0.070 |
| Carbohydrate and energy metabolism (7) | ||||||||
| P12783 | Phosphoglycerate kinase, cytosolic (PGK) | T. aestivum | 42,121 | 5.64 | 0.671 ± 0.104 | 0.652 ± 0.093 | 0.185 ± 0.103* | 0.444 ± 0.081 |
| F2CS51 | Pyruvate kinase (PK) | H. vulgare var. distichum | 55,453 | 7.50 | 1.366 ± 0.151 | 1.092 ± 0.021 | 1.661 ± 0.108 | 1.907 ± 0.099* |
| F2CX32 | Pyruvate kinase (PK) | H. vulgare var. distichum | 57,436 | 6.48 | 1.038 ± 0.027 | 1.128 ± 0.073 | 1.692 ± 0.088* | 1.872 ± 0.097* |
| B6TRW8 | Dihydrolipoyllysine-residue succinyltransferase component of 2-oxoglutarate dehydrogenase complex (DLST) | Z. mays | 48,775 | 8.95 | 2.043 ± 0.864 | 1.660 ± 0.322 | 2.754 ± 0.376* | 2.030 ± 0.649 |
| Q84ZL6 | Os08g0154300 protein, containing cd00957 transaldolase domain* (TA) | O. sativa subsp. japonica | 43,019 | 5.17 | 2.309 ± 0.739 | 2.139 ± 0.305 | 3.391 ± 1.210* | 3.136 ± 1.042 |
| F2CYT1 | Predicted protein, sorbitol dehydrogenase* (SDH) | H. vulgare var. distichum | 38,905 | 6.27 | 1.517 ± 0.138 | 1.706 ± 0.022 | 1.915 ± 0.062* | 1.928 ± 0.201* |
| F2CWQ6 | ATP synthase gamma chain | H. vulgare var. distichum | 35,424 | 9.39 | 0.640 ± 0.004 | 0.662 ± 0.052 | 0.377 ± 0.051 | 0.302 ± 0.037* |
| Amino acid metabolism (5) | ||||||||
| F2DWA1 | Chorismate synthase (CS) | H. vulgare var. distichum | 54,896 | 8.22 | 0.512 ± 0.047 | 0.363 ± 0.066 | 0.277 ± 0.117* | 0.329 ± 0.053 |
| C5IW60 | Glutamine synthetase (GS) | L. perenne | 38,762 | 5.58 | 0.504 ± 0.078 | 0.249 ± 0.121* | 0.180 ± 0.092 | 0.283 ± 0.051* |
| Q25C96 | Aspartate aminotransferase (AAT) | H. vulgare | 45,173 | 5.75 | 0.986 ± 0.054 | 0.523 ± 0.031* | 0.363 ± 0.088* | 0.650 ± 0.088 |
| F2D9P4 | Predicted protein, O-acetylserine (thiol) lyase of cysteine synthase complex* (CysS) | H. vulgare var. distichum | 37,137 | 5.38 | 1.440 ± 0.206 | 1.343 ± 0.183 | 2.301 ± 0.015* | 2.341 ± 0.274 |
| Q5EI64 | Pyrroline-5-carboxylate reductase (P5CR) | T. aestivum | 29,353 | 8.88 | 2.704 ± 0.035 | 1.846 ± 0.060 | 4.131 ± 0.054* | 3.183 ± 0.269* |
| Fatty acid metabolism (3) | ||||||||
| D2KZ12 | 3-ketoacyl-CoA thiolase-like protein (KCT) | T. aestivum | 47,925 | 8.21 | 0.606 ± 0.186 | 0.540 ± 0.215 | 0.446 ± 0.112* | 0.628 ± 0.146 |
| A2WNV6 | Putative uncharacterized protein, ATP citrate lyase* (ACL) | O. sativa subsp. indica | 66,069 | 7.57 | 0.499 ± 0.016* | 0.520 ± 0.071 | 0.453 ± 0.000* | 0.545 ± 0.043* |
| F2E3J2 | Predicted protein, leukotriene A4 hydrolase* (LTA4H) | H. vulgare var. distichum | 67,816 | 4.99 | 0.692 ± 0.094 | 0.664 ± 0.133 | 0.477 ± 0.117* | 0.596 ± 0.066 |
| Other metabolisms (10) | ||||||||
| Q40062 | 2′-deoxymugineic-acid 2′-dioxygenase (IDS3) | H. vulgare | 37,732 | 5.94 | 0.506 ± 0.121 | 0.396 ± 0.046 | 0.128 ± 0.049* | 0.363 ± 0.024 |
| F2DIA8 | Predicted protein, containing pfam00150 cellulase domain* | H. vulgare var. distichum | 117,787 | 5.57 | 1.289 ± 0.166 | 1.370 ± 0.308 | 1.924 ± 0.466* | 1.552 ± 0.348 |
| F2DIZ2 | Predicted protein, coproporphyrinogen III oxidase* (CPOX) | H. vulgare var. distichum | 43,446 | 7.05 | 2.270 ± 0.309 | 1.971 ± 0.307 | 2.646 ± 0.412* | 2.292 ± 0.445 |
| F2CSU5 | N-acetyl-gamma-glutamyl-phosphate reductase (AGPR) | H. vulgare var. distichum | 44,838 | 8.55 | 2.120 ± 0.425 | 2.033 ± 0.395 | 2.496 ± 0.548* | 2.192 ± 0.355* |
| C5WVL6 | Putative uncharacterized protein Sb01g031870, containing PLN02343 allene oxide cyclase domain* (AOC) | S. bicolor | 29,368 | 9.45 | 2.607 ± 0.506 | 3.049 ± 0.919 | 4.135 ± 0.617* | 3.605 ± 0.328 |
| F2DUQ7 | Predicted protein, containing cd04727 pyridoxal 5′-phosphate synthase domain* (PdxS) | H. vulgare var. distichum | 33,247 | 6.60 | 1.767 ± 0.255 | 1.908 ± 0.372 | 2.781 ± 0.399* | 3.257 ± 1.182* |
| F2E2V8 | Predicted protein, containing cd08936 peroxisomal carbonyl reductase like, classical SDR domain* (CR) | H. vulgare var. distichum | 26,780 | 8.42 | 1.700 ± 0.122 | 1.649 ± 0.172 | 2.323 ± 0.076* | 2.103 ± 0.178 |
| F2DZ92 | Predicted protein, containing cd07572 nitrilase domain* | H. vulgare var. distichum | 33,407 | 5.82 | 2.732 ± 0.561* | 2.140 ± 0.468* | 3.945 ± 0.778* | 4.018 ± 0.857* |
| F2D9Z5 | Predicted protein, containing smart00835 cupin domain* | H. vulgare var. distichum | 38,148 | 5.65 | 1.571 ± 0.082 | 1.684 ± 0.077 | 2.188 ± 0.028* | 1.655 ± 0.140 |
| F2CT63 | Predicted protein, containing cd04899 C-terminal ACT domains of the bacterial signal-transducing uridylyltransferase/uridylyl-removing enzyme* (UUR) | H. vulgare var. distichum | 33,923 | 5.92 | 2.114 ± 0.337* | 1.136 ± 0.138 | 1.598 ± 0.254 | 1.479 ± 0.172 |
aDatabase accession numbers from UniProt. bThe names and functional categories of the proteins identified by iTRAQ-based proteomics analysis. Protein names marked with an asterisk (*) have been edited by us according to functional domain annotations from NCBI non-redundant protein database. The abbreviations for the protein names are indicated in the bracket after protein names. cThe plant species that the peptides matched from. d,eTheoretical mass (Da) (d) and pI (e) of identified proteins. fRelative protein abundances under 150 mM Na2CO3 for 12 h, 200 mM Na2CO3 for 12 h, 150 mM Na2CO3 for 24 h, and 200 mM Na2CO3 for 24 h compared with control condition, respectively. Most of the protein abundance changes were compared with control condition, but the abundance change of PK (Accession No. F2CS51) was compared with 150 mM Na2CO3 for 12 h, eIF3 and PGK were compared with 200 mM Na2CO3 for 12 h, and MAP was compared with 150 mM Na2CO3 for 24 h. The ratios were presented as means ± standard deviation. The asterisks indicate significant differences (p < 0.05).
Na2CO3-responsive signaling and metabolic processes revealed from protein patterns
Broadly, 72 Na2CO3-responsive proteins covered a wide range of molecular functions, including signaling, ROS scavenging, transportation, chromosome assembly, transcription, protein synthesis, protein processing, protein degradation, carbohydrate and energy metabolism, amino acid metabolism, fatty acid metabolism, and other metabolisms (Table 1 and Supplementary Table S2). Among them, protein synthesis-related proteins accounted for the largest group (26% of Na2CO3-responsive proteins) (Table 1). The abundance change patterns of Na2CO3-responsive proteins show that multiple signaling and metabolic pathways are modulated in roots to cope with stress.
Signaling transduction and vesicle trafficking are affected by Na2CO3 stress. Six Na2CO3-responsive proteins involved in signal transduction were identified, including four calcium binding proteins, protein kinase and phosphatase. Among them, two calcium binding proteins, developmentally regulated plasma membrane polypeptide (DREPP) and calreticulin (CRT)-like protein were increased, but the other two proteins (CRT and calmodulin) were decreased under Na2CO3 stress (Table 1). Calcium-dependent protein kinase (CDPK) and serine/threonine-protein phosphatase (STPP) involved in protein phosphorylation and dephosphorylation were increased in Na2CO3-stressed roots. In addition to calcium signaling, ROS also work as signal molecules for alkali-responsive regulation. The increases of two ROS scavenging-related proteins, 2-Cys peroxiredoxin (PrxR) and GR, were detected under Na2CO3 stress (Table 1). Both PrxR and GR are responsible for H2O2 reduction, which is supposed to help maintain cellular ROS homeostasis. In addition, four Na2CO3-responsive proteins involved in cellular transportation were decreased in roots under stress (Table 1). They were PM H+-ATPase (P-ATPase) involved in proton transport, vesicle-associated membrane protein (VAMP) involved in membrane fusion, dynamin-related protein (DRP) associated with membrane trafficking, and mitochondrial phosphate transporter (MPT) in charge of Pi uptake. However, Rab1 related to cellular trafficking was increased in Na2CO3-stressed roots (Table 1).
Under the stress, gene expression and protein synthesis are altered in roots. We found four proteins involved in chromosome assembly and transcription were affected by Na2CO3 stress (Table 1). Among them, Na2CO3-decreased histone H2B and Na2CO3-increased nucleosome assembly protein (NAP) were involved in chromosome assembly. In addition, Na2CO3-increased transcription factor purine-rich alpha 1 (PURα1) and RNA recognition motif (RRM) could contribute to enhance specific gene transcription under stress condition. Importantly, we also identified thirty proteins involved in protein synthesis and turnover in Na2CO3-treated roots (Table 1). Among them, seventeen out of nineteen proteins related to protein synthesis were ribosomal protein subunits. They were all decreased in roots under Na2CO3 stress (Table 1). However, eukaryotic initiation factor 2b (eIF2b) involved in the initiation phase of eukaryotic translation was increased under Na2CO3 stress, and eIF3 was increased by Na2CO3 treatment for 24 h when compared with 12 h. In addition, four protein folding and processing-related proteins, nascent polypeptide-associated complex (NAC), chaperonin 60 (CPN60), T-complex protein 1 (TCP1), and Hsp90 were all decreased after Na2CO3 treatments. Furthermore, seven alkali-responsive proteins involved in protein degradation were also identified (Table 1). Among them, 26S protease regulatory subunit 4 (P26S4) and 6B (P26S6B), and 26S protease regulatory subunit-like protein (P26SLP) were decreased, but 26S proteasome regulatory subunit S2 (P26S2) and proteasome subunit alpha type (PSA), as well as ATPase family associated with various cellular activities (AAA+ ATPase) were increased in roots under Na2CO3. Additionally, methionine aminopeptidase (MAP) was decreased in roots treated with 150 mM and 200 mM Na2CO3 for 12 h when compared with 150 mM Na2CO3 for 24 h. This implies that protein synthesis, processing, and turnover are generally inhibited under Na2CO3.
Seven Na2CO3-responsive proteins involved in carbohydrate and energy metabolism were detected (Table 1). Among them, phosphoglycerate kinase was decreased under 150 mM Na2CO3 for 24 h when compared with 200 mM Na2CO3 for 12 h. In addition, pyruvate kinase (accession number F2CS51) was increased under 200 mM Na2CO3 for 24 h when compared with 150 mM Na2CO3 for 12 h. However, another pyruvate kinase (accession number F2CX32) was increased in Na2CO3-stressed roots. Besides, dihydrolipoyllysine-residue succinyltransferase component of 2-oxoglutarate dehydrogenase complex in the tricarboxylic acid (TCA) cycle, transaldolase in the pentose phosphate pathway, and sorbitol dehydrogenase associated with sugar metabolism were increased under Na2CO3 stress. While, ATP synthase gamma chain was decreased under Na2CO3 stress.
We identified five Na2CO3-responsive proteins involved in amino acid metabolism (Table 1). Chorismate synthase, glutamine synthetase, and aspartate aminotransferase were decreased, but O-acetylserine (thiol) lyase of cysteine synthase complex and pyrroline-5-carboxylate reductase were increased. Moreover, three fatty acid metabolism-related proteins were decreased, including 3-ketoacyl-CoA thiolase-like protein, ATP citrate lyase, and leukotriene A4 hydrolase. Additionally, we also found ten proteins involved in other metabolisms, most of which were increased in P. tenuiflora roots under Na2CO3 stress (Table 1).
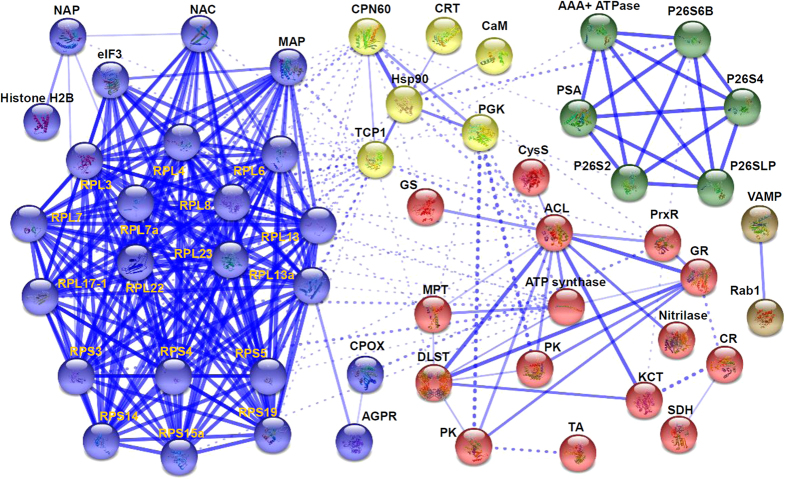
Protein-protein interaction (PPI) network
PPI network for Na2CO3-responsive proteins was visualized using STRING analysis based on homologous proteins in Arabidopsis (Fig. 6; Supplementary Table S2). Out of the 72 proteins, 53 proteins were depicted in the STRING database based on published literature, genome analysis of domain fusion, phylogenetic profiling/homology, gene neighborhood, co-occurrence, co-expression, and other experimental evidence. In the protein networks, stronger associations are represented by thicker lines (Fig. 6). Four main interactive clusters were formed among these proteins (Fig. 6). In Model 1 (yellow nodes), proteins involved in calcium signaling (CRT and calmodulin), protein processing (CPN60, TCP1, and Hsp90), and glycolysis (phosphoglycerate kinase) appeared close links (Fig. 6). This indicates that active protein turnover is crucial for signal transduction in Na2CO3-stressed roots. Model II (red nodes) included fifteen proteins belonging to ROS scavenging (PrxR and GR), transportation (MPT), carbohydrate metabolism (pyruvate kinase, dihydrolipoyllysine-residue succinyltransferase, transaldolase, sorbitol dehydrogenase, and ATP synthase), amino acid metabolism (glutamine synthetase and O-acetylserine (thiol) lyase of cysteine synthase complex). Additionally, four proteins participating in other metabolisms were also assigned in Model 2 (Fig. 6). Moreover, chromosome assembly proteins (histone H2B and NAP) and the members of protein synthesis machine (eIF3 and ribosomal proteins), as well as ribosome-associated chaperone NAC and aminopeptidase MAP were closely linked in Model III (blue nodes) (Fig. 6). In addition, five subunits of 26S proteasome (P26S2, P26S4, P26S6B, P26SLP, and PSA) and AAA+ ATPase were assigned in Model IV (green nodes). These indicate that protein synthesis and turnover play important roles in roots under alkali stress. Besides, interaction between two transportation-related proteins, VAMP and Rab1, was also predicted in the network.
Figure 6. Visualization of protein-protein interaction (PPI) network of differentially abundant proteins in Puccinellia tenuiflora roots using STRING analysis (confidence mode).
A total of 53 differentially abundant proteins represented by homologous proteins from Arabidopsis are shown in PPI network. The nodes represent proteins, and different protein groups are indicated in different colors. The lines represent the predicted functional associations. Strong associations are represented by thicker lines. Detailed information on protein names and abbreviations can be found in Table 1.
Homologous gene expression of Na2CO3-responsive proteins
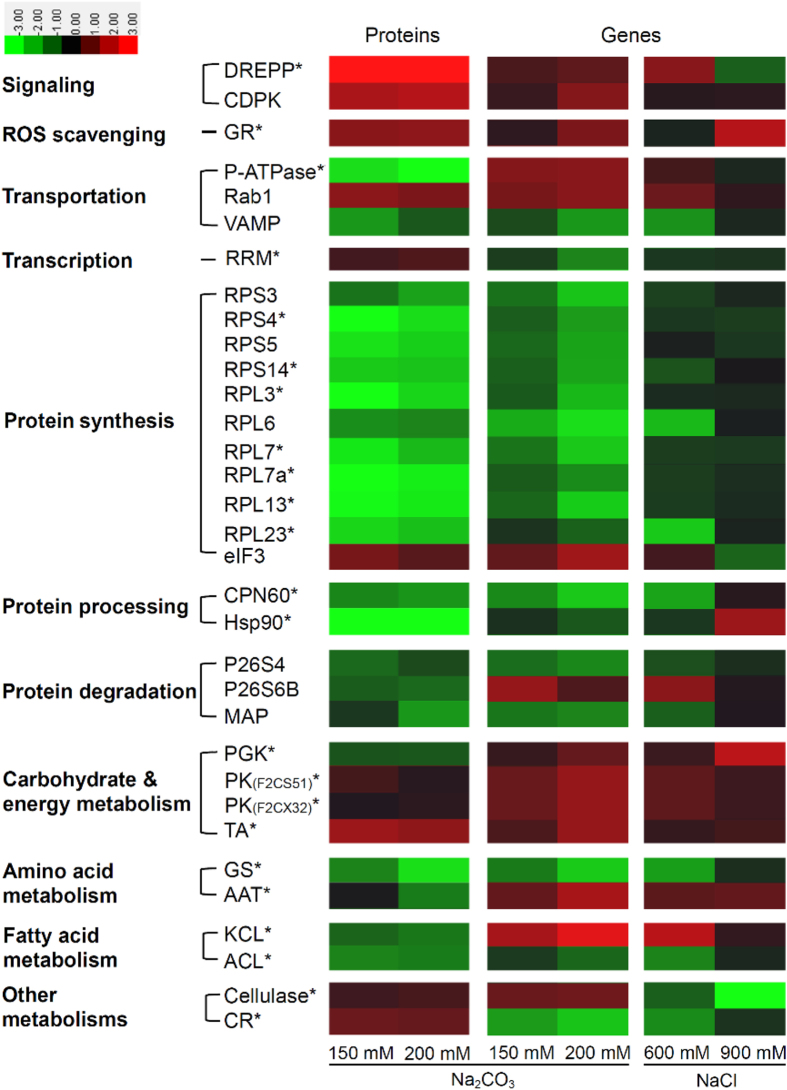
After sequence alignment analysis using TBLASTN algorithm, 69 homologous genes of Na2CO3-responsive proteins were found in the cDNA library of P. tenuiflora treated with 100 mM Na2CO3. Among them, 23 homologous genes were found to be differentially expressed at more than two-fold in seedlings under NaCl (600 mM and 900 mM for 12 h) and Na2CO3 (150 mM and 200 mM for 12 h) treatments based on microarray analysis (Supplementary Table S3). In addition, another ten differentially expressed genes in the microarray results were supposed to have similar function with the encoding genes of Na2CO3-responsive proteins based on protein functional domain analysis (Supplementary Table S3). Altogether, the correlation between 33 Na2CO3-responsive proteins and their corresponding genes were evaluated based on the comparison of proteomic and microarray results (Fig. 7). The results showed that nine proteins appeared in the increasing trends consistent with their corresponding genes, including two signal transduction-related proteins (DREPP and CDPK), a ROS scavenging enzyme (GR), a transportation-related Rab1, an eIF3 for protein synthesis, three carbohydrate/energy metabolic enzymes (two pyruvate kinases and a transaldolase), and a cell wall dynamics-related cellulase (Fig. 7). Furthermore, 17 Na2CO3-decreased proteins have similar trends as the corresponding genes, such as VAMP, ten ribosome proteins, two protein processing-related CPN60 and Hsp90, two enzymes for protein degradation (P26S4 and MAP), a glutamine synthetase for amino acid metabolism, as well as an ATP citrate lyase for fatty acid metabolism (Fig. 7). However, seven proteins showed opposite expression trends with their corresponding genes. Among them, five alkali-decreased proteins (i.e., P-ATPase, P26S6B, phosphoglycerate kinase, aspartate aminotransferase, and 3-ketoacyl-CoA thiolase-like protein) appeared induced at the transcriptional level, and the gene expression of two alkali-increased proteins (RRM and carbonyl reductase) were reduced. Interestingly, most of these genes, except for the cellulase and eIF3, showed similar expression trends in response to various Na2CO3 and NaCl treatments (Fig. 7). All these results indicate that the levels of most Na2CO3-responsive proteins were consistent with the corresponding gene expression levels in P. tenuiflora seedlings.
Figure 7. Expression pattern of 33 Na2CO3-responsive proteins and their corresponding genes under alkaline and salt stresses.
The columns represent different treatment conditions. They were 150 mM and 200 mM Na2CO3 for 12 h, as well as 600 mM and 900 mM NaCl for 12 h. The rows represent individual proteins and corresponding genes. Abbreviations of protein names and metabolic pathways are listed on the left side. The scale bar indicates log2 transformed relative expression levels of proteins and genes. The increased and decreased abundances of proteins and genes are represented in red and green, respectively. The color intensity increases with increasing abundant differences. Protein name marked with an asterisk represents the protein has homologous gene in cDNA dataset of Puccinellia tenuiflora. Accession numbers of two isoforms of pyruvate kinase were indicated in the brackets. Please see Table 1 for protein name abbreviations. Detailed information can be found in Supplementary Table S3.
Discussion
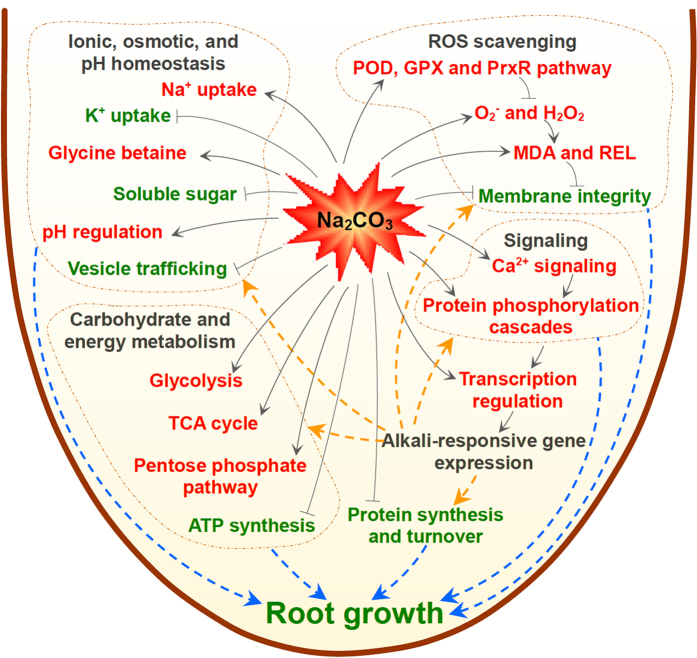
Alkali salt is more likely to cause serious stress than neutral salt
The activity of root system under stress conditions is critical for the plant survival and optimal growth34. P. tenuiflora is a monocotyledonous halophyte with high tolerance to alkaline stress. P. tenuiflora plants grow well under 50 mM Na2CO3 (pH 11.0) and could tolerate up to 150 mM Na2CO3 (pH 11.0) for 6 days17. The relative growth rate of P. tenuiflora roots was increased under 60 mM mixed alkali salt stress (NaHCO3 and Na2CO3) for 7 days, but it was decreased at higher concentrations (120–240 mM)4. Consistently, we found that the root growth was slightly inhibited under 150 mM Na2CO3 for 24 h and 200 mM Na2CO3 for 12 h and 24 h (Fig. 1A,B). These results suggest that the root growth of P. tenuiflora might be promoted under alkali stress with as high as 60 mM Na+, but inhibited at higher alkali concentrations. Different from halophyte P. tenuiflora, root growth of glycophyte sunflower was reduced under 5–15 mM Na2CO3 stress7. This implies that halophyte roots probably exhibit higher alkali tolerance than glycophyte.
In addition to alkali stress, P. tenuiflora can tolerate up to 600 mM NaCl for 6 days, but 900 mM NaCl would lead to the death of P. tenuiflora17. The relative growth rate of P. tenuiflora root was increased after 60 mM and 120 mM mixed neutral salt stress (NaCl and Na2SO4)4. However, the root biomass of P. tenuiflora was decreased after treated with 150 and 200 mM NaCl for 7 days2, and the relative growth rate of roots was significantly inhibited after 240 mM mixed salt stress (NaCl and Na2SO4)4. These results indicate that P. tenuiflora can tolerate higher level of neutral salts when compared with the level of alkali salts. This is probably because alkali stress causes an additional high pH stress to plants in addition to ionic and osmotic stresses35. In order to survive from such a severe stress, the activation and cooperation of multiple salt-resistant pathways are required to support optimal growth under alkali stress.
Ca2+ signaling and reversible protein phosphorylation are crucial for Na2CO3 response in roots
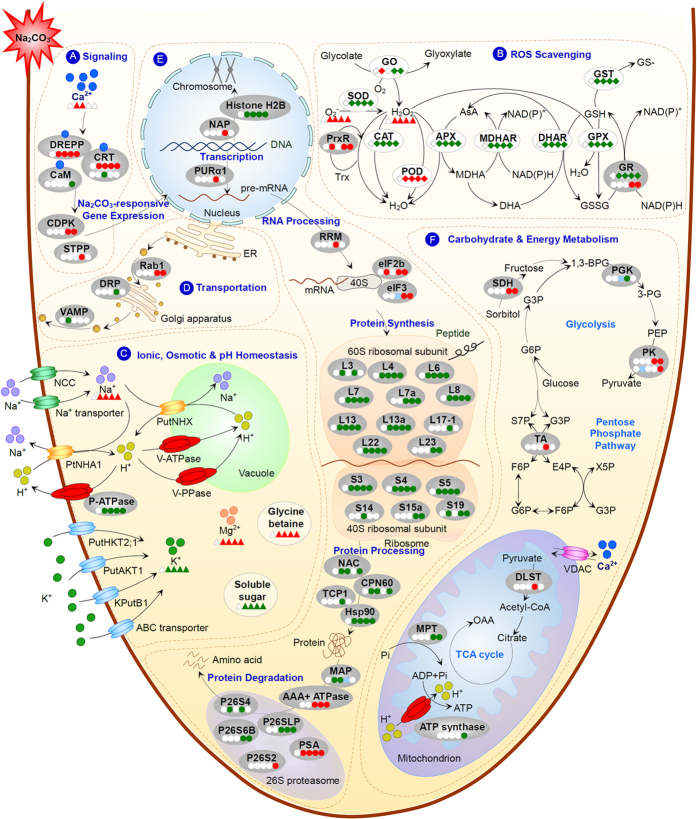
In the complicated salt-responsive signaling networks, the alteration of intracellular Ca2+ levels, the Ca2+ interaction with calcium-binding proteins, and the activation of Ca2+-regulated protein phosphorylation cascades are all vital for modulating specific salt-responsive gene expression36. Our data here provide important information for underlying Na2CO3-responsive Ca2+ signaling pathways in P. tenuiflora roots (Fig. 8A).
Figure 8. Na2CO3-responsive mechanism in roots of Puccinellia tenuiflora revealed by iTRAQ-based proteomics.
The solid line indicates single-step reaction, and the dashed line indicates multi-step reactions. Relative protein abundances, enzyme activities, and substrate contents in corresponding treatments compared with control are marked with circles, diamonds, and triangles in white (unchanged), red (increased), and green (decreased), respectively. Most of the protein abundance changes were compared with control condition (the left white circle), but the abundance changes of eIF3, PGK, PK, and MAP were compared with other treatment conditions which were marked with blue circles. Five circles/diamonds/triangles from left to right represent different treatment conditions including control, 150 mM Na2CO3 for 12 h, 200 mM Na2CO3 for 12 h, 150 mM Na2CO3 for 24 h, and 200 mM Na2CO3 for 24 h, respectively. (A) signaling; (B) ROS scavenging; (C) ionic, osmotic, and pH homeostasis; (D) transportation; (E) protein synthesis and turnover; (F) carbohydrate and energy metabolism. Abbreviations: 1,3-BPG, 1,3-bisphosphoglycerate; 3-PG, 3-phosphoglycerate; ABC transporter, ATP-binding cassette transporter; AKT, Arabidopsis K+ transporter; APX, ascorbate peroxidase; AsA, ascorbate; CAT, catalase; DHA, dehydroascorbate; DHAR, dehydroascorbate reductase; E4P, erythrose 4-phosphate; ER, endoplasmic reticulum; F6P, fructose 6-phosphate; G3P, glyceraldehyde 3-phosphate; G6P, glucose 6-phosphate; GO, glycolate oxidase; GPX, glutathione peroxidase; GSH, glutathione; GSSG, oxidized glutathione; GST, glutathione S-transferase; HKT, high-affinity K+ transporter; KPutB, K+ channel β subunit from Puccinellia tenuiflora; MDHA, monodehydroascorbate; MDHAR, monodehydroascorbate reductase; NCC, nonselective cation channel; NHA, Na+/H+ antiporter; NHX, Na+/H+ exchanger; OAA, oxaloacetic acid; PEP, phosphoenolpyruvate; POD, peroxidase; S7P, sedoheptulose 7-phosphate; SOD, superoxide dismutase; TCA, tricarboxylic acid; Trx, thioredoxin; V-ATPase, vacuolar-type H+-transporting ATPase; VDAC, voltage-dependent anion channel protein; V-PPase, vacuolar proton-inorganic pyrophosphatase; X5P, xylulose 5-phosphate. Please see Table 1 for abbreviations of proteins identified in this study.
In general, transient increase in cytosolic Ca2+ is considered to be an early response to Na+ increase in roots8. We found that Ca2+ content was increased in P. tenuiflora roots under 150 mM and 200 mM Na2CO3 for 12 h, but it was unchanged after 24 h treatment (Fig. 2D). Interestingly, in roots of halophyte Kosteletzkya virginica, Ca2+ level also did not change under 100 mM NaCl for 26 days37. This implies that the variation of Ca2+ level in halophyte roots transmits an important transient signal to trigger certain alkali-/salt- responsive gene expression.
Consistent with the transient increase of Ca2+ level, our proteomic results revealed that two calcium-binding proteins, DREPP and CRT-like protein, which involved in receiving Ca2+ signal, were increased under various Na2CO3 stress conditions (Table 1). Besides, a homologous gene of DREPP was induced in P. tenuiflora in response to alkali and salt stresses17 (Fig. 7). Similarly, DREPP was also increased in rice roots under NaCl stress38. This might facilitate the transduction of calcium signal for initiating downstream alkali-/salt- responsive gene expression39. However, DREPP gene expression was decreased in wild soybean roots under 50 mM NaHCO312, indicating the different alkali-responsive patterns of DREPP at the gene and protein levels. Besides, we found CRT was decreased under 200 mM Na2CO3 for 12 h (Table 1). Previous transcriptomic investigations have reported the diverse expression patterns of CRT genes in response to alkali stress. For example, the majority of CRT genes were induced in wild soybean roots under 50 mM NaHCO312. However, 50 mM Na2CO3 treatment for 5 h led to the down-regulation of a CRT gene in maize roots11. All these results suggest that CRTs in roots are sensitive to alkali stresses, being regulated at both gene and protein levels in different plant species. Moreover, calmodulin, another Ca2+ signal transducer, was decreased in P. tenuiflora roots under Na2CO3 stress (Table 1). The down-regulation of calmodulin genes was also found in roots of halophyte Limonium bicolor40 and wild soybean12 under NaHCO3 stress. Moreover, the NaCl-decreased calmodulin was detected in roots of rice38, maize41, and tomato (Solanum lycopersicum)42. This suggests that calmodulin is a common member in salt- and alkali-responsive signaling pathways in roots.
It has been proposed that reversible protein phosphorylation cascades play important roles in Ca2+ signaling under salt stress36. CDPK, which can be activated directly by the binding of Ca2+ to its calmodulin-like domain43, is considered as one of the major conserved players in coupling inorganic Ca2+ signal to specific protein phosphorylation cascade36. In our results, CDPK was increased by Na2CO3 in P. tenuiflora roots (Table 1). Consistently, a CDPK gene was also induced in P. tenuiflora seedlings under Na2CO317 (Fig. 7). A large amount of gain-of-function and loss-of-function studies have proved that various CDPK genes, such as OsCDPK744, OsCPK1245, OsCPK2146, AtCPK647 and ZoCDPK148, were all positive regulators involved in plant salt tolerance. Among them, the OsCDPK744 and AtCPK647 are considered as the homologous genes of PtCDPK (Contig720)17, because their encoded proteins showed 61% and 62.1% identities, respectively, on the basis of amino acid sequence analysis (Supplementary Fig. S2). It has been proved that over-expressing OsCDPK744 and AtCPK647 enhanced the rice and Arabidopsis tolerance to salt stress. Thus, the Na2CO3-induced PtCDPK at protein and gene levels would function in enhancement of alkali tolerance. Similarly, a CDPK gene was up-regulated in roots of halophyte L. bicolor under 400 mM NaHCO3 for 48 h40. Three out of four CDPK genes identified in halophyte T. hispida roots were also up-regulated under 300 mM NaHCO3 for 12 h and 48 h13. However, in wild soybean roots, only about half of CDPK genes were up-regulated under 50 mM NaHCO312, which might be due to its less alkali tolerance when compared with the halophytes mentioned above. Besides, STPP containing protein phosphatase type 1 (PP1) and keltch like domains, was also increased in P. tenuiflora roots under Na2CO3 treatment (Table 1). Interestingly, a PP1 isoform 2 gene was increased while PP1 genes were decreased under 300 mM NaHCO3 for 12 h and 24 h in roots of woody halophyte T. hispida13. In addition, the majority of STPP genes in wild soybean roots were decreased under 50 mM NaHCO312. The diverse patterns of STPP in roots under various alkali stresses suggest that the rapid switch between protein phosphorylation and dephosphorylation happens transiently for modulating corresponding gene expression in the roots to cope with stress.
Specific ROS scavenging pathways are employed in roots under Na2CO3
In salinity-stressed roots, the ROS level is dramatically elevated in cytosol, mitochondrion, peroxisome, and apoplast33. The accumulated ROS play a dual role in salt response, as toxic molecules causing oxidative damage and signaling molecules in the regulation of stress-responsive gene expression. Thus, the balance between ROS production and ROS scavenging is crucial to root growth under stress condition. In roots, ROS is mainly produced from over-reduction of the electron transduction chain in mitochondrion, while ROS detoxification depends on various ROS scavenging enzymes and antioxidants (e.g., AsA and GSH)33,49.
In this study, we found that oxidative stress triggered by Na2CO3 disrupted cellular membrane system and normal metabolism in roots. The O2− and H2O2 levels were increased dramatically with the increase of Na2CO3 levels (Fig. 4A). The cell membrane appeared to be damaged by ROS, as evidenced from the increased root MDA content and REL (Fig. 3B,C). All these indicate that P. tenuiflora roots undergo serious oxidative stress when subjected to 150 mM and 200 mM Na2CO3. Importantly, among ten important enzymes in ROS scavenging system, only the activities of POD and GPX were increased, but those of SOD, CAT, APX, MDHAR, DHAR, GR, and GST were all decreased with the increasing Na2CO3 stress (Fig. 4B–F). This implies that most ROS scavenging pathways are inhibited under Na2CO3 stress (Fig. 8B). Thus, in these cases, the accumulated H2O2 could not be efficiently scavenged through the CAT pathway and AsA-GSH cycle, which were catalyzed by APX, MDHAR, DHAR, and GR (Fig. 8B). Considering the increased level of PrxR revealed from our proteomic results (Table 1), the extra H2O2 might be eliminated mainly through the POD, PrxR, and GPX pathways to cope with Na2CO3 stress. In addition, the decreased activity of GST was speculated to accelerate GSH accumulation for active GPX pathway in the roots (Fig. 8B). Similarly, the pathways of POD, PrxR, and GPX have been reported to be alkali-increased in roots of other plant species. For example, POD abundance was increased in tomato roots under 50 mM NaHCO342, and several genes encoding PODs were induced in roots of woody halophyte T. hispida13 and wild soybean12 under NaHCO3. In addition, PrxRs were up-regulated in wild soybean roots under 50 mM NaHCO312. All these indicate that the enhancement of POD, PrxR, and GPX pathways would facilitate the scavenging of ROS in alkali-stressed roots.
The Na2CO3-inhibited pathways found in this study, such as SOD pathway, CAT pathway, and AsA-GSH cycle, were also known to be alkali-/salt-inhibited in roots of other plants. For example, the NaHCO3-reduced genes of CAT, APX, MDHAR, and GR were reported in roots of T. hispida13 and wild soybean12. Generally, the protein abundances and/or activities of oxidative enzymes were inhibited under higher concentration or longer duration of stress. For example, the activity of GO was increased in P. tenuiflora roots under 150 mM Na2CO3 for 12 h, but decreased under 24 h treatment (Fig. 4B). GO acts as a H2O2 generator through catalyzing the oxidation of glycolate to glyoxylate. Our results indicate that the oxidation of glycolate is initially induced under 150 mM Na2CO3 for 12 h, but it is inhibited under severe Na2CO3 stress, leading to the accumulation of glycolate and reduction of H2O2 production (Fig. 8B). Similarly, under NaHCO3 stress, most GSTs in wild soybean roots were up-regulated at 3–6 h, but down-regulated after 12 h12.
Interestingly, our results revealed that the abundance of GR was increased (Table 1), which is consistent with the induced homologous GR gene in P. tenuiflora17 (Fig. 7), but its activity was decreased in P. tenuiflora roots under Na2CO3 stress (Figs 4E and 8B). This implies that the dynamic of ROS scavenging system and redox status in roots are transient, compartmental, and complicated in coping with the alkali stress. Studies of protein abundance, enzyme activities, and protein redox modulation at organelle level may facilitate a deep understanding of ROS scavenging and redox regulation.
Modulation of ionic, osmotic, and pH homeostasis in roots under Na2CO3
Under alkali stress conditions, extra Na+ enters roots through nonselective cation channels and Na+ transporters, but the uptake of K+ is inhibited simultaneously8,50 (Fig. 8C). Especially, high pH has an additional influence on ionic and osmotic balance in roots35. Halophytes have developed diverse mechanisms to maintain intracellular ion homeostasis in roots, including maintaining K+ uptake, limiting Na+ entry, and enhancing Na+ exclusion and compartmentalization35. In P. tenuiflora roots, three genes encoding K+ transporters/channels have been characterized, including PutHKT2;1, PutAKT1, and KPutB1 (Fig. 8C). Among them, PutHKT2;1 encoding a PM-localized high-affinity K+ transporter was expressed mainly in roots, mediating a substantial K+ uptake under low external K+ concentration and in the presence of elevated Na+ 20. Moreover, the PM-localized PutAKT1 encoding a hyperpolarization-activated K+-selective inward-rectifying channel, was also predominantly expressed in roots under both normal condition and NaCl stress22. The function of PutAKT1 in salt tolerance was demonstrated from the enhanced cellular K+ uptake and reduced Na+ accumulation in the PutAKT1 over-expressed Arabidopsis seedlings under salt stress22. In addition, KPutB1 encoding a K+ channel β subunit was preferentially expressed in roots, and can be induced under 300 mM NaCl for 6–24 h51. Arabidopsis plants over-expressing KPutB1 showed lower Na+ content and higher K+/Na+ ratio than that in the control plants under 75 mM NaCl51. Importantly, KPutB1 can interact with PutAKT1 and the yeast co-expressing PutAKT1 and KPutB1 showed better growth and higher K+ uptake ability than yeast expressing PutAKT1 alone51. Besides, several Na2CO3-responsive ATP-binding cassette (ABC) transporter genes revealed from transcriptomic analysis may play a role in K+ transportation in P. tenuiflora seedlings17 (Fig. 8C). Furthermore, previous proteomic studies revealed several K+ transporters, such as voltage-gated potassium channel52, cyclic nucleotide-gated channel53, and ABC transporters52,53, were increased in roots of NaCl-stressed wheat (Triticum aestivum). All these highlight that enhancement of K+ uptake is a vital strategy in modulating ion homeostasis in roots to cope with salt and alkaline stresses. However, we found 150 mM and 200 mM Na2CO3 stress resulted in dramatic Na+ accumulation, K+ decline, and K+/Na+ ratio decrease in P. tenuiflora roots (Fig. 2A–C). This is different with our previous findings that the K+ content was increased in P. tenuiflora seedlings under 95 mM Na2CO3 for 7 days25. It needs to be further investigated whether it is the K+ uptake into roots were inhibited or the K+ in roots were rapidly transported to leave under higher concentration of Na2CO3.
In addition to the irreplaceable K+ required for diverse enzymatic processes, we found that the uptake of Ca2+ and Mg2+ was not inhibited in P. tenuiflora roots (Fig. 2D,E) and seedlings25 under Na2CO3. The increased Ca2+ in P. tenuiflora roots and seedlings under Na2CO3 and NaCl stresses24,25 might facilitate cell wall rigidity and PM integrity apart from its secondary messenger role54. Ca2+ transporters play a key role in regulating cellular Ca2+ levels to cope with salt and alkali stresses. An important Ca2+ transporter, voltage-dependent anion channel protein (VDAC) located in the mitochondrial outer membrane was reported to be affected by Na2CO3 and NaCl stresses (Fig. 8F). For instance, the expression of two genes encoding VDACs were inhibited in P. tenuiflora seedlings under Na2CO3 stress17. However, proteomic studies have revealed that VDACs were increased in NaCl-stressed roots of maize41 and wild tomato (Solanum chilense)55. We didn’t find the abundance change of VDAC in Na2CO3-stressed roots of P. tenuiflora, whether it was inhibited needs further investigation. Moreover, the increased Mg2+ content in Na2CO3-stressed P. tenuiflora roots and the constant levels in seedlings under 50 mM and 150 mM NaCl24 would benefit chlorophyll synthesis, enzyme activation, and the stabilization of nucleotides and nucleic acids to cope with alkali/salt stresses54 (Fig. 8C).
In roots, Na+ exclusion is a vital strategy to cope with salt and alkali stress. Na+ can be exported out of cells by Na+/H+ antiporter driven by the transmembrane proton electrochemical gradient generated by P-ATPase56. In P. tenuiflora, a PM Na+/H+ antiporter encoding gene has been identified as PtNHA121. PtNHA1 was preferentially expressed in roots and up-regulated under 75–300 mM NaCl and 300 mM NaHCO321,27 (Fig. 8C). Arabidopsis over-expressing PtNHA1 displayed NaCl tolerance with less Na+ and more K+ accumulations when compared to wild type plants21. In addition, alkali response of P-ATPase at gene and protein levels was also studied in other plants. For example, genes encoding P-ATPase isoforms were down-regulated in roots of woody halophyte T. hispida under 300 mM NaHCO313, but the protein abundance of P-ATPase was increased in roots of glycophyte tomato under 50 mM NaHCO342. This implies that different mechanisms of Na+ exclusion through P-ATPase under alkali stress might lie between glycophytes and halophytes. In P. tenuiflora, although a homologous gene of P-ATPase was Na2CO3-induced in seedlings17, the protein abundance of P-ATPase was decreased in roots under Na2CO3 stress (Figs 7 and 8C; Table 1). The Na2CO3-inhibited P-ATPase can lead to low proton driving force in the PM of roots, then reduce Na+ efflux through Na+/H+ antiporters. This probably can be explained by the previous notion that lower Na+ accumulation in P. tenuiflora is mainly contributed from the restriction of unidirectional Na+ influx rather than enhancement of Na+ efflux when compared with what happened in wheat seedlings under NaCI stress2.
Na+ remaining in root cells can be sequestered into vacuoles by vacuolar Na+/H+ antiporters8. In P. tenuiflora, a gene encoding vacuolar Na+/H+ antiporter has been identified as PutNHX involved in Na+ compartmentalization into vacuole27. The expression level of PutNHX in P. tenuiflora roots under NaHCO3 was significantly higher than that under NaCl, indicating that vacuolar Na+/H+ antiporter may be specifically involved in pH regulation under alkaline conditions27 (Fig. 8C). Different from NaCl, NaHCO3 and Na2CO3 stresses generate higher intracellular pH environment, imposing severe damage on plants. Therefore, intracellular pH homeostasis is usually modulated through Na+ compartmentalization under alkaline stress. Vacuolar-type H+-transporting ATPase (V-ATPase) and vacuolar proton-inorganic pyrophosphatase (V-PPase) can provide proton driven force for vacuolar Na+/H+ antiporter57. It was found that over-expressing the P. tenuiflora V-ATPase c subunit (VHA-c) encoding gene PutVHA-c in transgenic Arabidopsis resulted in better growth phenotypes under salt stress58.
In P. tenuiflora seedlings, two genes encoding V-ATPase and a gene encoding V-PPase were up-regulated under Na2CO3 stress17 (Fig. 8C). The increased V-ATPase was also found in NaHCO3-treated tomato roots42, as well as in NaCl-stressed roots of several plants (e.g., rice, wheat, maize, pea (Pisum sativum), sugar beet (Beta vulgaris), and cucumber (Cucumis sativus))59. The alkali-/salt-induced PutNHX, V-ATPase, and V-PPase indicate that the ability of Na+ compartmentalization in P. tenuiflora is enhanced to cope with stress. More importantly, these proteins also function as H+-transporters contributing to the promotion of intracellular pH homeostasis under alkaline conditions. Furthermore, to balance the osmotic pressure in vacuoles resulted from compartmentalized Na+, the accumulation of various osmolytes in cytosol is required8. In this study, we found soluble sugar content was decreased, while the content of glycine betaine was increased in P. tenuiflora roots under Na2CO3 stress (Fig. 8C). This suggests that the accumulated glycine betaine may play a key role in maintaining cellular osmotic balance in P. tenuiflora roots under alkali stress.
Vesicle trafficking in roots under Na2CO3
In roots, the dynamics of endomembrane system and vesicle trafficking are very sensitive to ionic and osmotic imbalance resulted from salt/alkaline stress. Previous proteomic studies have found some NaCl-responsive vesicle trafficking-related proteins in roots of various plant species, including annexin, soluble N-ethylmaleimide-sensitive factor attachment protein (SNAP), SNAP receptor, vacuolar-sorting receptor 1, and protein transport protein sec159. These proteins function in vesicle trafficking by regulating the processes of tethering/docking and membrane fusion to enhance salt tolerance60,61,62. In this study, we found more players, such as Na2CO3-increased Rab1, and Na2CO3- decreased DRP, VAMP, and MPT (Fig. 8D,F; Table 1), which participate in the trafficking from the ER to the Golgi apparatus, vesicle trafficking, endocytosis/exocytosis, and Pi influx to mitochondrion, respectively63,64,65,66. Consistently, transcriptomic analysis revealed that VAMP was down-regulated in Na2CO3-stressed P. tenuiflora seedlings17 (Fig. 7) and NaHCO3-stressed T. hispida roots13. In addition, Rab1a and Rab1b in wild soybean roots12 and DRP in roots of T. hispida13 were down-regulated under NaHCO3. MPT was NaHCO3-induced at gene and protein levels in roots of T. hispida13 and tomato42, respectively. Furthermore, VAMP and DRP displayed diverse expression levels in wild soybean roots under NaHCO312. These results indicate that dynamic modulation of cellular transport system is required for maintaining cellular homeostasis under alkaline stress, and the changes of aforementioned players in this system are modulated transiently and dependent on plant species and stress conditions.
Regulation of Na2CO3-responsive gene expression, protein processing and destination
Alkali-induced gene expression is regulated by specific chromosome dynamics and transcription factors (Fig. 8E). Proteins involved in gene expression and protein fate were grouped together with strong associations in PPI network (Fig. 6). Among them, histone H2B was decreased, but its chaperone, NAP, was increased in P. tenuiflora roots under Na2CO3 stress, respectively (Table 1). The down-regulated histone H2B gene was also found in T. hispida roots under NaHCO3 for 48 h13. This indicates that dynamic chromosome assembly exists in alkali-stressed roots, which would facilitate chromosome remodeling for alkali-specific gene expression regulation67. In our results, transcription factors PURα1 and RRM were increased in Na2CO3-stressed roots (Table 1). PURα has been proposed to participate in the regulation of sucrose synthase 1 gene expression in rice68, and the NaHCO3-induced PURα1 was found in wild soybean roots12. RRM was reported to be involved in almost all post-transcriptional events, especially plastid RNA editing in plants69. The alkali-increased PURα1 and RRM may contribute to specific alkali-responsive gene expression. Additionally, eIF3 was increased in P. tenuiflora roots under 24 h of Na2CO3 compared with 12 h of stress (Table 1), and eIF2b was increased under Na2CO3 stress. This is consistent with up-regulated homologous eIF3 gene in P. tenuiflora seedlings under Na2CO317 (Fig. 7) and in T. hispida roots under NaHCO313, implying the enhanced alkali-responsive gene expression.
Previous transcriptomic analysis has shown that the down-regulated genes are often over-represented in alkali-stressed roots. For example, 62.9% of alkali-responsive genes in maize roots under Na2CO311 and over 70% alkali-responsive genes in roots of wild soybean12 and woody halophyte T. hispida under NaHCO313 were down-regulated. In P. tenuiflora seedlings, 69.5% (260 out of 374) and 63.8% (510 out of 799) alkali-responsive genes were down-regulated under 150 mM and 200 mM Na2CO3 for 12 h, respectively17. Consistent with gene expression, our proteomic results presented 57.3% (39 out of 68) alkali-responsive proteins were decreased in P. tenuiflora roots under Na2CO3 stress (Table 1). Among them, 17 ribosomal proteins were alkali-decreased (Table 1). This was consistent with the transcriptomic results that almost all the genes involved in protein synthesis were down-regulated in P. tenuiflora seedlings under Na2CO3 stress17 (Fig. 7). This implies that the protein synthesis machinery in P. tenuiflora roots is inhibited by Na2CO3 stress. Similarly, ribosomal proteins also presented NaHCO3 and NaCl-decreased abundances in roots of tomato42 and Arabidopsis70. However, in roots of 300 mM NaHCO3-stressed T. hispida, most genes encoding ribosomal proteins were down-regulated at 12 h, but up-regulated at 48 h13. Ribosomal proteins were increased in roots of sugar beet71 and cucumber72 after 7 days of 50 mM and 500 mM NaCl stress. These results indicate that protein synthesis machinery in roots tends to be inhibited by short-term alkali/salt stress, but it can be activated after a long-term period of stress.
Protein processing in P. tenuiflora roots is also affected by Na2CO3 stress, as shown by the decreased abundances of NAC, CPN60, TCP1, and Hsp90 (Fig. 8E; Table 1). Consistently, NAC in rice roots and NAC gene in wild soybean roots were decreased under 150 mM NaCl73 and 50 mM NaHCO312, respectively. In addition, homologous CPN60 and Hsp90 were down-regulated in P. tenuiflora seedlings under 150 mM and 200 mM Na2CO3 for 12 h17 (Fig. 7), and Hsp90 was decreased in NaCl-stressed roots of sugar beet71 and creeping bentgrass (Agrostis stolonifera)74. NAC acts as a component of ribosome-associated chaperones. It can associate with ribosome, interact with nascent proteins and protect them from proteolysis, and facilitate their folding75. Besides, CPN60 and TCP1 belong to different groups of chaperonin. CPN60 was found in the mitochondrion and plastid, and TCP1 was localized in cytosol, being involved in assisting the folding of newly synthesized and translocated proteins76. In addition, Hsp90 functions as a molecular chaperone for assisting protein folding and protein complex formation in many processes, such as signal transduction, cell-cycle control, protein degradation and protein trafficking76,77. The four proteins (i.e., NAC, CPN60, TCP1, and Hsp90) being decreased in roots implies that the processing/folding of nascent peptides, proteins in different subcellular locations (e.g., mitochondrion, plastid, and cytosol), and protein complexes are all inhibited under salt or alkali stress.
Selective protein degradation in roots is also regulated by Na2CO3. We found five subunits of 26S proteasome (P26S2, P26S4, P26S6B, P26SLP, and PSA) in P. tenuiflora roots were affected by Na2CO3 stress (Fig. 8E). Some alkali-responsive genes of 26S proteasome subunits were also affected in Na2CO3-stressed P. tenuiflora seedlings17 (Fig. 7) and NaHCO3-treated wild soybean roots12. Moreover, proteomic results showed that AAA+ ATPase, which appeared strong association with proteasome proteins in PPI network (Fig. 6), was increased under Na2CO3 stress. In addition, MAP was decreased in P. tenuiflora roots under Na2CO3 for 12 h compared with 24 h of treatments, while the MAP gene was down-regulated in P. tenuiflora seedlings under 200 mM Na2CO3 for 12 h17 (Fig. 7). Considering all these aforementioned results, it becomes evident that protein synthesis and turnover in P. tenuiflora roots are inhibited by Na2CO3. This can account for the reduction of root growth under alkali stress.
Carbohydrate and energy metabolism was affected under Na2CO3 stress
Carbohydrate and energy metabolism plays a vital role in root salt/alkali response. It was reported that a number of enzymes involved in glycolysis, TCA cycle, electron transport chain, and pentose phosphate pathway in roots were affected by NaCl stress59. In this study, we found that pyruvate kinase (accession number F2CX32) involved in glycolysis was increased in P. tenuiflora roots under Na2CO3 stress (Fig. 8F; Table 1). This correlates well with the previous result that a homologous gene of pyruvate kinase was up-regulated in P. tenuiflora seedlings under Na2CO317 (Fig. 7). Besides, sorbitol dehydrogenase, catalyzing the oxidation of sorbitol to fructose, which can also enter into glycolysis, was increased in P. tenuiflora roots under Na2CO3 (Fig. 8F; Table 1). In addition, dihydrolipoyllysine-residue succinyltransferase, a vital component of 2-oxoglutarate dehydrogenase complex involved in TCA cycle, was increased in roots under Na2CO3 stress (Fig. 8F; Table 1). Moreover, we found transaldolase, an enzyme of the non-oxidative phase of the pentose phosphate pathway, was increased in P. tenuiflora roots under Na2CO3 (Fig. 8F; Table 1). This is consistent with the Na2CO3- and NaHCO3-induction of homologous genes of transaldolase in P. tenuiflora seedlings17 (Fig. 7) and wild soybean roots12, respectively. These results indicate that glycolysis, TCA cycle, and pentose phosphate pathway are all probably enhanced in alkali-stressed roots to provide energy, carbon skeletons, and NADPH for cellular metabolism.
Besides of these processes, ATP synthase acts as an important enzyme to provide energy for the cells through the synthesis of ATP. A previous study found that RMtATP6 gene encoding mitochondrial ATP synthase 6 kDa subunit was increased in rice roots under NaCl, NaHCO3, and Na2CO3, respectively1. However, in the present study, ATP synthase was decreased in P. tenuiflora roots under Na2CO3 stress (Table 1). This indicates that ATP synthase in P. tenuiflora roots is sensitive to alkali stress, leading to the decrease of energy supply through ATP synthase under Na2CO3 stress.
Common and specific alkali-responsive strategies between roots and leaves
Plant root functions as the first site for sensing and transducing alkali stress signal. Our Na2CO3-responsive proteomic studies provided important information for understanding the common and specific alkali-responsive strategies in roots and leaves from P. tenuiflora25. Nine common alkali-responsive proteins were found in both roots and leaves of P. tenuiflora when exposed to Na2CO3 treatment25. Among them, DREPP, eIF, proteasome subunit, and AAA+ ATPase were Na2CO3-increased in both roots and leaves, indicating that certain processes in signal transduction and proteins synthesis/processing/turnover were usually enhanced in roots and leaves. However, RRM, ribosomal protein, phosphoglycerate kinase, ATP synthase, and aspartate aminotransferase showed different abundance changes between roots and leaves. These diverse protein patterns imply that the processes of energy supplying, gene expression, as well as protein synthesis and amino acid metabolism are modulated in roots and leaves to cope with alkali stress, but different strategies are depended on different organs and various treatment conditions (i.e., alkali concentration and treatment time). Besides of these common proteins in roots and leaves, the physiological and proteomic analyses also highlighted some common pathways, such as Ca2+ signal transduction, ROS scavenging system, ion compartmentation, and carbohydrate metabolism, were all induced in roots and leaves under Na2CO3 stress25. At the same time, several specific mechanisms were revealed in roots and leaves25. For example, the reduction of light absorption, exudation of salts through stomata, increase of thermal dissipation, and enhance of energy supply were supposed to be employed in leaves25, while the enhanced pH modulation was taken as an positive strategy in roots of P. tenuiflora to cope with alkali stress.
Conclusion
The signaling and metabolic molecular mechanisms for alkali-tolerance are fine-tuned and sophisticated in roots. Halophytes are supposed to have unique pathways/strategies to cope with alkalinity. In this work, by integrating analysis of the physiological and iTRAQ-based proteomic data, we found multiple Na2CO3-responsive strategies in P. tenuiflora roots (Fig. 9). It mainly includes (1) the activation of Ca2+-mediated signaling pathway, (2) specific ROS scavenging pathways (e.g., POD, GPX, and PrxR pathways), (3) modulation of Na+ influx restriction, Na+ compartmentalization, H+-transport, glycine betaine accumulation, and vesicle trafficking, contributing to intracellular pH, ionic and osmotic homeostasis, (4) down-regulation of gene expression, transcription, and protein processing and destination for specific alkali-responsive pathways, as well as (5) induced pathways of glycolysis, TCA and pentose phosphate. These results provide new information and insights into the underlying alkali-responsive mechanism in roots.
Figure 9. Schematic presentation of systematic Na2CO3 tolerance mechanisms in roots of Puccinellia tenuiflora.
Na2CO3 stress activates the modulation of Na+ influx restriction, Na+ compartmentalization, H+ transportation, glycine betaine accumulation, and vesicle trafficking in roots, which contribute to intracellular pH, ionic, and osmotic homeostasis. In addition, alkali stress leads to ROS burst in roots, resulting in the damages of root cell membrane. To alleviate ROS toxicity, specific ROS scavenging pathways (e.g., POD, GPX, and PrxR pathways) are induced in roots. Na2CO3 induces glycolysis, TCA cycle, and pentose phosphate pathway, providing energy, carbon skeletons, and NADPH for cellular metabolism in stressed roots. Importantly, Na2CO3 stress increases the Ca2+-mediated signaling pathway, activates the protein phosphorylation cascades, and subsequently triggers alkali-responsive gene expression. However, the protein synthesis, processing and destination are inhibited in roots under Na2CO3 stress. Solid line with arrow and “T” shape line represent stimulation and inhibition, respectively. The red words and green words indicate Na2CO3-induced and Na2CO3-reduced cellular processes, respectively. Dashed lines indicate indirect regulations. Abbreviations: GPX, glutathione peroxidase; MDA, malondialdehyde; POD, peroxidase; PrxR, peroxiredoxin; REL, relative electrolyte leakage; ROS, reactive oxygen species; TCA, tricarboxylic acid.
Methods
Plant growth conditions and Na2CO3 treatment
Seeds of Puccinellia tenuiflora (Turcz.) scribn. et Merr. were sown on pearlite and grown hydroponically in Hoagland solution under fluorescent light (300 μM·m−2·s−1, 13 h light/11 h dark) at 25 °C and 75% relative humidity in a growth chamber24. The nutrient solution was renewed every other day for a stable nutrient supply. Fifty-day-old seedlings were treated with 150 mM and 200 mM Na2CO3 (pH 11.0) in Hoagland solution for 12 h and 24 h, respectively. After Na2CO3 treatments, roots of control and Na2CO3-treated plants were excised, washed gently and briefly in deionized water, and then blotted dry on filter paper. The collected roots were used fresh or immediately frozen in liquid nitrogen and stored at −80 °C. At least three biologically independent replicates for each treatment were collected.
Root biomass measurement
Root length and Fw were measured immediately after harvesting. Roots were floated on deionized water for 24 h, and then the turgid weight (Tw) was quickly measured. Root Dw was determined after oven-dried at 80 °C for 2 h followed by 60 °C to a constant weight. The RWC was calculated as: RWC = [(Fw-Dw)/(Tw-Dw)] × 100%78.
Ion content analysis
Root tissue was ground into powder after oven-dried at 80 °C for 2 h followed by 60 °C to a constant weight. The powder was digested in nitric acid and perchloric acid solution (5:1, v/v) and incubated at room temperature overnight. The mixture was boiled on an electric stove until the turbid solution became pellucid. After 6 M HCl was added, the solution was diluted to the appropriate concentration with deionized water for ion analysis. The contents of Na+, K+, Ca2+ and Mg2+ were assayed using an atomic absorption spectrophotometer AAnalyst 800 (Perkin Elmer, Wellesley, MA, USA) at 589 nm, 766.5 nm, 422.7 nm and 285.2 nm, respectively.
Total soluble sugar, glycine betaine, MDA and REL measurement
The content of total soluble sugar was determined using a sulfuric acid-anthrone method79. For the glycine betaine assay, root tissue was ground with the 60% methanol and 25% chloroform solution. The homogenate was incubated for 24 h, and then centrifuged at 15,000 g for 15 min at 20 °C. The supernatant was collected and filtered through a 0.45-μm-pore-size cellulose acetate filter. The filtrate was dried and then resuspended in distilled water. After 0.15% reinecke salt solution was added, the reaction solution was incubated at 4 °C for 2 h. The supernatant was collected after centrifuged at 1,500 g for 15 min at 4 °C. After aether was added, the mixed solution was centrifuged at 1,500 g for 15 min at 4 °C. The supernatant was collected, dried, and redissolved in 70% acetone. The absorbance was detected under 525 nm using a UV-1800 spectrophotometer (Shimadzu, Tokyo, Japan). The glycine betaine content was calculated from the standard curve. The MDA content and REL were determined according to a previous method80.
ROS measurement and enzyme activity assay
To evaluate the levels of ROS in roots, H2O2 content and O2− generation rate were measured. Root tissue was ground with 0.1% trichloroacetic acid. The homogenate was centrifuged at 15,000 g for 15 min at 4 °C and the supernatant was collected for H2O2 measurement. H2O2 content was determined spectrophotometrically at 390 nm after reacting with potassium iodide81.
To determine O2− generation rate and antioxidant enzyme activities, root tissue was ground in extraction buffer containing 50 mM phosphate buffer solution (pH 7.8), 2% polyvinylpyrrolidone-40, and 2 mM AsA (for APX activity assay) at 4 °C. The homogenate was centrifuged at 20,000 g for 15 min at 4 °C, and the supernatant was collected for analysis. O2− generation rate was measured using a hydroxylamine oxidization method82. The activities of SOD, GO, APX, GR, and GST were determined according to our previous methods24. The activities of CAT, MDHAR, POD, and DHAR were measured by monitoring H2O2 consumption at 240 nm, monodehydroascorbate reduction at 340 nm, production of tetraguaiacol at 470 nm, and dehydroascorbate reduction at 265 nm, respectively83. Their activities were expressed as the amount of H2O2 reduced, NADH oxidized, and products of tetraguaiacol and AsA per minute per milligram protein, respectively. GPX activity was determined using a Cellular GPX Assay Kit (Beyotime, Shanghai, China) according to the manufacturer’s instructions. The activity of GPX was expressed as the amount of NADPH oxidized per minute per milligram protein. In all the enzymatic preparations, protein content was determined using the Bradford method84.
Protein extraction for proteomics
Total root protein was extracted from three biological replicate samples for each treatment using a phenol extraction protocol80. Proteins were dissolved in a lysis buffer (7 M urea, 2 M thiourea, 4% CHAPS). The protein content was determined using a EZQ Protein Quantitation Kit (Molecular Probes, Eugene, OR, USA) according to the manufacturer’s instructions.
Trypsin digestion, iTRAQ labeling, and protein fractionation
An aliquot (100 μg) of proteins was precipitated with cold acetone. The pellet was dissolved in 0.5 M triethylammonium bicarbonate (pH 8.5), reduced with 50 mM tris-(2-carboxyethyl)-phosphine, and alkylated with 200 mM methyl methanethiosulfonate (MMTS) using the iTRAQ reagent kit (AB Sciex, Framingham, MA, USA). The proteins were then digested by trypsin (Promega, Madison, WI, USA) and labeled with 8-plex iTRAQ reagents according to the manufacturer’s instructions (AB Sciex, Framingham, MA, USA) using iTRAQ tags 113, 114, 115, 116, and 117 for samples under control condition, 150 mM Na2CO3 treated for 12 h, 200 mM Na2CO3 treated for 12 h, 150 mM Na2CO3 treated for 24 h, and 200 mM Na2CO3 treated for 24 h, respectively. After labeling, the samples were combined and lyophilized. The peptide mixture was dissolved in 0.1% formic acid and desalted on a Macrospin Vydac Silica C18 column (The Nest Group, Southborough, MA, USA). After desalting, the peptides were dried down and dissolved in strong cation exchange solvent A (25% (v/v) acetonitrile, 10 mM ammonium formate, pH 2.8). The peptides were fractionated on an Agilent high-performance liquid chromatography 1260 infinity system (Agilent Technologies, Palo Alto, CA, USA) with a polysulfoethyl A column (2.1 × 100 mm, 5 μm, 300 Å, PolyLC, Columbia, MD, USA). Peptides were eluted at a flow rate of 200 μl/min with a linear gradient of 0–20% solvent B (25% (v/v) acetonitrile, 500 mM ammonium formate) over 80 min followed by ramping up to 100% solvent B in 5 min and holding for 10 min. The absorbance at 214 nm was monitored, and a total of 13 fractions were collected, lyophilized and dissolved in 0.1% formic acid for LC-MS/MS analysis.
Mass spectrometry (MS) analysis
An aliquot from each fraction was submitted to a TripleTOF 5600 system (AB Sciex, Framingham, MA, USA) coupled to an Ultra 2D Plus nanoflow ultra-performance LC with a cHiPLC Nanoflex microchip device (Eksigent Technologies, Redwood City, CA, USA). The online trapping, desalting, and analytical separation were conducted using the microfluidic traps and columns packed with ChromXP C18 (3 μm, 120 Å) of the Nanoflex system. Solvent A and B were composed of water/acetonitrile/formic acid (A, 98/2/0.1%; B, 2/98/0.1%). After peptide loading, trapping and desalting were carried out at 2 μL/min for 10 min with 100% solvent A. At a flow rate of 300 nL/min, the analytical separation was established by increasing solvent B from 5% to 10% in 0.1 min, and a linear gradient to 26% solvent B in 60 min. Then the gradient was increased to 50% solvent B in 25 min, kept increasing to 80% solvent B in 1 min, and maintained at 80% solvent B for 4 min. Initial chromatographic condition was restored in 0.1 min and maintained for 10 min. Data were acquired using an ion spray voltage of 2.3 kV, curtain gas of 30, nebulizer gas of 6, and an interface heater temperature of 150 °C. The MS was operated with a resolution of 30,000fwhm for time-of-flight MS scans. For information-dependent acquisition, survey scans were acquired in 250 ms and as many as 30 product ion scans with 100 ms accumulation time were collected if they exceeded a threshold of 150 counts per second (counts/s) and with a 2+ to 5+ charge state. The total cycle time was fixed to 3.3 s. Four time bins were summed for each scan at a pulse frequency value of 11 kHz through monitoring the 40 GHz multichannel detector with four-anode/channel detection. A sweeping collision energy setting of 35 (15 eV) was applied to all precursor ions for collision-induced dissociation. Dynamic exclusion was set for 1/2 of peak width (∼ 18 s), and then the precursor was refreshed off the exclusion list.
Protein identification and quantification
The MS/MS data were analyzed for protein identification and quantification by searching against a UniProt database (taxonomy Viridiplantae, 2, 313, 498 entries, downloaded on 14 May 2013) using ProteinPilot Software 4.5 (AB Sciex, Framingham, MA, USA). The false discovery rate was determined to be 1.0% with the integrated Proteomics System Performance Evaluation Pipeline tool in the ProteinPilot Software. Search parameters included iTRAQ 8-plex quantification, cysteine modified with MMTS, trypsin digestion, an ID focus of biological modifications and amino acid substitutions, thorough searching mode and minimum protein threshold of 95% confidence (unused protein score ≥1.3). To avoid the redundancy when combining proteins from three replicates, the proteins with similar protein family name and amino acid sequence but identified only in one replicate were taken as one unique protein in the final dataset. For protein relative quantification, only MS/MS spectra unique to a particular protein and for which the sum of the signal-to-noise ratio for all of the peak pairs greater than nine were used for quantification (software default settings). For a differentially abundant protein, it had to be quantified with at least three spectra (allowing generation of a p value), a p value < 0.05, and a ratio fold change ≥1.5 in at least two independent replicates. Only the significant ratios from the replicates were used to calculate the average ratio for the protein85.
Protein classification and PPI analysis
Protein functional classification of the identified proteins were performed manually by searching against the NCBI non-redundant protein database (http://www.ncbi.nlm.nih.gov/) using PSI and PHI-BLAST programs (http://www.ncbi.nlm.nih.gov/BLAST/) for protein functional domain annotation. The biological function of protein was obtained from the KEGG pathway database (http://www.kegg.jp/kegg/), UniProt database (http://www.ebi.uniprot.org/), and the Gene Ontology protein database (http://geneontology.org). Besides, the conservative protein function during salt/alkali tolerance was predicted from previous publications on the salt-/alkali-responsive mechanism in plant root. Finally, by integrative analysis of all the information collected from aforementioned processes, proteins were classified into different categories.
The PPIs were predicted using Search Tool for the Retrieval of Interacting Gene (STRING, version 9.1) (http://string-db.org). The differentially abundant proteins homologs in Arabidopsis were found by sequence BLASTing in TAIR database (http://www.arabidopsis.org/Blast/index.jsp). The homologs were subjected to STRING for creating the proteome-scale interaction network. Parameters for species and confidence were “Arabidopsis thaliana” and “medium confidence (0.400)”, respectively. STRING analysis was based on “confidence” mode, and disconnected nodes were hidden.
Correlation analysis of Na2CO3-responsive proteins and corresponding genes
To find homologous genes, each Na2CO3-responsive protein sequence was aligned to a cDNA library of P. tenuiflora containing 4,982 unigenes17 using TBLASTN algorithm (NCBI Blast 2.3.31+). Besides, the genes in cDNA library encoding similar functional domains with Na2CO3-responsive proteins were also selected for correlation analysis on the basis of functional domain analysis using BLASTP program (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The expression levels of the selected genes under Na2CO3 and NaCl stresses were evaluated by microarray assay17. The correlation of Na2CO3-responsive proteins and their corresponding genes were analyzed using cluster 3.0 (http://bonsai.hgc.jp/~mdehoon/software/cluster/software.htm).
Statistical analysis
All results were given as means ± standard deviation of at least three replicates. The data were subjected to one-way analysis of variance using SPSS 17.0 software (SPSS, Chicago, IL, USA). A p value smaller than 0.05 was considered to be statistically significant.
Additional Information
How to cite this article: Zhao, Q. et al. Na2CO3-responsive mechanisms in halophyte Puccinellia tenuiflora roots revealed by physiological and proteomic analyses. Sci. Rep. 6, 32717; doi: 10.1038/srep32717 (2016).
Supplementary Material
Acknowledgments
The project was supported by the National Natural Science Foundation of China (No. 31270310) and Fundamental Research Funds for the Central Universities (No. 2572014EA04), China to S. Dai, and by the Special Fund for Forestry-Scientific Research in the Public Interest (No. 201104066) to Z. Zhou.
Footnotes
Author Contributions Q.Z. and S.D. designed the study and wrote the manuscript. Q.Z., J.S., Y.J., X.M., Z.Y., Y.Z., J.L., W.J. and X.Z. participated in experiments and analyses. Q.Z., S.C., T.W., Z.Z. and S.D. discussed the results and modified the manuscript. All authors have read and approved the final manuscript.
References
- Zhang X., Takano T. & Liu S. Identification of a mitochondrial ATP synthase small subunit gene (RMtATP6) expressed in response to salts and osmotic stresses in rice (Oryza sativa L.). J. Exp. Bot. 57, 193–200 (2006). [DOI] [PubMed] [Google Scholar]
- Wang C. M. et al. Puccinellia tenuiflora maintains a low Na+ level under salinity by limiting unidirectional Na+ influx resulting in a high selectivity for K+ over Na+. Plant Cell Environ. 32, 486–496 (2009). [DOI] [PubMed] [Google Scholar]
- Chen W. et al. Comparative effects of salt and alkali stresses on organic acid accumulation and ionic balance of seabuckthorn (Hippophae rhamnoides). Ind. Crops Prod. 30, 351–358 (2009). [Google Scholar]
- Guo L. Q., Shi D. C. & Wang D. L. The key physiological response to alkali stress by the alkali-resistant halophyte Puccinellia tenuiflora is the accumulation of large quantities of organic acids and into the rhyzosphere. J. Agron. Crop Sci. 196, 123–135 (2010). [Google Scholar]
- Peng Y. L. et al. Eco-physiological characteristics of alfalfa seedlings in response to various mixed salt-alkaline stresses. J. Integr. Plant Biol. 50, 29–39 (2008). [DOI] [PubMed] [Google Scholar]
- Fan X. D. et al. Gene expression profiling of soybean leaves and roots under salt, saline-alkali and drought stress by high-throughput Illumina sequencing. Gene 512, 392–402 (2013). [DOI] [PubMed] [Google Scholar]
- Manivannan P. et al. Mineral uptake and biochemical changes in Helianthus annuus under treatment with different sodium salts. Colloids Surf. B Biointerfaces 62, 58–63 (2008). [DOI] [PubMed] [Google Scholar]
- Munns R. & Tester M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 59, 651–681 (2008). [DOI] [PubMed] [Google Scholar]
- Dinneny J. R. Analysis of the salt-stress response at cell-type resolution. Plant Cell Environ. 33, 543–551 (2010). [DOI] [PubMed] [Google Scholar]
- Galvan-Ampudia C. S. & Testerink C. Salt stress signals shape the plant root. Curr. Opin. Plant Biol. 14, 296–302 (2011). [DOI] [PubMed] [Google Scholar]
- Zhang L. M. et al. Early transcriptomic adaptation to Na2CO3 stress altered the expression of a quarter of the total genes in the maize genome and exhibited shared and distinctive profiles with NaCl and high pH stresses. J. Integr. Plant Biol. 55, 1147–1165 (2013). [DOI] [PubMed] [Google Scholar]
- Ge Y. et al. Global transcriptome profiling of wild soybean (Glycine soja) roots under NaHCO3 treatment. BMC Plant Biol. 10, 1–14 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. et al. Comprehensive transcriptional profiling of NaHCO3-stressed Tamarix hispida roots reveals networks of responsive genes. Plant Mol. Biol. 84, 145–157 (2014). [DOI] [PubMed] [Google Scholar]
- Liu D., Zhang X., Cheng Y., Takano T. & Liu S. rHsp90 gene expression in response to several environmental stresses in rice (Oryza sativa L.). Plant Physiol. Biochem. 44, 380–386 (2006). [DOI] [PubMed] [Google Scholar]
- Liu S. et al. Expression of an NADP-malic enzyme gene in rice (Oryza sativa. L) is induced by environmental stresses, over-expression of the gene in Arabidopsis confers salt and osmotic stress tolerance. Plant Mol. Biol. 64, 49–58 (2007). [DOI] [PubMed] [Google Scholar]
- Wang Y. et al. Identification of expressed sequence tags in an alkali grass (Puccinellia tenuiflora) cDNA library. J. Plant Physiol. 164, 78–89 (2007). [DOI] [PubMed] [Google Scholar]
- Zhang X., Wei L., Wang Z. & Wang T. Physiological and molecular features of Puccinellia tenuiflora tolerating salt and alkaline-salt stress. J. Integr. Plant Biol. 55, 262–276 (2013). [DOI] [PubMed] [Google Scholar]
- Peng Y. H. et al. Alkali grass resists salt stress through high [K+] and an endodermis barrier to Na+. J. Exp. Bot. 55, 939–949 (2004). [DOI] [PubMed] [Google Scholar]
- Zhang C., Shunsaku N., Liu S. & Takano T. Characterization of two plasma membrane protein 3 genes (PutPMP3) from the alkali grass, Puccinellia tenuiflora, and functional comparison of the rice homologues, OsLti6a/b from rice. BMB Rep. 41, 448–454 (2008). [DOI] [PubMed] [Google Scholar]
- Ardie S. W., Xie L., Takahashi R., Liu S. & Takano T. Cloning of a high-affinity K+ transporter gene PutHKT2, 1 from Puccinellia tenuiflora and its functional comparison with OsHKT2, 1 from rice in yeast and Arabidopsis. J. Exp. Bot. 60, 3491–3502 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. et al. Functional characterization of a plasma membrane Na+/H+ antiporter from alkali grass (Puccinellia tenuiflora). Mol. Biol. Rep. 38, 4813–4822 (2011). [DOI] [PubMed] [Google Scholar]
- Ardie S. W., Liu S. & Takano T. Expression of the AKT1-type K+ channel gene from Puccinellia tenuiflora, PutAKT1, enhances salt tolerance in Arabidopsis. Plant Cell Rep. 29, 865–874 (2010). [DOI] [PubMed] [Google Scholar]
- Sun G. et al. Does Puccinelia tenuiflora have the ability of salt exudation? Colloids Surf. B Biointerfaces 46, 197–203 (2005). [DOI] [PubMed] [Google Scholar]
- Yu J. et al. Physiological and proteomic analysis of salinity tolerance in Puccinellia tenuiflora. J. Proteome Res. 10, 3852–3870 (2011). [DOI] [PubMed] [Google Scholar]
- Yu J., Chen S., Wang T., Sun G. & Dai S. Comparative proteomic analysis of Puccinellia tenuiflora leaves under Na2CO3 stress. Int. J. Mol. Sci. 14, 1740–1762 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Zhang X., Takano T. & Liu S. Characterization of a PutCAX1 gene from Puccinellia tenuiflora that confers Ca2+ and Ba2+ tolerance in yeast. Biochem. Biophys. Res. Commun. 383, 392–396 (2009). [DOI] [PubMed] [Google Scholar]
- Kobayashi S., Abe N., Yoshida K. T., Liu S. & Takano T. Molecular cloning and characterization of plasma membrane and vacuolar-type Na+/H+ antiporters of an alkaline-salt-tolerant monocot, Puccinellia tenuiflora. J. Plant Res. 125, 587–594 (2012). [DOI] [PubMed] [Google Scholar]
- Wang Y. et al. Effects of Na2CO3 and NaCl stresses on the antioxidant enzymes of chloroplasts and chlorophyll fluorescence parameters of leaves of Puccinellia tenuiflora (Turcz.) scribn.et Merr. Acta Physiol. Plant. 30, 143–150 (2008). [Google Scholar]
- Guan Q., Wang Z., Wang X., Takano T. & Liu S. A peroxisomal APX from Puccinellia tenuiflora improves the abiotic stress tolerance of transgenic Arabidopsis thaliana through decreasing of H2O2 accumulation. J. Plant Physiol. 175, 183–191 (2015). [DOI] [PubMed] [Google Scholar]
- Zhang M., Takano T., Liu S. & Zhang X. Abiotic stress response in yeast and metal-binding ability of a type 2 metallothionein-like protein (PutMT2) from Puccinellia tenuiflora. Mol. Biol. Rep. 41, 5839–5849 (2014). [DOI] [PubMed] [Google Scholar]
- Kobayashi S. et al. Transcriptional responses of a bicarbonate-tolerant monocot, Puccinellia tenuiflora, and a related bicarbonate-sensitive species, Poa annua, to NaHCO3 stress. Int. J. Mol. Sci. 16, 496–509 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Yang C., Liu G. & Jiang J. Development of a cDNA microarray to identify gene expression of Puccinellia tenuiflora under saline-alkali stress. Plant Physiol. Biochem. 45, 567–576 (2007). [DOI] [PubMed] [Google Scholar]
- Miller G., Suzuki N., Ciftci-Yilmaz S. & Mittler R. Reactive oxygen species homeostasis and signaling during drought and salinity stresses. Plant Cell Environ. 33, 453–467 (2010). [DOI] [PubMed] [Google Scholar]
- Den Herder G., Van Isterdael G., Beeckman T. & De Smet I. The roots of a new green revolution. Trends Plant Sci. 15, 600–607 (2010). [DOI] [PubMed] [Google Scholar]
- Shi D. C. & Yin L. J. Difference between salt (NaCl) and alkaline (Na2CO3) stresses on Pucinellia tenuiflora (Griseb.) Scribn. et Merr. plants. Acta Bot. Sin. 35, 144–149 (1993). [Google Scholar]
- Xiong L., Schumaker K. S. & Zhu J. K. Cell signaling during cold, drought, and salt stress. Plant Cell 14, S165–S183 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmond Ghanem M. et al. Mucilage and polysaccharides in the halophyte plant species Kosteletzkya virginica: localization and composition in relation to salt stress. J. Plant Physiol. 167, 382–392 (2009). [DOI] [PubMed] [Google Scholar]
- Cheng Y. et al. New changes in the plasma-membrane-associated proteome of rice roots under salt stress. Proteomics 9, 3100–3114 (2009). [DOI] [PubMed] [Google Scholar]
- Jia X. Y., He L. H., Jing R. L. & Li R. Z. Calreticulin: conserved protein and diverse functions in plants. Physiol. Plant. 136, 127–138 (2009). [DOI] [PubMed] [Google Scholar]
- Wang Y. et al. Generation and analysis of expressed sequence tags from a NaHCO3-treated Limonium bicolor cDNA library. Plant Physiol. Biochem. 46, 977–986 (2008). [DOI] [PubMed] [Google Scholar]
- Zörb C., Schmitt S. & Mühling K. H. Proteomic changes in maize roots after short-term adjustment to saline growth conditions. Proteomics 10, 4441–4449 (2010). [DOI] [PubMed] [Google Scholar]
- Gong B. et al. Identification of NaCl and NaHCO3 stress responsive proteins in tomato roots using iTRAQ-based analysis. Biochem. Biophys. Res. Commun. 446, 417–422 (2014). [DOI] [PubMed] [Google Scholar]
- Harper J. F., Breton G. & Harmon A. Decoding Ca2+ signals through plant protein kinases. Annu. Rev. Plant Biol. 55, 263–288 (2004). [DOI] [PubMed] [Google Scholar]
- Saijo Y., Hata S., Kyozuka J., Shimamoto K. & Izui K. Over-expression of a single Ca2+-dependent protein kinase confers both cold and salt/drought tolerance on rice plants. Plant J. 23, 319–327 (2000). [DOI] [PubMed] [Google Scholar]
- Asano T. et al. A rice calcium-dependent protein kinase OsCPK12 oppositely modulates salt-stress tolerance and blast disease resistance. Plant J. 69, 26–36 (2012). [DOI] [PubMed] [Google Scholar]
- Asano T. et al. Functional characterisation of OsCPK21, a calcium-dependent protein kinase that confers salt tolerance in rice. Plant Mol. Biol. 75, 179–191 (2011). [DOI] [PubMed] [Google Scholar]
- Xu J. et al. AtCPK6, a functionally redundant and positive regulator involved in salt/drought stress tolerance in Arabidopsis. Planta 231, 1251–1260 (2010). [DOI] [PubMed] [Google Scholar]
- Vivek P. J., Tuteja N. & Soniya E. V. CDPK1 from ginger promotes salinity and drought stress tolerance without yield penalty by improving growth and photosynthesis in Nicotiana tabacum. PLoS One 8, e76392 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittova V., Guy M., Tal M. & Volokita M. Salinity up-regulates the antioxidative system in root mitochondria and peroxisomes of the wild salt-tolerant tomato species Lycopersicon pennellii. J. Exp. Bot. 55, 1105–1113 (2004). [DOI] [PubMed] [Google Scholar]
- Tester M. & Davenport R. Na+ tolerance and Na+ transport in higher plants. Ann. Bot. 91, 503–527 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardie S. W., Nishiuchi S., Liu S. & Takano T. Ectopic expression of the K+ channel β subunits from Puccinellia tenuiflora (KPutB1) and rice (KOB1) alters K+ homeostasis of yeast and Arabidopsis. Mol. Biotechnol. 48, 76–86 (2011). [DOI] [PubMed] [Google Scholar]
- Peng Z. et al. A proteomic study of the response to salinity and drought stress in an introgression strain of bread wheat. Mol. Cell. Proteomics 8, 2676–2686 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M. C. et al. Proteomic analysis on a high salt tolerance introgression strain of Triticum aestivum/Thinopyrum ponticum. Proteomics 8, 1470–1489 (2008). [DOI] [PubMed] [Google Scholar]
- Maathuis F. J. Physiological functions of mineral macronutrients. Curr. Opin. Plant Biol. 12, 250–258 (2009). [DOI] [PubMed] [Google Scholar]
- Zhou S. P., Sauvé J., Liu Z., Reddy S. & Bhatti S. Identification of salt-induced changes in leaf and root proteomes of the wild tomato, Solanum chilense. J. Am. Soc. Hortic. Sci. 136, 288–302 (2011). [Google Scholar]
- Gévaudant F. et al. Expression of a constitutively activated plasma membrane H+-ATPase alters plant development and increases salt tolerance. Plant Physiol. 144, 1763–1776 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apse M. P., Aharon G. S., Snedden W. A. & Blumwald E. Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 285, 1256–1258 (1999). [DOI] [PubMed] [Google Scholar]
- Zhou A., Bu Y., Takano T., Zhang X. & Liu S. Conserved V-ATPase c subunit plays a role in plant growth by influencing V-ATPase-dependent endosomal trafficking. Plant Biotechnol. J. 14, 271–283 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q., Zhang H., Wang T., Chen S. & Dai S. Proteomics-based investigation of salt-responsive mechanisms in plant roots. J. Proteomics 82, 230–253 (2013). [DOI] [PubMed] [Google Scholar]
- Gerke V. & Moss S. E. Annexins: from structure to function. Physiol. Rev. 82, 331–371 (2002). [DOI] [PubMed] [Google Scholar]
- Han S. Y., Park D. Y., Park S. D. & Hong S. H. Identification of Rab6 as an N-ethylmaleimide-sensitive fusion protein-binding protein. Biochem. J. 352, 165–173 (2000). [PMC free article] [PubMed] [Google Scholar]
- Assaad F. F., Huet Y., Mayer U. & Jürgens G. The cytokinesis gene KEULE encodes a Sec1 protein that binds the syntaxin KNOLLE. J. Cell Biol. 152, 531–543 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batoko H., Zheng H. Q., Hawes C. & Moore I. A rab1 GTPase is required for transport between the endoplasmic reticulum and Golgi apparatus and for normal Golgi movement in plants. Plant Cell 12, 2201–2218 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Z. et al. A unified nomenclature for Arabidopsis dynamin-related large GTPases based on homology and possible functions. Plant Mol. Biol. 53, 261–265 (2003). [DOI] [PubMed] [Google Scholar]
- Hoepflinger M., Hametner C., Ueda T. & Foissner I. Vesicular trafficking in characean green algae and the possible involvement of a VAMP72-family protein. Plant Signal. Behav. 9, e28466 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takabatake R. et al. Isolation and characterization of cDNAs encoding mitochondrial phosphate transporters in soybean, maize, rice, and Arabidopsis. Plant Mol. Biol. 40, 479–486 (1999). [DOI] [PubMed] [Google Scholar]
- Wang W. et al. Overexpression of Camellia sinensis H1 histone gene confers abiotic stress tolerance in transgenic tobacco. Plant Cell Rep. 33, 1829–1841 (2014). [DOI] [PubMed] [Google Scholar]
- Chang J. C., Liao Y. C., Yang C. C. & Wang A. Y. The purine-rich DNA-binding protein OsPurα participates in the regulation of the rice sucrose synthase 1 gene expression. Physiol. Plant. 143, 219–234 (2011). [DOI] [PubMed] [Google Scholar]
- Sun T. et al. An RNA recognition motif-containing protein is required for plastid RNA editing in Arabidopsis and maize. Proc. Natl. Acad. Sci. USA 110, E1169–E1178 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Yang B., Harris N. S. & Deyholos M. K. Comparative proteomic analysis of NaCl stress-responsive proteins in Arabidopsis roots. J. Exp. Bot. 58, 3591–3607 (2007). [DOI] [PubMed] [Google Scholar]
- Yang L., Ma C., Wang L., Chen S. & Li H. Salt stress induced proteome and transcriptome changes in sugar beet monosomic addition line M14. J. Plant Physiol. 169, 839–850 (2012). [DOI] [PubMed] [Google Scholar]
- Du C. X., Fan H. F., Guo S. R., Tezuka T. & Li J. Proteomic analysis of cucumber seedling roots subjected to salt stress. Phytochemistry 71, 1450–1459 (2010). [DOI] [PubMed] [Google Scholar]
- Yan S., Tang Z., Su W. & Sun W. Proteomic analysis of salt stress-responsive proteins in rice root. Proteomics 5, 235–244 (2005). [DOI] [PubMed] [Google Scholar]
- Xu C., Sibicky T. & Huang B. Protein profile analysis of salt-responsive proteins in leaves and roots in two cultivars of creeping bentgrass differing in salinity tolerance. Plant Cell Rep. 29, 595–615 (2010). [DOI] [PubMed] [Google Scholar]
- Preissler S. & Deuerling E. Ribosome-associated chaperones as key players in proteostasis. Trends Biochem. Sci. 37, 274–283 (2012). [DOI] [PubMed] [Google Scholar]
- Wang W., Vinocur B., Shoseyov O. & Altman A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 9, 244–252 (2004). [DOI] [PubMed] [Google Scholar]
- Makhnevych T. & Houry W. A. The role of Hsp90 in protein complex assembly. Biochim. Biophys. Acta 1823, 674–682 (2012). [DOI] [PubMed] [Google Scholar]
- Guo G. et al. Comparative proteomic analysis of salt response proteins in seedling roots of two wheat varieties. J. Proteomics 75, 1867–1885 (2012). [DOI] [PubMed] [Google Scholar]
- Li H. S., Sun Q., Zhao S. J. & Zhang W. H. Principles and Techniques of Plant Physiological Biochemical Experiment (ed. Wang W.) 195–197 ( Higher Education Press, 2000). [Google Scholar]
- Wang X. et al. Desiccation tolerance mechanism in resurrection fern-ally Selaginella tamariscina revealed by physiological and proteomic analysis. J. Proteome Res. 9, 6561–6577 (2010). [DOI] [PubMed] [Google Scholar]
- Ibrahim M. H. & Jaafar H. Z. Primary, secondary metabolites, H2O2, malondialdehyde and photosynthetic responses of Orthosiphon stimaneus Benth. to different irradiance levels. Molecules 17, 1159–1176 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan B., Dai Q., Liu X., Huang S. & Wang Z. Flooding-induced membrane damage, lipid oxidation and activated oxygen generation in corn leaves. Plant Soil 179, 261–268 (1996). [Google Scholar]
- Chaparzadeh N., D’Amico M. L., Khavari-Nejad R. A., Izzo R. & Navari-Izzo F. Antioxidative responses of Calendula officinalis under salinity conditions. Plant Physiol. Biochem. 42, 695–701 (2004). [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976). [DOI] [PubMed] [Google Scholar]
- Zhu M., Dai S., McClung S., Yan X. & Chen S. Functional differentiation of Brassica napus guard cells and mesophyll cells revealed by comparative proteomics. Mol. Cell. Proteomics 8, 752–766 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.