Abstract
Background
While multiple prognostic variables have been proposed for Ewing sarcoma (EWS), little work has been done to further categorize these variables into prognostic groups for risk classification.
Procedure
We derived initial prognostic groups from 2,124 patients with EWS in the SEER database. We constructed a multivariable recursive partitioning model of overall survival using the following covariates: age; stage; race/ethnicity; sex; axial primary; pelvic primary; and bone or soft tissue primary. Based on this model, we identified risk groups and estimated 5-year overall survival for each group using Kaplan-Meier methods. We then applied these groups to 1,680 patients enrolled on COG clinical trials.
Results
A multivariable model identified 5 prognostic groups with significantly different overall survival: 1) Localized, age < 18 years, non-pelvic primary; 2) Localized, age < 18, pelvic primary or Localized, age ≥ 18, White, non-Hispanic; 3) Localized, age ≥ 18, all races/ethnicities other than White, non-Hispanic; 4) Metastatic, age < 18; and 5) Metastatic, age ≥ 18. These 5 groups were applied to the COG dataset and showed significantly different overall and event-free survival based upon this classification system (p < 0.0001). A sub-analysis of COG patients treated with ifosfamide and etoposide as a component of therapy evaluated these findings in patients receiving contemporary therapy.
Conclusions
Recursive partitioning analysis yields discrete prognostic groups in EWS that provide valuable information for patients and clinicians in determining an individual patient's risk of death. These groups may enable future clinical trials to adjust EWS treatment according to individualized risk.
Keywords: Ewing sarcoma, recursive partitioning, prognostic groups
INTRODUCTION
Ewing sarcoma (EWS) is a cancer of bone or soft tissues most prevalent in children and young adults. Multiple studies have identified clinical prognostic factors in EWS. The presence of metastases at diagnosis is consistently the strongest adverse clinical prognostic factor [1-4]. Larger tumor size and older age are also consistently associated with poor prognosis [1-10]. While many studies have associated older age with inferior survival, the optimal cut-point that defines “older” patients remains undefined. The studies referenced highlight this issue as the definitions for older age ranged from greater than 14 to 40 years old at diagnosis.
Many other prognostic factors have also been evaluated, including tumor site, tissue origin (bone or soft tissue), and race/ethnicity. However, no clear consensus on their independent prognostic value has been reached [1-4,7,11-13].
While these prior studies have identified factors that individually impact prognosis in EWS, few publications have described combining these prognostic factors into discrete risk groups that might guide future risk-adapted clinical trials and eventually clinical care. Moreover, prior studies focused on individual prognostic factors have largely ignored potential interactions between factors, such that one variable may be prognostic within one group and not another. One study used Cox proportional hazards methods to identify four distinct groups: 1) favorable risk (age <14 years with localized, non-pelvic tumors); 2) intermediate risk (localized and age ≥ 14 years old or pelvic tumors at any age); 3) unfavorable pulmonary (isolated lung metastases); and 4) unfavorable extrapulmonary (extrapulmonary metastases) [1]. These potential risk groups have not been validated.
In order to address these gaps in our knowledge, we utilized recursive partitioning to identify discrete prognostic groups in EWS using data derived from the NCI Surveillance Epidemiology and End Results (SEER) database. Recursive partitioning is a statistical method that uses decision trees in an attempt to correctly classify participants based on multiple, independent variables.[14] We then applied these groups using data from patients treated on Children's Oncology Group clinical trials.
METHODS
Patients
This retrospective cohort study utilized data from the SEER database to define prognostic groups and applied them to patient information from the COG. Data from the SEER system are derived from ~26% of the US population [15]. The SEER population tends to be more urban and have a higher proportion of foreign-born people, but is otherwise comparable to the general US population. The SEER program provides data on cancer incidence, patient demographics, primary tumor site, tumor morphology, stage at diagnosis, and survival. We identified 3,180 patients with a histologic diagnosis of EWS, Askin tumor, or peripheral primitive neuroectodermal tumor (PNET) diagnosed between 1988-2008. We excluded key groups of patients in the SEER database in order to align the SEER cohort with known characteristics of the COG cohort. We excluded 779 patients because their tumor arose within the central nervous system (while primary central nervous system tumors have the same name as peripheral PNET, they are not thought to be related biologically) and 96 patients because they had secondary EWS. Age was reported as whole integers without decimal places for partial years in SEER. 181 patients who were diagnosed at age 50 years or greater were excluded to align the SEER population with the COG cohort which only enrolled patients up until the age of 50. The remaining 2,124 patients formed the analytic SEER cohort (Supplemental Figure 1). Only the 1,945 patients that had complete variable data were included in the recursive partitioning models.
The COG cohort included all eligible patients enrolled on the following COG clinical trials for newly diagnosed metastatic or localized EWS: INT-0091 (enrolled December 1988 to November 1992); INT-0154 (enrolled May 1995 – September 1998); CCG-7951 (enrolled February 1996 to November 1998); AEWS0031 (enrolled May 2001 – August 2005); and AEWS02P1 (enrolled March 2004 – April 2008) [9,16-19]. INT-0154 and AEWS0031 included only patients who were non-metastatic at the time of diagnosis. AEWS02P1 and CCG-7951 included only patients who were metastatic at the time of diagnosis. INT-0091 included patients with metastatic or localized disease. Patients enrolled in INT-0091, INT-0154, and CCG-7951 were ≤ 30 years at the time of diagnosis and those enrolled in AEWS02P1 and AEWS0031 were ≤ 50 years at the time of diagnosis. INT-0091 and CCG-7951 included only patients with Ewing sarcoma of bone whereas INT-0154, AEWS0031, and AEWS02P1 included patients with either bone or soft tissue primaries. 1,690 patients were identified from these studies; however ten of these patients were excluded because of missing tumor site data leaving a total of 1,680 patients in the COG cohort (Supplemental Figure 1). As patients in the SEER cohort were de-identified, adjustment for potential overlap between the SEER and COG cohorts was not possible.
Analytical Methods
Using the SEER cohort, a univariate recursive partitioning model of overall survival was used to identify the optimal prognostic threshold for age [20,21]. The recursive partitioning model uses martingale residuals of a Cox model to calculate (approximate) chi-square values for all possible cut-points of variables included in the model [21]. The model was only allowed to choose a cut-point if the resulting two-sided p-value was less than 0.01 and the resulting group had a minimum of 10 subjects. A hazard ratio for death for each group was then calculated using a Cox proportional hazards model with the resulting age groups from the recursive partitioning model used as the only variable.
Following identification of the optimal age cut-point, a multivariable recursive partitioning model was constructed using this age cut-point as a binary variable. The following additional potential covariates were included: stage (localized vs. metastatic); self-reported race/ethnicity (White, non-Hispanic vs. Other); sex; primary site (axial vs. appendicular); primary site (pelvic vs. non-pelvic); and bone vs. soft tissue primary. The model was again constrained to only choose a cut-point if the resulting two-sided p-value was less than 0.01 and the resulting group had a minimum of 10 subjects. Based on this model, we identified discrete risk groups and estimated 5-year overall survival for each risk group using Kaplan-Meier methods. Overall survival was defined as the time from diagnosis to the time of death. An overall log-rank test was done to compare the survival distributions. We also used a Cox proportional hazards model to calculate a hazard ratio for death in each risk group. The Cox model used risk group as its only variable and was set so that the lowest risk group served as the reference for the remaining groups. Groups were ordered according to their increasing hazard ratio and an unadjusted log rank statistic was calculated for each pair of groups considered adjacent to that ranking. Adjusted p-values accounting for multiple comparisons between adjacent groups were also calculated using the Bonferroni method.
We then applied these prognostic groups to the COG cohort and used Kaplan-Meier methods to determine overall survival and event-free survival (EFS) for each group. Event-free survival was defined as the time from enrollment onto the COG trial to the time of death, relapse or development of a secondary malignancy. An overall log-rank test and pair-wise log-rank tests between groups were calculated for each outcome. We also looked exclusively at patients in the COG cohort who received ifosfamide and etoposide as a component of their therapy in an attempt to evaluate the modern, North American standard treatment.
The SEER database was accessed using SEER*Stat version 7.0.5. All statistical analyses were performed using STATA, version 12. All recursive partitioning work was done using Wim van Putten's ado file for STATA [20]. This study was Institutional Review Board (IRB) exempt as no patient identifying information was used.
RESULTS
Patient Characteristics
Characteristics of patients in the SEER and COG cohorts are shown in Table I. The median age of patients in the SEER cohort was 17. In the COG cohort the median age was 13. In COG studies that limited age to patients ≤ 30 years, 4 (0.4%) patients were between the ages of 18 and 30. In COG studies that limited age to patients ≤ 50, 4 (0.7%) patients were between the ages of 18 and 50. There were several other differences between the two groups. The SEER cohort had more participants from minority populations and with metastatic disease. We also found differences in tissue origin (soft tissue vs. bone), with the SEER cohort having a higher proportion of patients with EWS of soft tissue origin.
Table I.
Patient characteristics for the SEER and COG cohorts
| Characteristic | SEER Cohort | COG Cohort | p-value |
|---|---|---|---|
| Total Number of Subjects, n | 2,124 | 1,680 | |
| Sex, n (%) | |||
| Male | 1,279 (60) | 930 (55) | 0.003 |
| Age at Diagnosis in Years | |||
| Median | 17 | 13 | <0.0001 |
| Range | 0 – 50 | 0 – 50 | |
| Race, n (%) | |||
| White, non-Hispanic | 1,431 (67) | 1,205 (72) | <0.001 |
| Other | 657 (31) | 228 (13) | |
| Unknown | 36 (2) | 247(15) | |
| Primary Tumor Site, n (%) | |||
| Pelvic | 477 (22) | 347 (21) | 0.34 |
| Non pelvic | 1,647 (78) | 1,296 (77) | |
| Axial | 1,263 (59) | 1,006 (60) | 0.28 |
| Appendicular | 861 (41) | 637 (38) | |
| Unknown | 0 (0) | 37 (2) | |
| Tissue Origin, n (%) | |||
| Bone | 1,283 (60) | 827 (49) | <0.001 |
| Soft tissue | 841 (40) | 213 (13) | |
| Unknown | 0 (0) | 640 (38) | |
| Stage, n (%) | |||
| Localized | 1,374 (65) | 1,436 (85) | <0.001 |
| Metastatic | 599 (28) | 244 (15) | |
| Unknown | 151 (7) | 0 (0) | |
| COG Treatment Protocol | N/A | N/A | |
| INT-0091 | 573 | ||
| INT-0154 | 472 | ||
| CCG-7951 | 32 | ||
| AEWS-0031 | 568 | ||
| AEWS-02P1 | 35 | ||
Abbreviations: SEER Surveillance Epidemiology and End Results; COG Children's Oncology Group
Determination of Optimal Age Threshold in the SEER Cohort
The results of a univariate recursive partitioning model of overall survival using age as the only potential covariate identified the initial age cut-point. All potential whole integer age cut-points were considered, however 18 years was selected as the best cut based on the parameters outlined in the methods (Supplemental Figure 2). Patients who were diagnosed between 18 and 50 years had a hazard ratio for death of 1.96 [95% confidence interval (CI) 1.71-2.25] compared to those who were diagnosed between the ages of 1 and 17 years. Patients who were < 1 year at the time of diagnosis also did worse with a hazard ratio of 2.12 (95% CI 1.22-3.69). Given the relatively small number of infants in the SEER cohort (n = 29) and that EWS is predominately a disease of teenagers and young adults and rarely seen in infants, we proceeded with a binary age at diagnosis cut-point (<18 years of age vs. ≥ 18 years) in subsequent analyses.
Identification of Prognostic Groups in the SEER Cohort
A multivariable recursive partitioning model of overall survival revealed 6 potential risk groups (Figure 1). An overall log-rank test to compare the survival distributions of the six groups demonstrated significant differences in overall survival (p < 0.0001). We next evaluated pair-wise log-rank tests between adjacent groups. The group that was defined as localized, age < 18 years, pelvic primary and the group defined as localized, age ≥ 18 years, white, non-Hispanic were found to have nearly identical overall survival distributions (unadjusted p = 0.99; Supplemental Figure 3). Given this, these two groups were combined post-hoc and a total of 5 groups were defined (Group 1: localized, age < 18, non-pelvic primary; Group 2: localized, age < 18, pelvic primary or localized age ≥ 18, White, non-Hispanic; Group 3: localized, age ≥ 18 years; other races; Group 4: metastatic, age < 18 years; Group 5: metastatic, age ≥ 18). Estimates of the 5-year overall survival for each group are shown in Table II. The resulting overall survival distribution and pair-wise log rank tests between adjacent groups are shown in Figure 2A and Table 3 respectively. Pair-wise log rank tests demonstrated that the survival distribution for each of the remaining five groups was significantly different from that of its adjacent group, except the comparison between Groups 3 and 4 was only significantly different using the unadjusted p-value. Hazard ratios were calculated according to group, with Group 1 forming the reference group. Hazard ratios for death in the other groups were 1.92 (Group 2), 3.01 (Group 3), 4.11 (Group 4), and 7.13 (Group 5).
Figure 1.
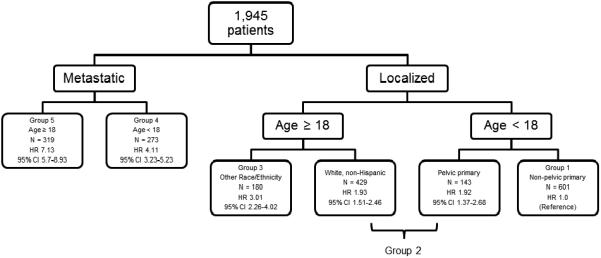
Outcome of survival recursive partitioning analysis that included stage, age, race/ethnicity, sex, axial primary, pelvic primary, and tissue origin as potential prognostic variables for selection in a model of overall survival with newly diagnosed EWS in SEER. 179 patients were excluded from the multivariate tree analysis as they had incomplete data. Abbreviations: N, number of subjects per group; HR, hazard ratio for death (using patients diagnosed under the age of 18 years with localized, non-pelvic disease as a reference); 95% CI 95% confidence interval for hazard ratio
Table II.
Prognostic subgroups derived from survival tree analysis in patients with newly diagnosed Ewing sarcoma
| Group | Prognostic Variables | SEER 5 yr % OS (95% CI) |
COG 5 yr % OS (95% CI) |
COG 5 yr % EFS (95% CI) |
COG I/E 5 yr % OS (95% CI) |
|---|---|---|---|---|---|
| 1 | Localized; Age < 18; Non-pelvic |
79 (74 – 82) N = 601 |
79 (76 – 81) N = 1,041 |
72 (69 – 75) N = 1,041 |
81 (78 – 83) N = 921 |
| 2 | Localized; Age < 18; Pelvic primary |
64 (60 – 69) N = 572 |
64 (59 – 69) N = 373 |
57 (52 – 62) N = 373 |
68 (62 – 72) N = 317 |
| Localized; Age ≥ 18; White, non-Hispanic | |||||
| 3 | Localized; Age ≥ 18; Other Races |
49 (40 – 57) N = 180 |
53 (27 – 74) N = 22 |
47 (24 – 68) N = 22 |
53 (27 – 74) N = 20 |
| 4 | Metastatic; Age < 18 |
39 (33 – 45) N = 273 |
38 (30 – 45) N = 198 |
25 (19 – 32) N = 198 |
40 (31 – 49) N = 147 |
| 5 | Metastatic; Age ≥ 18 |
21 (16 – 26) N = 319 |
21 (8 – 37) N = 46 |
13 (5 – 26) N = 46 |
16 (4 – 36) N = 36 |
Abbreviations: SEER Surveillance Epidemiology and End Results; COG Children's Oncology Group; yr year; CI confidence interval; I/E ifosfamide and etoposide received as part of chemotherapy; N = number of participants in that group; OS overall survival; EFS event-free survival
Figure 2.
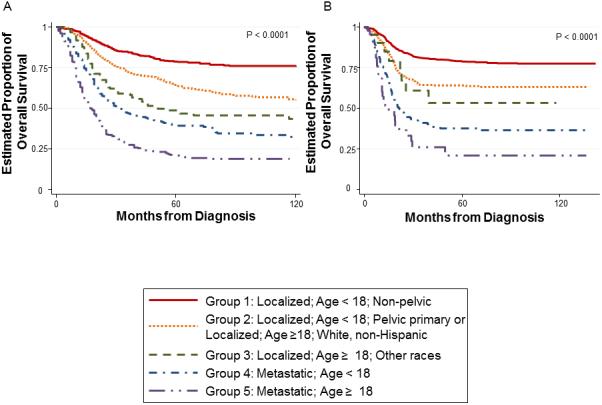
Kaplan-Meier estimates of overall survival for (A) SEER cohort and (B) COG cohort according to prognostic group
Table III.
Pair-wise log-rank tests between discrete prognostic groups in Ewing sarcoma.
| Comparison | SEER OS p-value (adjusted)* |
COG OS p-value (adjusted)* |
COG EFS p-value (adjusted)* |
|---|---|---|---|
| Group 1 vs Group 2 | <0.0001 (<0.001) | <0.0001 (<0.001) | <0.0001 (<0.001) |
| Group 2 vs Group 3 | 0.0005 (0.005) | 0.02 (0.19) | 0.55 (1.0) |
| Group 3 vs Group 4 | 0.02 (0.19) | <0.0001 (<0.001) | <0.0001 (<0.001) |
| Group 4 vs Group 5 | <0.0001 (<0.001) | <0.0001 (<0.001) | <0.0001 (<0.001) |
Abbreviations: vs versus; SEER Surveillance Epidemiology and End Results; COG Children's Oncology Group; OS overall survival; EFS event free survival
numbers in the parenthesis represent the p-value after adjustment for multiple comparisons using a Bonferroni correction.
Application of Prognostic Groups to the COG Cohort
We applied the five prognostic groups in Table II to the COG cohort and calculated the resulting overall survival and EFS distributions per group. An overall log-rank test to compare overall survival distributions showed a significant difference according to group assignment (p < 0.0001; Figure 2B). Pairwise log-rank tests were again done between adjacent groups and are reported in Table III. We observed significant differences in the overall survival distributions of each group and its adjacent group, except the comparison between Groups 2 and 3 was only significantly different using the unadjusted p-value. 5-year estimates of overall survival for each group are shown in Table II and were similar to estimates derived from the SEER cohort.
Unlike the SEER cohort in which only overall survival could be estimated, EFS could be estimated for the COG cohort. We repeated the above steps using EFS as the endpoint of interest. A log-rank test to compare the overall survival distribution again showed a significant difference according to group (p < 0.0001; Figure 3A). Pairwise log-rank tests (reported in Table III) also found significant differences between each group, with the exception of between Group 2 and Group 3.
Figure 3.
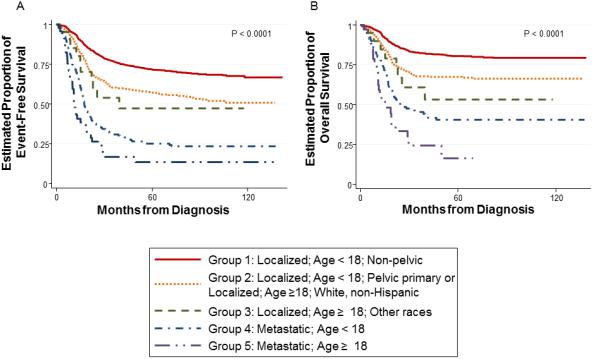
Kaplan-Meier estimates of (A) EFS in the overall COG cohort and (B) OS in only those who received ifosfamide and etoposide in the COG cohort according to prognostic group
Finally, we performed a sensitivity analysis focusing exclusively on patients in the COG cohort who received ifosfamide and etoposide as a component of their therapy. Since type of chemotherapy administered was not available for the SEER cohort, this analysis provides an assessment of the applicability of the prognostic groups among patients receiving a more contemporary chemotherapy regimen. The overall survival analyses was again significant (Figure 3B; p<0.0001). The estimates of 5-year overall survival for the sensitivity analysis are shown in Table II.
DISCUSSION
This is the first analysis to use survival recursive partitioning techniques to identify potential risk groups in Ewing sarcoma. We identified 5 discrete groups that encompass all newly diagnosed patients and we were able to evaluate these groups with a secondary data set from the Children's Oncology Group.
Identification of these risk groups may provide a backbone to explore potential biologic differences in the tumor and/or hosts that might influence outcome. These risk groups also provide valuable information for clinicians counseling individual patients and important data as the field develops risk-based strategies. In addition, they provide an adjustment strategy for comparing across published results. For example, if a new treatment appears to have superior results, presentation of the results within these groups would allow the reader to better understand whether the treatment is truly superior or whether the results instead reflect that the treatment was given only to lower risk patients.
Little previous work has been published identifying risk groups within Ewing sarcoma and no validated risk groups have been identified. One prior study identified four distinct risk groups utilizing the variables age, stage, and tumor location. In addition, within stage they identified that those with isolated lung metastasis had better outcomes than those with any extrapulmonary metastases [1]. The finding that location of metastases impacts outcome was also found in the Euro-Ewing 99 Trial [22]. Our groups are similar in that metastatic disease and older age conferred worse outcome and are key variables in defining risk groups. We also found tumor location (pelvic vs non-pelvic tumors) to be group-defining in younger patients with localized tumors at diagnosis. We differ however in how we define our highest risk groups. Within our metastatic patients, we subsequently use age to further characterize risk (those being ≥ 18 years having a higher risk of death) whereas the previous group used metastatic location (isolated lung metastases versus extrapulmonary metastases) to further delineate risk. We were not able to evaluate the role of metastatic type in our data, as this information was not available in the SEER cohort.
While recursive partitioning has not previously been applied to outcomes in EWS, this technique has been used to study other pediatric cancers. Cohn et al. utilized recursive partitioning in neuroblastoma to classify patients into risk categories [23]. The COG soft tissue sarcoma committee also utilized recursive partitioning to develop a staging algorithm in rhabdomyosarcoma [24]. In addition, recursive partitioning has been utilized to identify risk groups in glioblastoma [25]. Recursive partitioning provides a valuable statistical tool to explore the complex relationships that exist between potential prognostic variables when attempting to define risk groups and treatment algorithms. The resulting groups can then easily be applied to all patients and are intuitive for practitioners to use.
There were several differences noted between the two cohorts. The SEER cohort had more participants from minority populations. This is expected since SEER intentionally over-samples minority populations. It is also possible that that there may be an under-representation of minorities in the COG due to poorly understood factors, such as cultural or societal issues with enrolling on a study. There were also more patients with metastatic disease in the SEER cohort, as COG studies INT-0154 and AEWS-0031 (which represent 62% of the COG cohort) only enrolled patients with localized disease. We also found differences in tissue origin (soft tissue vs. bone) between cohorts, likely representing that COG studies INT-0091 and CCG-7951 enrolled only patients with EWS of boney origin. Despite these differences, similar results were seen in the two cohorts suggesting that the prognostic groups may generalize to other cohorts.
Our findings are consistent with previous work demonstrating that older age is associated with a worse outcome in EWS [2,3,7,8]. We identified that patients 18 years or older had a higher risk for poor outcome than other age categorizations with the exception of infants. Ewing sarcoma, however, is primarily a disease of adolescents and young adults. It is noteworthy that the cut-point chosen by the model is at the interface of pediatric and adult medicine. Previous work has suggested that the adverse effect of age on survival in EWS is mitigated in older patients who are treated on pediatric protocols [26-28]. However, application of our age-based prognostic model to the COG cohort yielded distinct survival outcomes and all such patients were treated on pediatric protocols. Whether differences in outcomes between adults and children with EWS are due to treatment differences, tumor biologic differences, or host differences is an area of active investigation.
We also found that race/ethnicity impacted outcome, with two prognostic groups defined in part by race/ethnicity. It should be noted that in our work, we used race/ethnicity as a binary variable (White non-Hispanic vs. other races/ethnicities). This was done based on previous work utilizing the SEER database that suggests that White non-Hispanic patients have better outcomes than Black, Asian and White Hispanic patients [13]. We acknowledge that differences based solely upon ethnicity may be obscured by combining these variables. Interestingly, race/ethnicity was only a significant factor in localized patients over the age of 18, perhaps highlighting that Black patients tend to be older than White non-Hispanic patients [13]. Racial and ethnic differences in survival have been noted in Ewing sarcoma before, though, as with the effect of age, it is unclear whether this reflects true biologic differences or differential access to medical care [4,13]. Our findings, however, were consistent within the COG in which all patients would have received intensive, multi-modal treatment regardless of race/ethnicity. This finding suggests underlying biologic differences might mediate these differential outcomes.
Ewing sarcoma is a rare disease, making large cohorts for detailed statistical analysis difficult to assemble [29]. In our study, we were fortunate to have two cohorts each with more than 1,000 cases. In addition, a sensitivity analysis including only patients who received the current North American standard therapy (upfront treatment with ifosfamide/etoposide) continued to show that our defined groups were applicable in the setting of modern therapy. We note a 5-year OS in Group 1 of 79% in the entire COG cohort and 81% in the COG ifosfamide/etoposide cohort. This finding is likely due to the fact that there were only 200 patients (12%) in the COG cohort who did not receive ifosfamide and etoposide. Therefore, there is a relatively small number of patients in each prognostic subgroup who did not receive ifosfamide/etoposide and our power to detect a difference in outcome based upon chemotherapy regimen is low. This finding does not supersede previous findings from a randomized control trial that clearly demonstrated the benefit of the addition of ifosfamide and etoposide.[9]
Some limitations should be noted with our data. First, multiple studies indicate the prognostic value of tumor size [1-6]. We were unable to include tumor size in our primary model since these data are missing from 39% of cases in the SEER registry, were not collected on COG trial AEWS0031 (34% of COG cohort), and were missing from a subset of the other COG patients. While we acknowledge this limitation, we also note that measuring tumor size in primary bone malignancies can be variable between radiologists [30,31]. In contrast, the variables in our final model can be reliably reported and therefore are more amenable to use across multiple centers. Our analysis was also limited by the small number of COG patients in group 3 (n = 22). Given this, it is difficult to make any firm recommendations or statements regarding this particular group. Moreover, it should be noted that our analysis assumed that the SEER and COG cohorts were independent, but there is likely some overlap between the two cohorts. Given our inability to quantify the overlap between the COG dataset and the SEER dataset, the COG dataset could not be used as a validation or confirmatory cohort. As one of the main goals of this work was to aid in risk stratification for patients enrolling on clinical trials, we applied the model derived from the SEER dataset to patients enrolled on Children Oncology Group (COG) clinical trials. An additional benefit of using the COG cohort was that treatment regimens were known. This allowed us to evaluate whether these groups were still relevant using modern therapy that included ifosfamide and etoposide. Likewise, while we have extensive clinical data, no tumor biology data were able to be included in our model. We used overall survival as our primary clinical endpoint since data on overall survival were available in both SEER and COG. Finally, our emphasis was on prognostic factors available at initial diagnosis and our models do not include initial response to therapy as a potential prognostic marker.
In conclusion, survival recursive partitioning analysis yields discrete prognostic groups in EWS. These groups provide valuable information for patients and clinicians in determining an individual patient's risk of death based upon a composite of their individual risk factors. Like many tumors, the clinical characteristics and demographics of patients with EWS can be variable. These prognostic groups highlight these differences and acknowledge the likely biologic differences in the tumor and/or in the hosts within these groups. To continue to make advances in the treatment of EWS we need to acknowledge these differences and continue to strive to understand the underlying biologic and molecular differences between patients. Perhaps most importantly in rare diseases like EWS, we need to continue to foster international relationships and develop cross-continental clinical trials to explore risk-based treatment strategies.
Supplementary Material
Acknowledgments
Grant Sponsor:
NIH/NCI 1K23CA154530 (Steven G. DuBois)
NIH T32 Training Grant 5T32CA128583-05 (Erin E. Karski)
Campini Foundation (Steven G. DuBois)
Alex's Lemonade Stand Foundation (Erin E. Karski)
COG Chair's Grant CA98543-08 (Mark Krailo and Elizabeth Mcilvaine)
COG Statistics and Data Center Grant CA98413-08 (Mark Krailo and Elizabeth Mcilvaine)
Nick Currey Foundation at CureSearch for Children's Cancer (Mark Krailo and Elizabeth Mcilvaine)
Daniel P. Sullivan Fund (Richard B. Womer)
Footnotes
Conflicts of Interest: None
References
- 1.Rodriguez-Galindo C, Liu T, Krasin MJ, Wu J, Billups CA, Daw NC, Spunt SL, Rao BN, Santana VM, Navid F. Analysis of prognostic factors in ewing sarcoma family of tumors: review of St. Jude Children's Research Hospital studies. Cancer. 2007;110(2):375–384. doi: 10.1002/cncr.22821. [DOI] [PubMed] [Google Scholar]
- 2.Lee J, Hoang BH, Ziogas A, Zell JA. Analysis of prognostic factors in Ewing sarcoma using a population-based cancer registry. Cancer. 2010;116(8):1964–1973. doi: 10.1002/cncr.24937. [DOI] [PubMed] [Google Scholar]
- 3.Cotterill SJ, Ahrens S, Paulussen M, Jürgens HF, Voûte PA, Gadner H, Craft AW. Prognostic factors in Ewing's tumor of bone: analysis of 975 patients from the European Intergroup Cooperative Ewing's Sarcoma Study Group. J Clin Oncol. 2000;18(17):3108–3114. doi: 10.1200/JCO.2000.18.17.3108. [DOI] [PubMed] [Google Scholar]
- 4.Jawad MU, Cheung MC, Min ES, Schneiderbauer MM, Koniaris LG, Scully SP. Ewing sarcoma demonstrates racial disparities in incidence-related and sex-related differences in outcome: an analysis of 1631 cases from the SEER database, 1973-2005. Cancer. 2009;115(15):3526–3536. doi: 10.1002/cncr.24388. [DOI] [PubMed] [Google Scholar]
- 5.Sauer R, Jurgens H, Burgers JM, Dunst J, Hawlicek R, Michaelis J. Prognostic factors in the treatment of Ewing's sarcoma. The Ewing's Sarcoma Study Group of the German Society of Paediatric Oncology CESS 81. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 1987;10(2):101–110. doi: 10.1016/s0167-8140(87)80052-x. [DOI] [PubMed] [Google Scholar]
- 6.Ahrens S, Hoffmann C, Jabar S, Braun-Munzinger G, Paulussen M, Dunst J, Rübe C, Winkelmann W, Heinecke A, Göbel U, Winkler K, Harms D, Treuner J, Jürgens H. Evaluation of prognostic factors in a tumor volume-adapted treatment strategy for localized Ewing sarcoma of bone: the CESS 86 experience. Cooperative Ewing Sarcoma Study. Medical and pediatric oncology. 1999;32(3):186–195. doi: 10.1002/(sici)1096-911x(199903)32:3<186::aid-mpo5>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 7.Gupta AA, Pappo A, Saunders N, Hopyan S, Ferguson P, Wunder J, O'Sullivan B, Catton C, Greenberg M, Blackstein M. Clinical outcome of children and adults with localized Ewing sarcoma: impact of chemotherapy dose and timing of local therapy. Cancer. 2010;116(13):3189–3194. doi: 10.1002/cncr.25144. [DOI] [PubMed] [Google Scholar]
- 8.Baldini EH, Demetri GD, Fletcher CD, Foran J, Marcus KC, Singer S. Adults with Ewing's sarcoma/primitive neuroectodermal tumor: adverse effect of older age and primary extraosseous disease on outcome. Ann Surg. 1999;230(1):79–86. doi: 10.1097/00000658-199907000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grier HE, Krailo MD, Tarbell NJ, Link MP, Fryer CJ, Pritchard DJ, Gebhardt MC, Dickman PS, Perlman EJ, Meyers PA, Donaldson SS, Moore S, Rausen AR, Vietti TJ, Miser JS. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. The New England journal of medicine. 2003;348(8):694–701. doi: 10.1056/NEJMoa020890. [DOI] [PubMed] [Google Scholar]
- 10.Karski EE, Matthay KK, Neuhaus JM, Goldsby RE, Dubois SG. Characteristics and outcomes of patients with Ewing sarcoma over 40 years of age at diagnosis. Cancer Epidemiol. 2012 doi: 10.1016/j.canep.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marina N GH, Womer R, Granowetter L, Krailo M. Prognostic factors in Ewing sarcoma family of tumors treated in consecutive children's oncology group studies: A report of 1444 patients [abstract].. Connective Tissue Oncology Society Annual Meeting.; Paris, France. 2010. [Google Scholar]
- 12.Applebaum MA, Worch J, Matthay KK, Goldsby R, Neuhaus J, West DC, Dubois SG. Clinical features and outcomes in patients with extraskeletal Ewing sarcoma. Cancer. 2011;117(13):3027–3032. doi: 10.1002/cncr.25840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Worch J, Matthay KK, Neuhaus J, Goldsby R, DuBois SG. Ethnic and racial differences in patients with Ewing sarcoma. Cancer. 2010;116(4):983–988. doi: 10.1002/cncr.24865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strobl C, Malley J, Tutz G. An introduction to recursive partitioning: rationale, application, and characteristics of classification and regression trees, bagging, and random forests. Psychological methods. 2009;14(4):323–348. doi: 10.1037/a0016973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Surveillance E, and End Results (SEER) Program (www.seer.cancer.gov) Research Data (1973-2008), National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2011, based on the November 2010 submission.
- 16.Granowetter L, Womer R, Devidas M, Krailo M, Wang C, Bernstein M, Marina N, Leavey P, Gebhardt M, Healey J, Shamberger RC, Goorin A, Miser J, Meyer J, Arndt CA, Sailer S, Marcus K, Perlman E, Dickman P, Grier HE. Dose-intensified compared with standard chemotherapy for nonmetastatic Ewing sarcoma family of tumors: a Children's Oncology Group Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(15):2536–2541. doi: 10.1200/JCO.2008.19.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyers PA, Krailo MD, Ladanyi M, Chan KW, Sailer SL, Dickman PS, Baker DL, Davis JH, Gerbing RB, Grovas A, Herzog CE, Lindsley KL, Liu-Mares W, Nachman JB, Sieger L, Wadman J, Gorlick RG. High-dose melphalan, etoposide, total-body irradiation, and autologous stem-cell reconstitution as consolidation therapy for high-risk Ewing's sarcoma does not improve prognosis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001;19(11):2812–2820. doi: 10.1200/JCO.2001.19.11.2812. [DOI] [PubMed] [Google Scholar]
- 18.Womer RB, West DC, Krailo MD, Dickman PS, Pawel BR, Grier HE, Marcus K, Sailer S, Healey JH, Dormans JP, Weiss AR. Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: a report from the Children's Oncology Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(33):4148–4154. doi: 10.1200/JCO.2011.41.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Felgenhauer JL, Nieder ML, Krailo MD, Bernstein ML, Henry DW, Malkin D, Baruchel S, Chuba PJ, Sailer SL, Brown K, Ranganathan S, Marina N. A pilot study of low-dose anti-angiogenic chemotherapy in combination with standard multiagent chemotherapy for patients with newly diagnosed metastatic Ewing sarcoma family of tumors: A Children's Oncology Group (COG) Phase II study NCT00061893. Pediatric blood & cancer. 2013;60(3):409–414. doi: 10.1002/pbc.24328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Putten W. Classification and Regression Tree Analysis for STATA. Daniel den Hoed Cancer Center; Rotterdam, Netherlands: 2004. [Google Scholar]
- 21.Keles S, Segal MR. Residual-based tree-structured survival analysis. Statistics in medicine. 2002;21(2):313–326. doi: 10.1002/sim.981. [DOI] [PubMed] [Google Scholar]
- 22.Ladenstein R, Potschger U, Le Deley MC, Whelan J, Paulussen M, Oberlin O, van den Berg H, Dirksen U, Hjorth L, Michon J, Lewis I, Craft A, Jürgens H. Primary disseminated multifocal Ewing sarcoma: results of the Euro-EWING 99 trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(20):3284–3291. doi: 10.1200/JCO.2009.22.9864. [DOI] [PubMed] [Google Scholar]
- 23.Cohn SL, Pearson AD, London WB, Monclair T, Ambros PF, Brodeur GM, Faldum A, Hero B, Iehara T, Machin D, Mosseri V, Simon T, Garaventa A, Castel V, Matthay KK. The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(2):289–297. doi: 10.1200/JCO.2008.16.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiss AR, Lyden ER, Anderson JR, Hawkins DS, Spunt SL, Walterhouse DO, Wolden SL, Parham DM, Rodeberg DA, Kao SC, Womer RB. Histologic and Clinical Characteristics Can Guide Staging Evaluations for Children and Adolescents With Rhabdomyosarcoma: A Report From the Children's Oncology Group Soft Tissue Sarcoma Committee. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(26):3226–3232. doi: 10.1200/JCO.2012.44.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scott JG, Bauchet L, Fraum TJ, Nayak L, Cooper AR, Chao ST, Suh JH, Vogelbaum MA, Peereboom DM, Zouaoui S, Mathieu-Daudé H, Fabbro-Peray P, Rigau V, Taillandier L, Abrey LE, DeAngelis LM, Shih JH, Iwamoto FM. Recursive partitioning analysis of prognostic factors for glioblastoma patients aged 70 years or older. Cancer. 2012;118(22):5595–5600. doi: 10.1002/cncr.27570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pieper S, Ranft A, Braun-Munzinger G, Jurgens H, Paulussen M, Dirksen U. Ewing's tumors over the age of 40: a retrospective analysis of 47 patients treated according to the International Clinical Trials EICESS 92 and EURO-E.W.I.N.G. 99. Onkologie. 2008;31(12):657–663. doi: 10.1159/000165361. [DOI] [PubMed] [Google Scholar]
- 27.Bacci G, Ferrari S, Comandone A, Zanone A, Ruggieri P, Longhi A, Bertoni F, Forni C, Versari M, Rimondini S. Neoadjuvant chemotherapy for Ewing's sarcoma of bone in patients older than thirty-nine years. Acta Oncol. 2000;39(1):111–116. doi: 10.1080/028418600431076. [DOI] [PubMed] [Google Scholar]
- 28.Bacci G, Balladelli A, Forni C, Ferrari S, Longhi A, Bacchini P, Alberghini M, Fabbri N, Benassi M, Briccoli A, Picci P. Adjuvant and neoadjuvant chemotherapy for Ewing sarcoma family tumors in patients aged between 40 and 60: report of 35 cases and comparison of results with 586 younger patients treated with the same protocols in the same years. Cancer. 2007;109(4):780–786. doi: 10.1002/cncr.22456. [DOI] [PubMed] [Google Scholar]
- 29.Esiashvili N, Goodman M, Marcus RB., Jr Changes in incidence and survival of Ewing sarcoma patients over the past 3 decades: Surveillance Epidemiology and End Results data. Journal of pediatric hematology/oncology. 2008;30(6):425–430. doi: 10.1097/MPH.0b013e31816e22f3. [DOI] [PubMed] [Google Scholar]
- 30.Erasmus JJ, Gladish GW, Broemeling L, Sabloff BS, Truong MT, Herbst RS, Munden RF. Interobserver and intraobserver variability in measurement of non-small-cell carcinoma lung lesions: implications for assessment of tumor response. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21(13):2574–2582. doi: 10.1200/JCO.2003.01.144. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki C, Torkzad MR, Jacobsson H, Aström G, Sundin A, Hatschek T, Fujii H, Blomqvist L. Interobserver and intraobserver variability in the response evaluation of cancer therapy according to RECIST and WHO-criteria. Acta Oncol. 2010;49(4):509–514. doi: 10.3109/02841861003705794. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


