Abstract
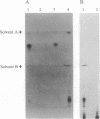
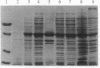
Erwinia herbicola, a nonphotosynthetic bacterium, is yellow colored due to the accumulation of unusually polar carotenoids, primarily mono- and diglucosides of zeaxanthin. We have cloned and expressed the gene for the enzyme that catalyzes the glucosylation of zeaxanthin. The enzyme has an apparent molecular mass of 45 kDa on an SDS/polyacrylamide gel, which is consistent with its calculated molecular mass. In vitro enzymatic activity was demonstrated using UDP-[14C]glucose and zeaxanthin as substrates. The product zeaxanthin diglucoside and its intermediate monoglucoside were identified by thin layer chromatography. The optimum pH and temperature ranges of the enzyme are 7.0-7.5 and 32-37 degrees C, respectively. A hydropathy plot indicates no apparent membrane-spanning regions, and biochemical experiments suggest that the enzyme is weakly membrane-associated. The amino acid sequence derived from the zeaxanthin glucosyltransferase gene shows a small region of high similarity with other glucuronosyl- and glucosyltransferases that use either UDP-activated glucuronic acid or a sugar as one of their substrates. Based on these similarities, we propose that this conserved sequence is part of the UDP binding site.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar-Peled M., Lewinsohn E., Fluhr R., Gressel J. UDP-rhamnose:flavanone-7-O-glucoside-2''-O-rhamnosyltransferase. Purification and characterization of an enzyme catalyzing the production of bitter compounds in citrus. J Biol Chem. 1991 Nov 5;266(31):20953–20959. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brutlag D. L., Dautricourt J. P., Maulik S., Relph J. Improved sensitivity of biological sequence database searches. Comput Appl Biosci. 1990 Jul;6(3):237–245. doi: 10.1093/bioinformatics/6.3.237. [DOI] [PubMed] [Google Scholar]
- Hundle B. S., Beyer P., Kleinig H., Englert G., Hearst J. E. Carotenoids of Erwinia herbicola and an Escherichia coli HB101 strain carrying the Erwinia herbicola carotenoid gene cluster. Photochem Photobiol. 1991 Jul;54(1):89–93. doi: 10.1111/j.1751-1097.1991.tb01989.x. [DOI] [PubMed] [Google Scholar]
- Jackson M. R., McCarthy L. R., Harding D., Wilson S., Coughtrie M. W., Burchell B. Cloning of a human liver microsomal UDP-glucuronosyltransferase cDNA. Biochem J. 1987 Mar 1;242(2):581–588. doi: 10.1042/bj2420581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T., Owens I. S. Mouse UDP glucuronosyltransferase. cDNA and complete amino acid sequence and regulation. Eur J Biochem. 1987 Nov 2;168(3):515–521. doi: 10.1111/j.1432-1033.1987.tb13448.x. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loh E. Y., Elliott J. F., Cwirla S., Lanier L. L., Davis M. M. Polymerase chain reaction with single-sided specificity: analysis of T cell receptor delta chain. Science. 1989 Jan 13;243(4888):217–220. doi: 10.1126/science.2463672. [DOI] [PubMed] [Google Scholar]
- Mackenzie P. I. Rat liver UDP-glucuronosyltransferase. Identification of cDNAs encoding two enzymes which glucuronidate testosterone, dihydrotestosterone, and beta-estradiol. J Biol Chem. 1987 Jul 15;262(20):9744–9749. [PubMed] [Google Scholar]
- Misawa N., Nakagawa M., Kobayashi K., Yamano S., Izawa Y., Nakamura K., Harashima K. Elucidation of the Erwinia uredovora carotenoid biosynthetic pathway by functional analysis of gene products expressed in Escherichia coli. J Bacteriol. 1990 Dec;172(12):6704–6712. doi: 10.1128/jb.172.12.6704-6712.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowat A. P., Arias I. M. Partial purification of hepatic UDP-glucuronyltransferase. Studies of some of its properties. Biochim Biophys Acta. 1970 Jul 15;212(1):65–78. doi: 10.1016/0005-2744(70)90179-8. [DOI] [PubMed] [Google Scholar]
- Nybraaten G., Liaaen-Jensen S. Bacterial carotenoids. VLIV. Zeaxanthin mono- and dirhamnoside. Acta Chem Scand B. 1974;28(10):1219–1224. doi: 10.3891/acta.chem.scand.28b-1219. [DOI] [PubMed] [Google Scholar]
- O'Reilly D. R., Miller L. K. A baculovirus blocks insect molting by producing ecdysteroid UDP-glucosyl transferase. Science. 1989 Sep 8;245(4922):1110–1112. doi: 10.1126/science.2505387. [DOI] [PubMed] [Google Scholar]
- Perry K. L., Simonitch T. A., Harrison-Lavoie K. J., Liu S. T. Cloning and regulation of Erwinia herbicola pigment genes. J Bacteriol. 1986 Nov;168(2):607–612. doi: 10.1128/jb.168.2.607-612.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfander H., Hodler M. Carotinoid-Glycoside. I. Mitteilung Partialsynthese und Charakterisierung von Zeaxanthin Mono- und Diglucosid. Helv Chim Acta. 1974;57(6):1641–1651. doi: 10.1002/hlca.19740570617. [DOI] [PubMed] [Google Scholar]
- Ralston E. J., English J. J., Dooner H. K. Sequence of three bronze alleles of maize and correlation with the genetic fine structure. Genetics. 1988 May;119(1):185–197. doi: 10.1093/genetics/119.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Ury A., Benveniste P., Bouvier-Navé P. Phospholipid-Dependence of Plant UDP-Glucose Sterol beta-d-Glucosyl Transferase : IV. Reconstitution into Small Unilamellar Vesicles. Plant Physiol. 1989 Oct;91(2):567–573. doi: 10.1104/pp.91.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]