Abstract
Novel action beta‐blockers combine many different pharmacological effects. The espindolol exhibits effects through β and central 5‐HT1α receptors to demonstrate pro‐anabolic, anti‐catabolic, and appetite‐stimulating actions. In the ACT‐ONE trial, espindolol reversed weight loss and improved handgrip strength in patients with cachexia due to non‐small cell lung cancer or colorectal cancer. With this trial, another frontier of cachexia management is in sight. Nonetheless, more efficacy and safety data is needed before new therapeutic indications for novel action beta‐blockers can be endorsed.
Keywords: Beta‐blockers, Espindolol, Cancer, Cachexia, Muscle
It was in 1965 when John Black reported on propranolol properties.1 This was the start of beta‐blockers that have made it to a standard of care for many clinical conditions, primarily of cardiovascular origin. The way, however, was not an easy stroll as many challenges needed to be met. The heart failure serves as a good example: beta‐blockers were initially contraindicated as it was considered that blocking the actions of activated sympathetic nervous system would be detrimental for the patients. Yet, chronic over‐activation may be even more harmful, the Swedish researchers hypothesized. Thus, they have started to investigate this concept2, 3 and pursued it into landmark trials that transformed beta‐blockers into a well‐established therapy for heart failure with reduced ejection fraction.4 Although beta‐blockers are rather heterogenous drug group, the benefit was demonstrated for several agents.4 Moreover, next to other cardiovascular conditions, patients with non‐cardiac chronic disease might benefit as well. In observational studies, the signal was extended to conditions like chronic obstructive pulmonary disease, which initially was considered as a contraindication to beta‐blocker therapy.5, 6
Keeping the heart failure as an example of chronic disease, we can state that the management for a significant proportion of patients is well established.4 However, despite relevant advances in management of heart failure, many patients progress into stage of body wasting and cachexia, a state that actually represents a public health problem.7, 8 Herein, it needs to be emphasized that body wasting is generalized, and it also involves the heart muscle.9, 10 From this point, it is a small but dare step to cross the boundary with other chronic disease, the malignant conditions in our case. Some interesting concepts have been proposed, and likely, there is significant rationale that cancer per se also induces cardiovascular changes that eventually manifests clinically as heart failure.11, 12 This actually opened an avenue for new management strategies for cachexia of different aetiology, including cancer.13, 14, 15, 16, 17
The potential role of beta‐blockers in preventing and treating cancer cachexa has emerged during the last decade. In fact, the major contributory role of the central and peripheral nervous systems in the pathogenesis and phenotype of cancer cachexia has been recognized since long.18 Recently, Springer et al.19 and Toledo et al.20 demonstrated in different experimental models of cancer cachexia that the use of beta‐blockers contributes to the prevention of cardiac and muscle wasting, respectively. Translating this information into clinical reality, it is important to note that Watkins et al.21 have recently published a multicentre review of 1425 patients with ovarian cancer and demonstrated that the use of beta‐blockers significantly extends either overall or disease‐specific survival. In particular, the use of non‐selective beta‐blockers confers the best survival advantage.
Along with these lines, Coats et al. report about the findings of the ACT‐ONE trial that tested espindolol in patients with non‐small cell lung cancer and colorectal cancer that have developed cachexia.22, 23 With this trial, they actually bring beta‐blockers to next frontier. The selection of an agent to be tested in the trial is not a play of chance. Pindolol is a well‐known beta‐blocker,24 but there are several bits that need to be appreciated in context of the trial. Primarily, in the ACT‐ONE trial, espindolol was tested. Pindolol exist in a racemic mixture that combines R‐pindolol and S‐pindolol, but they differ in pharmacological properties. In fact, the espindolol (S‐pindolol—that has been used in the trial) has quite unique effects as it combines actions through β and central 5‐HT1α receptors; this cumulatively translates into a combination of pro‐anabolic (β2 stimulating), anti‐catabolic (β1 blocking), and completely unique appetite stimulating (5‐HT1α receptors) actions that carry significant potential for wasting conditions like cancer cachexia (Figure 1). Effectively, Coats et al. have combined existing pathophysiological facts of (cancer) cachexia with pharmacodynamics profile of espindolol that has translated into clinically meaningful patient‐related outcomes.23, 25 The observed benefits in terms of reversed weight loss as manifested through maintained fat mass and an increase in fat free mass, with a simultaneous improvement in handgrip strength, are viable patient‐related outcomes that withstand scrutiny of clinical relevance.25 In this context, it should be emphasized that handgrip strength testing was done in a much more elaborative way than previously. Investigators by far exceeded the standards of most previous trials that usually test dominant hand only; the ACT‐ONE investigators, however, took much more comprehensive approach as they have tested both hands three times and then measured the handgrip strength of a stronger hand in the fourth run. Whether this is going to be adopted as a new standard in clinical trials remains open, yet it certainly is a more natural and likely less biased way of assessment. It may also be that this is the reason why ACT‐ONE demonstrated an improvement in the handgrip strength while other trials have failed.
Figure 1.
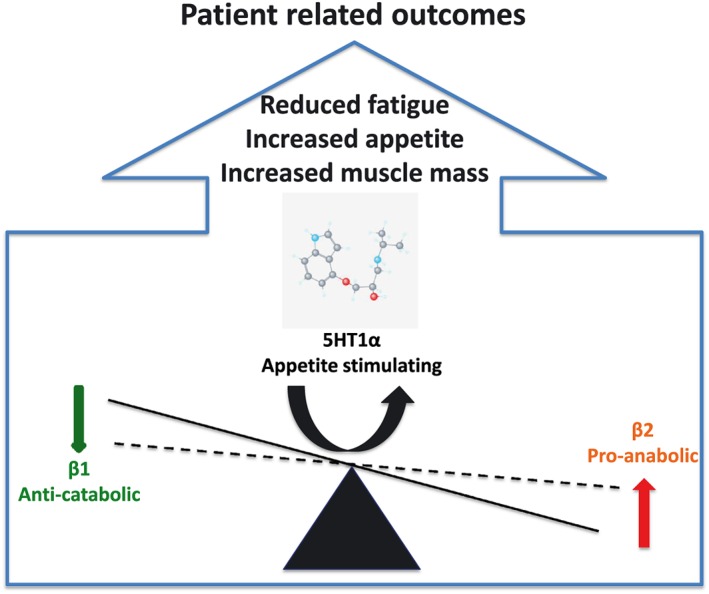
Espindolol pharmacological actions and implications in cachexia.
The current study by Coats et al.22 is, amongst other issues, crucial due to transdisciplinary impact. The field of cardio‐oncology has already entered the clinical arena, but implications remain rather limited to cardiovascular damage of cancer therapy and protective effects of heart failure agents.26 With their approach out of the box, Coats et al. 22 showed us how chronic disease management should combine available knowledge over many disciplines. Novel action beta‐blockers, the espindolol in this case, is aiming for a new frontier. Initial findings are encouraging, but before therapy is embraced, more efficacy and safety data is needed. One of the aspects that deserve attention is drug dosing and pharmacokinetics. Body wasting and cachexia modulate not only chemotherapy regimens but have the potential to influence drug metabolism in general.27, 28, 29 Whether the longer survival under beta‐blocker therapy is related to improved nutritional status remains to be assessed. Nevertheless, the results of the ACT‐ONE trial strengthen the link between the neural system and the systemic manifestations of cancer cachexia and suggest that beta‐blocker may contribute to its prevention and treatment. With initial evidence at hand, it should be easier to conduct more studies, potentially in cachexia of various aetiologies or at least in several forms of cancer. Combining patient, clinical, scientific, and regulatory perspectives, high‐quality data is expected to accumulate and address open issues. Through pharmacodynamic profile and history of persistence in other fields, novel action beta‐blockers are strong contender to break another paradigm and to conquer new frontier.
Conflict of interest
Mitja Lainscak and Alessandro Laviano declare that they have no conflict of interest.
Acknowledgement
The authors verify that they comply with the ethical guidelines for the authorship and publishing of the Journal of Cachexia, Sarcopenia, and Muscle.30
Lainscak, M. , and Laviano, A. (2016) ACT‐ONE ‐ ACTION at last on cancer cachexia by adapting a novel action beta‐blocker. Journal of Cachexia, Sarcopenia and Muscle, 7: 400–402. doi: 10.1002/jcsm.12136.
References
- 1. Black JW, Duncan WA, Shanks RG. Comparison of some properties of pronethanol and propranolol. Brit J Pharmacol Chemother 1965;25:577–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Waagstein F, Hjalmarson A, Varnauskas E, Wallentin I. Effect of chronic beta‐adrenergic receptor blockade in congestive cardiomyopathy. Br Heart J 1975;37:1022–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Swedberg K, Hjalmarson A, Waagstein F, Wallentin I. Prolongation of survival in congestive cardiomyopathy by beta‐receptor blockade. Lancet 1979;1:1374–1376. [DOI] [PubMed] [Google Scholar]
- 4. Ponikowski P, Voors A, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur J Heart Fail 2016, in press 2016. [Google Scholar]
- 5. Rutten FH, Zuithoff NP, Hak E, Grobbee DE, Hoes AW. Beta‐blockers may reduce mortality and risk of exacerbations in patients with chronic obstructive pulmonary disease. Arch Intern Med 2010;170:880–887. [DOI] [PubMed] [Google Scholar]
- 6. Lainscak M, Podbregar M, Kovacic D, Rozman J, von Haehling S. Differences between bisoprolol and carvedilol in patients with chronic heart failure and chronic obstructive pulmonary disease: a randomized trial. Respir Med 2011;105:S44–S49. [DOI] [PubMed] [Google Scholar]
- 7. Farkas J, von Haehling S, Kalantar‐Zadeh K, Morley JE, Anker SD, Lainscak M. Cachexia as a major public health problem: frequent, costly, and deadly. J Cachexia Sarcopenia Muscle 2013;4:173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. von Haehling S, Anker SD. Prevalence, incidence and clinical impact of cachexia: facts and numbers‐update 2014. J Cachexia Sarcopenia Muscle 2014;4:261–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Florea VG, Moon J, Pennell DJ, Doehner W, Coats AJ, Anker SD. Wasting of the left ventricle in patients with cardiac cachexia: a cardiovascular magnetic resonance study. Int J Cardiol 2004;97:15–20. [DOI] [PubMed] [Google Scholar]
- 10. Molfino A, Papa A, Gasperini‐Zacco ML, Muscaritoli M, Amoroso A, Cascino A, et al. Left ventricular mass correlates with lean body mass in patients with disease‐associated wasting. J Cachexia Sarcopenia Muscle 2014;5:251–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cramer L, Hildebrandt B, Kung T, Wichmann K, Springer J, Doehner W, et al. Cardiovascular function and predictors of exercise capacity in patients with colorectal cancer. J Am Coll Cardiol 2014;64:1310–1319. [DOI] [PubMed] [Google Scholar]
- 12. Schünemann M, Anker SD, Rauchhaus M. Cancer fatigue syndrome reflects clinically non‐overt heart failure: an approach towards onco‐cardiology. Nat Clin Pract Oncol 2008;5:632–633. [DOI] [PubMed] [Google Scholar]
- 13. von Haehling S, Anker SD. Treatment of cachexia: an overview of recent developments. Int J Cardiol 2015;184:736–742. [DOI] [PubMed] [Google Scholar]
- 14. Morley JE, von Haehling S, Anker SD. Are we closer to having drugs to treat muscle wasting disease? J Cachexia Sarcopenia Muscle 2014;5:83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Loncar G, Omersa D, Cvetinovic N, Arandjelovic A, Lainscak M. Emerging biomarkers in heart failure and cardiac cachexia. Int J Mol Sci 2014;15:23878–23896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Loncar G, Springer J, Anker M, Doehner W, Lainscak M. Cardiac Cachexia: hic et nunc. Int J Cardiol 2015;201:e1–e12. [DOI] [PubMed] [Google Scholar]
- 17. Lainscak M, Keber I, Anker SD. Body composition changes in patients with systolic heart failure treated with beta blockers: a pilot study. Int J Cardiol 2006;106:319–322. [DOI] [PubMed] [Google Scholar]
- 18. Laviano A, Inui A, Marks DL, Meguid MM, Pichard C, Rossi Fanelli F, Seelaender M. Neural control of the anorexia‐cachexia syndrome. Am J Physiol Endocrinol Metab 2008;295:E1000–E1008. [DOI] [PubMed] [Google Scholar]
- 19. Springer J, Tschirner A, Haghikia A, von Haehling S, Lal H, Grzesiak A, et al. Prevention of liver cancer cachexia‐induced cardiac wasting and heart failure. Eur Heart J 2014;35:932–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Toledo M, Penna F, Oliva F, Luque M, Betancourt A, Marmonti E, et al. A multifactorial anti‐cachectic approach for cancer cachexia in a rat model undergoing chemotherapy. J Cachexia Sarcopenia Muscle 2016;7:48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Watkins JL, Thaker PH, Nick AM, Ramondetta LM, Kumar S, Urbauer DL, Matsuo K, Squires KC, et al. Clinical impact of selective and nonselective beta‐blockers on survival in patients with ovarian cancer. Cancer 2015;121:3444–3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stewart Coats AJ, Srinivasan V, Surendran J, Chiramana H, Vangipuram SR, Bhatt NN, et al. The ACT‐ONE trial, a multicentre, randomised, double‐blind, placebo‐controlled, dose‐finding study of the anabolic/catabolic transforming agent, MT‐102 in subjects with cachexia related to stage III and IV non‐small cell lung cancer and colorectal cancer: study design. J Cachexia Sarcopenia Muscle 2011;2:201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Coats AJS, Ho GF, Prabhash K, von Haehling S, Tilson J, Brown R, et al. Espindolol for the treatment and prevention of cachexia in patients with stage III / IV non‐small cell lung cancer or colorectal cancer: a randomised, double‐blind, placebo‐controlled, international multi‐centre phase II study (the ACT‐ONE trial). J Cachexia Sarcopenia Muscle 2016;7:355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Frishman WH. Pindolol: a new β‐adrenoceptor antagonists with partial agonist activity. N Engl J Med 1983;308:940–944. [DOI] [PubMed] [Google Scholar]
- 25. Fearon KCH, Argiles JM, Baracos VE, Bernabei R, Coats AJS, Crawford J, et al. Request for regulatory guidance for cancer cachexia intervention trials. J Cachexia Sarcopenia Muscle 2015;6:272–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Curigliano G, Cardinale D, Dent S, Criscitiello C, Aseyev O, Lenihan D, et al. Cardiotoxicity of anticancer treatments: epidemiology, detection, and management. CA Cancer J Clin 2016. in press. [DOI] [PubMed] [Google Scholar]
- 27. Laviano A, Rianda S, Rossi FF. Sarcopenia and chemotherapy dosing in obese patients. Nat Rev Clin Oncol 2013;10:664. [DOI] [PubMed] [Google Scholar]
- 28. Trobec K, Kerec Kos M, von Haehling S, Springer J, Anker SD, Lainscak M. Pharmacokinetics of drugs in cachectic patients: a systematic review. PLoS One 2013;8: e79603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cvan Trobec K, Kerec Kos M, Trontelj J, Grabnar I, Tschirner A, Palus S, et al. Influence of cancer cachexia on drug liver metabolism and renal elimination in rats. J Cachexia Sarcopenia Muscle 2015;6:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2015. J Cachexia Sarcopenia Muscle 2015;6:315–316. [DOI] [PMC free article] [PubMed] [Google Scholar]


