Abstract
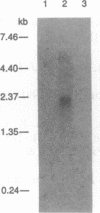
A cDNA encoding UDP-GlcNAc:Gal beta 1-3GalNAc-R (GlcNAc to GalNAc) beta 1-6GlcNAc transferase (EC 2.4.1.102), which forms critical branches in O-glycans, has been isolated by an expression cloning approach using Chinese hamster ovary (CHO) cells. Increased activity of this enzyme and the concomitant occurrence of the O-glycan core 2 structure [Gal beta 1-3(GlcNAc beta 1-6)GalNAc] has been observed in a variety of biological processes, such as T-cell activation and immunodeficiency due to the Wiskott-Aldrich syndrome and AIDS. Since CHO cells do not express this enzyme, CHO cell lines were established to stably express polyoma large tumor (T) antigen, which enables transient expression cloning. Because the antibody used was found to detect most efficiently the oligosaccharide products attached to leukosialin, the CHO cells were also stably transfected with leukosialin cDNA. By using this particular CHO cell line, a cDNA that encodes a protein determining the formation of the core 2 structure was isolated from an HL-60 cDNA library. The cDNA sequence predicts a protein with type II membrane topology, as has been found for all other mammalian glycosyltransferases cloned to date. The expression of the presumed catalytic domain as a fusion protein with the IgG binding domain of protein A enabled us to demonstrate unequivocally that the cDNA encodes the core 2 beta-1,6-N-acetylglucosaminyltransferase, the enzyme responsible for the formation of Gal beta 1-3(GlcNAc beta 1-6)GalNAc structures. No activity with this enzyme was detected toward the acceptors for other beta 1-6GlcNAc transferases.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brockhausen I., Kuhns W., Schachter H., Matta K. L., Sutherland D. R., Baker M. A. Biosynthesis of O-glycans in leukocytes from normal donors and from patients with leukemia: increase in O-glycan core 2 UDP-GlcNAc:Gal beta 3 GalNAc alpha-R (GlcNAc to GalNAc) beta(1-6)-N-acetylglucosaminyltransferase in leukemic cells. Cancer Res. 1991 Feb 15;51(4):1257–1263. [PubMed] [Google Scholar]
- Brockhausen I., Matta K. L., Orr J., Schachter H. Mucin synthesis. UDP-GlcNAc:GalNAc-R beta 3-N-acetylglucosaminyltransferase and UDP-GlcNAc:GlcNAc beta 1-3GalNAc-R (GlcNAc to GalNAc) beta 6-N-acetylglucosaminyltransferase from pig and rat colon mucosa. Biochemistry. 1985 Apr 9;24(8):1866–1874. doi: 10.1021/bi00329a010. [DOI] [PubMed] [Google Scholar]
- Carlsson S. R., Fukuda M. Isolation and characterization of leukosialin, a major sialoglycoprotein on human leukocytes. J Biol Chem. 1986 Sep 25;261(27):12779–12786. [PubMed] [Google Scholar]
- Carlsson S. R., Sasaki H., Fukuda M. Structural variations of O-linked oligosaccharides present in leukosialin isolated from erythroid, myeloid, and T-lymphoid cell lines. J Biol Chem. 1986 Sep 25;261(27):12787–12795. [PubMed] [Google Scholar]
- Cepko C. L., Roberts B. E., Mulligan R. C. Construction and applications of a highly transmissible murine retrovirus shuttle vector. Cell. 1984 Jul;37(3):1053–1062. doi: 10.1016/0092-8674(84)90440-9. [DOI] [PubMed] [Google Scholar]
- Cummings R. D., Trowbridge I. S., Kornfeld S. A mouse lymphoma cell line resistant to the leukoagglutinating lectin from Phaseolus vulgaris is deficient in UDP-GlcNAc: alpha-D-mannoside beta 1,6 N-acetylglucosaminyltransferase. J Biol Chem. 1982 Nov 25;257(22):13421–13427. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M., Carlsson S. R., Klock J. C., Dell A. Structures of O-linked oligosaccharides isolated from normal granulocytes, chronic myelogenous leukemia cells, and acute myelogenous leukemia cells. J Biol Chem. 1986 Sep 25;261(27):12796–12806. [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Heffernan M., Dennis J. W. Polyoma and hamster papovavirus large T antigen-mediated replication of expression shuttle vectors in Chinese hamster ovary cells. Nucleic Acids Res. 1991 Jan 11;19(1):85–92. doi: 10.1093/nar/19.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kukowska-Latallo J. F., Larsen R. D., Nair R. P., Lowe J. B. A cloned human cDNA determines expression of a mouse stage-specific embryonic antigen and the Lewis blood group alpha(1,3/1,4)fucosyltransferase. Genes Dev. 1990 Aug;4(8):1288–1303. doi: 10.1101/gad.4.8.1288. [DOI] [PubMed] [Google Scholar]
- Kumar R., Yang J., Larsen R. D., Stanley P. Cloning and expression of N-acetylglucosaminyltransferase I, the medial Golgi transferase that initiates complex N-linked carbohydrate formation. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9948–9952. doi: 10.1073/pnas.87.24.9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N., Wang W. C., Fukuda M. Granulocytic differentiation of HL-60 cells is associated with increase of poly-N-acetyllactosamine in Asn-linked oligosaccharides attached to human lysosomal membrane glycoproteins. J Biol Chem. 1990 Nov 25;265(33):20476–20487. [PubMed] [Google Scholar]
- Muller W. J., Naujokas M. A., Hassell J. A. Isolation of large T antigen-producing mouse cell lines capable of supporting replication of polyomavirus-plasmid recombinants. Mol Cell Biol. 1984 Nov;4(11):2406–2412. doi: 10.1128/mcb.4.11.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palcic M. M., Ripka J., Kaur K. J., Shoreibah M., Hindsgaul O., Pierce M. Regulation of N-acetylglucosaminyltransferase V activity. Kinetic comparisons of parental, Rous sarcoma virus-transformed BHK, and L-phytohemagglutinin-resistant BHK cells using synthetic substrates and an inhibitory substrate analog. J Biol Chem. 1990 Apr 25;265(12):6759–6769. [PubMed] [Google Scholar]
- Pallant A., Eskenazi A., Mattei M. G., Fournier R. E., Carlsson S. R., Fukuda M., Frelinger J. G. Characterization of cDNAs encoding human leukosialin and localization of the leukosialin gene to chromosome 16. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1328–1332. doi: 10.1073/pnas.86.4.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson J. C., Colley K. J. Glycosyltransferases. Structure, localization, and control of cell type-specific glycosylation. J Biol Chem. 1989 Oct 25;264(30):17615–17618. [PubMed] [Google Scholar]
- Piller F., Cartron J. P., Maranduba A., Veyrières A., Leroy Y., Fournet B. Biosynthesis of blood group I antigens. Identification of a UDP-GlcNAc:GlcNAc beta 1-3Gal(-R) beta 1-6(GlcNAc to Gal) N-acetylglucosaminyltransferase in hog gastric mucosa. J Biol Chem. 1984 Nov 10;259(21):13385–13390. [PubMed] [Google Scholar]
- Piller F., Le Deist F., Weinberg K. I., Parkman R., Fukuda M. Altered O-glycan synthesis in lymphocytes from patients with Wiskott-Aldrich syndrome. J Exp Med. 1991 Jun 1;173(6):1501–1510. doi: 10.1084/jem.173.6.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piller F., Piller V., Fox R. I., Fukuda M. Human T-lymphocyte activation is associated with changes in O-glycan biosynthesis. J Biol Chem. 1988 Oct 15;263(29):15146–15150. [PubMed] [Google Scholar]
- Prieels J. P., Monnom D., Dolmans M., Beyer T. A., Hill R. L. Co-purification of the Lewis blood group N-acetylglucosaminide alpha 1 goes to 4 fucosyltransferase and an N-acetylglucosaminide alpha 1 goes to 3 fucosyltransferase from human milk. J Biol Chem. 1981 Oct 25;256(20):10456–10463. [PubMed] [Google Scholar]
- Ropp P. A., Little M. R., Cheng P. W. Mucin biosynthesis: purification and characterization of a mucin beta 6N-acetylglucosaminyltransferase. J Biol Chem. 1991 Dec 15;266(35):23863–23871. [PubMed] [Google Scholar]
- Saitoh O., Piller F., Fox R. I., Fukuda M. T-lymphocytic leukemia expresses complex, branched O-linked oligosaccharides on a major sialoglycoprotein, leukosialin. Blood. 1991 Apr 1;77(7):1491–1499. [PubMed] [Google Scholar]
- Sanchez-Lopez R., Nicholson R., Gesnel M. C., Matrisian L. M., Breathnach R. Structure-function relationships in the collagenase family member transin. J Biol Chem. 1988 Aug 25;263(24):11892–11899. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar M., Hull E., Nishikawa Y., Simpson R. J., Moritz R. L., Dunn R., Schachter H. Molecular cloning and expression of cDNA encoding the enzyme that controls conversion of high-mannose to hybrid and complex N-glycans: UDP-N-acetylglucosamine: alpha-3-D-mannoside beta-1,2-N-acetylglucosaminyltransferase I. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):234–238. doi: 10.1073/pnas.88.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H., Bothner B., Dell A., Fukuda M. Carbohydrate structure of erythropoietin expressed in Chinese hamster ovary cells by a human erythropoietin cDNA. J Biol Chem. 1987 Sep 5;262(25):12059–12076. [PubMed] [Google Scholar]
- Schachter H., Brockhausen I., Hull E. High-performance liquid chromatography assays for N-acetylglucosaminyltransferases involved in N- and O-glycan synthesis. Methods Enzymol. 1989;179:351–397. doi: 10.1016/0076-6879(89)79138-2. [DOI] [PubMed] [Google Scholar]
- Seed B., Aruffo A. Molecular cloning of the CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. Proc Natl Acad Sci U S A. 1987 May;84(10):3365–3369. doi: 10.1073/pnas.84.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sportsman J. R., Park M. M., Cheresh D. A., Fukuda M., Elder J. H., Fox R. I. Characterization of a membrane surface glycoprotein associated with T-cell activation. J Immunol. 1985 Jul;135(1):158–164. [PubMed] [Google Scholar]
- Stanley P. Glycosylation mutants of animal cells. Annu Rev Genet. 1984;18:525–552. doi: 10.1146/annurev.ge.18.120184.002521. [DOI] [PubMed] [Google Scholar]
- Weston B. W., Nair R. P., Larsen R. D., Lowe J. B. Isolation of a novel human alpha (1,3)fucosyltransferase gene and molecular comparison to the human Lewis blood group alpha (1,3/1,4)fucosyltransferase gene. Syntenic, homologous, nonallelic genes encoding enzymes with distinct acceptor substrate specificities. J Biol Chem. 1992 Feb 25;267(6):4152–4160. [PubMed] [Google Scholar]
- Williams M. A., Fukuda M. Accumulation of membrane glycoproteins in lysosomes requires a tyrosine residue at a particular position in the cytoplasmic tail. J Cell Biol. 1990 Sep;111(3):955–966. doi: 10.1083/jcb.111.3.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yayon A., Klagsbrun M., Esko J. D., Leder P., Ornitz D. M. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991 Feb 22;64(4):841–848. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]
- Yousefi S., Higgins E., Daoling Z., Pollex-Krüger A., Hindsgaul O., Dennis J. W. Increased UDP-GlcNAc:Gal beta 1-3GaLNAc-R (GlcNAc to GaLNAc) beta-1, 6-N-acetylglucosaminyltransferase activity in metastatic murine tumor cell lines. Control of polylactosamine synthesis. J Biol Chem. 1991 Jan 25;266(3):1772–1782. [PubMed] [Google Scholar]