Abstract
The incidence of anaphylactic/anaphylactoid reactions has been reported to vary between 1 : 3500 and 1 : 20,000 cases with a mortality rate ranging from 3 to 9%. Clinical signs present as skin rash, urticaria, angioedema, bronchospasm, tachycardia, bradycardia, and hypotension. Rapid identification and treatment are crucial to overall patient prognosis, as delayed intervention is associated with increased mortality. Diagnosis may be confirmed with clinical presentation, serum tryptase levels, and skin test results. While the main causative agents in anesthetic practice are typically neuromuscular blocking agents (NMBs), latex, and antibiotics, this review aims to discuss recognition, management, and preventive measures in perioperative anaphylactic/anaphylactoid reactions from benzodiazepine administration.
Key Words: Benzodiazepine allergy, Anaphylaxis, Anaphylactoid reactions, Diagnosis of anaphylaxis, Management of anaphylaxis
Serious complications during surgery have been shown to occur rather infrequently (0.4% of 83,844 cases), but anesthesia-related complications contribute to more than one third of these events.1 Allergic reactions to medications are among the major factors affecting morbidity and mortality peri- and postoperatively.1,2 The relative allergenicity of benzodiazepines, arguably the most commonly used anxiolytic premedication and a cornerstone in moderate to deep sedation, is explored in an attempt to quantify its allergic history.
Benzodiazepines are sedative hypnotic agents that have been in clinical use since the 1960s for sedation, anterograde amnesia, anxiolysis, as well as treatment of seizures, substance withdrawal states, insomnia, and drug-associated agitation. Allergic reactions are rare, with few cases reported in literature.3,4 Amid these sparse reports, a lack of consistent methods for diagnosis stimulates investigation into identification of the causative agent and classification of the observed reactions.
BENZODIAZEPINE ALLERGY: REVIEW OF LITERATURE
A thorough investigation was conducted in an attempt to provide a comprehensive summary of published reports involving anaphylactic or anaphylactoid reactions to benzodiazepines.
The earliest reports (over 17 total) of reactions to benzodiazepines, dating from 1960 to 1975 describe numerous signs of cutaneous allergic reactions such as urticaria, angioedema, macular erythematous rash, photoallergy, purpura, and erythema multiforme among others.5 In most cases the offending agent was predominately chlordiazepoxide followed by diazepam and flurazepam. In 1977, what was considered to be the first true anaphylaxis to diazepam was published in the British Medical Journal. The mechanism was attributed to a common metabolite, desmethyldiazepam, the antigenic moiety for cross-allergenicity in benzodiazepines.6
Years later, 1 report of diazepam allergy describes a case involving a healthy 28-year-old nurse with no significant past medical history or allergies presenting for gastroscopy. Here, a relatively brief and nonspecific case of hypersensitivity was reported including only that the patient showed signs of “generalized urticaria and shock,” requiring treatment. The report also mentions positive wheal and flare reactions to diazepam on skin prick test, as well as intradermal test.7
Martinez-Tadeo and Perez-Rodriguez4 reported a case of urticaria upon oral intake of tetrazepam that later yielded a negative skin prick test result. Subsequently, a single-blinded, placebo-controlled challenge oral test was performed using two 5-mg doses and one 15-mg dose of tetrazepam with 30-minute interval dosing. This study demonstrated that the patient began to show signs of mild allergic reaction 50 minutes after total intake of tetrazepam 25 mg. Full strength skin prick and intradermal tests were performed with both midazolam and diazepam yielding negative results. The failure to demonstrate cross-reactivity suggests a selective pattern of sensitization.4
The earliest published report of allergy to midazolam, released in 1992, documented 2 accounts of angioedema on the same medically compromised patient. Midazolam was suspected as the common offending agent despite the use of multiple drugs. Unfortunately, follow-up testing was not available for confirmation.8 Previously midazolam has been linked to effects such as respiratory depression, laryngospasm, urticaria, and cardiac dysrhythmia; however, identification of midazolam's primary metabolite, 1-hydroxymidazolam, suggests an alternative mechanism of allergy.9
In 1994, Fujita et al10 reported a case in which a 38-year-old male with medical history significant for positive reaction to antibiotics was seen for C4-C5 fusion.10 Upon administration of midazolam 10 mg, the patient presented with severe hypotension (57 mmHg systolic) and tachycardia (135 bpm) resolving after epinephrine administration (3 intravenous [IV] doses of 50 mcg).
Another suspected case of anaphylactoid reaction published in the Japanese Journal of Anesthesia describes a 37-year-old healthy male with no significant medical history or known drug allergies exhibiting pruritus, chest tightness, hypotension, bradycardia, and hypoxia shortly after administration of midazolam 2 mg with lactated Ringer's solution. In this study, follow-up testing revealed elevated serum tryptase levels in addition to positive intradermal testing 3 months later.11
The Korean Journal of Anesthesiology published a case of suspected midazolam hypersensitivity in which concurrent administration of midazolam and antibiotic precipitated events resembling hypersensitivity reaction rather than anaphylaxis. Positive intradermal reaction to midazolam coupled with negative reaction to antibiotic confirmed the hypothesis.12 While in this study no serum tryptase or histamine levels were evaluated, elevated total IgE levels further support midazolam hypersensitivity.
Another report published in the Indian Journal of Anesthesia described a case of apparent anaphylaxis occurring 2 minutes after IV midazolam administration with lactated Ringer's solution.13 Described is a 26-year-old male with no significant medical history and no drug or food allergies presenting for cervical lymph node biopsy. Within 2 minutes of IV midazolam, the patient experienced pruritus over his right forearm and trunk with urticarial wheals, severe hypotension, and bradycardia. No stridor or wheezing was noted ruling out airway involvement, but prompt treatment was initiated. Serum tryptase levels were elevated upon measurement and later skin prick tests revealed positive result with midazolam confirming anaphylaxis.
In 2014, Shin et al9 published a report documenting a case of midazolam anaphylaxis involving a 53-year-old woman during an esophagogastroduodenoscopy (EGD) procedure.9 Ten years prior, the patient had presented to the emergency medical center for allergic urticaria of unknown cause, but otherwise had no significant medical history. Four minutes following IV administration of midazolam 5 mg, the patient's pulse could not be palpated and her SpO2 dropped to 75%. Two doses of flumazenil (0.3 and 0.2 mg) were administered at which point blood pressure and pulse could not be assessed. Tachycardia was observed on the monitor, but no wheezing or stridor was evident. Norepinephrine (8 mcg/min) and epinephrine (1 mg) were administered, and the patient's pulse rate was then measured at 130 bpm. She developed a generalized rash all over her body at which point dexamethasone (5 mg) and an antihistamine were administered and symptoms resolved. Subsequently, arterial blood gas analysis and chemical analyses revealed no abnormal results except an elevated lactate dehydrogenase level of 370 IU/L and an elevated blood tryptase level to 14.8 mcg/L, which was used to presume the occurrence of anaphylaxis. Skin prick test was considered for confirmation; however, the test was considered high risk and patient consent could not be obtained.
Most recently, a report published in Anesthesia Progress describes a case of life-threatening hypotension, bronchoconstriction, and edema in a 59-year-old woman undergoing resection of a mandibular tumor.14 Because her medical history was significant for skin reactions to several antibiotics and contrast medium, an initial small test dose of midazolam, 0.5 mg (0.01 mg/kg), was administered prior to induction with midazolam 5 mg (0.1 mg/kg). The patient suddenly developed hypotension with a systolic pressure of 65 mmHg, associated wheezing, and widespread flush on the body. Because anaphylactoid reaction was suspected, epinephrine 50 mcg and methylprednisolone 1000 mg were injected intravenously followed by dopamine for sustained hypotension and nitroglycerine for ischemic ST depression. Unfortunately, follow-up allergy tests did not reveal clear evidence of allergic drug reaction. The surgery was postponed and completed without incident a week later under general anesthesia with sevoflurane.
Further discussion on benzodiazepine allergy will follow a basic concepts review for anaphylaxis including clinical features, diagnosis, allergy testing, and management.
ANAPHYLAXIS
Anaphylaxis is a life-threatening condition marked by cardiovascular collapse, generalized interstitial edema, and bronchospasm. It may occur through immune mediated and nonimmune mediated (anaphylactoid) mechanisms. Anaphylaxis is a Gell and Coombs Type I, IgE-mediated hypersensitivity reaction involving release of vasoactive mediators (Table 1). Diagnosis is primarily based on clinical signs and symptoms and is considered highly likely in patients who experience acute onset of symptoms with skin and/or mucosal tissue involvement, respiratory compromise, and significant reduction in blood pressure following exposure to an allergen (Table 2).15 In anaphylaxis there is initial exposure of an antigen with subsequent production of specific IgE antibodies. Upon re-exposure to the same or chemically similar antigen, antigen-antibody interactions cause marked degranulation of mast cells and basophils. Anaphylactoid reactions are caused by direct nonimmune mediated release of mediators from mast cells and basophils, or by direct complement activation and do not require prior exposure for sensitization. However, both anaphylaxis and anaphylactoid reactions exhibit similar clinical manifestations.16
Table 1. .
Vasoactive Mediators Released During Anaphylaxis
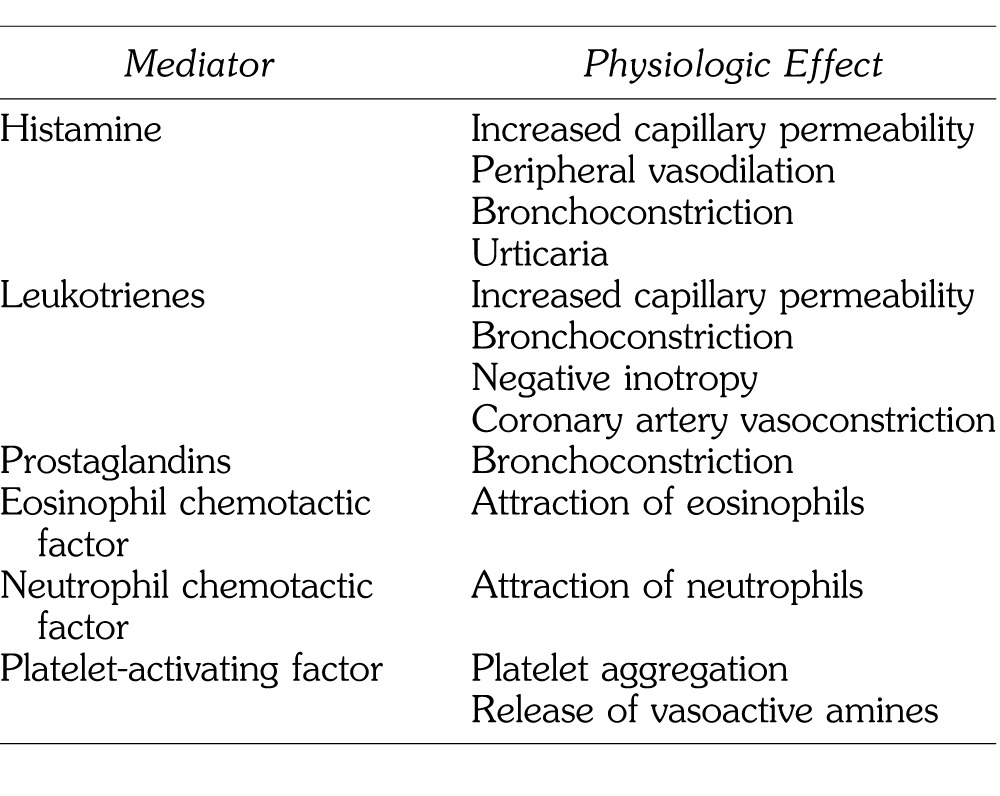
Table 2. .
Clinical Criteria for Diagnosing Anaphylaxis

COMMON CAUSATIVE AGENTS
Among the myriad of drugs and substances used in the provision of an anesthetic, neuromuscular blockers are the number one cause of reported cases of anaphylaxis with an incidence of 69.2%.2 Latex (12.1%) is the second leading cause with antibiotics (8%), hypnotics (3.7%), colloids (2.7%), opioids (1.4%), and other substances (2.9%) such as propacetamol, aprotonic, chymopapain, and bupivacaine accounting for the remainder of reported cases. Some anesthetic induction agents have been implicated in producing anaphylactic reactions, the majority of which are attributed to thiopental and propofol with incidence rates 1 : 30,000 and 1 : 60,000, respectively.2 Benzodiazepines, etomidate, and ketamine account for the remainder with incidence rates that have not been estimated. Reactions to benzodiazepines specifically are exceedingly rare.17
CLINICAL FEATURES
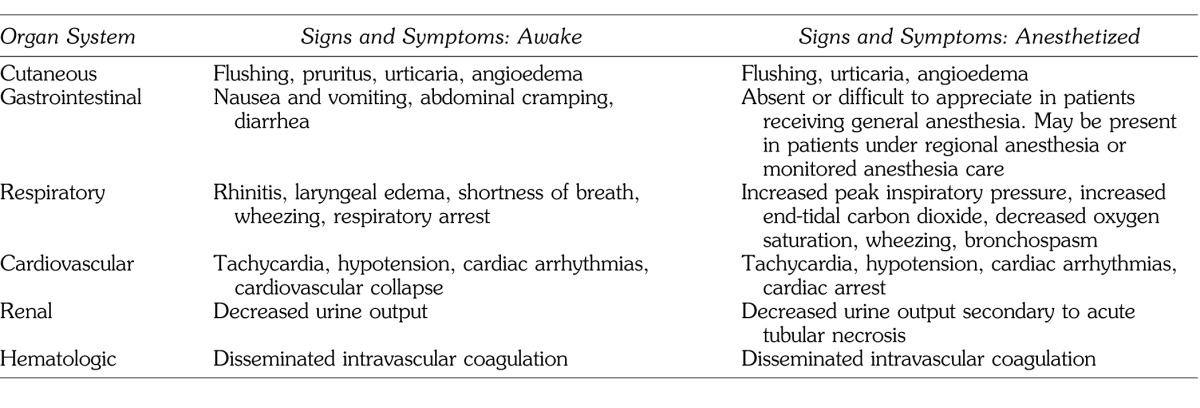
Clinical manifestations of anaphylactoid reactions are often indistinguishable from anaphylaxis. The reactions are rapid in onset and occur within seconds to minutes of exposure to the offending agent. Symptoms progress rapidly and can affect most organ systems, including skin (pruritus, flushing, urticaria, and angioedema), eyes (conjunctivitis), the upper airway (rhinitis and angioedema), lower airway (bronchoconstriction with wheezing and dyspnea, and cyanosis), the intestinal tract (abdominal pain, nausea, vomiting, and diarrhea), and the cardiovascular system (tachycardia, hypotension, and shock). The latter may lead to cardiovascular collapse and death. Airway edema and/or bronchospasm may lead to inability to ventilate, hypoxia, and hypercarbia with subsequent cardiovascular collapse as well. In the dental perioperative setting, patients are sedated, draped, and usually fully clothed concealing early cutaneous signs. Thus, the earliest recognizable signs may be ventilation impairment, overt bronchoconstriction, and/or cardiovascular collapse. Signs and symptoms in the awake versus anesthetized patient are displayed in Table 3.
Table 3. .
Comparison of Signs and Symptoms: Awake Versus Anesthetized
MANAGEMENT OF ANAPHYLAXIS
The treatment of anaphylaxis begins similarly to any emergent condition with assessment, maintenance of airway, breathing, and circulation followed by definitive treatment.18 Specific management of intraoperative anaphylaxis consists of withdrawal of the offending agent, if known, interrupting the effects of preformed mediators and blocking further mediator release. Early recognition and intervention is key given the possible life-threatening nature of anaphylaxis.
Epinephrine
Epinephrine is the first-line drug for anaphylaxis. Epinephrine may be administered intramuscularly, subcutaneously, or intravenously with dosages determined by the amount necessary for symptom control and blood pressure maintenance.18
Intramuscular (IM) epinephrine is dosed at 0.01 mg/kg (maximum dose 0.5 mg for anaphylaxis) every 5–15 minutes. Studies have indicated more rapid absorption and higher plasma levels of epinephrine in individuals receiving IM injections in the anterior-lateral thigh than those receiving subcutaneous injections or IM injections in the deltoid.19,20
IV administration of epinephrine may be considered as well by experienced providers, and may be preferred for patients with severe hypotension or severe bronchospasm needing immediate treatment or unresponsive to IM epinephrine doses and fluid resuscitation. IV epinephrine is preferred for cardiac arrest. In likely anaphylaxis, IV epinephrine should be administered at 10–25 mcg bolus doses initially as needed for hypotension or bronchospasm, with gradually escalating doses as required. Initial treatment with 0.1–0.5 mg epinephrine doses is practical in the presence of cardiovascular collapse.18 In some instances, continuous low-dose epinephrine infusions may be appropriate to avoid lethal arrhythmias associated with large boluses.
Airway Management
Edema of the airway is an immediate concern of the anesthesiologist. If the patient is not intubated for the surgical procedure, endotracheal intubation is indicated and should be accomplished sooner rather than later, unless the reaction is confirmed as mild and not progressing. It should be realized that tissue edema is not reversed by epinephrine and progressive airway edema may make intubation difficult or impossible. Therefore, depending on patient presentation, endotracheal intubation and epinephrine administration may both be primary life-saving treatments.
Adjunctive Management
One-hundred percent oxygen should be administered to correct hypoxia, and Trendelenburg positioning of the patient may help to increase stroke volume and cardiac output by shifting fluids centrally. Volatile agents and propofol should be discontinued in the setting of hemodynamic collapse. Additionally, aggressive fluid resuscitation of up to 30 mL/kg in the first hour may compensate for peripheral vasodilation and fluid extravasation and help to manage persistent hypotension.2,18 Further treatment has been explored with use of other vasopressors and IV glucagon; however, neither mechanism has been proven in the context of systemic anaphylaxis. For example, vasopressin is able to enhance endogenous catecholamine-induced vasoconstriction in cases of resistance to the catecholamine vasopressor effect. Vasopressin inhibits inducible nitric oxide synthase, which is a major contributor to vasodilatation and resistance to catecholamine-induced vasoconstriction.21,22
Glucagon has been reported to be effective in improving the airway complications of anaphylaxis in patients on beta-blockers who are resistant to epinephrine. It acts to increase cyclic-adenosine monophosphate levels, which in turn relaxes smooth muscle in the airway. Glucagon also has positive inotropic and chronotropic effects on the heart.23
Following initial management as noted above, antihistamines (H1 and H2 antagonists) may be considered for symptomatic treatment of urticaria, angioedema, and pruritus. Studies indicate treatment with diphenhydramine (1 mg/kg up to 50 mg) used in combination with ranitidine (1 mg/kg up to 50 mg) or famotidine (0.5 mg/kg up to 20 mg slowly) is more effective than treatment with H1-antagonists alone. In addition, high doses of corticosteroids such as hydrocortisone (2–5 mg/kg up to 250 mg) may treat symptoms of anaphylaxis by decreasing airway swelling but its effectiveness is delayed.
Definitive Treatment
If surgery is rendered in the office or ambulatory surgery center setting, early activation of Emergency Medical Services (EMS-911) is important. The patient will need to be transferred to the emergency department and, especially if intubated, require intensive care unit (ICU) admission. Transfer to the ICU is likely for in-hospital surgery.
DIAGNOSIS AND TESTING
The initial diagnosis of anaphylaxis relies on thorough history and physical examination. Assessment of clinical history assists with identification of increased risk factors such as symptoms of allergic reaction in previous anesthetics, diagnosed allergy to drugs likely to be used in anesthetic regimen, repeated exposure to latex, and/or allergic manifestations following ingestion of foods with high frequency of cross-reactivity to latex.24 Retrospective diagnosis is based on both in vivo and in vitro biochemical tests.
In Vivo Biochemical Tests
Plasma histamine is an inflammatory mediator stored in mast cells and basophils, which peaks immediately upon anaphylactic reaction, while serum tryptase is a mast cell protease, which increases to levels above 25 mcg/L within 2 hours of suspected anaphylaxis suggesting IgE mediated mechanism.24 Histamine and tryptase concentrations typically correlate with severity of clinical reactions and are increased in anaphylaxis. However, distinguishing between anaphylactic and anaphylactoid reactions solely with serum tryptase levels remains controversial, necessitating additional methods of verification.2
Dermal Tests
Skin testing remains the standard in determining the pathophysiologic mechanism of clinical anaphylaxis, identifying the agent, assessing drug-related cross-reactivity, and exploring alternative drugs for further procedures.25 However, routine skin testing of all patients undergoing anesthesia is not recommended in the absence of prior history due to potential subclinical IgE sensitization.26,27 Also, testing is not without consequences and should not be performed within 6 weeks of expected exposure to anaphylaxis triggering agents due to possible mast cell and basophil mediator depletion. Furthermore, in the general population, 9.3% have a positive skin test for specific IgE quaternary ammonium ions as in neuromuscular blockers even in the absence of clinical symptoms.2 Also, although the risk of eliciting anaphylaxis with skin testing is minimal (<0.1% for antibiotics), the presence of trained personnel with resuscitation equipment should be readily available.
There are two commonly utilized skin tests for allergy identification. The skin prick technique triggers superficial dermal mast cells and nonspecific irritation is its main limitation. A positive test has a high predictive value in the patient with a history of anaphylaxis; however, the polypharmacy of the typical anesthetic has many potential offending agents.2 A graded challenge is required in certain cases including local anesthetics, in which skin prick tests are usually negative.28 Intradermal allergy testing involves injection of small amounts of suspected allergens under the surface of the skin. Similarly to the skin prick test, patients are examined after 15–20 minutes for a reaction at injection site. Regardless of results, testing should be verified with clinical symptoms for diagnosis of anaphylaxis.
In Vitro Biochemical Tests
Serum IgE measurements specific to some allergy inducing agents have been used to confirm anaphylaxis. In vitro immunoassays through radioallergosorbent tests (RASTs) are commercially available for agents such as neuromuscular blockers, beta-lactam antibiotics, morphine, chlorhexidine, protamine, and latex among others.16,25 While there are indications for which in vitro testing may be indicated (Table 4), many in vitro tests are arguably less sensitive and specific when compared to skin testing and not as readily available. As mentioned previously, biochemical test results should always be correlated with clinical history.
Table 4. .
Indications for In Vitro Versus Skin Prick Testing

ALTERNATIVES TO BENZODIAZEPINES
Benzodiazepines are most commonly utilized in anesthesia as anxiolytic premedication. Goals of preoperative administration include sedation anxiolysis, amnesia, improved patient cooperation, and improved patient satisfaction.29 However, when administering benzodiazepines to potentially allergic individuals, alternatives should be considered.
In the pediatric population, behavioral modalities have shown some anxiolytic efficacy including use of music specialists, communication styles, and trained distraction tactics.30 Evidence has even shown that teaching families to expose their child to facemask and distract them on day of surgery may produce better anxiety control than parental presence or midazolam. Interestingly, in 2013, Kerimoglu et al30 published a study of nearly 100 children, in which the use of video glasses was determined to be as effective as midazolam for preoperative anxiolysis.
Pharmacological alternatives to benzodiazepines are also available. Preanesthetic intranasal administration of the alpha-2 agonist, dexmedetomidine, has shown similar levels of anxiety relief and sedation as midazolam.31 In some instances, children receiving dexmedetomidine have also demonstrated less perioperative stimulation and less postoperative pain. Another study exploring dexmedetomidine administration versus midazolam in patients undergoing flexible bronchoscopy demonstrated superior patient comfort and tolerance in the group receiving the alpha-2 agonist.32 Oral alpha-2 agonists, such as clonidine, as well as sedating antihistamines such as hydroxyzine could also be considered. IM ketamine is a common agent used in office-based dental anesthesia as well.
In adults, oral alpha-2 agonists such as clonidine or tizanidine can also be considered. Alternatively, after IV access, propofol or barbiturates could be considered for sedation. For the needle phobic adult, sevoflurane mask induction can be considered.
An older, now rarely used anticholinergic agent, scopolamine, has been employed to provide some degree of sedation and amnesia when administered intravenously. As a tertiary amine that has the ability to cross the blood-brain barrier, scopolamine offers amnestic, anticholinergic, and antiemetic properties that may have utility in dental and oral surgical procedures.
In choosing any anesthetic agent, providers should consider goals of administration in the setting of risks and benefits.
CONCLUSION
Benzodiazepine hypersensitivity is difficult to assess in the typical polypharmacy anesthetic because the cause and classification of reactions are not always apparent. The multitude of potential offending agents complicates follow-up testing and interpretation of results. The elevated serum tryptase levels noted in some reports support evidence of allergic reaction, but do not differentiate between anaphylactic and anaphylactoid reactions, or establish the causative drug(s).
The current literature on benzodiazepine allergy remains inconclusive and consists primarily of sparse case reports of suspected reactions, typically involving multiple drug administration, without definitive follow-up allergy testing to determine the true causative agent. Challenge testing remains the gold standard for diagnosis, but given the potentially severe manifestation of allergic reaction, it is not routinely pursued. Ultimately, thorough assessment of clinical history along with identification of early signs and symptoms in the perioperative period are important for managing anaphylactic/anaphylactoid reactions from benzodiazepine administration.
CONTINUING EDUCATION QUESTIONS
This continuing education (CE) program is designed for dentists who desire to advance their understanding of pain and anxiety control in clinical practice. After reading the designated article, the participant should be able to evaluate and utilize the information appropriately in providing patient care.
The American Dental Society of Anesthesiology (ADSA) is accredited by the American Dental Association and Academy of General Dentistry to sponsor CE for dentists and will award CE credit for each article completed. You must answer 3 of the 4 questions correctly to receive credit.
Submit your answers online at www.adsahome.org. Click on ‘‘On Demand CE.''
CE questions must be completed within 3 months and prior to the next issue.
-
1.
Which of the following is the drug of choice for treatment of anaphylaxis?
-
A.
Albuterol
-
B.
Epinephrine
-
C.
Hydrocortisone
-
D.
IV crystalloid
-
A.
-
2.
What are the 3 most common causative agents of anaphylaxis during the perioperative period?
-
A.
Latex, colloids, and antibiotics
-
B.
Propofol, benzodiazepines, and opioids
-
C.
Neuromuscular blocking drugs, latex, and antibiotics
-
D.
Local anesthetics, barbiturates, and volatile agents
-
A.
-
3.
Clinical manifestations of anaphylactoid reactions are often indistinguishable from anaphylaxis.
-
A.
True
-
B.
False
-
A.
-
4.
Anaphylaxis is what type of immune mediated allergic reaction based on Gell and Coombs classification?
-
A.
Type I: Immunoglobulin IgE mediated hypersensitivity reaction
-
B.
Type II: IgG, IgM, and complement mediated cytotoxicity
-
C.
Type III: Immune-complex formation and deposition leading to tissue damage
-
D.
Type IV: T-lymphocyte cell-mediated delayed hypersensitivity reaction
-
A.
REFERENCES
- 1. Fasting S, Gisvold SE. Serious intraoperative problems: a five-year review of 83,844 anesthetics. Can J Anaesth. 2002; 49: 545– 553. [DOI] [PubMed] [Google Scholar]
- 2. Hepner DL, Castells MC. Anaphylaxis during the perioperative period. Anesth Analg. 2003; 97: 1381– 1395. [DOI] [PubMed] [Google Scholar]
- 3. Miller RD, Manuel P, Stoelting RK. Basics of Anesthesia. 6th ed. Philadelphia, PA: Elsevier/Saunders; 2011. [Google Scholar]
- 4. Martinez-Tadeo JA, Perez-Rodriguez E. Anaphylaxis caused by tetrazepam without cross-reactivity with other benzodiazepines. Ann Allergy Asthma Immunol. 2012; 108: 284– 285. [DOI] [PubMed] [Google Scholar]
- 5. Ghosh JS. Allergy to diazepam and other benzodiazepines. Br Med J. 1977; 1: 902– 903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Majumdar SK. Allergy to diazepam. Br Med J. 1977; 1: 444. [PMC free article] [PubMed] [Google Scholar]
- 7. Asero R. Hypersensitivity to diazepam. Allergy Net. 2002; 57: 1209– 1215. [DOI] [PubMed] [Google Scholar]
- 8. Yakel DL, Whittaker SE, Elstad MR. Midazolam-induced angioedema and bronchoconstriction. Crit Care Med. 1992; 20: 307– 308. [DOI] [PubMed] [Google Scholar]
- 9. Shin JG, Hwang JH, Lee BS, et al. A case of midazolam anaphylaxis. Clinical Endoscopy Clin Endosc. 2014; 47: 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fujita Y, Ishikawa H, Yokota K. Anaphylactoid reaction to midazolam. Anesth Analg. 1994; 79: 811– 812. [DOI] [PubMed] [Google Scholar]
- 11. Shrivastava S. An experience with midazolam anaphylactoid reaction. Jap Soc of Anesth. 2012; 26: 642– 643. [DOI] [PubMed] [Google Scholar]
- 12. Hwang JY, Jeon YT, Na HS, et al. Midazolam hypersensitivity during transportation to theater: a case report. Kor Jour of Anesth. 2010; 59: S1– S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. George C, Williams A. Anaphylaxis with midazolam: our experience. Ind Jour of Anesth. 2011; 55: 630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ayuse T, Kurata S, Ayuse T. Anaphylactoid-like reaction to midazolam during oral and maxillofacial surgery. Anesthesia Prog. 2015; 62: 64– 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim H, Fischer D. Anaphylaxis. Allergy Asthma Clin Immunol. 2011: 7(suppl 1):S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rinder CS. Diseases related to immune system dysfunction . In: Stoelting RK, Hines Marschall KE. Stoelting's Anesthesia and Co-existing Disease. Philadelphia, PA: Churchill Livingstone/Elsevier; 2008: 526– 528. [Google Scholar]
- 17. Nitti JT, Nitti GJ. Anesthetic complications. : EG Morgan, Mikhail MS, Murray MJ. Clinical Anesthesiology. 5th ed. New York, NY: Lange Medical/McGraw Hill Medical Pub; 2006: 973. [Google Scholar]
- 18. Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second Symposium on the Definition and Management of Anaphylaxis: Summary Report—Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network Symposium. Ann Emerg Med. 2006; 47: 373– 380. [DOI] [PubMed] [Google Scholar]
- 19. Simons FE, Roberts JR, Gu X, et al. Epinephrine absorption in children with a history of anaphylaxis. J Allergy Clin Immunol. 1998; 101: 33– 37. [DOI] [PubMed] [Google Scholar]
- 20. Simons FE, Gu X, Simons KJ. Epinephrine absorption in adults: intramuscular versus subcutaneous injection. J Allergy Clin Immunol. 2001; 108: 871– 873. [DOI] [PubMed] [Google Scholar]
- 21. Wakatsuki T, Nakaya Y, Inoue I. Vasopressin modulates K+- channel activities of cultured smooth muscle cells from porcine coronary artery. Am J Physiol. 1992; 263: H491– H496. [DOI] [PubMed] [Google Scholar]
- 22. Schummer W, Schummer C, Wippermann J, Fuchs J. Anaphylactic shock: is vasopressin the drug of choice? Anesthesiology. 2004; 101: 1025– 1027. [DOI] [PubMed] [Google Scholar]
- 23. Tang A. A practical guide to anaphylaxis. Am Fam Physician. 2003; 68: 1325– 1333. [PubMed] [Google Scholar]
- 24. Galvao VR, Giavina-Bianchi P, Castells M. Perioperative anaphylaxis. Curr Allergy Asthma Rep. 2014: 452–462. [DOI] [PubMed] [Google Scholar]
- 25. Dewachter P, Mouton-Faivre C, Hepner DL. Perioperative anaphylaxis: what should be known? Curr Allergy Asthma Rep. 2015; 15: 21– 31. [DOI] [PubMed] [Google Scholar]
- 26. Laxenaire MC, Mertes PM, Benabes B, et al. Anaphylaxis during anaesthesia: results of a two-year survey in France. Br J Anaesth. 2001; 87: 549– 558. [DOI] [PubMed] [Google Scholar]
- 27. Vervloet D, Magnan A, Birnbaum J, et al. Allergic emergencies seen in surgical suites. Clin Rev Allergy Immunol. 1999; 17: 459– 467. [DOI] [PubMed] [Google Scholar]
- 28. Wasserfallen JB, Frey PC. Long term evaluation of usefulness of skin and incremental challenge tests in patients with history of adverse reaction to local anesthetics. Allergy. 1995; 50: 162– 165. [DOI] [PubMed] [Google Scholar]
- 29. Bauer KP, Dom PM, Ramirez AM, O'Flaherty JE. Preoperative intravenous midazolam: benefits beyond anxiolysis. J Clin Anesthesia. 2004; 16: 177– 183. [DOI] [PubMed] [Google Scholar]
- 30. Kerimoglu B, Neuman A, Paul J, Stefanov DG, Twersky R. Anesthesia induction using video glasses as a distraction tool for the management of preoperative anxiety in children. Anesthesia Analgesia. 2013; 117: 1373– 1379. [DOI] [PubMed] [Google Scholar]
- 31. Schmidt AP, Valinetti EA, Bandeira D, Bertacchi MF, Simões CM, Auler J. Effects of preanesthetic administration of midazolam, clonidine, or dexmedetomidine on postoperative pain and anxiety in children. Pediatr Anesthesia. 2007; 17: 667– 674. [DOI] [PubMed] [Google Scholar]
- 32. Goneppanavar U, Magazine R, Janardhana BP, Achar SK. Intravenous dexmedetomidine provides superior patient comfort and tolerance compared to intravenous midazolam in patients undergoing flexible bronchoscopy. Pulmon Med. 2015;2015: 1– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]


