Abstract
Background and study aims
To demonstrate the clinical use of a multimodal endoscope with a targeted fluorescently labeled peptide for quantitative detection of Barrett’s neoplasia.
Patients and methods
We studied 50 patients with Barrett’s esophagus using a prototype multimodal endoscope with a fluorescently labeled peptide. Co-registered fluorescence and reflectance images were converted to ratios to correct for differences in distance and geometry over the image field of view. The ratio images were segmented using a unique threshold that maximized the variance between high and low intensities to localize regions of high grade dysplasia (HGD) and esophageal adenocarcinoma (EAC).
Results
Early neoplasia (HGD and EAC) was identified with 94% specificity and 96% positive predictive value at a threshold of 1.49. The mean results for HGD and EAC were significantly greater than those for squamous/Barrett’s esophagus and low grade dysplasia by one-way analysis of variance (ANOVA). The receiver operator characteristic curve for detection of early neoplasia had an area under the curve of 0.884. No adverse events associated with the endoscope or peptide were found.
Conclusion
A multimodal endoscope can quantify fluorescence images from targeted peptides to localize early Barrett’s neoplasia. (ClinicalTrials.gov number NCT01630798.)
Introduction
Wide-field imaging techniques that rapidly visualize large mucosal surface areas for the detection of early Barrett’s neoplasia are needed to address the rapid rise in the incidence of esophageal adenocarcinoma (EAC). Advanced imaging methods, including chromoendoscopy, narrow-band imaging (NBI), and autofluorescence imaging (AFI), are based on nonspecific contrast mechanisms [1–3]. Molecular imaging is an emerging technology being developed to detect disease on the basis of biological changes that are specific to cancer.
We have previously identified the peptide ASY-NYDA and demonstrated its specific binding to early Barrett’s neoplasia in vivo with confocal endomicroscopy [4]. We labeled this peptide with fluorescein isothiocyanate (FITC), hereafter called ASY*-FITC, because fluorescein has an excellent safety profile and is approved for human use by the US Food and Drug Administration (FDA).
In this study, we aimed to assess the feasibility of using the ASY*-FITC peptide for the collection of wide-field fluorescence images of Barrett’s neoplasia, including high grade dysplasia (HGD) and EAC, in vivo using a multimodal endoscope that can produce quantified images.
More detailed information on the rationale for this study is available in Appendix e1 (available online).
Patients/material and methods
Study population
Consecutive patients referred to the University of Michigan for the evaluation of therapy for Barrett’s neoplasia (HGD/EAC) were enrolled. All patients had been on proton pump inhibitor (PPI) therapy for at least 2 weeks. Inclusion criteria included: (i) history of Barrett’s esophagus/EAC; (ii) medically cleared for endoscopy; and (iii) age over 18. Exclusion criteria included: (i) known allergy to FITC/fluorescein; (ii) history of esophagectomy; and (iii) being on an active chemotherapy or radiation protocol.
The study was approved by the FDA (investigational new drug [IND] #110,444; sponsor D.K.T.) and the institutional review board. All patients provided written informed consent. The clinical study was registered online at ClinicalTrials.gov (NCT01630798).
Multimodal endoscope
The filter set in an AFI endoscope (GIF-Y0029; Olympus Medical Systems Corp., Tokyo, Japan) was modified to match the fluorescence spectrum of FITC (Fig. 1a). A xenon short-arc lamp delivers white-light illumination (Fig. 1b), and either blue or green light is provided for fluorescence or reflectance, respectively. The imaging mode is determined by a push-button on the endoscope handle that moves a rotating filter wheel in front of the light source. All modes are delivered through two standard fiberoptic guides (Fig. 1c–e). Separate standard definition detectors are used to collect white-light and fluorescence/reflectance images. The parameters of the long-pass filter (LPF) were chosen to match FITC (Table 1). All images are collected at 20 frames per second (fps). Both objectives have a maximum light divergence angle of 140°. The depth of field (distance over which the image is in focus) is 7–100mm and 5–100mm for white light and fluorescence, respectively. The entire system is contained within a portable instrument cart (Fig. 1f).
Fig. 1.
The molecular endoscope. a A standard definition charge-coupled device (CCD) detector (labeled WL in the diagram) is used for white-light imaging. A separate detector (F/R) is used to collect either fluorescence or reflectance images that are co-registered. A long-pass filter (LPF) blocks excitation light and allows fluorescence and reflectance to pass. The biopsy channel (B) is used to pass the spray catheter for peptide administration. b A xenon short-arc lamp provides white-light illumination (400 – 700 nm). Operated by a button on the endoscope handle, a rotating filter wheel moves in front of this lamp to produce either excitation for fluorescence (450 – 490nm) or illumination for reflectance (540 – 560nm) at 20 frames per second (fps). c–e Multimodal imaging is performed using: c white light; d fluorescence; and e reflectance. f The system, including endoscope, digitizer (D), video processor (VP), and light source (LS), is contained on a portable instrument cart.
Table 1.
The multimodal endoscope, a prototype upper gastrointestinal endoscope that collects wide-field images in three modes: white light, and fluorescence and reflectance, which are collected using separate standard definition detectors.
| Optics | White light | Fluorescence | Reflectance |
|---|---|---|---|
| Illumination, nm | 400 – 700 | 450 – 490 | 540 – 560 |
| Collection, nm | 400 – 700 | 520 – 600 | 540 – 560 |
| Imaging speed, fps | 20 | 20 | 20 |
| Maximum divergence | 140° | 140° | 140° |
| Depth of field, mm | 7 – 100 | 5 – 100 | 5 – 100 |
| Light source | Xenon short-arc lamp | ||
| Insertion tube | |||
| Measurements, mm | |||
| Outer diameter | 11 | ||
| Working length | 1030 | ||
| Total length | 1345 | ||
| Angulation range | |||
| Up | 210° | ||
| Down | 90° | ||
| Right | 100° | ||
| Left | 100° | ||
A device master file (#1888) for the endoscope was registered with the FDA and the use of this instrument in the clinical study was referenced in the IND application.
Imaging procedure
The study was performed by three experienced therapeutic endoscopists (C.P., B.J.E., R.S.K.). Imaging was performed first with white-light endoscopy (WLE). The length of the Barrett’s esophagus segment was measured using the Prague criteria [5] (Table 2). Careful inspection was performed for visible lesions, and the macroscopic descriptions were recorded using the Paris classification [6].
Table 2.
Clinicopathological data for the 50 patients with Barrett’s esophagus who underwent examination with the multimodal endoscope followed by resection of the imaged areas for pathological examination.
| Patient number | Age; sex | Race | BMI | Prague criteria1 | Paris class2 | Macroscopic description on white-light endoscopy | Therapy | Pathology | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Squamous | Barrett’s esophagus | GEJ | LGD | HGD | EAC | ||||||||
| 1 | 56; male | White | 25 | C0M2 | 0 – Is | Focal nodularity | EMR/biopsy | X | X | X | X | ||
| 2 | 58; male | White | 28 | C0M6 | 0 – IIb | Nodularity, ulceration | EMR/biopsy | X | X | X | |||
| 3 | 59; male | White | 30 | C1M5 | 0 – IIb | No nodularity | Biopsy | X | X | ||||
| 4 | 57; male | White | 18 | C6M6 | 0 – IIb | Some nodularity | EMR/biopsy | X | X | ||||
| 5 | 70; male | White | 21 | C2M4 | 0 – IIb | Some nodularity | EMR/biopsy | X | X | X | |||
| 6 | 63; female | White | 37 | C0M1 | 0 – IIa | Focal nodularity | Biopsy | X | X | ||||
| 7 | 86; male | White | 26 | C0M0 | 0 – IIb | No nodularity | Biopsy | X | |||||
| 8 | 80; male | White | 38 | C2M3 | 0 – IIb | No nodularity | Biopsy | X | |||||
| 9 | 75; male | White | 25 | C0M1 | 0 – IIb | No nodularity | Biopsy | X | X | ||||
| 10 | 67; male | White | 22 | C0M0 | 0 – IIb | Scant nodularity | Biopsy | X | |||||
| 11 | 59; male | White | 28 | C9M9 | 0 – IIb | Focal nodularity | EMR/biopsy | X | X | ||||
| 12 | 82; male | White | 33 | C1M11 | 0 – IIb | Barrett’s esophagus islands | EMR/biopsy | X | X | ||||
| 13 | 56; male | White | 29 | C0M1 | 0 – llb | No visible abnormalities | Biopsy | X | X | ||||
| 14 | 62; male | White | 32 | C0M1 | 0 – IIa | Variable nodularity | EMR | X | |||||
| 15 | 63; male | White | 24 | C0M1 | 0 – Is | Nodularity, ulceration | Biopsy | X | |||||
| 16 | 79; male | White | 26 | C0M0 | 0 – IIb | Subtle nodularity | EMR/biopsy | X | |||||
| 17 | 68; female | White | 27 | C0M1 | 0 – Is | Medium-sized nodule | EMR | X | X | X | X | ||
| 18 | 81; male | White | 40 | C10M10 | 0 – IIa | Nodularity, erosion | EMR/biopsy | X | X | ||||
| 19 | 70; male | White | 23 | C0M6 | 0 – IIb | Nodularity, Barrett’s esophagus islands | Biopsy | X | X | ||||
| 20 | 75; male | White | 34 | C0M1 | 0 – IIa | Flat nodule | EMR/biopsy | X | X | X | X | ||
| 21 | 59; male | White | 23 | C1M2 | 0 – IIb | Nodularity | EMR/biopsy | X | X | X | X | ||
| 22 | 69; male | White | 25 | C1M1 | 0 – IIa | Focal nodularity | EMR/biopsy | X | X | ||||
| 23 | 40; male | White | 23 | C1M2 | 0 – Is | Focal mass | EMR/biopsy | X | X | X | |||
| 24 | 84; male | White | 35 | C7M7 | 0 – IIb | No nodularity | Biopsy | X | X | ||||
| 25 | 63; male | White | 37 | C0M0.5 | 0 – IIb | Mild mucosal irregularity | EMR/biopsy | X | X | X | X | ||
| 26 | 55; male | White | 53 | C3M5 | 0 – IIc | Nodularity | EMR/biopsy | X | X | ||||
| 27 | 53; female | White | 24 | C0M1 | 0 – IIb | Subtle nodularity | Biopsy | X | |||||
| 28 | 69; female | White | 24 | C0M6 | 0 – IIa | Subtle nodularity | EMR/biopsy | X | X | X | X | ||
| 29 | 56; male | White | 30 | C1M2 | 0 – IIa | Somewhat nodular | EMR/biopsy | X | X | ||||
| 30 | 61; male | White | 20 | C1M4 | 0 – IIb | Island of nodular mucosa | EMR | X | X | X | |||
| 31 | 69; male | White | 34 | C5M6 | 0 – IIb | No visible abnormalities | EMR/biopsy | X | X | X | X | ||
| 32 | 76; male | White | 39 | C8M10 | 0 – IIa | Nodularity | Biopsy | X | |||||
| 33 | 66; female | White | 24 | C0M4 | 0 – IIa | Barrett’s esophagus segments × 3 | EMR/biopsy | X | X | ||||
| 34 | 65; male | White | 33 | C8M8 | 0 – IIa | Several areas of nodularity | EMR/biopsy | X | X | ||||
| 35 | 56; male | White | 31 | C0.5M1 | 0 – IIb | Nodularity | EMR | X | |||||
| 36 | 51; male | Unknown | 29 | C0M1 | 0 – IIb | No visible abnormalities | EMR/biopsy | X | X | ||||
| 37 | 37; male | White | 32 | C0M2 | 0 – IIa | Frondy nodularity | EMR/biopsy | X | X | X | |||
| 38 | 71; male | White | 29 | C1M4 | 0 – IIa | Nodularity | EMR/biopsy | X | X | X | |||
| 39 | 63; male | White | 39 | C5M6 | 0 – IIc | Barrett’s esophagus islands, nodularity | EMR | X | X | ||||
| 40 | 70; male | White | 34 | C0M1 | 0 – IIa | Focal nodularity | EMR | X | X | X | |||
| 41 | 67; male | White | 29 | C0M1 | 0 – IIb | No visible abnormalities | EMR | X | X | X | X | X | |
| 42 | 64; male | White | 34 | C0M1 | 0 – IIb | Flat tongue of BE | EMR | X | X | X | |||
| 43 | 65; male | White | 25 | C1M3 | 0 – IIb | Barrett’s esophagus island, no nodularity | EMR | X | X | X | |||
| 44 | 71; male | White | 33 | C3M5 | 0 – IIa | Nodularity around scar | EMR/biopsy | X | X | X | |||
| 45 | 52; male | White | 26 | C8M8 | 0 – IIb | Ulcer, nodularity | Biopsy | X | X | ||||
| 46 | 70; male | White | 30 | C0.5M1.5 | 0 – IIb | Focal nodularity | Biopsy | X | X | ||||
| 47 | 75; male | White | 26 | C4M5 | 0 – IIb | Mild mucosal distortion | EMR/biopsy | X | |||||
| 48 | 75; male | White | 28 | C9M9 | 0 – IIc | Nodularity | EMR/biopsy | X | X | X | |||
| 49 | 76; male | White | 26 | C0M0.5 | 0 – IIa | Focal, flat Barrett’s esophagus | EMR | X | X | ||||
| 50 | 71; female | White | 33 | C0M0.1 | 0 – IIb | No nodularity | EMR | X | X | X | |||
BMI, body mass index; GEJ gastroesophageal junction; LGD low grade dysplasia; HGD high grade dysplasia; EAC esophageal adenocarcinoma; EMR, endoscopic mucosal resection.
The anatomic extent of the imaged Barrett’s segment (C, circumferential; M, maximum) was defined by the Prague criteria.
The Paris classification was used to provide a macroscopic description of the level of mucosal elevation: 0–Is protruded; 0–IIa elevated; 0–IIb flat; 0–IIc depressed.
Overlying mucous, debris, and bubbles were removed with water. Autofluorescence images were collected before peptide administration to assess the background (Video 1). The ASY*-FITC peptide (see Appendix e2) was then topically administered at a concentration of 100 μM in 5mL to all patients. After 5 minutes’ incubation, the unbound peptides were rinsed away. White-light, fluorescence, and reflectance videos were collected from the Barrett’s segment (Video 2). The average time required to perform the peptide spraying and imaging was less than 10 minutes.
Video 1.

Autofluorescence. Real-time white-light, fluorescence, and reflectance images of Barrett’s esophagus on molecular endoscopy prior to peptide administration shows minimal background. Online content including video sequences viewable at: http://dx.doi.org/10.1055/s-0034-1392803
Video 2.

Barrett’s esophagus. Real-time white light, fluorescence, and reflectance images of Barrett’s esophagus on molecular endoscopy following topical administration of peptide shows minimal fluorescence intensity. Online content including video sequences viewable at: http://dx.doi.org/10.1055/s-0034-1392803
The endoscopist then used endoscopic mucosal resection (EMR) to remove the Barrett’s segment that had been imaged (Fig. e2a). After resection, dabs of four different colored inks were applied to the edges of each specimen to allow for correct alignment of the image and the specimen (Fig. e2b). The tissue was sectioned in rows by the pathologist along the length of the specimen at 2-mm intervals. For small regions not amenable to endoscopic resection, biopsies were taken with forceps.
Two gastrointestinal pathologists (H.D.A., S.R.O.), who were blinded to the imaging results, classified the pathology as squamous, Barrett’s esophagus, gastroesophageal junction (GEJ), low grade dysplasia (LGD), HGD, and EAC. For sections with mixed histology, the most advanced grade was assigned.
All videos were recorded in high definition (1920 ×1080-pixel resolution) in MP4 format with 8-bit intensity (0–255 gray levels) using an image digitizer (IMH-10; Olympus). Video streams of fluorescence with >20 consecutive images (1 second) and negligible motion artifacts (blurring) were used to identify co-registered fluorescence and reflectance images (540×540 pixels). An intensity of 12 gray levels was subtracted from each image, corresponding to the detector read-out with no illumination (dark frame).
Because of the large optical divergence angle used to collect the wide-field images [7], we used a ratio of the fluorescence and reflectance intensities for each pixel to correct for differences in distance between the detector and the various points imaged on the mucosa (see Appendix e3, Fig. e3, available online). These ratio images were segmented to localize regions of HGD and EAC using a unique threshold that maximized the variance between high and low intensities (Fig. e4) [8].
For details of the statistical analysis performed see Appendix e4 (available online).
Results
Study population
A total of 55 patients gave their consent for entry into this study. At the discretion of the attending physician, imaging was not performed in five patients who showed physical signs of difficulty tolerating the procedure. Patient characteristics are shown in detail in Table 2 and summarized in Table 3.
Table 3.
Summary of clinico-pathological data for the 50 patients with Barrett’s esophagus who underwent examination with the multimodal endoscope followed by resection of the imaged areas for pathological examination.
| Sex, n (%) | |
| Male | 44 (88 %) |
| Female | 6 (12 %) |
| Age, mean ± SD (range), years | 65.6 ± 10.2 (37 – 86) |
| Body mass index (BMI), mean ± SD, kg/m2 | 29.5 ± 6.3 |
| Length of Barrett’s esophagus segment measured by Prague criteria, mean ± SD (range), cm | |
| Circumferential length (C) | 1.8 ± 2.8 (0 – 9) |
| Maximum length (M) | 3.6 ± 3.1 (0 – 11) |
| Macroscopic description of imaged segment according to Paris classification, n (%) | |
| 0 – Is protruded | 3 (6 %) |
| 0 – IIa elevated | 16 (32 %) |
| 0 – IIb flat | 28 (56 %) |
| 0 – IIc depressed | 3 (6 %) |
| Most advanced grade of pathology found on imaged segment, n (%) | |
| Squamous epithelium | 3 (6 %) |
| Barrett’s esophagus | 3 (6 %) |
| Gastroesophageal junction | 4 (8 %) |
| Low grade dysplasia | 7 (14 %) |
| High grade dysplasia | 21 (42 %) |
| Esophageal adenocarcinoma | 12 (24 %) |
Imaging procedure
For squamous and Barrett’s esophagus, we observed minimal signal before (background) and after the peptide was administered. Representative fluorescence images are shown in Fig. e5a–c. The corresponding white-light images are shown in Fig. e5d–f.
As an example of HGD, a representative set of white-light, fluorescence, and reflectance images are shown in Fig. 6a–c. On white-light imaging, Barrett’s esophagus can be identified by a salmon-red appearance. No structural abnormalities were visible in this region (Paris 0–IIb) to suggest the presence of neoplasia. A region of increased intensity was observed on fluorescence that was found to be HGD on pathological examination of the resected specimen.
Fig. 6.
In vivo multimodal images of early neoplasia (high grade dysplasia [HGD]). a White-light image showing several areas of Barrett’s esophagus (labeled BE in the diagram), identified by salmon-red mucosa, surrounded by squamous epithelium but no macroscopically visible structural abnormalities to suggest the presence of neoplasia. b Fluorescence image showing a region of increased intensity (surrounding dashed line) found later to be HGD; the squamous epithelium shows a minimal background level. c Reflectance image, which is co-registered with the fluorescence image of part b. The reflectance and fluorescence images of parts b and c are combined to give d, the ratio image, which corrects for differences in detected fluorescence intensity caused only by distance. d Ratio image showing enhancement of the signal from HGD. e Intensities from fluorescence, reflectance, and ratio images along the dashed line shown in b – d, showing a peak at the location of the HGD. f Corresponding pathology showing histological features of HGD on a background of Barrett’s esophagus (BE), identified by the presence of goblet cells. (GEJ, gastroesophageal junction.)
We combined co-registered fluorescence and reflectance as a ratio to correct for differences in distance to the mucosal surface caused by viewing down the esophageal lumen at large angles. The quantified image intensities are shown in pseudocolor (Fig. 6d). The intensities along the dashed line passing through the lesion in Fig. 6b – d are shown in Fig. 6e. A peak is seen on fluorescence at the location of HGD. Histological features of HGD are seen next to the adjacent squamous epithelium (Fig. 6f). A few residual goblet cells are present, reflecting the Barrett’s esophagus background.
Adverse events of this study included bleeding at the site of the resection after completion of EMR (n=2), fever of 102 °F (n=1), abdominal pain and nausea (n=1). All of these events resolved spontaneously and are known complications of the EMR procedure.
Image evaluation
“Red flag” contours of LGD (Video 3), HGD (Video 4), and EAC (Video 5) produced with the segmentation algorithm are shown on representative fluorescence images in Fig. 7a – c. On the corresponding white-light images, these lesions are indistinguishable from Barrett’s esophagus (Fig. 7d–f). Note: that the fluorescence and white-light images are not co-registered because they are collected by different detectors (Fig. 1a). Pathology of the specimens removed by EMR from the imaged Barrett’s segments is shown in Fig. 7g–i.
Video 3.
Low grade dysplasia (LGD). Real-time white-light, fluorescence, and reflectance images of LGD on molecular endoscopy following topical peptide administration shows patchy regions of increased fluorescence intensity that were found to contain LGD on histology after endoscopic mucosal resection. Online content including video sequences viewable at: http://dx.doi.org/10.1055/s-0034-1392803
Video 4.

High grade dysplasia (HGD). Real-time white-light, fluorescence, and reflectance images of HGD on molecular endoscopy following topical peptide administration shows a single region of increased fluorescence intensity that was found to contain HGD on histology after endoscopic mucosal resection. Online content including video sequences viewable at: http://dx.doi.org/10.1055/s-0034-1392803
Video 5.

Esophageal adenocarcinoma (EAC). Real-time white-light, fluorescence, and reflectance images of EAC on molecular endoscopy following topical peptide administration shows several regions of increased fluorescence intensity that were found to contain EAC on histology after endoscopic mucosal resection. Online content including video sequences viewable at: http://dx.doi.org/10.1055/s-0034-1392803
Fig. 7.
Localization of early neoplasia. a – c Red flag” contours (dashed red lines) were identified by segmenting the ratio images and are shown on representative fluorescence images of: a low grade dysplasia (LGD); BE, Barrett’s esophagus; b high grade dysplasia (HGD); c esophageal adenocarcinoma (EAC). The mean target/background ratio was measured from these regions for each patient. d–f Corresponding white-light images showing that these lesions appear flat in morphology and patchy in distribution, being difficult to distinguish from non-neoplastic Barrett’s esophagus. g–i Corresponding histology (stained with hematoxylin and eosin [H&E]) showing: g LGD with metaplastic tubules lined by atypical epithelium with enlarged and hyperchromatic nuclei that remain oriented toward the base of the epithelium; h HGD with several irregularly shaped tubules lined by epithelium with cytological features of HGD (arrow) and a few adjacent tubules that contain residual goblet cells reflecting the Barrett’s esophagus background (arrowhead); i EAC with a complex, back-to-back arrangement of tubules that are lined by markedly atypical epithelium with enlarged, crowded, and hyperchromatic nuclei with negligible mucus production and total lack of epithelial maturation toward the mucosal surface. In a few places, small clusters of dysplastic cells can be seen “budding” from larger tubules and infiltrating the lamina propria (arrow).
Imaging performance
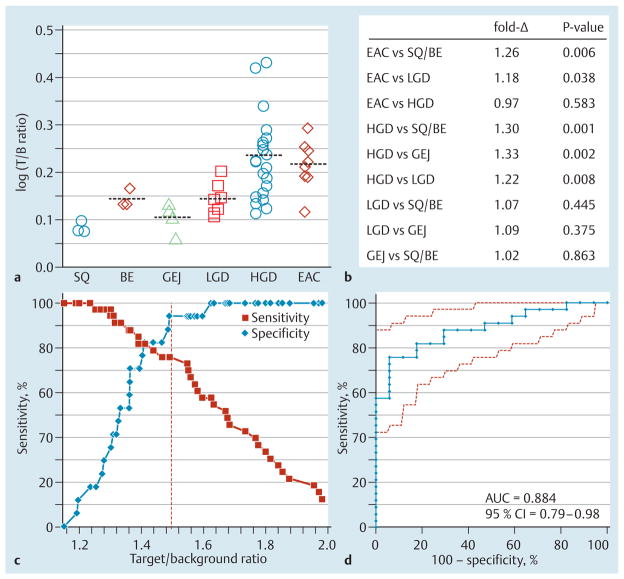
For each patient, the mean target/background ratio from the fluorescence intensities of the “red flag” regions was measured, log-transformed, and classified by the highest grade found on pathology (Fig. 8a). On one-way analysis of variance (ANOVA), the mean results for either EAC or HGD were significantly greater than those for squamous/Barrett’s esophagus, GEJ, and LGD (Fig. 8b). The mean results for LGD were slightly greater than those for non-neoplasia (Barrett’s esophagus/squamous), but without reaching statistical significance.
Fig. 8.
Imaging performance. a The log-transformed mean target/background (T/B) ratios from the “red flag” contours for individual patients, grouped by the most advanced grade found on pathology (SQ, squamous; BE, Barrett’s esophagus; GEJ, gastroesophageal junction; LGD, low grade dysplasia; HGD, high grade dysplasia; EAC, esophageal adenocarcinoma), are shown on a log2 scale. The mean ± standard deviation values were 1.21 ± 0.03 for squamous (n= 3), 1.39 ±0.06 for Barrett’s esophagus (n=3), 1.27 ± 0.09 for GEJ (n=4), 1.39 ± 0.11 for LGD (n=7), 1.73± 0.39 for HGD (n=21), and 1.65 ± 0.23 for EAC (n=12). b A pairwise comparison of classifications showing the estimated fold-change and P values by one-way analysis of variance (ANOVA). c At a T/B ratio of 1.49 (dashed line), specificity was 94 % and positive predictive value (PPV) was 96 %; sensitivity was 76 % but increased with lower T/B ratios. d The receiver operating characteristic (ROC) curve for distinguishing early neoplasia (HGD and EAC) from the other classifications (squamous, Barrett’s esophagus, GEJ, and LGD) has an area under the curve (AUC) of 0.884 (95% confidence interval [CI] 0.793–0.975).
At a target/background ratio of 1.49, we found 25 true positives, 1 false positive, 16 true negatives, and 8 false negatives for identifying neoplasia, resulting in 94% specificity (Fig. 8c). The sensitivity was 76% but increased at lower target/background ratios. The receiver operating characteristic (ROC) curve comparing the target/background ratios for early neoplasia (HGD and EAC) with those for the other classifications (squamous, Barrett’s esophagus, LGD, and GEJ) had an area under the curve (AUC) of 0.884 (Fig. 8d). The result of a two-sided Wilcoxon’s rank sum test comparing these two groups was P<0.0001. The 95% confidence intervals for sensitivity, specificity, and the positive and negative likelihood ratios are shown in Fig. e9.
Discussion
Here, we demonstrate the first clinical use of a multimodal endoscope to collect fluorescence images in vivo using a specific peptide to identify early Barrett’s neoplasia, including 28 flat lesions (Paris 0–IIb) that were poorly visualized with WLE. Molecular endoscopy represents a new technique in advanced imaging that uses differences in biological expression to distinguish diseased from normal tissues. This innovation can minimize procedure time and reduce risks from sedation, bleeding, and perforation. Using this endoscope, we were able to collect adequate signal in vivo over clinically relevant distances from the mucosal surface. High definition rather than standard definition detectors could be used in the future to improve image resolution. For a more in depth discussion of this and other relevant studies see Appendix e5 (available online).
This approach addresses an important unmet clinical need for improved methods for the early detection of neoplasia in the esophagus and other epithelial-derived cancers, where early lesions are often flat in appearance, patchy in distribution, and difficult to visualize with WLE alone.
Supplementary Material
Acknowledgments
We thank B. R. Reisdorph and K. J. Weatherwax for regulatory support; M. Tuck, C. Breakey, and E. Feinberg for clinical support; T. Hirakawa, J. Ito, T. Ozawa, T. Watanabe, Y. Chen, and J. Taylor for technical support.
This study was supported in part by the National Institutes of Health U54 CA13642, U54 CA163059, and the Doris Duke Charitable Foundation Clinical Scientist Development Award #2006068 (T. D. W) and 2UL1TR000433 (Michigan Institute for Clinical & Health Research [MICHR]).
Footnotes
Competing interests: N.D. is an employee of Olympus Medical Systems Corp. S.L. and T.D.W. are co-inventors on intellectual property for the peptide. The other authors have no conflicts to declare.
References
- 1.Longcroft-Wheaton G, Duku M, Mead R, et al. Acetic acid spray is an effective tool for the endoscopic detection of neoplasia in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol. 2010;8:843–847. doi: 10.1016/j.cgh.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 2.Sharma P, Hawes RH, Bansal A, et al. Standard endoscopy with random biopsies versus narrow band imaging targeted biopsies in Barrett’s oesophagus: a prospective, international, randomised controlled trial. Gut. 2013;62:15–21. doi: 10.1136/gutjnl-2011-300962. [DOI] [PubMed] [Google Scholar]
- 3.Boerwinkel DF, Holz JA, Kara MA, et al. Effects of autofluorescence imaging on detection and treatment of early neoplasia in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol. 2014;12:774–781. doi: 10.1016/j.cgh.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Sturm MB, Joshi BP, Lu S, et al. Targeted imaging of esophageal neoplasia with a fluorescently labeled peptide: first-in-human results. Sci Transl Med. 2013;5:184ra61. doi: 10.1126/scitranslmed.3004733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma P, Dent J, Armstrong D, et al. The development and validation of an endoscopic grading system for Barrett’s esophagus: the Prague C & M criteria. Gastroenterology. 2006;131:1392–1399. doi: 10.1053/j.gastro.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 6.The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3–S43. doi: 10.1016/s0016-5107(03)02159-x. [DOI] [PubMed] [Google Scholar]
- 7.Wang TD, Janes GS, Wang Y, et al. Mathematical model of fluorescence endoscopic image formation. Appl Opt. 1998;37:8103–8111. doi: 10.1364/ao.37.008103. [DOI] [PubMed] [Google Scholar]
- 8.Otsu N. A threshold selection method from gray-level histograms. Automatica. 1975;11:285–296. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.