Introduction
Colorectal cancer is one of the most common causes of cancer-related deaths worldwide. The incidence continues to rise steadily because of increasing obesity and greater adoption of Western diets by developing countries.1 While white light colonoscopy is widely used for screening, up to 25% of adenomas may be missed.2 Moreover, proximal lesions are more likely to be non-polypoid (flat) in morphology and difficult to visualize.3 A number of advanced imaging methods based on non-specific detection mechanisms have been developed but have not demonstrated a clear improvement.4 Peptides have been used intravenously to target detection of adenomas with fiber optic colonoscopes that have limited resolution and are no longer used.5 With topical administration, delivery of exogenous imaging agents can be localized to known segments of colon that are at risk to maximize image contrast, minimize background, reduce quantity of agent needed, and increase safety. Here, we demonstrate clinical use of a novel, multi-modal video colonoscope to detect non-polypoid adenomas in the proximal colon that is sensitive to fluorescent imaging agents.
Description of Technology
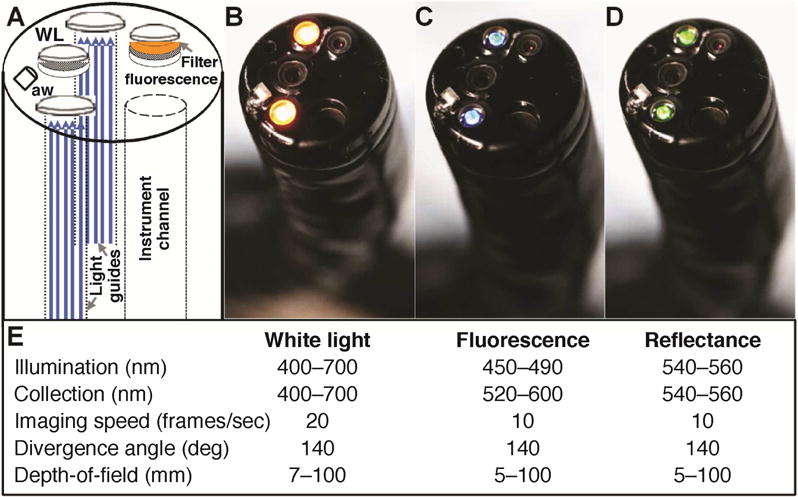
The multi-modal video colonoscope (CF-Y0012, Olympus) was designed for forward viewing with two separate standard definition detectors to collect either white light (WL) or fluorescence images, shown in schematic, Fig. 1A. All modes of illumination are delivered through two standard fiber optic light guides. A filter (520–600 nm) is located between the fluorescence objective and detector to allow light from FITC to pass. Reflectance images (540–560 nm) can be collected that are co-registered with fluorescence. The white light and fluorescence/reflectance images are collected at 20 and 10 frames/sec, respectively, Fig. 1B–D, and all images are displayed on the monitor at 30 frames/sec. A summary of the optical and instrument parameters is shown, Fig. 1E. The maximum divergence angle and depth-of-field of all imaging modes are similar to that of a conventional colonoscope and allow for the proximal colon to be rapidly visualized. A standard nozzle (aw) provides air for insufflation and water to clear the lens. The dimensions of the distal end (14.8 mm), working length (1680 mm), and total length (2010 mm) are identical to that of a conventional video colonoscope. Videos are recorded in MP4 format with 8-bit intensity (0–255 gray levels) using an image digitizer (IMH-10, Olympus). The entire system, including the endoscope, light source, video processor, and image digitizer, is contained on a portable instrument cart.
Fig. 1. Multi-modal video colonoscope.
A) Schematic of prototype instrument. Separate standard definition detectors are used to collect either B) white light (WL) or C) fluorescence images. A filter located in between the fluorescence objective and detector passes light from FITC. D) Reflectance images collected with the same detector are co-registered with fluorescence. E) Summary of optical parameters. White light and fluorescence/reflectance images are collected at 20 and 10 frames/sec, respectively. aw – air/water nozzle.
Video description
We performed a clinical demonstration of this novel instrument during surveillance colonoscopy in a 74 year old male with a history of polyps in the proximal colon. This procedure was approved by the University of Michigan IRB and the FDA (IND #116,907). A cap was placed on the distal end of the multi-modal video colonoscope to flatten colonic folds for improved visualization of non-polypoid lesions. The instrument was used first in the white light mode to identify regions of mucosa that appear suspicious for harboring disease. The white light video shows a 12 mm non-polypoid adenoma in the ascending colon that may easily have been missed on routine surveillance, Fig. 2A. The peptide KCCFPAQ, shown previously to bind rapidly and specifically to colonic adenomas,6 was labeled with FITC (fluorescein isothiocyanate) to match the detection parameters of the fluorescence filter. Fluorescein has a well-known safety profile and is FDA approved for human use. The peptide was topically administered to the mucosa in the proximal colon at a concentration of 76.4 μM (1.2 mg in 10 mL of 0.9% NaCl) via a spray catheter passed through the instrument channel.
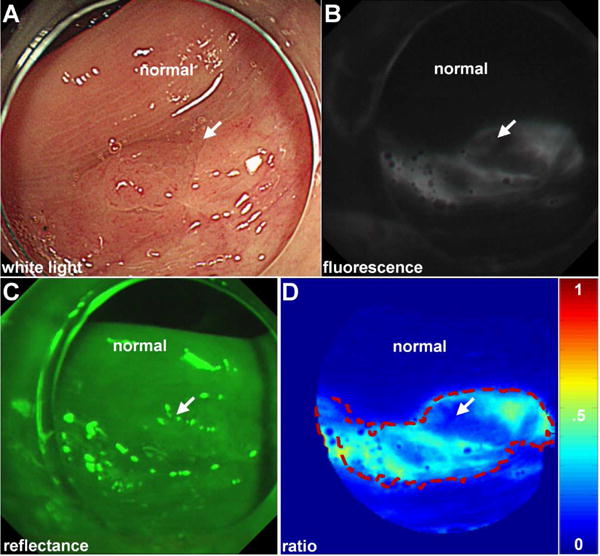
Fig. 2. Detection of non-polpoid adenoma.
A) A cap is placed on the distal end of the multi-modal video colonoscope to flatten mucosal folds for improved visualization. On white light, a non-polypoid lesion (arrow) is seen in the proximal colon that could easily be missed. B) After topical peptide administration, the lesion is clearly visualized with fluorescence. Normal colon shows minimal background. C, D) The registered reflectance image was used to produce a ratio-image, shown in pseudo-color, that corrects for distance and geometry. Intensities can be quantified to outline the extent of the lesion (dashed red line) and guide resection.
After 5 min for incubation, water was used to rinse away the unbound peptides. The imaging mode was changed to fluorescence, and the resulting high contrast images show the lesion with increased intensity from peptide binding, Fig. 2B. There is minimal background from surrounding normal mucosa. A push button on the endoscope handle is used to change the imaging mode to reflectance, Fig. 2C. These images are co-registered with fluorescence and are used in a ratio to correct for differences in distance and geometry between the tissue and detector over the image field-of-view. This step allows for the image intensities to be quantified and is particularly useful for detecting lesions at large angles.7 Although not as fast as white light, the fluorescence videos showed minimal motion artifacts (blurring). Co-registered fluorescence and reflectance images (540×540 pixels) were processed by subtracting an intensity of 12 gray levels from either image that corresponds to the detector read-out with no illumination (dark frame). The ratio images were segmented using a unique threshold that maximized the variance between high and low intensities to assign pixels with greater fluorescence intensities as “diseased.”8 This process defines a “red flag” region (dashed red line) that can be shown as an overlay on the white light image to help guide physician biopsy, Fig. 2D. The resected lesion was found on pathology to be a sessile serrated adenoma. No adverse events associated with either the prototype instrument or peptide occurred.
Take home message
A multi-modal video colonoscope can collect in vivo real time wide-field images of non-polypoid colonic adenomas using fluorescently-labeled peptides. Targeted detection is based on molecular expression rather than morphological changes, and may be effective for improved early detection of colorectal cancers found in the proximal colon that arise from flat pre-malignant lesions that are difficult to visualize. This instrument has similar appearance, dimensions, and handling to that of a conventional colonoscope, and can be translated seamlessly into the clinic.
Supplementary Material
Acknowledgments
We thank BR Reisdorph and KJ Weatherwax for regulatory support; M Tuck, E Feinberg, B Kleiner, and K Gonzales for clinical support.
Funding Support: Funding was provided in part by the Rose and Lawrence C. Page Foundation (DKT), Mary L. Petrovich (TDW), and the National Institutes of Health R01 CA142750, R01 CA200007, and R01 EB020644 (TDW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: Study concept and design: BPJ, TDW, DKT. Acquisition of data: AP, AP, EJW, RSK, GHE. Analysis and interpretation of data: XD, SRO, HDA. Drafting of the manuscript: BPJ, TDW, DKT. Obtained funding: TDW, DKT.
Conflicts of Interest: BPJ and TDW are inventors on U.S. Patent No. 8,901,276 filed by the University of Michigan on the peptide used in this study. All other authors report no conflicts.
References
- 1.Ferlay J, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Leufkens AM, et al. Factors influencing the miss rate of polyps in a back-to-back colonoscopy study. Endoscopy. 2012;44:470–5. doi: 10.1055/s-0031-1291666. [DOI] [PubMed] [Google Scholar]
- 3.Rondagh EJ, et al. Endoscopic appearance of proximal colorectal neoplasms and potential implications for colonoscopy in cancer prevention. Gastrointest Endosc. 2012;75:1218–25. doi: 10.1016/j.gie.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Dik VK, et al. Endoscopic innovations to increase the adenoma detection rate during colonoscopy. World J Gastroenterol. 2014;20:2200–11. doi: 10.3748/wjg.v20.i9.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burggraaf J, et al. Detection of colorectal polyps in humans using an intravenously administered fluorescent peptide targeted against c-Met. Nat Med. 2015;21:955–61. doi: 10.1038/nm.3641. [DOI] [PubMed] [Google Scholar]
- 6.Miller SJ, et al. Targeted detection of murine colonic dysplasia in vivo with flexible multispectral scanning fiber endoscopy. J Biomed Opt. 2012;17:021103. doi: 10.1117/1.JBO.17.2.021103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang TD, et al. Mathematical model of fluorescence endoscopic image formation. Appl Opt. 1998;37:8103–11. doi: 10.1364/ao.37.008103. [DOI] [PubMed] [Google Scholar]
- 8.Joshi BP, et al. Multimodal endoscope can quantify wide-field fluorescence detection of Barrett’s neoplasia. Endoscopy. 2015 doi: 10.1055/s-0034-1392803. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


