Abstract
Tec family kinases, which include tyrosine kinase expressed in hepatocellular carcinoma (TEC), Bruton’s tyrosine kinase (BTK), interleukin (IL)-2-inducible T-cell kinase (ITK), tyrosine-protein kinase (TXK), and bone marrow tyrosine kinase on chromosome X (BMX), are the second largest group of non-receptor tyrosine kinases and have a highly conserved carboxyl-terminal kinase domain. BMX was identified in human bone marrow cells, and was demonstrated to have been expressed in myeloid hematopoietic lineages cells, endothelial cells, and several types of cancers. Significant progress in this area during the last decade revealed an important role for BMX in inflammation and oncologic disorders. This review focuses on BMX biology, its role in inflammation and possible signaling pathways, and the potential of selective BMX inhibitors.
Keywords: BMX, inflammation, tyrosine kinase
Introduction
The response of cells to extracellular stimuli is in part mediated by a number of intracellular kinases. Tec family kinases, as the second largest group of non-receptor tyrosine kinases, are of critical importance to the biology of lymphocytes and other cell lineages derived from the bone marrow.[1] Five distinct subgroups within the Tec kinase family have been described. These include (1) tyrosine kinase expressed in hepatocellular carcinoma (TEC), (2) Bruton’s tyrosine kinase (BTK), (3) interleukin (IL)-2-inducible T-cell kinase (ITK), (4) tyrosine-protein kinase (TXK), and (5) bone marrow tyrosine kinase on chromosome X (BMX).[2] Tec family kinases have been the focus of immunological interest ever since their discovery.
| Access this article online | |
|---|---|
Quick Response Code: 
|
Website: www.burnstrauma.com |
| DOI: 10.4103/2321-3868.135483 | |
While BTK, ITK and TXK show selective expression in cells of bone marrow origin,[3] the expression patterns of BMX and TEC are broader and extends to certain normal somatic cells — such as the cardiac endothelium as a response to ischemia and pressure overload.[4] Specifically, BMX is expressed in hematopoietic cells of the myeloid lineage like granulocytes and monocytes.[5,6] Besides, BMX expression has also been demonstrated in glioblastoma cancer stem cells and several solid tumors, such as prostate and breast cancer. BMX has been suggested to have a role in differentiation, motility and cell survival.[7] The endothelial cells, granulocytes and monocytes play critical roles in the inflammation. This review will examine BMX biology, its role in inflammation and possible signaling pathway, and the potential of selective BMX inhibitors.
Structure and function of BMX
Like many other kinase families, members of the Tec kinase have a typical array of regulatory domains and a highly conserved carboxyl-terminal kinase domain [Figure 1].[8] As such, BMX has Src homology (SH)3 and SH2 domains and a carboxyl-terminal kinase domain.[9] The aminoterminus contains a membrane localization module that is a characteristic feature of the TEC kinases and sets them apart from other non-receptor tyrosine kinases.[10] An amino-terminal pleckstrin homology (PH) domain, which binds to phosphatidylinositols during the process of membrane localization,[11] is followed by a zinc-binding BTK homology (BH) motif and a proline-rich region, which shows a low degree of conservation to the other family members.[7] The SH2, SH3 and BH domains all mediate inter- and intramolecular protein interactions that are likely to regulate kinase activity and substrate access.[7]
Figure 1:
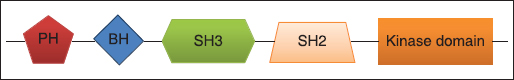
Schematic representation of bone marrow tyrosine kinase on chromosome X (BMX) structural domains. PH = pleckstrin homology, BH = BTK homology, SH = Src homology.
BMX was the latest identified one among the 5 human TEC kinases. In 1994, the human BMX gene was first identified and cloned in bone marrow cells by Tamagnone et al.[12] The BMX gene is located in chromosomal band X p22.2 between the DXS197 and DXS207 loci.[12] The BTK gene, the closest relative of BMX, is also located in chromosome X. The BMX gene encodes a protein with 675 amino acids, of which 70% are identical with BTK.[12] Mutations in BTK gene are responsible for X-linked agammaglobulinemia (XLA) in humans or X-linked immunodeficiency (XID) in mice.[8] However, diseases-associated BMX gene mutations have not been described yet.
BMX in inflammation
Inflammation is a necessary and rapid, yet coordinated response that is induced by microbial infection or tissue injury.[13] Triggers capable of inducing an inflammatory response include tissue damage and infection by pathogenic and nonpathogenic microbes.[13] Undue prolongation of inflammation can be very destructive or even initiate the systemic inflammatory response syndrome, multiple organ failure and death.[14] The inflammatory cytokines, which affect various and numerous physiologic activities, play a significant role in the pathogenesis of inflammation. In the previous studies, tumor necrosis factor (TNF)-α, IL-1β and IL-6 have been demonstrated to be the core of the cytokine-network and play a critical role in the inflammatory response.[15,16] During the immune response, the cytokine IL-8 functions as a potent neutrophil attractant and activator leads to the recruitment of neutrophils from blood, penetration of these cells through the vessel wall, and their directed migration to inflammatory sites and contributes to the advance of inflammation by releasing superoxide anion, matrix metalloproteinase, leukotriene B(4) and platelet-activating factor.[17–19]
There is emerging evidence that non-receptor tyrosine kinase BMX is involved in the pathogenesis of inflammatory disorders, such as rheumatoid arthritis (RA).[7,20,21] An siRNA against BMX-inhibited lipopolysaccharide (LPS)-induced IL-6 secretion in synovial fibroblasts.[22] In macrophages and synovial fibroblasts from RA patients, overexpression of BMX mediates an increase in LPS-induced stabilization of the IL-6 mRNA.[20,21] In the absence of LPS, overexpression of BMX failed to induce IL-6 mRNA expression. Further study revealed that transient depletion of BMX strongly reduced secretion of IL-8 in human fibroblasts stimulated by TNF-α and IL-1β.[7] In neuronal injury induced by H2O2 or ischemia, BMX is activated and suppressing BMX activity protects against neurodegeneration.[23] Altogether, an essential role for the tyrosine kinase BMX in cytokine signaling and inflammation has been established.
The stimulation of Toll-like receptor (TLR)4 by LPS induces the release of critical pro-inflammatory cytokines that result in systemic inflammation and sepsis.[16,24] TLR4 signaling has been divided into MyD88-dependent and MyD88-independent pathways. Signaling through the MyD88-dependent pathway leads to the activation of p38 mitogen-activated protein kinase (MAPK), c-Jun NH(2)-terminal kinase (JNK), and nuclear factor-κB (NF-κB).[25,26] Such signaling activation consequently lead to the release of pro-inflammatory cytokines including TNF-α and IL-1β.[27,28]
An siRNA against BMX strongly reduces secretion of IL-8 in cell lines and HUVECs stimulated by TLR4 agonist, suggesting that BMX could play a role in inflammatory-signaling pathways.[7] Study performed on RA synovial fibroblasts demonstrated that BMX is activated by TLR agonists and that it functionally and physically interacts with the components of the MyD88-dependent TLR pathway MyD88 and Mal as shown by co-immunoprecipitation.[22] More specifically, BMX was required for phosphorylation of p38 MAPK and JNK, as well as activation of NF-κB.[7,11,29] An epistasis analysis indicated that BMX functionally regulates the complex transforming growth factor (TGF)-β activated kinase (TAK)1-binding protein complex.[7] The possible role of BMX in TLR4 signaling is shown in Figure 2. Studies performed on COS cells and HEK293 cells revealed that BMX induced the tyrosine phosphorylation and DNA binding activity of all the Stat factors tested, including STAT1, STAT3 and STAT5.[30] Further study demonstrated that BMX is a critical mediator of Src-induced cell transformation and STAT3 activation.[31,32] However, the upstream localization of BMX kinase relative to the adaptor molecules and the exact mechanism of BMX-dependent regulation of cytokine gene expressions warrants further studies.
Figure 2:
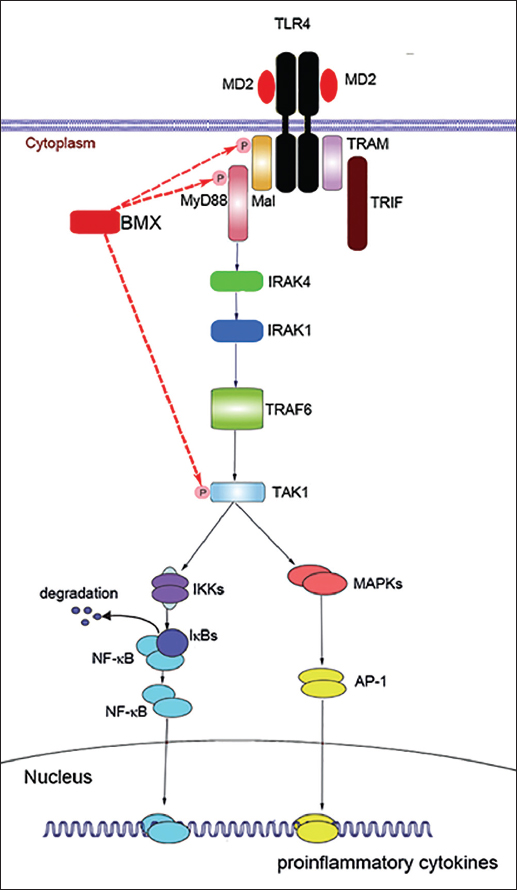
Role of bone marrow tyrosine kinase on chromosome X (BMX) in Toll-like receptor (TLR)4 signaling. BMX has been reported to interact with adaptor molecules (MyD88, or Mal) and transforming growth factor (TGF)-β activated kinase (TAK)1, leading to the activation of the NF-κB and MAPK-signaling pathways. This process results in inflammatory cytokine production. MD = myeloid differentiation protein, TRIF = Toll/interleukin-1 receptor domain-containing adaptor protein inducing interferon-β, TRAM = TRIF-related adaptor molecule, IRAK = interleukin-1 receptor-associated kinase, TRAF = TNF receptor-associated factor, MAPK = mitogen-activated protein kinases, IKK = inhibitor kappa B kinase, AP = activator protein.
BMX kinase inhibitors
As shown in the previously mentioned data, BMX is an important molecule involved in the inflammatory processes. Modulating BMX activity might control the excessive inflammatory response. Prerequisite for the treatment of inflammation by modifying BMX activity is to use a specific, non-toxic inhibitor.[33]
In order to develop irreversible BMX inhibitors, Liu and his colleagues developed compounds capable of targeting cysteine (Cys) 496. Kinome-wide sequence alignment reveals that all 5 TEC family kinases have the conserved cysteine. Dr. Liu introduced an electrophilic acrylamide moiety targeting Cys 496 and first successfully synthesized a selective BMX inhibitor, called BMX-IN-1.[34] The chemical structure is shown in Figure 3. In RV-1 cells, 1 µM of BMX-IN-1 is sufficient to inhibit BMX autophosphorylation.[34]
Figure 3:
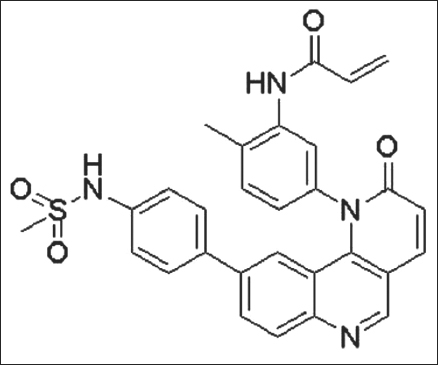
The chemical structure of BMX-IN-1, an irreversible BMX inhibitor. [Figure is from Liu F, et al. ACS Chem Biol 2013;8(7):1423-8.][33]
Ibrutinib (PCI-32765), a reported irreversible inhibitor of BTK, is also a potent inhibitor of BMX and other TEC family kinases.[35,36] Primary data showed that ibrutanib is beneficial in models of arthritis and patients with relapsed or refractory chronic lymphocytic leukemia.[36,37]
Whether these inhibitors could provide significant beneficial effects in inflammatory diseases remains to be established.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant No. 81372050 and 81000836) and the Natural Science Foundation of Anhui Province (Grant No. 1208085MH135).
Footnotes
How to cite this article: Qiu L, Wang F, Liu S, Chen X. Current understanding of tyrosine kinase BMX in infl ammation and its inhibitors. Burn Trauma 2014;2:121–4.
Source of Support: Supported by the National Natural Science Foundation of China (Grant No. 81372050 and 81000836) and the Natural Science Foundation of Anhui Province (Grant No. 1208085MH135). Conflict of Interest: None declared.
References
- 1.Horwood NJ, Urbaniak AM, Danks L. Tec family kinases in inflammation and disease. Int Rev Immunol. 2012;31:87–103. doi: 10.3109/08830185.2012.670334. [DOI] [PubMed] [Google Scholar]
- 2.Faris M, Bot A. In this issue: Tec kinases in the crosshairs. Int Rev Immunol. 2012;31:85–6. doi: 10.3109/08830185.2012.671692. [DOI] [PubMed] [Google Scholar]
- 3.Block H, Zarbock A. The role of the tec kinase Bruton’s tyrosine kinase (Btk) in leukocyte recruitment. Int Rev Immunol. 2012;31:104–18. doi: 10.3109/08830185.2012.668982. [DOI] [PubMed] [Google Scholar]
- 4.Ekman N, Lymboussaki A, Vastrik I, Sarvas K, Kaipainen A, Alitalo K. Bmx tyrosine kinase is specifically expressed in the endocardium and the endothelium of large arteries. Circulation. 1997;96:1729–32. doi: 10.1161/01.CIR.96.6.1729. [DOI] [PubMed] [Google Scholar]
- 5.Kaukonen J, Lahtinen I, Laine S, Alitalo K, Palotie A. BMX tyrosine kinase gene is expressed in granulocytes and myeloid leukaemias. Br J Haematol. 1996;94:455–60. [PubMed] [Google Scholar]
- 6.Weil D, Power MA, Smith SI, Li CL. Predominant expression of murine Bmx tyrosine kinase in the granulo-monocytic lineage. Blood. 1997;90:4332–40. [PubMed] [Google Scholar]
- 7.Gottar-Guillier M, Dodeller F, Huesken D, Iourgenko V, Mickanin C, Labow M, et al. The tyrosine kinase BMX is an essential mediator of inflammatory arthritis in a kinase-independent manner. J Immunol. 2011;186:6014–23. doi: 10.4049/jimmunol.1002813. [DOI] [PubMed] [Google Scholar]
- 8.Boucheron N, Ellmeier W. The role of Tec family kinases in the regulation of T-helper-cell differentiation. Int Rev Immunol. 2012;31:133–54. doi: 10.3109/08830185.2012.664798. [DOI] [PubMed] [Google Scholar]
- 9.August A, Ragin MJ. Regulation of T-cell responses and disease by tec kinase Itk. Int Rev Immunol. 2012;31:155–65. doi: 10.3109/08830185.2012.668981. [DOI] [PubMed] [Google Scholar]
- 10.Buggy JJ, Elias L. Bruton tyrosine kinase (BTK) and its role in B-cell malignancy. Int Rev Immunol. 2012;31:119–32. doi: 10.3109/08830185.2012.664797. [DOI] [PubMed] [Google Scholar]
- 11.Cenni B, Gutmann S, Gottar-Guillier M. BMX and its role in inflammation, cardiovascular disease, and cancer. Int Rev Immunol. 2012;31:166–73. doi: 10.3109/08830185.2012.663838. [DOI] [PubMed] [Google Scholar]
- 12.Tamagnone L, Lahtinen I, Mustonen T, Virtaneva K, Francis F, Muscatelli F, et al. BMX, a novel nonreceptor tyrosine kinase gene of the BTK/ITK/TEC/TXK family located in chromosome Xp22.2. Oncogene. 1994;9:3683–8. [PubMed] [Google Scholar]
- 13.Barton GM. A calculated response: Control of inflammation by the innate immune system. J Clin Invest. 2008;118:413–20. doi: 10.1172/JCI34431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fry DE. Sepsis, systemic inflammatory response, and multiple organ dysfunction: The mystery continues. Am Surg. 2012;78:1–8. [PubMed] [Google Scholar]
- 15.Chen XL, Xia ZF, Wei D, Han S, Ben DF, Wang GQ. Role of p38 mitogen-activated protein kinase in Kupffer cell secretion of the proinflammatory cytokines after burn trauma. Burns. 2003;29:533–9. doi: 10.1016/S0305-4179(03)00147-5. [DOI] [PubMed] [Google Scholar]
- 16.Chen XL, Sun L, Guo F, Wang F, Liu S, Liang X, et al. High-mobility group box-1 induces proinflammatory cytokines production of Kupffer cells through TLRs-dependent signaling pathway after burn injury. PLoS One. 2012;7:e50668. doi: 10.1371/journal.pone.0050668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakagome K, Matsushita S, Nagata M. Neutrophilic inflammation in severe asthma. Int Arch Allergy Immunol. 2012;158(1):96–102. doi: 10.1159/000337801. [DOI] [PubMed] [Google Scholar]
- 18.Allen TC, Kurdowska A. Interleukin 8 and acute lung injury. Arch Pathol Lab Med. 2014;138:266–9. doi: 10.5858/arpa.2013-0182-RA. [DOI] [PubMed] [Google Scholar]
- 19.Pichert A, Schlorke D, Franz S, Arnhold J. Functional aspects of the interaction between interleukin-8 and sulfated glycosaminoglycans. Biomatter. 2012;2:142–8. doi: 10.4161/biom.21316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palmer CD, Mutch BE, Page TH, Horwood NJ, Foxwell BM. Bmx regulates LPS-induced IL-6 and VEGF production via mRNA stability in rheumatoid synovial fibroblasts. Biochem Biophys Res Commun. 2008;370:599–602. doi: 10.1016/j.bbrc.2008.03.142. [DOI] [PubMed] [Google Scholar]
- 21.Palmer CD, Mutch BE, Workman S, McDaid JP, Horwood NJ, Foxwell BM. Bmx tyrosine kinase regulates TLR4-induced IL-6 production in human macrophages independently of p38 MAPK and NFkapp. Blood. 2008;111:1781–8. doi: 10.1182/blood-2007-07-102343. [DOI] [PubMed] [Google Scholar]
- 22.Semaan N, Alsaleh G, Gottenberg JE, Wachsmann D, Sibilia J. Etk/BMX, a Btk family tyrosine kinase, and Mal contribute to the cross-talk between MyD88 and FAK pathways. J Immunol. 2008;180:3485–91. doi: 10.4049/jimmunol.180.5.3485. [DOI] [PubMed] [Google Scholar]
- 23.Chen KY, Wu CC, Chang CF, Chen YH, Chiu WT, Lou YH, et al. Suppression of Etk/Bmx protects against ischemic brain injury. Cell Transplant. 2012;21:345–54. doi: 10.3727/096368911X582741. [DOI] [PubMed] [Google Scholar]
- 24.Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145–51. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 25.Chen XL, Xia ZF, Ben DF, Wang GQ, Wei D. Role of p38 mitogen-activated protein kinase in lung injury after burn trauma. Shock. 2003;19:475–9. doi: 10.1097/01.shk.0000055242.25446.84. [DOI] [PubMed] [Google Scholar]
- 26.Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009;22:240–73. doi: 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang QW, Mou L, Lv FL, Zhu PF, Wang ZG, Jiang JX, et al. Novel TLR4-antagonizing peptides inhibit LPS-induced release of inflammatory mediators by monocytes. Biochem Biophys Res Commun. 2005;329:846–54. doi: 10.1016/j.bbrc.2005.01.162. [DOI] [PubMed] [Google Scholar]
- 28.Liang X, Wang RS, Wang F, Liu S, Guo F, Sun L, et al. Sodium butyrate protects against severe burn-induced remote acute lung injury in rats. PLoS One. 2013;8:e68786. doi: 10.1371/journal.pone.0068786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo L, Guo Y, Xiao S. Expression of tyrosine kinase Etk/Bmx and its relationship with AP-1- and NF-kappa B-associated proteins in hepatocellular carcinoma. Oncology. 2007;72:410–6. doi: 10.1159/000113491. [DOI] [PubMed] [Google Scholar]
- 30.Saharinen P, Ekman N, Sarvas K, Parker P, Alitalo K, Silvennoinen O. The Bmx tyrosine kinase induces activation of the Stat signaling pathway, which is specifically inhibited by protein kinase Cdelta. Blood. 1997;90:4341–53. [PubMed] [Google Scholar]
- 31.Tsai YT, Su YH, Fang SS, Huang TN, Qiu Y, Jou YS, et al. Etk, a Btk family tyrosine kinase, mediates cellular transformation by linking Src to STAT3 activation. Mol Cell Biol. 2000;20:2043–54. doi: 10.1128/MCB.20.6.2043-2054.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guryanova OA, Wu Q, Cheng L, Lathia JD, Huang Z, Yang J, et al. Nonreceptor tyrosine kinase BMX maintains self-renewal and tumorigenic potential of glioblastoma stem cells by activating STAT3. Cancer Cell. 2011;19:498–511. doi: 10.1016/j.ccr.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jarboe JS, Dutta S, Velu SE, Willey CD. Mini-review: Bmx kinase inhibitors for cancer therapy. Recent Pat Anticancer Drug Discov. 2013;8:228–38. doi: 10.2174/15748928113089990043. [DOI] [PubMed] [Google Scholar]
- 34.Liu F, Zhang X, Weisberg E, Chen S, Hur W, Wu H, et al. Discovery of a selective irreversible BMX inhibitor for prostate cancer. ACS Chem Biol. 2013;8:1423–8. doi: 10.1021/cb4000629. [DOI] [PubMed] [Google Scholar]
- 35.Burger JA, Buggy JJ. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) Leuk Lymphoma. 2013;54:2385–91. doi: 10.3109/10428194.2013.777837. [DOI] [PubMed] [Google Scholar]
- 36.Chang BY, Huang MM, Francesco M, Chen J, Sokolove J, Magadala P, et al. The Bruton tyrosine kinase inhibitor PCI-32765 ameliorates autoimmune arthritis by inhibition of multiple effector cells. Arthritis Res Ther. 2011;13:R115. doi: 10.1186/ar3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Advani RH, Buggy JJ, Sharman JP, Smith SM, Boyd TE, Grant B, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol. 2013;31:88–94. doi: 10.1200/JCO.2012.42.7906. [DOI] [PMC free article] [PubMed] [Google Scholar]


