Abstract
Background
The acquisition of inappropriate migratory feature is crucial for tumor metastasis. Rho-family GTPases including RhoA are molecular switches that play critical roles in regulating cell movement. We investigated the molecular mechanism underlying CD147 induced RhoA deactivation in hepatocellular carcinoma (HCC) cells.
Methods
Wound-healing assay was performed to study the cell motility. Analysis of RhoA activation in living cells was conducted using RhoA biosensor. Changes in the expression of certain genes were determined by quantitative real-time PCR. The expression of proteins was evaluated by Western blot. Cytoskeleton reorganization and focal adhesion formation were observed by immunofluorescence staining. Further investigation on the correlation between CD147 and p190-B RhoGAP (p190-B) in HCC tissues was performed by immunological histological chemistry analysis.
Results
CD147 promoted cell movement and suppressed RhoA activation. p190-B, a negative regulator of RhoA activity, was upregulated by CD147 at both mRNA and protein levels. This regulatory relationship was further confirmed by analyzing the expression pattern of CD147 and p190-B in human HCC tissues. Silencing of p190-B caused the increased formation of stress fiber and focal adhesion and blunted the impact of CD147 overexpression on cell movement, indicating that the regulatory effect of CD147 on cell movement is mediated, at least partially, by p190-B.
Conclusions
These findings indicated that p190-B, a negative regulator of RhoA, is positively regulated by CD147 and contributes to the regulation of cell movement in HCC. CD147 plays critical roles in the motility of cancer cells and may be therefore a valuable drug target for anti-cancer therapy.
Keywords: CD147, Cell movement, Hepatocellular carcinoma, p190-B RhoGAP, RhoA
Background
The majority of deaths associated with cancer are due to the metastasis of the original tumor cells [1]. The acquisition of inappropriate migratory and invasive characteristics is a common feature of all metastatic cancer cells. Rho family GTPases are intracellular signaling molecules that play critical roles in regulating cytoskeleton reorganization and cell movement. The activities of most Rho family members including RhoA depend on a delicate balance between the GTP-bound, active state and the GDP-bound, inactive state. The cycling between these two states is positively controlled by guanine exchange factors (GEFs), and negatively controlled by GTPase-activating proteins (GAPs) [2]. In the activated form, they are competent in binding to a large number of effector proteins, which leads to the activation of myriad numerous downstream signals. >80 RhoGEFs and >70 RhoGAPs have been discovered to regulate the activities of RhoGTPases in mammals [3, 4]. The overabundance of RhoGEFs and RhoGTPases versus Rho GTPases substrates allows the regulators to function in a tissue/cell type-specific manner [5].
CD147 also known as extracellular matrix metalloproteinase inducer (EMMPRIN), is a type I transmembrane glycoprotein. Previous studies showed that CD147 plays important roles in cellular processes of HCC progression, including adhesion [6], migration [7–9], invasion and metastasis [10]. Of note, CD147 interacts directly with annexin A2 and suppresses RhoA activity [7]. Also, CD147 enhances Src activity and promotes mesenchymal-type cell movement by up-regulating RacGEF DOCK8 [8], suggesting that CD147 may regulate cell motility via downregulation of RhoA and upregulation of Rac1, however, the underlying mechanisms are far from clear.
p190-B RhoGAP (p190-B) is a 190 kDa multidomain protein. The N-terminal domain of p190-B contains several motifs characteristic of a GTPase domain, while its C terminus contains a Rho GAP domain. p190-B is among the most important regulators of RhoA and the actin cytoskeleton. Mice lacking p190-B display defects in cell size, thymus and lung during fetal development. Also, p190-B loss in embryonic mesenchymal/fibroblasts leads to an imbalance in adipogenesis/myogenesis cell fate determination [11–13]. Loss of p190-B enhances hematopoietic stem cell (HSC) self-renewal ability [14] and p190-B is critical for the constitution of a functional mesenchymal-derived hematopoietic niche [15]. In cells, p190-B co-localizes with the α5β1 integrin receptor for fibronectin and is thought to be a negative regulator of RhoA activity [16, 17]. The junctional localization of p190-B can be inhibited by centralspindlin, leading to increased RhoA activity during cytokinesis in interphase cells at the zonula adherens [18]. All these results suggest that p190-B functions mainly via regulating RhoA activity. We hypothesized that if p190-B is critical for promoting RhoA deactivation it might also play an important role during cancer cell movement in HCC.
In this study, we provide evidence that CD147 promotes cell movement while inhibiting RhoA activity. CD147 enhances p190-B expression at both mRNA and protein levels and increased p190-B results in reduced RhoA activation and upregulation of cell movement in HCC cells.
Methods
Cell lines and transfection
Human SMMC-7721 hepatoma cell line was obtained from Chinese Academy of Medical Sciences. Huh-7 cells were obtained from the Japanese Collection of Research Bioresources. HepG2 cells were obtained from the American Type Culture Collection. All cell lines were routinely cultured using standard protocols. Transient transfection of plasmids was performed using Lipofectamine 2000 (Life Technologies, US) according to the manufacturer’s protocol.
Tissue specimens and immunological histological chemistry (IHC) analysis
Forty-seven tissue specimens of HCC were collected from the Department of Hepatobiliary & Pancreas Surgery, Xijing Hospital, which is affiliated with the Fourth Military Medical University (FMMU) from 2008 to 2009 and were histological confirmed by staining with hematoxylin and eosin (H and E). All individuals provided written informed consent, and the study was approved by the hospital Ethics Committee. For IHC analysis, sections were incubated with primary antibodies and developed with the Histostain®-Plus Kit (Invitrogen, Carlsbad, CA). The expression level was independently evaluated by two senior pathologists according to the proportion and intensity of positive cells. The following criteria was used to score each specimen: 0 (no staining), 1 (any percentage with weak intensity or <30 % with strong intensity), 2 (>30 % with strong intensity).
Antibodies, plasmids and biosensor
CD147-specific antibody HAb18 was produced by our lab; α-tubulin antibody (sc-8035) was purchased from Santa Cruz; p190-A antibody (2513) was obtained from Cell Signaling Technology; p190-B antibody (611612) and paxillin antibody (610620) were obtained from BD biosciences; Alexa 594-conjugated goat anti-mouse IgG and Alexa Fluor 488 phalloidin were purchased from Invitrogen. A siRNA-resistant CD147 replacement vector was generated based on a previously reported CD147 expression plasmid [19] using the following primer: forward 5′-TCATGAACGGCTCCGAGAGCAGATTTTTTGTTTCATCCTCGCAGGGCCGGTCAGAGC-3′, reverse 5′-GCTCTGACCGGCCCTGCGAGGATGAAACAAAAAATCTGCTCTCGGAGCCGTTCATGA-3′. The RhoA biosensor was obtained from Addgene.
Western blot
Cell pellets were lysed with RIPA buffer (Beyotime, China) containing protease inhibitor (Roche, Switzerland). Blots were probed with the appropriate antibodies and developed using ECL kit (Pierce, US).
RNA interference
Cells were transfected with a pool of siRNAs using HiPerFect Transfection Reagent according to the manufacturer’s instruction (Qiagen). siRNA mixes targeting CD147 or p190-B were designed and synthesized by Shanghai GenePharma Co. (Shanghai, China). Silencer negative control siRNA (siCtrl) was used as a negative control under similar conditions. The sequence for siRNA targeting CD147 used in rescue experiment was 5′-GUUCUUCGUGAGUUCCUCtt-3′.
Quantitative real-time PCR analysis
Total RNA was extracted using the TRIzol reagents (OMEGA Bio-Tek). Reverse transcription was performed using the PrimeScript RT reagent Kit (TaKaRa Biotechnology). All primers were synthesized by BGI (BGI, Shenzhen, China). Primer sequences were listed in Table X. Real-time PCR was performed using the SYBR Premix Ex Taq II Kit (TaKaRa Biotechnology).
In vitro wound-healing assay
In vitro wound-healing assay was performed as described previously [8]. Briefly, 24 h after treatment, the cells were harvested and seeded in 12-well plates until confluent. A pipette tip was used to scratch the monolayer. The cells were then washed with serum-free medium. Photomicrographs were obtained at two time points (0 and 24 h), and the relative migration distance was calculated using the following formula: the relative migration distance (%) = 100 (AX−BX)/(A blank−B blank), where A is the width of the cell wound before incubation, and B is the width of the cell wound after incubation.
Fluorometric analysis of the RhoA activation
Cells were prepared as previously reported [20]. Images were obtained using an A1 confocal microscopy (Nikon, Japan). For emission ratio imaging, the filter sets were used as previously reported [21]. NIS-Elements software (Nikon, Japan) was used to analyze the images following previously described methods [20]. Briefly, images were dark-current and background-subtracted. Binary masks generated through intensity thresholding were applied to each emission channels, and the matched FRET and donor image sets were ratioed to depict RhoA activation throughout the cell. A linear pseudocolour lookup table was applied.
Immunofluorescence
Immunofluorescence was performed as described previously [8]. Briefly, cells were harvested and allowed to attach for 24 h to fibronectin-pre-coated cell culture dishes with glass bottoms (801002, NEST Biotechnology Co., LTD.). After washing twice with PBS, the cells were fixed in paraformaldehyde in PBS, permeabilized with 0.1 % Triton X-100, and blocked with 1 % BSA in PBS for 1 h. The dishes were first incubated with the indicated antibodies for 1 h, washed twice with PBS, and then incubated with Alexa 488-phalloidin solution and the corresponding FITC-conjugated secondary antibodies for 30 min in the dark. Cell nuclei were dyed with DAPI (Vector Labs). After washing, the cells were visualized using an A1R-A1 confocal laser microscope system (Nikon, Japan).
Statistical analysis
The results were expressed as the mean value ± SD of individual experiments. Comparisons of means were conducted using student’s t test or one-way ANOVA (GraphPad Prism, version 6.0). Spearman correlation analysis was used to assess the correlation between CD147 and p190-B in tissues. P values less than 0.05 were considered as statistically significant.
Results
CD147 promotes cell movement and inhibits RhoA activation
We began by determining the effects of CD147 silencing on HCC cells motility. The expression of CD147 in cells transfected with siRNAs targeting CD147 (siCD147) was significantly reduced compared to the cells transfected with control siRNA (siCtrl) (Fig. 1a). Wound-healing assay was used to investigate the role of CD147 in cell movement in HCC cells. A significant decrease was observed in SMMC-7721 cells transfected with siCD147 compared to the control cells (Fig. 1b). To exclude the possibility of off-target effect of siRNA-mediated CD147 interference, a siRNA-resistant CD147 replacement vector was generated. The expression of CD147 in cells cotransfected with siCD147 and the CD147 replacement vector was rescued (Fig. 1a) and cell motility was also rescued (Fig. 1b), indicating that CD147 exerts positive regulation over hepatoma cells. RhoA, one of the most extensively studied members of the Rho family of small GTPases, is most readily recognized for its contributions to actin-myosin contractility and stress fiber formation mainly by activating effector proteins such as Rho kinase, which promotes myosin-2 activation [22]. Thus, we determined the effects of CD147 silencing on RhoA activation. We found that CD147 silencing led to increased RhoA activation (Fig. 1c), indicating that CD147 may regulate cell movement via inhibiting RhoA activation.
Fig. 1.
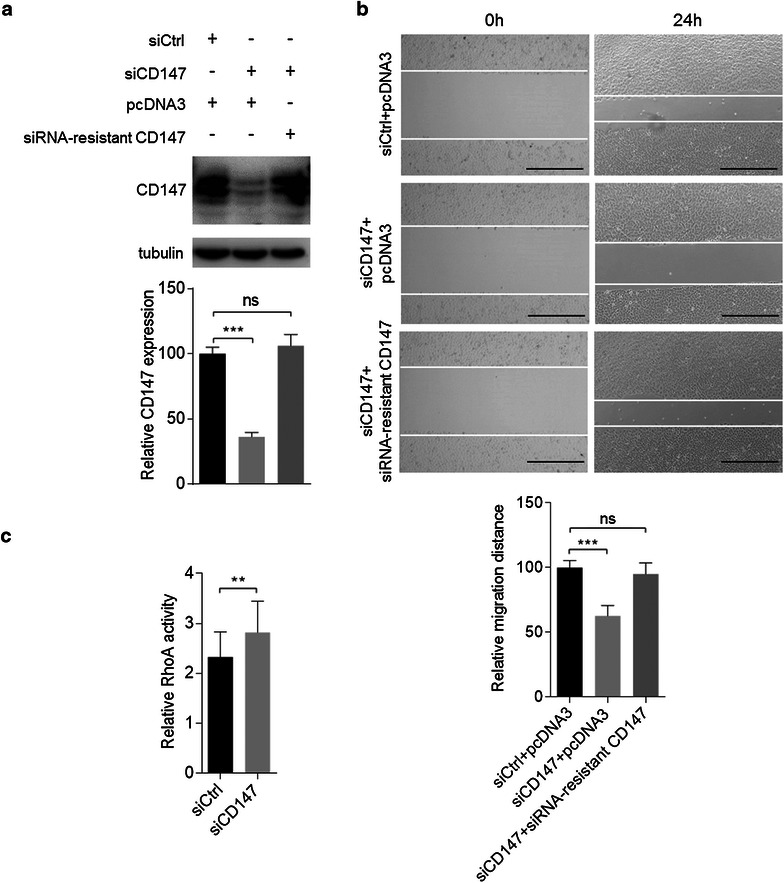
CD147 promotes cell movement and inhibits RhoA activation. a The expression of CD147 was measured by Western blot at 48 h after transfection with the indicated constructs. b Quantitative analysis of relative migration distance of SMMC-7721 cells transfected with the indicated constructs. c Quantification of RhoA activation at 48 h after transfection. **p < 0.01, ***p < 0.001 by student’s t test. Error bars indicate the standard deviations (SD) from at least triplicate determinations
CD147 promotes p190-B expression in HCC cells
To determine the mechanism underlying the downregulation of RhoA activation by CD147 silencing, we examined the expression of p190 RhoGAPs. Strikingly, we found that p190-B, but not p190-A, was decreased in HCC cells transfected with siCD147 both at mRNA (Fig. 2a, b) and protein levels (Fig. 2c, d). Also, forced expression of CD147 in HCC cells resulted in increased expression of p190-B both at mRNA (Fig. 3a, c) and protein levels (Fig. 3d), indicating that CD147 promotes p190-B expression both at mRNA and protein levels in HCC cells.
Fig. 2.
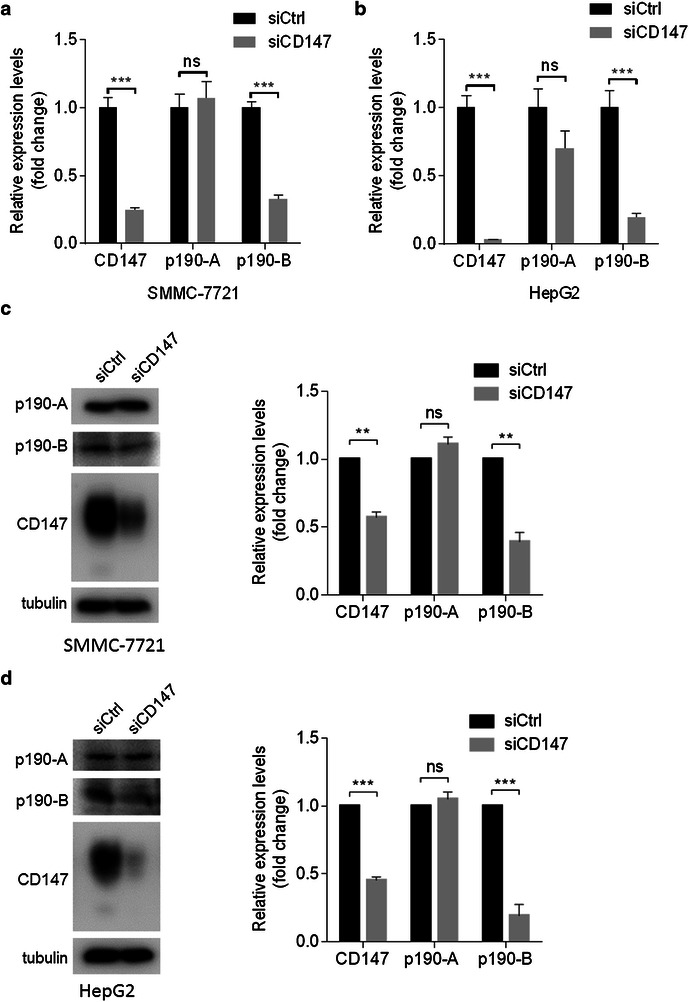
Silencing CD147 leads to decreased p190-B expression. a, b Relative quantitative real-time RT-PCR analysis of p190 RhoGAP family in indicated cells transfected with siCD147 or siCtrl at 24 h after transfection. ***p < 0.001, ns p > 0.05 by student’s t test. c, d The expression of p190-A or p190-B in indicated cells transfected with siCD147 or siCtrl was determined by Western blot at 48 h after transfection
Fig. 3.
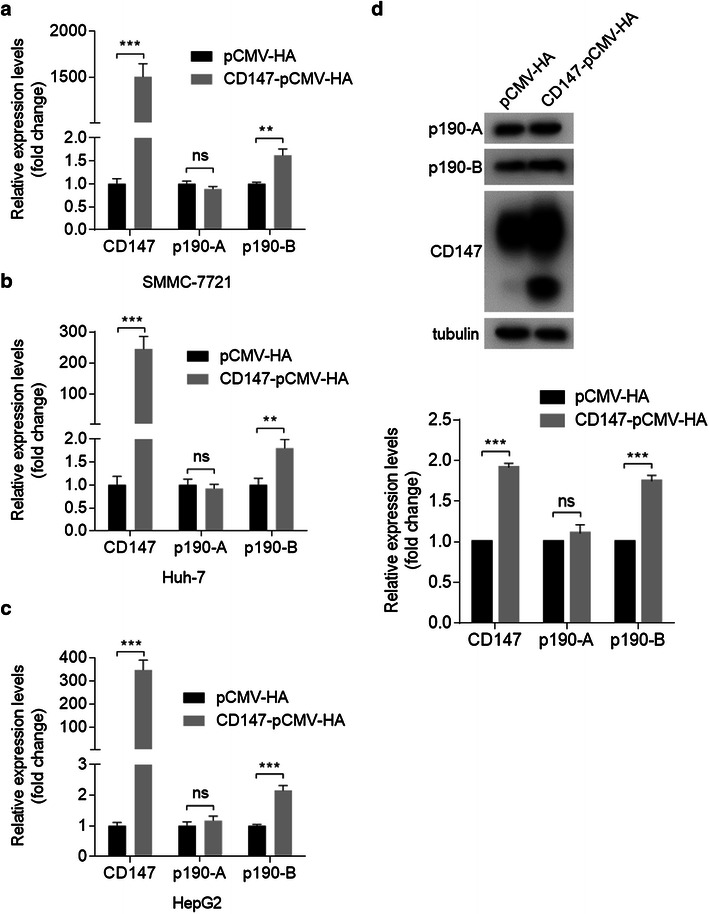
Overexpression of CD147 results in upregulation of p190-B expression. a–c Relative quantitative real-time RT-PCR analysis of p190 RhoGAP family in indicated cells transfected with pCMV-HA or CD147-pCMV-HA at 24 h after transfection. ***p < 0.001, **p < 0.01, ns p > 0.05 by student’s t test. d The expression of indicated molecules in SMMC-7721 cells transfected with pCMV-HA or CD147-pCMV-HA was determined by Western blot at 48 h after transfection. Error bars indicate SD from at least triplicate determinations
p190-B expression is positively correlated with the expression of CD147 in HCC tissues
To further illuminate the regulatory role of CD147 in p190-B expression, the expression of CD147 and p190-B in HCC tissues was evaluated by IHC. As shown in Fig. 4a–c and Table 1, most samples exhibited similar expression patterns of CD147 and p190-B. Spearman correlation analysis based on IHC score indicated a significant positive correlation between the expression levels of the two proteins (Fig. 4d). Together, these findings identify p190-B as a major RhoGAP regulated by CD147 in HCC.
Fig. 4.
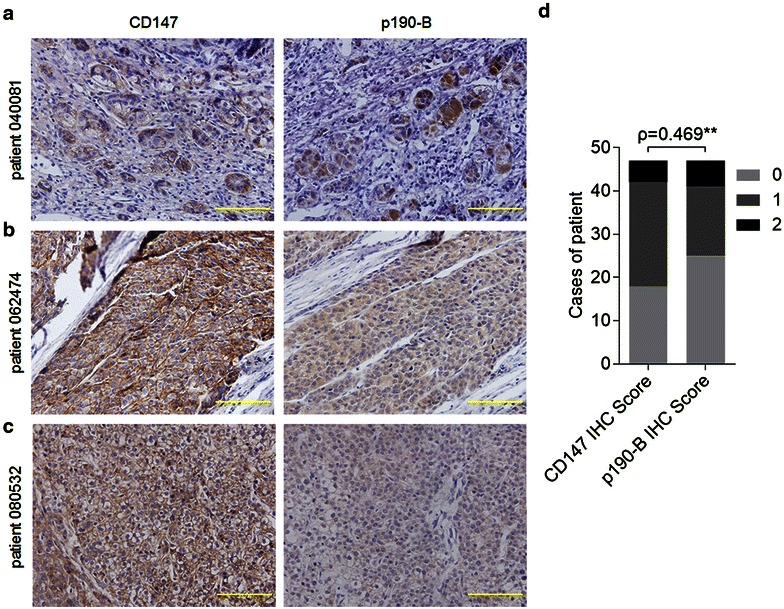
Immunohistochemical analysis of CD147 and p190-B expression in the resected tumor. a–c Representative images of immunohistochemical staining for CD147 and p190-B in paired HCC tissues. The scores for CD147 in patient 040081, 062474 and 080532 were grade 1, grade 2 and grade 2, respectively. The scores for p190-B in the indicated patients were grade 2, grade 2 and grade 1, respectively. The scale bar represents 10 μm. d The relationship between CD147 and p190-B expression. **p = 0.001 by Spearman correlation
Table 1.
Correlation between CD147 and p190-B expression in HCC tissues
| CD147 expression | p190-B expression | Total, N | ||
|---|---|---|---|---|
| – | + | ++ | ||
| – | 15 | 3 | 0 | 18 |
| + | 8 | 12 | 4 | 24 |
| ++ | 2 | 1 | 2 | 5 |
| Total, N | 25 | 16 | 6 | 47 |
Using the Spearman correlation analysis, ρ = 0.469, p = 0.001
p190-B promotes cell movement and inhibits RhoA activation
To better understand the role of p190-B in cell movement, we determined the effects of p190-B silencing on cell movement and RhoA activation in HCC cells. We found that p190-B silencing resulted in decreased cell movement (Fig. 5a) and increased RhoA activation (Fig. 5b), indicating that p190-B may regulate cell movement via inhibiting RhoA activation. As RhoA regulates cell movement via stimulating cytoskeleton rearrangement, we examined whether p190-B could affect stress fiber distribution and focal adhesion formation. We found that p190-B silencing resulted in increased stress fiber and focal adhesion (Fig. 5c).
Fig. 5.
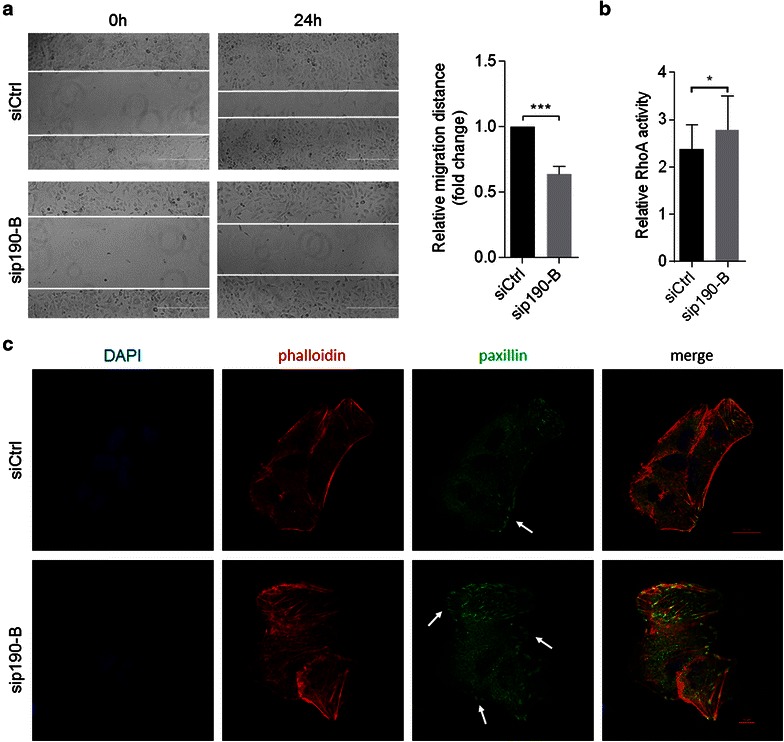
p190-B promotes cell movement and inhibits RhoA activation. SMMC-7721 cells were transfected with a pool of siRNAs targeting p190-B or siCtrl. a Quantitative analysis of relative migration distance by wound-healing assay. ***p < 0.001 by student’s t test. b Quantification of RhoA activation in indicated cells at 48 h after transfection. *p < 0.05 by student’s t test. c Cells were stained with DAPI, rhodamine-conjugated phalloidin and an antibody against paxillin and imaged by confocal microscopy. Arrows indicate focal adhesions. Error bars indicate SD from at least triplicate determinations
CD147 promotes cell movement by enhancing p190-B expression
We have demonstrated that CD147 promotes cell movement and p190-B expression. We next tried to investigate whether p190-B-mediated RhoA deactivation was involved in the regulation of cell movement by CD147. This hypothesis predicted that reducing p190-B should impair the impact of CD147 overexpression on cell motility and RhoA deactivation. Accordingly, we transfected SMMC-7721 cells with CD147-pCMV-HA, and then selectively depleted p190-B by a pool of siRNAs (Fig. 6a). Cell motility was increased in cells transfected with CD147-pCMV-HA together with control siRNAs. Strikingly, however, CD147 overexpression did not enhance cell motility to the same level in p190-B knockdown cells as it did in control cells (Fig. 6b). This implies that p190-B contributes to the regulation of cell movement by CD147.
Fig. 6.
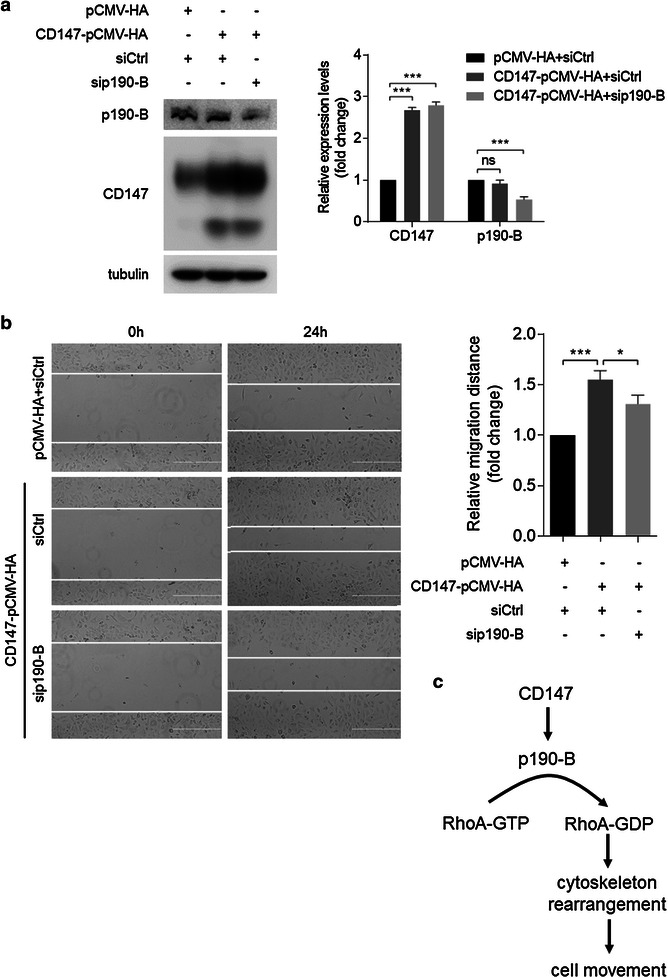
CD147 inhibits RhoA activation through enhancing p190-B expression. SMMC-7721 cells were transfected with the indicated constructs. a The expression of CD147 and p190-B was analyzed by Western blot. b Quantitative analysis of relative migration distance by wound-healing assay. *p < 0.05, ***p < 0.001 by ANOVA. c Schematic representation of major mechanisms of CD147 in regulating cancer cell movement via promoting p190-B expression. Error bars indicate SD from at least triplicate determinations
Discussion
In this study we found that CD147 promotes p190-B expression both at mRNA and protein levels. p190-B was further identified as a RhoA GAP and was able to inhibit RhoA activation and to promote cell movement in HCC cells. Also, we demonstrated that the regulation of RhoA activation and cell movement by CD147 was mediated, at least partially, by p190-B in HCC (Fig. 6c), indicating that RhoA is an indirect target of CD147.
Members of the Rho GTPase family including RhoA operate as molecular switches that affect downstream signaling pathways and play a critical role in cytoskeleton dynamics and cell migration [23]. p190-B is a negative regulator of RhoA activity [16], which is consistent with our finding reported here. Aside from the canonical Rho GTPase RhoA, three isoforms have been identified. RhoA, RhoB and RhoC have high amino acid sequence homology. However, they differ in many biochemical characteristics and cellular functions. The specificity of p190B and the role of RhoB and RhoC in CD147-regulated cell movement are still far from clear. Overexpression of p190-B in MCF-10A cells results in reduced actin stress fiber network and exhibit circumferential staining for actin [24] and knockdown of p190-B decreases cell spread and migration in Huh-7 cells [17], suggesting a role for p190-B in regulating the signaling pathways that influence cancer cell migration and invasion. In vivo analysis using transgenic mice indicates that p190-B has pro-tumorigenic functions that enhance MMTV-Neu induced mammary tumor formation and metastasis [25]. Here, we show that siRNA-mediated silencing of p190-B in HCC cells causes reduced RhoA activation and increases formation of stress fiber and focal adhesion, leading to decreased cell movement. Furthermore, CD147, a key regulator of HCC progression, is able to promote p190-B expression both at mRNA and protein levels and exerts positive influence on cancer cell motility mediated at least partially by p190-B. This regulatory relationship was further verified by immunohistochemistry staining of CD147 and p190-B in HCC tissues. Therefore, p190-B is involved in the regulation of cell movement by CD147 as a major GAP for RhoA in HCC cells.
CD147 has been reported to play important roles in HCC progression, including migration and invasion [10, 19, 26]. Of note, CD147 interacts with integrin β1, enhances expression and phosphorylation of FAK and paxillin, and subsequently leads to cytoskeletal rearrangement and morphological changes [27, 28]. Also, it is believed that CD147 plays a role in mediating epithelial-mesenchymal transition (EMT) in the process of HCC progression, providing a slight clue to the function of CD147 in cytoskeleton rearrangement [29, 30]. Recently, we reported that CD147 promotes cell motility by regulating Annexin A2-activated RhoA [7], enhances Src activity and promotes mesenchymal-type cell movement by up-regulating RacGEF DOCK8 [8], is able to promote WAVE2-mediated lamellipodia formation and cell movement [9]. All these results, together with our data reported here, suggest that CD147 regulates cytoskeleton rearrangement and cell movement at multiple levels and through different mechanisms, however, how to coordinate these mechanisms during regulating cell movement still awaits further investigation and it is still difficult to dissect to what extent each mechanism contributes to regulation of cell movement under diverse circumstances. Above all, targeting CD147 could be a promising strategy to reduce migration and metastasis of tumor cells.
Conclusions
The results of this study do support the regulatory role of CD147 on cytoskeleton rearrangement in HCC. p190-B, a negative regulator of RhoA, contributes to the regulation of RhoA activation and cell movement by CD147. CD147 could be a promising target molecule for developing new anti-cancer drugs.
Authors’ contributions
HYC and JLJ conceived and designed the study. RC, SJW and RH carried out the study. YZ analyzed the data. HYC wrote the manuscript. JLJ revised the manuscript. All authors read and approved the final manuscript.
Acknowledgements
This work was supported and funded by the National Natural Science Foundation of China (31371405 and 81201774).
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The data of this manuscript have been presented in the main paper.
Ethics approval and consent to participate
All individuals provided written informed consent, and the study was approved by the Ethics Committee of the Xijing Hospital, which is affiliated with the Fourth Military Medical University.
Funding
This work was supported by the National Natural Science Foundation of China (31371405 and 81201774).
Abbreviations
- p190-B
p190-B GTPase activating protein
- HCC
hepatocellular carcinoma
- RhoA
ras homolog gene family, member A
- GEF
guanine exchange factor
- GAP
GTPase-activating protein
- DOCK
dedicator of cytokinesis
- HSC
hematopoietic stem cell
- IHC
immunological histological chemistry
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- FRET
fluorescence resonance energy transfer
- FITC
fluorescein isothiocyanate
- DAPI
4′,6-diamidino-2-phenylindole
- FAK
focal adhesion kinase
- EMT
epithelial-mesenchymal transition
- siRNA
small interfering RNA
- SD
standard deviation
- ANOVA
analysis of variance
Footnotes
Ruo Chen and Shi-Jie Wang contributed equally to this work
Contributor Information
Ruo Chen, Email: novababe@sina.com.
Shi-Jie Wang, Email: kola_519@163.com.
Yang Zhang, Email: zhangzzy@fmmu.edu.cn.
Rong Hou, Email: 18700101670@163.com.
Jian-Li Jiang, Email: jiangjl@fmmu.edu.cn.
Hong-Yong Cui, Email: cui-hongyong@163.com.
References
- 1.Spano D, Heck C, De Antonellis P, Christofori G, Zollo M. Molecular networks that regulate cancer metastasis. Semin Cancer Biol. 2012;22(3):234–249. doi: 10.1016/j.semcancer.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Schaefer A, Reinhard NR, Hordijk PL. Toward understanding RhoGTPase specificity: structure, function and local activation. Small GTPases. 2014;5(2):6. doi: 10.4161/21541248.2014.968004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6(2):167–180. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- 4.Tcherkezian J, Lamarche-Vane N. Current knowledge of the large RhoGAP family of proteins. Biol Cell Auspices Eur Cell Biol Organ. 2007;99(2):67–86. doi: 10.1042/BC20060086. [DOI] [PubMed] [Google Scholar]
- 5.Shang X, Marchioni F, Evelyn CR, Sipes N, Zhou X, Seibel W, Wortman M, Zheng Y. Small-molecule inhibitors targeting G-protein-coupled Rho guanine nucleotide exchange factors. Proc Natl Acad Sci USA. 2013;110(8):3155–3160. doi: 10.1073/pnas.1212324110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang Q, Han Q, Huang W, Nan G, Xu BQ, Jiang JL, Chen ZN. HAb18G/CD147 regulates vinculin-mediated focal adhesion and cytoskeleton organization in cultured human hepatocellular carcinoma cells. PLoS One. 2014;9(7):e102496. doi: 10.1371/journal.pone.0102496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao P, Zhang W, Wang SJ, Yu XL, Tang J, Huang W, Li Y, Cui HY, Guo YS, Tavernier J, et al. HAb18G/CD147 promotes cell motility by regulating annexin II-activated RhoA and Rac1 signaling pathways in hepatocellular carcinoma cells. Hepatology. 2011;54(6):2012–2024. doi: 10.1002/hep.24592. [DOI] [PubMed] [Google Scholar]
- 8.Wang SJ, Cui HY, Liu YM, Zhao P, Zhang Y, Fu ZG, Chen ZN, Jiang JL. CD147 promotes Src-dependent activation of Rac1 signaling through STAT3/DOCK8 during the motility of hepatocellular carcinoma cells. Oncotarget. 2015;6(1):243–257. doi: 10.18632/oncotarget.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui HY, Wang SJ, Miao JY, Fu ZG, Feng F, Wu J, Yang XM, Chen ZN, Jiang JL. CD147 regulates cancer migration via direct interaction with annexin A2 and DOCK3-beta-catenin-WAVE2 signaling. Oncotarget. 2016;7(5):5613–5629. doi: 10.18632/oncotarget.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu J, Xu HY, Zhang Q, Song F, Jiang JL, Yang XM, Mi L, Wen N, Tian R, Wang L, et al. HAb18G/CD147 functions in invasion and metastasis of hepatocellular carcinoma. Mol Cancer Res MCR. 2007;5(6):605–614. doi: 10.1158/1541-7786.MCR-06-0286. [DOI] [PubMed] [Google Scholar]
- 11.Chakravarty G, Hadsell D, Buitrago W, Settleman J, Rosen JM. p190-B RhoGAP regulates mammary ductal morphogenesis. Mol Endocrinol. 2003;17(6):1054–1065. doi: 10.1210/me.2002-0428. [DOI] [PubMed] [Google Scholar]
- 12.Sordella R, Classon M, Hu KQ, Matheson SF, Brouns MR, Fine B, Zhang L, Takami H, Yamada Y, Settleman J. Modulation of CREB activity by the Rho GTPase regulates cell and organism size during mouse embryonic development. Dev Cell. 2002;2(5):553–565. doi: 10.1016/S1534-5807(02)00162-4. [DOI] [PubMed] [Google Scholar]
- 13.Sordella R, Jiang W, Chen GC, Curto M, Settleman J. Modulation of Rho GTPase signaling regulates a switch between adipogenesis and myogenesis. Cell. 2003;113(2):147–158. doi: 10.1016/S0092-8674(03)00271-X. [DOI] [PubMed] [Google Scholar]
- 14.Xu H, Eleswarapu S, Geiger H, Szczur K, Daria D, Zheng Y, Settleman J, Srour EF, Williams DA, Filippi MD. Loss of the Rho GTPase activating protein p190-B enhances hematopoietic stem cell engraftment potential. Blood. 2009;114(17):3557–3566. doi: 10.1182/blood-2009-02-205815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raman R, Kumar RS, Hinge A, Kumar S, Nayak R, Xu J, Szczur K, Cancelas JA, Filippi MD. p190-B RhoGAP regulates the functional composition of the mesenchymal microenvironment. Leukemia. 2013;27(11):2209–2219. doi: 10.1038/leu.2013.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burbelo PD, Miyamoto S, Utani A, Brill S, Yamada KM, Hall A, Yamada Y. p190-B, a new member of the Rho GAP family, and Rho are induced to cluster after integrin cross-linking. J Biol Chem. 1995;270(52):30919–30926. doi: 10.1074/jbc.270.52.30919. [DOI] [PubMed] [Google Scholar]
- 17.Gen Y, Yasui K, Zen K, Nakajima T, Tsuji K, Endo M, Mitsuyoshi H, Minami M, Itoh Y, Tanaka S, et al. A novel amplification target, ARHGAP5, promotes cell spreading and migration by negatively regulating RhoA in Huh-7 hepatocellular carcinoma cells. Cancer Lett. 2009;275(1):27–34. doi: 10.1016/j.canlet.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 18.Ratheesh A, Gomez GA, Priya R, Verma S, Kovacs EM, Jiang K, Brown NH, Akhmanova A, Stehbens SJ, Yap AS. Centralspindlin and alpha-catenin regulate Rho signalling at the epithelial zonula adherens. Nat Cell Biol. 2012;14(8):818–828. doi: 10.1038/ncb2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang JL, Zhou Q, Yu MK, Ho LS, Chen ZN, Chan HC. The involvement of HAb18G/CD147 in regulation of store-operated calcium entry and metastasis of human hepatoma cells. J Biol Chem. 2001;276(50):46870–46877. doi: 10.1074/jbc.M108291200. [DOI] [PubMed] [Google Scholar]
- 20.Pertz O, Hodgson L, Klemke RL, Hahn KM. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature. 2006;440(7087):1069–1072. doi: 10.1038/nature04665. [DOI] [PubMed] [Google Scholar]
- 21.Fritz RD, Letzelter M, Reimann A, Martin K, Fusco L, Ritsma L, Ponsioen B, Fluri E, Schulte-Merker S, van Rheenen J, et al. A versatile toolkit to produce sensitive FRET biosensors to visualize signaling in time and space. Sci Signal. 2013;6(285):rs12. doi: 10.1126/scisignal.2004135. [DOI] [PubMed] [Google Scholar]
- 22.O’Connor K, Chen M. Dynamic functions of RhoA in tumor cell migration and invasion. Small GTPases. 2013;4(3):141–147. doi: 10.4161/sgtp.25131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ensign SPF, Mathews IT, Symons MH, Berens ME, Tran NL. Implications of Rho GTPase signaling in glioma cell invasion and tumor progression. Front Oncol. 2013;3:241. doi: 10.3389/fonc.2013.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chakravarty G, Roy D, Gonzales M, Gay J, Contreras A, Rosen JM. P190-B, a Rho-GTPase-activating protein, is differentially expressed in terminal end buds and breast cancer. Cell Growth Differ Mol Biol J Am Assoc Cancer Res. 2000;11(7):343–354. [PubMed] [Google Scholar]
- 25.McHenry PR, Sears JC, Herrick MP, Chang P, Heckman-Stoddard BM, Rybarczyk M, Chodosh LA, Gunther EJ, Hilsenbeck SG, Rosen JM, et al. P190B RhoGAP has pro-tumorigenic functions during MMTV-Neu mammary tumorigenesis and metastasis. Breast Cancer Res BCR. 2010;12(5):R73. doi: 10.1186/bcr2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang JL, Chan HC, Zhou Q, Yu MK, Yao XY, Lam SY, Zhu H, Ho LS, Leung KM, Chen ZN. HAb18G/CD147-mediated calcium mobilization and hepatoma metastasis require both C-terminal and N-terminal domains. Cell Mol Life Sci CMLS. 2004;61(16):2083–2091. doi: 10.1007/s00018-004-4146-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang J, Wu YM, Zhao P, Yang XM, Jiang JL, Chen ZN. Overexpression of HAb18G/CD147 promotes invasion and metastasis via α3β1 integrin mediated FAK-paxillin and FAK-PI3K-Ca2+ pathways. Cell Mol Life Sci CMLS. 2008;65(18):2933–2942. doi: 10.1007/s00018-008-8315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Wu J, Song F, Tang J, Wang SJ, Yu XL, Chen ZN, Jiang JL. Extracellular membrane-proximal domain of HAb18G/CD147 binds to metal ion-dependent adhesion site (MIDAS) motif of integrin β1 to modulate malignant properties of hepatoma cells. J Biol Chem. 2012;287(7):4759–4772. doi: 10.1074/jbc.M111.277699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu J, Ru NY, Zhang Y, Li Y, Wei D, Ren Z, Huang XF, Chen ZN, Bian H. HAb18G/CD147 promotes epithelial-mesenchymal transition through TGF-beta signaling and is transcriptionally regulated by Slug. Oncogene. 2011;30(43):4410–4427. doi: 10.1038/onc.2011.149. [DOI] [PubMed] [Google Scholar]
- 30.Ru NY, Wu J, Chen ZN, Bian H. HAb18G/CD147 is involved in TGF-β-induced epithelial-mesenchymal transition and hepatocellular carcinoma invasion. Cell Biol Int. 2015;39(1):44–51. doi: 10.1002/cbin.10341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data of this manuscript have been presented in the main paper.


