Abstract
Background
Exercise testing devices for evaluating cardiopulmonary fitness in patients with severe disability after stroke are lacking, but we have adapted a robotics-assisted tilt table (RATT) for cardiopulmonary exercise testing (CPET). Using the RATT in a sample of patients after stroke, this study aimed to investigate test-retest reliability and repeatability of CPET and to prospectively investigate changes in cardiopulmonary outcomes over a period of four weeks.
Methods
Stroke patients with all degrees of disability underwent 3 separate CPET sessions: 2 tests at baseline (TB1 and TB2) and 1 test at follow up (TF). TB1 and TB2 were at least 24 h apart. TB2 and TF were 4 weeks apart. A RATT equipped with force sensors in the thigh cuffs, a work rate estimation algorithm and a real-time visual feedback system was used to guide the patients’ exercise work rate during CPET. Test-retest reliability and repeatability of CPET variables were analysed using paired t-tests, the intraclass correlation coefficient (ICC), the coefficient of variation (CoV), and Bland and Altman limits of agreement. Changes in cardiopulmonary fitness during four weeks were analysed using paired t-tests.
Results
Seventeen sub-acute and chronic stroke patients (age 62.7 ± 10.4 years [mean ± SD]; 8 females) completed the test sessions. The median time post stroke was 350 days. There were 4 severely disabled, 1 moderately disabled and 12 mildly disabled patients. For test-retest, there were no statistically significant differences between TB1 and TB2 for most CPET variables. Peak oxygen uptake, peak heart rate, peak work rate and oxygen uptake at the ventilatory anaerobic threshold (VAT) and respiratory compensation point (RCP) showed good to excellent test-retest reliability (ICC 0.65–0.94). For all CPET variables, CoV was 4.1–14.5 %. The mean difference was close to zero in most of the CPET variables. There were no significant changes in most cardiopulmonary performance parameters during the 4-week period (TB2 vs TF).
Conclusions
These findings provide the first evidence of test-retest reliability and repeatability of the principal CPET variables using the novel RATT system and testing methodology, and high success rates in identification of VAT and RCP: good to excellent test-retest reliability and repeatability were found for all submaximal and maximal CPET variables. Reliability and repeatability of the main CPET parameters in stroke patients on the RATT were comparable to previous findings in stroke patients using standard exercise testing devices. The RATT has potential to be used as an alternative exercise testing device in patients who have limitations for use of standard exercise testing devices.
Keyword: Cardiopulmonary exercise testing, Robotics-assisted tilt table, Stroke, Test-retest reliability, Repeatability
Background
Stroke is one of the leading causes of adult disability worldwide [1, 2]. Stroke affects not only the neurological system but also influences pulmonary and cardiovascular health [3, 4]. The effect of stroke on the cardiovascular system can result from: existing comorbid conditions, e.g. coronary artery disease; a direct effect of stroke on impairments in cardiovascular regulation, e.g. stroke patients frequently have electrocardiographic abnormalities such as T-wave abnormalities, prolonged QTc interval and arrhythmia [5]; or, indirectly from weakness, fatigue or spasticity leading to a sedentary lifestyle and resulting in a further decline in cardiopulmonary fitness [6].
Cardiopulmonary exercise testing (CPET) is important to determine a patient’s cardiopulmonary fitness and to accurately prescribe an individualised exercise programme [7]. The most commonly used devices, i.e. a treadmill or a cycle ergometer, cannot be used in all stroke patients – their use is limited to mildly or moderately impaired stroke patients [8]. Post stroke impairments such as spasticity, muscle weakness, coordination problem or reduced joint range of motion may preclude some moderately to severely impaired stroke patients from performing CPET on these devices [9, 10]. Recent systematic reviews showed that suitable methods to measure cardiopulmonary fitness and to provide appropriate exercise training in stroke patients with severe disability are still lacking [11, 12]. This problem needs to be addressed soon because stroke incidence is projected to increase as a result of a higher proportion of older people in the population in the future [13] and because people who live with disability after stroke have a longer survival than formerly [14].
In this study, a robotics-assisted tilt table (RATT), which is used clinically for early neurorehabilitation, was adapted for CPET. This device was considered to have potential to be used in severely disabled patients because it has a harness to provide body support and it incorporates thigh cuffs and foot plates to secure the legs [15]. As the patient is well supported, the risk of falls or exercise related injuries is minimised. The RATT was augmented with force sensors, a work rate calculation algorithm and a visual feedback system to facilitate exercise testing and training [16]. A previous validity and reliability study in healthy individuals found that peak oxygen uptake and submaximal exercise thresholds on the RATT were approximately 20 % lower than for a cycle ergometer [17]. Test-retest reliability of maximal and submaximal exercise thresholds on the RATT were comparable to standard exercise devices [17, 18]. Furthermore, a feasibility study in stroke showed that patients with severe disability could successfully undergo CPET and exercise training on the RATT [15].
Test-retest reliability of peak and submaximal cardiopulmonary exercise parameters, which are normally used as the main outcomes for exercise intervention studies, require careful analysis prior to future clinical uptake of the RATT. Although reliability data in healthy individuals have been established, this form of analysis in stroke patients with various degrees of disability is still lacking.
The aims of this study were twofold: to investigate test-retest reliability and repeatability of CPET on the RATT in stroke patients with all degrees of disability; and to prospectively investigate changes in cardiopulmonary outcomes in this sample over a period of four weeks.
Methods
Study design and participants
This prospective study was approved by the Ethics Review Committee of Canton Aargau, Switzerland (Ref. EKNZ 2014–296). All subjects gave their written informed consent before participating in the study.
Sub-acute and chronic stroke patients were recruited from Reha Rheinfelden, a rehabilitation centre in the north-west of Switzerland, from December 2014 to January 2016. Patient inclusion criteria were: (1) a diagnosis of first-ever stroke, either ischaemic or intracerebral haemorrhagic; (2) ≥ 18 years old; and (3) willing to cooperate in the study and able to attend all testing sessions. Exclusion criteria were: (1) any contraindications to maximal exercise testing according to the American College of Sports Medicine guidelines [19]; (2) any contraindications for the RATT based on guidelines from the manufacturer, e.g. body mass greater than 135 kg, bone instability, or open skin lesions in the area of the lower limbs and/or back; (3) severe perceptual or communication problems; (4) Mini Mental State Examination (MMSE) score ≤ 17 [20]; (5) concurrent neurological diseases, e.g. Parkinson’s disease; and (6) severe concurrent cardiac or pulmonary disease. A cardiologist evaluated the cardiac status of all patients before giving approval for participation.
Study protocol
Patients underwent 3 separate CPET sessions: 2 tests at baseline (TB1 and TB2) and 1 test at follow up (TF). TB2 was conducted as soon as possible after TB1, but at least 24 h later. TB2 and TF were 4 weeks apart. Patients were instructed to avoid strenuous activity within the 24 h before each test session, not to consume a large meal 3 h before, and to refrain from caffeine and nicotine 12 h prior to testing.
Patients’ characteristics
Gender, age, height, body mass, body mass index (BMI), type of stroke, time of stroke diagnosis, side of weakness, comorbidity and current medication were recorded prior to the first test (Table 1).
Table 1.
Characteristics and demographic data of subjects
| Characteristic (n = 17) | Value | Range |
|---|---|---|
| Age (years) | 62.7 ± 10.4 | 41.0–78.0 |
| Sex, n (%) | ||
| Male | 9 (52.9 %) | |
| Female | 8 (47.1 %) | |
| Height (cm) | 169.5 ± 6.6 | 160.0–183.0 |
| Body mass (kg) | 72.3 ± 10.0 | 57.5–90.0 |
| Body mass index (kg/m2) | 25.2 ± 3.2 | 20.2–33.1 |
| Type of stroke, n (%) | ||
| Ischaemic | 15 (88.2 %) | |
| Haemorrhagic | 2 (11.8 %) | |
| Hemiparetic side, n (%) | ||
| Left | 7 (41.2 %) | |
| Right | 10 (58.8 %) | |
| Stage, n (%) | ||
| Sub-acute | 6 (35.3 %) | |
| Chronic | 11 (64.7 %) | |
| Days post stroke, | 485.3 (535.5) | 21–1810 |
| median (IQR) | 350 (788) | |
| FAC | 3.6 ± 2.1 | 0–5 |
| NIHSS | 3.1 ± 3.2 | 0–11 |
| MMSE score | 28.1 ± 2.3 | 21–30 |
| Modified Rankin Scale | ||
| 0–2 | 12 (70.6 %) | |
| 3 | 1 (5.9 %) | |
| 4–5 | 4 (23.5 %) | |
| Comorbidities, n (%) | ||
| Hypertension | 9 (52.9 %) | |
| Diabetes mellitus | 1 (5.9 %) | |
| Dyslipidemia | 4 (23.5 %) | |
| None | 4 (23.5 %) | |
| Antihypertensive medications, n (%) | ||
| β-blocker | 2 (11.9 %) | |
| ACE inhibitors | 3 (17.6 %) | |
| Calcium channel blockers | 3 (17.6 %) | |
| None | 9 (52.9 %) | |
Values are mean ± SD unless otherwise indicated
Abbreviations: n number; SD standard deviation; MMSE Mini Mental State Examination; IQR interquartile range; FAC functional ambulation category; ACE angiotensin-converting-enzyme
Prior to cardiopulmonary exercise testing, functional measures were assessed using functional ambulation category (FAC) [21], the National Institutes of Health Stroke Scale (NIHSS) [22] and a 6-min walk test (6 MWT) [23]. The 6 MWT was performed indoors along a straight 50 m walking course. Standardized instructions were given to the patients according to the guidelines. The six-minute walk distance (6MWD) was recorded.
Cardiopulmonary fitness assessment
Cardiopulmonary exercise testing (CPET) was performed using a robotics-assisted tilt table (RATT; model Erigo, Hocoma AG, Switzerland; Fig. 1). Each test session had the same format. The patient was first transferred and secured using the body harness, thigh cuffs and foot plates according to the instruction of the manufacturer. Then, the patient was tilted upwards to 70 ° and the formal CPET protocol described below was initiated. During the passive and incremental exercise phases, the stepping cadence was set at 80 steps/min, which is the maximal cadence allowed by the device.
Fig. 1.
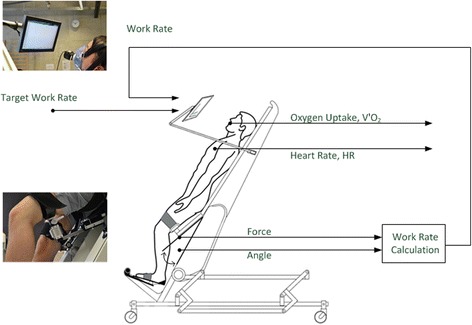
Robotics-assisted tilt table (RATT) with visual feedback system. The visual feedback screen shows the target work rate and the subject’s work rate. The latter was calculated from the forces in the thigh cuffs and the angular velocities. Adapted from Saengsuwan et al., 2015 [18]
The CPET methodology was previously described in detail elsewhere [15] and it is briefly summarized in the following. CPET consisted of the following phases: (1) a 3-min rest phase, where the patient lay without leg movement on the RATT; (2) a passive phase, where the RATT moved the patient’s legs for 5 min and the measured relative work rate was adjusted to 0 W; (3) a ramp phase, where the rate of work rate increase was in the range 1.25 to 4.5 W/min – the work rate ramp was estimated by patients’ gender, age, level of weakness and comorbidity, e.g. the ramp rate for a 70 year old woman with severe disability was set to 1.25 W/min – the aim of the individualised ramp rate was to bring the patient to their limit of functional capacity within 8–12 min of ramp onset. The individual ramp increment rates stayed the same for 13 patients in TB1 and TB2. The ramp rates were adjusted in four patients in TB2 to enable the patients to reach their maximal effort in 8–12 min; and (4) a recovery phase, where the patient remained passive while the RATT moved their legs for 5 min. The termination criteria for the ramp phase followed the American College of Sports Medicine guidelines [19]. Additionally, blood pressure (BP) was used as a termination criterion: systolic BP > 210 mmHg or diastolic BP > 115 mmHg [24].
Prior to the incremental exercise testing, the researcher showed and explained the Borg CR 10 scale to the patients. The instructions were as follows: “This scale aims to measure your feeling of difficulty in breathing and leg fatigue. The range of the scale is from 0 to 10. The number 0 means that you feel no difficulty at all. The scale progresses to number 10 where you feel that you have maximal difficulty with breathing/leg effort.” During the incremental exercise test the patients rated the Borg CR 10 for dyspnea every 3 min. At the end of exercise, the patients were asked to rate the Borg CR 10 for both dyspnea and leg effort.
Metabolic gas exchange was recorded throughout using a breath-by-breath system (MetaMax 3B, Cortex Biophysik GmbH, Germany); the data were analysed using the associated Metasoft software. Prior to each test, standardized pressure, volume and precision gas calibration were performed according to the manufacturer’s instructions. Heart rate was continuously recorded using a chest strap (model T34, Polar Electro Oy, Finland). Blood pressure was measured by manual sphygmomanometry every 2 min during the tests.
Outcome measures
The primary outcome measures were:
- Maximal outcomes:
- Peak oxygen uptake, denoted V'O2peak. This was determined as the maximum of a 15-breath average during the ramp phase.
- Peak heart rate, HRpeak: the highest value of HR during the ramp phase.
- Peak respiratory exchange ratio, RERpeak: the 15-breath average at the time of V'O2peak.
- Peak work rate, WRpeak. This was calculated as the highest work rate achieved.
- Submaximal outcomes:
- Oxygen uptake at the ventilatory anaerobic threshold VAT (V'O2VAT): the method for determination of VAT is summarized below.
- Oxygen uptake at the respiratory compensation point RCP (V'O2RCP), described below.
The secondary outcome measures were:
Rating of perceived exertion (RPE) for dyspnea and leg effort at the time of peak exercise (Borg CR10) [25].
Heart rate at the VAT (HRVAT) and heart rate at the RCP (HRRCP).
The value of the ventilatory equivalent of oxygen (V'E/V'O2) at VAT and ventilatory equivalent of carbon dioxide (V'E/V'CO2) at RCP, where V'E is minute ventilation and V'CO2 is carbon dioxide output, and the slope of V'E-vs-V'CO2 from the start of the ramp phase to the RCP.
The ventilatory anaerobic threshold (VAT) and the respiratory compensation point (RCP) were determined as the averages from two independent raters (JS and LB) provided that the difference in the V'O2 values of the corresponding points between two raters was less than 100 mL/min [26]. In the case of any discrepancy, a third experienced rater (KH) rated the point, and the VAT or RCP was taken as the average of the 2 closest values. The methods used to determine the VAT and RCP were those described by Binder et al. [27] and summarised in the following.
The VAT was determined using the combination of these criteria: (1) the point where the V'E/V'O2 reaches its minimum or starts to increase without an increase in the V'E/V'CO2; (2) the point at which the partial pressure of end-tidal oxygen tension (PETO2) reaches a minimum or starts to increase without a decline in the partial pressure of end-tidal carbon dioxide tension (PETCO2); and, (3) the point of deflection of V'CO2versus V'O2 (V-slope method). The first two criteria were prioritized in the case that the 3 criteria gave different results.
The RCP was determined by: (1) the minimal value or nonlinear rise of V'E/V'CO2; (2) the point that PETCO2 starts to decline; and, (3) the point of deflection of V'E versus V'CO2. Again, the first two criteria were prioritized.
Statistical analysis
Continuous variables are presented as mean ± standard deviation (SD). Categorical variables are presented as frequencies and percentages. Test-retest reliability of submaximal parameters on each device was analysed using an intraclass correlation coefficient (ICC3,1) [28]. For the interpretation of results, 0.40 ≤ ICC < 0.75 was considered as fair to good reliability and ICC ≥ 0.75 was considered excellent reliability [29]. The within-subject coefficients of variation (CoV) were also calculated [30].
Repeatability was analysed using the Bland and Altman limits of agreement (LoA). Heteroscedasticity was examined by calculating Pearson’s correlation coefficient (r) between the absolute difference and the corresponding means. When r > 0.1 was found, the data were considered heteroscedastic. The heteroscedastic data were log transformed using base 10 and r was recalculated. If r decreased, the data were analysed using log-transformation. The limits of agreement for heteroscedastic data were transformed back and displayed in the Bland Altman plots as a linear function ± bx̄ calculated by the method described by Euser et al. [31], where x̄ is the data mean and b is the slope of the LoA. If the data were homoscedastic, or the data were heteroscedastic but the log-transformed data did not reduce the correlation coefficient, the limits of agreement were reported as the standard mean difference (MD) ± 1.96 SD of the difference [32].
Two-sided paired t-tests were used to test differences between TB1 and TB2, as well as between TB2 and TF, if the difference between the tests was normally distributed (Shapiro Wilk test). Otherwise, the Wilcoxon-signed rank test was used. The significance level was set at 0.05. The analyses were performed using SPSS (Version 20.0, IBM Corp., Armonk, NY).
Results
General observations
Seventeen patients (8 females, 9 males) aged 62.7 ± 10.4 years (mean ± SD), see Table 1, completed all three sessions and are included in the data analysis. The median time post stroke was 350 days. There were 4 severely disabled, 1 moderately disabled and 12 mildly disabled patients. Three further patients were recruited but did not complete all measurement sessions because of, respectively, severe hypertension, new onset atrial fibrillation, or due to automatic shutdown of the RATT caused by inappropriate forces. These three patients were not included in the data analysis
There were no complications or serious adverse events. The ramp-phase duration was 8 min 53 s ± 2 min 17 s. Of the total of 51 completed exercise test sessions (17 patients x 3 sessions each), 49 sessions were terminated at the patient’s own volition (functional capacity reached). The most common reasons for volitional exercise termination were leg fatigue (45.1 %), generalized fatigue (17.6 %) and inability to maintain the target work rate (15.7 %). One session was terminated because blood pressure reached the upper limit (SBP > 210 mmHg) and 1 session because of pain due to a tension headache.
Overall, the VAT and RCP were identifiable in 49 of 51 tests (96.1 %) and 39 of 51 tests (76.5 %), respectively: this allowed 16 paired comparisons of V'O2VAT to be done for TB1 vs TB2 (test-retest) and for TB2 vs TF (four-week changes); 12 paired comparisons of V'O2RCP were able to be done for TB1 vs TB2 and 11 comparisons for TB2 vs TF (Fig. 2, Tables 2 and 3). There was one measurement problem for submaximal heart rate recording in TB1, so the pairwise comparison for HRVAT and HRRCP were 1 pair lower than for the submaximal V'O2 pairs (test-retest).
Fig. 2.
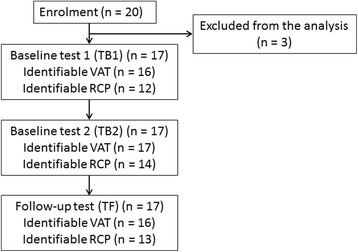
Study flow chart. Reasons for the three exclusions are detailed in results
Table 2.
Test-retest reliability of the cardiopulmonary performance parameters
| TB1 (mean ± SD) | TB2 (mean ± SD) | p-value | MD | 95%CI | SDD | 95 % LoA | CoV (%) | ICC (95 % CI) | |
|---|---|---|---|---|---|---|---|---|---|
| Absolute V'O2peak (mL/min) | 1130.5 ± 349.4 | 1156.4 ± 376.6 | 0.60 | 25.8 | −77.4, 129.1 | 200.8 | −0.26x̄, 0.26x̄ | 11.6 | 0.85 (0.64, 0.94) |
| Relative V'O2peak (mL/kg/min) | 15.7 ± 4.7 | 16.0 ± 4.9 | 0.65 | 0.3 | −1.1, 1.7 | 2.8 | −0.26x̄, 0.26x̄ | 11.6 | 0.84 (0.61, 0.94) |
| HRpeak (beats/min) | 120.9 ± 26.3 | 124.2 ± 25.2 | 0.26 | 3.2 | −2.6, 9.0 | 11.3 | −18.9, 25.3 | 6.9 | 0.90 (0.76, 0.96) |
| HRpeak% of APMHR | 78.0 ± 15.0 | 80.1 ± 13.6 | 0.26 | 2.04 | −1.6, 5.7 | 7.2 | −12.1, 16.2 | 6.9 | 0.87 (0.69, 0.95) |
| RERpeak | 1.06 ± 0.09 | 1.08 ± 0.08 | 0.10 | 0.02 | −0.01, 0.52 | 0.06 | −0.1, 0.1 | 4.1 | 0.78 (0.49, 0.91) |
| WRpeak (W) | 28.0 ± 12.4 | 29.0 ± 13.3 | 0.36 | 1.07 | 1.4, 3.5 | 4.6 | −7.9, 10.1 | 13.4 | 0.94 (0.83, 0.98) |
| Absolute V'O2VAT (mL/min) (n = 16) | 575.4 ± 105.9 | 635.5 ± 177.3 | 0.045 | 60.1 | −1.4, 118.9 | 110.2 | −0.34x̄, 0.34x̄ | 14.5 | 0.67 (0.27, 0.87) |
| Relative V'O2VAT (mL/kg/min) | 8.0 ± 1.6 | 8.8 ± 2.4 | 0.050 | 0.8 | 0.0, 1.6 | 1.5 | −0.34x̄, 0.34x̄ | 14.5 | 0.68 (0.29, 0.88) |
| V'O2VAT% of V'O2peak (%) | 50.3 ± 9.4 | 53.1 ± 9.1 | 0.27 | 2.8 | −2.4, 8.2 | 10 | −16.8, 22.4 | 15.4 | 0.41 (−0.07, 0.74) |
| HRVAT (beats/min) (n = 15) | 89.5 ± 9.3 | 91.9 ± 9.7 | 0.17 | 2.4 | −1.1, 6.0 | 6.5 | −10.3, 15.1 | 5.5 | 0.76 (0.44, 0.91) |
| HRVAT % of HR reserve | 32.6 ± 9.2 | 35.1 ± 11.5 | 0.33 | 2.5 | −2.8, 7.8 | 9.5 | −16.1, 21.1 | 19.5 | 0.58 (0.13, 0.84) |
| HRVAT % of APMHR | 57.3 ± 5.4 | 58.8 ± 5.2 | 0.20 | 1.5 | −0.9, 3.8 | 4.3 | −0.62x̄, 0.62x̄ | 5.5 | 0.67 (0.28, 0.87) |
| Absolute V'O2RCP (mL/min) (n = 12) | 956.5 ± 197.9 | 1002.9 ± 179.4 | 0.33 | 46.3 | −54.1, 146.6 | 157.9 | −263.2, 355.8 | 11.6 | 0.65 (0.17, 0.88) |
| Relative V'O2RCP (mL/kg/min) | 13.4 ± 2.5 | 14.1 ± 2.5 | 0.28 | 0.7 | −0.7, 2.1 | 2.1 | −3.4, 4.8 | 11.6 | 0.77 (0.24, 0.87) |
| V'O2RCP% of V'O2peak (%) | 77.2 ± 8.8 | 82.1 ± 6.8 | 0.035 | 4.9 | 0.4, 9.5 | 7.1 | −9.0, 18.8 | 8.3 | 0.50 (−0.02, 0.82) |
| HRRCP (beats/min) (n = 11) | 112.5 ± 18.2 | 113.6 ± 17.7 | 0.42 | 1.1 | −5.3, 7.4 | 9.4 | −17.3, 19.5 | 5.8 | 0.87 (0.59, 0.96) |
| HRRCP % of HR reserve | 70.2 ± 16.2 | 73.6 ± 10.8 | 0.48 | 3.5 | −7.0, 14.0 | 15.6 | −27.1, 34.1 | 18.4 | 0.37 (−0.28, 0.78) |
| HRRCP % of APMHR | 71.3 ± 12.1 | 71.9 ± 11.7 | 0.72 | 0.7 | −3.2, 4.6 | 5.8 | −10.7, 12.1 | 5.8 | 0.89 (0.64, 0.97) |
| RPE dyspnea | 5.1 ± 2.0 | 5.2 ± 2.7 | 0.93 | 0.1 | −0.8, 0.9 | 1.7 | −3.2, 3.4 | 28.4 | 0.76 (0.44, 0.91) |
| RPE leg effort | 6.8 ± 2.1 | 7.5 ± 1.6 | 0.23 | 0.8 | −0.3, 1.8 | 2 | −3.1, 4.7 | 29.5 | 0.41 (−0.04, 0.73) |
| V'E/V'O2 at VAT | 31.8 ± 4.8 | 32.8 ± 5.0 | 0.28 | 0.9 | −0.8, 2.7 | 3.3 | −5.6, 7.4 | 7.3 | 0.77 (0.48, 0.91) |
| V'E/V'CO2 at RCP | 33.8 ± 3.7 | 33.8 ± 3.7 | 0.91 | 0.1 | −1.2, 1.3 | 2.0 | −3.9, 4.0 | 4.5 | 0.87 (0.60, 0.96) |
| V'E-vs-V'CO2 slope to RCP | 32.2 ± 4.8 | 33.0 ± 5.5 | 0.36 | 0.8 | −1.0, 2.6 | 2.8 | −4.7, 6.3 | 6.1 | 0.85 (0.58,0.96) |
| 6 MWD (n = 12) | 502.5 ± 113.4 | 528.3 ± 113.1 | 0.010 | 25.8 | 7.5, 44.1 | 28.7 | −30.5, 82.1 | 5.6 | 0.95 (0.65, 0.99) |
n = 17, unless otherwise indicated
TB1 baseline test 1, TB2 baseline test 2, APMHR age-predicted maximal heart rate, VAT ventilatory anaerobic threshold, RCP respiratory compensation point, 6 MWD 6 min-walk distance, MD mean difference, SDD standard deviation of difference, LoA limits of agreement, CoV coefficient of variation, ICC intraclass correlation coefficient, CI confidence interval, x̄ individual average
Table 3.
Changes in cardiopulmonary fitness during 4 weeks
| TB2 | TF | MD (95 % CI) (TF-TB2) | p-value | % changes (95 % CI) | |
|---|---|---|---|---|---|
| Absolute V'O2peak (mL/min) | 1156.4 ± 376.6 | 1170.0 ± 397.5 | 13.6 (−102.9, 130.2) | 0.81 | 2.8 (−6.3, 11.9) |
| Relative V'O2peak (mL/kg/min) | 16.0 ± 4.9 | 16.2 ± 5.0 | 0.2 (−1.3, 1.6) | 0.80 | 2.8 (−6.3, 11.9) |
| HRpeak (beats/min) | 124.2 ± 25.2 | 124.5 ± 23.5 | 0.4 (−4.7, 5.4) | 0.88 | 0.9 (−3.1, 5.0) |
| RERpeak | 1.08 ± 0.08 | 1.08 ± 0.10 | 0.001 (−0.02, 0.03) | 0.89 | 0.2 (−2.3, 2.6) |
| WRpeak (W) (n = 16) | 29.0 ± 13.3 | 30.0 ± 14.0 | 1.0 (−1.8, 3.8) | 0.46 | 2.6 (−7.3, 12.6) |
| Absolute V'O2VAT (mL/min) (n = 16) | 637.4 ± 173.3 | 569.3 ± 151.3 | −68.1 (−107.5, −28.7) | 0.002 | −9.6 (−15.1, 4.0) |
| Relative V'O2VAT (mL/kg/min) | 8.8 ± 2.3 | 7.9 ± 2.0 | −0.9 (−1.5, −0.4) | 0.002 | −9.6 (−15.1, 4.0) |
| HRVAT (beats/min) | 93.8 ± 11.5 | 91.1 ± 7.4 | −2.6 (−6.8, 1.5) | 0.20 | −2.1 (−6.2, 2.0) |
| Absolute V'O2RCP (mL/min) (n = 11) | 1018.7 ± 179.1 | 1055.4 ± 199.4 | 36.7 (−80.4, 153.7) | 0.50 | 4.5 (−5.9, 14.9) |
| Relative V'O2RCP (mL/kg/min) | 14.2 ± 2.5 | 14.7 ± 2.5 | 0.5 (−1.0, 1.9) | 0.48 | 4.5 (−5.9, 14.9) |
| HRRCP (beats/min) | 118.2 ± 16.8 | 116.8 ± 19.0 | −1.3 (−8.8, 6.1) | 0.69 | −1.0 (−7.7, 5.7) |
| RPE dyspnea | 5.2 ± 2.7 | 4.5 ± 1.9 | −0.7 (−1.9, 0.5) | 0.25 | −0.7 (−25.2, 23.8) |
| RPE leg effort | 7.5 ± 1.6 | 6.1 ± 2.2 | −1.4 (−2.8, −0.2) | 0.028 | −16.4 (−32.4, 0.4) |
| V'E/V'O2 at VAT (n = 16) | 32.4 ± 4.6 | 32.4 ± 4.2 | 0.1 (−1.2, 1.3) | 0.93 | 0.5 (−3.2, 4.3) |
| V'E/V'CO2 at RCP (n = 11) | 33.7 ± 3.8 | 33.5 ± 4.4 | −0.1 (−1.7, 1.4) | 0.86 | −0.4 (−5.2, 4.5) |
| V'E-vs-V'CO2 slope to RCP (n = 11) | 32.8 ± 5.7 | 32.0 ± 4.6 | −0.8 (−3.4, 1.9) | 0.53 | −1.3 (−8.4, 5.8) |
| 6MWD (n = 12) | 528.3 ± 113.1 | 526.7 ± 114.5 | −1.7 (−26.1 to 22.8) | 0.89 | 0.1 (−5.0 to 5.2) |
n = 17, unless otherwise indicated
TB2 baseline test 2, TF follow up test, MD mean difference, CI confidence interval
Test-retest reliability (TB1 vs TB2)
The first and second baseline tests (TB1 and TB2) were 2.1 ± 2.1 days apart. The primary outcomes (V'O2peak, HRpeak, RERpeak, WRpeak, V'O2VAT and V'O2RCP) showed good to excellent test-retest reliability with ICCs in the range of 0.65 to 0.94 (Table 2). Overall, peak exercise performance parameters showed higher reliability compared to submaximal exercise parameters: V'O2peak had a better reliability and repeatability shown by a higher ICC, a lower or equal coefficient of variation and a smaller mean difference (ICC 0.85 [95 % CI 0.64-0.94], CoV 11.6 %, MD 25.8 mL/min) compared to V'O2VAT (ICC 0.67 [95%CI 0.27–0.87], CoV 14.5 %, MD 60.1 mL/min) and V'O2RCP (ICC 0.65 [95%CI 0.17–0.88], CoV 11.6 %, MD 46.3 mL/min). Most cardiopulmonary exercise parameters were slightly higher in TB2: a statistically significant difference was found between the tests only for V'O2VAT (575.4 mL/min in TB1 vs 635.5 mL/min in TB2, p = 0.045). For the Bland and Altman analysis, the variables V'O2peak, V'O2VAT and HRVAT as a percentage of HR reserve were found to be heteroscedastic and the limits of agreements were calculated from the log-transformed data (Table 2, Fig. 3).
Fig. 3.
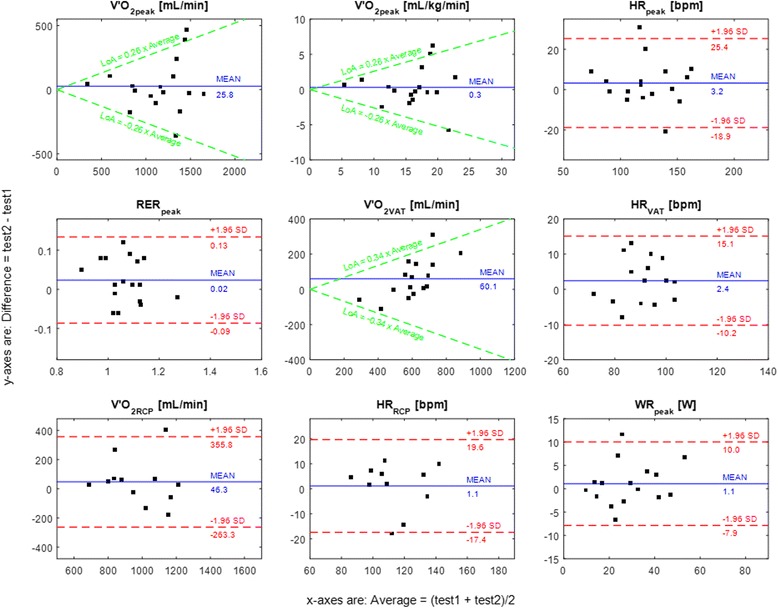
Bland-Altman plots for the main outcome measures. The differences between test 2 (TB2) and test 1 (TB1) are plotted against the average values of TB1 and TB2
Amongst the secondary outcomes, HRVAT, HRRCP, V'E/V'O2 at VAT, V'E/V'CO2 at RCP and V'E-vs-V'CO2 slope showed excellent reliability with ICC from 0.76 to 0.87 and CoV less than 8 %. The least reliable parameters as classified by the highest CoV (28.4 and 29.5 %) were the patients’ subjective rating of perceived exertion (RPE), both for leg effort and dyspnea.
Changes in cardiopulmonary fitness after 4 weeks (TB2 vs TF)
The follow up test (TF) was 30.4 ± 10.2 days after the second baseline measurement (TB2). No significant changes were found in V'O2peak, HRpeak, RERpeak, WRpeak, or V'O2RCP (Table 3), but V'O2VAT showed a statistically significant decrease (637.4 mL/min in TB2 vs 569.3 mL/min in TF, p = 0.002). Regarding secondary outcomes, mean RPE for leg effort in TF was lower than for TB2 but the other variables were comparable (Table 3).
Discussion
This study had two aims: to investigate test-retest reliability and repeatability of CPET on the RATT in stroke patients with all degrees of disability; and to prospectively investigate changes in cardiopulmonary outcomes in this sample over a period of four weeks.
Test-retest reliability
Good to excellent reliability was found in V'O2peak, HRpeak, RERpeak, WRpeak, V'O2VAT and V'O2RCP. Overall, there were slightly higher cardiopulmonary performance parameter values in test 2, but, amongst the formal outcome measures, only the difference in V'O2VAT reached statistical significance.
The test-retest reliability of the peak cardiopulmonary exercise parameters observed here in stroke patients (V'O2peak ICC of 0.85 and CoV of 11.6 %) was lower than values seen previously in healthy individuals (ICC of 0.97 and CoV of 4.1 %) exercising on the RATT [17]. The lower reliability may be explained by the higher variation in day-to-day levels of motor performance, levels of fatigue and motivation in stroke patients.
Comparing the test-retest reliability results with other devices used in stroke patients is difficult as ICCs are often reported by different ICC methods. Additionally, test-retest reliability as calculated by other measures such as CoV are scarce in the stroke literature. Results from various previous studies found that V'O2peak obtained from a cycle ergometer, a semi-recumbent cycle ergometer, a treadmill and a robotics-assisted treadmill in both sub-acute and chronic stroke yielded excellent reliability with ICC ranging from 0.82 to 0.98 [10, 33–38]. However, one study in sub-acute stroke patients using a semi-recumbent cycle ergometer showed poor reliability: there was an increase of 10 % in V'O2peak in the second test and an ICC for V'O2peak of 0.50 [24]; the reason for this discrepancy is unclear.
Additional maximal exercise parameters showed good to excellent test-retest reliability in previous reports. The ICC of 0.90 for HRpeak in the present study is comparable to previous reports (0.74 to 0.97) for a cycle ergometer, a treadmill and a robotics-assisted treadmill [10, 24, 33, 35–38]. By way of comparison, and bearing in mind the differences in degrees of disability and testing devices, the ICCs for RERpeak and WRpeak of 0.78 and 0.94 found here can be contrasted with other reports in chronic stroke patients with mild to moderate disability using a cycle ergometer and a treadmill (0.72 for RERpeak and 0.99 for work rate) [36, 37].
For the Bland and Altman analysis, we found that V'O2peak, V'O2VAT and HRVAT as a percentage of HR reserve were heteroscedastic (Fig. 3). The finding of heteroscedasticity in V'O2peak was also previously reported in stroke patients and patients with cardiac and pulmonary problems [33, 39, 40]. This means that patients with low V'O2peak have lower variation in the absolute test-retest difference compared to patients with higher V'O2peak. This finding is important as it implies that, for future studies, it would be necessary to plan the test-retest reliability protocol for specific levels of exercise capacity (i.e. V'O2peak) to get the most accurate results because variations in the test-retest data are not uniform.
For submaximal exercise thresholds, the reliability of V'O2VAT and V'O2RCP were lower than for V'O2peak: ICC was 0.67 and CoV was 14.5 % for V'O2VAT; ICC was 0.65 and CoV was 11.6 % for V'O2RCP. These values, again, reflect lower reliability compared to healthy individuals on the same device: the ICC was 0.92 for both V'O2VAT and V'O2RCP and the CoV was 5.9 and 6.5 % for V'O2VAT and V'O2RCP, respectively, for healthy individuals [18]. The finding of lower reliability for V'O2VAT and V'O2RCP compared to V'O2peak was not unexpected as this was previously documented in healthy individuals, and in patients with cardiac and pulmonary problems [17, 18, 39]. This was thought to be because of day-to-day biological variability, which may be related to intrinsic factors such as daily haemodynamic fluctuations [39]. Additionally, the lower reliability may be caused by intra and inter-rater variability in the determination of the submaximal exercise thresholds. It was found that the intra or inter-rater variability was 5 to 12 % in patients with congestive heart failure and in normal subjects [41, 42].
HRVAT and HRRCP were shown to have excellent reliability. This suggests that the use of HRVAT and HRRCP to set the recommended intensity for exercise training could be implemented in practice. RPE showed the lowest reliability and repeatability. The findings in the present study support previous evidence that RPE is not an appropriate indicator of exercise intensity in stroke patients at a high-intensity exercise level [43]. V'E/V'O2 at VAT, V'E/V'CO2 at RCP, and the V'E-vs-V'CO2 slope showed excellent test-retest reliability with CoVs comparable to healthy individuals (CoV of 4.5 to 7.3 % in stroke patients vs CoV of 2.5 to 6 % in healthy individuals) [18].
In summary, based on this evidence, reliability and repeatability of the main CPET parameters obtained from the RATT are comparable to previous findings reported in stroke patients using standard exercise testing devices. However, the reliability and repeatability levels found here in stroke patients were generally lower than for healthy individuals on the RATT.
Changes in cardiopulmonary fitness after 4 weeks
Apart from V'O2VAT, the main cardiopulmonary performance parameters did not demonstrate any statistically significant differences over the four week period. This may be because the follow up time was short and because more than half of the patients who participated in this study were in the chronic phase following stroke. The non-significant change in mean V'O2peak from 16.0 to 16.2 mL/kg/min is comparable to a study in a control group of chronic stroke patients: 15.1 to 15.2 mL/kg/min in a 10-week period [37]. This reflects that without any specific exercise intervention, changes in V'O2peak are unlikely to be seen over a short period.
There was a significant difference (decrease) in V'O2VAT over the 4-week period (TB2 vs TF). However, based on the large and significant difference in V'O2VAT between tests TB1 and TB2 (mean difference of +60.1 mL/min) and the fact that V'O2VAT from TB1 was only 575.4 mL/min (Table 2), this is considered not to be clinically significant.
The observation of no changes in cardiopulmonary fitness over 4 weeks might serve as a baseline for future studies which implement a training intervention using the RATT.
General comments
V'O2peak averaged over the three tests was 16 mL/kg/min. This is in line with previous reports and shows that cardiopulmonary fitness in stroke patients is low (8 to 22 mL/kg/min, 26 to 87 % of age and sex-matched prediction) [8].
The cardiopulmonary fitness values obtained in this study need to be interpreted with caution. The V'O2peak and V'O2 at submaximal exercise thresholds are device specific: it was found that peak and submaximal V'O2 were approximately 20 % lower on the RATT than the cycle ergometer in healthy individuals [17, 18]. The HR response is also device specific: the HRpeak of 80 % of age predicted maximal heart rate (APMHR) obtained from CPET on the RATT in this study is at the lower end of the range for HRpeak reported for a recumbent cycle ergometer, an upright cycle ergometer and a treadmill in chronic ambulatory stroke patients (78.2 to 94.7 % of APMHR) [34, 44, 45]. The lower values for V'O2peak and HRpeak on the RATT compared to other devices may be because of more body support provided and a difference in the movement pattern of exercise on the RATT [17].
An RERpeak of > 1.0 is a recommended corroboratory criterion for detection of maximal effort in stroke patients [46]. This criterion was satisfied here in 82.4 % of all tests, even though severely disabled patients were included. This is considered a high proportion compared to previous reports of only 17.6 to 62.1 % in patients with sub-acute or chronic ambulatory stroke tested on a treadmill [44, 47, 48], 44 % of sub-acute stroke patients tested on a semi-recumbent cycle ergometer [24], and 78.9 % of sub-acute stroke patients using a cycle ergometer [49]. These differences in the proportion of patients who achieved the criterion for maximal effort points to the importance of the device employed for CPET. Patients may experience difficulty exercising on a treadmill at higher speeds because of balance problems or fear of falling and they may have problems with leg control during cycling that prevent them from reaching their maximal exercise capacity. The RATT, in contrast, provides a body harness, thigh cuffs and foot plates to secure the patients, thus enabling them to exercise to a higher intensity safely and securely.
Overall, the VAT was identified in 16/17 (94.1 %) (TB1), 17/17 (100 %) (TB2) and 16/17 (94.7 %) (TF) patients. The RCP was identified in 12/17 (70.6 %) (TB1), 14/17 (82.4 %) (TB2) and 13/17 (76.5 %) (TF) patients. In contrast, the VAT was previously demonstrated to be identifiable in only 67.3 to 83.5 % of chronic stroke patients exercising on a semi-recumbent cycle ergometer, an upright cycle ergometer or a treadmill [45]. To the best of our knowledge, there are no other studies in stroke patients that reported identification of an RCP, which is a point of substantially higher exercise intensity than the VAT, apart from our own feasibility study [15]. The finding of high proportions of patients who were able to exercise on the RATT to a sufficiently high level of intensity to allow identification of both the VAT and the RCP is regarded as an important finding. These submaximal exercise thresholds provide additional information regarding a patient’s fitness status [23, 50] and they can be used to follow up after an exercise intervention [51, 52]. Moreover, these submaximal exercise thresholds could be adopted for the prescription of individualised exercise programmes because the exercise intensity between the VAT and RCP reflects the recommended individualized moderate to high intensity exercise regime [53].
HRVAT in this study was lower than the value reported in mild to moderate chronic stroke on a treadmill: here, HRVAT was approximately 59 % of APMHR and in a study of Bosch et al. (n = 8) it was 66 % of APMHR [26]. This difference may be due to the device specific responses to exercise as mentioned above.
Limitations
The strict patient eligibility criteria described in Methods and the exclusion of patients who had cardiac problems or who were not approved by the cardiologist for CPET led to the small sample size in this study, which may limit generalizability of the findings. Further investigation in a large cohort of stroke patients from the principal target population, that is, patients with severe disability, is warranted.
Conclusions
These findings provide the first evidence of test-retest reliability and repeatability of the principal CPET variables using the novel RATT system and testing methodology, together with evidence of high success rates in identification of submaximal CPET threshold variables. Good to excellent test-retest reliability and repeatability were found for all submaximal and maximal CPET variables. Reliability and repeatability of the main CPET parameters in stroke patients on the RATT were comparable to previous findings in stroke patients using standard exercise testing devices. The RATT has potential to be used as an alternative exercise testing device in patients who have limitations for use of standard exercise testing devices.
Acknowledgements
The authors are grateful to Lukas Bichsel and Matthias Schindelholz who developed and implemented the force sensors, work-rate estimation algorithm and the visual feedback system for the RATT. We thank Sophie Chen, Tanja Spycher and Sandra Küng for assistance with patient recruitment. We thank Dr Bernhard Spoendlin for cardiac examination. The authors are grateful to all patients for their time and motivation to participate in the study.
Funding
Not applicable.
Availability of data and material
The datasets supporting the conclusions of this article are included within the article.
Authors’ contributions
All authors made substantial contributions to the study and to manuscript preparation. JS, KH, CS and TN contributed to the design of the study. JS, LB and CS screened patients for inclusion and recruited the patients. JS, LB and CS carried out the assessments. JS, LB and KH performed the data analysis. JS, LB, CS, TN and KH participated in drafting and critical review of the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This prospective study was approved by the Ethics Review Committee of Canton Aargau, Switzerland (Ref. EKNZ 2014–296). All subjects gave their written informed consent before participating in the study.
Abbreviations
- 6 MWD
Six-minute walk distance
- APMHR
Age-predicted maximal heart rate
- CoV
Coefficient of variation
- CPET
Cardiopulmonary exercise testing
- HR
Heart rate
- HRpeak
Peak heart rate
- ICC
Intraclass correlation coefficient
- LoA
Limits of agreement
- MD
Mean difference
- PETCO2
Partial pressure of end-tidal carbon dioxide tension
- PETO2
Partial pressure of end-tidal oxygen tension
- RATT
Robotics-assisted tilt table
- RCP
Respiratory compensation point
- RERpeak
Peak respiratory exchange ratio
- RPE
Rating of perceived exertion
- VAT
Ventilatory anaerobic threshold
- V'CO2
Carbon dioxide output
- V'E
Minute ventilation
- V'E/V'CO2
Ventilatory equivalent of carbon dioxide
- V'E/V'O2
Ventilatory equivalent of oxygen
- V'O2
Oxygen uptake
- V'O2peak
Peak oxygen uptake
- WRpeak
Peak work rate
Contributor Information
Jittima Saengsuwan, Email: sjittima@kku.ac.th.
Lucia Berger, Email: L.Berger@reha-rhf.ch.
Corina Schuster-Amft, Email: C.Schuster@reha-rhf.ch.
Tobias Nef, Email: tobias.nef@artorg.unibe.ch.
Kenneth J. Hunt, Email: kenneth.hunt@bfh.ch
References
- 1.Adamson J, Beswick A, Ebrahim S. Is stroke the most common cause of disability? J Stroke Cerebrovasc Dis. 2004;13:171–7. doi: 10.1016/j.jstrokecerebrovasdis.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 3.Teasell RW. Long-term sequelae of stroke: How should you handle stroke complications? Can Fam Physician. 1992;38:381–8. [PMC free article] [PubMed] [Google Scholar]
- 4.Billinger SA, Coughenour E, Mackay-Lyons MJ, Ivey FM. Reduced cardiorespiratory fitness after stroke: biological consequences and exercise-induced adaptations. Stroke Res Treat. 2012;2012:959120. doi: 10.1155/2012/959120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Togha M, Sharifpour A, Ashraf H, Moghadam M, Sahraian MA. Electrocardiographic abnormalities in acute cerebrovascular events in patients with/without cardiovascular disease. Ann Indian Acad Neurol. 2013;16:66–71. doi: 10.4103/0972-2327.107710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Myers J, Nieman D, editors. ACSM’s resources for clinical exercise physiology: musculoskeletal, neuromuscular, neoplastic, immunologic, and hematologic conditions. 2. Philadelphia: Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 7.Billinger S, Arena R, Bernhardt J, Eng J, Franklin B, Johnson C, et al. Physical activity and exercise recommondations for stroke survivors: a statement for health care professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2532–53. doi: 10.1161/STR.0000000000000022. [DOI] [PubMed] [Google Scholar]
- 8.Smith AC, Saunders DH, Mead G. Cardiorespiratory fitness after stroke: a systematic review. Int J Stroke. 2012;7:499–510. doi: 10.1111/j.1747-4949.2012.00791.x. [DOI] [PubMed] [Google Scholar]
- 9.Chu KS, Eng JJ, Dawson AS, Harris JE, Ozkaplan A, Gylfadottir S. Water-based exercise for cardiovascular fitness in people with chronic stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2004;85:870–4. doi: 10.1016/j.apmr.2003.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yates JS, Studenski S, Gollub S, Whitman R, Perera S, Lai SM, Duncan PW. Bicycle ergometry in subacute-stroke survivors: feasibility, safety, and exercise performance. J Aging Phys Act. 2004;12:64–74. doi: 10.1123/japa.12.1.64. [DOI] [PubMed] [Google Scholar]
- 11.Stoller O, de Bruin ED, Knols RH, Hunt KJ. Effects of cardiovascular exercise early after stroke: systematic review and meta-analysis. BMC Neurol. 2012;12:45. doi: 10.1186/1471-2377-12-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marsden DL, Dunn A, Callister R, Levi CR, Spratt NJ. Characteristics of exercise training interventions to improve cardiorespiratory fitness after stroke: a systematic review with meta-analysis. Neurorehabil Neural Repair. 2013;27:775–88. doi: 10.1177/1545968313496329. [DOI] [PubMed] [Google Scholar]
- 13.Truelsen T, Piechowski-Jóźwiak B, Bonita R, Mathers C, Bogousslavsky J, Boysen G. Stroke incidence and prevalence in Europe: a review of available data. Eur J Neurol. 2006;13:581–98. doi: 10.1111/j.1468-1331.2006.01138.x. [DOI] [PubMed] [Google Scholar]
- 14.Boysen G, Marott JL, Grønbaek M, Hassanpour H, Truelsen T. Long-term survival after stroke: 30 years of follow-up in a cohort, the Copenhagen City Heart Study. Neuroepidemiology. 2009;33:254–60. doi: 10.1159/000229780. [DOI] [PubMed] [Google Scholar]
- 15.Saengsuwan J, Huber C, Schreiber J, Schuster-Amft C, Nef T, Hunt KJ. Feasibility of cardiopulmonary exercise testing and training using a robotics-assisted tilt table in dependent-ambulatory stroke patients. J Neuroeng Rehabil. 2015;12:88. doi: 10.1186/s12984-015-0078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bichsel L, Sommer M, Hunt KJ. Development of a biofeedback system for controlling the patients work rate, heart rate and oxygen uptake during robot-assisted tilt table therapy. Automatisierungstechnik. 2011;59:622–8. doi: 10.1524/auto.2011.0953. [DOI] [Google Scholar]
- 17.Saengsuwan J, Nef T, Laubacher M, Hunt KJ. Comparison of peak cardiopulmonary performance parameters on a robotics-assisted tilt table, a cycle and a treadmill. PLoS One. 2015;10(4):e0122767. doi: 10.1371/journal.pone.0122767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saengsuwan J, Nef T, Laubacher M, Hunt KJ. Submaximal cardiopulmonary thresholds on a robotics-assisted tilt table, a cycle and a treadmill: a comparative analysis. Biomed Eng Online. 2015;14:104. doi: 10.1186/s12938-015-0099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pescatello LS, Arena R, Riebe D, Thompson PD, editors. ACSM’s guidelines for exercise testing and prescription. 9. Philadelphia: Lippincott Williams & Wilkins; 2014. [DOI] [PubMed] [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Holden MK, Gill KM, Magliozzi MR, Nathan J, Piehl-Baker L. Clinical gait assessment in the neurologically impaired. Reliability and meaningfulness. Phys Ther. 1984;64:35–40. doi: 10.1093/ptj/64.1.35. [DOI] [PubMed] [Google Scholar]
- 22.Brott T, Adams HP, Jr, Olinger CP, Marler JR, Barsan WG, Biller J, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864–70. doi: 10.1161/01.STR.20.7.864. [DOI] [PubMed] [Google Scholar]
- 23.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 24.Tang A, Sibley KM, Thomas SG, McIlroy WE, Brooks D. Maximal exercise test results in subacute stroke. Arch Phys Med Rehabil. 2006;87:1100–5. doi: 10.1016/j.apmr.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 25.Borg G. Psychophysical scaling with applications in physical work and the perception of exertion. Scand J Work Environ Health. 1990;16(Suppl 1):55–8. doi: 10.5271/sjweh.1815. [DOI] [PubMed] [Google Scholar]
- 26.Bosch PR, Holzapfel S, Traustadottir T. Feasibility of measuring ventilatory threshold in adults with stroke-induced hemiparesis: implications for exercise prescription. Arch Phys Med Rehabil. 2015;96:1779–84. doi: 10.1016/j.apmr.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 27.Binder RK, Wonisch M, Corra U, Cohen-Solal A, Vanhees L, Saner H, et al. Methodological approach to the first and second lactate threshold in incremental cardiopulmonary exercise testing. Eur J Cardiovasc Prev Rehabil. 2008;15:726–34. doi: 10.1097/HJR.0b013e328304fed4. [DOI] [PubMed] [Google Scholar]
- 28.Weir JP. Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J Strength Cond Res. 2005;19:231–40. doi: 10.1519/15184.1. [DOI] [PubMed] [Google Scholar]
- 29.Rosner B. Fundamentals of biostatistics. 7. Boston: Brooks/Cole Cengage learning; 2010. [Google Scholar]
- 30.Bland JM, Altman DG. Measurement error proportional to the mean. BMJ. 1996;313(7049):106. doi: 10.1136/bmj.313.7049.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Euser AM, Dekker FW, le Cessie S. A practical approach to Bland-Altman plots and variation coefficients for log transformed variables. J Clin Epidemiol. 2008;61:978–82. doi: 10.1016/j.jclinepi.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. doi: 10.1016/S0140-6736(86)90837-8. [DOI] [PubMed] [Google Scholar]
- 33.Stoller O, de Bruin ED, Schindelholz M, Schuster-Amft C, de Bie RA, Hunt KJ. Cardiopulmonary exercise testing early after stroke using feedback-controlled robotics-assisted treadmill exercise: test-retest reliability and repeatability. J Neuroeng Rehabil. 2014;11:145. doi: 10.1186/1743-0003-11-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eng JJ, Dawson AS, Chu KS. Submaximal exercise in persons with stroke: Test-retest reliability and concurrent validity with maximal oxygen consumption. Arch Phys Med Rehabil. 2004;85:113–8. doi: 10.1016/S0003-9993(03)00436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olivier C, Doré J, Blanchet S, Brooks D, Richards CL, Martel G, et al. Maximal cardiorespiratory fitness testing in individuals with chronic stroke with cognitive impairment: practice test effects and test-retest reliability. Arch Phys Med Rehabil. 2013;94:2277–82. doi: 10.1016/j.apmr.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 36.Dobrovolny CL, Ivey FM, Rogers MA, Sorkin JD, Macko RF. Reliability of treadmill exercise testing in older patients with chronic hemiparetic stroke. Arch Phys Med Rehabil. 2003;84:1308–12. doi: 10.1016/S0003-9993(03)00150-3. [DOI] [PubMed] [Google Scholar]
- 37.Potempa K, Lopez M, Braun LT, Szidon JP, Fogg L, Tincknell T. Physiological outcomes of aerobic exercise training in hemiparetic stroke patients. Stroke. 1995;26:101–5. doi: 10.1161/01.STR.26.1.101. [DOI] [PubMed] [Google Scholar]
- 38.Duncan P, Studenski S, Richards L, Gollub S, Lai SM, Reker D, et al. Randomized clinical trial of therapeutic exercise in subacute stroke. Stroke. 2003;34:2173–80. doi: 10.1161/01.STR.0000083699.95351.F2. [DOI] [PubMed] [Google Scholar]
- 39.Bensimhon DR, Leifer ES, Ellis SJ, Fleg JL, Keteyian SJ, Piña IL, et al. Reproducibility of peak oxygen uptake and other cardiopulmonary exercise testing parameters in patients with heart failure (from the Heart Failure and A Controlled Trial Investigating Outcomes of exercise traiNing) Am J Cardiol. 2008;102:712–7. doi: 10.1016/j.amjcard.2008.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barron A, Dhutia N, Mayet J, Hughes AD, Francis DP, Wensel R. Test-retest repeatability of cardiopulmonary exercise test variables in patients with cardiac or respiratory disease. Eur J Prev Cardiol. 2014;21:445–53. doi: 10.1177/2047487313518474. [DOI] [PubMed] [Google Scholar]
- 41.Myers J, Goldsmith RL, Keteyian SJ, Brawner CA, Brazil DA, Aldred H, et al. The ventilatory anaerobic threshold in heart failure: a multicenter evaluation of reliability. J Card Fail. 2010;16:76–83. doi: 10.1016/j.cardfail.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 42.Simonton CA, Higginbotham MB, Cobb FR. The ventilatory threshold: quantitative analysis of reproducibility and relation to arterial lactate concentration in normal subjects and in patients with chronic congestive heart failure. Am J Cardiol. 1988;62:100–7. doi: 10.1016/0002-9149(88)91372-0. [DOI] [PubMed] [Google Scholar]
- 43.Sage M, Middleton LE, Tang A, Sibley KM, Brooks D, McIlroy W. Validity of rating of perceived exertion ranges in individuals in the subacute stage of stroke recovery. Top Stroke Rehabil. 2013;20:519–27. doi: 10.1310/tsr2006-519. [DOI] [PubMed] [Google Scholar]
- 44.Ovando AC, Michaelsen SM, Carvalho T, Herber V. Evaluation of cardiopulmonary fitness in individuals with hemiparesis after cerebrovascular accident. Arq Bras Cardiol. 2011;96:140–7. doi: 10.1590/S0066-782X2011005000001. [DOI] [PubMed] [Google Scholar]
- 45.Marzolini S, Oh P, McIlroy W, Brooks D. The feasibility of cardiopulmonary exercise testing for prescribing exercise to people after stroke. Stroke. 2012;43:1075–81. doi: 10.1161/STROKEAHA.111.635128. [DOI] [PubMed] [Google Scholar]
- 46.van de Port IG, Kwakkel G, Wittink H. Systematic review of cardiopulmonary exercise testing post stroke: Are we adhering to practice recommendations? J Rehabil Med. 2015;47:881–900. doi: 10.2340/16501977-2031. [DOI] [PubMed] [Google Scholar]
- 47.Kelly JO, Kilbreath SL, Davis GM, Zeman B, Raymond J. Cardiorespiratory fitness and walking ability in subacute stroke patients. Arch Phys Med Rehabil. 2003;84:1780–5. doi: 10.1016/S0003-9993(03)00376-9. [DOI] [PubMed] [Google Scholar]
- 48.Mackay-Lyons MJ, Makrides L. Exercise capacity early after stroke. Arch Phys Med Rehabil. 2002;83:1697–702. doi: 10.1053/apmr.2002.36395. [DOI] [PubMed] [Google Scholar]
- 49.Chen JK, Chen TW, Chen CH, Huang MH. Preliminary study of exercise capacity in post-acute stroke survivors. Kaohsiung J Med Sci. 2010;26:175–81. doi: 10.1016/S1607-551X(10)70026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maciejczyk M, Szymura J, Cempla J, Gradek J, Wiecek M, Bawelski M. Respiratory compensation point during incremental test in overweight and normoweight boys: is it useful in assessing aerobic performance? A longitudinal study. Clin Physiol Funct Imaging. 2014;34:56–63. doi: 10.1111/cpf.12064. [DOI] [PubMed] [Google Scholar]
- 51.Wisløff U, Støylen A, Loennechen JP, Bruvold M, Rognmo Ø, Haram PM, et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation. 2007;115:3086–94. doi: 10.1161/CIRCULATIONAHA.106.675041. [DOI] [PubMed] [Google Scholar]
- 52.Sullivan MJ, Higginbotham Michael B, Cobb FR. Exercise training in patients with chronic heart failure delays ventilatory anaerobic threshold and improves submaximal exercise performance. Circulation. 1989;79:6. doi: 10.1161/01.CIR.79.2.324. [DOI] [PubMed] [Google Scholar]
- 53.Mezzani A, Hamm LF, Jones AM, McBride PE, Moholdt T, Stone JA, et al. Aerobic exercise intensity assessment and prescription in cardiac rehabilitation: a joint position statement of the European Association for Cardiovascular Prevention and Rehabilitation, the American Association of Cardiovascular and Pulmonary Rehabilitation and the Canadian Association of Cardiac Rehabilitation. Eur J Prev Cardiol. 2013;20:442–67. doi: 10.1177/2047487312460484. [DOI] [PubMed] [Google Scholar]


