Abstract
The Serratia plymuthica strains 3Rp8 and 3Re4-18 are motile, Gram-negative, non-sporulating bacteria. Strain 3Rp8 was isolated from the rhizosphere of Brassica napus L. and strain 3Re4-18 from the endorhiza of Solanum tuberosum L. Studies have shown in vitro activity against the soil-borne fungi Verticillium dahliae Kleb., Rhizoctonia solani Kühn, and Sclerotinia sclerotiorum. Here, we announce and describe the complete genome sequence of S. plymuthica 3Rp8 consisting of a single circular chromosome of 5.5 Mb that encodes 4954 protein-coding and 108 RNA-only encoding genes and of S. plymuthica 3Re4-18 consisting of a single circular chromosome of 5.4 Mb that encodes 4845 protein-coding and 109 RNA-only encoding genes. The whole genome sequences and annotations are available in NCBI under the locus numbers CP012096 and CP012097, respectively. The genome analyses revealed genes putatively responsible for the promising plant growth promoting and biocontrol properties including predicting factors such as secretion systems, iron scavenging siderophores, chitinases, secreted proteases, glucanases and non-ribosomal peptide synthetases, as well as unique genomic islands.
Keywords: Serratia plymuthica, Biocontrol, Plant growth promotion, Secretion systems, Antagonistic rhizosphere bacteria
Introduction
Serratia species are well known for their potential as biocontrol agents with broad-spectrum antagonistic activities against common phytopathogens and their plant growth-promoting abilities. Serratia plymuthica 3Rp8 was isolated as an indigenous colonizer of oilseed rape (Brassica napus L.) rhizosphere and is an in vitro antagonist of the soil-borne fungal phytopathogens Verticillium dahliae Kleb., Rhizoctonia solani Kühn and Sclerotinia sclerotiorum [1] which can cause severe yield losses in a large number of different crops. Chitinase and protease activity were demonstrated by plate assays and the production of N-acylhomoserine lactones was detected using bioluminescent sensor plasmid pSB403 [1, 2]. Serratia plymuthica 3Re4-18 was isolated from the endorhiza of a potato plant (Solanum tuberosum L.) and was identified as the most effective isolate in an in vitro study screening potato-associated bacterial communities for antagonistic functions against plant pathogenic fungi [3]. Both strains were sequenced to augment current studies targeting novel biotechnological applications for seed and root treatment since the strains represent promising candidates for biological control. In this report, we summarize the complete genome sequences and annotations of S. plymuthica 3Rp8 and 3Re4-18 and describe their genomic properties. Analysis of the genomes of 3Rp8 and 3Re4-18 will provide a framework for further studies of their rhizosphere competence, biocontrol properties, and plant growth promoting activity. 3Rp8 and 3Re4-18 are deposited in the strain collection of antagonistic microorganisms at Graz University of Technology, Institute of Environmental Biotechnology, Austria.
Organism information
Classification and features
S. plymuthica 3Rp8 and 3Re4-18 are motile, Gram-negative, non-sporulating Enterobacteriaceae. Colonies appear yellow-beige opaque, domed and moderately mucoid with smooth margins on Luria-Bertani (LB) solid media and form colonies within 24 h at 20 °C (Fig. 1a-b). Both strains grow in standard complex media such as LB, potato dextrose agar (PDA), Waksman agar (WA) and nutrient agar (NA) [4] as well as in minimal medium such as Standard Succinate Medium (SSM). The standard growth temperature is at 30 °C, but both strains can replicate in liquid LB at 5 °C and at 40 °C as well. Both strains do not show a production of red pigments on the media mentioned above. The rod-shaped cells are approximately 0.5 μm in width and 2.0 μm in length (Fig. 1c-d).
Fig. 1.
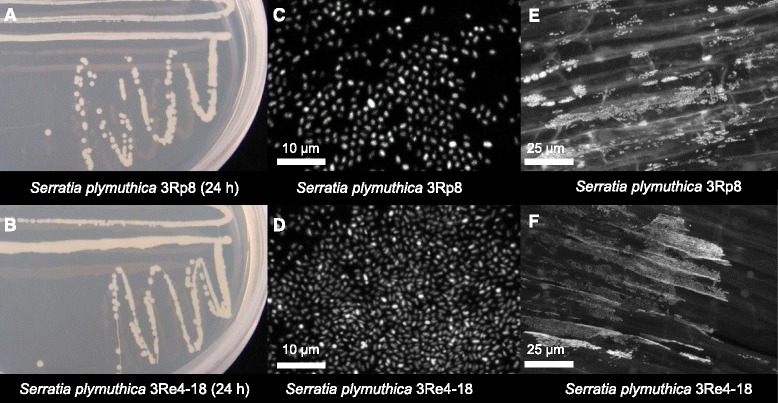
S. plymuthica 3Rp8 and 3Re4-18 on solid media and Confocal Laser Scanning Microscopy micrographs. a-b S. plymuthica 3Rp8 and 3Re4-18 grown on LB solid media after 24 h at 30 °C. Confocal Laser Scanning Microscopy micrographs: c and d show the cell morphology of pure cultures of 3Rp8 and 3Re4-18 after SYTO 9 green-fluorescent staining. e-f Fluorescence in situ hybridized 3Rp8 and 3Re4-18 colonizing the roots of young lettuce seedlings 1 week after inoculation in a gnotobiotic plant growth approach
3Rp8 was isolated from the roots of oilseed rape cultivar Express grown for a field trial in Braunschweig (Germany) in 1998 [1, 5]. 3Re4-18 was isolated from the endorhiza of an early senescent Solanum tuberosum L. cultivar Cilena at the experimental station of the Institute for Plant Diseases, Bonn University in Bonn-Poppelsdorf (Germany) in 2001 [3].
Both bacterial strains are efficient colonizer of oilseed rape and cauliflower [4], lettuce and pumpkin roots (unpublished data) and do not cause any obvious negative effects to those hosts. Priming of oilseed rape and cauliflower seeds with the S. plymuthica 3Rp8 and 3Re4-18 strains had a significant PGP effect on the root weights of the oilseed rape seedlings [4]. Figure 1e-f shows 3Rp8 and 3Re4-18 colonizing the roots of young lettuce seedlings 1 week after inoculation in a gnotobiotic plant growth approach. The strains have natural resistance to Cefuroxime, Cefuroxime Axetil and Cefoxitin (minimal inhibitory concentration (MIC) > = 64 mg/L) as well as Fosfomycin (MIC > = 256 mg/L). Minimum Information about the Genome Sequences (MIGS) of S. plymuthica 3Rp8 and 3Re4-18 are summarized in Table 1, and their phylogenetic position is shown in Figs. 2 and 3. Average nucleotide identity (ANI) data were calculated with Gegenees [6] version 2.2.1 by using a fragmented all against all comparison. The data are illustrated as heat-plot in Fig. 4.
Table 1.
Classification and general features of Serratia plymuthica 3Rp8 and 3Re4-18 according to the MIGS recommendations [20]
| MIGS ID | Property | Term | Evidence codea |
|---|---|---|---|
| Classification | Domain Bacteria | TAS [21] | |
| Phylum Proteobacteria | TAS [22] | ||
| Class Gammaproteobacteria | TAS [23, 24] | ||
| Order “Enterobacteriales” | TAS [25] | ||
| Family Enterobacteriaceae | TAS [26–28] | ||
| Genus Serratia | TAS [26, 29, 30] | ||
| Species Serratia plymuthica | TAS [26, 31] | ||
| Strain Serratia plymuthica 3Rp8 | TAS [1] | ||
| Strain Serratia plymuthica 3Re4-18 | TAS [3] | ||
| Gram stain | Gram-negative | TAS [30] | |
| Cell shape | Rod-shaped | IDA | |
| Motility | Motile | IDA | |
| Sporulation | Non-spore forming | IDA | |
| Temperature range | 5-40 °C | IDA | |
| Optimum temperature | 30 °C | IDA | |
| pH range; Optimum | 5–9; 6 | IDA | |
| Carbon source | Heterotrophic | IDA, TAS [1, 3, 4] | |
| MIGS-6 | Habitat | Root-associated | TAS [1, 3] |
| MIGS-6.3 | Salinity | 3Rp8 - 0.5 %-8 % NaCl (w/v) 3Re4-18 - 0.5 %-9 % NaCl (w/v) |
IDA |
| MIGS-22 | Oxygen requirement | Facultative anaerobe | TAS [30, 32] |
| MIGS-15 | Biotic relationship | 3Rp8 - Rhizospheric 3Re4-18 - Root endophytic |
IDA, TAS [1] IDA, TAS [3] |
| MIGS-14 | Pathogenicity | Non-pathogenic | NAS, TAS [30, 33] |
| MIGS-4 | Geographic location | 3Rp8 - North Germany 3Re4-18 - West Germany |
TAS [1] TAS [3] |
| MIGS-5 | Sample collection | 3Rp8 - 1998 3Re4-18 - 2001 |
TAS [1] TAS [3] |
| MIGS-4.1 | Latitude | 3Rp8 - ~52.27 N 3Re4-18 - ~50.72 N |
NAS |
| MIGS-4.2 | Longitude | 3Rp8 - ~10.57 E 3Re4-18 - ~7.09 E |
NAS |
| MIGS-4.4 | Altitude | 3Rp8 - ~72 m.a.s.l. 3Re4-18 - ~63 m.a.s.l. |
NAS |
aEvidence codes - IDA: Inferred from Direct Assay; TAS: Traceable Author Statement (i.e., a direct report exists in the literature); NAS: Non-traceable Author Statement (i.e., not directly observed for the living, isolated sample, but based on a generally accepted property for the species, or anecdotal evidence). These evidence codes are from the Gene Ontology project [34]
Fig. 2.
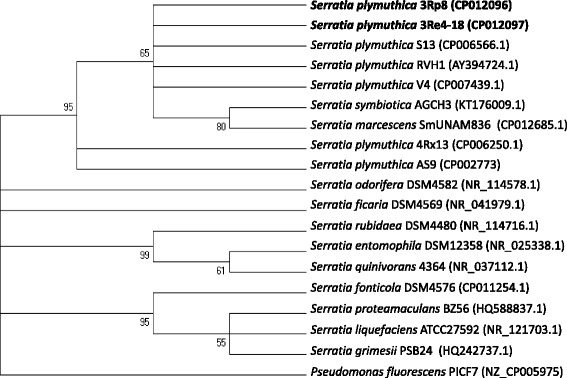
Maximum likelihood 16S rDNA phylogenetic tree indicating the phylogenetic relationship of sequenced isolates. The phylogenetic relationships inferred from the alignment of 1532 bp of 16S rDNA highlighting the positions of S. plymuthica 3Rp8 and 3Re4-18 relative to their closest Serratia strains for which 16S rDNA sequences are publicly available. A representative rhizosphere bacterium from the genera Pseudomonas was used as outgroup. The evolutionary history was inferred by using the Maximum Likelihood method based on the Tamura-Nei model [35]. The percentage of trees in which the associated taxa clustered in the bootstrap test (1000 replicates) is shown next to the branches [36]. Evolutionary analyses were conducted in MEGA7 [37]
Fig. 3.
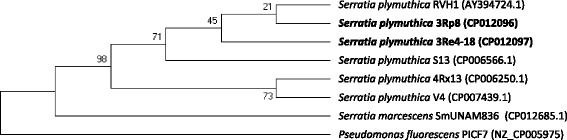
Maximum likelihood phylogenetic tree inferred from three housekeeping genes. The phylogenetic relationships inferred from the alignment of 8077 bp of concatenated DNA from three housekeeping genes highlighting the positions of S. plymuthica 3Rp8 and 3Re4-18 relative to their closest Serratia strains for which complete genomes are publicly available. A representative rhizosphere bacterium from the genera Pseudomonas was used as outgroup. For the construction of the tree, the protein-coding house-keeping genes gyrB (2420 bp), rpoP (4146 bp) and nusA (1511 bp) were concatenated and aligned. Then the evolutionary history was inferred by using the Maximum Likelihood method based on the Tamura-Nei model [35]. The percentage of trees in which the associated taxa clustered in the bootstrap test (1000 replicates) is shown next to the branches [36]. Evolutionary analyses were conducted in MEGA7 [37]
Fig. 4.
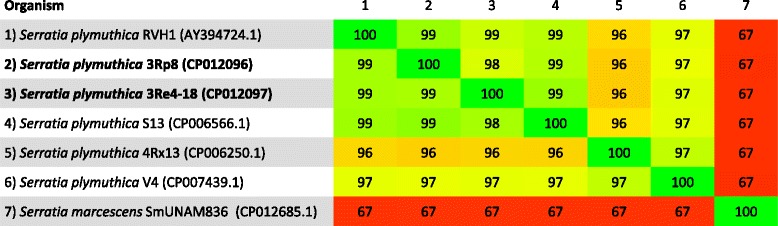
Phylogenomic overview using ANI data calculated from whole genome sequences. The heat-plot was compiled in Gegenees [6] and is based on a fragmented alignment using BLASTN made with settings 200/100 (accurate calculation). The cutoff threshold for non-conserved material was set to 30 %
Genome sequencing information
Genome project history
The strains S. plymuthica 3Rp8 and 3Re4-18 were selected for sequencing due to their in vitro activity against V. dahliae and R. solani, their production of hydrolytic enzymes and their root-associated lifestyle on plants [1, 3, 4]. The sequence data will help to reveal genetic features responsible for their plant growth promoting effects and their ability to protect seeds against fungal threats during germination. The genome project is deposited in the NCBI BioProject database under ID 289082 with the Biosample UIDs 3841799 and 3841798, respectively. The finished genome sequences are deposited in GenBank under the accession numbers CP012096 and CP012097, respectively. A summary of the project information is shown in Table 2.
Table 2.
Project information
| MIGS ID | Property | Term |
|---|---|---|
| MIGS 31 | Finishing quality | Finished |
| MIGS 28 | Libraries used | PacBio RS libraries with inserts of 8 to 20 kb |
| MIGS 29 | Sequencing platforms | PacBio RS II |
| MIGS 31.2 | Fold coverage | 3Rp8 - 81 x 3Re4-18 - 110 x |
| MIGS 30 | Assemblers | Celera Assembler + Hierarchical genome assembly process v. 2.2.0 |
| MIGS 32 | Gene calling method | NCBI Prokaryotic Genome Annotation Pipeline, Glimmer gene prediction |
| Locus Tag | 3Rp8 - ADP72 3Re4-18 - ADP73 |
|
| Genbank ID | 3Rp8 - CP012096 3Re4-18 - CP012097 |
|
| GenBank Date of Release | June 15, 2016 | |
| GOLD ID | 3Rp8 - Gp0137065 3Re4-18 - Gp0131532 |
|
| BIOPROJECT | PRJNA289082 | |
| MIGS 13 | Source Material Identifier | 3Rp8 - SAMN03841799 3Re4-18 - SAMN03841798 |
| Project relevance | Agricultural, Environmental |
Growth conditions and genomic DNA preparation
3Rp8 and 3Re4-18 were grown in 50 ml of nutrient broth II (NB II) (Sifin, Berlin, Germany) medium and incubated for 20 h at 30 °C. 0.5 ml was then centrifuged at 2500 x g for 5 min at 4 °C and genomic DNA was extracted using the MasterPure DNA purification kit (Epicentre, Madison, WI, USA). DNA quality and quantity were checked by agarose gel electrophoresis and spectrophotometry using a UV-Vis spectrophotometer (NanoDrop 2000c, Thermo Fisher Scientific, Waltham, MA USA). Total genomic DNA of 3Rp8 (50.7 μg; 0.8 μg μL-1) and of 3Re4-18 (102.8 μg; 1.7 μg μL-1) was sent on dry ice to the sequencing service.
Genome sequencing and assembly
PacBio RS libraries with inserts of 8 to 20 kb were constructed and sequenced at GATC Biotech (Konstanz, Germany) using single molecule, real-time (SMRT) sequencing. Assemblies were completed with the Hierarchical Genome Assembly Process v. 2.2.0 (HGAP) algorithm implemented in the PacBio SMRT Analysis software (Pacific Biosciences, Menlo Park, CA, USA). The assembly of the 3Rp8 genome was based on 119,662 quality reads with a mean length of 4581 bp resulting in a single circular chromosome consisting of 5,546,041 bp with 81-fold overall coverage. For assembling the genome of 3Re4-18, 127,834 quality reads with a mean length of 5358 bp were used resulting in a single circular chromosome of 5,439,574 bp with 110-fold overall coverage.
Genome annotation
Automatic annotation was performed using the NCBI Prokaryotic Genome Annotation Pipeline (released 2013). Additional annotation for using the automated assignment of clusters of orthologous groups (COG)-functions to protein-coding genes was completed on the BASys Web server using Glimmer gene prediction [7–9]. Prediction of Pfam domains, signal peptides and transmembrane helices were calculated using BASys Web Server [7–9], SignalP [10, 11] and TMHMM [12, 13], respectively.
Genome properties
The genome of S. plymuthica strain 3Rp8 is composed of one circular chromosome consisting of 5,546,041 bp with an average GC content of 56.07 % (Table 3 and Fig. 5a). Among the 5130 predicted genes, 4954 (96.57 %) were identified as protein coding genes, 68 (1.33 %) were designated as pseudo genes, 22 (0.43 %) as rRNAs, 85 (1.66 %) as tRNAs and one (0.02 %) as ncRNA. 21 (0.41 %) genes were frameshifted.
Table 3.
Genome statistics
| 3Rp8 | 3Re4-18 | |||
|---|---|---|---|---|
| Attribute | Value | % of Totala | Value | % of Totala |
| Genome size (bp) | 5,546,041 | 100.00 | 5,439,574 | 100.00 |
| DNA coding (bp) | 4,745,098 | 85.56 | 4,683,982 | 86.11 |
| DNA G + C (bp) | 3,109,696 | 56.07 | 3,058,992 | 56.24 |
| DNA scaffolds | 1 | - | 1 | - |
| Total genes | 5130 | 100.00 | 5005 | 100.00 |
| Protein coding genes | 4954 | 96.57 | 4845 | 96.80 |
| RNA genes | 108 | 2.11 | 109 | 2.18 |
| Pseudo genes | 68 | 1.33 | 51 | 1.02 |
| Genes in internal clusters | NA | - | NA | - |
| Genes with function prediction | 4278 | 83.39 | 4239 | 84.70 |
| Genes assigned to COGs | 4077 | 79.47 | 4017 | 80.26 |
| Genes with Pfam domains | 3829 | 74.64 | 3780 | 75.52 |
| Genes with signal peptides | 499 | 9.73 | 489 | 9.77 |
| Genes with transmembrane helices | 1239 | 24.15 | 1213 | 24.24 |
| CRISPR repeats | 0 | 0 | 0 | 0 |
aThe total is based on either the size of the genome in base pairs or the total number of genes in the annotated genome
Fig. 5.
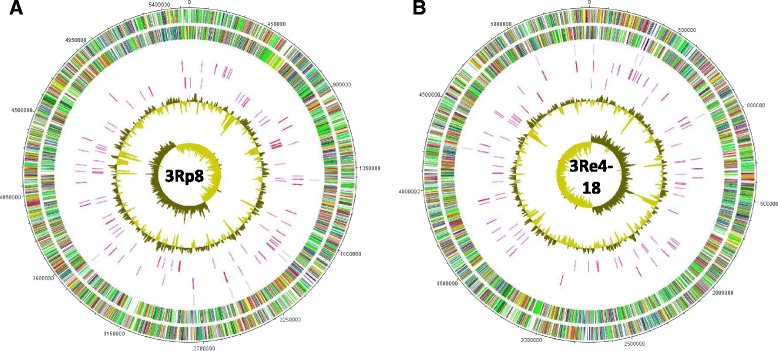
Graphical map of the chromosome of 3Rp8 (a) and 3Re4-18 (b). The outer scale is marked every 10 kb. Circles range from 1 (outer circle) to 7 (inner circle). Circle 1 and 2, ORFs encoded by leading and lagging strand respectively, with color code for functions: salmon, translation, ribosomal structure and biogenesis; aquamarine, RNA processing and modification; light blue, transcription; cyan, DNA replication, recombination and repair; tan, chromatin structure and dynamics; turquoise, cell division; dark orange, defense mechanisms; deep pink, post-translational modification, protein turnover and chaperones; dark olive green, cell envelope biogenesis; purple, cell motility and secretion; lavender, intracellular trafficking, secretion, and vesicular transport; forest green, inorganic ion transport and metabolism; pink, signal transduction; red, energy production; sienna, carbohydrate transport and metabolism; yellow, amino acid transport; orange, nucleotide transport and metabolism; gold, co-enzyme transport and metabolism; cornflower blue, lipid metabolism; blue, secondary metabolites, transport and catabolism; gray, general function prediction only; yellow green, unknown function; black, function unclassified or unknown. Circle 3 and 4, distributions of tRNA genes and rrn operons respectively. Circle 5, distribution of pseudogenes. Circle 6 and 7, G + C content and GC skew (G-C/G + C) respectively
The genome of S. plymuthica strain 3Re4-18 is composed of one circular chromosome of 5,439,574 bp with an average GC content of 56.24 % (Table 3 and Fig. 5b). Among the 5005 predicted genes, 4845 (96.80 %) were identified as protein coding genes, 51 (1.02 %) were designated as pseudo genes, 22 (0.44 %) as rRNAs, 86 (1.72 %) as tRNAs and one (0.02 %) as ncRNA. 19 (0.38 %) genes were frameshifted.
The GC contents of both strains are similar to that of other S. plymuthica strains. The classification of CDSs into functional categories according to the COG database [14, 15] is summarized in Table 4 on BASys gene prediction.
Table 4.
Number of genes associated with general COG functional categories
| Code | 3Rp8 | 3Re4-18 | Description | ||
|---|---|---|---|---|---|
| Value | %age | Value | %age | ||
| J | 169 | 2.90 | 167 | 2.97 | Translation, ribosomal structure and biogenesis |
| A | 1 | 0.02 | 1 | 0.02 | RNA processing and modification |
| K | 441 | 7.57 | 445 | 7.92 | Transcription |
| L | 170 | 2.92 | 152 | 2.70 | Replication, recombination and repair |
| B | 1 | 0.02 | 1 | 0.02 | Chromatin structure and dynamics |
| D | 27 | 0.46 | 28 | 0.50 | Cell cycle control, cell division, chromosome partitioning |
| V | 58 | 1.00 | 56 | 1.00 | Defense mechanisms |
| T | 141 | 2.42 | 146 | 2.60 | Signal transduction mechanisms |
| M | 256 | 4.40 | 256 | 4.55 | Cell wall/membrane biogenesis |
| N | 99 | 1.70 | 90 | 1.60 | Cell motility |
| U | 54 | 0.93 | 49 | 0.87 | Intracellular trafficking and secretion |
| O | 153 | 2.63 | 148 | 2.63 | Posttranslational modification, protein turnover, chaperones |
| C | 261 | 4.48 | 259 | 4.61 | Energy production and conversion |
| G | 412 | 7.08 | 406 | 7.22 | Carbohydrate transport and metabolism |
| E | 442 | 7.59 | 433 | 7.70 | Amino acid transport and metabolism |
| F | 89 | 1.53 | 90 | 1.60 | Nucleotide transport and metabolism |
| H | 144 | 2.47 | 145 | 2.58 | Coenzyme transport and metabolism |
| I | 150 | 2.58 | 138 | 2.45 | Lipid transport and metabolism |
| P | 246 | 4.22 | 246 | 4.38 | Inorganic ion transport and metabolism |
| Q | 96 | 1.65 | 91 | 1.62 | Secondary metabolites biosynthesis, transport and catabolism |
| R | 383 | 6.58 | 385 | 6.85 | General function prediction only |
| S | 284 | 4.88 | 285 | 5.07 | Function unknown |
| - | 1746 | 29.98 | 1605 | 28.55 | Not in COGs |
Insights from the genome sequence
Both strains share a collection of genes that may be important contributors to biological control with other S. plymuthica strains already published, like genes annotated as secretion systems, iron scavenging siderophores (locus tags ADP72_19185, ADP73_16995), chitinases (e.g. locus tags ADP72_04805, ADP73_00825), secreted proteases (e.g. locus tags ADP72_11930, ADP73_24375), glucanases (e.g. locus tags ADP72_10355, ADP73_00890) and non-ribosomal peptide synthetases (e.g. locus tags ADP72_05100, ADP73_05800). Additionally, genes predicting plant growth promotion, like spermidine synthases (e.g. locus tags ADP72_15170, ADP73_11985), indole-3-pyruvate decarboxylases (locus tags ADP72_18190, ADP73_17980) or diacetyl-reductase (locus tags ADP72_19475, ADP73_16745) were detected. Unique genomic islands were identified in both strains with IslandViewer 3 software [16–18]. In 3Rp8 coding regions containing high similarities on DNA-level with a region in Photorhabdus luminescens TT01 [19] as well as a region annotated as type IV/VI secretion system were found. In 3Re4-18 unique coding regions for proteins related to type VI secretion systems as well as other islands with putatively phage origin were detected.
Conclusions
Here, we announce the complete genome sequences of Serratia plymuthica 3Rp8 and 3Re4-18, two enterobacteria that were originally isolated in Germany from oilseed rape rhizosphere and from endorhiza of potato, respectively. Both strains were selected for sequencing based on their ability to control soil-borne plant-pathogenic fungi. Such properties likely have origins in a repertoire of genes probably involved in fungal cell wall degradation expressed by chitinases, proteases or non-ribosomal peptide synthetases. They also share a collection of genes known to be responsible for specific PGP features and both carry unique genomic islands with interesting genes for agricultural applications. Further functional studies and comparative genomics with related isolates will greatly enhance the understanding of biocontrol and PGP features.
Acknowledgements
The authors thank NAWI Graz for providing technical devices for confocal laser scanning microscopy, Kathrin Hölzl (Graz) for antibiotic resistance characterization of the strains, John Hunter Allan (Dundee) for growth experiments and Christin Zachow (Graz) for proofreading of the manuscript.
Funding
This Project was supported by grant id 836466 (FFG project “Novel biotechnological processes for seed and root applications”) from the Austrian Research Promotion Agency to GB, that was co-funded by Biotenzz GmbH.
Authors’ contributions
EA wrote the manuscript, participated in the design of the study and performed the statistical analysis and annotation. HM conceived the study, participated in its design and coordination, and carried out the molecular genetic experiments and the sequence alignment. AE provided the photographs and microscopic images. EA, HM, AE and GB commented on the manuscript at all stages. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Abbreviations
- MIC
Minimal inhibitory concentration
- PGP
Plant growth promoting
- SMRT
Single molecule, real-time
References
- 1.Berg G, Roskot N, Steidle A, Eberl L, Zock A, Smalla K. Plant-dependent genotypic and phenotypic diversity of antagonistic rhizobacteria isolated from different Verticillium host plants. Appl Environ Microbiol. 2002;68(7):3328–3338. doi: 10.1128/AEM.68.7.3328-3338.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winson MK, Swift S, Fish L, Throup JP, Jørgensen F, Ram S. Construction and analysis of luxCDABE-based plasmid sensors for investigating N-acyl homoserine lactone-mediated quorum sensing. FEMS Microbiol Lett. 1998;163:185–192. doi: 10.1111/j.1574-6968.1998.tb13044.x. [DOI] [PubMed] [Google Scholar]
- 3.Berg G, Krechel A, Ditz M, Sikora RA, Ulrich A, Hallmann J. Endophytic and ectophytic potato-associated bacterial communities differ in structure and antagonistic function against plant pathogenic fungi. FEMS Microbiol Ecol. 2005;51(2):215–229. doi: 10.1016/j.femsec.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Rybakova D, Schmuck M, Wetzlinger U, Varo-Sarez A, Murgu O, Müller H, et al. Kill or cure? The interaction between endophytic Paenibacillus and Serratia strains and the host plant is shaped by plant growth conditions. Plant Soil. 2015 [Google Scholar]
- 5.Smalla K, Wieland G, Buchner A, Zock A, Parzy J, Kaiser S, et al. Bulk and rhizosphere soil bacterial communities studied by denaturing gradient gel electrophoresis: plant-dependent enrichment and seasonal shifts revealed. Appl Environ Microbiol. 2001;67(10):4742–4751. doi: 10.1128/AEM.67.10.4742-4751.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ågren J, Sundström A, Håfström T, Segerman B. Gegenees: fragmented alignment of multiple genomes for determining phylogenomic distances and genetic signatures unique for specified target groups. PLoS One. 2012;7(6):e39107. doi: 10.1371/journal.pone.0039107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Domselaar GH, Stothard P, Shrivastava S, Cruz JA, Guo A, Dong X, et al. BASys: a web server for automated bacterial genome annotation. Nucleic Acids Res. 2005;33:W455–W459. doi: 10.1093/nar/gki593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delcher AL, Harmon D, Kasif S, White O, Salzberg SL. Improved microbial gene identification with GLIMMER. Nucleic Acids Res. 1999;27:4636–4641. doi: 10.1093/nar/27.23.4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salzberg SL, Delcher AL, Kasif S, White O. Microbial gene identification using interpolated Markov models. Nucleic Acids Res. 1998;26:544–548. doi: 10.1093/nar/26.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods. 2011;8(10):785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- 11.Emanuelsson O, Brunak S, von Heijne G, Nielsen H. Locating proteins in the cell using TargetP, SignalP and related tools. Nat Protoc. 2007;2:953–971. doi: 10.1038/nprot.2007.131. [DOI] [PubMed] [Google Scholar]
- 12.Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol. 2001;305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 13.TMHMM Server v. 2.0. Prediction of transmembrane helices in proteins. Center for Biological Sequence Analysis. Technical University of Denmark DTU, Lyngby. 2015. www.cbs.dtu.dk/services/TMHMM/. Accessed 22 Feb 2016.
- 14.Tatusov RL, Koonin EV, Lipman DJ. A genomic perspective on protein families. Science. 1997;278:631–637. doi: 10.1126/science.278.5338.631. [DOI] [PubMed] [Google Scholar]
- 15.Clusters of Orthologous Groups. NCBI. 2015. http://www.ncbi.nlm.nih.gov/COG. Accessed 26 Feb 2016.
- 16.Dhillon B, Chiu T, Laird M, Langille M, Brinkman F. IslandViewer update: improved genomic island discovery and visualization. Nucleic Acids Res. 2013;41(W1):129–132. doi: 10.1093/nar/gkt394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhillon B, Laird M, Shay J, Winsor G, Lo R, Nizam F, et al. IslandViewer 3: more flexible, interactive genomic island discovery, visualization and analysis. Nucleic Acids Res. 2015;43(1):W104–W108. doi: 10.1093/nar/gkv401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langille M, Brinkman F. IslandViewer: an integrated interface for computational identification and visualization of genomic islands. Bioinformatics. 2009;25(5):664–665. doi: 10.1093/bioinformatics/btp030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duchaud E, Rusniok C, Frangeul L, Buchrieser C, Givaudan A, Taourit S, et al. The genome sequence of the entomopathogenic bacterium Photorhabdus luminescens. Nat Biotechnol. 2003;21:1307–1313. doi: 10.1038/nbt886. [DOI] [PubMed] [Google Scholar]
- 20.Field D, Garrity G, Gray T, Morrison N, Selengut J, Sterk P, et al. The minimum information about a genome sequence (MIGS) specification. Nat Biotechnol. 2008;26:541–547. doi: 10.1038/nbt1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woese CR. Towards a natural system of organisms: Proposal for the domains Archea, Bacteria and Eucarya. Proc Natl Acad Sci U S A. 1990;87:4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garrity GM, Bell JA, Lilburn T. Phylum XIV. Proteobacteria phyl. nov. In: Garrity GM, Brenner DJ, Krieg NR, Staley JT, editors. Bergey’s Manual of Systematic Bacteriology, 2nd ed, Volume 2, Part B. New York: Springer; 2005: p. 1.
- 23.Garrity GM, Bell JA, Lilburn T. Class III. Gammaproteobacteria class. nov. In: Garrity GM, Brenner DJ, Krieg NR, Staley JT, editors. Bergey’s Manual of Systematic Bacteriology, 2nd ed, Volume 2, Part B. New York: Springer; 2005: p. 1.
- 24.Validation of publication of new names and new combinations previously effectively published outside the IJSEM. List no. 106. Int J Syst Evol Microbiol. 2005; 55:2235–8. [DOI] [PubMed]
- 25.Garrity GM, Holt JG. Taxonomic outline of the Archaea and Bacteria. In: Garrity GM, Boone DR, Castenholz RW, editors. Bergey’s Manual of Systematic Bacteriology. 2. New York: Springer; 2001. pp. 155–166. [Google Scholar]
- 26.Skerman VBD, McGowan V, Sneath PHA. Approved lists of bacterial names. Int J Syst Bacteriol. 1980;30:225–420. doi: 10.1099/00207713-30-1-225. [DOI] [PubMed] [Google Scholar]
- 27.Brenner DJ, Farmer JJ. Family I. Enterobacteriaceae Rahn 1937. In: Garrity GM, Brenner DJ, Krieg NR, Staley JT, editors. Bergey’s Manual of Systematic Bacteriology, 2nd ed, Volume 2, Part B. New York: Springer; 2005. p. 587.
- 28.Rahn O. New principles for the classification of bacteria. Zentralblatt für Bakteriologie, Parasitenkunde, Infektionskrankheiten und Hygiene. Abteilung II. 1937; 96:273–86.
- 29.Bizio B. Lettera di Bartolomeo Bizio al chiarissimo canonico Angelo Bellani sopra il fenomeno della polenta porporina. Biblioteca Italiana o sia Giornale di Letteratura. [Anno VIII] Scienze e Arti. 1823;30:275–295. [Google Scholar]
- 30.Grimont F, Genus GPAD, XXXIV . Serratia Bizio 1823, 288AL. In: Garrity GM, Brenner DJ, Krieg NR, Staley JT, editors. Bergey’s Manual of Systematic Bacteriology, Volume 2, Part B. 2. New York: Springer; 2005. pp. 799–811. [Google Scholar]
- 31.Breed RS, Murray EGD, Hitchens AP. In: Breed RS, Murray EGD, Hitchens AP, editors. Bergey’s Manual of Determinative Bacteriology. 6th ed. Baltimore: Williams and Wilkins Co; 1948: p. 481–2.
- 32.Neupane S, Högberg N, Alström S, Lucas S, Han J, Lapidus A, et al. Complete genome sequence of the rapeseed plant-growth promoting Serratia plymuthica strain AS9. Stand Genomic Sci. 2012;6:54–62. doi: 10.4056/sigs.2595762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zachow C, Pirker H, Westendorf C, Tilcher R, Berg G. The Caenorhabditis elegans assay: a tool to evaluate the pathogenic potential of bacterial biocontrol agents. Eur J Plant Pathol. 2009;125(3):367–376. doi: 10.1007/s10658-009-9486-3. [DOI] [Google Scholar]
- 34.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. Gene Ontology: tool for the unification of biology. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol. 1993;10:512–526. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- 36.Felsenstein J. Confidence limits on phylogenies – an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.2307/2408678. [DOI] [PubMed] [Google Scholar]
- 37.Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016. in press. [DOI] [PMC free article] [PubMed]


