Abstract
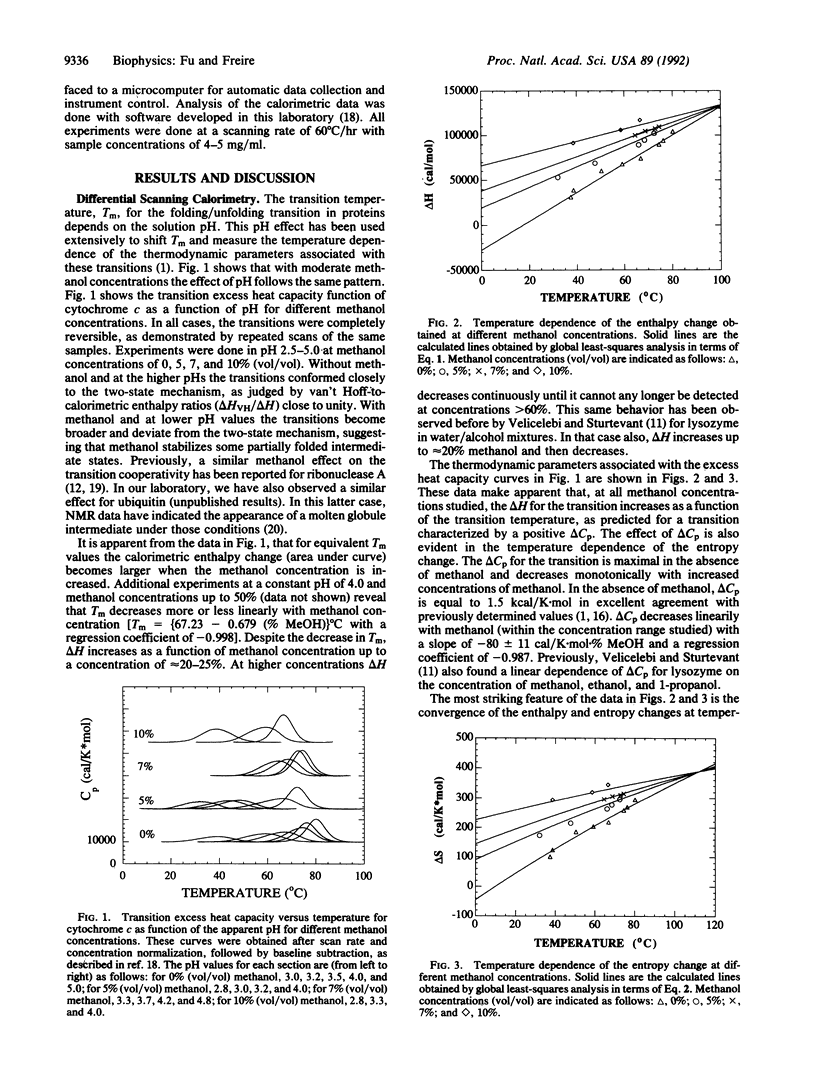
Temperature dependence of the thermodynamics of folding/unfolding for cytochrome c has been determined as a function of moderate [0-10% (vol/vol)] concentrations of methanol. Heat capacity change (delta Cp) for unfolding decreases with increased concentrations of methanol, consistent with a higher solvent hydrophobicity. For a given transition temperature, this effect results in higher experimental enthalpy (delta H) and entropy (delta S) changes with increased methanol concentrations. When the enthalpy or entropy data sets obtained at different methanol concentrations are plotted as a function of temperature, they are seen to converge and assume common values around 100 degrees C for delta H and 112 degrees C for delta S. These convergence temperatures are similar to those obtained for different proteins in aqueous solution when delta H and delta S are normalized with respect to number of residues. It has been previously hypothesized that these convergence temperatures correspond to the temperatures at which the hydrophobic contributions to delta H and delta S are zero; the results presented here agree with this viewpoint.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin R. L. Temperature dependence of the hydrophobic interaction in protein folding. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8069–8072. doi: 10.1073/pnas.83.21.8069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandts J. F., Hu C. Q., Lin L. N., Mos M. T. A simple model for proteins with interacting domains. Applications to scanning calorimetry data. Biochemistry. 1989 Oct 17;28(21):8588–8596. doi: 10.1021/bi00447a048. [DOI] [PubMed] [Google Scholar]
- Brandts J. F., Hunt L. The thermodynamics of protein denaturation. 3. The denaturation of ribonuclease in water and in aqueous urea and aqueous ethanol mixtures. J Am Chem Soc. 1967 Sep 13;89(19):4826–4838. doi: 10.1021/ja00995a002. [DOI] [PubMed] [Google Scholar]
- Fink A. L., Painter B. Characterization of the unfolding of ribonuclease A in aqueous methanol solvents. Biochemistry. 1987 Mar 24;26(6):1665–1671. doi: 10.1021/bi00380a027. [DOI] [PubMed] [Google Scholar]
- Franks F., Eagland D. The role of solvent interactions in protein conformation. CRC Crit Rev Biochem. 1975 Aug;3(2):165–219. doi: 10.3109/10409237509102556. [DOI] [PubMed] [Google Scholar]
- Freire E., Murphy K. P. Molecular basis of co-operativity in protein folding. J Mol Biol. 1991 Dec 5;222(3):687–698. doi: 10.1016/0022-2836(91)90505-z. [DOI] [PubMed] [Google Scholar]
- Freire E., Murphy K. P., Sanchez-Ruiz J. M., Galisteo M. L., Privalov P. L. The molecular basis of cooperativity in protein folding. Thermodynamic dissection of interdomain interactions in phosphoglycerate kinase. Biochemistry. 1992 Jan 14;31(1):250–256. doi: 10.1021/bi00116a034. [DOI] [PubMed] [Google Scholar]
- Harding M. M., Williams D. H., Woolfson D. N. Characterization of a partially denatured state of a protein by two-dimensional NMR: reduction of the hydrophobic interactions in ubiquitin. Biochemistry. 1991 Mar 26;30(12):3120–3128. doi: 10.1021/bi00226a020. [DOI] [PubMed] [Google Scholar]
- Johnson M. L. Evaluation and propagation of confidence intervals in nonlinear, asymmetrical variance spaces. Analysis of ligand-binding data. Biophys J. 1983 Oct;44(1):101–106. doi: 10.1016/S0006-3495(83)84281-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B. Isoenthalpic and isoentropic temperatures and the thermodynamics of protein denaturation. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5154–5158. doi: 10.1073/pnas.88.12.5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhatadze G. I., Privalov P. L. Heat capacity of proteins. I. Partial molar heat capacity of individual amino acid residues in aqueous solution: hydration effect. J Mol Biol. 1990 May 20;213(2):375–384. doi: 10.1016/S0022-2836(05)80197-4. [DOI] [PubMed] [Google Scholar]
- Murphy K. P., Gill S. J. Solid model compounds and the thermodynamics of protein unfolding. J Mol Biol. 1991 Dec 5;222(3):699–709. doi: 10.1016/0022-2836(91)90506-2. [DOI] [PubMed] [Google Scholar]
- Murphy K. P., Privalov P. L., Gill S. J. Common features of protein unfolding and dissolution of hydrophobic compounds. Science. 1990 Feb 2;247(4942):559–561. doi: 10.1126/science.2300815. [DOI] [PubMed] [Google Scholar]
- Privalov P. L., Gill S. J. Stability of protein structure and hydrophobic interaction. Adv Protein Chem. 1988;39:191–234. doi: 10.1016/s0065-3233(08)60377-0. [DOI] [PubMed] [Google Scholar]
- Privalov P. L., Khechinashvili N. N. A thermodynamic approach to the problem of stabilization of globular protein structure: a calorimetric study. J Mol Biol. 1974 Jul 5;86(3):665–684. doi: 10.1016/0022-2836(74)90188-0. [DOI] [PubMed] [Google Scholar]
- Privalov P. L., Khechinashvili N. N. Kalorimetricheskoe issledovanie teplovoi denaturatsii tsitokhroma C. Biofizika. 1974 Jan-Feb;19(1):14–18. [PubMed] [Google Scholar]
- Timasheff S. N., Inoue H. Preferential binding of solvent components to proteins in mixed water--organic solvent systems. Biochemistry. 1968 Jul;7(7):2501–2513. doi: 10.1021/bi00847a009. [DOI] [PubMed] [Google Scholar]
- Velicelebi G., Sturtevant J. M. Thermodynamics of the denaturation of lysozyme in alcohol--water mixtures. Biochemistry. 1979 Apr 3;18(7):1180–1186. doi: 10.1021/bi00574a010. [DOI] [PubMed] [Google Scholar]
- Watt G. D., Sturtevant J. M. The enthalpy change accompanying the oxidation of ferrocytochrome c in the pH range 6-11 at 25 degrees. Biochemistry. 1969 Nov;8(11):4567–4571. doi: 10.1021/bi00839a050. [DOI] [PubMed] [Google Scholar]
- Xie D., Bhakuni V., Freire E. Calorimetric determination of the energetics of the molten globule intermediate in protein folding: apo-alpha-lactalbumin. Biochemistry. 1991 Nov 5;30(44):10673–10678. doi: 10.1021/bi00108a010. [DOI] [PubMed] [Google Scholar]


