Abstract
In addition to being a rich source of several essential vitamins and minerals, mono- and polyunsaturated fatty acids, and fiber, most tree nuts provide an array of phytochemicals that may contribute to the health benefits attributed to this whole food. Although many of these constituents remain to be fully identified and characterized, broad classes include the carotenoids, hydrolyzable tannins, lignans, naphthoquinones, phenolic acids, phytosterols, polyphenols, and tocopherols. These phytochemicals have been shown to possess a range of bioactivity, including antioxidant, antiproliferative, anti-inflammatory, antiviral, and hypocholesterolemic properties. This review summarizes the current knowledge of the carotenoid, phenolic, and tocopherol content of tree nuts and associated studies of their antioxidant actions in vitro and in human studies. Tree nuts are a rich source of tocopherols and total phenols and contain a wide variety of flavonoids and proanthocyanidins. In contrast, most tree nuts are not good dietary sources of carotenoids and stilbenes. Phenolic acids are present in tree nuts but a systematic survey of the content and profile of these compounds is lacking. A limited number of human studies indicate these nut phytochemicals are bioaccessible and bioavailable and have antioxidant actions in vivo.
Keywords: tree nuts, phytochemicals, flavonoids, resveratrol, antioxidants
INTRODUCTION
Tree nuts appear to have an impact on antioxidant status1,2 and inflammatory pathways3,4 that may underlie, in part, their putative benefits in reducing the risk of cardiovascular disease,5,6 type 2 diabetes,7,8 and some types of cancer.9 Evidence for this relationship has been sufficiently compelling that nuts have been incorporated into recommended dietary guidelines in the United States, Canada, and Spain10 and a qualified health claim for reducing the risk of heart disease is allowed by the U.S. Food and Drug Administration.11 As the nutrient composition of different tree nuts can vary markedly, there is unlikely to be a single or even a few constituents that contribute principally to these outcomes. Thus, it would be worthwhile to consider the potential interactions between the essential nutrients (including amino acids, fatty acids, minerals, and vitamins), fiber, and phytochemicals (including carotenoids, phytosterols, and polyphenols like flavonoids and stilbenes) most commonly present in tree nuts.12 Recently, several review articles have been published describing the health benefits and related aspects of tree nuts.1,2,7,13,14 This review focuses on carotenoids, tocopherols, and the polyphenols found in tree nuts and their impact on antioxidant capacity.
CAROTENOIDS
Carotenoids are polyisoprenoids with differing degrees of conjugated double bonds. They are abundant in most colorful plant foods, e.g., with 7.6 and 6.6 mg/100 g β-carotene and lutein found in carrots and spinach, respectively. In contrast, tree nuts contain very low amounts of carotenoids with α- and β-carotene, β-cryptoxanthin, lutein, and zeaxanthin found in μg/100 g concentrations when present at all. Pistachios present a modest exception with a mean β-carotene and lutein content of 0.21 and 4.4 mg/100 g dry weight, respectively.15
TOCOPHEROLS
Tree nuts are among the richest sources of vitamin E, the principal lipid-soluble vitamin, along with seeds and vegetable oils. Table 1 lists the α- and γ-tocopherol content of tree nuts. Traces of δ-tocopherol at <0.5 mg/100 g (except for walnuts at <4 mg/100 g extracted oil) are found in many tree nuts. Barreira et al.16 report the presence of tocotrienols in chestnuts, particularly γ-tocotrienol at 0.03 mg/100 g. Kornsteiner et al.15 report a several-fold range in vitamin E content of some types of tree nuts, though it is not clear whether this result is from differences in the harvest year, source, variety or agricultural and environmental factors. It is noteworthy that the absorption of vitamin E is influenced by the amount of fat in a meal or snack and the food matrix,17 making nuts with their high fat content, a particularly bioavailable food source. Jambazian et al18 found increasing almond intake from 0 to 10 and 20% energy significantly elevated plasma α-tocopherol status by 12 and 15%, respectively.
Table 1.
| Tree Nut | α-Tocopherol | γ-Tocopherol |
|---|---|---|
| Almonds | 25.9 | 0.9 |
| Brazils | 5.7 | 7.9 |
| Cashews | 0.9 | nd† |
| Chestnuts | 0.005 | 0.4 |
| Hazelnuts | 15.0 | nd |
| Macadamia | 0.6 | nd |
| Pecans | 4.1 | 8.1 |
| Pines | 9.3 | 11.2 |
| Pistachios | 1.9 | 22.5 |
| Walnuts | 0.7 | 20.8 |
nd: not detected
POLYPHENOLS
The classes of polyphenols identified in tree nuts include anthocyanins, flavonoids, lignans, naphthoquinones, phenolic acids, proanthocyanidins, stilbenes, and hydrolyzable tannins. However, a substantial number of nut polyphenols remain to be identified. Importantly, the profiles and content of polyphenols are affected not only by the type of nut and its cultivar but also the harvest year, orchard location, processing steps, and storage. The dietary sources and intake of polyphenols is of interest because of their putative contribution to health promotion and the reduction of risk of chronic disease. However, limited information is available regarding the intake of polyphenols from tree nuts. In a Spanish population, Saura-Calixto et al.19 estimated an intake of 102-121 mg polyphenols from daily consumption of 5.9 g nuts. They calculated that 107 mg of these polyphenols were bioaccessible. Almond polyphenols have been found to be bioaccessible, bioavailable, and subsequently metabolized by phase 2 conjugating enzymes and gut microflora.20 Various studies suggest that it is the biotransformed polyphenols that are the bioactive compounds.
Total phenols
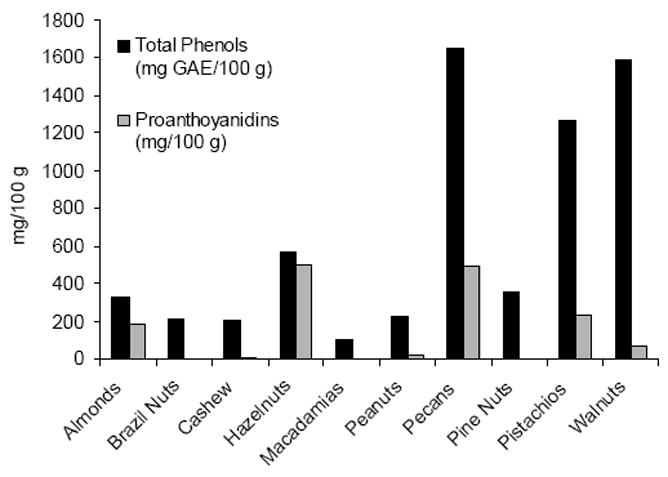
The Folin-Ciocalteu reaction has been widely used to estimate of the content of total phenols in plant foods, including nuts. The phenolic content of nuts ranges from 103-1650 mg Gallic Acid Equivalents (GAE)/100 g, with pecans, walnuts, and pistachios having the highest values15,21 (Figure 1). The total phenols in nuts are within the range of fruit such as blueberries (531 mg GAE/100 g), plums (367 mg GAE/100 g), and raisins (1065 mg GAE/100 g).22
Figure 1.
Proanthocyanidins
Proanthocyanidins are flavan-3-ol oligomers linked through carbon-carbon bonds. Type A proanthocyanidins consist of a C4 to C6 or C8 interflavan bond and a C2-ether bond to the flavanol extension. Type B proanthocyanidins have single C4 to C6 or C8 interflavan bonding. Nut proanthocyanidins mainly consist of (+)-catechin and (-)-epicatechin, but also include afzelechin (almonds and peanuts) and epigallocatechin (hazelnuts, pecans, pistachios).23-25 To date, the A-type proanthocyanidins have been found only in almonds and peanuts. The majority of nut proanthocyanidins are highly polymerized (>10-mers).24 However cashews and peanuts contain proanthocyanidins of 1-4 mers.24,26 Proanthocyanidins are the predominant polyphenols in almonds, hazelnuts, peanuts, pecans, and pistachios. Hazelnuts and pecans have the highest proanthocyanidin content with 501 and 494 mg/100 g, respectively.24 No proanthocyanidins have been reported in Brazil nuts, macadamias or pine nuts.24 Roasting generally decreases the proanthocyanidin content of nuts. Roasting decreased peanut skin proanthocyanidin monomers, trimers, and tetramers 54-29%, but increased monomers 29% from raw skin.26 Toasting pistachios decreases their proanthocyanidin to 12% of that found in the raw nut.27
Flavonoids
Flavonoids have been identified in all nuts except macadamias and Brazil nuts. Flavan-3-ols, flavonols, and anthocyanins are the main flavonoids in nuts, with flavanones and isoflavones found in lesser amounts (Table 2). Pecans have the highest total flavonoid content among nuts at 34 mg/100 g, consisting mostly of flavan-3-ols and anthocyanins.28 Hazelnuts and almonds also have an appreciable content of flavonoids with 18 and 15 mg/100 g, respectively. To date, flavanones have only been found in almonds. Almonds and pistachios are the only nuts that contain flavonols, mainly as isorhamnetin and quercetin. Pistachios have the highest isoflavone content of nuts at 3.63 mg/100 g, more than 100-fold greater than levels of other nuts; in contrast, peanuts contain 0.26 mg isoflavones/100 g.29
Table 2.
Flavonoid content of nuts (mg/100 g)28
| Tree Nut | Flavan-3-ols | Flavanones | Flavonols | Anthocyanins | Isoflavones | Sum |
|---|---|---|---|---|---|---|
| Almonds | 4.47 | 0.38 | 7.93 | 2.46 | 0.01 | 15.25 |
| Brazils | nd† | nd | nd | nd | nd | nd |
| Cashews | 1.98 | nd | nd | nd | 0.01 | 1.99 |
| Chestnuts | 0.01 | nd | nd | nd | -‡ | 0.01 |
| Hazelnuts | 5.25 | nd | nd | 6.71 | 0.03 | 11.99 |
| Macadamias | nd | nd | nd | nd 0 | - | nd |
| Peanut | 0.66 | nd | nd | nd | 0.26 | 0.68 |
| Pecans | 15.99 | nd | nd | 18.02 | nd | 34.01 |
| Pines | 0.49 | nd | nd | nd | - | 0.49 |
| Pistachios | 6.85 | nd | 1.46 | 6.06 | 3.63 | 18.00 |
| Walnuts (English) | nd | nd | nd | 2.71 | 0.03 | 2.74 |
nd, not detected.
- not determined
Resveratrol
The stilbene resveratrol is found in pistachios and peanuts at comparable levels. Resveratrol in peanuts ranges from 3 to 192 μg/100 g, with Cerezlik cultivars as the highest producers.30 In pistachio, resveratrol content ranges from 9 to 167 μg/100 g, with the most produced in Ohadi and Siirt cultivars.30 In comparison, the resveratrol content of red wine ranges from 98 to 1800 μg/100 mL.31 Recent research suggests that resveratrol may contribute to neuro-protection, immune regulation, and cancer chemoprevention.32
Other Phenolic Compounds
Tree nuts contain a variety of other phenolic compounds which may contribute their bioactivity. Phenolic acids are present in almonds33 but a systematic survey of the content and profile of these compounds in nuts is lacking. Walnuts contain considerable amounts of syringic acid (34 mg/100 g) and the naphthoquinone juglone (12 mg/100 g).34 Ellagitannins are also found in walnut, hazelnut, and cashews.35 Nuts also contain lignans, which have phytoestrogenic activity. Pistachios have the highest amount of lignans among nuts, with 0.2 mg/100 g.36
ANTIOXIDANT CAPACITY OF TREE NUTS IN VITRO
In vitro assessment of the antioxidant capacity of tree nuts has largely been conducted by examining the ability of extracts to increase the resistance of human plasma or low density lipoprotein (LDL) to oxidation. Extracts of walnuts,37 almond and almond skins,38-40 pistachios,27 and hazelnuts41 have been found to increase the lag time to oxidation of LDL. Walnut extracts have been reported to inhibit lipid peroxidation reactions as well in human plasma.37 Pistachios have also been shown to inhibit lipid peroxidation in bovine liver microsomes.27 Protection of DNA from oxidative injury has also been demonstrated in vitro for extracts of almonds40 and hazelnuts.41
Several in vitro assays that are particularly sensitive to polyphenols have been developed to assess “total antioxidant capacity” in foods. Four assays have been applied to testing tree nuts, the Oxygen Radical Absorbance Capacity (ORAC), Ferric Reducing Antioxidant Power (FRAP), Total Radical-trapping Antioxidant Parameter (TRAP), and Trolox Equivalent Antioxidant Capacity (TEAC) (Table 3). The relevance of these assays in whole foods to in vivo antioxidant actions is not always clear as they cannot account for factors such as bioaccessibility, bioavailability, metabolism, and distribution of the phytochemical ingredients. However, these assays may prove useful in experiments directed to the understanding the potential interactions between nut components and constituents and other nutrients.
Table 3.
| Tree Nut | ORAC (L + H) † μmol TE‡/g | FRAP μmol Fe2+/g | TRAP μmol TE/g | TEAC μmol TE/g |
|---|---|---|---|---|
| Almonds | 44.5 | 41.3 | 6.3 | 13.4 |
| Brazils | 14.2 | -§ | - | - |
| Cashews | 20.0 | - | - | - |
| Hazelnuts | 96.5 | 42.3 | 6.9 | 12.0 |
| Macadamias | 17 | - | - | - |
| Pecans | 179.4 | - | - | - |
| Pines | 7.2 | 13.4 | 1.5 | 5.3 |
| Pistachios | 79.8 | 192.7 | 25.9 | 61.5 |
| Walnuts | 135.4 | 453.9 | 31.9 | 137.0 |
Lipophilic (L) and hydrophilic (H) ORAC assay values combined.
Trolox Equivalents.
not determined.
ANTIOXIDANT ACTIONS OF TREE NUTS IN HUMAN STUDIES
Data from large observational studies show that regular nut consumption is associated with a reduced risk of several conditions in which oxidative stress may play a role, including coronary heart disease,42-44 hypertension,45 type 2 diabetes,46 inflammation and endothelial dysfunction.3,47 To date, no observational studies have directly correlated nut intake with plasma total antioxidant activity or biomarkers of oxidative stress as these measures are not routinely assessed in large cohorts.
The acute bioavailability of polyphenols from both walnuts and almonds, as well as a concomitant reduction in plasma lipid peroxidation and increased antioxidant capacity following their consumption, was demonstrated by Torabian et al.48 in a randomized, placebo-controlled crossover study of 13 healthy adults. The total phenolic content of plasma significantly increased 30 min after consuming either 81 g walnuts or 91 g almonds (blended in water), while no changes were observed following an isocaloric control meal. Peak phenolic concentrations coincided with a significant reduction at 90 min in plasma thiobarbituric acid reactive substances, a measure of lipid peroxidation. Values from the FRAP and both lipophilic and hydrophilic ORAC assays also increased significantly following the consumption of both nut meals, with peak capacity observed at 150 min. No changes in plasma total antioxidant capacity were observed following the control meal.
The effects of nut consumption on antioxidant status and biomarkers of oxidative stress have been reported in a limited number of human intervention studies. Lopez-Uriarte et al.2 and Ros13 reviewed a total of 21 clinical studies in which the potential effects of tree nuts on biomarkers of oxidation or antioxidant activity were evaluated. As most of these studies used whole nuts, rather than nut components (such as the skins which contain much of the polyphenol content or the kernels where the tocopherols are found), or their individual phytochemical constituents, the contribution of these bioactive compounds to these results is unknown. However, when the reported outcomes of these nut studies are considered, it is clear that their effects cannot be due entirely to their polyunsaturated fatty acid (PUFA) or monounsaturated fatty acid (MUFA) content alone.
Early intervention studies comparing a walnut-enriched, high PUFA diet with a walnut-free, lower PUFA diet showed no differences in the prevention of LDL oxidation49-52 or total antioxidant capacity53 between diets. While the shift to more PUFA in the diet and plasma with walnut consumption could be predicted to increase plasma biomarkers of lipid peroxidation, the absence of an effect may be due to the concurrent intake and bioavailability of walnut phytochemicals, including phenolic compounds and tocopherols. More recently, Davis et al.54 reported no change in plasma antioxidant capacity following the consumption of 63-108 g/d walnuts or cashews for 8 wk in metabolic syndrome patients. Interestingly, a significant reduction in erythrocyte lipid peroxidation was observed in subjects at increased risk for cardiovascular disease following a diet with 21.4 g/d walnuts for 5 wk, when compared to a control diet,55 but this effect was modulated according to the subjects’ particular paraoxonase (PON1) polymorphism.56
According to Ros,13 the available evidence suggests that while PUFA-rich nuts confer a neutral or minimal effect on oxidative status, the effects of MUFA-rich nuts are more moderate. Indeed, Fito et al.57 reported a significant reduction in circulating oxidized LDL levels among asymptomatic adults, age 55-80 y, 3 mo after consuming a Mediterranean diet including 30 g/d whole nuts mixed at 50% walnuts, 25% almonds, and 25% hazelnuts. Moreover, chronic feeding studies using low PUFA nuts, including pecans,58 hazelnuts,59 macadamia nuts,60 pistachios,61 almonds,62,63 and brazil nuts64 all showed either an improvement in oxidation status or increased antioxidant enzyme activity. It is plausible that with less PUFA intake, the need to protect this oxidizable substrate is reduced and a higher proportion of the nut bioactives are available for other functions.
Other studies show the antioxidant effects of nuts are not limited to reduced lipid peroxidation and improved plasma antioxidant capacity. Jenkins et al.65 reported a significant postprandial increase in serum protein thiol concentrations, reflecting less oxidative damage to proteins, following a meal with 60 g almonds in a study of 15 healthy young adults. Jia et al.66 and Li et al.67 both reported significant reductions in oxidative DNA damage among smokers following 4 wk of almond consumption at 84 g/d. Li et al.67 evaluated the effects of almonds vs. pork (120 g/d) in a cohort of 60 male smokers serving in the Chinese army, and compared these effects with 30 nonsmokers who consumed the control (pork) diet. After 4 wk, the amount of DNA strand breaks and urinary 8-hydroxy-deoxyguanosine were significantly lower in the almond-supplemented smokers, compared with the pork-supplemented smokers. Significantly lower urinary malondialdehyde, a biomarker of lipid peroxidation, and higher serum α-tocopherol status and activities of super-oxide dismutase and glutathione peroxidase were also observed in the almond group. No changes in these biomarkers of oxidative stress were observed in the pork-supplemented nonsmokers. Although the authors did not measure the specific contribution of the nut polyphenols or antioxidants to these outcomes, this study does suggest that almonds confer an antioxidant benefit.
SUMMARY
Tree nuts are a complex whole food containing an array of essential nutrients as well as phytochemicals, including carotenoids, polyphenols, and tocopherols that possess antioxidant functions as well as other bioactivity. These compounds may contribute to the health benefits associated with nut consumption but elucidating this relationship requires additional research that characterizes their content as well as the impact of agricultural practices and food processing in addition to human studies investigating their bioaccessibility, bioavailability, metabolism, and functional impact in vivo.
Acknowledgments
Support was provided by the US Department of Agriculture (USDA) Agricultural Research Service under Cooperative Agreement No. 58-1950-7-707. Dr Bolling was supported by award K12GM074869 from the National Institute of Medical Sciences.
Footnotes
AUTHOR DISCLOSURES
The authors declare no conflicts of interest.
References
- 1.Chen CY, Lapsley K, Blumberg JB. A nutrition and health perspective on almonds. J Sci Food Agric. 2006;86:2245–50. [Google Scholar]
- 2.Lopez-Uriarte P, Bullo M, Casas-Agustench P, Babio N, Salas-Salvado J. Nuts and oxidation: a systematic review. Nutr Rev. 2009;67:497–508. doi: 10.1111/j.1753-4887.2009.00223.x. [DOI] [PubMed] [Google Scholar]
- 3.Jiang R, Jacobs DR, Jr, Mayer-Davis E, Szklo M, Herrington D, Jenny NS, Kronmal R, Barr RG. Nut and seed consumption and inflammatory markers in the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2006;163:222–31. doi: 10.1093/aje/kwj033. [DOI] [PubMed] [Google Scholar]
- 4.Rajaram S, Connell KM, Sabaté J. Effect of almond-enriched high-monounsaturated fat diet on selected markers of inflammation: a randomised, controlled, crossover study. Br J Nutr. doi: 10.1017/S0007114509992480. (In press) [DOI] [PubMed] [Google Scholar]
- 5.Cortes B, Nunez I, Cofan M, Gilabert R, Perez-Heras A, Casals E, Deulofeu R, Ros E. Acute effects of high-fat meals enriched with walnuts or olive oil on postprandial endothelial function. J Am Coll Cardiol. 2006;48:1666–71. doi: 10.1016/j.jacc.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 6.Kris-Etherton PM, Hu FB, Ros E, Sabaté J. The role of tree nuts and peanuts in the prevention of coronary heart disease: multiple potential mechanisms. J Nutr. 2008;138:1746S–51S. doi: 10.1093/jn/138.9.1746S. [DOI] [PubMed] [Google Scholar]
- 7.Jenkins DJA, Hu FB, Tapsell LC, Josse AR, Kendall CWC. Possible benefit of nuts in type 2 diabetes. J Nutr. 2008;138:1752S–6S. doi: 10.1093/jn/138.9.1752S. [DOI] [PubMed] [Google Scholar]
- 8.Casas-Agustench P, López-Uriarte P, Bullo M, Ros E, Cabré-Vila JJ, Salas-Salvadó J. Effects of one serving of mixed nuts on serum lipids, insulin resistance and inflammatory markers in patients with the metabolic syndrome. Nutr Metab Cardiovasc Dis. doi: 10.1016/j.numecd.2009.08.005. (In press) [DOI] [PubMed] [Google Scholar]
- 9.Jenab M, Ferrari P, Slimani N, Norat T, Casagrande C, Overad K, et al. Association of nut and seed intake with colorectal cancer risk in the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev. 2004;13:1595–603. [PubMed] [Google Scholar]
- 10.Sabate J, Ros E, Salas-Salvadó J. Nuts: nutrition and health outcomes. Br J Nutr. 2006;96:S1–S2. doi: 10.1017/bjn20061857. [DOI] [PubMed] [Google Scholar]
- 11.US Food and Drug Administration. Food labeling: health claims: nuts & heart disease. Washington, DC: US FDA; 2003. (Federal Register Docket No 02P-0505) [Google Scholar]
- 12.Allen LH. Priority areas for research on the intake, composition, and health effects of tree nuts and peanuts. J Nutr. 2008;138:1763S–5S. doi: 10.1093/jn/138.9.1763S. [DOI] [PubMed] [Google Scholar]
- 13.Ros E. Nuts and novel biomarkers of cardiovascular disease. Am J Clin Nutr. 2009;89:1649S–56S. doi: 10.3945/ajcn.2009.26736R. [DOI] [PubMed] [Google Scholar]
- 14.Raw D, Lockwood B. The health benefits of nuts. Nutra Foods. 2009;8:7–14. [Google Scholar]
- 15.Kornsteiner M, Wagner K-H, Elmadfa I. Tocopherols and total phenolics in 10 different nut types. Food Chem. 2006;98:381–7. [Google Scholar]
- 16.Barreira JC, Alves RC, Casal S, Ferreira IC, Oliveira MB, Pereira JA. Vitamin E profile as a reliable authenticity discrimination factor between chestnut (Castanea sativa Mill.) cultivars. J Agric Food Chem. 2009;57:5524–8. doi: 10.1021/jf900435y. [DOI] [PubMed] [Google Scholar]
- 17.Jeanes YM, Hall WL, Ellard S, Lee E, Lodge JK. The absorption of vitamin E is influenced by the amount of fat in a meal and the food matrix. Br J Nutr. 2004;92:575–9. doi: 10.1079/bjn20041249. [DOI] [PubMed] [Google Scholar]
- 18.Jambazian PR, Haddad E, Rajaram S, Tanzman J, Sabatè J. Almonds in the diet simultaneously improve plasma α-tocopherol concentrations and reduce plasma lipids. J Am Diet Assoc. 2005;105:449–54. doi: 10.1016/j.jada.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Saura-Calixto F, Serrano J, Goni I. Intake and bioaccessibility of total polyphenols in a whole diet. Food Chem. 2007;101:492–501. [Google Scholar]
- 20.Urpi-Sarda M, Garrido I, Monagas M, Gómez-Cordovés C, Medina-Remón A, Andres-Lacueva C, Bartolomé B. Profile of plasma and urine metabolites after the intake of almond [Prunus dulcis (Mill.) D.A. Webb] polyphenols in humans. J Agric Food Chem. 2009;57:10134–42. doi: 10.1021/jf901450z. [DOI] [PubMed] [Google Scholar]
- 21.Wu X, Beecher GR, Holden JM, Haytowitz DB, Gebhardt SE, Prior RL. Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. J Agric Food Chem. 2004;52:4026–37. doi: 10.1021/jf049696w. [DOI] [PubMed] [Google Scholar]
- 22. [2009/12/15];USDA Oxygen Radical Absorbance capacity (ORAC) of Selected Foods, 2007. 2007 Nov; Available from: http://www.ars.usda.gov/SP2UserFiles/-Place/12354500/Data/ORAC/ORAC07.pdf.
- 23.Gu L, Kelm MA, Hammerstone JF, Zhang Z, Beecher G, Holden J, Haytowitz D, Prior RL. Liquid chromatographic/ electrospray ionization mass spectrometric studies of proanthocyanidins in foods. J Mass Spectrom. 2003;38:1272–80. doi: 10.1002/jms.541. [DOI] [PubMed] [Google Scholar]
- 24.Gu L, Kelm MA, Hammerstone JF, Beecher G, Holden J, Haytowitz D, Gebhardt S, Prior RL. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J Nutr. 2004;134:613–7. doi: 10.1093/jn/134.3.613. [DOI] [PubMed] [Google Scholar]
- 25.Monagas M, Garrido I, Lebrón-Aguilar R, Bartolome B, Gómez-Cordovés C. Almond (Prunus dulcis (Mill.) D.A. Webb) skins as a potential source of bioactive polyphenols. J Agric Food Chem. 2007;55:8498–507. doi: 10.1021/jf071780z. [DOI] [PubMed] [Google Scholar]
- 26.Yu J, Ahmedna M, Goktepe I, Dai J. Peanut skin procyanidins: composition and antioxidant activities as affected by processing. J Food Comp Anal. 2006;19:364–71. [Google Scholar]
- 27.Gentile C, Tesoriere L, Butera D, Fazzari M, Monastero M, Allegra M, Livrea MA. Antioxidant activity of Sicilian pistachio (Pistacia vera L. var. Bronte) nut extract and its bioactive components. J Agric Food Chem. 2007;55:643–8. doi: 10.1021/jf062533i. [DOI] [PubMed] [Google Scholar]
- 28. [2009/12/15];USDA Database for the Flavonoid Content of Selected Foods, Release 2.1, 2007. 2007 Jan 31; Available from: http://www.nal.usda.gov/fnic/foodcomp/-Data/Flav/Flav02-1.pdf.
- 29. [2009/12/15];USDA Database for the Isoflavone Content of Selected Foods, Release 2.0, 2008. 2008 Sep 23; Available from: http://www.ars.usda.gov/SP2UserFiles/-Place/12354500/Data/isoflav/Isoflav_R2.pdf.
- 30.Tokusoglu O, Unal MK, Yemis F. Determination of the phytoalexin resveratrol (3,5,4’-trihydroxystilbene) in peanuts and pistachios by high-performance liquid chromatographic diode array (HPLC-DAD) and gas chromatography (GC-MS) J Agric Food Chem. 2005;53:5003–9. doi: 10.1021/jf050496+. [DOI] [PubMed] [Google Scholar]
- 31.Burns J, Yokota T, Ashihara H, Lean MEJ, Crozier A. Plant foods and herbal sources of resveratrol. J Agric Food Chem. 2002;50:3337–40. doi: 10.1021/jf0112973. [DOI] [PubMed] [Google Scholar]
- 32.Pervaiz S, Holme AL, Aggarwal BB, Anekonda TS, Baur JA, Gojkovic-Bukarica L, Ragione FD, Kim AL, Pirola L, Saiko P. Resveratrol: its biological targets and functional activity. Antioxid Redox Signal. 2009;11:2851–97. doi: 10.1089/ars.2008.2412. [DOI] [PubMed] [Google Scholar]
- 33.Milbury PE, Chen CY, Donikowski GG, Blumberg JB. Determination of flavonoids and phenolics and their distribution in almonds. J Agric Food Chem. 2006;54:5027–33. doi: 10.1021/jf0603937. [DOI] [PubMed] [Google Scholar]
- 34.Colaric M, Veberic R, Solar A, Hudina M, Stampar F. Phenolic acids, syringaldehyde, and juglone in fruits of different cultivars of Juglans regia L. J Agric Food Chem. 2005;53:6390–6. doi: 10.1021/jf050721n. [DOI] [PubMed] [Google Scholar]
- 35.Clifford MN, Scalbert A. Ellagitannins- nature, occurrence and dietary burden. J Sci Food Agric. 2000;80:1118–25. [Google Scholar]
- 36.Thompson LU, Boucher BA, Liu Z, Cotterchio M, Kreiger N. Phytoestrogen content of foods consumed in Canada, including isoflavones, lignans, and coumestan. Nutr Cancer. 2006;54:184–201. doi: 10.1207/s15327914nc5402_5. [DOI] [PubMed] [Google Scholar]
- 37.Anderson KJ, Teuber SS, Gobeille A, Cremin P, Waterhouse AL, Steinberg FM. Walnut polyphenolics inhibit in vitro human plasma and LDL oxidation. J Nutr. 2001;131:2837–42. doi: 10.1093/jn/131.11.2837. [DOI] [PubMed] [Google Scholar]
- 38.Chen CY, Milbury PE, Lapsley K, Blumberg JB. Flavonoids from almond skins are bioavailable and act synergistically with vitamins C and E to enhance hamster and human LDL resistance to oxidation. J Nutr. 2005;135:1366–73. doi: 10.1093/jn/135.6.1366. [DOI] [PubMed] [Google Scholar]
- 39.Chen CY, Milbury PE, Chung SK, Blumberg J. Effect of almond skin polyphenolics and quercetin on human LDL and apolipoprotein B-100 oxidation and conformation. J Nutr Biochem. 2007;18:785–94. doi: 10.1016/j.jnutbio.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 40.Shahidi F, Alasalvar C, Liyana-Pathirana CM. Antioxidant phytochemicals in hazelnut kernel (Corylus avellana L.) and hazelnut byproducts. J Agric Food Chem. 2007;55:1212–20. doi: 10.1021/jf062472o. [DOI] [PubMed] [Google Scholar]
- 41.Wijeratne SS, Abou-Zaid MM, Shahidi F. Antioxidant polyphenols in almond and its coproducts. J Agric Food Chem. 2006;54:312–8. doi: 10.1021/jf051692j. [DOI] [PubMed] [Google Scholar]
- 42.Hu FB, Stampfer MJ. Nut consumption and risk of coronary heart disease: a review of epidemiologic evidence. Curr Atheroscler Rep. 1999;1:204–9. doi: 10.1007/s11883-999-0033-7. [DOI] [PubMed] [Google Scholar]
- 43.Fraser GE. Nut consumption, lipids, and risk of a coronary event. Clin Cardiol. 1999;22(7 Suppl):III11–5. doi: 10.1002/clc.4960221504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kris-Etherton PM, Zhao G, Binkoski AE, Coval SM, Etherton TD. The effects of nuts on coronary heart disease risk. Nutr Rev. 2001;59:103–11. doi: 10.1111/j.1753-4887.2001.tb06996.x. [DOI] [PubMed] [Google Scholar]
- 45.Djousse L, Rudich T, Gaziano JM. Nut consumption and risk of hypertension in US male physicians. Clin Nutr. 2009;28:10–4. doi: 10.1016/j.clnu.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang R, Manson JE, Stampfer MJ, Liu S, Willett WC, Hu FB. Nut and peanut butter consumption and risk of type 2 diabetes in women. JAMA. 2002;288:2554–60. doi: 10.1001/jama.288.20.2554. [DOI] [PubMed] [Google Scholar]
- 47.Nettleton JA, Steffen LM, Mayer-Davis EJ, Jenny NS, Jiang R, Herrington DM, Jacobs DR., Jr Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Clin Nutr. 2006;83:1369–79. doi: 10.1093/ajcn/83.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Torabian S, Haddad E, Rajaram S, Banta J, Sabate J. Acute effect of nut consumption on plasma total polyphenols, antioxidant capacity and lipid peroxidation. J Hum Nutr Diet. 2009;22:64–71. doi: 10.1111/j.1365-277X.2008.00923.x. [DOI] [PubMed] [Google Scholar]
- 49.Zambon D, Sabate J, Munoz S, Campero B, Casals E, Merlos M, Laguna JC, Ros E. Substituting walnuts for monounsaturated fat improves the serum lipid profile of hypercholesterolemic men and women. A randomized crossover trial. Ann Intern Med. 2000;132:538–46. doi: 10.7326/0003-4819-132-7-200004040-00005. [DOI] [PubMed] [Google Scholar]
- 50.Ros E, Nunez I, Perez-Heras A, Serra M, Gilabert R, Casals E, Deulofeu R. A walnut diet improves endothelial function in hypercholesterolemic subjects: a randomized crossover trial. Circulation. 2004;109:1609–14. doi: 10.1161/01.CIR.0000124477.91474.FF. [DOI] [PubMed] [Google Scholar]
- 51.Iwamoto M, Imaizumi K, Sato M, Hirooka Y, Sakai K, Takeshita A, Kono M. Serum lipid profiles in Japanese women and men during consumption of walnuts. Eur J Clin Nutr. 2002;56:629–37. doi: 10.1038/sj.ejcn.1601400. [DOI] [PubMed] [Google Scholar]
- 52.Munoz S, Merlos M, Zambon D, Rodriguez C, Sabate J, Ros E, Laguna JC. Walnut-enriched diet increases the association of LDL from hypercholesterolemic men with human HepG2 cells. J Lipid Res. 2001;42:2069–76. [PubMed] [Google Scholar]
- 53.Tapsell LC, Gillen LJ, Patch CS, Batterham M, Owen A, Bare M, Kennedy M. Including walnuts in a low-fat/modified-fat diet improves HDL cholesterol-to-total cholesterol ratios in patients with type 2 diabetes. Diabetes Care. 2004;27:2777–83. doi: 10.2337/diacare.27.12.2777. [DOI] [PubMed] [Google Scholar]
- 54.Davis L, Stonehouse W, Loots DT, Mukuddem-Petersen J, van der Westhuizen FH, Hanekom SM, Jerling JC. The effects of high walnut and cashew nut diets on the antioxidant status of subjects with metabolic syndrome. Eur J Nutr. 2007;46:155–64. doi: 10.1007/s00394-007-0647-x. [DOI] [PubMed] [Google Scholar]
- 55.Canales A, Benedi J, Nus M, Librelotto J, Sánchez-Montero JM, Sánchez-Muniz FJ. Effect of walnut-enriched restructured meat in the antioxidant status of overweight/obese senior subjects with at least one extra CHD-risk factor. J Am Coll Nutr. 2007;26:225–32. doi: 10.1080/07315724.2007.10719605. [DOI] [PubMed] [Google Scholar]
- 56.Nus M, Frances F, Librelotto J, Canales A, Corella D, Sanchez-Montero JM, Sanchez-Muniz FJ. Arylesterase activity and antioxidant status depend on PON1-Q192R and PON1-L55M polymorphisms in subjects with increased risk of cardiovascular disease consuming walnut-enriched meat. J Nutr. 2007;137:1783–8. doi: 10.1093/jn/137.7.1783. [DOI] [PubMed] [Google Scholar]
- 57.Fito M, Guxens M, Corella D, Saez G, Estruch R, de la Torre R, et al. PREDIMED Study Investigators. Effect of a traditional Mediterranean diet on lipoprotein oxidation. Arch Intern Med. 2007;167:1195–203. doi: 10.1001/archinte.167.11.1195. [DOI] [PubMed] [Google Scholar]
- 58.Haddad E, Jambazian P, Karunia M, Tanzman J, Sabate J. A pecan-enriched diet increases γ-tocopherol/cholesterol and decreases thiobarbituric acid reactive substances in plasma of adults. Nutr Res. 2006;26:397–402. [Google Scholar]
- 59.Durak I, Koksal I, Kacmaz M, Buyukkocak S, Cimen BMY, Ozturk HS. Hazelnut supplementation enhances plasma antioxidant potential and lowers plasma cholesterol levels. Clin Chim Acta. 1999;284:113–5. doi: 10.1016/s0009-8981(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 60.Garg ML, Blake RJ, Wills RBH, Clayton EH. Macadamia nut consumption modulates favourably risk factors for coronary artery disease in hypercholesterolemic subjects. Lipids. 2007;42:583–7. doi: 10.1007/s11745-007-3042-8. [DOI] [PubMed] [Google Scholar]
- 61.Kocyigit A, Koylu AA, Keles H. Effects of pistachio nuts consumption on plasma lipid profile and oxidative status in healthy volunteers. Nutr Metab Cardiovasc Dis. 2006;16:202–9. doi: 10.1016/j.numecd.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 62.Jenkins DJ, Kendall CW, Marchie A, Parker TL, Connelly PW, Qian W, et al. Dose response of almonds on coronary heart disease risk factors: blood lipids, oxidized low density lipoproteins, lipoprotein(a), homocysteine, and pulmonary nitric oxide: a randomized, controlled, crossover trial. Circulation. 2002;106:1327–32. doi: 10.1161/01.cir.0000028421.91733.20. [DOI] [PubMed] [Google Scholar]
- 63.Jenkins DJA, Kendall CWC, Marchie A, Josse AR, Nguyen TH, Faulkner DA, Lapsley KG, Blumberg J. Almonds reduce biomarkers of lipid peroxidation in older hyperlipidemic subjects. J Nutr. 2008;138:908–13. doi: 10.1093/jn/138.5.908. [DOI] [PubMed] [Google Scholar]
- 64.Thomson CD, Chisholm A, McLachlan SK, Campbell JM. Brazil nuts: a effective way to improve selenium status. Am J Clin Nutr. 2008;87:379–84. doi: 10.1093/ajcn/87.2.379. [DOI] [PubMed] [Google Scholar]
- 65.Jenkins DJA, Kendall CWC, Josse AR, Salvatore S, Brighenti F, Augustin LSA, Ellis PR, Vidgen E, Rao AV. Almonds decrease postprandial glycemia, insulinemia, and oxidative damage in healthy individuals. J Nutr. 2006;136:2987–92. doi: 10.1093/jn/136.12.2987. [DOI] [PubMed] [Google Scholar]
- 66.Jia X, Li N, Zhang W, Zhang X, Lapsley K, Huang G, Blumberg J, Ma G, Chen J. A pilot study on the effects of almond consumption on DNA damage and oxidative stress in smokers. Nutr Cancer. 2006;54:179–83. doi: 10.1207/s15327914nc5402_4. [DOI] [PubMed] [Google Scholar]
- 67.Li N, Jia X, Chen CYO, Blumberg JB, Song Y, Zhang W, Zhang X, Guansheng M, Chen J. Almond consumption reduces oxidative damage and lipid peroxidation in male smokers. J Nutr. 2007;137:2717–22. doi: 10.1093/jn/137.12.2717. [DOI] [PubMed] [Google Scholar]
- 68.U.S. Department of Agriculture, Agricultural Research Service. [2010/1/19];USDA National Nutrient Database for Standard Reference, Release 22. 2009 Nov 20; Available from: http://www.ars.usda.gov/ba/bhnrc/ndl.
- 69.Pellegrini N, Serafini M, Salvatore S, Del Rio D, Bianchi M, Brighenti F. Total antioxidant capacity of spices, dried fruits, nuts, pulses, cereals and sweets consumed in Italy assessed by three different in vitro assays. Mol Nutr Food Res. 2006;50:1030–8. doi: 10.1002/mnfr.200600067. [DOI] [PubMed] [Google Scholar]


