Abstract
The extent to which sample dilution factor (DF) affects total antioxidant capacity (TAC) values is poorly understood. Thus, we examined the impact of DF on the ORAC, FRAP, DPPH, and total phenols (TP) assays using pomegranate juice (PJ), grape juice (GJ), selected flavonoids, ascorbic acid, and ellagic acid. For ORAC, GJ was comparable to PJ at DF 750, but at DF 2000, the ORAC value of GJ was 40% more than PJ. Increasing DF increased GJ and PJ, DPPH, TP, and FRAP values 11% and 14%, respectively. Increased test concentrations of quercetin and catechin resulted in 51% and 126% greater ORAC values, but decreased naringenin by 68%. Flavonoids, but not ellagic acid or ascorbic acid, may contribute to the dilution effect on the variation of final TAC values. Thus, reporting TAC or TP using a single DF may introduce uncertainty about the confidence of TAC assay values, especially when comparing different juices. These results underscore the importance of using compatible test standards for reporting TAC values.
Keywords: antioxidant, dilution, grape, polyphenol, pomegranate
Introduction
The potential health benefits of dietary antioxidants is suggested by an extensive body of data from observational studies showing an inverse association of intake with reduced risk of chronic diseases such as cancer, cardiovascular disease, cataract, Alzheimer disease, age-related macular degeneration, and rheumatoid arthritis (Cerhan and others 2003; Hagfors and others 2003; van Leeuwen and other 2005; Khan and others 2008; Stoner and others 2008; Tan and others 2008; Hooper and others 2008; Devore and others 2010). As a result, the antioxidant content of fruit and vegetables and related products, including juices, has been increasingly used to promote their putative health benefits to consumers.
Several chemical assays of total antioxidant capacity (TAC) have been developed to more readily assess and rank overall antioxidant capacity of foods and beverages. These include the oxygen radical absorbance capacity (ORAC), ferric reducing antioxidant power (FRAP), Trolox equivalent antioxidant capacity (TEAC), 2,2-diphenyl-1-picrylhydrazyl (DPPH), total radical-trapping antioxidant parameter (TRAP), and 2,2’-azino-di[3-ethylbenzthiazoline-6-sulfonate] (ATBS) assays (Huang and others 2005). TAC values have also been used as a collective measure to quantify dietary antioxidant intake (Rautiainen and others 2008; Talegawkar and others 2009; Floegel and others 2010; Psaltopoulou and others 2011).
TAC assays of foods do not address their ultimate biological actions in vivo as they cannot account for factors such as bioavailability, metabolism, distribution, and elimination of their nutrients or effect modifiers such as food matrix, genetics, background nutritional status, and nutrient–nutrient interactions (Sies 2007). Further, the chemical TAC assays do not take into account the impact of study antioxidants on endogenous antioxidant defenses. For instance, the DPPH assay is based on the reduction of the stable DPPH radical, which do not occur in humans (Brand-Williams and others 1995). Notably, in vitro cell culture methods have been developed to begin to address the limitations of chemical TAC assays in assessment of the interaction between study antioxidants and endogenous antioxidant defenses. For example, the cellular antioxidant activity (CAA) assay measures prevention of peroxyl-radical induced oxidation of 2’7’-dichlorofluorescin in HepG2 cells (Wolfe and Liu 2007, 2008).
While TAC assays might not be appropriate to assess the health effects of antioxidants, relevant chemistry may occur in foods and they could be used to assess antioxidant function for preservation and extended shelf life and other new applications for food products (Mermelstein 2010). Further, TAC may be useful as an index for enrichment of potentially bioactive components of foods. Thus, there is a need for greater understanding and improved methodology for TAC assays.
Relatively little effort has been undertaken to compare and correlate these TAC measures. In general, there appears to be a moderate correlation between the results of these assays (Murakami and others 2002; Pellegrini and others 2003). For example, the FRAP assay was correlated to the TEAC (r = 0.917) and TRAP (r = 0.744) (Pellegrini and others 2003). The discordance in ranking order can be partly attributed to different probes, reaction milieus, and assay mechanisms (electron transferring, hydrogen donating, and redox potential) utilized in different TAC assays (Huang and others 2005).
For routine determinations of TAC, beverages or extracts are typically diluted or dissolved in a buffer or solvent, with the goal of diluting the antioxidant capacity of the samples to be within the linear range of a standard curve constructed with a reference compound. Samples are commonly diluted 1- to 1000-fold before introduction to a TAC assay (Bolling and others 2009). The final TAC value is obtained with adjustment for the arbitrarily selected dilution factor. For more comprehensive characterization, a series of doses for a sample are tested to attain an IC50 value. Thus, the IC50 approach may be impractical for large screening studies.
Synergistic or antagonistic interactions of 2 or more antioxidants have been documented in biological and model systems. For example, a polyphenol-rich almond skin extract acted synergistically with vitamin E to enhance LDL resistance against ex vivo Cu2+-induced oxidation (Chen and others 2005, 2007). Certain citrus flavonoids exhibited antagonistic or synergistic interactions in the ORAC assay (Freeman and others 2010). Importantly, we also found these interactions to be concentration-dependent (Chen and others 2008). Therefore, the magnitude of the interaction between antioxidants in TAC assays could be modulated by test concentrations. We hypothesized that sample dilution prior to determination of TAC has a significant impact on the final TAC value. The main objective of this study was to examine the effect of dilution on the ORAC, FRAP, DPPH, and total phenols (TP) values of polyphenol-rich pomegranate juice (PJ) and grape juice (GJ).
Materials and Methods
Materials
Commercial samples of POM Wonderful brand pomegranate nectarine juice, Trader Joe’s brand organic grape juice, and 100% pomegranate juice were provided by POM Wonderful Inc (Los Angeles, Calif., U.S.A.). 2–2’-Azobis (2-amidinopropane) dihydrochloride (AAPH) was from Wako Chemicals U.S.A. (Richmond, Va., U.S.A.). Sodium hydroxide, hydrochloric acid, and methanol were from Fisher Scientific (Pittsburg, Pa., U.S.A.). All other chemicals and flavonoids were acquired from Sigma (St. Louis, Mo., U.S.A.).
Dilution of juices and standards
The juices were diluted to selected concentrations immediately prior to each TAC assay with respective assay buffers or solvents. Test compounds, consisting of the flavonoids quercetin, catechin, luteolin, naringenin, and daidzein, as well as ascorbic acid and ellagic acid (EA) were chosen for further evaluation, as they are present in a wide variety of foods and beverages. Quercetin, catechin, and luteolin, were dissolved in methanol at 1 mg/mL, naringenin at 5 mg/mL, and daidzein at 0.5 mg/mL. These flavonoid stocks were stored at −80 °C. Before use in the TAC and TP assays, flavonoid stock solutions, ascorbic acid, or EA were diluted with respective assay buffers or solvents to appropriate test concentrations. The ranges of test concentrations for samples were chosen to fall on the upper and lower limits of linearity and midpoint for the standard Trolox in the DPPH, FRAP, and ORAC assays or gallic acid for TP.
ORAC assay
The ORAC assay employs AAPH as a peroxyl radical generator and fluorescein as a target molecule to detect the ability of the added antioxidant to inhibit their interaction relative to Trolox standard according to Ou and others (2001). The assay method provides an integrated and quantitative determination of TAC by employing the area under the curve (AUC) of the magnitude and time of inhibition of peroxyl radicals attack against fluorescein. Fluorescence at 485/520 nm was monitored using a FLUOstar OPTIMA plate reader (BMG LABTECH Inc., Cary, N.C., U.S.A.). Trolox from 5 to 50 μmol/L were used as an assay standard. Intra- and inter-assay coefficients of variation (CV) for the ORAC assay were 3% and 7.3%, respectively.
FRAP assay
The FRAP assay determines the capability of antioxidants as electron transferring reductants in a redox-linked colorimetric reaction of the reduction of Fe3+-2,4,6-tri-pyridyl-S-triazine to a blue-colored Fe2+ complex at pH 3.5 (Benzie and Strain 1996; Chen and others 2010). Fruit juice was incubated at room temperature with the FRAP reagent for 1 h, and the absorbance at 593 nm was then recorded. FRAP values were calculated from standard curves using Trolox at 31.25 to 500 μmol/L. Intra- and inter-assay CV were 0.7% and 4.2%, respectively.
DPPH assay
Determination of DPPH scavenging activity was performed according to Brand-Williams and others (1995). Briefly, 900 μL of 100 μmol/L DPPH in ethanol was mixed with 100 μL of juice sample and the absorbance at 520 nm was then measured after 30 min of incubation at room temperature in the dark. DPPH values were calculated from standard curves using Trolox at 31.25 to 500 μmol/L. Intra- and inter-assay CV were 1.4% and 7.6%, respectively.
TP assay
Total phenol of fruit juices was determined according to Singleton and others (1999) with results expressed as micromol per liter gallic acid equivalents (GAE). Intra- and inter-assay CV were 1.6% and 4.9%, respectively.
Statistical methods
Data are presented as mean ± SD of at least duplicate determinations. Statistical significance and regression analysis was determined using GraphPad Software (version 5.00, San Diego, Calif., U.S.A.) at the P < 0.05 level by a 2-tailed t-test or one-way analysis of variance (ANOVA), followed by Tukey’s honestly significant difference test.
Results and Discussion
Measures of TAC have been widely used to characterize antioxidant-rich foods, botanicals, and beverages, and, with extrapolation provide an estimate of total antioxidant intake. Synergistic or antagonistic interactions between antioxidant constituents in foods that are highly concentration-dependent in assay milieus might affect final TAC values. Thus, we examined the effect of dilution factor on TAC (ORAC, FRAP, and DPPH) and TP assays in pomegranate and grape juice.
Responses of standards in TAC and TP assays
The response of Trolox as a standard in the DPPH, FRAP, and ORAC assays was linear from 5 to 50 μmol/L. Likewise, the gallic acid response was linear in the TP assay from 0.18 to 2.94 mmol/L. EA, ascorbic acid, and the flavonoids catechins, daidzein, luteolin, naringenin, and quercetin also had linear responses of untransformed data in TP and TAC assays.
Transformation of EA to gallic acid or Trolox equivalents (TE) resulted in consistent TAC values expressed as μmol GE or TE/μmol (Figure 1), as well as did ascorbic acid (data not shown). In contrast, transformation of flavonoids to gallic acid or TE yielded varied TAC values in all cases (Table 1). FRAP, DPPH, and ORAC values of quercetin increased at greater concentrations by 105%, 59%, and 51%, respectively. In contrast, TP, DPPH, and FRAP values of luteolin decreased by 98%, 38%, and 33%, respectively, when assayed concentrations were increased. At greater concentrations, ORAC values of catechin increased by 126%, but FRAP and DPPH values decreased by 16% and 51%, respectively. The FRAP value of daidzein also decreased 82% when tested at higher concentrations.
Figure 1.
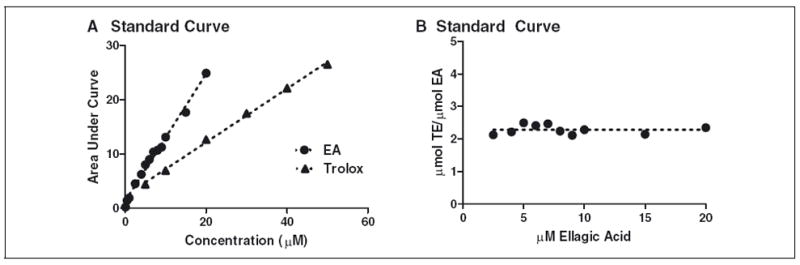
A. Linear regression of ORAC area under the curve verse concentration for EA and Trolox. B. Transformation of EA to TE results in a constant value.
Table 1.
The concentration of flavonoids introduced into the TP and TAC assays changes TAC values of flavonoid standards.
| Assay | Compound | Dilution range (μmol/L) | Standard equivalents μmol/μmol | % difference in TAC between concentrationsb |
|---|---|---|---|---|
| TP | Catechin | 70 to 340 | 3.6 | −4 |
| Daidzein | 1000 to 4000 | 0.38 | 7 | |
| Luteolin | 146 to 1747 | 1.7 | −98 | |
| Naringenin | 367 to 3060 | 1.0 | −43 | |
| Quercetin | 170 to 660 | 1.8 | 8 | |
| DPPHa | Catechin | 34 to 450 | 2.16 | −51 |
| Luteolin | 30 to 220 | 2.9 | −38 | |
| Quercetin | 66 to 330 | 1.36 | 105 | |
| FRAP | Catechin | 34 to 340 | 3.3 | −16 |
| Daidzein | 250 to 7900 | 0.13 | −82 | |
| Luteolin | 28 to 440 | 2.5 | −33 | |
| Naringenin | 11 to 92 | 11 | −34 | |
| Quercetin | 66 to 660 | 1.2 | 59 | |
| ORAC | Catechin | 8.3 to 19 | 14 | 126 |
| Daidzein | 0.82 to 2.5 | 15 | −3 | |
| Luteolin | 1.1 to 2.9 | 18 | −6 | |
| Naringenin | 1.0 to 8.3 | 37 | −68 | |
| Quercetin | 0.83 to 6.6 | 11 | 51 |
Daidzein and naringenin did not have a measurable responses in the DPPH assay.
The percent change is the maximum difference between final TAC values of the tested flavonoid concentrations observed experimentally using the routine assay conditions.
The inconsistencies in final flavonoid TAC values expressed as μmol GE or TE/μmol appeared to occur when the values are transformed to gallic acid or TE. Further, these inconsistencies were present within the optimized linear range of TP and TAC assays in plots of gallic acid or TE against flavonoid concentrations. For example, in the TP assay, naringenin and luteolin had larger Y-intercepts than quercetin and catechin (Figure 2). Therefore, transformation of TP and TAC values to gallic acid or TE may unpredictably introduce systematic variation. While these 2 standards have been commonly employed to evaluate TP content and TAC values of plant foods and derived products and botanicals, selection of standards most closely reflecting the antioxidant or radical scavenging nature of crude extracts may help reduce this variation.
Figure 2.
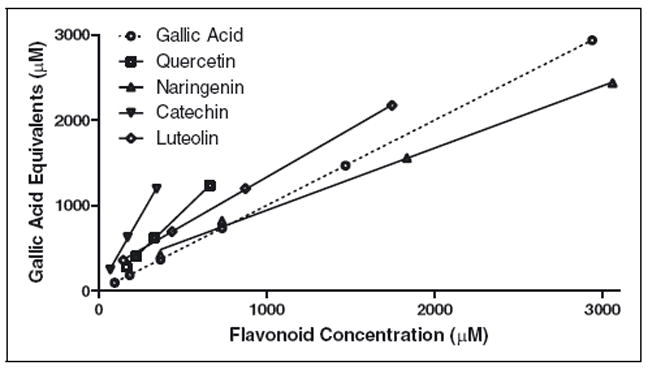
GAE of flavonoid standards assessed by the TP assay.
Responses of polyphenol-rich juices in TAC and TP assays
Our ORAC and TP values for PJ and GJ are in range of those reported by Seeram and others (2008). However, Seeram and others (2008) reported DPPH and FRAP values of PJ and GJ using different standard equivalents and so are not comparable to the present study. This discrepancy could arise from different assay conditions between laboratories, suggesting the need to standardize protocols of common TAC assays. However, selecting the most suitable standards to diminish the abovementioned variation will be challenging. Plant foods and botanicals contain many polyphenol and non-polyphenol antioxidants at various concentrations. Anthocyanins, proanthocyanidins, or hydroxycinnamic acids may be appropriate for certain foods, but ascorbic acid for others. Thus, it will be necessary to identify multiple standards appropriate for different groups of foods and products.
Dilution effect on TE or GAE values of juices
Transformation of raw responses of diluted juices in TAC assays to either TE or GAE did not yield constant final TAC values over a range of dilution factors (Figure 3). The plots of juice response against concentration values or dilution factors were apparently nonlinear, but could not be readily modeled by standard nonlinear functions. Modeling the raw data of standard and juice responses by nonlinear functions also did not result in consistent TAC values. ORAC Trolox and sample responses were previously modeled using quadratic functions (Wu and others 2004). Modeling the Trolox and juice ORAC raw data by the quadratic function did not provide a statistically significant advantage when comparing curve fits (P > 0.05). Thus, transformation of quadratic functions to TE also did not yield consistent TAC values by concentration.
Figure 3.
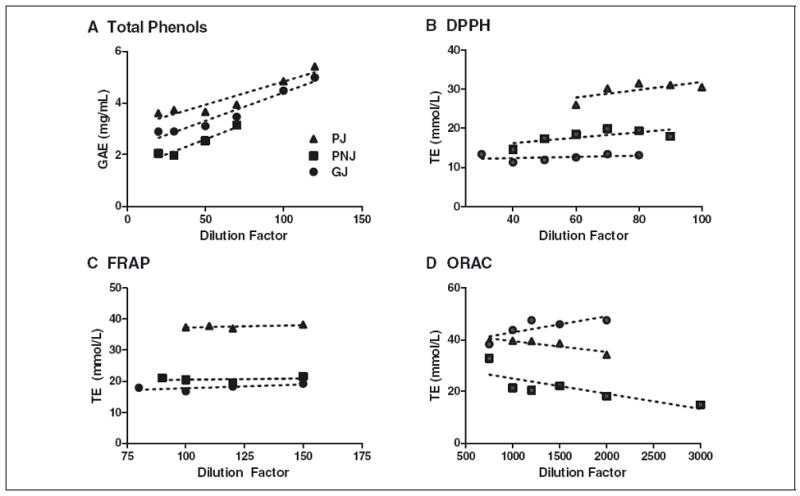
GAE or TE values of polyphenol-rich juices are constants values over a range of dilution factors in the (A) TP, (B) DPPH, (C) FRAP, and (D) ORAC assays. PJ, pomegranate juice; PNJ, pomegranate nectarine juice; GJ, grape juice.
Nonlinear functions better modeled the deviation from a constant TAC value by concentration. PJ, PNJ, and GJ TAC deviation in the TP, FRAP, and ORAC values were best modeled by one-phase decay functions (R2 = 0.9695 to 0.9997). In the DPPH assay, PJ and PNJ changes in TAC values by concentration were best modeled by quadratic functions, R2 = 0.9720 and 0.9711, respectively, and GJ by an exponential growth function with R2 = 0.9646.
Linear functions were used to model juice dilution responses to show the magnitude of deviation from a constant TAC value (Figure 3). Linear TAC trends for juices were apparent for DPPH and FRAP assays (R2 = 0.05 to 0.53). However, changing test concentrations (DF) resulted in changes in final juice TAC values. At increasing DFs, juice FRAP values increased 3% to 14%. In the DPPH assay, PJ and PNJ TE values increased 37% and 21%, respectively, while GJ declined 16%. ORAC and TP values of juices were also markedly affected by DF. In the ORAC assay, when PJ and PNJ DF was increased, their TAC values declined by 55% and 14%, respectively. GJ ORAC values were proportional to dilution, and increased by 24% over the range of the assay (Figure 3). At comparable ORAC TE, the diluted GJ and PJ exhibited a similar antioxidative kinetic pattern in term of lag time and slop of decay phase (data not shown). TP values of all juices increased with dilution, with strong linear correlations of plots of GAE values against DF (R2 = 0.89 to 0.95). The increases in the TP value of the juices ranged from 50% to 72% upon increasing DF, and rates of change were not statistically different.
The inconsistent results of TAC assays of polyphenol-rich juices using TP, DPPH, FRAP, and ORAC are in contrast to the general assumption that the TAC value of a food or beverage is independent of the quantity of sample introduced to the TAC assays. The effect of DF may be attributed to physical and chemical interactions between constituents in the juices, such as molecular hindrance, noncompetitive inhibition to radical reduction or scavenging, or antioxidant sparing and recycling. Changes in TAC may also arise from concentration-dependent synergy or antagonism between antioxidant constituents. Both synergistic and antagonistic interactions between combinations of citrus polyphenols occurred in the ORAC assay, possibly attributed to the coupling of different redox potentials of the antioxidants (Freeman and others 2010). However, whether the magnitude of polyphenol redox coupling is dependent on dilution remains to be examined. Importantly, Balk (2009) reported that the result of antioxidant competition assays, such as ORAC, is dependent on the relative concentration of reactive oxidant species to antioxidants. This relationship could provide one of possible underlying mechanism for changes in final TAC value at different sample dilutions in the ORAC assay. It also suggests the importance of selecting appropriate dilution factor in TAC assays based on antioxidant competition.
Selective choice of dilution confounds TAC and TP values
PJ and GJ had significant (P < 0.05), but negligible differences in the TP assay when tested at DF 120 (Figure 4). When GJ and PJ were analyzed at DF of 20 and 120, respectively, GJ had 47% less TP than PJ, but at DF 120 and 20, respectively, GJ had 38% more TP than PJ. In the ORAC assay, GJ and PJ at DF 750 had indistinguishable TE. However, at DF 2000, GJ had 39% more TE than PJ. Combination of high and low DF for GJ and PJ also resulted in 12% or 21% differences in ORAC value. In contrast, DPPH and FRAP values were more stable with selective choice of DF because the impact of dilution on final DPPH and FRAP values was much smaller (Figure 3).
Figure 4.
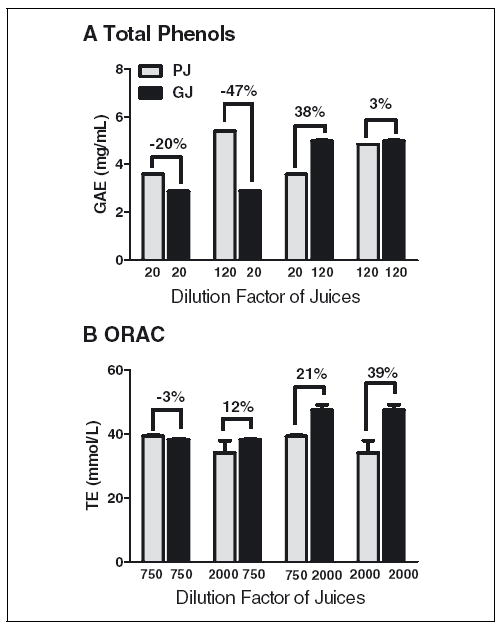
The dilution factors employed in the assays had a significant effect on final TAC and TP values, (A) TP and (B) ORAC. Values between bars are the % difference of pomegranate juice. PJ, pomegranate juice; PNJ, pomegranate nectarine juice; GJ, grape juice. P-values for 2-tailed t-test comparisons for TP are P = 0.0003 for 20/20 DF; P < 0.0001 for 120/20 DF; P < 0.0001 for 20/120 DF; P = 0.0051 for 120/120 DF; and for ORAC are P = 0.5467 for 750/750 DF; P = 0.0016 for 2000/750 DF; P = 0.0799 for 750/2000 DF; P = 0.0446 2000/2000 DF.
Because of the nonlinearity of TAC assays, the selective choice of single DF for PJ and GJ confounds TAC values, most strikingly in the TP and ORAC assays, 2 of most common assays employed to estimate phenolic content and TAC, respectively, in foods and beverages. Thus, our results indicate that DF should be seriously considered in the design of TAC assays and interpretation of the results. The effort to quantify simply the dietary intake of phenolics and antioxidants has led to an establishment of ORAC and TP (USDA 2005) and FRAP databases (Carlsen and others 2010). Our study suggests the accuracy of rankings generated particularly from ORAC and TP may be compromised when polyphenol-rich products are screened and ranked without characterizing the impact of DF. Further study will be necessary to characterize the degree to which DF may confound TP and TAC values of other foods, beverages, and botanicals.
Alternative methods to calculate TAC values are unable to compensate for DF effect
With the knowledge that final TAC values may not be constant over a range of DF, a variety of approaches were considered to approximate TAC values. Figure 5 depicts 3 potential approaches directed to diminish the impact of the dilution effect. For example, when multiple dilutions over the range of the linear standard curve of assays are employed, an effective concentration (EC) at a predefined potency (TE) could be obtained, similar to calculation of an IC50 value, commonly employed in radical scavenging assays, such as superoxide scavenging assay. An EC is defined as the assay response (in TE or GAE) of a sample at a predetermined standard concentration (n), for example, 20 μmol/L Trolox. ECn is the TAC of a test sample, which is the multiplication product of a predefined Trolox concentration (n) and the calculated dilution factor. The calculated dilution factor is obtained using a regression plot of raw responses of at least 3 diluted samples falling in the range of the standard curve and corresponding dilution factors when the response of the diluted sample at the calculated dilution factor is equal to the target response of a predefined Trolox concentration. Thus, an EC could represent a low, medium, or high TAC. It should be noted that this definition of an EC varies slightly from previous definitions. For example, Adom and Liu (2005) define the EC50 value for the peroxyl radical scavenging assay as the dose of a compound to cause a 50% inhibition of oxidation of the fluorescent probe and Wolfe and Liu (2008) define the EC50 value as the median effective dose derived from logarithmic plots of dose and treatment responses. In this case, we base the ECn value on the response of a standard in the ORAC assay. Alternatively, an average of the TAC value at multiple dilutions or the TAC at one predetermined dilution factor for all test samples could be used.
Figure 5.
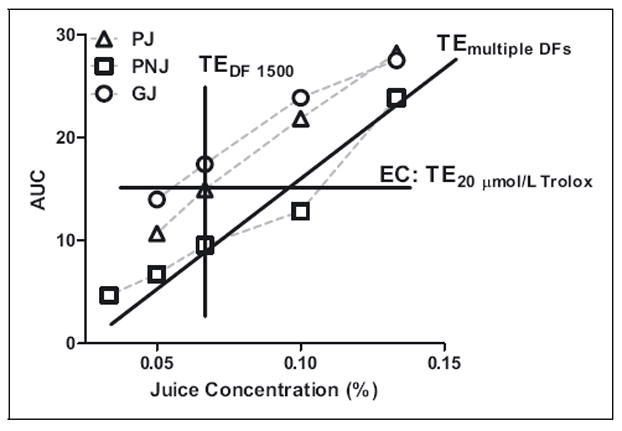
Potential strategies to report TAC as TE in the ORAC assay for fruit juices. EC: effective concentration, defined as the assay response (in TE) of a sample at a predetermined standard concentration, for example, 20 μmol/L Trolox. ECn is the TAC of a test sample, which is the multiplication product of a predefined Trolox concentration (n) and the calculated dilution factor. The calculated dilution factor is obtained using a regression plot of raw responses of at least three diluted samples falling in the range of the standard curve and corresponding dilution factors when the response of the diluted sample at the calculated dilution factor is equal to the target response of a predefined Trolox concentration. DF, Dilution Factor; PJ, pomegranate juice; PNJ, pomegranate nectarine juice; GJ, grape juice.
Using these 3 methods, TAC values were calculated for TP, DPPH, FRAP, and ORAC assays (Table 2). Choice of the 3 methods had a divergent impact on the test assays. Use of an EC approach resulted in 14% and 30% greater FRAP values for PJ and PNJ than single or multiple DF, respectively. On the other hand, use of an EC method resulted in 17% and 18% lower DPPH values for PJ and GJ, respectively. Likewise, use of an EC resulted in 17% and 9% lower ORAC values for GJ and PJ than use of single DF, respectively. For TP, use of multiple DF resulted in the highest TAC values for PNJ and GJ.
Table 2.
The divergent impact of selection of quantification method on TP and TAC values of polyphenol-rich fruit juices.
| Assay (units) | Methoda | Juice TAC
|
||
|---|---|---|---|---|
| PJ | PNJ | GJ | ||
| TP (mg GAE/mL) | single DF | 3.57 ± 0.01 | 2.02 ± 0.01 | 2.86 ± 0.01 |
| multiple DFs | 3.73 ± 0.15 | 2.42 ± 0.53 | 3.09 ± 0.27 | |
| EC | 3.69 | 2.06 | 2.98 | |
| DPPH (μmol TE/L) | single DF | 31.5 ± 0.3 | 19.4 ± 0.8 | 13.1 ± 0.2 |
| multiple DFs | 29.8 ± 2.2 | 17.9 ± 1.9 | 12.6 ± 0.9 | |
| EC | 26.3 | 18.5 | 10.7 | |
| FRAP (μmol TE/L) | single DF | 38.2 ± 0.8 | 21.5 ± 0.2 | 19.2 ± 0.3 |
| multiple DFs | 37.6 ± 0.5 | 20.6 ± 0.9 | 18.0 ± 1.0 | |
| EC | 43.4 | 28 | 19.5 | |
| ORAC (μmol TE/L) | single DF | 41.9 ± 2.7 | 20.9 ± 0.4 | 49.1 ± 1.8 |
| multiple DFs | 38.0 ± 2.5 | 20.6 ± 1.7 | 46.2 ± 1.8 | |
| EC | 34.5 | 21.2 | 44.5 | |
Methods of determination of final TAC value as explained in Figure 5.
DF, dilution factor; EC, effective concentration; PJ, pomegranate juice; PNJ, pomegranate nectarine juice; GJ, grape juice.
Our data suggested that the choice of single or multiple DF is arbitrary and may impose an insurmountable challenge in creating a validated TAC and TP database. Establishing a widely acceptable method that minimizes the DF-induced inter-laboratory variation in TAC and TP assays would require a pre-determined EC to avoid arbitrary concentration ranges. We suggest that the pre-defined standard concentration could be set at the midpoint of assay linearity, as to approximate an IC50 value. As dilution series are already employed to determine IC50 values, the EC method could be adopted to standardize inter-laboratory comparisons. A consensus of appropriate reference standards, standard concentration, and assay conditions would need to be established to facilitate TAC comparisons. At best, any TAC assay is an approximation of actual antioxidant capacity with EC appearing to be a preferred means of expressing its value. However, the EC approach would likely prove incompatible with high throughput screening of large sample sets.
Conclusions
Changes in polyphenol-rich juice or flavonoid test concentrations even within the optimized linear range of TP, DPPH, FRAP, and ORAC may unintentionally lead to variation in final TAC values. Reporting TAC or TP values obtained with a single DF do not fully account for confounding DF effects on synergistic and/or antagonistic interactions between antioxidant constituents, thereby decreasing confidence about the accuracy of final TAC values, especially used for ranking different food products. Thus, using a single TAC value for mixtures of polyphenols in foods and beverages may not provide an effective comparison. Careful selection of standards that represent the type of major antioxidants in test foods and products is recommended to reduce error when transforming data to TAC values. Chemical TAC assays of foods and beverages cannot address their ultimate biological actions in vivo, but may have utility for assessing diet or food quality. Finally, to be useful for database applications, there is an urgent need to establish standardized TAC and TP protocols that take into account DF and EC calculations.
Practical Application.
Total antioxidant capacity (TAC) values such as the ORAC assay are increasingly used for comparison of polyphenol-rich foods and beverages. Choice of standards and test concentrations, even within the linear range of standards, may introduce variation probably due to synergy/antagonism between antioxidant and thereby, confound final TAC values. Thus, test concentration or dilution factors of samples should be considered in the design of TAC assays and interpretation of their results.
Acknowledgments
Supported by POM Wonderful, LLC, and U.S. Dept. of Agriculture (USDA)/Agricultural Research Service under Cooperative Agreement nr 58–1950-7–707. BWB was supported by NIGMS K12GM074869. The authors are grateful to Desire Kelley for technical assistance.
Footnotes
Author disclosures: The contents of this publication do not necessarily reflect the views or policies of the USDA nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. government.
Contributor Information
Bradley W. Bolling, Dept. of Nutritional Sciences, Univ. of Connecticut, 3624 Horsebarn Rd. Unit 4017, Storrs, CT, 06269, U.S.A
Ya-Yen Chen, School of Nutrition and Health Sciences, Taipei Medical Univ., Taipei, Taiwan.
Alison G. Kamil, Antioxidants Research Lab., Jean Mayer USDA Human Nutrition Research Center on Aging, Tufts Univ., 711 Washington St., Boston, MA, 02111, U.S.A
C-Y. Oliver Chen, Antioxidants Research Lab., Jean Mayer USDA Human Nutrition Research Center on Aging, Tufts Univ., 711 Washington St., Boston, MA, 02111, U.S.A.
References
- Adom KK, Liu RH. Rapid peroxyl radical scavenging capacity (PSC) assay for assessing both hydrophilic and lipophilic antioxidants. J Agric Food Chem. 2005;53:6572–80. doi: 10.1021/jf048318o. [DOI] [PubMed] [Google Scholar]
- Balk J. Evaluation of the accuracy of antioxidant competition assays: incorrect assumptions with major impact. Free Rad Biol Med. 2009;47:135–44. doi: 10.1016/j.freeradbiomed.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP Assay. Anal Biochem. 1996;239:70–6. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. Leb Wis Tech. 1995;28:25–30. [Google Scholar]
- Bolling BW, Blumberg JB, Chen C-YO. Extraction methods determine the antioxidant capacity and induction of quinone reductase by soy products in vitro. Food Chem. 2009;116:351–5. doi: 10.1016/j.foodchem.2009.01.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen M, Halvorsen B, Holte K, Bohn S, Dragland S, Sampson L, Willey C, Senoo H, Umezono Y, Sanada C, Barikmo I, Berhe N, Willett W, Phillips K, Jacob D, Jr, Blomhoff R. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr J. 2010 doi: 10.1186/1475-2891-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerhan JR, Saag KG, Merlino LA, Mikuls TR, Criswell LA. Antioxidant micronutrients and risk of rheumatoid arthritis in a cohort of older women. Am J Epidemiol. 2003;157:345–54. doi: 10.1093/aje/kwf205. [DOI] [PubMed] [Google Scholar]
- Chen C-YO, Blumberg JB. In vitro activity of almond skin polyphenols for scavenging free radicals and inducing quinone reductase. J Agric Food Chem. 2008;56:4427–34. doi: 10.1021/jf800061z. [DOI] [PubMed] [Google Scholar]
- Chen C-YO, Milbury PE, Lapsley K, Blumberg JB. Flavonoids from almond skins are bioavailable and act synergistically with vitamins C and E to enhance hamster and human LDL resistance to oxidation. J Nutr. 2005;135:1366–73. doi: 10.1093/jn/135.6.1366. [DOI] [PubMed] [Google Scholar]
- Chen C-YO, Milbury P, Chung S, Blumberg JB. Effect of almond skin polyphenolics and quercetin on human LDL and apolipoprotein B-100 oxidation and conformation. J Nutr Biochem. 2007;18:785–94. doi: 10.1016/j.jnutbio.2006.12.015. [DOI] [PubMed] [Google Scholar]
- Chen C-YO, Ribaya-Mercado JD, McKay DL, Croom E, Blumberg JB. Differential antioxidant and quinone reductase inducing activity of American, Asian, and Siberian ginseng. Food Chem. 2010;119:445–51. [Google Scholar]
- Devore E, Grodstein F, van Rooij F, Hofman A, Stampfer M, Witteman J, Breteler M. Dietary antioxidants and long-term risk of dementia. Arch Neurol. 2010;67:819–25. doi: 10.1001/archneurol.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floegel A, Kim DO, Chung SJ, Song WO, Fernandez ML, Bruno RS, Koo SI, Chun OK. Development and validation of an algorithm to establish a total antioxidant capacity database of the US diet. Int J Food Sci Nutr. 2010;61:600–23. doi: 10.3109/09637481003670816. [DOI] [PubMed] [Google Scholar]
- Freeman BL, Eggett DL, Parker TL. Synergistic and antagonistic interactions of phenolic compounds found in navel oranges. J Food Sci. 2010;75:C570–C6. doi: 10.1111/j.1750-3841.2010.01717.x. [DOI] [PubMed] [Google Scholar]
- Hagfors L, Leanderson P, Skoldstam L, Andersson J, Johansson G. Antioxidant intake, plasma antioxidants and oxidative stress in a randomized, controlled, parallel, Mediterranean dietary intervention study on patients with rheumatoid arthritis. Nutr J. 2003 doi: 10.1186/1475-2891-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper L, Kroon PA, Rimm EB, Cohn JS, Harvey I, Le Cornu KA, Ryder JJ, Hall WL, Cassidy A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2008;88:38–50. doi: 10.1093/ajcn/88.1.38. [DOI] [PubMed] [Google Scholar]
- Huang D, Ou B, Prior RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005;53:1841–56. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- Khan N, Afaq F, Mukhtar H. Cancer chemoprevention through dietary antioxidants: progress and promise. Antioxid Redox Signal. 2008;10:475–510. doi: 10.1089/ars.2007.1740. [DOI] [PubMed] [Google Scholar]
- Mermelstein N. Dealing with antioxidant assay issues. Food Technol. 2010;64(5):72–5. [Google Scholar]
- Murakami M, Yamaguchi T, Takamura H, Matoba T. A comparative study on the various in vitro assays of active oxygen scavenging activity in foods. J Food Sci. 2002;67:539–41. [Google Scholar]
- Ou B, Hampsch-Woodill M, Prior RL. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J Agric Food Chem. 2001;49:4619–26. doi: 10.1021/jf010586o. [DOI] [PubMed] [Google Scholar]
- Pellegrini N, Serafini M, Colombi B, Del Rio D, Salvatore S, Bianchi M, Brighenti F. Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J Nutr. 2003;133:2812–9. doi: 10.1093/jn/133.9.2812. [DOI] [PubMed] [Google Scholar]
- Psaltopoulou T, Panagiotakos DB, Pitsavos C, Chrysochoou C, Detopoulou P, Skoumas J, Stefanadis C. Dietary antioxidant capacity is inversely associated with diabetes biomarkers: the ATTICA study. Nutr Metab Cardiovasc Dis. 2011;21:561–7. doi: 10.1016/j.numecd.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Rautiainen S, Serafini M, Morgenstern R, Prior RL, Wolk A. The validity and reproducibility of food-frequency questionnaire-based total antioxidant capacity estimates in Swedish women. Am J Clin Nutr. 2008;87:1247–53. doi: 10.1093/ajcn/87.5.1247. [DOI] [PubMed] [Google Scholar]
- Seeram NP, Aviram M, Zhang Y, Henning SM, Feng L, Dreher M, Heber D. Comparison of antioxidant potency of commonly consumed polyphenol-rich beverages in the United States. J Agric Food Chem. 2008;56:1415–22. doi: 10.1021/jf073035s. [DOI] [PubMed] [Google Scholar]
- Sies H. Total antioxidant capacity: appraisal of a concept. J Nutr. 2007;137:1493–5. doi: 10.1093/jn/137.6.1493. [DOI] [PubMed] [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Ravent RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Meth Enzym. 1999;299:152–78. [Google Scholar]
- Stoner GD, Wang LS, Casto BC. Laboratory and clinical studies of cancer chemoprevention by antioxidants in berries. Carcinogenesis. 2008;29:1665–74. doi: 10.1093/carcin/bgn142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talegawkar S, Beretta G, Yeum KJ, Johnson E, Carithers T, Taylor H, Jr, Russell M, Tucker K. Total antioxidant performance is associated with diet and serum antioxidants in participants of the diet and physical activity substudy of the Jackson Heart Study. J Nutr. 2009;139:1964–71. doi: 10.3945/jn.109.107870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AG, Mitchell P, Flood VM, Burlutsky G, Rochtchina E, Cumming RG, Wang JJ. Antioxidant nutrient intake and the long-term incidence of age-related cataract: the Blue Mountains Eye Study. Am J Clin Nutr. 2008;87:1899–905. doi: 10.1093/ajcn/87.6.1899. [DOI] [PubMed] [Google Scholar]
- U.S. Dept. of Agriculture ARS. [Nov 20, 2011];Oxygen radical absorbance capacity (orac) of selected foods, release 2. 2005 Available from: http://www.ars.usda.gov/nutrientdata/orac.
- van Leeuwen R, Boekhoorn S, Vingerling JR, Witteman JC, Klaver CC, Hofman A, de Jong PT. Dietary intake of antioxidants and risk of age-related macular degeneration. JAMA. 2005;294:3101–7. doi: 10.1001/jama.294.24.3101. [DOI] [PubMed] [Google Scholar]
- Wolfe KL, Liu RH. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J Agric Food Chem. 2007;55:8896–907. doi: 10.1021/jf0715166. [DOI] [PubMed] [Google Scholar]
- Wolfe KL, Liu RH. Structure–activity relationships of flavonoids in the cellular antioxidant activity assay. J Agric Food Chem. 2008;56:8404–11. doi: 10.1021/jf8013074. [DOI] [PubMed] [Google Scholar]
- Wu XL, Beecher GR, Holden JM, Haytowitz DB, Gebhardt SE, Prior RL. Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. J Agric Food Chem. 2004;52:4026–37. doi: 10.1021/jf049696w. [DOI] [PubMed] [Google Scholar]


