Abstract
Mechanical forces trigger biological responses in bone cells that ultimately control osteoblastogenesis and bone program. Although several mechanosensors have been postulated, the precise mechanotransduction pathway remains obscure as the initial mechanosensing event has not yet been identified. Studies in kidney cells have shown that polycystin-1 (PC1), via its extracellular N-terminal part, may function as an “antenna-like” protein providing a linkage between environmental cues and their conversion into biochemical responses that regulate various cellular processes via the calcineurin/NFAT pathway. Here we explored the involvement of PC1 in mechanical load (stretching)-induced signaling cascades that control osteoblastogenesis/bone formation. FACS and TransAM Transcription Factor ELISA analyses employing extracts from primary human osteoblast-like, PC1 expressing cells subjected to mechanical stretching (0–6 h) revealed that unphosphorylated/DNA-binding competent NFATc1 increased at 0.5–1 h and returned to normal at 6 h. In accordance with the activation mechanism of NFATc1, stretching of cultured cells pre-treated with cyclosporin A (CsA, a specific inhibitor of the calcineurin/NFAT pathway) abrogated the observed decrease in the abundance of the cytoplasmic pNFATc1 (phosphorylated/inactive) species. Furthermore, stretching of osteoblastic cells pre-treated with an antibody against the mechanosensing N-terminal part of PC1 also abrogated the observed decrease in the cytoplasmic levels of the inactive pNFATc1 species. Importantly, under similar conditions (pre-incubation of stretched cells with the inhibitory anti-PC1 antibody), the expression of the key osteoblastic, NFATc1-target gene runx2 decreased compared to untreated cells. Therefore, PC1 acts as chief mechanosensing molecule that modulates osteoblastic gene transcription and hence bone-cell differentiation through the calcineurin/NFAT signaling cascade.
Keywords: Polycystin-1 (PC1), Human osteoblastic cells, Mechanotransduction, Calcineurin/NFAT pathway, Runx2
Introduction
Bone is a dynamic tissue that is renewed through the balanced processes of resorption and formation in response to the mechanical forces that it experiences. If loading on bone increases, the bone will remodel itself by becoming stronger in order to resist to the increased load forced upon it [1]. On the contrary, in cases of decreased loading, there is no stimulus for remodeling and bone becomes weaker. Therefore, tight control of bone resorption and formation is required for a healthy skeleton, whereas loss of this coupling results in serious skeletal pathologies.
Mechanoregulation of bone involves a complex interconnection between the mechanical stimulus applied, the specific response of the mechanosensing bone cells and ultimately the cascade of molecular events that follow [2]. Upon a mechanical cue, bone cells respond to this extracellular stimulus by “translating” it into a biochemical signal [3, 4]. As a clinical application, targeted load application of specific rate can enhance bone formation and reverse the decreased osteoblastic activity that occurs in many bone diseases. Thus, clinicians could use mechanical stimulation to increase osteoblastogenesis as a non-pharmacological approach in various bone pathophysiologies. For example, osteoporosis, a very common disease in women, is caused by an imbalance between bone formation and resorption, leading to low bone mass and fractures. Current treatment includes administration of drugs such as bisphosphonates, selective estrogen receptor modulators (SERMs), calcitonin, and parathyroid hormone (PTH) analogues [5, 6]. A novel means to decrease or revert bone loss in this condition is to mechanically stimulate the bone-forming cells. New physical therapy procedures and strengthening exercises have been introduced in patients with osteoporosis to achieve bone mass gain and reduce the risk of falls and fractures [7–9]. Overall, the therapeutic value of applying mechanical forces to induce bone formation is of high importance since it provides a possible non-invasive method to treat many skeletal disorders.
The primary aim of our research group over the last years has been the elucidation of molecular mechanisms implicated in mechanical stimulation of human osteoblasts and the identification of specific osteoblastic gene targets. An optimal and highly reproducible experimental in vitro model for application of mechanical stretching in osteoblast-like cells was generated that allowed us to determine the induction of several osteoblastic genes after mechanical stimulation [10–17]. Amongst them, activator protein-1 (AP-1) and Runx2, the major regulator of osteoblastic differentiation present important targets of mechanical stimulation through activation of the extracellular signal-regulated kinase (ERK) mitogen-activated protein kinase (MAPK) [14, 17]. Since Runx2 controls osteoblastic differentiation and the rate of bone formation, identifying stimuli that augment its expression and/or potency in these cells may lead to novel therapeutic treatments of bone loss diseases.
Although several mechanosensitive molecules have been proposed for transmission of mechanical signals into bone cells [such as integrins, G protein-coupled receptors (GPCRs), protein tyrosine kinases and stretch-activated Ca2+ channels] [3, 4], the initial mechanosensing event/molecule that converts the mechanical cue into a biochemical signal leading to osteoblastogenesis has not yet been identified.
Research evidence from autosomal dominant polycystic kidney disease (ADPKD), caused by mutations in polycystin genes PKD1 (85 % of families) or PKD2 (15 % of families), points towards the role of the proteins encoded by these genes as major mechanosensor molecules [18–20]. Polycystins (PCs) comprise a family of eight transmembrane proteins with polycystin-1 (PC1) and polycystin-2 (PC2) being the mostly studied in ADPKD. PC1 is a 4,303-amino-acid cell surface-expressed protein that bears a large N-terminal extracellular domain, 11 transmembrane domains and a short ~200-amino-acid C-terminal cytoplasmic tail [19]. It interacts with PC2 through a coiled-coil domain in the C-terminal region and forms a Ca2+-permeable mechanosensitive ion channel. Studies in kidney cells have shown that PC1 might act as a mechanosensor, receiving signals from primary cilia via its extracellular N-terminus, and transducing them into cellular responses that regulate proliferation, adhesion, differentiation, and cell morphology [19, 20]. Compelling evidence exists as to whether PC1–PC2 complex formation is necessary for proper sensing of mechanical stimuli at the primary cilium of renal epithelial cells [20].
Based on these data, it was conceivable to seek for PC1’s presence in (pre) osteoblasts where it may also act as a primary mechanosensor molecule, responding to mechanical stimuli through its N-terminal domain and possibly via complex formation with PC2 to translate them into biochemical events affecting osteoblastogenesis.
Consistent with this hypothesis, two studies by Xiao et al. [21, 22] demonstrated that mouse osteoblasts and osteocytes express transcripts of PC1, PC2, and the ciliary proteins, Tg737 and Kif3a. In addition, homozygous mutant mice for pkd1 demonstrated delayed endochondral and intramembranous bone formation, as well as significant reductions in endogenous Runx2 expression, osteoblastic markers and differentiation capacity ex vivo [21, 22]. Analyses of pkd1- and runx2-II-deficient mice verified the functional linkage between PC1, Runx2-II expression, and skeletal development, and showed that PC1 regulates the osteoblast-specific enhancer element in the runx2-II P1 promoter through intracellular Ca2+-dependent mechanisms [22]. In accordance, a recent study of mice possessing a conditional deficiency for PC1 in neural crest cells and subsequent analysis of osteoblasts or chondrocytes after midpalatal suture expansion demonstrated an essential role of PC1 in the response of osteochondroprogenitor cells to mechanical stress [23].
Among the main molecular networks implicated in bone mechanobiology and one of the earliest recorded responses of osteoblast cells to mechanical stimulation is the rapid influx of extracellular and/or the mobilization on intracellular Ca2+ [24–27]. The cellular target for sustained Ca2+ increases is calcineurin, a ubiquitous serine–threonine phosphatase and its intracellular substrate nuclear factor of activated T-cells (NFAT). The inactive hyperphosphorylated form of NFAT (pNFAT) is localized in the cytosol being dephosphorylated upon calcineurin activation following increased Ca2+ influx. NFAT translocation into the nucleus influences the regulation of target genes, often at composite NFAT/AP-1 elements [28–30]. Termination of NFAT signaling occurs through rephosphorylation of NFAT by glycogen synthase kinase-3β (GSK-3β) and relocation back to the cytoplasm. The immunosuppressive drugs cyclosporin A (CsA) and FK506 inhibit calcineurin and hence nuclear translocation of NFAT. Activation of calcineurin/NFAT signaling has been shown to control cell differentiation, apoptosis, and cellular adaptation in a wide variety of cell types and tissues, and to regulate the non-canonical Wnt/Ca2+ pathway during embryonic development [30]. It is thus conceivable that the calcineurin/NFAT axis may be activated by mechanical forces exerted upon the cell. Furthermore, regulation of intracellular Ca2+ has been previously associated with the function of PC1 and PC2 [31]. Therefore, it is tempting to speculate that PC1, upon stimulation by mechanical cues, might trigger the calcineurin/NFAT signaling pathway ultimately leading to modulation of NFAT target gene expression.
The purpose of our study was to explore the involvement of PCs in mechanical load (short-term mechanical stretching)-induced signaling cascades that control human osteoblastogenesis/bone formation.
Materials and methods
General
Cell culture media (including fetal bovine serum, FBS) and all tissue culture reagents were obtained from Invitrogen (Carlsbad, CA, USA). The MDCK-PKD1 stable cell line and the anti-CT rabbit polyclonal antibody were kindly provided by Dr. G.G. Germino [32]. The inhibitory anti-Ig-PKD1 antibody was a kind gift from Dr. O. Ibraghimov-Beskrovnaya (Genzyme, Cambridge, MA, USA) [33, 34]. Antibodies against PC1 (sc-130554), PC2 (sc-28331), pERK1/2 (sc-7383; reactive with phosphorylated ERK1 and ERK2), and pNFATc1 (sc-32979) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The mouse monoclonal NFATc1 antibody was obtained from BD Biosciences (BD-556602, Franklin Lakes, NJ, USA) and the mouse monoclonal actin antibody was purchased from Millipore (MAB-1501, Bedford, MA, USA). The secondary antibodies used for Western immunoblotting were purchased from Thermo Scientific (Ontario, Canada) and Millipore (Bedford, MA, USA) (goat anti-mouse 31430 and goat anti-rabbit AP132P, respectively) and those used for immunofluorescence [goat anti-rabbit (sc-2780) and goat anti-mouse (sc-2010)] were from Santa Cruz Biotechnology. The calcineurin inhibitor CsA and DAPI were from Sigma-Aldrich Chemie (Germany). The oligonucleotides were custom ordered from Invitrogen. The NFATc1-TransAm kit was purchased from Active Motif (Carlsbad, CA, USA). The iScript cDNA synthesis kit was from Bio-Rad (170-890, Hercules, CA, USA) and the RT-PCR reagents were all purchased from Fermentas (Maxima RT EP0741, Hot Start Green PCR Master Mix K1069, Ontario, Canada). Finally, all FACS reagents were from BD Biosciences (Franklin Lakes, NJ, USA) and the FACS Calibur (BD Biosciences) was used for analysis.
Cell cultures
Human PDL fibroblasts were obtained from explant cultures of PDL tissues as detailed previously [10, 14]. Human PDL cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10 % FBS; all experiments were carried out with cells from the third to the sixth passage after being checked for their osteoblastic characteristics.
Mechanical stretch of cultured hPDL cells and preparation of cell extracts
Approximately 4 × 105 cells were seeded onto 50 mm dishes with a flexible, hydrophilic growth surface (SARSTEDT, Germany) and cultivated until they reached complete confluence. The medium was then changed to Dulbecco’s modified Eagle’s medium supplemented with 0.1 % FBS, to remain quiescent. Twenty-four hours later, the hPDL cell cultures were stretched continuously for 30, 60, and 360 min using the stretching apparatus, as described previously [10–14]. This stretching system allows mechanical stimulation of hPDL cells to be quantitated by calculating the percent expansion of the membrane at the bottom surface of the culture dishes using a specific formula previously described [10, 13, 15, 16]. The percentage of stretch applied to hPDL cells in our study was 2.5 %, an amount that closely resembles the strain forces exerted on PDL cells in vivo [10, 12].
Control (unstretched) cultures were incubated under the same conditions for the maximum period of stretch application. Cells were treated with the inhibitors (CsA, anti-Ig-PKD1 antibody) for 3 h prior to stretching in starvation medium (CsA: 5 μg/ml working solution, inhibitory anti-Ig-PKD1 antibody: 1:50 dilution).
After stretch application, cells were washed with ice-cold phosphate-buffered saline and whole-cell or nuclear lysates were obtained as follows. Total cell extracts were prepared in SDS sample buffer as described [35–37]. Nuclear extracts were prepared according to Schreiber et al. [36] except that the nuclear pellet was extracted with 20 mM HEPES–NaOH, pH 7.5, 20 % glycerol, 0.38 M NaCl, 2 mM MgCl2, 10 mM NaF, 10 mM [Na2]-β-glycerophosphate, 10 mM [Na2] p-nitrophenyl phosphate, 0.1 mM Na3VO4, 2 mM phenylmethylsulfonyl fluoride, 5 μg/ml aprotinin, 2 μg/ml pepstatin, 2 μg/ml leupeptin, and 2 mM dithiothreitol. All protein extracts were aliquoted and stored at −80 °C until use.
Western immunoblotting
Protein extracts were resolved on SDS polyacrylamide gels followed by electrotransfer onto polyvinylidene fluoride membranes (Amersham Biosciences, Germany). For PC1, proteins were run on a 6 % gel, for PC2 on a 10 % gel, and for pERK1/2 and actin on a 15 % gel. Membranes were blocked for 1 h at room temperature with 5 % milk in Tris-buffered saline with Tween (TBST; 10 mM Tris–HCl, pH 7.4, 150 mM NaCl, 0.1 % Tween 20) and probed with the primary antibody overnight at 4 °C. After incubation with a horseradish peroxidase-conjugated secondary antibody (Sigma-Aldrich, Germany), immunoreactive bands were visualized by the enhanced chemiluminescence (ECL) kit (Amersham Biosciences, Germany).
The primary antibodies were used in the following dilutions: anti-CT (1:5,000), anti-PC2 (1:250), anti-pERK1/2 (1:500), anti-actin (1:5,000). Both secondary antibodies (goat anti-mouse IgG, goat anti-rabbit IgG) were used at a 1:2,500 dilution.
RNA isolation and RT-PCR analysis
Human PDL cells were serum-deprived in medium containing 0.1 % FBS for 24 h and exposed to mechanical stretch for 1/2, 1, and 6 h, as well as treated with CsA or inhibitory anti-Ig-PKD1 antibody. Total RNA was isolated using the RNeasy Mini Kit (QIAGEN, Valencia, CA, USA) according to the manufacturers’ instructions and cDNA was synthesized using the Fermentas Reverse Transcriptase (Maxima RT, EP0741, Fermentas, Ontario, Canada). RT-PCR analysis was performed for human pkd1, pkd2, and runx2 using the Maxima Hot Start Green PCR Master Mix (K1069, Fermentas, Ontario, Canada) based on the manufacturers’ instructions. Primers used were:
for human pkd1: forward, 5′-CGCCGCTTCACTAGCTTCGAC-3′; reverse, 5′-ACGCTCCAGAGGGAGTCCAC-3′
for human pkd2: forward, 5′-GCGAGGTCTCTGGGGAAC-3′; reverse, 5′-TACACATGGAGCTCATCATGC-3′
and for human runx2: forward, 5′-CCCCACGACAACCGCACCAT-3′; reverse 5′-GTCCACTCCGGCCCACAAATC-3′.
The semi-quantitative PCR approach was performed in parallel by assaying human actin mRNA levels as a reference gene, as described previously [38].
PCR for each gene was performed as follows: for pkd1: 35 cycles and 58 °C annealing; for pkd2: 30 cycles and 53 °C annealing; for runx2: 32 cycles and 59 °C annealing; and for actin: 28 cycles and 57 °C annealing. Reaction products were confirmed on a 2 % agarose gel with size markers (New England Biolabs, MA, USA) and stained with ethidium bromide. The intensity of density area was analyzed using the Quantity-One Programme (Bio-Rad, Hercules, CA, USA). The final PCR product was expressed as the ratio to actin used for scanning analysis.
Immunofluorescence imaging
Seeded hPDL cells on cover slips (10 × 10 mm) in a 24-well plate were grown in DMEM + 10 % to over-confluency and then starved with 0.1 % FBS for 24 h. Cells were fixed for 15 min in a 3.7 % paraformaldehyde solution, washed three times with PBST (0.05 % Tween-20 in PBS) and blocked with 5 % BSA in PBST at room temperature for 1 h. The cells were then incubated with the primary antibodies at a 1:50 dilution for 1 h in PBST+BSA. They were then washed three times in PBST and incubated with the secondary antibodies for 1 h (1:500). Following three washes with PBST, cells were stained with 0.2 μg/ml DAPI in PBS for 1 min and then washed three times in PBS. Cells were mounted in MOWIOL Reagent (Calbiochem, Germany) on glass slides. The slides were examined using a Leica TCS-SPS confocal microscope. Sequential scanning between channels was used to separate fluorescence emission from different fluorochromes and to completely eliminate bleed-through between channels. Typically, 9–15 confocal sections from three independent experiments were quantified with Image-Pro Plus software (Media Cybernetics, Bethesda, MD, USA).
Flow cytometry analysis
Human PDL cells were treated as described in previous sections and exposed to mechanical stretch. After the set time points, cells were harvested and fixed with 1 % paraformaldehyde for 15 min at room temperature. After washing with PBS and blocking for 20 min with 5 % BSA in PBST (0.01 % Tween-20 in PBST), samples were further incubated with the secondary antibody for another hour at room temperature (1:250). Finally, cells were washed twice with PBST and twice with PBS, resuspended in PBS, and subjected to analysis. A total of 10,000 events were analyzed per sample. All antibody incubations were performed at room temperature. Flow cytometry analyses were performed in a FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
TransAM
Nuclear extraction was performed as described previously [36]. Five micrograms of nuclear extract was tested for NFATc1 DNA-binding activity by using TransAM NFATc1 kit, using a wild-type sequence (5′-T/AGGAAA-3′) and a specific NFATc1 antibody, according to the manufacturers’ instructions (Active Motif, Carlsbad, CA, USA). The results (optical density measured at 450 nm) were expressed as percentage increase over basal (untreated cells).
Image and data analysis
For densitometry quantification analysis, three independent Western-blot and PCR results were analyzed using the Quantity One Programme (Bio-Rad, Hercules, CA, USA). Statistical analysis was performed using the SPSS V16.0. Differences between two groups were assessed using analysis of variances followed by a Student’s t test (*p < 0.05, n.s. not significant).
Results
Detection of PC1 and PC2 protein expression in human osteoblastic cells
In order to detect PC1 and PC2 expression in osteoblastic cells, we used primary cultures of fibroblasts isolated from human periodontium with well-established osteoblast-like properties, as verified by the intense immunohistochemical staining for alkaline phosphatase and vimentin, a highly reliable marker of cells originating from the mesenchyme (data not shown) [10–14].
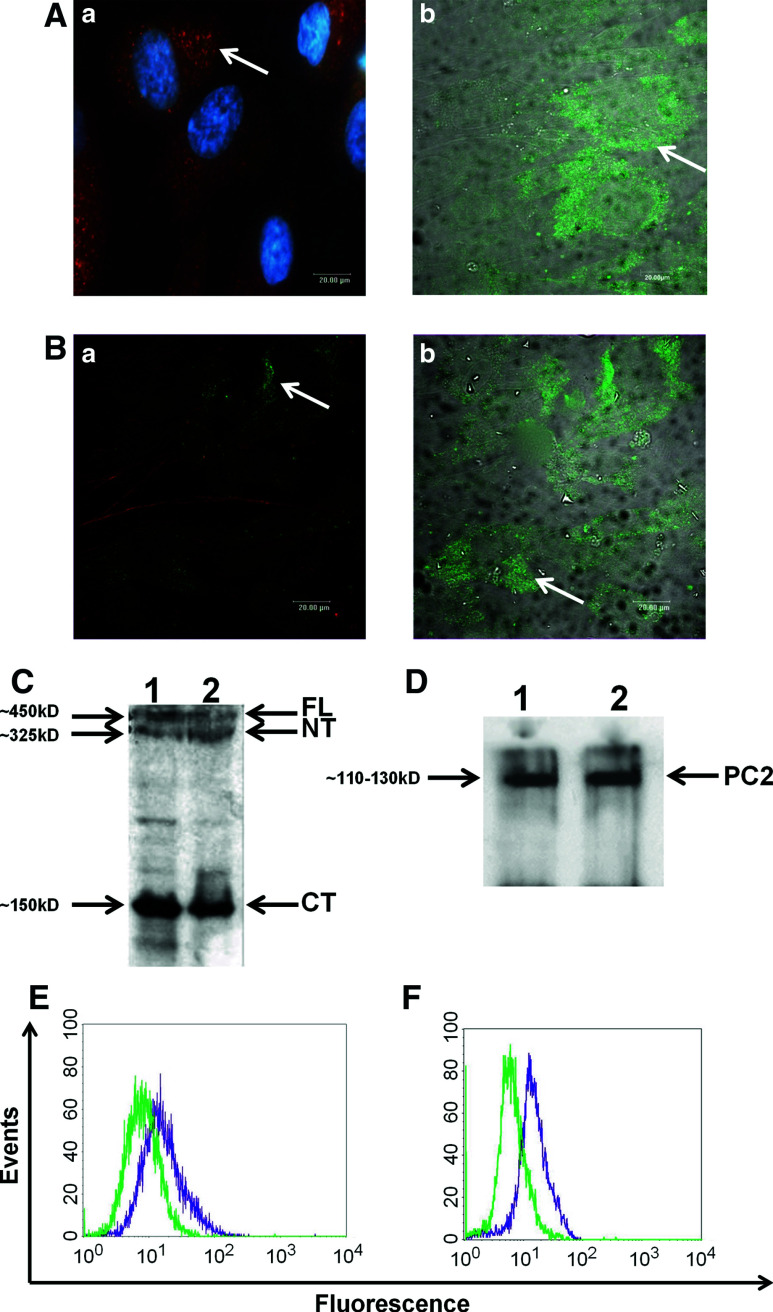
Evidence regarding PCs presence in osteoblasts is rather limited, with only two recent studies demonstrating the presence of PC1–PC2 complex in mouse osteoblasts and osteocytes, further suggesting a possible functional role of PC1 in anabolic signaling of these cells [21, 22]. Inspired by this observation, we sought to determine whether PCs are expressed in human osteoblast-like cells such as periodontal ligament fibroblasts (hPDL cells). Employing immunofluorescence and confocal microscopy, we demonstrated that confluent hPDL cell cultures express both PC1 and PC2 proteins (Fig. 1A, B). For PC1, we used two different antibodies against different epitopes of the protein; a polyclonal antibody against the entire protein (Fig. 1Aa) and a monoclonal antibody against the N-terminal part (Fig. 1Ab). Furthermore, by Western immunoblotting we detected protein expression of both PC1 and PC2 in confluent hPDL cells and positive control cells (Fig. 1C, D). A stably transfected for PC1 MDCK cell line (MDCK-PKD1) previously characterized by Qian et al. [32] was used as a positive control for the aforementioned experiments. Subsequently, the expression of PCs in quiescent hPDL confluent cell cultures was also demonstrated by using flow cytometric analysis (Fig. 1E, F).
Fig. 1.
(A and B) Immunofluorescent images showing expression levels of PC1 and PC2 in human PDL (hPDL) cells. Cells were grown to over-confluency on glass cover slips, starved for 24 h and fixed with 3.7 % PFA. Anti-CT, a polyclonal antibody against PC1 and DAPI stain was used (Aa) as well as a commercially available monoclonal anti-PC1 antibody (scale bar 20 μm) (Ab). Human PDL cells were immunostained with an anti-PC2 antibody as described in the Materials and methods section, as well as with phalloidin stain (scale bar 20 μm) (Ba and Bb). C, D Western immunoblotting showing PC1 and PC2 protein levels in hPDL and MDCK-PKD1 (positive control) cells. Whole-cell protein extracts were isolated as described in the Materials and methods section. An amount of 10–20 μg of protein samples were analyzed on an SDS–polyacrylamide gel (6 % for PC1, 10 % for PC2). Membranes were blotted with anti-CT (1:5000) for PC1 protein detection (lane 1: hPDL cells, lane 2: MDCK-PKD1 cells). Arrows indicate the various detectable forms of PC1 (full length at ~450 kD, N-terminal at ~325 kD and C-terminal at ~150 kD) (C). Membranes were blotted with anti-PC2 antibody (lane 1: hPDL cells, lane 2: MDCK cells) and PC2 protein was detected at ~110–130 kD (D). E, F Flow cytometric analyses indicating basal expression levels of PC1 and PC2 proteins in hPDL cells. Cells were grown to over-confluency and treated for FACS analysis as described in the Materials and methods section. Cell extracts were incubated for 1 h at room temperature with anti-PC1 (E) and anti-PC2 (F) primary antibodies at a dilution of 1:50, followed by a FITC-conjugated secondary antibody (Green: isotype control, purple: PC1/PC2-FITC)
Expression levels of PC1 and PC2 after mechanical stretching of human osteoblastic cells
Previous mechanotransduction studies in our laboratory and others have shown that hPDL cells respond to mechanical stretch and several molecules have been proposed as mechanosensors in bone cells [10–14, 17]. Moreover, PC1 has been attributed a mechanosensor function in the kidney cell line, HEK293T [31]. Consistently, we investigated whether PCs expression in hPDL cells may be affected by mechanical stretching both at mRNA and/or protein level. To address this question, a previously established in vitro system for application of calibrated low-level continuous mechanical stretch in cultured cells was used [10–13, 15, 16]. Confluent hPDL cell cultures grown on Flexi-Perm tissue culture plates were incubated overnight with starvation medium and then subjected to continuous mechanical stretching for various time points, as described in the Materials and methods section. In order to verify the response of hPDL cells to mechanical stretch, the kinetics of ERK1/2 activation [phosphorylated (p) ERK1/2] in hPDL cell protein lysates were monitored by Western immunoblotting and compared to quiescent lysates at different time points (Fig. 2A) [13, 14].
Fig. 2.
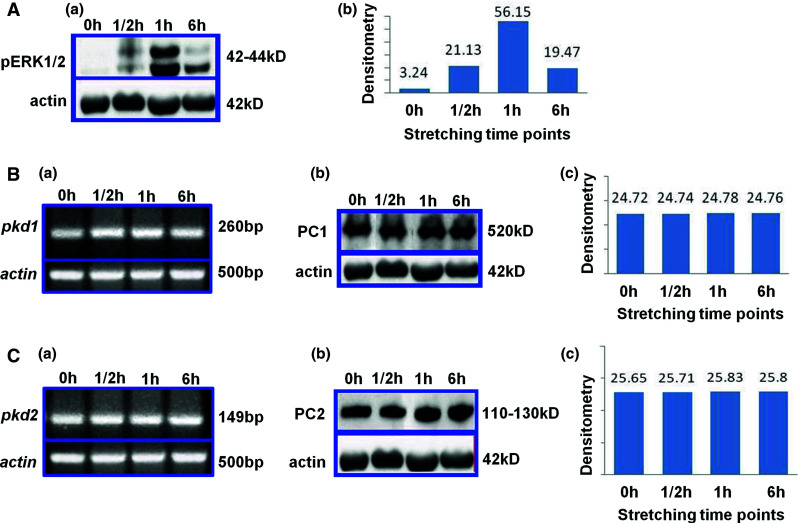
Effect of mechanical stretching on protein and mRNA PC1 and PC2 expression levels at different time points. A Effect of mechanical stretching on pERK1/2 protein levels (Aa) and corresponding densitometric analysis (Ab). B PC1 mRNA levels (Ba), protein levels (Bb) and densitometric analysis of Western-blot results (Bc). C PC2 mRNA levels (Ca) protein levels (Cb) and densitometric analysis of Western-blot results (Cc). Human PDL cells were grown to confluency, subjected to mechanical stretching for different time points (0–6 h) and isolated either for PCR or Western-blot analyses as described in the Materials and methods section. For both, methods, actin was used as the reference gene for quantification. Expected protein sizes are as follows: pERK1/2: 42–44 kD, actin: 42 kD, PC1: ~450 kD and PC2: 110–130 kD. Expected PCR product sizes are as follows: human pkd1: 260 bp, human pkd2: 149 bp and human actin: 500 bp
Total RNA was also isolated from hPDL cells mechanically stretched for different time points (0–6 h) and subjected to RT-PCR analysis. As depicted in Fig. 2Ba, Ca, unstretched cells and cells after continuously applied mechanical load for various time points exhibited marginally equal levels of pkd1 and pkd2 mRNA. Total RNA isolated from the MDCK-PKD1 stably transfected cell line was used as positive control.
To verify the above result, similar stretching experiments using hPDL cell lysates were analyzed by Western immunoblotting using specific antibodies against PC1 and PC2, respectively (Fig. 2Bb, Cb). Densitometric analysis data revealed that PC1 (Fig. 2Bc) and PC2 (Fig. 2Cc) expression levels were not altered significantly after exposure of cells to mechanical load.
Mechanical stretching activates the calcineurin/NFAT signaling pathway in human osteoblastic cells via PC1
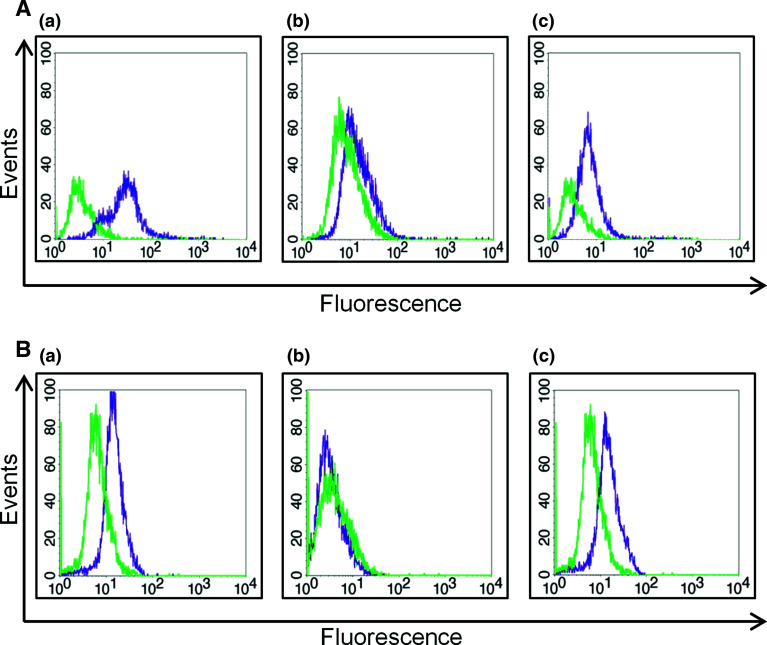
Previous work in HEK293T cells indicated that PC1 signaling leads to activation of the calcineurin/NFAT axis [31]. To determine potential interplay between mechanotransduction and PC1 modulation of Ca2+ influx, we investigated variation in PC1, NFAT and pNFAT expression levels after mechanical stretching of hPDL cells. Specifically, PC1-mediated dephosphorylation and nuclear translocation of NFAT following mechanical stimulation was monitored using FACS analysis and the relative expression levels of PC1 (Fig. 3Aa), NFAT (Fig. 3Ab), and pNFAT (Fig. 3Ac) in extracts from quiescent and mechanically stretched cells were assayed (Fig. 3B–D). PC1 levels remained equally elevated at all time points during mechanical stretching (Fig. 3Ba), whereas NFATc1 increased at 1/2 h and 1 h and returned to normal levels at 6 h (Fig. 3Bb). Inversely, pNFATc1 decreased at 1/2 h and 1 h and increased again at 6 h (Fig. 3Bc). These data demonstrate that mechanical load can induce PC1-mediated activation of the calcineurin/NFAT signaling pathway.
Fig. 3.
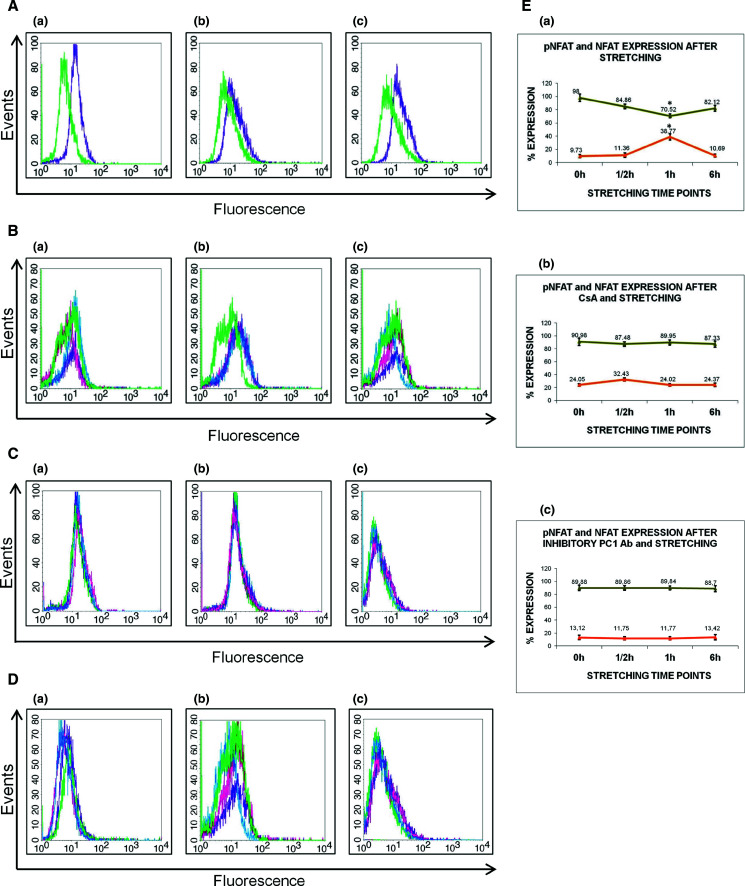
Flow cytometry analyses of baseline expression levels of PC1 (Aa), NFAT (Ab), and pNFAT (Ac). Green depicts the isotype control and purple the respective protein expression. B–D Human PDL cells were grown to over-confluency in Lumon dishes, followed by overnight starvation (DMEM + 0.1 % FBS), treated as need with inhibitors, subjected to mechanical stretching and collected for FACS analysis. FACS of differences in expression levels of PC1, NFAT and pNFAT after mechanical stretching in untreated cells (B), stretching with CsA treatment (5 μg/ml for 3 h in starvation medium) (C) and stretching with inhibitory anti-Ig-PKD1 treatment (1:50 dilution for 3 h in starvation medium) (D). Ten thousand events were analyzed per sample. Green line: negative isotype control; pink line: protein expression at 1/2 h time point; blue line: protein expression at 1 h time point; purple line: protein expression at 6 h time point. E Quantitative estimation of NFAT and pNFAT expression levels following the above treatments. In the graphs, depicted with green color is the pNFAT expression levels and with orange color is the NFAT expression levels. Results display data from one representative experiment, repeated at least three independent times under the same conditions for statistical analysis (*p < 0.05)
In order to validate our results, cells were treated with a well-known specific inhibitor of the calcineurin/NFAT pathway. CsA has been shown to inhibit calcineurin and its substrate NFAT in bone cells in a dose-dependent manner [39]. In accordance with the activation mechanism of NFAT, stretching for 1/2 h of cultured hPDL cells pre-treated with CsA abrogated the observed decrease in the abundance of the cytoplasmic pNFATc1 species (Fig. 3Cc). CsA treatment of hPDL cells did not seem to have an effect on PC1 expression, but did in fact reverse the effect of NFAT activation (Fig. 3Cb).
We next pre-treated mechanically stretched hPDL cells with anti-Ig-PKD, a previously characterized PC1 inhibitory antibody [33], and again we noticed significant abrogation of the observed increase in NFAT levels (Fig. 3D). As shown in Fig. 3Da, the anti-Ig-PKD1 antibody inhibited significantly PC1 expression. To summarize, Fig. 3E displays quantitation graphs from NFAT and pNFAT expression levels following the aforementioned treatments. At the 1/2 h stretching time point, the percent increase in NFAT is analogous to the percent decrease in pNFAT expression levels, with a statistical significance (p < 0.05). Also, the small percent changes in NFAT and pNFAT expression levels in the experiments with either CsA or anti-Ig-PKD1 treatments were not statistically significant (p value n.s.).
PC1-mediated activation of the calcineurin/NFAT signaling pathway in human osteoblastic cells is mechanical stretch-dependent
To determine whether PC1-mediated activation of the calcineurin/NFAT signaling pathway is directly dependent on mechanical stretch, we examined if inhibition of PC1 affects the equilibrium of NFAT/pNFAT expression levels in hPDL cells, in the absence of mechanical stimulation. We used FACS analysis to compare baseline expression levels of NFAT and pNFAT in hPDL cells to cells pre-treated with the PC1 inhibitory antibody. Indeed, the shift in fluorescence intensity indicating baseline PC1expression levels (Fig. 4Aa) was significantly reduced (p < 0.05) when cells were pre-incubated with the anti-IgPKD antibody (Fig. 4Ba). Additionally, NFAT and pNFAT expression levels displayed no difference between the quiescent untreated (Fig. 4Ab, Ac) and anti-Ig-PKD1-treated cells (Fig. 4Bb, Bc) in the absence of mechanical stretching (p value n.s.). This result suggests that the mechanical load stimulus is needed in order for PC1 to activate the calcineurin/NFAT signaling pathway.
Fig. 4.
FACS analysis of PC1, NFAT, and pNFAT expression levels in quiescent hPDL cells (Aa) compared to pre-treated cells with the inhibitory anti-Ig-PKD1 antibody (PC1-fluorescence: purple line, isotype control: green line). A significant inhibition of PC1 expression (*p < 0.05) was observed when cells were incubated with anti-Ig-PKD1 for 3 h prior to collection (Ba) compared to the expression levels of PC1 in untreated cells (Aa). NFAT and pNFAT expression levels did not differ between the quiescent untreated (Ab, Ac) and quiescent anti-Ig-PKD1-treated cells (Bb, Bc) in the absence of mechanical stretching. Statistical analysis was performed and data are representative of three independent experiments
Mechanical stretching induces activation of the NFAT transcription factor in human osteoblastic cells
To further investigate the specific activation of NFAT transcription factor under conditions of mechanical load applied to hPDL cells, the TransAM NFAT cell-based assay was employed. The TransAM methodology allows sensitive, non-radioactive determination of specific transcription factor activation in mammalian tissues and cell extracts (Fig. 5A).
Fig. 5.
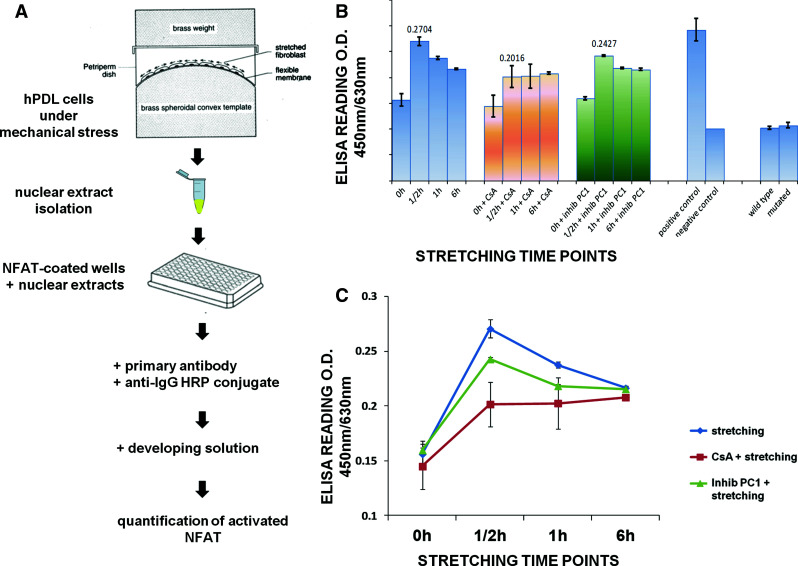
Schematic representation of the TransAM ELISA kit used for detection of NFAT transcription factor activation in mechanically-stretched hPDL cells (A). Cells were grown to over-confluency, subjected to mechanical stress for various time points (0–6 h) and nuclear protein extracts were carefully isolated as described in the Materials and methods section. B, C Quantified colorimetric measurements by spectrophotometry using the TransAM kit for the activation of the NFAT transcription factor in mechanically stretched hPDL cells for various time points and different treatments (blue: stretched + untreated cells; orange: stretched + CsA-treated cells; green: stretched + anti-Ig-PKD1-treated cells). Results are representative of three independent experiments (*p < 0.05)
Following the same conditions of mechanical stretching previously applied for FACS analysis (Fig. 3), untreated and pre-treated with either CsA or the anti-Ig-PKD1 antibody hPDL cells were subjected to mechanical stretch for set time points. Cells were collected and nuclear extracts isolated for assaying NFAT activity following mechanostimulation. Indeed, our data revealed a statistically significant activation (p < 0.05) of NFAT in nuclear extracts from hPDL cells after 1/2 h of mechanical stretch (Fig. 5B, C). This activation gradually decreased at the 1- and 6-h time points. Furthermore, pre-treatment of cells with the two inhibitory agents (CsA and anti-Ig-PKD1) partially inhibited this activation. This finding demonstrates the PC1-triggered activation of NFAT after mechanical stretching of hPDL cells.
Modulation of runx2 gene expression by PC1-mediated mechanical stimulation of human osteoblastic cells
Previous work in our laboratory has shown that exposure of hPDL cells to short-term mechanical stretch affects osteoblastic gene expression and specifically runx2 mRNA and protein expression levels [14, 17]. Consequently, we asked whether PC1-mediated mechanical stimulation can potentially influence runx2 transcription. Based on computational analysis (Genomatix, TFSEARCH) and recent Ref. [40] putative NFATc1-binding sites (5′-GGAAA-3′) are present in the 5′ regulatory region of the runx2 promoter near the transcription start site, and in proximity with the Runx2-binding motifs previously studied in our laboratory [14]. To this end, untreated hPDL cells (Fig. 6A) and cells pre-treated with the inhibitory anti-Ig-PKD1 antibody (Fig. 6B) were subjected to mechanical stretch at different time points (0–6 h) and total RNA was isolated. As depicted in Fig. 6A, unstretched cells (0 h time point) exhibit low levels of runx2 mRNA. These levels are increased by greater than twofold after 1/2 h of continuously applied mechanical load and remain elevated until 6 h (Fig. 6A). By contrast, mechanically stretched hPDL cells pre-incubated with the PC1 inhibitory antibody continued to exhibit low levels of runx2 mRNA at the 1/2 h time point with a slight increase thereafter (Fig. 6B). These results demonstrate that in hPDL cells, PC1-mediated mechanostimulation induces the expression of the osteoblast-specific transcriptional regulator Runx2.
Fig. 6.
Effect of mechanical stretching on runx2 gene expression levels. Quiescent hPDL cells were subjected to mechanical stretching and treated as described in the materials and methods section, at the indicated time points. cDNA was synthesized from total RNA and runx2 gene expression levels were studied for (A) stretched untreated cells (the 1/2 h time point showed greater than twofold increase compared to quiescent cells—0 h time point) and (B) stretched cells treated with the inhibitory anti-Ig-PKD1 antibody (the 1/2 h time point showed no change compared to quiescent cells—0 h time point). Human actin was used as the reference gene for normalization. Images are representative of one experiment. Statistical analysis was performed on data from three independent experiments (*p < 0.05)
Discussion
Osteoblasts are key components of the bone multicellular unit and have a seminal role in bone program, the skeleton’s essential function for the maintenance of its structural integrity and metabolic capacity. The coordinated function of these skeletal effector cells is regulated by a number of hormones, growth factors, and mechanical cues that act via interconnected signaling networks. Mechanical signals, in the form of fluid flow stress and compressive force, induce anabolic response of bone cells and this is of pivotal importance in the field of biomechanics for the treatment of osteodegenerative diseases. Here we investigated (and introduce for the first time) an important role of PC1 as a mechanosensor molecule in human osteoblastic cells, capable of modulating intracellular signaling pathways culminating to regulation of osteoblast differentiation and ultimately bone formation.
PCs have been previously attributed a mechanosensor role in kidney cells where experimental data have shown PC1’s reception of signals from primary cilia through its N-terminus and subsequent transduction into cellular responses regulating proliferation, adhesion, differentiation, and cell morphology [19, 24–27]. Studies in renal epithelial cells provide compelling evidence on the necessity of PC2’s presence and complex formation with PC1 located at the primary cilium of the cell for mechanosensation properties [27].
PCs presence in human osteoblasts is documented for the first time in the present study being consistent with the limited evidence that exists for mouse chondrocytes, osteocytes, and osteoblasts, where a link between PC1 and osteoblast differentiation and bone development is suggested [21–23]. Our data demonstrate the expression of both PCs at the mRNA as well as the protein level in primary osteoblast-like cell cultures.
Furthermore, we provide evidence for a novel role of PC1 in responding to mechanical load applied to bone cells. Using an optimal tissue culture system and a highly efficient method of applying short-term mechanical stretching on osteoblast-like cells, previously established in our laboratory, we demonstrate PC1 and PC2 expression (both at mRNA and protein level) in hPDL cells subjected to mechanical stretch for various time points. This finding is in accordance with other studies reporting the ability of alternative types of mechanical force (with different magnitude, frequency, and duration) to stimulate a mechanosensor molecule such as PC1 and regulate skeletal tissue homeostasis [23, 27, 41]. Indeed, previous work from our group has shown that low-level continuous mechanical strain of hPDL cells rapidly induces the principal constituents of the transcription factor AP-1 via ERK/c-Jun N-terminal kinase (JNK) signaling, enhancing its DNA-binding activity on osteoblast-specific genes such as runx2, hence modulating their expression rate [14, 17]. These data provided an important molecular link between mechanotransduction and bone-specific transcription factor function in human osteoblasts [13, 14]. Moreover, they urged us to search for novel intracellular molecules and associated signaling pathways that can be affected by mechanical stimuli and ultimately direct bone cell differentiation. Recent work by Xiao et al. [21, 22] applied mouse genetic approaches to create combined PC1 and Runx2-II-deficient mice in order to demonstrate a functional linkage between PC1, Runx2-II expression and skeletal development in vivo. Their findings suggested that PC1 can act as a potential regulator of skeletal development through the selective regulation of Runx2-II [21, 22]. In accordance to these data, our study revealed modulation of runx2 gene expression by PC1-mediated mechanical stimulation. mRNA analysis of mechanically stretched hPDL cells in the presence or absence of a PC1-specific inhibitor unveiled a link between PC1 and runx2 expression levels.
Searching for the signaling pathway that may link PCs induction after mechanical stimulation with runx2 activation in osteoblastic cells, we were directed towards the calcineurin/NFAT pathway since this is a vital signal transduction cascade implicated in the control of cell differentiation, apoptosis, and cellular adaptation in a broad gamut of cell types and tissues [28–30]. Therefore, it was rational to investigate the possible activation of calcineurin/NFAT axis by mechanical forces exerted upon the cell. In addition, regulation of intracellular Ca2+ has been previously associated with the function of PC1 and PC2 [24–27, 31, 42]. It was thus suggested that PC1, upon stimulation by a mechanical stimulus, might potentiate the calcineurin/NFAT signaling pathway ultimately leading to modulation of NFAT target genes activity. Indeed, our data showed that even though PC1 levels remained equally elevated during mechanical stretching, NFATc1 increased at 1/2 and 1 h and returned to normal levels at 6 h. Inversely, pNFATc1 decreased at 1/2 and 1 h and increased again at 6 h. To further strengthen this observation, we treated mechanically stretched cells with either an inhibitor of the calcineurin/NFAT pathway (CsA), or an inhibitor of PC1 (anti-Ig-PKD antibody). In both cases, the effect on the NFAT/pNFAT levels was reversed, demonstrating that mechanical stretch can induce PC1-mediated activation of the calcineurin/NFAT signaling pathway. This observation is in accordance with the study of Puri et al. [31] where it was demonstrated that the C-tail of PC1 can activate the calcineurin/NFAT signaling pathway based on in vitro data from HEK293T cells. Therefore, our study is the first to show PC1-mediated activation of the calcineurin/NFAT signaling axis in human osteoblastic cells and further link this activation to mechanical stimulation.
Furthermore, the expression of PC2 was detectable in human osteoblast-like cells for the first time with limited, however, evidence regarding its functional role in the induction of Ca2+ signaling. Experiments with stress activation of hPDL cells in the absence of extracellular Ca2+ are being designed to investigate whether and to what extend PC2 contributes to the observed NFAT activation.
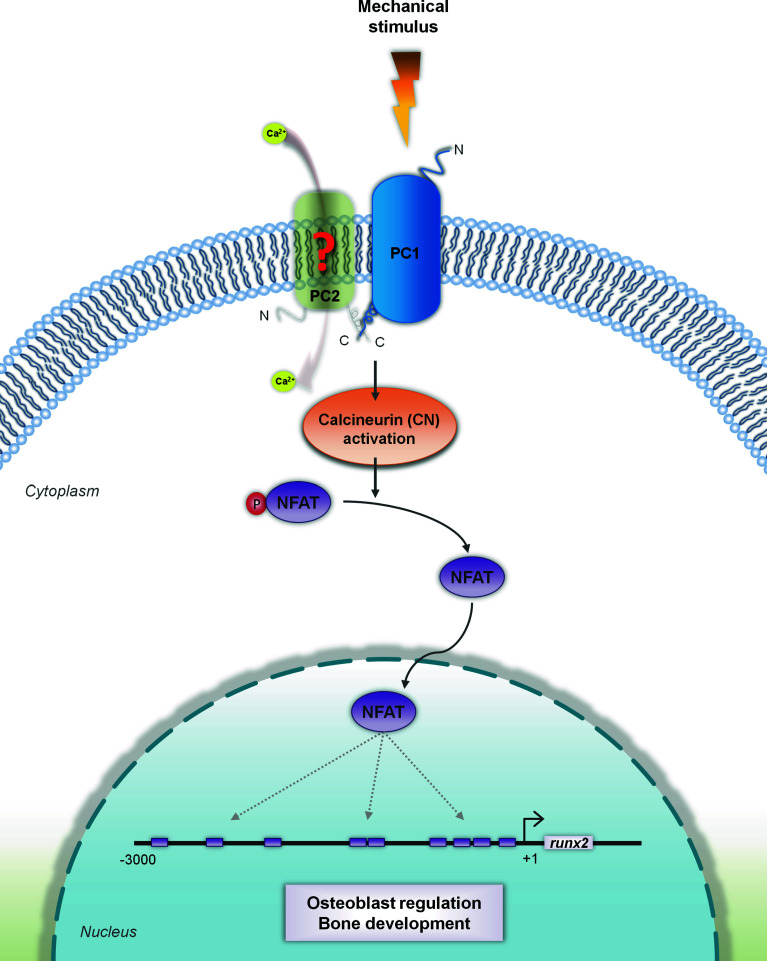
Taken together, our results can be integrated into a schematic model that links PC1 to induction of human osteoblastic gene expression via potentiation of the calcineurin/NFAT signaling axis under mechanical stimulation (Fig. 7). According to this model, the extracellular N-terminal part of PC1 acts as a mechanosensor that responds to the external mechanical signal applied on the cell by activating the calcineurin/NFAT pathway via dephosphorylation of NFAT. The active form of NFAT is then translocated to the nucleus were it can modulate runx2 gene expression [40].
Fig. 7.
Schematic representation of the proposed crosstalk between PC1 and calcineurin/NFAT signaling in hPDL cells under mechanical stimulation. The extracellular N-terminal part of PC1 acts as a mechanosensor that responds to the external mechanical stimulus applied onto the cell. As a result, PC1 activates the calcineurin (CN)/NFAT pathway by allowing the dephosphorylation of NFAT [42]. Activation of the NFAT pathway may possibly involve interaction and complex formation between PC1 and PC2 (depicted as green shadow), requiring, however, further experimental verification (red question mark). The active form of NFAT then translocates to the nucleus were it can modulate runx2 gene expression, a crucial osteoblastic gene that influences osteoblast differentiation. Putative NFAT-binding sites in the runx2 promoter based on literature [40] and computational analysis are shown. PC1: petrol blue; PC2: green shadow; mechanical stimulus: orange lightning; pNFAT (phosphorylated, inactive form): purple oval with attached red circle indicating phosphate group; NFAT (dephosphorylated, active form): purple oval; runx2 promoter: black line with purple rectangles demarcating putative NFAT-binding sites
In conclusion, our study uncovers an important role of PC1 as a key molecule that can sense environmental cues such as mechanical strain and regulate signaling pathways leading to modulation of osteoblastic gene expression and control of differentiation and bone program.
Acknowledgments
We are grateful to Dr. G.G. Germino for the anti-CT antibody and the stably transfected MDCK-PKD1 cell line. We thank Dr. Oxana Ibraghimov-Beskrovnaya for the inhibitory anti-Ig-PKD1 antibody.
Abbreviations
- ADPKD
Autosomal dominant polycystic kidney disease
- CsA
Cyclosporin A
- FACS
Fluorescent-activated cell sorting
- hPDL cells
Human periodontal ligament cells
- NFAT
Nuclear factor of activated T cells
- NFATc1
Nuclear factor of activated T cells, cytoplasmic 1
- PC1
Polycystin-1
- PC2
Polycystin-2
- Pkd ½
Polycystic kidney disease 1/2 (polycystin 1/2) gene
- pNFAT
Phosphorylated nuclear factor of activated T cells
- Runx2
Runt-related transcription factor 2
Footnotes
G. Dalagiorgou and C. Piperi contributed equally to this study.
References
- 1.Chen JH, Liu C, You L, Simmons CA. Boning up on Wolff’s law: mechanical regulation of the cells that make and maintain bone. J Biomech. 2010;43:108–118. doi: 10.1016/j.jbiomech.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 2.Marie PJ. Transcription factors controlling osteoblastogenesis. Arch Biochem Biophys. 2008;473:98–105. doi: 10.1016/j.abb.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 3.Papachristou DJ, Papachroni KK, Basdra EK, Papavassiliou AG. Signaling networks and transcription factors regulating mechanotransduction in bone. BioEssays. 2009;31:794–804. doi: 10.1002/bies.200800223. [DOI] [PubMed] [Google Scholar]
- 4.Papachroni KK, Karatzas DN, Papavassiliou KA, Basdra EK, Papavassiliou AG. Mechanotransduction in osteoblast regulation and bone disease. Trends Mol Med. 2009;15:208–216. doi: 10.1016/j.molmed.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Chien MY, Wu YT, Hsu AT, Yang RS, Lai JS. Efficacy of a 24-week aerobic exercise programme for osteopenic postmenopausal women. Calcif Tissue Int. 2000;67:443–448. doi: 10.1007/s002230001180. [DOI] [PubMed] [Google Scholar]
- 6.Iwamoto J, Takeda T, Ichimura S. Effect of exercise training and detraining on bone mineral density in postmenopausal women with osteoporosis. J Orthop Sci. 2001;6:128–132. doi: 10.1007/s007760100059. [DOI] [PubMed] [Google Scholar]
- 7.Sinaki M, Brey RH, Hughes CA, Larson DR, Kaufman KR. Significant reduction in risk of falls and back pain in osteoporotic-kyphotic women through a Spinal Proprioceptive Extension Exercise Dynamic (SPEED) Programme. Mayo Clin Proc. 2005;80:849–855. doi: 10.4065/80.7.849. [DOI] [PubMed] [Google Scholar]
- 8.Kemmler W, Lauber D, Weineck J, Hensen J, Kalender W, Engelke K. Benefits of 2 years of intense exercise on bone density, physical fitness, and blood lipids in early postmenopausal osteopenic women: results of the Erlangen Fitness Osteoporosis Prevention Study (EFOPS) Arch Intern Med. 2004;164:1084–1091. doi: 10.1001/archinte.164.10.1084. [DOI] [PubMed] [Google Scholar]
- 9.Todd JA, Robinson RJ. Osteoporosis and exercise. Postgrad Med J. 2003;79:320–323. doi: 10.1136/pmj.79.932.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basdra EK, Papavassiliou AG, Huber LA. Rab and rho GTPases are involved in specific response of periodontal ligament fibroblasts to mechanical stretching. Biochim Biophys Acta. 1995;1268:209–213. doi: 10.1016/0167-4889(95)00090-F. [DOI] [PubMed] [Google Scholar]
- 11.Basdra EK, Komposch G. Osteoblast-like properties of human periodontal ligament cells: an in vitro analysis. Eur J Orthod. 1997;19:615–621. doi: 10.1093/ejo/19.6.615. [DOI] [PubMed] [Google Scholar]
- 12.Kletsas D, Basdra EK, Papavassiliou AG. Mechanical stress induces DNA synthesis in PDL fibroblasts by a mechanism unrelated to autocrine growth factor action. FEBS Lett. 1998;430:358–362. doi: 10.1016/S0014-5793(98)00695-4. [DOI] [PubMed] [Google Scholar]
- 13.Peverali FA, Basdra EK, Papavassiliou AG. Stretch-mediated activation of selective MAPK subtypes and potentiation of AP-1 binding in human osteoblastic cells. Mol Med. 2001;7:68–78. [PMC free article] [PubMed] [Google Scholar]
- 14.Ziros PG, Gil AP, Georgakopoulos T, Habeos I, Kletsas D, Basdra EK, Papavassiliou AG. The bone-specific transcriptional regulator Cbfa1 is a target of mechanical signals in osteoblastic cells. J Biol Chem. 2002;277:23934–23941. doi: 10.1074/jbc.M109881200. [DOI] [PubMed] [Google Scholar]
- 15.Yousefian JZ, Ngan PW, Miller B, Shanfeld J, Davidovitch Z. Effect of different types of stress on human periodontal ligament cells in vitro. In: Davidovitch Z, editor. Biological mechanisms of tooth movement and craniofacial adaptation. Colombus: The Ohio State University, College of Dentistry; 1992. pp. 319–329. [Google Scholar]
- 16.Naruse K, Sokabe M. Involvement of stretch-activated ion channels in Ca2+ mobilisation to mechanical stretch in endothelial cells. Am J Physiol. 1993;264:C1037–C1044. doi: 10.1152/ajpcell.1993.264.4.C1037. [DOI] [PubMed] [Google Scholar]
- 17.Ziros PG, Basdra EK, Papavassiliou AG. Runx2: of bone and stretch. Int J Biochem Cell Biol. 2008;40:1659–1663. doi: 10.1016/j.biocel.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 18.Reeders ST, Breuning MH, Davies KE, Nicholls RD, Jarman AP, Higgs DR, Pearson PL, Weatherall DJ. A highly polymorphic DNA marker linked to adult polycystic kidney disease on chromosome 16. Nature. 1985;317:542–544. doi: 10.1038/317542a0. [DOI] [PubMed] [Google Scholar]
- 19.Zhou J. Polycystins and primary cilia: primers for cell cycle progression. Annu Rev Physiol. 2009;71:83–113. doi: 10.1146/annurev.physiol.70.113006.100621. [DOI] [PubMed] [Google Scholar]
- 20.Dalagiorgou G, Basdra EK, Papavassiliou AG. Polycystin-1: function as a mechanosensor. Int J Biochem Cell Biol. 2010;42:1610–1613. doi: 10.1016/j.biocel.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 21.Xiao Z, Zhang S, Mahlios J, Zhou G, Magenheimer BS, Guo D, Dallas SL, Maser R, Calvet JP, Bonewald L, Quarles LD. Cilia-like structures and polycystin-1 in osteoblasts/osteocytes and associated abnormalities in skeletogenesis and Runx2 expression. J Biol Chem. 2006;281:30884–30895. doi: 10.1074/jbc.M604772200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao Z, Zhang S, Magenheimer BS, Luo J, Quarles LD. Polycystin-1 regulates skeletogenesis through stimulation of the osteoblast-specific transcription factor RUNX2-II. J Biol Chem. 2008;283:12624–12634. doi: 10.1074/jbc.M710407200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hou B, Kolpakova-Hart E, Fukai N, Wu K, Olsen BR. The polycystic kidney disease 1 (Pkd1) gene is required for the responses of osteochondroprogenitor cells to midpalatal suture expansion in mice. Bone. 2009;44:1121–1133. doi: 10.1016/j.bone.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanaoka K, Qian F, Boletta A, Bhunia AK, Piontek K, Tsiokas L, Sukhatme VP, Guggino WB, Germino GG. Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature. 2000;408:990–994. doi: 10.1038/35050128. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez-Perrett S, Kim K, Ibarra C, Damiano AE, Zotta E, Batelli M, Harris PC, Reisin IL, Arnaout MA, Cantiello HF. Polycystin-2, the protein mutated in autosomal dominant polycystic kidney disease (ADPKD), is a Ca2+-permeable nonselective cation channel. Proc Natl Acad Sci USA. 2001;98:1182–1187. doi: 10.1073/pnas.98.3.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koulen P, Cai Y, Geng L, Maeda Y, Nishimura S, Witzgall R, Ehrlich BE, Somlo S. Polycystin-2 is an intracellular calcium release channel. Nat Cell Biol. 2002;4:191–197. doi: 10.1038/ncb754. [DOI] [PubMed] [Google Scholar]
- 27.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. J Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 28.Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 29.Flanagan WM, Corthesy B, Bram RJ, Crabtree GR. Nuclear association of a T-cell transcription factor blocked by FK-506 and cyclosporine A. Nature. 1991;352:803–807. doi: 10.1038/352803a0. [DOI] [PubMed] [Google Scholar]
- 30.Zayzafoon M. Calcium/calmodulin signaling controls osteoblast growth and differentiation. J Cell Biochem. 2006;97:56–70. doi: 10.1002/jcb.20675. [DOI] [PubMed] [Google Scholar]
- 31.Puri S, Magenheimer BS, Maser RL, Ryan EM, Zien CA, Walker DD, Wallace DP, Hempson SJ, Calvet JP. Polycystin-1 activates the calcineurin/NFAT (nuclear factor of activated T-cells) signaling pathway. J Biol Chem. 2004;53:55455–55564. doi: 10.1074/jbc.M402905200. [DOI] [PubMed] [Google Scholar]
- 32.Qian F, Boletta A, Bhunia AK, Xu H, Liu L, Ahrabi AK, Watnick TJ, Zhou F, Germino GG. Cleavage of polycystin-1 requires the receptor for egg jelly domain and is disrupted by human autosomal-dominant polycystic kidney disease 1-associated mutations. Proc Natl Acad Sci USA. 2002;99:16981–16986. doi: 10.1073/pnas.252484899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ibraghimov-Beskrovnaya O, Bukanov NO, Donohue LC, Dackowski WR, Klinger KW, Landes GM. Strong homophilic interactions of the Ig like domains of polycystin-1, the protein product of an autosomal dominant polycystic kidney disease gene, PKD1. Hum Mol Genet. 2000;9:1641–1649. doi: 10.1093/hmg/9.11.1641. [DOI] [PubMed] [Google Scholar]
- 34.Streets AJ, Wagner BE, Harris PC, Ward CJ, Ong AC. Homophilic and heterophilic polycystin 1 interactions regulate E-cadherin recruitment and junction assembly in MDCK cells. J Cell Sci. 2009;122:1410–1417. doi: 10.1242/jcs.045021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marais R, Wynne J, Treisman R. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell. 1993;73:381–393. doi: 10.1016/0092-8674(93)90237-K. [DOI] [PubMed] [Google Scholar]
- 36.Schreiber E, Matthias P, Muller MM, Schaffner W. Rapid detection of octamer binding proteins with ‘min-extracts’, prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujii S, Maeda H, Wada N, kano Y, Akamine A. Establishing and characterizing human periodontal ligament fibroblasts immortalized by SV40T-antigen and hTERT gene transfer. Cell Tissue Res. 2006;324:117–125. doi: 10.1007/s00441-005-0101-4. [DOI] [PubMed] [Google Scholar]
- 38.Farmaki E, Mkrtchian S, Papazian I, Papavassiliou AG, Kiaris H. ERp29 regulates response to doxorubicin by a PERK-mediated mechanism. Biochim Biophys Acta. 2011;1813:1165–1171. doi: 10.1016/j.bbamcr.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 39.Yeo H, Beck LH, McDonald JM, Zayzafoon M. Cyclosporin A elicits dose-dependent biphasic effects on osteoblast differentiation and bone formation. Bone. 2007;40:1502–1516. doi: 10.1016/j.bone.2007.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Penolazzi L, Lisignoli G, lambertini E, Torreggiani E, Manferdini C, Lolli A, Vecchiatini R, Ciardo F, Gabusi E, Facchini A, Gambari R, Piva R. Transcription factor decoy against NFATc1 in human primary osteoblasts. Int J Mol Med. 2011;28:199–206. doi: 10.3892/ijmm.2011.701. [DOI] [PubMed] [Google Scholar]
- 41.Kolpakova-Hart E, McBratney-Owen B, Hou B, Fukai N, Nicolae C, Zhou J, Olsen BR. Growth of cranial synchondroses and sutures requires polycystin-1. Dev Biol. 2008;321:407–419. doi: 10.1016/j.ydbio.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aguiari G, Trimi V, Bogo M, Magnolini A, Szabadkai G, Pinton P, Witzgall R, Harris PC, Borea PA, Del Senno L. Novel role of polycystin-1 in modulating cell proliferation trough calcium oscillations in kidney cells. Cell Prolif. 2008;41:554–573. doi: 10.1111/j.1365-2184.2008.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]