Abstract
River‐dominated continental shelf environments are active sites of air‐sea CO2 exchange. We conducted 13 cruises in the northern Gulf of Mexico, a region strongly influenced by fresh water and nutrients delivered from the Mississippi and Atchafalaya River system. The sea surface partial pressure of carbon dioxide (pCO2) was measured, and the air‐sea CO2 flux was calculated. Results show that CO2 exchange exhibited a distinct seasonality: the study area was a net sink of atmospheric CO2 during spring and early summer, and it was neutral or a weak source of CO2 to the atmosphere during midsummer, fall, and winter. Along the salinity gradient, across the shelf, the sea surface shifted from a source of CO2 in low‐salinity zones (0≤S<17) to a strong CO2 sink in the middle‐to‐high‐salinity zones (17≤S<33), and finally was a near‐neutral state in the high‐salinity areas (33≤S<35) and in the open gulf (S≥35). High pCO2 values were only observed in narrow regions near freshwater sources, and the distribution of undersaturated pCO2 generally reflected the influence of freshwater inputs along the shelf. Systematic analyses of pCO2 variation demonstrated the importance of riverine nitrogen export; that is, riverine nitrogen‐enhanced biological removal, along with mixing processes, dominated pCO2 variation along the salinity gradient. In addition, extreme or unusual weather events were observed to alter the alongshore pCO2 distribution and to affect regional air‐sea CO2 flux estimates. Overall, the study region acted as a net CO2 sink of 0.96 ± 3.7 mol m−2 yr−1 (1.15 ± 4.4 Tg C yr−1).
Keywords: carbon cycle, gas exchange, river plume
Key Points:
pCO2 varied along the salinity gradient, showing low values in middle salinities
This study region was generally a CO2 sink or was neutral (relative to the air)
Biological and mixing processes dominated sea surface pCO2 variations
1. Introduction
The continental shelf accounts for about ∼30% of oceanic net ecosystem production [Ducklow and McCallister, 2005] and 15–21% of the net annual carbon dioxide (CO2) sink of the global ocean [Borges, 2011; Cai, 2011; Cai et al., 2006; Chen and Borges, 2009] even though it accounts for only 7% of the area of the sea surface. Despite continental margins' importance in the global carbon budget and the fact that they are sensitive to impacts of both anthropogenic perturbation and climate‐related changes, insufficient attention has been given to carbon cycle research in continental shelf systems [Bauer et al., 2013]. Past studies have shown substantial spatial and temporal heterogeneity of air‐sea CO2 fluxes on continental shelves that experience moderate river impacts [Jiang et al., 2008; Salisbury et al., 2008]. For continental shelves receiving large freshwater discharge and nutrient fluxes, coastal eutrophication has been shown to affect the CO2 system [Borges and Gypens, 2010; Cai et al., 2011; Chou et al., 2011; Chen et al., 2012]. Moreover, circulation can be complex on continental shelves [Lentz and Fewings, 2012], and thus sharp variations in partial pressure of CO2 (pCO2) are expected in river plumes and surrounding areas due to strong gradients in biological and physical processes [Cai, 2003; Chou et al., 2013; Cooley et al., 2007; Kortzinger, 2003; Ternon et al., 2000; Tseng et al., 2011; Tsunogai et al., 1997; Zhai and Dai, 2009]. Therefore, a better understanding of CO2 distributions and air‐sea CO2 fluxes in river‐dominated continental shelves is needed to improve global carbon budgets.
The Mississippi and Atchafalaya River System is one of the world's largest rivers, ranking sixth in freshwater discharge (18,400 m3 s−1) [Milliman and Meade, 1983]. Seventy percent of the discharge is through the Mississippi River to the northern Gulf of Mexico, while the remaining ∼30% is via the Atchafalaya River (Figure 1) [Walker et al., 2005]. Each river contributes similar amounts of freshwater discharge to the Louisiana shelf and adjacent areas that are the focus of this study, because all freshwater from the Atchafalaya River flows onto the shelf, but only about half of the freshwater discharge from the Mississippi River (approximately 35% of the total) is transported westward to the Louisiana shelf [Dinnel and Wiseman, 1986; Etter et al., 2004; Lehrter et al., 2013]. Overall, the Mississippi and Atchafalaya River System delivers 1 to 1.5 Tg (Tg = 1012 g) of nitrogen, about two‐thirds of it as nitrate N [Goolsby et al., 2000; Lehrter et al., 2013] and ∼17 Tg as bicarbonate C annually to the northern Gulf of Mexico [Raymond et al., 2008; Lohrenz et al., 2013].
Figure 1.
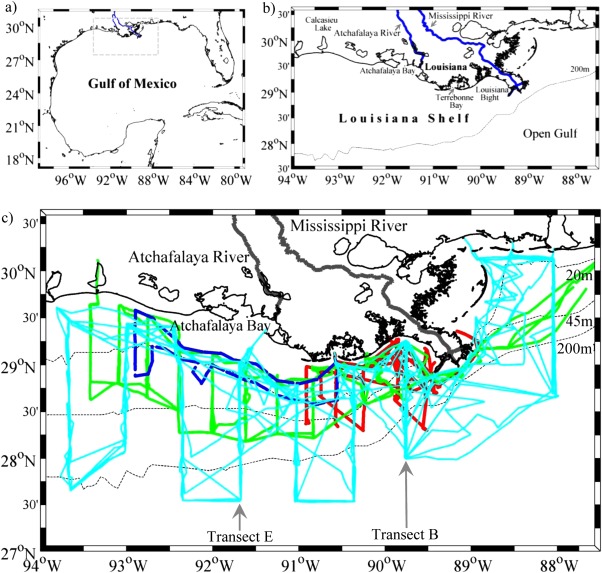
Study area and cruise tracks. The study area was in the northern Gulf of Mexico (the gray rectangular in plot) which was enlarged in plot (b) to display geographic setting of the Mississippi and Atchafalaya River system and various coastal bays and estuaries. The cross‐shelf area (light blue lines) was surveyed during 2009–2010 (c). The shelf‐wide area (green lines) was surveyed during June 2006–2007 (c), and this area was used as a reference area to unify the shelf‐wide and cross‐shelf survey areas when discussing air‐sea CO2 fluxes. The Louisiana Bight and its adjacent areas (red lines) were surveyed from 2004 to June 2006 (c). The inner shelf (dark blue lines) was surveyed in 2008 (c).
The large freshwater discharge and nitrogen loading contribute to distinct river‐ocean mixing dynamics that are associated with enhanced biological production, both leading to low pCO2 in the plume and the adjacent coastal system [Cooley and Yager, 2006; Salisbury et al., 2008; Cai et al., 2013; Tseng et al., 2011]. Prior studies in this region provide evidence of strong nutrient enhancement of biological production [Dagg et al., 2007; Green et al., 2008; Murrell et al., 2013; Turner and Rabalais, 2013] that contribute to strong drawdown of CO2 in the moderate salinity region coinciding with the location of maximum nutrient removal [Guo et al., 2012; Huang et al., 2012].
Previous measurements have shown a strong cross‐shelf pCO2 gradient with the inner shelf being undersaturated with respect to atmospheric CO2 and the outer shelf being oversaturated [Cai, 2003; Lohrenz and Cai, 2006; Lohrenz et al., 2010], along with a distinct temporal variation of air‐sea CO2 fluxes near the Mississippi River delta [Lohrenz et al., 2010]. Air‐sea CO2 fluxes in the Gulf of Mexico reported in “The First State of the Carbon Cycle Report (SOCCR)” [Chavez et al. 2007] were based on very limited observations and were insufficient to characterize seasonal variations of sea surface pCO2 and to quantify the annual air‐sea CO2 flux. Thus, more comprehensive field measurements with sufficient seasonal and shelf coverage are needed to more fully describe the pCO2 variations over the northern Gulf of Mexico and to understand the role of riverine nitrogen in sea surface pCO2 variations.
The major objectives of this study were to determine the spatial and temporal distributions of surface water pCO2, to identify surface pCO2 features along the salinity gradient or across the shelf, and to quantify the air‐sea CO2 fluxes over this study area. We also discuss the factors controlling pCO2 and provide a surface CO2 budget for the study area. In a companion paper, we will examine surface water CO2 and oxygen dynamics.
2. Methods
2.1. Field Measurement
Data for this study were acquired from thirteen cruises conducted in the northern Gulf of Mexico (Figure 1 and Table 1). Four shelf‐wide cruises (2006–2007) covered the area very likely to develop summer bottom‐water hypoxia (dissolved oxygen concentration less than 2 mg L−1) (Figure 1), similar to the region sampled during a hypoxia study in the past 30 years (http://www.gulfhypoxia.net/). A wider area across the shelf was surveyed during five cruises from 2009 to 2010 (Figure 1). Three earlier cruises (April 2006 and before) also surveyed the shelf, with a focus on the Louisiana Bight and the adjacent area immediately west of the Mississippi River birdfoot delta (Figure 1). Finally, one cruise (August 2008) surveyed the inner Louisiana shelf (Figure 1).
Table 1.
Summary of Survey Cruises
| Year | Datea | Ship | Survey Regionb | Coverage Area | River Dischargec | NO3+NO2 Fluxc | Air CO2 |
|---|---|---|---|---|---|---|---|
| 103km2 | 103m3 s−1 | 109 g N month−1 | μatm | ||||
| 2004 | 9 Aug to 12 Aug | Pelican | LAB | 9.2 | 12.9 | 42.5 | 380 |
| 2005 | 4 Oct to 7 Octd | Pelican | LAB | 4.4 | 7.9 | 19.9 | 387.6 |
| 2006 | 27 Apr to 1 May | Pelican | LAB | 7.2 | 17.3 | 96.0 | 375 |
| 2006 | 6 Jun to 11 Jun | Bold | nGOM | 34.1 | 12.2 | 60.2 | 386 |
| 2006 | 6 Sep to 11 Sep | Bold | nGOM | 34.7 | 7.5 | 19.7 | 369.5 |
| 2007 | 2 May to 7 May | Bold | nGOM | 39.1 | 25.4 | 148 | 379 |
| 2007 | 18 Aug to 24 Aug | Bold | nGOM | 41.4 | 12.1 | 31.2 | 384 |
| 2008 | 17 Jul to 20 Jul | Pelican | in‐LAs | 14.9 | 28.4 | 138.0 | 385 |
| 2009 | 9 Jan to 20 Jan | Cape Hatteras | nGOM | 41.4 | 23.9 | 92.6 | 371.7 |
| 2009 | 20 Apr to 1 May | Cape Hatteras | nGOM | 41.4 | 30.9 | 151.0 | 386 |
| 2009 | 19 Jul to 29 Juld | Cape Hatteras | nGOM | 41.4 | 18.9 | 73.5 | 387.3 |
| 2009 | 28 Oct to 7 Nov | Hugh R. Sharp | nGOM | 41.4 | 25.6 | 52.4 | 371.8 |
| 2010 | 9 Mar to 21 Mard | Cape Hatteras | nGOM | 40.9 | 27.8 | 121.0 | 379 |
Spring was from March to May, summer was from June to August, fall was from September to November, and winter was from December to February in this study.
LAB: the Louisiana Bight and its adjacent area; nGOM: the northern Gulf of Mexico; in‐LAs: the inner Louisiana shelf.
River discharge and monthly NO3+NO2 flux data were in Mississippi and Atchafalaya Rivers. Data from the U.S. Geological Survey (USGS) “Hypoxia in the Gulf of Mexico studies.”
Cruise affected by weather events.
Throughout the course of our sampling, freshwater discharge and nitrate plus nitrite fluxes (U.S. Geological Survey, USGS data) were seasonally variable: they were generally high during spring and low during late summer and fall (Figure 2). Note that the October 2005 cruise coincided with a period of low discharge and was also about 1 month after Hurricane Katrina (23–30 August 2005) and 2 weeks after Hurricane Rita (18–26 September 2005).
Figure 2.
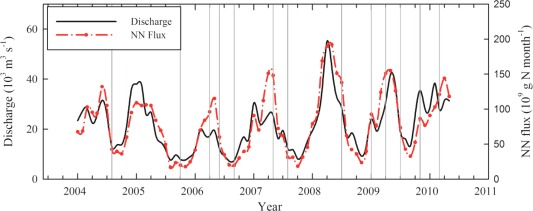
The variations of monthly river discharge and nitrate plus nitrite fluxes for the Mississippi and Atchafalaya rivers from 2004 to May 2010. River discharge (black line) and nitrate plus nitrite fluxes (red line) were usually low in fall and high in winter or spring. The vertical gray line indicates the first day of each survey cruise. Data source: U.S. Geological Survey (USGS, “Hypoxia in the Gulf of Mexico studies”, http://toxics.usgs.gov/hypoxia/index.html).
Surface water pCO2 was measured by pumping surface water continuously through an underway pCO2 analyzer installed in the shipboard laboratory. On cruises from 2003 to 2007, the system included a gas‐water equilibrator and CO2 detection via an infrared gas analyzer (LI‐COR® 7000) as described by Jiang et al. [2008]. On cruises from 2008 to 2010, we used an upgraded system that included a Global Positioning System, a Seabird thermosalinograph (SBE‐45), and a water‐gas equilibrator with an improved spray head, a temperature sensor, and a pressure sensor (Setra 270). This underway pCO2 system (AS‐P2, Apollo SciTech, USA) also had an improved water vapor trap via sequential Peltier cooling and Nafion dryers. The upgraded system yielded more reliable and consistent results and was similar to the NOAA (National Oceanic and Atmospheric Administration) system [Pierrot et al., 2009]. However, both systems had similar accuracy and precision (better than 2 ppm). Periodically, the CO2 analyzer was calibrated against certificated CO2 gas standards of 0, 197.5, 400.6, and 594.7, and 975.3 ppm referenced against standards traceable to those of the National Institute of Standards and Technology. Atmospheric CO2 values were measured every 6 or 12 h on cruises from 2004 to 2007, and every 3 to 4 h during the 2008–2010 cruises. Sea surface salinity (SSS) and temperature (SST) were measured using a Seabird SBE‐45 flow‐through thermosalinograph from 2004 to 2010.
2.2. Air‐Sea CO2 Flux Calculation
The instrument records the mole fraction of CO2 in the dry air flow (xCO2), which then is converted to the pCO2 inside the equilibrator at 100% water saturation via the equation:
| (1) |
where xCO2(eq) is the mole fraction (ppm) of CO2 in the dried sample gas; Pb is the barometric or total pressure inside the equilibrator; and Pweq is the equilibrium water vapor pressure at the equilibrated temperature, Teq, (°C) and salinity [Weiss and Price, 1980]. The pCO2(eq) was further converted to pCO2 in water at SST [Takahashi et al., 1993] using:
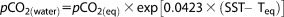 |
(2) |
For data from before 2008, we estimated that the equilibrator temperature was 0.4°C lower than sea surface temperature from a comparison with water column temperature data from the ship's CTD (conductivity, temperature, and depth) package. The atmospheric pCO2 (pCO2air) was corrected by the water vapor pressure with:
| (3) |
where Pw is the surface seawater vapor pressure at SST and SSS [Weiss and Price, 1980].
To calculate the air‐sea CO2 exchange flux, SSS, SST, pCO2(water), and pCO2(air) were gridded at a resolution of 0.1° × 0.1° (by applying the grid data function in Matlab®). The pCO2(air) value was set at a uniform value for each cruise over the Louisiana shelf from 2004 to 2007, as we have insufficient data to quantify its full spatial and temporal variability. After 2008, we used intensively measured pCO2(air) data to interpolate pCO2(air) values for each pCO2(water).
Finally, air‐sea CO2 flux (F) was calculated as:
| (4) |
where k is the gas transfer velocity of CO2; K0 is the CO2 solubility coefficient at the ambient temperature and salinity [Weiss, 1974]. To calculate k, we adopted the updated coefficient given by Ho et al. [2006], which is very close to the commonly used coefficients given by Wanninkhof [1992] and the more recent coefficients recommended by Sweeney et al. [2007] and Wanninkhof et al. [2009] (i.e., the coefficient is 0.27, 0.31, 0.26, and 0.25, respectively, in the equation: k ≈ a × , where a is the coefficient and U10 is the wind speed at 10 m). Monthly satellite products QuikSCAT wind data (12.5 km resolution) were adopted (from a Live Access Server provided by NOAA Coastwatch and SWFSC/Environmental Research Division, http://las.pfeg.noaa.gov/) and were interpolated to 0.1° by 0.1° to calculate the areal‐integrated air‐sea CO2 fluxes. In addition, we followed the approach given by Jiang et al. [2008] who suggested using instantaneous wind speeds from coastal buoy stations to deal with the non‐Gaussian distribution of wind speeds over a month [Wanninkhof et al., 2002]. By this approach, a nonlinearity coefficient, C2 (roughly 1.2), was calculated from buoy wind data and the gas transfer velocity equation is expanded to k ≈ a × C2 × , where Umonthly is the monthly average wind speed. Buoy wind data were taken from the following stations: 42362, 42364, PSTL, LUML1, FGBL1, CAPL1, 42002, 42001, 42039, 42035 in the northern Gulf of Mexico. Thus, the buoy stations provided high temporal resolution for a limited area, while satellite products provided lower temporal resolution and high spatial resolution.
The monthly mean air‐sea CO2 flux was averaged from the areal‐integrated CO2 fluxes interpolated to 0.1° × 0.1° grid. To systematically compare these monthly mean CO2 fluxes among surveys, we designated a reference area for comparison based on the smaller area surveyed during the period between June 2006 and August 2007 (Figure 1), as the area surveyed during 2009–2010 were larger. Results from the three surveys focusing on the Louisiana Bight from 2004 to April 2006 were not used to estimate a shelf‐wide CO2 flux, but instead only the CO2 flux for the Louisiana Bight and its adjacent areas. The inner shelf region had larger spatial variations in sea surface salinity, temperature, and pCO2, and thus also had more highly variable CO2 fluxes than the outer shelf. To better account for this variability, an additional inner shelf alongshore track was added in later cruises.
2.3. The Uncertainties in Air‐Sea CO2 Fluxes
As we have no precise way to assess the uncertainties of the calculated air‐sea CO2 flux, we adopt the methods described by Jiang et al. [2008] and consider three uncertainties. For the first of these, we calculated the standard deviation of the CO2 flux using six available gas transfer velocities (including Ho et al. [2006], Liss and Merlivat [1986]; Wanninkhof [1992]; Wanninkhof and McGillis [1999]; Nightingale et al., [2000a]; Nightingale et al., [2000b]). This approach resulted in 11%–15% relative variability in monthly mean gas transfer velocity, with an average of 12%, in this study. This variation of 12% is reasonable under usual wind speed distributions, but can be as much as doubled when wind speeds are extremely high or low [Ho et al., 2006; Wanninkhof et al., 2009]. The second source of uncertainty was assessed as the standard deviation due to the spatial and temporal variation of atmospheric CO2 (6 μatm) that was based on our measurements during 2008 and 2010. For the third of the three uncertainties considered, we determined the error associated with a small temperature difference (deltaT) between the equilibrator and surface seawater during 2006 and 2007. The above three uncertainties were then used to calculate the uncertainty of air‐sea CO2 flux for each cruise [Taylor, 1997]:
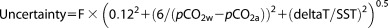 |
(5) |
Thus, we determined the average uncertainty for each cruise was in the range ± 0.05 to ± 2.98 mmol m−2 d−1 (the average is ± 1.15 mmol m−2 d−1), similar to the uncertainty estimated in the South Atlantic Bight [Jiang et al., 2008].
2.4. Data Analysis
We use two scenarios to examine mixing and biological effects on pCO2 variations: conservative mixing (pCO2Mix), and conservative mixing plus biological removal (pCO2Mix+Bio). We first simulated the abiotic mixing of pCO2 along the salinity gradient between the Mississippi River and seawater. The fraction from each end‐member was solved by equations (6) and (7) to estimate the conservative mixing values of total alkalinity (TA) and dissolved inorganic carbon (DIC) as equations (8) and (9) in one‐unit salinity interval (end‐members were listed in supporting information, Table S1):
| (6) |
| (7) |
| (8) |
| (9) |
where Sal is our desired salinity, S is the salinity and f is the fraction of end‐members from the Mississippi River (subscripted R) and seawater (subscripted S), respectively, “Mix” represents the conservative mixing values for its affiliated parameters. These TAMix and DICMix values were converted to pCO2 (pCO2Mix) at the measured temperature by using CO2SYS program (from Carbon Dioxide Information Analysis Center, http://cdiac.ornl.gov/ftp/co2sys/, K1, K2 from Mehrbach et al. [1973] refit by Dickson and Millero [1987] were adopted). To simulate the effects of biotic activity on pCO2 along the salinity gradient, we estimated the net biological NO3 removal (NO3Bio) by the difference between the measured NO3 data (NO3) during our cruises (data from Lehrter et al, [2013]) and the conservative mixing values (NO3Mix) derived from the river and ocean end‐members (supporting information, Table S1):
| (10) |
| (11) |
We converted net biological NO3 removal to estimate TAMix+Bio by the following equation;
| (12) |
and also to estimate DIC consumption by assuming that Redfield stoichiometry [Redfield, 1958] is applicable in this river dominated shelf [Huang et al., 2012] as the following equation:
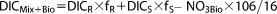 |
(13) |
TAMix+Bio and DICMix+Bio were converted to pCO2 (pCO2Mix+Bio) by using CO2SYS. Furthermore, to examine the effect of temperature, pCO2Mix+Bio was modeled for both binned temperature and the seasonal average temperature along the salinity gradient. Finally, as air‐sea gas exchanges also affect pCO2 variation (we will discuss this effect later), this estimated biological effect is only a conservative result to demonstrate the importance of biological uptake of CO2 in determining plume pCO2.
3. Results
3.1. pCO2 in Lower Rivers and Bays
The Mississippi River and Atchafalaya Bay waters had characteristically highly supersaturated pCO2 values ranging between 977 and 2220 μatm (Table 2) with noticeable seasonality, being lower in the winter and higher in the summer, with the highest value in July 2009. The pCO2 values decreased rapidly from extremely high values in rivers and bays to below the atmospheric value along the river‐ocean mixing gradient extending out onto the shelf. Water pCO2 values in Terrebonne Bay and Calcasieu Lake, which only have limited freshwater inputs, however, were much lower than values in the two major river systems (less than 800 μatm) (Table 2). The lower pCO2 values in these bays are likely caused by a lower turbidity and water mixing rates, allowing biological removal of CO2, in them than the two river mouths. Furthermore, water pCO2 values in Calcasieu Lake showed a larger variation (171.5 and 768.8 μatm) than the change in Terrebonne Bay (257–532 μatm) (Table 2). While these differences are interesting, we cease the discussion about them as the CO2 exchange fluxes from these bays had minor contributions to the overall regional flux due to their small area.
Table 2.
Surface Water pCO2 Values on the Lower Mississippi and Atchafalaya Rivers, Terrebonne Bay and Calcasieu Lake
| Year | Month | Salinity | pCO2 (μatm) | |||
|---|---|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | n | ||
| Lower Mississippi River | ||||||
| 2004 | August | 2.0 | 0.2 | 1628.3 | 18.0 | 32 |
| 2005 | October | 5.9 | 0.4 | 1348.8 | 32.3 | 62 |
| 2006 | June | 1.9 | 0.7 | 1708.6 | 281.4 | 153 |
| 2006 | September | 6.2 | 0.8 | 1209.8 | 52.9 | 72 |
| 2007 | May | 1.2 | 1.0 | 1732.9 | 39.2 | 74 |
| 2007 | August | 5.0 | 0.7 | 1362.7 | 103.6 | 143 |
| 2009 | January | 0.1 | 0.0 | 977.4 | 77.7 | 46 |
| 2009 | April | 0.1 | 0.1 | 1663.2 | 53.2 | 86 |
| 2009 | July | 1.4 | 0.4 | 2222.4 | 57.2 | 91 |
| 2009 | November | 0.2 | 0.1 | 1611.9 | 78.5 | 63 |
| 2010 | March | 0.2 | 0.1 | 1407.5 | 164.6 | 76 |
| Atchafalaya Bay | ||||||
| 2009 | January | 0.1 | 0.1 | 1361.6 | 142.6 | 55 |
| 2009 | April | 1.2 | 1.7 | 1310.3 | 553.8 | 42 |
| 2009 | July | 0.5 | 0.7 | 2072.8 | 402.4 | 84 |
| 2009 | November | 0.6 | 0.8 | 1813.7 | 208.2 | 23 |
| 2010 | March | 0.1 | 0.0 | 1682.4 | 111.9 | 45 |
| Terrebonne Bay | ||||||
| 2009 | January | 30.7 | 1.0 | 532.4 | 95.3 | 55 |
| 2009 | April | 26.1 | 0.6 | 257.3 | 15.0 | 46 |
| 2009 | July | 28.6 | 0.3 | 486.4 | 52.5 | 66 |
| 2009 | November | 27.0 | 1.0 | 314.4 | 29.7 | 44 |
| 2010 | March | 23.1 | 0.7 | 293.6 | 23.7 | 56 |
| Calcasieu Lake | ||||||
| 2007 | May | 22.8 | 0.1 | 171.5 | 5.0 | 20 |
| 2007 | August | 17.9 | 0.9 | 768.8 | 264.7 | 228 |
3.2. The Plume Trajectory Reflected by Sea Surface Salinity
Distributions of sea surface salinity demonstrated an alongshore Mississippi‐and‐Atchafalaya‐Rivers plume trajectory in most of our surveys (Figure 3). Such an alongshore freshwater trajectory was similar to those described in previous studies, which documented low salinities close to the Mississippi River mouth, increasing salinities along the Louisiana coast toward Texas, and a localized region of freshwater near the Atchafalaya Bay. An exception was in October 2005 when both low and high salinities were observed in the Louisiana Bight and the adjacent area shortly after two hurricanes (Figure 3b). Another exception was in July 2009, when the freshwater plume was largely confined to the eastern Louisiana and Mississippi shelf regions (Figure 3k). In March 2010, the freshwater plume was widely dispersed, extending across the shelf southward of 28°N (Figure 3m), and low temperature waters (less than 19°C) were also observed offshore. These weather‐related exceptions will be discussed later.
Figure 3.
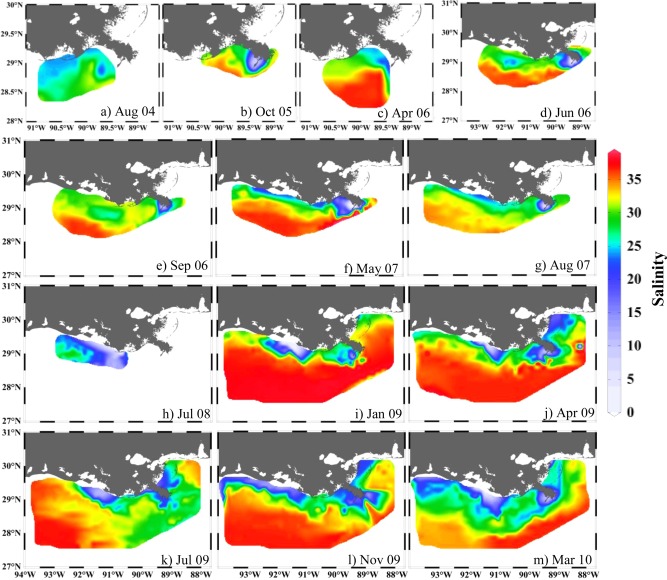
Sea surface salinity distributions. Contour maps are adopted to present the freshwater distribution in August 2004 (a), October 2005 (b), April 2006 (c), June 2006 (d), September 2006 (e), May 2007 (f), August 2007 (g), July 2008 (h), January 2009 (i), April 2009 (j), July 2009 (k), November 2009 (l), and March 2010 (m). The freshwater plume was usually distributed alongshore, but it was confined to the eastern shelf in July 2009, and extended to the open gulf in March 2010.
3.3. Distributions of Shelf pCO2 and Air‐Sea CO2 Flux
3.3.1. CO2 Gradients Across Inner, Middle, and Outer Shelves
The sea surface pCO2 distribution (Figure 4) revealed sharp gradients from inner to outer shelves. To illustrate this variation better, we considered two example transects (Figure 1): Transect B, starting from the northern Louisiana Bight along longitude 89.7°W (Figure 5a); and Transect E, from Atchafalaya Bay along longitude 91.6°W (Figure 5b). In general, both transects displayed a similar pattern across the shelf: near the coast, water pCO2 decreased sharply from highly oversaturated to undersaturated with respect to the atmospheric CO2 across the inner shelf, and pCO2 increased gradually to near atmospheric values when moving toward open ocean waters. The locations of lowest pCO2 varied among these cruises from inner shelf to middle shelf. Furthermore, higher variability in surface water pCO2 values was observed on the inner shelf (± 200 ppm) compared to the outer shelf (± 100 ppm) for both transects (Figures 5a and 5b).
Figure 4.
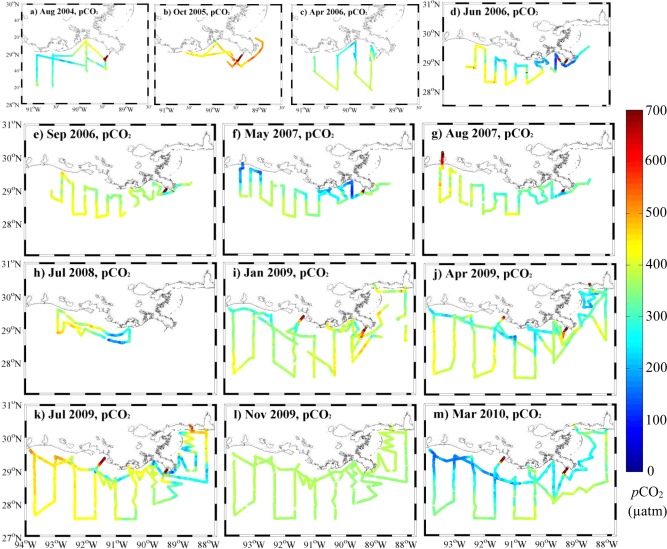
Sea surface pCO2 distributions. pCO2 values are presented along the cruise tracks for the corresponding plots shown in Figure 3. Under saturated pCO2 usually mirrored the freshwater distribution along the plume trajectories, whether the trajectories were alongshore (in most cases) or not (in July 2009 and March 2010). The exception was in November 2009, when we observed pCO2 values generally close to the atmospheric values.
Figure 5.
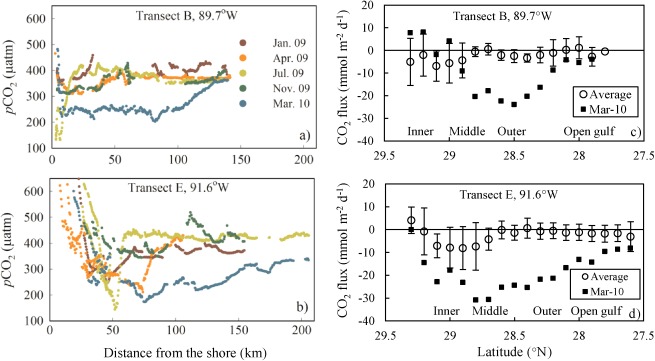
Variations of sea surface pCO2 values and air‐sea CO2 fluxes across the shelf. For both transects (labeled in Figure 1) B, on longitude 89.7°W (a), and E, on longitude 91.6°W (b), water pCO2 showed larger variations on the inner shelf than the outer shelf during 2009 to 2010. Air‐sea CO2 fluxes are binned by 0.1° latitude, and error bars represent the standard deviation in each 0.1° latitude bin. Larger variations of CO2 fluxes were observed on the inner shelf than on the middle, outer shelves, and the open gulf (c,d). Water pCO2 was not only under saturated on the inner shelf but also on the middle and outer shelves in March 2010 (c,d), resulting a significantly strong sink of CO2 with respect to the atmosphere in the northern Gulf of Mexico.
Our overall estimates of air‐sea CO2 flux further revealed that the inner shelf (where bottom depth was less than 20 m but excluding river mouths and bays) and the middle shelf (20–45 m depth) acted as atmospheric CO2 sinks (−3.35 mmol m−2 d−1 and −2.14 mmol m−2 d−1, respectively), while the outer shelf (45–200 m) and the open gulf (depth deeper than 200 m) varied from neutral to weak sources (i.e., 0.08 mmol m−2 d−1 and 1.46 mmol m−2 d−1, respectively). The specific CO2 fluxes for different subregions in each cruise are given in Table 3. Transects B and E illustrate the cross‐shelf variation of the CO2 flux: the inner shelf was not only an atmospheric CO2 sink, but it also showed larger air‐sea CO2 flux variations than the outer shelf (Figures 5c and 5d).
Table 3.
Monthly Air‐Sea CO2 Fluxes in the Northern Gulf of Mexico (unit: mmol m−2 d−1)a
| Year | Month | Survey Area | Reference Area | Inner | Middle | Outer | Open Gulf | Louisiana Bight |
|---|---|---|---|---|---|---|---|---|
| 2004 | August | −5.9 | −6.2 | −6.1 | −6.3 | N.A. | N.A. | −5.6 |
| 2005 | October | 13.1 | 13.0 | 13.0 | N.A. | N.A. | N.A. | 10.1 |
| 2006 | April | −4.5 | −6.4 | −8.7 | −3.5 | N.A. | N.A. | −6.6 |
| 2006 | June | −3.2 | −3.4 | −6.3 | −1.3 | 2.1 | N.A. | −7.7 |
| 2006 | September | −0.8 | −0.9 | −1.6 | −0.5 | 1.5 | N.A. | −2.0 |
| 2007 | May | −8.1 | −8.3 | −12.8 | −4.0 | −2.5 | N.A. | −14.3 |
| 2007 | August | −2.0 | −2.0 | −1.8 | −2.2 | −0.7 | N.A. | −4.0 |
| 2008 | July | −2.3 | −2.3 | −0.2 | −5.3 | N.A. | N.A. | N.A. |
| 2009 | January | −2.3 | −2.4 | −0.6 | −4.0 | −5.0 | −10.8 | −0.2 |
| 2009 | April | −4.8 | −6.5 | −6.8 | −6.6 | 0.1 | −1.3 | −11.4 |
| 2009 | July | 0.4 | 1.4 | 1.5 | 1.4 | 1.1 | 0.8 | −0.6 |
| 2009 | November | 0.2 | −0.1 | 0.5 | −0.6 | −1.2 | −0.6 | −4.8 |
| 2010 | March | −14.3 | −17.7 | −17.3 | −18.3 | −15.3 | −4.1 | −12.4 |
CO2 fluxes were calculated for various regions: for the cruise survey area, the reference area (the gray area in Figure 1), the inner shelf (bottom depth less than 20 m), the middle shelf (20 to 45 m), the outer shelf (45 to 200 m), and the open gulf (depth deeper than 200 m), and the Louisiana Bight and its adjacent area. N.A.: no available data.
3.3.2. Overall Spatial and Temporal Distribution
In addition to the cross‐shelf variation, an important aspect of the complex spatial pCO2 variation was that the distribution of waters undersaturated in pCO2 generally mirrored the freshwater distribution on shelf‐wide scales. For example, the plume trajectories were alongshore for the majority of surveys (Figures 4d, 4e, 4f, 4g, 4i, and 4j) and undersaturated pCO2 values were also observed in those regions. When the freshwater was confined to the east in July 2009, undersaturated pCO2 values were also observed on the eastern shelf but not the western shelf (Figure 4k). For the wide plume extending across the shelf in March 2010, undersaturated pCO2 values also expanded to the off‐shore and open gulf (Figure 4m). Among these varied plume trajectories, there was a gradient in pCO2 values, where they increased progressively as the low‐salinity plume traveled downstream to middle and to high salinities. We will discuss this pCO2 versus salinity pattern in the next section. An exception to this pattern occurred in November (Figure 4l), when pCO2 values remained close to the atmospheric level along the entire trajectory of fresh water. To sum up, three types of pCO2 spatial variation were observed, including high oversaturated values in river end‐members, clear cross‐shelf variations when plume trajectories were alongshore, and undersaturated values that mirrored the freshwater distribution over the shelf.
Monthly averaged properties for 0.1°×0.1° pixels gridded over the entire study area displayed seasonality in various environmental variables, including water temperature, wind speed, pCO2, and air‐sea CO2 flux (Figure 6). Water temperature was lower in winter and early spring, and it could be as high as 30°C in summer and fall (Figure 6a). Stronger wind speed was observed in winter and spring, and weaker wind speed was observed in summer and fall (Figure 6b). pCO2 was generally <300 μatm during spring and summer, and it increased to oversaturation during fall (Figure 6c). Finally, this study area acted as a strong sink for atmospheric CO2 in spring, a weak sink in summer, a source in fall, and was nearly neutral in winter (Figure 6d). Specifically, the strong sink from spring to June was mainly due to the inner and middle shelf contributions (Table 3). Moreover, two extreme conditions were observed, comprising a strong CO2 source in October 2005 and a strong sink in March 2010. These unusual periods are considered further in sections 4.2 and 4.3, respectively. Overall, this study area acted as a sink of atmospheric CO2 (overall mean plus standard deviation of −0.963 ± 3.7 mmol m−2 d−1) and within it, the Louisiana Bight and the adjacent area acted as a particularly strong sink (−4.89 mmol m−2 d−1).
Figure 6.
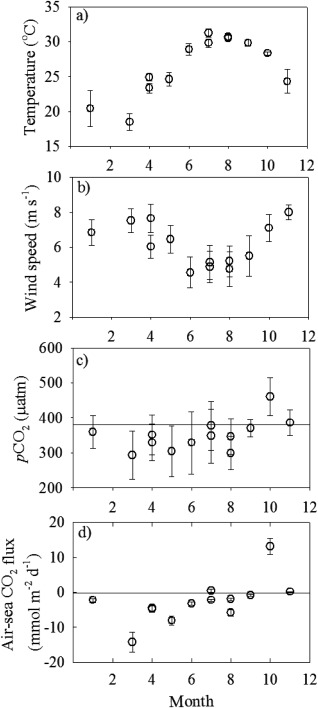
Seasonal variations of average (a) temperature, (b) wind speed, (c) sea surface pCO2, and (d) air‐sea CO2 flux in the reference area. The error bars in Figures 6a–6c showed ±1 standard deviation of each data point. In Figure 6, the bar showed the uncertainties of the data point. The line on Figure 6c represents the atmospheric CO2 value.
3.3.3. CO2 Distribution Along the Salinity Gradient
To further examine the pattern of pCO2 variations along the plume trajectory, we describe the variation of pCO2 along the salinity gradient. By binning pCO2 values into salinity intervals, it became evident that pCO2 versus salinity exhibited a concave upward curve and varied seasonally (Figure 7). In spring, low and undersaturated pCO2 was observed at moderate salinities around 20–25 and gradually increased toward saturation with increasing salinity (Figure 7a). In summer, undersaturated pCO2 values were observed over a wider salinity range from 9 to 31 (Figure 7b). In fall, undersaturated pCO2 values were restricted to a salinity range smaller than that in spring or summer (Figure 7c). Finally, pCO2 values were close to atmospheric values along the salinity gradient in winter (Figure 7d). To systematically organize the data and describe larger features, we further group pCO2 and air‐sea CO2 fluxes by five salinity zones: 0 ≤ S <17, 17 ≤ S < 25, 25 ≤ S < 33, 33 ≤ S < 35, and S ≥ 35 for each cruise (Table 4) and for each season (Table 5). Note that whereas the low‐salinity region (0≤S< 17) acted as a strong CO2 source, it only covered a relatively small proportion of the study area and thus its contribution to the overall regional flux is small. In contrast, the two middle salinity regions (17 ≤ S < 33) with undersaturated pCO2 values covered the majority of this area (Table 4). Thus, the northern Gulf of Mexico as a whole was still a CO2 sink.
Figure 7.
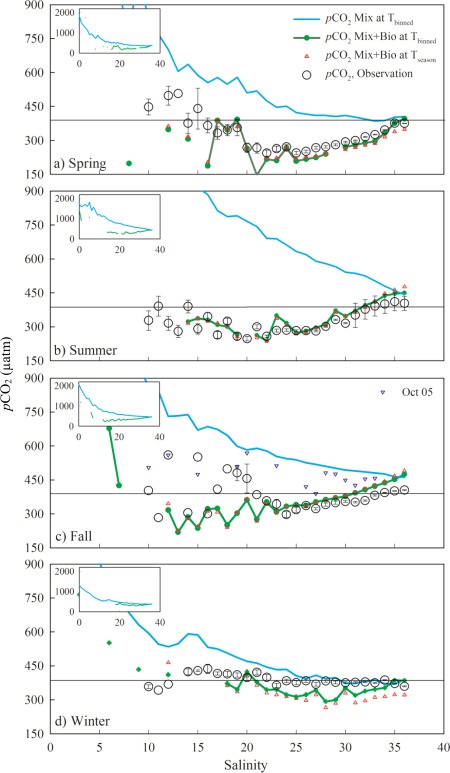
Observed and modeled relationships between pCO2 and salinity in each season. The measured pCO2 values are binned by 1‐unit salinity intervals (circle) and the error bars indicate ±1 standard deviation of the point in each interval. Each plot focuses on pCO2 < 900 μatm, but the inserted plots show the plots' full scales. The comparison of mixing only (blue lines) and mixing plus biology (green lines) under the same temperature (the binned temperature in each salinity interval) suggests that biological activity is the dominant factor controlling pCO2 while abiotic mixing could not be ignored in spring (a), summer (b), and fall (c). Mixing became important in winter at higher salinities (d). The comparison of two temperatures under mixing plus biology, i.e., the binned temperature in each 1‐ unit salinity interval (green lines) versus the seasonal average (red triangle markers), suggests that temperature is less important than biological and mixing processes. The observations in October 2005 (blue triangle markers in (c) affected by the remaining effects of hurricanes were close to abiotic mixing. Black lines represent the atmospheric CO2 value.
Table 4.
Average pCO2, Area, and CO2 flux on the Louisiana Shelf During Each Cruisea
| Salinity | Aug 2004 | Oct 2005 | Apr 2006 | Jun 2006 | Sep 2006 | May 2007 | Aug 2007 | Jul 2008 | Jan 2009 | Apr 2009 | Jul 2009 | Nov 2009 | Mar 2010 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average pCO2 (μatm) | |||||||||||||
| 0 ≤ S <17 | N.A. | 506.9 | N.A. | 305.3 | 297.2 | 316.2 | 616.0 | 286.1 | 375.6 | 465.1 | 440.7 | 431.3 | 427.1 |
| 17≤ S<25 | 285.6 | 539.2 | 283.0 | 199.0 | 323.8 | 199.5 | 390.9 | 350.7 | 367.3 | 300.2 | 299.0 | 405.4 | 262.6 |
| 25≤ S<33 | 304.3 | 445.6 | 287.7 | 295.4 | 364.6 | 253.0 | 328.1 | 410.1 | 387.2 | 303.3 | 347.5 | 371.0 | 267.4 |
| 33≤ S<35 | N.A. | 457.4 | 332.3 | 380.6 | 394.6 | 336.7 | 380.8 | N.A. | 386.4 | 353.9 | 423.6 | 376.5 | 326.0 |
| 35≤ S<37 | N.A. | N.A. | 373.9 | 423.7 | 402.8 | 364.6 | N.A. | N.A. | 343.9 | 379.6 | 435.8 | 393.3 | 358.8 |
| Area (103 km2) | |||||||||||||
| 0 ≤ S <17 | N.A. | 0.4 | N.A. | 0.9 | 0.5 | 1.4 | 0.3 | 4.1 | 1.4 | 2.1 | 2.1 | 2.3 | 2.8 |
| 17≤ S<25 | 2.8 | 0.4 | 1.0 | 3.5 | 0.5 | 4.3 | 1.8 | 7.0 | 3.1 | 9.2 | 6.9 | 12.9 | 15.6 |
| 25≤ S<33 | 7.2 | 3.5 | 3.9 | 18.4 | 27.3 | 13.3 | 28.8 | 3.8 | 21.1 | 28.4 | 54.7 | 31.6 | 53.7 |
| 33≤ S<35 | N.A. | 0.3 | 1.3 | 5.8 | 2.9 | 7.6 | 10.5 | N.A. | 13.8 | 9.5 | 19.9 | 18.7 | 19.9 |
| 35≤ S<37 | N.A. | N.A. | 4.5 | 8.1 | 4.8 | 13.7 | N.A. | N.A. | 63.9 | 50.4 | 16.4 | 40.6 | 12.3 |
| Average CO2 flux (mmol m−2 d−1) | |||||||||||||
| 0 ≤ S <17 | N.A. | 24.2 | N.A. | −5.9 | −9.3 | −8.1 | 27.8 | −6.0 | −0.3 | 12.7 | 3.4 | 6.8 | 7.3 |
| 17≤ S<25 | −8.4 | 23.0 | −8.9 | −10.3 | −5.2 | −20.4 | 0.8 | −2.0 | −1.9 | −11.7 | −5.2 | 2.5 | −20.0 |
| 25≤ S<33 | −4.9 | 10.4 | −8.0 | −5.1 | −1.2 | −13.1 | −3.1 | 1.4 | −0.2 | −11.4 | −1.1 | −2.2 | −17.9 |
| 33≤ S<35 | N.A. | 13.8 | −3.8 | 0.0 | 1.0 | −4.1 | −0.2 | N.A. | 0.2 | −4.6 | 3.0 | −1.2 | −9.1 |
| 35≤ S<37 | N.A. | N.A. | −0.9 | 2.0 | 1.7 | −1.8 | N.A. | N.A. | −4.7 | −0.8 | 3.8 | 1.5 | −4.2 |
N.A.: no available data.
Table 5.
Average Air‐Sea CO2 Flux (mmol m−2 d−1) by Season and Salinity Region
| Spring | Summer | Fall | Winter | Annual | Area (103 km2) | Annual (109g C yr−1) | |
|---|---|---|---|---|---|---|---|
| 0 ≤ S <17 | 9.6 | −4.1 | 11.1 | 4.1 | 5.2 | 1.7 | 38.7 |
| 17≤ S<25 | −16.6 | −8.0 | −2.7 | 1.7 | −6.4 | 6.7 | −189.6 |
| 25≤ S<33 | −15.3 | −3.6 | −1.8 | −1.4 | −5.5 | 29.5 | −713.4 |
| 33≤ S<35 | −7.5 | 0.9 | 0.4 | −0.6 | −1.7 | 12.2 | −90.9 |
| 35≤ S<37 | −1.4 | 1.4 | 1.7 | −2.3 | −0.1 | 24.7 | −14.6 |
4. Discussion
4.1. Factors Controlling pCO2 Variations
From the river mouth to the inner shelf, the outer shelf, and beyond, both physical and biological conditions changed greatly. Mechanistic analysis in sections 2.4 and 3.3.3 demonstrates that biological removal and mixing were the two dominant factors influencing pCO2 along the salinity gradient. As predicted from carbon dynamics during river‐to‐sea mixing, the conservative mixing lines of pCO2 are concave upward along the salinity gradient [Cai et al., 2013] (Figure 7). Along this concave‐upward curve, the pCO2 difference between salinities 18 and 36 (salinity regions which covered the majority of this study area) was 124, 346, 185, and 117 μatm in spring, summer, fall, and winter, respectively. However, the majority of pCO2 values calculated from conservative mixing were still much higher than observed values, implying mixing is not the only factor controlling pCO2. The exception was in October 2005, when observed pCO2 values were close to mixing‐only simulated results (Figure 7c). We believe this represented an extreme case when the water column was well mixed following two major storm events.
Simulations that included biological activity as well as mixing produced results much closer to our observed pCO2 values (Figure 7). From salinities 18–36, the maximum pCO2 values induced by biological removal (i.e., the difference between pCO2Mix and pCO2Mix+Bio at the salinity binned temperature) were 322, 527, 397, and 141 μatm in spring, summer, fall, and winter, respectively. The strong biological carbon uptake inferred from nitrate removal along the salinity gradient in pCO2Mix+Bio was consistent with that observed during our previous studies on the Louisiana Bight and the adjacent region [Cai 2003; Guo et al., 2012; Huang et al., 2012]. While the majority of pCO2 observations agreed with pCO2Mix+Bio in spring, summer, and fall (Figures 7a, 7b, and 7c), a few pCO2 observations fell midway between the mixing‐only and mixing‐plus‐biology simulated values in fall and winter (Figures 7c and 7d). Some observed data in winter between salinities 28 and 35 also agreed with mixing‐only values (Figure 7d). The above two facts led us to conclude that along salinities 18–36, biological activity drove pCO2 variation and was more important than mixing. But mixing was a nonnegligible factor to estimate biological removal (equations (6–13)) in spring, summer, and fall and mixing became a dominant factor in winter at higher salinities.
In the carbonate system, pCO2 variation is theoretically related to variations of DIC, TA, and temperature [Sarmiento and Gruber, 2006]. To explore the role of mixing and biology, we have set our simulations at the salinity binned temperature. To explore the role of temperature, we also run the pCO2Mix+Bio simulation at the seasonal average temperature (Figure 7). The maximum differences of pCO2Mix+Bio between the two temperatures were only 41, 27, 16, and 64 μatm for spring, summer, fall, and winter, respectively. These pCO2Mix+Bio differences due to temperature variations were much smaller than the differences due to biological uptake between pCO2Mix and pCO2Mix+Bio at the same temperature. The temperature effect was only notable when mixing dominated in the moderate to high salinities (27 to 36) during winter (Figure 7d). This result highlights the importance of non‐temperature effects, e.g., biological activity and mixing, on pCO2 variation in this river‐influenced system.
Finally, air‐sea CO2 exchange also plays a role in determining plume pCO2 values although it is generally minor. For example, in the Mississippi River plume, net community production (NCP) estimated by DIC deficient were 83 to −233 mmol m−2 d−1 (1 to −2.8 g C m−2 d−1) in salinity ranges 0–18, 275–616 mmol m−2 d−1 (3.3–7.4 g C m−2 d−1) in salinity ranges 18–27, 32–80 mmol m−2 d−1 (0.38–0.96 g C m−2 d−1) in salinity ranges 27–32, and 25–48 mmol m−2 d−1 (0.3–0.53 g C m−2 d−1) in salinity ranges 32–34.5 [Guo et al., 2012]. Mean CO2 gas exchange rates in similar salinity ranges (Table 5) were only <6.2%, <2.3%, 6.8–17%, and <6.8% of these estimated NCP values, respectively. Thus, the effect of air‐sea exchanges on pCO2 variations was rather small in this enhanced primary production area.
4.2. Disturbance of River Plume Trajectories by Weather Events
Weather events may have a strong influence on spatial distributions of pCO2 in this study area. Two weather events considered here included wind‐driven shifts in plume trajectory in March 2010 and July 2009. When northerly winds and high river discharge dominated in March 2010 [Huang et al., 2013], the trajectory of the alongshore plume became wider and extended over the area between 28°N and 25°N (Figure 3m), and as a result, low pCO2 values also extended to the middle and outer shelves (Figures 4m, 5a and 5b). In this case, the extensive strong CO2 uptake in the middle and outer shelves encompassed areas that would be normally nearly net zero CO2 uptake and resulted in a strong CO2 sink in March 2010 than the averages along transects B and E (Figures 5c and 5d).
The plume trajectory and wind forcing in July 2009 were unusual [Zhang et al., 2012; Feng et al., 2014]. Our observations also showed high surface salinity on the western inner shelf while the low‐salinity plume was confined to the eastern side (Figure 3k). Under these conditions, pCO2 was oversaturated west of 90°W, especially on the inner shelf which typically had low pCO2; areas east of 90°W were undersaturated, especially on the outer shelf (Figure 4k). This pattern can also be seen along the two transects in Figure 5; pCO2 values were undersaturated in July 2009 on the outer shelf in Transect B and were oversaturated in the middle and outer shelves in Transect E. Thus, the entire shelf shifted from a normally weak CO2 sink to a strong source in July 2009. To sum up, plume trajectory can be strongly influenced by wind forcing [Cochrane and Kelly, 1986; Schiller et al., 2011; Walker et al., 2005; Zhang et al., 2012], and it can affect the spatial distributions of primary production [Chen et al., 2000] and other biogeochemical processes [Huang et al., 2013].
4.3. Disturbance by Hurricanes
The anomalously high surface water pCO2 values observed in October 2005 were attributed to mixing effects of hurricanes Katrina (23–30 August 2005) and Rita (18–29 September 2005), as the water column was still nearly completely mixed during the survey as indicated by CTD and DIC data (not presented). The October pCO2 values were higher than the mean values of the September 2006 and November 2009 surveys, which were considered to represent typical fall conditions (Figure 7c). As in situ pCO2 measurements were limited on the inner shelf during this cruise, Lohrenz et al. [2010] extrapolated them across the Louisiana Bight and its adjacent area (∼9000 km2) using remote sensing techniques and estimated an air‐sea CO2 flux of 5.4 mmol m−2 d−1 (0.21 Tg C yr−1). Compared to the CO2 fluxes from nonstorm months in September 2006 and November 2009 (average of 1.79 mmol m−2 d−1 or 0.07 Tg C yr−1), the higher CO2 flux (3.61 mmol m−2 d−1 or 0.14 Tg C yr−1) in October 2005 can arguably be attributed to effects of storm‐enhanced terrestrial inputs and bottom sediment resuspension by hurricanes [Lohrenz et al., 2010]. In addition, winds were relatively high in October 2005 and the uncertainty of gas transfer velocity was large under high wind speeds [Wanninkhof et al., 2009], resulting in larger uncertainty in air‐sea CO2 fluxes. Nonetheless, it is apparent, and perhaps not a surprising conclusion, that hurricanes can exert dramatic changes to normal sea surface pCO2 distribution and thus the flux of air‐sea CO2 exchange.
4.4. Conceptual Model for CO2 Dynamics in the River Plume
We conclude this paper with a conceptual model and a summary for the surface water inorganic carbon budget and dynamics of this river plume in the northern Gulf of Mexico (Figure 8). Annually, the Mississippi and Atchafalaya Rivers exported about 17 Tg C of bicarbonate to the coastal ocean [Cai et al., 2008; Lohrenz et al., 2013] while they also transported 1.57 Tg N of inorganic nitrogen [Goolsby et al., 2000]. In the subregion where salinity was 0–17 in the river plume, water transit time was short (2 days) [Green et al., 2006] and high turbidity inhibited light penetration that would support phytoplankton growth [Dagg et al., 2003; Lohrenz et al., 1990], with the result that this subregion was a intensive source of CO2 to the atmosphere (5.2 mmol m−2 d−1, 39 ×109 g C yr−1). Surface waters of salinity 17–24 and 25–33 covered a relatively large portion of the shelf and were characterized by reduced turbidity and thus higher light penetration, resulting in high primary production and nonconservative behavior in nutrients consistent with biological uptake [Dagg et al., 2007; Lohrenz et al., 1990; Lohrenz et al., 1999]. DIC mass balance analyses also pointed to strong biological DIC removal only in the middle salinity plume (salinity 18–27) [Guo et al., 2012]. Nitrate removal along the salinity gradient also showed patterns proportional to DIC removal [Cai 2003; Guo et al., 2012; Huang et al., 2012 ; Lehrter et al., 2013], supporting our pCO2 simulations based on nitrate plus nitrite removal in section 3.5. These middle salinity regions coincided with strong sinks of atmospheric CO2 (6.4 mmol m−2 d−1, 190 × 109 g C yr−1) for waters with salinity 17–24, and 5.5 mmol m−2 d−1, 713 × 109 g C yr−1 for waters with salinity 25–33). Net DIC removal was close to zero at the high‐salinity end of these intermediate salinity regions (S>32) [Guo et al., 2012]. In our high‐salinity subregion (33 ≤ S < 35), surface waters shifted from a CO2 sink to a near neutral status, and this subregion was a weak sink of 90 × 109 g C yr−1 (1.7 mmol m−2 d−1).
Figure 8.
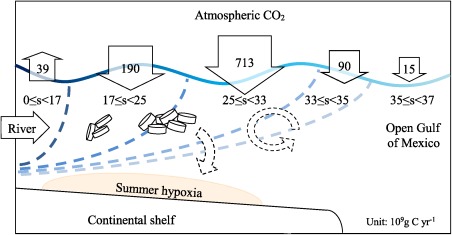
The conceptual model of the CO2 budget in the river plume. Blue‐dashed lines separate the plume into five salinity divisions: 0 ≤ salinity <17, 17≤ salinity <25, 25≤ salinity <33, 33≤ salinity <35, and 35≤ salinity <37.
5. Summary
This study described spatial and seasonal variations of surface water pCO2 in the northern Gulf of Mexico. The spatial distribution was characterized by: (1) water pCO2 values of 1000–2000 μatm in the Mississippi and Atchafalaya river sources, (2) a strong cross‐shelf CO2 gradient, where the inner to middle shelf acted as a sink of atmospheric CO2 and the outer shelf was near a neutral status, and (3) a concave upward curve of pCO2 values versus salinity with high values at low salinities near the river sources, low at middle salinities, and increasing to near equilibrium or slight oversaturation in surface waters at high salinities. A quantitative budget to describe the CO2 flux along the salinity gradient was also presented. The average pCO2 values over this study area revealed seasonality: undersaturation in spring, near atmospheric CO2 values in summer, oversaturation in fall, and a return to near atmospheric values in winter. Overall, the northern Gulf of Mexico acted as a sink of CO2 with respect to the atmosphere, especially strong in the Louisiana Bight.
Analysis further indicated that variations in pCO2 distribution and air‐sea CO2 flux were strongly influenced by biological activity, and to a lesser extent mixing processes. Finally, air‐sea fluxes of CO2 were also affected by weather events, implying that future climate‐related changes in meteorological forcing may alter air‐sea CO2 fluxes on river‐dominated continental shelves.
Supporting information
Supporting Information:
Supporting Information S1
Acknowledgments
We thank the captains and crews on R/V Pelican, OSV Bold, R/V Cape Hatteras, and R/V Hugh R. Sharp for their support. We thank P. Yager, C. S. Hopkinson, and C. Meile for their valuable discussion and comments and two anonymous reviewers for their comments. Thank S. F. DiMarco for sharing the cruise in July 2008. We also thank the National Science Foundation (OCE‐0752110 and OCE‐ 0752254), the National Aeronautics and Space Administration (NNX10AU06G, NNX10AU78G, NNH14AY07I, NNX14AO73G), and the BP‐funded Gulf of Mexico Research Initiative RFP‐II award (GoMRI‐020) for providing funding. The majority of the data have been submitted to Surface Ocean CO2 Atlas (SOCAT) and Carbon Dioxide Information Analysis Center (CDIAC) and are available at these addresses: http://cdiac.ornl.gov/ftp/oceans/UG_GoM_UW_Data/2006.data, http://cdiac.ornl.gov/ftp/oceans/UG_GoM_UW_Data/2007.datam, http://cdiac.esd.ornl.gov/ftp/oceans/Cape_Hatteras_GM/.
Huang, W.‐J. , Cai W.‐J., Wang Y., Lohrenz S. E., and Murrell M. C. (2015), The carbon dioxide system on the Mississippi River‐dominated continental shelf in the northern Gulf of Mexico: 1. Distribution and air‐sea CO2 flux, J. Geophys. Res. Oceans, 120, 1429–1445, doi:10.1002/2014JC010498.
References
- Bauer, J. E. , Cai W.‐J., Raymond P. A., Bianchi T. S., Hopkinson C. S., and Regnier P. A. G. (2013), The changing carbon cycle of the coastal ocean, Nature, 504(7478), 61–70. [DOI] [PubMed] [Google Scholar]
- Borges, A. V. (2011), Present day carbon dioxide fluxes in the coastal ocean and possible feedbacks under global change, in Oceans and the atmospheric carbon content, edited by P. Duarte and J. M. Santana‐Casiano, pp. 47–77, Springer, Netherlands.
- Borges, A. V. , and Gypens N. (2010), Carbonate chemistry in the coastal zone responds more strongly to eutrophication than to ocean acidification, Limnol. Oceanogr., 55(1), 346–353. [Google Scholar]
- Cai, W.‐J. (2003), Riverine inorganic carbon flux and rate of biological uptake in the Mississippi River plume, Geophys. Res. Lett., 30(2), 1032, doi:10.1029/2002GL016312. [Google Scholar]
- Cai, W.‐J. (2011), Estuarine and coastal ocean carbon paradox: CO2 sinks or sites of terrestrial carbon incineration?, Annu. Rev. Mar. Sci., 3(1), 123–145. [DOI] [PubMed] [Google Scholar]
- Cai, W.‐J. , Dai M., and Wang Y. C. (2006), Air‐sea exchange of carbon dioxide in ocean margins: A province‐based synthesis, Geophys. Res. Lett., 33, L12603, doi:10.1029/2006GL026219. [Google Scholar]
- Cai, W.‐J. , Guo X., Chen C.‐T. A., Dai M., Zhang L., Zhai W., Lohrenz S. E., Yin K., Harrison P. J., and Wang Y. (2008), A comparative overview of weathering intensity and flux in the world's major rivers with emphasis on the Changjiang, Huanghe, Zhujiang (Pearl) and Mississippi Rivers, Cont. Shelf Res., 28(12), 1538–1549. [Google Scholar]
- Cai, W.‐J. , et al. (2011), Acidification of subsurface coastal waters enhanced by eutrophication, Nat. Geosci, 4(11), 766–770. [Google Scholar]
- Cai, W.‐J. , Chen C.‐T. A., Borges A. V. (2013), Carbon dioxide dynamics and fluxes in coastal waters influenced by river plumes, in Biogeochemical Dynamics at Major River‐Coastal Interfaces: Linkages With Global Change, edited by Bianchi T. S., Allison M. A., and Cai W.‐J., Cambridge Univ. Press, N. Y. [Google Scholar]
- Chavez, F.P. , Takahashi T., Cai W.‐J., Friederich G., Hales B., Wanninkhof R., and Feely R.A. (2007), Coastal oceans, Chap. 15, in The First State of the Carbon Cycle Report (SOCCR): The North American Carbon Budget and Implications for the Global Carbon Cycle, Synthesis and Assessment Product 2.2, Report by the U.S. Climate Change Science Program and the Subcommittee on Global Change Research, edited by A.W. King, et al., 157–166. [Available at http://cdiac.ornl.gov/SOCCR/pdf/SAP2.2_Entire_Report_March2007.pdf.]
- Chen, C.‐T. A. , and Borges A. V. (2009), Reconciling opposing views on carbon cycling in the coastal ocean: Continental shelves as sinks and near‐shore ecosystems as sources of atmospheric CO2 , Deep Sea Res., Part II, 56(8–10), 578–590. [Google Scholar]
- Chen, C.‐T. A. , Huang T.‐H., Fu Y.‐H., Bai Y., and He X. (2012), Strong sources of CO2 in upper estuaries become sinks of CO2 in large river plumes, Curr. Opin. Environ. Sustain., 4(2), 179–185. [Google Scholar]
- Chen, X. , Lohrenz S. E., and Wiesenburg D. A. (2000), Distribution and controlling mechanisms of primary production on the Louisiana‐Texas continental shelf, J. Mar. Syst., 25(2), 179–207. [Google Scholar]
- Chou, W.‐C. , Gong G.‐C., Tseng C.‐M., Sheu D. D., Hung C.‐C., Chang L.‐P., and Wang L.‐W. (2011), The carbonate system in the East China Sea in winter, Mar. Chem., 123(1–4), 44–55. [Google Scholar]
- Chou, W. C. , Gong G. C., Cai W. J., and Tseng C. M. (2013), Seasonality of CO2 in coastal oceans altered by increasing anthropogenic nutrient delivery from large rivers: Evidence from the Changjiang‐East China Sea system, Biogeosciences, 10(6), 3889–3899. [Google Scholar]
- Cochrane, J. D. , and Kelly F. J. (1986), Low‐frequency circulation on the Texas‐Louisiana continental shelf, J.Geophys. Res., 91(9), 10,645–10,659. [Google Scholar]
- Cooley, S. R. , and Yager P. L. (2006), Physical and biological contributions to the western tropical North Atlantic Ocean carbon sink formed by the Amazon River plume, J. Geophys. Res., 111, C08018, doi:10.1029/2005JC002954. [Google Scholar]
- Cooley, S. R. , Coles V. J., Subramaniam A., and Yager P. L. (2007), Seasonal variations in the Amazon plume‐related atmospheric carbon sink, Global Biogeochem. Cycles, 21, GB3014, doi:10.1029/2006GB002831. [Google Scholar]
- Dagg, M. J. , and Breed G. A. (2003), Biological effects of Mississippi River nitrogen on the northern Gulf of Mexico: A review and synthesis, J. Mar. Syst., 43(3/4), 133–152. [Google Scholar]
- Dagg, M. J. , Ammerman J., Amon R., Gardner W., Green R., and Lohrenz S. (2007), A review of water column processes influencing hypoxia in the northern Gulf of Mexico, Estuaries Coasts, 30(5), 735–752. [Google Scholar]
- Dickson, A. G. , and Millero F. J. (1987), A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media, Deep Sea Res. Part A. Oceanogr. Res. Pap., 34(10), 1733–1743, doi:10.1016/0198-0149(87)90021-5. [Google Scholar]
- Dinnel, S. P. , and Wiseman J. W. J. (1986), Fresh water on the Louisiana and Texas shelf, Cont. Shelf Res., 6(6), 765–784. [Google Scholar]
- Ducklow, H. W. , and McCallister S. L. (2005), The biogeochemistry of carbon dioxide in the coastal oceans, in The Global Coastal Ocean. Multiscale Interdisciplinary Processes. The Sea: Ideas and Observations on Progress in the Study of the Seas., edited by Robinson A. R. and Brink K. H., pp. 269–315, Harvard Univ. Press, Cambridge, Mass. [Google Scholar]
- Etter, P. C. , Howard M. K., and Cochrane J. D. (2004), Heat and freshwater budgets of the Texas‐Louisiana shelf, J. Geophys. Res., 109, C02024, doi:10.1029/2003JC001820. [Google Scholar]
- Feng, Y. , Fennel K., Jackson G. A., DiMarco S. F., and Hetland R. D. (2014), A model study of the response of hypoxia to upwelling‐favorable wind on the northern Gulf of Mexico shelf, J. Mar. Syst., 131(0), 63–73. [Google Scholar]
- Goolsby, D. A. , Battaglin W. A., Aulenbach B. T., and Hooper R. P. (2000), Nitrogen flux and sources in the Mississippi River Basin, Sci. Total Environ., 248(2–3), 75–86. [DOI] [PubMed] [Google Scholar]
- Green, R. E. , Bianchi T. S., Dagg M. J., Walker N. D., and Breed G. A. (2006), An organic carbon budget for the Mississippi River turbidity plume and plume contributions to air‐sea CO2 fluxes and bottom water hypoxia, Estuaries Coasts, 29(4), 579–597. [Google Scholar]
- Green, R. E. , Breed G. A., Dagg M. J., and Lohrenz S. E. (2008), Modeling the response of primary production and sedimetation to variable nitrate loading in the Mississippi River plume, Cont. Shelf Res., 28(12), 1451–1465. [Google Scholar]
- Guo, X. , et al. (2012), Carbon dynamics and community production in the Mississippi River plume, Limnol. Oceanogr., 57(1), 1–17. [Google Scholar]
- Ho, D. T. , Law C. S., Smith M. J., Schlosser P., Harvey M., and Hill P. (2006), Measurements of air‐sea gas exchange at high wind speeds in the Southern Ocean: Implications for global parameterizations, Geophys. Res. Lett., 33, L16611, doi:10.1029/2006GL026817. [Google Scholar]
- Huang, W.‐J. , Cai W.‐J., Powell R. T., Lohrenz S. E., Wang Y., Jiang L. Q., and Hopkinson C. S. (2012), The stoichiometry of inorganic carbon and nutrient removal in the Mississippi River plume and adjacent continental shelf, Biogeosciences, 9(7), 2781–2792. [Google Scholar]
- Huang, W.‐J. , Cai W.‐J., Castelao R. M., Wang Y., and Lohrenz S. E. (2013), Effects of a wind‐driven cross‐shelf large river plume on biological production and CO2 uptake on the Gulf of Mexico during spring, Limnol. Oceanogr., 58(5), 1727–1735. [Google Scholar]
- Jiang, L.‐Q. , Cai W.‐J., Wang Y., Wanninkhof R., and Lüger H. (2008), Air‐sea CO2 fluxes on the U.S. South Atlantic Bight: Spatial and seasonal variability, J. Geophys. Res., 113, C07019, doi:10.1029/2007JC004366. [Google Scholar]
- Kortzinger, A. (2003), A significant CO2 sink in the tropical Atlantic Ocean associated with the Amazon River plume, Geophys. Res. Lett., 30(24), 2287, doi:10.1029/2003GL018841. [Google Scholar]
- Lehrter, J. C. , Ko D. S., Murrell M. C., Hagy J. D., Schaeffer B. A., Greene R. M., Gould R. W., and Penta B. (2013), Nutrient distributions, transports, and budgets on the inner margin of a river‐dominated continental shelf, J. Geophys. Res., 118, 4822–4838, doi:10.1002/jgrc.20362. [Google Scholar]
- Lentz, S. J. , and Fewings M. R. (2012), The wind‐ and wave‐driven inner‐shelf circulation, Annu. Rev. Mar. Sci., 4(1), 317–343. [DOI] [PubMed] [Google Scholar]
- Liss, P. , and Merlivat L. (1986), Air‐sea gas exchange rates: Introduction and synthesis, in The Role of Air‐Sea Exchange in Geochemical Cycling, edited by Buat‐Menard P., pp. 113–129, Springer, Netherlands. [Google Scholar]
- Lohrenz, S. E. , and Cai W.‐J. (2006), Satellite ocean color assessment of air‐sea fluxes of CO2 in a river‐dominated coastal margin, Geophys. Res. Lett., 33, L01601, doi:10.1029/2005GL023942. [Google Scholar]
- Lohrenz, S. E. , Dagg M. J., and Whitledge T. E. (1990), Enhanced primary production at the plume/oceanic interface of the Mississippi River, Cont. Shelf Res., 10(7), 639–664. [Google Scholar]
- Lohrenz, S. E. , Fahnenstiel G. L., Redalje D. G., Lang G. A., Dagg M. J., Whitledge T. E., and Dortch Q. (1999), Nutrients, irradiance, and mixing as factors regulating primary production in coastal waters impacted by the Mississippi River plume, Cont. Shelf Res. 19, 1113–1141. [Google Scholar]
- Lohrenz, S. E. , Cai W.‐J., Chen F., Chen X., and Tuel M. (2010), Seasonal variability in air‐sea fluxes of CO2 in a river‐influenced coastal margin, J. Geophys. Res., 115, C10034, doi:10.1029/2009JC005608. [Google Scholar]
- Lohrenz, S. E. , Cai W.‐J., Chakraborty S., Gundersen K., and Murrell M. C. (2013), Nutrient and carbon dynamics in a large river‐dominated coastal ecosystem: The Mississippi‐Atchafalaya River system, in Biogeochemical Dynamics at Major River‐Coastal Interfaces: Linkages with Global Change, edited by Bianchi T. S., Allison M. A., and Cai W.‐J., Cambridge Univ. Press, N. Y. [Google Scholar]
- Mehrbach, C. , Culberson C. H., Hawley J. E., and Pytkowicx R. M. (1973), Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure, Limnol. Oceanogr., 18(6), 897–907. [Google Scholar]
- Milliman, J. D. , and Meade R. H. (1983), World‐wide delivery of river sediment to the oceans, J. Geol., 91(1), 1–21. [Google Scholar]
- Murrell, M. C. , Stanley R. S., Lehrter J. C., and Hagy Iii J. D. (2013), Plankton community respiration, net ecosystem metabolism, and oxygen dynamics on the Louisiana continental shelf: Implications for hypoxia, Cont. Shelf Res., 52(0), 27–38. [Google Scholar]
- Nightingale, P. D. , Liss P. S., and Schlosser P. (2000a), Measurements of air‐sea gas transfer during an open ocean algal bloom, Geophys. Res. Lett., 27(14), 2117–2120. [Google Scholar]
- Nightingale, P. D. , Malin G., Law C. S., Watson A. J., Liss P. S., Liddicoat M. I., Boutin J., and Upstill‐Goddard R. C. (2000b), In situ evaluation of air‐sea gas exchange parameterizations using novel conservative and volatile tracers, Global Biogeochem. Cycles, 14(1), 373–387. [Google Scholar]
- Pierrot, D. , Neill C., Sullivan K., Castle R., Wanninkhof R., Lüer H., Johannessen T., Olsen A., Feely R. A., and Cosca C. E. (2009), Recommendations for autonomous underway pCO2 measuring systems and data‐reduction routines, Deep Sea Res., Part II, 56(8–10), 512–522. [Google Scholar]
- Raymond, P. A. , Oh N.‐H., Turner R. E., and Broussard W. (2008), Anthropogenically enhanced fluxes of water and carbon from the Mississippi River, Nature, 451(7177), 449–452. [DOI] [PubMed] [Google Scholar]
- Redfield, A. C. (1958), The biological control of chemical factors in the environment, Am. Sci., 46, 205–221. [PubMed] [Google Scholar]
- Salisbury, J. E. , Vandemark D., Hunt C. W., Campbell J. W., McGillis W. R., and McDowell W. H. (2008), Seasonal observations of surface waters in two Gulf of Maine estuary‐plume systems: Relationships between watershed attributes, optical measurements and surface pCO2 , Estuarine Coastal Shelf Sci., 77(2), 245–252. [Google Scholar]
- Sarmiento, J. L. , and Gruber N. (2006), Ocean Biogeochemical Dynamics, Princeton Univ. Press, N. J. [Google Scholar]
- Schiller, R. V. , Kourafalou V. H., Hogan P., and Walker N. D. (2011), The dynamics of the Mississippi River plume: Impact of topography, wind and offshore forcing on the fate of plume waters, J. Geophys. Res., 116, C06029, doi:10.1029/2010JC006883. [Google Scholar]
- Sweeney, C. , Gloor E., Jacobson A. R., Key R. M., McKinley G., Sarmiento J. L., and Wanninkhof R. (2007), Constraining global air‐sea gas exchange for CO2 with recent bomb 14C measurements, Global Biogeochem. Cycles, 21, GB2015, doi:10.1029/2006GB002784. [Google Scholar]
- Takahashi, T. , Olafsson J., Goddard J. G., Chipman D. W., and Sutherland S. C. (1993), Seasonal variation of CO2 and nutrients in the high‐latitude surface oceans: A comparative study, Global Biogeochem. Cycles, 7(4), 843–878. [Google Scholar]
- Ternon, J. F. , Oudot C., Dessier A., and Diverres D. (2000), A seasonal tropical sink for atmospheric CO2 in the Atlantic ocean: The role of the Amazon River discharge, Mar. Chem., 68(3), 183–201. [Google Scholar]
- Taylor, J. R. (1997), An Introduction to Error Analysis: The Study of Uncertainties in Physical Measurements, 2nd ed., 327 pp., Univ. Sci. Books, Sausalito, Calif. [Google Scholar]
- Tseng, C.‐M. , Liu K. K., Gong G. C., Shen P. Y., and Cai W. J. (2011), CO2 uptake in the East China Sea relying on Changjiang runoff is prone to change, Geophys. Res. Lett., 38, L24609, doi:10.1029/2011GL049774. [Google Scholar]
- Tsunogai, S. , Watanabe S., Nakamura J., Ono T., and Sato T. (1997), A preliminary study of carbon system in the East China Sea, J. Oceanogr., 53(1), 9–17. [Google Scholar]
- Turner, R. E. , and Rabalais N. N. (2013), Nitrogen and phosphorus phytoplankton growth limitation in the northern Gulf of Mexico, Aquat. Microb. Ecol., 68(2), 159–169. [Google Scholar]
- Walker, N. D. , Wiseman W. J., Rouse L. J., and Babin A. (2005), Effects of river discharge, wind stress, and slope eddies on circulation and the satellite‐observed structure of the Mississippi River plume, J. Coastal Res., 21(6), 1228–1244. [Google Scholar]
- Wanninkhof, R. (1992), Relationship between wind speed and gas exchange over the ocean, J. Geophys. Res., 97(C5), 7373–7382. [Google Scholar]
- Wanninkhof, R. , and McGillis W. R. (1999), A cubic relationship between air‐sea CO2 exchange and wind speed, Geophys. Res. Lett., 26(13), 1889–1892. [Google Scholar]
- Wanninkhof, R. , Doney S. C., Takahashi T., and McGillis W. R. (2002), The effect of using time‐averaged winds on regional air‐sea CO2 fluxes, in Gas Transfer at Water Surfaces, Geophys. Monogr. Ser., vol. 127, edited by Donelan M. A. et al., pp. 351–356, AGU, Washington, D. C. [Google Scholar]
- Wanninkhof, R. , Asher W. E., Ho D. T., Sweeney C., and McGillis W. R. (2009), Advances in quantifying air‐sea gas exchange and environmental forcing*, Annu. Rev. Mar. Sci., 1(1), 213–244. [DOI] [PubMed] [Google Scholar]
- Weiss, R. F. (1974), Carbon dioxide in water and seawater: The solubility of a non‐ideal gas, Mar. Chem., 2(3), 203–215. [Google Scholar]
- Weiss, R. F. , and Price B. A. (1980), Nitrous oxide solubility in water and seawater, Mar. Chem., 8(4), 347–359, doi:10.1016/0304-4203(80)90024-9. [Google Scholar]
- Zhai, W. , and Dai M. (2009), On the seasonal variation of air‐sea CO2 fluxes in the outer Changjiang (Yangtze River) Estuary, East China Sea, Mar. Chem., 117(1–4), 2–10. [Google Scholar]
- Zhang, X. , Hetland R. D., Marta‐Almeida M., and DiMarco S. F. (2012), A numerical investigation of the Mississippi and Atchafalaya freshwater transport, filling and flushing times on the Texas‐Louisiana Shelf, J. Geophys. Res., 117, C11009, doi:10.1029/2012JC008108. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information:
Supporting Information S1


