Abstract
Constipation is a common and often debilitating condition in the elderly, which may be caused by underlying disease conditions, structural abnormalities in the bowel, and a variety of medications such as anticholinergics, antidepressants, and opiates. In this review, we focus on opioid-induced constipation (OIC), which is often underrecognized and undertreated in the elderly. When opioid therapy is initiated, healthcare providers are encouraged to evaluate risk factors for the development of constipation as part of a thorough patient history. To this end, the patient assessment should include the use of validated instruments, such as the Bristol Stool Scale and Bowel Function Index, to confirm the diagnosis and provide a basis for evaluating treatment outcomes. Healthcare providers should use a stepwise approach to the treatment of OIC in the elderly. Conventional laxatives are a first-line option and considered well tolerated with short-term use as needed; however, evidence is lacking to support their effectiveness in OIC. Moreover, because of the risk of adverse events and other considerations, such as chewing difficulties and swallowing disorders, conventional oral laxatives may be inappropriate for the treatment of OIC in the elderly. Thus, the availability of new pharmacologic agents such as the peripherally acting µ-opioid receptor antagonists methylnaltrexone and naloxegol, which target the underlying causes of OIC, and the secretagogue lubiprostone may provide more effective treatment options for elderly patients with OIC.
Key Points
| Constipation is a prevalent and often debilitating condition in the elderly, which may be caused by underlying disease conditions, structural abnormalities in the bowel, and a variety of medications that are commonly used in this age group. |
| Opioid-induced constipation (OIC), a debilitating adverse event resulting from the agonist actions of opioid medications at µ-opioid receptors, which are abundant throughout the gastrointestinal tract, is often underrecognized and undertreated in the elderly. |
| Healthcare providers should perform a thorough patient assessment to evaluate risk factors for the development of constipation in elderly patients, recognizing the potential impact of different care settings, underlying comorbidities (and medications for their treatment), and the differentiation of OIC from functional constipation as crucial aspects in guiding the choice of treatment option for effective management of OIC. |
Introduction
Constipation is a common and uncomfortable condition, affecting an estimated 2–27 % of the general population in the USA [1]. However, the incidence is much higher in the elderly and ranges from 20 to 74 % of patients, depending on the care setting [2–4], and negatively impacts quality of life [5, 6]. The etiology of constipation in the elderly is often multifactorial and may be associated with the presence of comorbidities [7, 8], the use of medications (e.g., anticholinergics and antidepressants) [9, 10], and sedentary lifestyles [7].
The use of opioid analgesics is often associated with the onset of opioid-induced bowel dysfunction (OBD), which comprises a constellation of gastrointestinal (GI) symptoms including abdominal bloating, gastrointestinal reflux, abdominal cramps, and constipation [11, 12]. The constipation resulting from opioid pain management alone can be debilitating and is estimated to affect 40–86 % of patients being treated for noncancer pain and cancer-related pain [12–18]. Although constipation in general is a well-recognized condition in adult patients, including the elderly [19], studies suggest that OIC in the elderly is often underrecognized and undertreated [20–23].
For these reasons, the present review aims to help healthcare providers to better understand the risk factors for constipation in elderly patients in the context of different care settings and underlying comorbidities for effective management of this condition, with particular focus on OIC.
Prevalence of Constipation and Pain in the Elderly
Constipation is generally considered a condition in which bowel movements (BMs) occur less often than usual and/or consist of hard, lumpy stools that are difficult or painful to pass [24]. Although it is difficult to define what represents “normal” bowel function across individuals [16], it is typical to consider an adult who has not had a BM in 3 days as constipated [25]. Consequently, the prevalence of constipation varies and has been estimated to range from approximately 2 to 27 % across studies in the general population of North America [1]. The prevalence of self-reported constipation also increases with age and differs by sex. Thus, the frequency of constipation tends to be greater in patients at least 80 years of age compared with younger individuals (66 vs 57 % of those aged <70 years) and is more common in women compared with men (63 vs 54 %); women at least 60 years of age are twice as likely as men to report being “always” or “mostly” constipated [26]. Moreover, the frequency of emergency department visits for constipation rose by approximately 13 % from 2006 to 2011 in patients ≥65 years of age [27], further underscoring the burden of illness and the importance of recognition and effective management of constipation in the elderly.
Different care settings impact the prevalence rates for constipation in older patients. For example, a lower prevalence of constipation has been reported among independent community-dwelling individuals (14–25 %) [2, 28, 29] than among patients in the hospitalized acute care (42–83 %) [30–32], hospice (45–70 %) [33–35], and long-term care settings (47–55 %) [36, 37]. In addition, a longitudinal survey conducted in the community setting revealed that >60 % of those who reported constipation at baseline continued to suffer from the condition at follow-up 10 years later [28].
The prevalence of chronic pain in the elderly ranges from 24 to 62 % in the community setting [38–41] and from 64 to 83 % in the long-term care setting [42, 43]. Chronic pain often persists for longer durations in the elderly compared with younger age groups [44, 45] and is associated with a more sedentary lifestyle [46, 47]. In addition to lower levels of physical activity, chronic pain in the elderly confers an additional disease burden, most commonly presenting as cardiac disorders (54 %), GI disorders (36 %), psychiatric disorders (33 %), and obesity (i.e., body mass index ≥30; 26 %) [41, 48]. Elderly women are twice as likely as men to experience chronic pain [41, 49, 50].
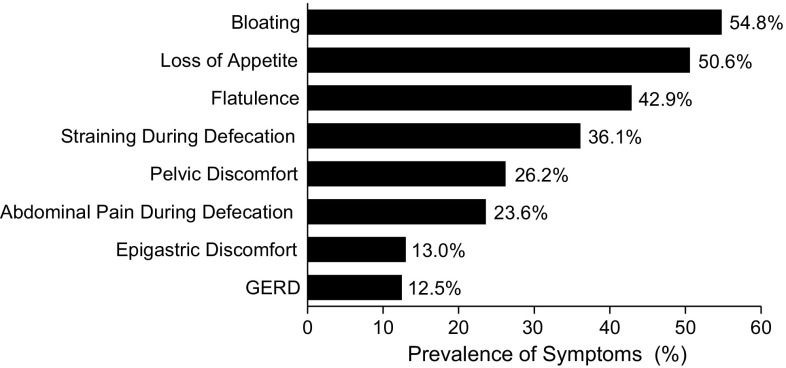
Opioid analgesics are recommended for the treatment of chronic pain in the elderly [51] and are prescribed to 36–90 % of adult patients for the treatment of chronic pain [52–54]. Although opioids provide effective pain management, 25–86 % of elderly patients taking these analgesics may have symptoms of OIC, and such patients frequently report additional GI symptoms (e.g., loss of appetite, gastroesophageal reflux) of OBD (Fig. 1) [14, 21].
Fig. 1.
Prevalence of gastrointestinal symptoms in elderly patients with opioid-induced constipation. GERD gastroesophageal reflux disease.
(Adapted from [14], reprinted by permission of Taylor & Francis Ltd)
Pathophysiology
Constipation can present as normal or slow colon transit constipation, either alone or in combination with defecatory disorders [55]. Constipation can also occur secondary to extrinsic factors, such as lack of dietary fiber or physical inactivity, and can be caused by systemic diseases, medications, or structural abnormalities in the bowel (Table 1) [56–59].
Table 1.
| Medical conditionsa | Medicationsa | Structural abnormalitiesb |
|---|---|---|
| Electrolyte disturbances | Analgesics (opioids, tramadol, NSAIDs) | Carcinomas (colon, rectum, pancreas, stomach) |
| Hypercalcemia | Antacids (calcium and aluminum) | Colonic stricture (ischemic, inflammatory) |
| Hypokalemia | Anticholinergics | Radiation fibrosis |
| Hypermagnesemia | Anticonvulsants | Surgical complications (adhesions) |
| Endocrine and metabolic disorders | Antihistamines | |
| Diabetes mellitus | Antiparkinsonian drugs (dopaminergic agents) | |
| Hypothyroidism | Antipsychotics (phenothiazine derivatives) | |
| Hyperparathyroidism | Bile acid binders | |
| Chronic renal disease | Calcium channel blockers | |
| Myopathic disorders | Calcium supplements | |
| Amyloidosis | Diuretics (furosemide, hydrochlorothiazide) | |
| Scleroderma | Iron supplements | |
| Neurologic disorders | Tricyclic antidepressants | |
| Autonomic neuropathy | ||
| Dementia | ||
| Multiple sclerosis | ||
| Parkinson disease | ||
| Spinal cord lesions | ||
| Other | ||
| Depression | ||
| General disability |
By definition, functional constipation has no specifically identifiable underlying pathophysiologic mechanism. OIC, on the other hand, is caused by opioid medications via direct agonism of µ-opioid receptors, which are abundant throughout the GI tract [60–62], the direct effect of which results in delayed GI transit, decreased secretion of electrolytes resulting in increased fluid absorption, and increased sphincter tone with impaired reflex relaxation following rectal distension [11, 12, 62]. Additionally, µ-opioid receptors are widely distributed throughout the central and peripheral nervous systems [63, 64]; therefore, it is important to strike a balance between the pain-relieving effects of opioid analgesics, which are primarily mediated by agonism at central µ-opioid receptors, and the risk of GI effects of opioids such as OIC, which can compromise the potential clinical benefits of opioid analgesics by patients choosing to decrease or stop the use of opioid medications to self-manage their OIC and facilitate a BM [13].
Clinical Evaluation of Constipation
Optimal patient management depends on differentiating functional constipation and secondary constipation caused by neurologic disorders or medications other than opioids (e.g., calcium channel antagonists, antidepressants) from other causes such as OIC, which may occur in patients receiving chronic opioid pharmacotherapy for noncancer pain and cancer-related pain [65]. According to the Rome III diagnostic criteria (Table 2), functional constipation is characterized by infrequent, incomplete, and difficult BMs without physiologic abnormalities that would explain the condition. It is also characterized by the presence of at least two of the following symptoms for at least 3 months: <3 BMs per week, stool hardness, straining, sensation of incomplete evacuation or anorectal blockage, or the requirement for manual maneuvers with at least 25 % of BMs [11, 24]. Patients’ self-reporting of functional constipation is frequently based on subjective impressions, such as difficulty in having a BM, the presence of hard stools, and a sensation of abdominal pain and bloating [26, 55].
Table 2.
Rome III diagnostic criteria for functional constipation
| Diagnostic criteria: specific symptomology |
|---|
| ≥2 of the following symptoms: |
| Straining during ≥25 % of BMs |
| Lumpy/hard stools in ≥25 % of BMs |
| Sensation of incomplete evacuation or anorectal obstruction/blockage in ≥25 % of BMs |
| Manual maneuvers to facilitate ≥25 % of defecationsa |
| <3 BMs/week |
| Loose stools rarely present without laxative use |
| Insufficient criteria for IBS |
Adapted with permission from [24]
BM bowel movement, IBS irritable bowel syndrome
aExamples include digital evacuation and pelvic floor support
To provide a standardized approach to the evaluation of OIC, a multidisciplinary working group developed a consensus definition of OIC as “a change when initiating opioid therapy from baseline bowel habits that is characterized by any of the following: reduced bowel movement frequency, development or worsening of straining to pass bowel movements, a sense of incomplete rectal evacuation, or harder stool consistency” [66]. Symptoms commonly reported by patients with OIC are similar to those reported by patients with functional constipation [13, 66, 67].
In addition to presenting as a new condition secondary to treatment with opioids, preexisting constipation can be aggravated by opioids [68, 69], even in patients who have received prophylactic treatment with osmotic or stimulant laxatives [69, 70]. Despite the availability of a consensus definition of OIC, the condition is often underrecognized and undertreated [9, 20, 21]. As a result, many patients with OIC continue to experience bothersome symptoms and often decrease or stop their use of opioid medications to reduce the symptoms of constipation, thereby compromising pain management and quality of life (QOL) [67, 71]. However, this is an unreliable approach to pain management because the constipation-inducing dose of opioid medication is typically 25 % of the dose that alleviates pain [72].
Opioid-Induced Constipation and Quality of Life
Although OIC may result in changes in opioid dosing in many patients, there is limited information on how this condition impacts the quality of life (QOL) burden in elderly patients with noncancer pain. In one study, patients (mean age 50 years) with OIC reported significantly worse scores on the both the mental (44.8 vs 41.6; P < 0.05) and physical components (34.9 vs 31.5; P < 0.05) of the Short Form-8 health-related QOL survey compared with patients (mean age 52 years) without OIC [73]. In another study in younger patients (mean age 53 years) with OIC and chronic noncancer pain, the EuroQOL-5D (EQ-5D; 1 = full health, 0 = death) score reported at baseline (mean ± SD 0.49 ± 0.29) was consistent with decreased QOL [67]. In a study that evaluated QOL in patients using opioids, patients with advanced illness and OIC (mean age >64 years) reported significantly worse scores on the Patient Assessment of Constipation-Quality of Life (PAC-QOL) survey (Fig. 2a). Patients with non-advanced illness and OIC (mean age 59 years) reported significantly worse scores on both the PAC-QOL survey (Fig. 2a) and the EQ-5D index (Fig. 2b) compared with patients (mean age 59 years) with non-advanced illness [74].
Fig. 2.
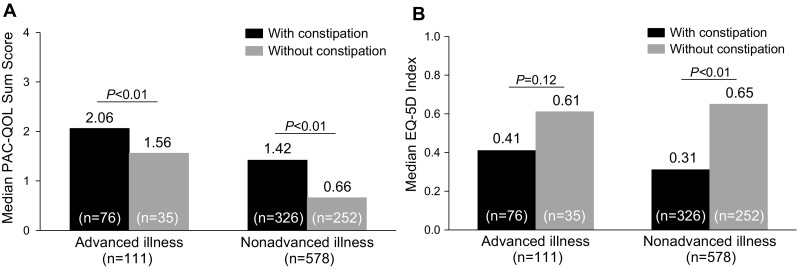
Quality of life in opioid-treated patients classified as having advanced illness (severe, non-curable disease and relatively short life-expectancy) or non-advanced illness (disabling but not life-threatening chronic condition) based on a PAC-QOL sum scores (higher scores indicate lower quality of life) and b the EQ-5D index (lower scores indicate lower quality of life). EQ-5D EuroQOL-5 Dimensions, PAC–QOL Patient Assessment of Constipation–Quality of Life.
(Adapted from [74], reprinted by permission of Informa Healthcare)
The impact of OIC over time on QOL in elderly patients with noncancer pain has not been fully evaluated. In one study, patients (mean age 61 years) with primarily noncancer pain and severe OIC reported a significantly worse QOL score (0 = worst possible, 10 = best possible) over a 6-month period (constipation score 3.8; P < 0.05) compared with patients with no (4.9), mild (4.9), and moderate (4.7) constipation [75]. In addition, when asked to rate their satisfaction with their pain treatment on a 10-point scale (0 = very dissatisfied, 10 = very satisfied) over a 6-month period, patients with severe constipation reported significantly less satisfaction with their pain treatment (mean satisfaction score 5.2; P < 0.05), compared with patients with no (6.6), mild (6.6), and moderate (6.2) constipation [75].
In another study, patients (mean age 54 years) with OIC and chronic noncancer pain [predominantly back pain (74.4 %) and joint pain (53.4 %)] reported a moderate impact of OIC on quality of life, based on PAC-QOL domain scores (range 0–4; higher scores indicate greater impact) for physical discomfort (mean ± SD 2.0 ± 0.9), psychological discomfort (1.3 ± 0.9), and worries and concerns (1.8 ± 1.0) [76]. Moreover, PAC-QOL domain scores remained relatively unchanged over a 24-week follow-up period despite sufficient laxative use by >80 % of patients in the 2 weeks before baseline and by >70 % of patients throughout the 24-week follow-up period. Taken together, these studies suggest that the burden of OIC on quality of life may be affected by the patient’s health status and persist over time despite sufficient laxative use.
Constipation in Different Care Settings
The risk of development or aggravation of constipation in the elderly is contingent on a number of factors, which may vary, depending on the care setting (Table 3). In the independent community setting, for example, significant risk factors for constipation are abdominal pain [2], lower urinary tract symptoms [77], body mass index ≥25 [77], use of acetaminophen (≥7 tablets/week) [78], use of opioid analgesics [28], and any use of aspirin or nonsteroidal anti-inflammatory drugs (NSAIDs) [78].
Table 3.
Risk factors for the development of constipation in the elderly in different care settings
| Community dwelling [2, 28, 77, 78] | Hospitalized acute care [79–83] | Long-term care [84–88] | Hospice care [89, 90] |
|---|---|---|---|
| Abdominal pain | Comorbidities | Chewing problems | Cancer (e.g., trachea, bronchus, lung) |
| BMI ≥ 25 | Acute exacerbation of COPD | Comorbidities | Dependence on caregivers |
| Lower urinary tract symptoms | Cerebrovascular events | Arthritis | Insufficient food and fluid intake |
| Medications | Chewing difficulties | Anorexia nervosa | Nonmalignant comorbidities |
| Acetaminophen ≥7 tablets/week | Spinal cord lesions | CV disease | Circulatory |
| Antiparkinsonian drugs | Medications | Cognitive impairment | Cardiac |
| Aspirin or NSAIDs | Antimuscarinic drugs | Parkinson disease | Last pain score ≥ mild |
| Diuretics | Antiparkinsonian drugs | Pneumonia | Respiratory |
| Opioid analgesics | Diuretics | Postoperative pain (immobility) | Poor performance status |
| Tricyclic antidepressants | Hypnotics | Presence of allergies | Toileting facilities (e.g., lack of privacy) |
| Muscle relaxants | Decreased fluid intake (<5 glasses/day) | ||
| NSAIDs | Dependence on caregivers | ||
| Opioids | Inadequate dietary fiber | ||
| Statins | Medications | ||
| Antacids | |||
| Acetaminophen | |||
| Anticholinergic drugs | |||
| Antidepressants | |||
| Calcium channel antagonistsa | |||
| Diuretics | |||
| Histamine H2 receptor antagonists | |||
| NSAIDs | |||
| Opioid analgesics | |||
| Polypharmacy (>5−7 drugs) | |||
| Poor nutritional assessment | |||
| Sedentary lifestyle | |||
| Toileting facilities (e.g., lack of privacy) |
BMI body mass index, COPD chronic obstructive pulmonary disease, CV cardiovascular, NSAID nonsteroidal anti-inflammatory drug
aOther than verapamil and nifedipine
In the hospitalized acute care setting, the use of medications such as opioids, NSAIDs, diuretics, hypnotics, muscle relaxants, statins, iron supplements, antimuscarinic drugs, and drugs for Parkinson disease confer risk of developing constipation (Table 1) [79–82]. Chewing difficulty, a history of cerebrovascular events, acute exacerbation of chronic obstructive pulmonary disease, or spinal cord lesions can also independently aggravate constipation [81, 83].
Elderly patients in the long-term care setting are at risk of developing constipation from a variety of factors. These include comorbidities such as Parkinson disease, pneumonia, the presence of allergies, and cognitive impairment [84, 85]; a sedentary lifestyle [84, 85]; decreased fluid intake (<5 glasses/day) [85], inadequate dietary fiber, and chewing problems [85]; poor nutritional assessment (i.e., Mini Nutritional Assessment score <17) [85]; polypharmacy (>5−7 drugs) [84, 85]; lack of privacy [86, 87]; and dependence on caregivers [88].
In the setting of hospice care, the majority of patients (54 %) with moderate to severe constipation at first assessment had a primary diagnosis of cancer (trachea, bronchus, and lung: 17 %; digestive organs and peritoneum: 14 %) or a nonmalignant medical condition (46 %) such as a circulatory (15 %), cardiac (11 %), or respiratory system disorder (7 %) [89]. In these patients, the most significant risk factors for development of constipation were insufficient food and fluid intake, a lack of privacy, dependence on caregivers, and poor performance status [90].
It is evident, therefore, that it is important to consider the patient’s care setting and the risk factors that could further exacerbate constipation to minimize the incidence of this disorder, especially in those receiving opioids.
Identification of Vulnerable Elderly Patients
Regardless of setting, there are common factors that increase susceptibility to constipation in general and to OIC. Compared with individuals <65 years of age, elderly individuals with constipation often report more frequent straining, hard stools, self-digitation, sensation of rectal blockage, and <2 BMs per week [29]. The elderly typically have more comorbidities that must be carefully monitored and considered when diagnosing and managing OIC. These include endocrine and metabolic disorders, such as diabetes mellitus and chronic renal disease, and neurologic disorders, including cerebrovascular disease, Parkinson disease, and spinal cord injury [57].
The patient’s functional status should also be taken into account; many elderly patients have sedentary lifestyles and cognitive impairments [57]. Specific medications including analgesics, tricyclic antidepressants, and anticholinergic agents (Tables 1, 3) can further exacerbate OIC [7, 10, 57, 78]. Lastly, insufficient intake of dietary fiber and fluids is a common problem in the elderly, awareness of which needs to be promoted among patients when commencing opioid therapy [7, 63, 90, 91].
Risk of Developing Complications from Untreated Constipation
As previously mentioned, several comorbid conditions can aggravate constipation in general, but studies further suggest that elderly individuals with constipation may be at increased risk of developing additional medical complications. For example, increased straining to have a BM has been associated with the onset of cardiovascular events, including congestive heart failure and myocardial infarction, and cerebrovascular events, including transient ischemic attacks and syncopal episodes [7, 92].
Elderly patients with chronic constipation may also be at risk of developing new GI comorbidities, including megacolon, volvulus, and anal fissures; and other comorbidities such as depression and mood disorders, iron deficiency anemia, and hypothyroidism [93]. In the nursing home setting, chronic untreated constipation is a risk factor for the development of fecal impaction, which can cause stercoral ulceration, leading to bowel perforation [94].
Physicians have expressed concern that, if not treated, OIC could have serious consequences in the elderly beyond the additional GI symptoms associated with OBD, including fecal impaction and bowel obstruction, which may contribute to increased patient morbidity (e.g., abdominal pain, nausea and vomiting) [13, 52, 95–98]. Thus, it is important that healthcare providers be aware of possible risk factors (e.g., anorexia, immobility, cognitive impairment, colonic neuromuscular disorders, urinary frequency) for fecal impaction and bowel obstruction, and monitor elderly patients accordingly [7, 99]. Reports of stercoral perforation of the bowel, a potentially fatal condition caused by fecal impaction, are rare in patients with OIC [100], and the risk of other medical complications, such as those previously mentioned in elderly patients with chronic constipation in general, has not been fully evaluated in elderly patients with OIC [101].
Clinical Management of Opioid-Induced Constipation
Although nonpharmacologic and pharmacologic agents are available for the management of constipation, most studies were not designed to address the issues associated with constipation in the elderly, including OIC. Treatment guidelines specifically for the management of elderly patients with OIC are not available. However, a review of the evidence in the literature for treatment of adult patients with OIC and noncancer pain recommends nonpharmacologic interventions (e.g., dietary measures, increased physical activity, biofeedback training) and use of over-the-counter laxatives followed by prescription opioid receptor antagonists if these fail [8, 63, 102].
Dietary Measures
Consistent with the association between insufficient food and fluid and increased risk of constipation in palliative care patients [90], increased food and fluid intake by patients in the community (N = 27; mean age 64 years) and nursing home settings (N = 23; > 60 years of age) was associated with significant improvements in Patient Assessment of Constipation–Symptoms total scores and abdominal, rectal, and stool symptom subscale scores [103, 104]. In addition to improving symptoms of constipation, dietary measures such a high fiber diet and increased fluid intake have been shown to increase stool weight and decrease colon transit time in some patients with constipation. Even so, it is unclear whether the effectiveness of dietary measures observed in patients with functional constipation can be extrapolated to elderly patients with OIC (Table 4) [8, 10, 11].
Table 4.
Interventions for the treatment of opioid-induced constipation and potential limitations for their use in elderly patients
| Intervention | Mechanism of action | Potential limitations in elderly patients | References |
|---|---|---|---|
| Lifestyle modification | |||
| Dietary [increased food/fluid intake] | Increases stool weight/hydration and decreases colonic transit time | Effectiveness of increased dietary fiber in OIC not established Poor response to dietary fiber ≥30 g/day in patients with slow-transit constipation and dyssynergic defecation Reluctance to increase fluid intake due to perceived risk of becoming incontinent May be ineffective in patients with chewing/swallowing disorders Failed to reduce laxative use or improve symptoms of constipation in acute care setting |
[4, 87, 142, 143] |
| Physical activity | Stimulates colonic activity after exercise | Chronic pain may limit patient’s ability to engage in physical activity | [63, 105] |
| Laxatives | |||
| Bulk-forming agent [psyllium fiber] | Increases stool bulk, distends colon, stimulates peristalsis | Risk of AEs: gas, bloating, and rectal bleeding May be unsuitable for treatment of OIC owing to prevention of peristalsis by opioids, which may result in exacerbation of abdominal pain The need to drink sufficient fluids to avoid mechanical obstruction may limit utility in frail, immobile patients Not recommended for relief of severe constipation in palliative care settings |
[10, 56, 63, 144] |
| Osmotic agent [PEG 3350, lactulose] | Increases fluid content of bowel lumen to hydrate and soften stool, leading to improved propulsion | Risk of AEs PEG 3350: rectal bleeding, diarrhea, nausea, vomiting, abdominal pain, bloating Lactulose: gaseous distention, belching, flatulence, borborygmi, abdominal discomfort Increased risk of aspiration of PEG-balanced electrolyte solution in elderly with supranuclear palsy or Parkinson disease PEG may increase risk of folate deficiency in frail elderly patients Sweet taste of lactulose disagreeable to some patients |
[10, 56, 63, 145, 146] |
| Stimulant [senna, bisacodyl] | Increases muscle contractions via enteric reflex | Risk of AEs Senna: diarrhea, abdominal pain, abdominal cramps Bisacodyl: stomach discomfort, faintness, cramps, rectal burning Slower onset of response (i.e., >8−12 h) in frail elderly Risk of electrolyte disturbances (e.g., hyperkalemia) at high doses in elderly |
[10, 147, 148] |
| Surfactant [docusate sodium] | Emulsifier facilitates admixture of fat and water in feces to soften the stool | Risk of rectal bleeding | [10, 63, 147] |
| Secretagogue [lubiprostone] | Chloride channel activator bypasses antisecretory effects of opiates to increase intestinal fluid secretion motility, facilitating passage of stool | Risk of nausea, diarrhea | [126] |
| PAMORAs | |||
| Methylnaltrexone | Functions as µ-opioid receptor antagonist in GI tract with limited ability to cross BBB; decreases constipating effects of opioids without compromising centrally mediated opioid analgesia | Risk of abdominal pain, nausea, diarrhea, hyperhidrosis, hot flush, tremor, chills | [115] |
| Naloxegol | Functions as µ-opioid receptor antagonist in GI tract; reduced permeability and increased efflux of naloxegol across BBB limits potential for interference with centrally mediated opioid analgesia | Risk of abdominal pain, diarrhea, nausea, flatulence, vomiting, headache, hyperhidrosis Contraindicated in patients with known/suspected GI obstruction or at increased risk of recurrent obstruction |
[114] |
| Biofeedback | Patients trained to relax pelvic floor muscles during straining to have BMs | Usefulness compromised in patients with cognitive impairment | [4, 10] |
AE adverse event, BBB blood–brain barrier, BM bowel movement, GI gastrointestinal, OIC opioid-induced constipation, PAMORA peripherally acting µ-opioid receptor antagonist, PEG polyethylene glycol
Physical Activity
Consistent with the ability of physical activity to increase colonic motility following exercise [105], a randomized study conducted in outpatients (N = 43) >45 years of age with chronic constipation demonstrated that a 12-week program of regular daily physical exercise improved several symptoms of constipation (e.g., incomplete BM, straining, hard stools) [106]. However, elderly patients (N = 224; mean age 81 years) with constipation in the long-term care setting showed no improvement in the frequency of BMs as a result of either resistance training or physical activity performed twice weekly for 6 months under the guidance of a trained physical therapist [107].
Biofeedback Therapy
Biofeedback therapy is a form of behavioral modification in which patients are trained to relax muscles of the anus and pelvic floor and use their abdominal muscles to create a pushing force that results in a BM [108]. Biofeedback therapy may be used in the elderly; one study showed long-term improvement in symptoms of chronic constipation in patients (some of whom were elderly) followed for up to nearly 4 years [10]. However, it is important to note that biofeedback therapy and physical activity as interventions for constipation in the elderly may not be useful in patients with diminished cognitive function and other comorbidities, including chronic pain [4, 10, 63, 109].
Laxatives
Laxatives commonly used for treatment of constipation include agents that inhibit fluid reabsorption, increase the fluid content in the bowel on the basis of hydrophilic and osmotic properties, or normalize contraction of the bowel (Table 4) [12, 66]. Laxatives recommended as first-line therapy in patients with OIC include stimulant laxatives and stool softeners [63, 66]. However, there is insufficient evidence from randomized clinical trials to determine whether individual laxatives are better than others for the management of constipation in the elderly [110], including those with OIC [66].
It is worth noting that proactive, prophylactic treatment of OIC is not routinely practiced in elderly patients. A survey in elderly patients in the ambulatory care setting revealed that only 1 % of patients received prescriptions for laxatives when initiating opioid therapy for chronic pain [111]. Moreover, a study in patients (mean age 53 years) with OIC and noncancer pain revealed an inadequate response to laxatives (e.g., osmotic laxatives, lactulose, lubiprostone, and methylnaltrexone) in 94 % of patients within the previous 2 weeks [67], indicating that such patients are undertreated by commonly used laxatives.
Importantly, healthcare providers should be cognizant of the fact that lifestyle interventions and laxative therapies that have been used successfully for the treatment of constipation in younger patients may be unsuccessful or unrealistic in an elderly population (Table 4).
Opioid-Induced Constipation–Targeted Pharmacotherapy
In elderly patients with OIC and chronic pain who do not respond to lifestyle interventions or laxatives, relief from symptoms of OIC may be achieved using agents that target the underlying causes of constipation, such as methylnaltrexone and naloxegol, and are indicated for the treatment of OIC [112–115].
Peripherally acting µ-opioid receptor antagonists (PAMORAs), such as methylnaltrexone and naloxegol, have a limited ability to penetrate the blood–brain barrier and selectively antagonize peripheral µ-opioid receptors in the GI tract, thereby decreasing the constipating effects of opioids while preserving centrally mediated opioid analgesia [112, 114, 115].
Methylnaltrexone
Methylnaltrexone is approved for use in adult patients with OIC and advanced disease when laxative response is insufficient in the palliative care setting, as well as for use in adult patients with chronic noncancer pain and OIC [115]. In one clinical trial, patients (median age 72 years) with OIC and advanced disease who received subcutaneous methylnaltrexone (0.15 mg/kg body weight) every other day (QOD) for 2 weeks reported a significantly higher rate of laxation within 4 h of the first dose (48 vs 15 %, P < 0.001) and within 4 h after two or more of the first four doses (52 vs 8 %, P < 0.001) compared with placebo, and there were no changes in pain scores, consistent with a peripheral mechanism of action [116].
A 2-week study showed that patients (mean age 66 years) with OIC, advanced disease (cancer in 66 %), and pain (median daily morphine equivalent dose 177 mg/day) who were randomized to receive fixed-dose subcutaneous methylnaltrexone (8 or 12 mg QOD based on body weight) reported a higher rate of rescue-free BMs (RFBMs) within 4 h after two or more of the first four doses (i.e., during the first week; 62.9 vs 9.6 %, P < 0.0001), an increased number of BMs within 24 h after dosing (week 1, 4.9 vs 3.0, P < 0.0001; week 2, 3.2 vs 2.2, P = 0.0083), a greater number of RFBMs within 24 h after dosing (week 1, 4.9 vs 2.7, P < 0.0001; week 2, 3.2 vs 2.0, P = 0.0024), and decreased use of rescue laxatives (27.2 vs 39.6 %, P = 0.002) compared with placebo [117]. The effectiveness observed during the 2-week period was maintained in a 10-week open-label extension (OLE) study [117]. In the aforementioned studies, analgesia was maintained, mean daily opioid doses remained stable, and fixed-dose subcutaneous methylnaltrexone was generally safe and well tolerated in patients with OIC and advanced disease [117].
Adult patients with OIC and chronic noncancer pain (primarily back pain) who received methylnaltrexone 12 mg once daily (QD) or QOD reported an improvement in rescue-free laxation (34.2 vs 9.9 %, P < 0.001). There was also an improvement in the number of injections resulting in rescue-free laxation (QD dosing, 28.9 vs 9.4 %, P < 0.001; QOD dosing, 30.2 vs 9.3 %, P < 0.001) within 4 h of the first dose of study medication. In addition, treatment with methylnaltrexone resulted in significant improvement in straining, completeness of evacuation, and Bristol Stool Form Scale scores compared with placebo, while maintaining opioid analgesia during the course of treatment [118].
In patients (median age 72 years) with noncancer pain, OIC and advanced disease who received subcutaneous methylnaltrexone (0.15 mg/kg body weight) QOD for 2 weeks, the incidence of overall adverse events (AEs) was similar in patients who received methylnaltrexone (81 % vs 80 %) compared with placebo [116]. However, AEs that occurred more frequently with methylnaltrexone compared with placebo included abdominal pain (17 vs 13 %), flatulence (13 vs 7 %), nausea (11 vs 7 %), increase in body temperature (8 vs 3 %), dizziness (8 vs 3 %), and diarrhea (6 vs 4 %) [116].
In patients (mean age 66 years) with OIC and advanced disease (cancer in 66 %) and pain who received fixed-dose subcutaneous methylnaltrexone (8 or 12 mg QOD) for 2 weeks, the overall incidence of AEs was greater with methylnaltrexone (randomized placebo-controlled trial [RCT], 81.9 %; OLE phase, 90.6 %) compared with placebo (73.7 %, RCT) [117]. The most common AEs that occurred more frequently with methylnaltrexone compared with placebo were abdominal pain (33.6 vs 16.7 %), back pain (7.8 vs 2.6 %), falling (7.8 vs 3.5 %), and flatulence (6.9 vs 4.4 %) in the 2-week RCT phase; abdominal pain (26.8 %), peripheral edema (17.4 %), diarrhea (16.1 %), confusional state (15.4 %), nausea (14.1 %), and falling (14.1 %) were reported by >10 % of patients in the 10-week OLE phase [117].
In adult patients with OIC and chronic noncancer pain who received methylnaltrexone 12 mg QD or QOD, the overall incidence of treatment-emergent adverse events (TEAEs) was greater in patients who received methylnaltrexone QD (49.3 %) or QOD (45.3 %) compared with placebo (38.3 %) [118]. GI AEs that were more frequent in the methylnaltrexone QD or QOD group compared with placebo included abdominal pain (methylnaltrexone QD and QOD, 19.3 and 15.5 %; placebo, 3.7 %), diarrhea (6.0 and 11.5 vs 3.7 %), and nausea (8.7 and 11.5 vs 6.2 %); the incidence of hyperhidrosis was also greater in patients receiving methylnaltrexone compared with placebo (6.0 and 6.1 vs 1.2 %) [118].
Maintenance of analgesia was observed, and subcutaneous methylnaltrexone was generally well tolerated in patients with chronic pain [118], including those with advanced illness [116, 117]. An oral formulation of methylnaltrexone is in development [119] and, if approved for use, may provide an alternative route of administration with potential clinical value for patients and healthcare providers.
Naloxegol
Naloxegol is a PEGylated derivative of the µ-opioid receptor antagonist naloxone for targeted oral treatment of OIC in adults [114, 120]. In two phase 3, double-blind studies of adults (11 % aged ≥65 and 2 % ≥75 years) with noncancer pain, patients treated with naloxegol 25 mg reported significantly higher 12-week response rates [≥3 spontaneous BMs (SBMs) per week and an increase from baseline of ≥1 SBM for ≥9 of 12 weeks and for ≥3 of the final 4 weeks] compared with placebo (study 1, 44.4 vs 29.4 %, P = 0.001; study 2, 39.7 vs 29.3 %, P = 0.02); response rates for the 12.5 mg dose versus placebo were significantly higher in study 1 (40.8 vs 29.4 %, P = 0.02) [114, 121]. Similar findings for response rates in patients with an inadequate response to laxatives were reported for naloxegol 25 mg versus placebo (study 1, 48.7 vs 28.8 %, P = 0.002; study 2, 46.8 vs 31.4 %, P = 0.01) and for naloxegol 12.5 mg versus placebo in study 1 (42.6 vs 28.8 %, P = 0.03) [121, 122]. In these studies, there were no differences in effectiveness between elderly patients ≥65 years of age and younger patients [114].
There was a reduction in rescue medication use for naloxegol 25 mg (study 1, 54.7 %; study 2, 57.3 %) and naloxegol 12.5 mg (study 1, 63.4 %; study 2, 57.3 %) compared with placebo (study 1, 72.0 %; study 2, 70.7 %) [121]. Patients also reported greater improvements in straining, stool consistency (Bristol Stool Scale scores), and percentage of days per week with a complete SBM for naloxegol 25 mg compared with placebo in both studies and for naloxegol 12.5 mg compared with placebo in study 2 (P < 0.05) [121].
Naloxegol has generally been well tolerated in clinical trials to date. A greater incidence of overall AEs was reported in the naloxegol 25 mg group (study 1, 61.2 %; study 2, 69.0 %) compared with the naloxegol 12.5 mg (study 1, 49.3 %; study 2, 59.6 %) and placebo (study 1, 46.9 %; study 2, 58.9 %) groups [121]. AEs that occurred more frequently in the naloxegol 25 mg group were primarily GI in nature, including abdominal pain (study 1, 12.6 %; study 2, 19.0 %), diarrhea (study 1, 9.3 %; study 2, 9.1 %), nausea (study 1, 7.5 %; study 2, 8.6 %), and flatulence (study 1, 5.6 %; study 2, 6.0 %) [121].
Elderly patients ≥65 years of age with noncancer pain and OIC were also evaluated in a pooled analysis of these two phase 3, double-blind, 12-week studies. The incidence of overall AEs reported in the naloxegol 25 mg group (56.6 %) was similar to that in the naloxegol 12.5 mg group (50.0 %) and the placebo group (62.0 %) [123]. AEs that occurred more frequently in the naloxegol 25 mg group were primarily GI and included diarrhea (11.3 %), nausea (11.3 %), abdominal pain (9.4 %), and vomiting (7.5 %) [123].
Patients with noncancer pain and OIC were also evaluated in a 52-week, open-label, randomized study of naloxegol 25 mg compared with usual care treatment, in which naloxegol was generally well tolerated [124]. The incidence of overall AEs was 81.8 % with naloxegol and 72.2 % with usual care [124]. TEAEs that occurred more frequently with naloxegol compared with usual care included abdominal pain (17.8 vs 3.3 %), diarrhea (12.9 vs 5.9 %), nausea (9.4 vs 4.1 %), headache (9.0 vs 4.8 %), flatulence (6.9 vs 1.1 %), and upper abdominal pain (5.1 vs 1.1 %) [124]. Among elderly patients ≥65 years of age in this 52-week study, the overall incidence of AEs was 86.7 % for naloxegol and 83.9 % for usual care [123]. TEAEs that occurred more frequently with naloxegol compared with usual care in elderly patients included diarrhea (13.3 vs 9.7 %), back pain (11.1 vs 6.5 %), headache (8.9 vs 0 %), nausea (6.7 vs 0 %), abdominal discomfort (6.7 vs 3.2 %), and sinusitis (6.7 vs 3.2 %) [123].
In both the 12-week and 52-week studies of naloxegol, analgesia was maintained, mean daily opioid doses remained stable, and signs of opioid withdrawal were infrequent [121, 124], consistent with the mechanism of action of naloxegol in antagonizing peripheral µ-opioid receptors located in the GI tract.
Lubiprostone
Lubiprostone is a chloride channel activator, which increases fluid secretion within the bowel lumen, thereby softening stools and promoting BMs [125]. It was approved in 2006 for the treatment of chronic idiopathic constipation in adults and in 2013 for the treatment of OIC in adult patients with chronic noncancer pain [126]. Patients with noncancer pain and OIC who were administered oral lubiprostone (24 µg twice daily [BID]) reported significant improvement from baseline in the frequency of SBMs after 8 weeks compared with placebo (3.3 vs 2.4 SBMs/week, P = 0.005) and in the overall change from baseline (2.2 vs 1.6 SBMs/week, P = 0.005) [127]. The number of patients reporting a first SBM within 24 h (P = 0.018) and 48 h (P = 0.05) was also greater for lubiprostone compared with placebo, and improvements in other constipation-related symptoms (e.g., stool consistency, constipation severity, straining) were observed [127].
In a multinational, phase 3 study in adult patients with OIC and noncancer pain, a significantly greater percentage of patients administered lubiprostone (24 µg BID) were overall responders [reporting at least a moderate response (≥1 SBM improvement over baseline for all treatment weeks for which observed data were available) as well as a full response (≥3 additional SBMs per week for at least 9 of the 12 treatment weeks after 12 weeks; 27.1 vs 18.9 %, P = 0.03)] compared with placebo. Significant improvements in straining, stool consistency, and constipation severity were also observed [128].
In an OLE of these two 12-week studies, treatment with lubiprostone (24 µg BID) maintained the improvement in mean SBM frequency (range 4.9–5.3/week vs 1.4/week at baseline) over the 9-month treatment period; significant improvements from baseline for SBM and BM frequency were reported at each month (P < 0.001, all months). Significant improvements in symptoms associated with constipation (straining, abdominal bloating, abdominal discomfort, constipation severity, stool consistency, bowel habit regularity) were observed at monthly intervals (months 1–9, P < 0.001, all months) [129].
In patients with OIC and noncancer pain who were administered oral lubiprostone (24 µg BID), the overall incidence of AEs was greater in patients who received lubiprostone (63.5 vs 54.4 %) compared with placebo [127]. AEs that occurred more frequently in the lubiprostone group compared with placebo were GI in nature and included nausea (16.8 vs 5.8 %), diarrhea (9.6 % vs 2.9 %), and abdominal distention (8.2 % vs 2.4 %) [127].
In a multinational phase 3 study in adult patients with OIC and noncancer pain who received oral lubiprostone (24 µg BID), the overall incidence of TEAEs was similar in patients who received lubiprostone (55.2 vs 49.5 %) compared with placebo [128]. TEAEs reported more commonly in the lubiprostone group compared with placebo included diarrhea (11.3 vs 3.8 %), nausea (9.9 vs 4.7 %), and abdominal pain (7.1 vs 0 %) [128].
In an OLE of two 12-week studies in adult patients with OIC who were administered lubiprostone (24 µg BID), the most common TEAEs during the 9-month treatment period were nausea (5.0 %), diarrhea (4.6 %), headache (1.6 %), and vomiting (1.4 %) [129].
Lubiprostone was generally well tolerated and did not interfere with opioid-induced analgesia, which is reflected in the stability of pain scores; however, in the 9-month open-label study, the mean morphine equivalent daily dose was not different at months 1–5 and month 9 (P ≥ 0.09) but was significantly increased at months 6–8 (P < 0.04) when compared with baseline [127–129].
Cost Effectiveness of Drugs for Treatment of Opioid-Induced Constipation
Opioid-induced constipation (OIC) increases direct and indirect healthcare costs, increases certain aspects of healthcare utilization, and negatively impacts work productivity [67, 73, 75, 130]. Moreover, healthcare costs are reportedly higher for patients with severe OIC compared with mild or moderate OIC [75]. Although effective management of OIC has the potential for reducing healthcare costs in elderly patients receiving opioids for chronic pain [131], limited information is available on the cost effectiveness of OIC treatments. Thus, longitudinal data are needed to better understand the cost effectiveness of drugs such as PAMORAs and lubiprostone in the management of OIC in elderly patients.
Clinical Guidance on Choice of Treatment in Elderly Patients
Patient Medical History
According to American Gastroenterological Association guidelines and recommendations by an American Academy of Pain Medicine consensus panel, when a healthcare provider suspects a defecatory disorder, patients should be evaluated for a change in bowel habits, specific symptoms of constipation, and the use of medications for the treatment of constipation [25, 113, 132]. In addition to the patient’s medical history (Fig. 3), one of the most crucial factors on which to base clinical management strategies in elderly patients is the information provided by caregivers, who may often know more about the patient’s symptoms and functional status than the patient does.
Fig. 3.
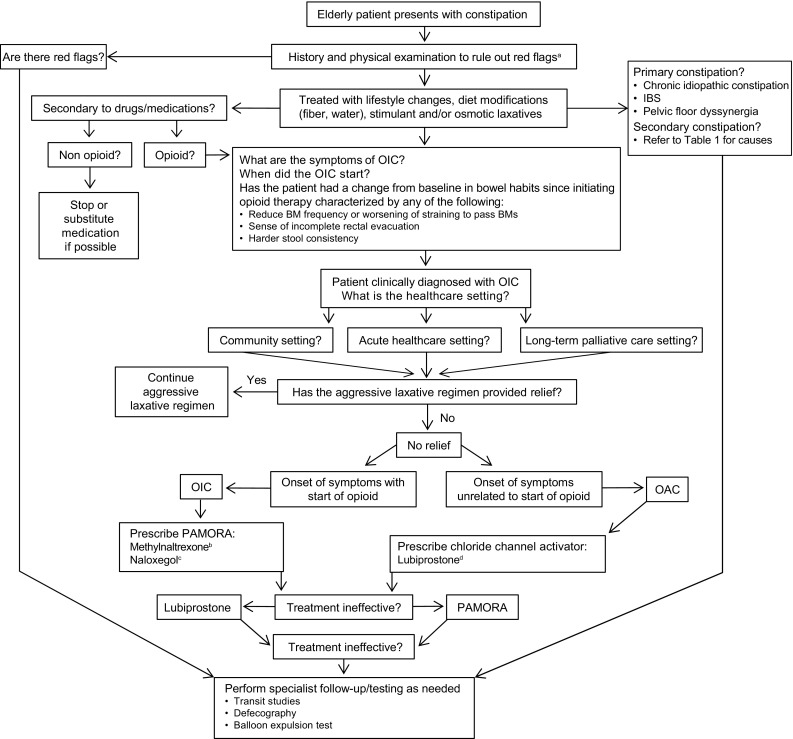
Stepwise management of constipation in the elderly. BM bowel movement, IBS irritable bowel syndrome, OAC opioid-aggravated constipation, OIC opioid-induced constipation, PAMORA peripherally acting µ-opioid receptor antagonist. aRed flags: history of unintentional weight loss, onset of constipation in older patient, family history of cancer or inflammatory bowel disease, bright red blood per rectum; physical examination: abnormal abdominal examination/digital rectal examination, positive fecal occult blood test, flexible sigmoidoscopy or colonoscopy (>50 years); initial laboratory values: decreased hemoglobin, increased white blood cells, increased erythrocyte sedimentation rate, increased thyroid-stimulating hormone, or abnormal potassium or calcium. bIndicated for adults with OIC and advanced disease in the palliative care setting when laxative response is insufficient; adults with chronic noncancer pain. cIndicated for adults with OIC and chronic noncancer pain (USA); adults with OIC when laxative response is inadequate (European Union). dIndicated for adults with OIC and chronic noncancer pain
The healthcare provider should use this history to rule out other causes of constipation, including medications, such as anticholinergics and tricyclic antidepressants, and comorbidities, such as diabetes mellitus and Parkinson disease (Tables 1, 3) [25, 113]. The medical history should also include any remedies (over-the-counter and prescription) the patient has tried that have not relieved symptoms of constipation [87, 113].
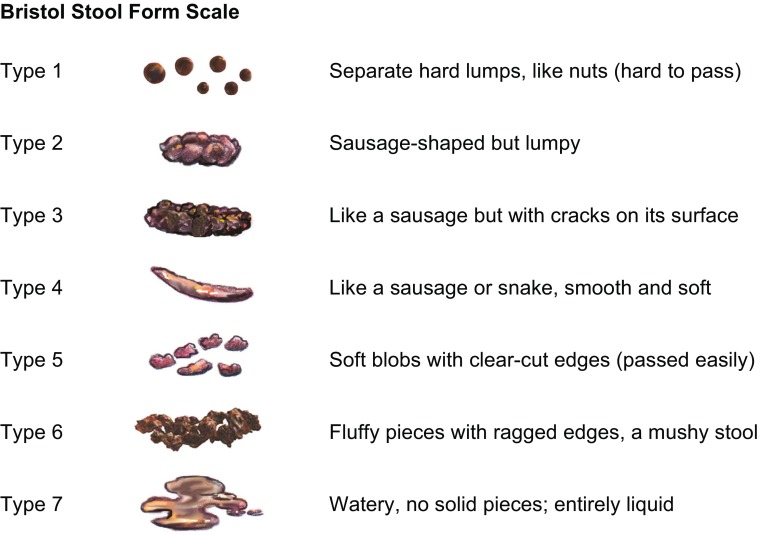
Because patients use variable definitions to describe the subjective experience of constipation, the healthcare provider should use validated assessment tools, such as the Bristol Stool Scale [133] (Fig. 4) and the Bowel Function Index [132, 134], to diagnose the presence and severity of constipation and establish a baseline for the assessment of treatment outcomes. Healthcare providers should also encourage patients to keep a diary of bowel habits for up to 2 weeks, using the Bristol Stool Chart to assess stool form and consistency (Fig. 4) [133] and a diary of food and fluid intake for at least 1 week to establish a baseline for monitoring treatment success [135].
Fig. 4.
Bristol Stool Form Scale [133]. Stool images from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
(Stool scale adapted from [133], reprinted by permission of Informa Healthcare)
Stepwise Approach to Patient Management
The management of constipation in elderly patients should be considered using a stepwise approach (Fig. 3). Healthcare providers should be aware that patients with OIC may continue to report symptoms of constipation, despite efforts to manage the condition by using natural remedies (e.g., increased fluids, fiber supplements), behavioral approaches, and conventional laxatives [67]. Therefore, it is important to monitor the response of elderly patients to conventional laxative approaches, particularly those patients who may be especially vulnerable to AEs associated with these agents (Table 4). In elderly patients with an inadequate response to conventional laxatives or who are not suitable candidates for laxative or lifestyle interventions, because of their specific medical status, treatment options may include targeted therapies (e.g., peripherally acting µ-opioid receptor antagonists) or lubiprostone (Table 4) [136].
To increase the potential for having a BM, elderly patients should also be trained in the mechanics of bowel evacuation. Patients should be instructed to sit on the toilet with the feet elevated, to lean forward, placing the elbows on the knees, and to bulge the abdomen and flatten the spine; this position straightens the anorectal angle and takes advantage of gravity, breathing, and diaphragmatic control to facilitate evacuation of the bowel [58, 135, 137, 138].
Specialist/Follow-Up Testing
When available treatment options fail, further tests in collaboration with specialists may be warranted, including transit studies (e.g., the SITZMARKS® test), defecography (i.e., to rule out diffuse GI dysmotility as the cause of constipation), and anorectal physiology testing (e.g., a balloon expulsion test to eliminate outlet obstruction as the cause of constipation) to more thoroughly evaluate and provide individualized treatment of the elderly patient with constipation [113, 139, 140].
Conclusions
Healthcare providers should be aware that constipation in the elderly may occur secondary to underlying disease conditions and use of medications other than those used to treat chronic pain [57]. Moreover, the use of prescription opioid analgesics for treatment of chronic pain in the elderly is commonly associated with the development of OIC [14, 21]. Thus, healthcare providers should obtain an exhaustive patient history to establish whether the constipation is a preexisting condition aggravated by medications or a new condition secondary to treatment with opioids. The patient assessment should include the use of validated assessment tools (e.g., Bristol Stool Scale, Bowel Function Index) not only to confirm the diagnosis but also to provide a basis for evaluating treatment outcomes.
Healthcare providers should take a stepwise approach when considering the various treatment options for OIC in the elderly. Although laxatives are a first-line treatment option in short-term use as needed and considered generally well tolerated, evidence is lacking to support their effectiveness in OIC [60, 63, 112, 141]. Moreover, it is important to note that conventional laxatives may be inappropriate for the treatment of OIC owing to the risk of AEs and other considerations (e.g., chewing/swallowing disorders).
Approaches to long-term pain management that depend on chronic use of opioid medications in elderly patients may decrease the likelihood that OIC will resolve. Therefore, long-term treatment with OIC-specific medication may be an appropriate option in elderly patients. The availability of the new pharmacologic agents methylnaltrexone, naloxegol, and lubiprostone may provide more effective treatment options for elderly patients with OIC and noncancer pain. Maintenance of opioid-induced analgesia is an important consideration in patients with OIC. Therefore, healthcare providers should be aware that methylnaltrexone [116–118] and naloxegol [121, 122, 124], because of their peripheral mechanism of action, do not interfere with opioid-induced, centrally mediated analgesia when administered to patients with noncancer pain and OIC [116–118, 121, 122, 124]. The complex multifactorial nature of constipation and its potentially negative impact in elderly patients with OIC and chronic pain, coupled with the availability of newer pharmacologic agents that target the underlying mechanisms of constipation (i.e., PAMORAs), and the locally acting secretagogue lubiprostone, provide an opportunity for healthcare providers to better manage their elderly patients with OIC and chronic pain.
Compliance with Ethical Standards
Funding
This work was funded by AstraZeneca Pharmaceuticals LP (Wilmington, DE, USA). Editorial support for this review was provided by Craig D. Albright, PhD, and Diane DeHaven-Hudkins, PhD, from Complete Healthcare Communications, LLC (Chadds Ford, PA, USA) and was funded by AstraZeneca Pharmaceuticals LP. Editorial support consisted of outline preparation and assistance with revisions to all subsequent versions of the manuscript under the direction of the authors.
Conflict of interest
S Chokhavatia, E.S. John, M.B. Bridgeman, and D. Dixit declare that they have no conflicts of interest.
References
- 1.Higgins PD, Johanson JF. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol. 2004;99:750–759. doi: 10.1111/j.1572-0241.2004.04114.x. [DOI] [PubMed] [Google Scholar]
- 2.Choung RS, Locke GR, 3rd, Schleck CD, et al. Cumulative incidence of chronic constipation: a population-based study 1988–2003. Aliment Pharmacol Ther. 2007;26:1521–1528. doi: 10.1111/j.1365-2036.2007.03540.x. [DOI] [PubMed] [Google Scholar]
- 3.Noguera A, Centeno C, Librada S, et al. Screening for constipation in palliative care patients. J Palliat Med. 2009;12:915–920. doi: 10.1089/jpm.2009.0054. [DOI] [PubMed] [Google Scholar]
- 4.Rao SS, Go JT. Update on the management of constipation in the elderly: new treatment options. Clin Interv Aging. 2010;5:163–171. doi: 10.2147/cia.s8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belsey J, Greenfield S, Candy D, et al. Systematic review: impact of constipation on quality of life in adults and children. Aliment Pharmacol Ther. 2010;31:938–949. doi: 10.1111/j.1365-2036.2010.04273.x. [DOI] [PubMed] [Google Scholar]
- 6.O’Keefe EA, Talley NJ, Zinsmeister AR, et al. Bowel disorders impair functional status and quality of life in the elderly: a population-based study. J Gerontol A Biol Sci Med Sci. 1995;50:M184–M189. doi: 10.1093/gerona/50a.4.m184. [DOI] [PubMed] [Google Scholar]
- 7.Gallagher P, O’Mahony D. Constipation in old age. Best Pract Res Clin Gastroenterol. 2009;23:875–887. doi: 10.1016/j.bpg.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Prichard D, Bharucha A. Management of opioid-induced constipation for people in palliative care. Int J Palliat Nurs. 2015;21:272–280. doi: 10.12968/ijpn.2015.21.6.272. [DOI] [PubMed] [Google Scholar]
- 9.Tamayo AC, Diaz-Zuluaga PA. Management of opioid-induced bowel dysfunction in cancer patients. Support Care Cancer. 2004;12:613–618. doi: 10.1007/s00520-004-0649-7. [DOI] [PubMed] [Google Scholar]
- 10.Bosshard W, Dreher R, Schnegg JF, et al. The treatment of chronic constipation in elderly people: an update. Drugs Aging. 2004;21:911–930. doi: 10.2165/00002512-200421140-00002. [DOI] [PubMed] [Google Scholar]
- 11.Dorn S, Lembo A, Cremonini F. Opioid-induced bowel dysfunction: epidemiology, pathophysiology, diagnosis, and initial therapeutic approach. Am J Gastroenterol Suppl. 2014;2:31–37. doi: 10.1038/ajgsup.2014.7. [DOI] [PubMed] [Google Scholar]
- 12.Pappagallo M. Incidence, prevalence, and management of opioid bowel dysfunction. Am J Surg. 2001;182:11S–18S. doi: 10.1016/s0002-9610(01)00782-6. [DOI] [PubMed] [Google Scholar]
- 13.Bell TJ, Panchal SJ, Miaskowski C, et al. The prevalence, severity, and impact of opioid-induced bowel dysfunction: results of a US and European Patient Survey (PROBE 1) Pain Med. 2009;10:35–42. doi: 10.1111/j.1526-4637.2008.00495.x. [DOI] [PubMed] [Google Scholar]
- 14.Abramowitz L, Béziaud N, Labreze L, et al. Prevalence and impact of constipation and bowel dysfunction induced by strong opioids: a cross-sectional survey of 520 patients with cancer pain: DYONISOS study. J Med Econ. 2013;16:1423–1433. doi: 10.3111/13696998.2013.851082. [DOI] [PubMed] [Google Scholar]
- 15.Kalso E, Edwards JE, Moore RA, et al. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain. 2004;112:372–380. doi: 10.1016/j.pain.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 16.McMillan SC. Assessing and managing opiate-induced constipation in adults with cancer. Cancer Control. 2004;11:3–9. doi: 10.1177/10732748040110S302. [DOI] [PubMed] [Google Scholar]
- 17.Tuteja AK, Biskupiak J, Stoddard GJ, et al. Opioid-induced bowel disorders and narcotic bowel syndrome in patients with chronic non-cancer pain. Neurogastroenterol Motil. 2010;22:424–430. doi: 10.1111/j.1365-2982.2009.01458.x. [DOI] [PubMed] [Google Scholar]
- 18.Cook SF, Lanza L, Zhou X, et al. Gastrointestinal side effects in chronic opioid users: results from a population-based survey. Aliment Pharmacol Ther. 2008;27:1224–1232. doi: 10.1111/j.1365-2036.2008.03689.x. [DOI] [PubMed] [Google Scholar]
- 19.Bharucha AE, Pemberton JH, Locke GR., III American Gastroenterological Association technical review on constipation. Gastroenterology. 2013;144:218–238. doi: 10.1053/j.gastro.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thorpe DM. Management of opioid-induced constipation. Curr Pain Headache Rep. 2001;5:237–240. doi: 10.1007/s11916-001-0037-7. [DOI] [PubMed] [Google Scholar]
- 21.Williams R, Bosnic N, Duncan AW, et al. Prevalence of opioid dispensings and concurrent gastrointestinal medications in an elderly population from Ontario, Canada. J Opioid Manag. 2008;4:193–200. doi: 10.5055/jom.2008.0025. [DOI] [PubMed] [Google Scholar]
- 22.Hunold KM, Esserman DA, Isaacs CG, et al. Side effects from oral opioids in older adults during the first week of treatment for acute musculoskeletal pain. Acad Emerg Med. 2013;20:872–879. doi: 10.1111/acem.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corrado-McKeon L, Saad M, Mir T, et al. Treating persistent pain in the elderly: how do we proceed? Consult Pharm. 2013;28:509–514. doi: 10.4140/TCP.n.2013.509. [DOI] [PubMed] [Google Scholar]
- 24.Rome Foundation. Rome III diagnostic criteria for functional gastrointestinal disorders. http://www.romecriteria.org/assets/pdf/19_RomeIII_apA_885-898.pdf. Accessed 10 May 2016. [PubMed]
- 25.American Gastroenterological Association. Bharucha AE, Dorn SD, et al. American Gastroenterological Association medical position statement on constipation. Gastroenterology. 2013;144:211–217. doi: 10.1053/j.gastro.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 26.Harari D, Gurwitz JH, Avorn J, et al. How do older persons define constipation? Implications for therapeutic management. J Gen Intern Med. 1997;12:63–66. doi: 10.1046/j.1525-1497.1997.12110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sommers T, Corban C, Sengupta N, et al. Emergency department burden of constipation in the United States from 2006 to 2011. Am J Gastroenterol. 2015;110:572–579. doi: 10.1038/ajg.2015.64. [DOI] [PubMed] [Google Scholar]
- 28.Werth BL, Williams KA, Pont LG. A longitudinal study of constipation and laxative use in a community-dwelling elderly population. Arch Gerontol Geriatr. 2015;60:418–424. doi: 10.1016/j.archger.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Talley NJ, Fleming KC, Evans JM, et al. Constipation in an elderly community: a study of prevalence and potential risk factors. Am J Gastroenterol. 1996;91:19–25. [PubMed] [Google Scholar]
- 30.Mok K, Smith RJ, Reid DA, et al. Changing clinical guidelines from delayed to early aperient administration for enterally fed intensive care patients was associated with increased diarrhoea: a before-and-after, intention-to-treat evaluation. Aust Crit Care. 2015;28:208–213. doi: 10.1016/j.aucc.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 31.Mostafa SM, Bhandari S, Ritchie G, et al. Constipation and its implications in the critically ill patient. Br J Anaesth. 2003;91:815–819. doi: 10.1093/bja/aeg275. [DOI] [PubMed] [Google Scholar]
- 32.Nassar AP, Jr, da Silva FM, de Cleva R. Constipation in intensive care unit: incidence and risk factors. J Crit Care. 2009;24(630):e9–e12. doi: 10.1016/j.jcrc.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 33.Loke SS, Rau KM, Huang CF. Impact of combined hospice care on terminal cancer patients. J Palliat Med. 2011;14:683–687. doi: 10.1089/jpm.2010.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McMillan SC. Presence and severity of constipation in hospice patients with advanced cancer. Am J Hosp Palliat Care. 2002;19:426–430. doi: 10.1177/104990910201900616. [DOI] [PubMed] [Google Scholar]
- 35.Braiteh F, El Osta B, Palmer JL, et al. Characteristics, findings, and outcomes of palliative care inpatient consultations at a comprehensive cancer center. J Palliat Med. 2007;10:948–955. doi: 10.1089/jpm.2006.0257. [DOI] [PubMed] [Google Scholar]
- 36.Harari D, Gurwitz JH, Avorn J, et al. Constipation: assessment and management in an institutionalized elderly population. J Am Geriatr Soc. 1994;42:947–952. doi: 10.1111/j.1532-5415.1994.tb06585.x. [DOI] [PubMed] [Google Scholar]
- 37.Phillips C, Polakoff D, Maue SK, et al. Assessment of constipation management in long-term care patients. J Am Med Dir Assoc. 2001;2:149–154. [PubMed] [Google Scholar]
- 38.Bergh I, Steen G, Waern M, et al. Pain and its relation to cognitive function and depressive symptoms: a Swedish population study of 70-year-old men and women. J Pain Symptom Manage. 2003;26:903–912. doi: 10.1016/s0885-3924(03)00329-4. [DOI] [PubMed] [Google Scholar]
- 39.Blyth FM, March LM, Brnabic AJ, et al. Chronic pain in Australia: a prevalence study. Pain. 2001;89:127–134. doi: 10.1016/s0304-3959(00)00355-9. [DOI] [PubMed] [Google Scholar]
- 40.Elliott AM, Smith BH, Penny KI, et al. The epidemiology of chronic pain in the community. Lancet. 1999;354:1248–1252. doi: 10.1016/s0140-6736(99)03057-3. [DOI] [PubMed] [Google Scholar]
- 41.McCarthy LH, Bigal ME, Katz M, et al. Chronic pain and obesity in elderly people: results from the Einstein aging study. J Am Geriatr Soc. 2009;57:115–119. doi: 10.1111/j.1532-5415.2008.02089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boerlage AA, van Dijk M, Stronks DL, et al. Pain prevalence and characteristics in three Dutch residential homes. Eur J Pain. 2008;12:910–916. doi: 10.1016/j.ejpain.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 43.Zanocchi M, Maero B, Nicola E, et al. Chronic pain in a sample of nursing home residents: prevalence, characteristics, influence on quality of life (QoL) Arch Gerontol Geriatr. 2008;47:121–128. doi: 10.1016/j.archger.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 44.Knauer SR, Freburger JK, Carey TS. Chronic low back pain among older adults: a population-based perspective. J Aging Health. 2010;22:1213–1234. doi: 10.1177/0898264310374111. [DOI] [PubMed] [Google Scholar]
- 45.Manchikanti L, Manchikanti KN, Cash KA, et al. Age-related prevalence of facet-joint involvement in chronic neck and low back pain. Pain Physician. 2008;11:67–75. [PubMed] [Google Scholar]
- 46.Palma R, de Conti MH, Quintino NM, et al. Functional capacity and its associated factors in the elderly with low back pain. Acta Ortop Bras. 2014;22:295–299. doi: 10.1590/1413-78522014220600890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stubbs B, Patchay S, Soundy A, et al. The avoidance of activities due to fear of falling contributes to sedentary behavior among community-dwelling older adults with chronic musculoskeletal pain: a multisite observational study. Pain Med. 2014;15:1861–1871. doi: 10.1111/pme.12570. [DOI] [PubMed] [Google Scholar]
- 48.Leong IY, Farrell MJ, Helme RD, et al. The relationship between medical comorbidity and self-rated pain, mood disturbance, and function in older people with chronic pain. J Gerontol A Biol Sci Med Sci. 2007;62:550–555. doi: 10.1093/gerona/62.5.550. [DOI] [PubMed] [Google Scholar]
- 49.Reitsma ML, Tranmer JE, Buchanan DM, et al. The epidemiology of chronic pain in Canadian men and women between 1994 and 2007: results from the longitudinal component of the National Population Health Survey. Pain Res Manag. 2012;17:166–172. doi: 10.1155/2012/875924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ray L, Lipton RB, Zimmerman ME, et al. Mechanisms of association between obesity and chronic pain in the elderly. Pain. 2011;152:53–59. doi: 10.1016/j.pain.2010.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pergolizzi J, Böger RH, Budd K, et al. Opioids and the management of chronic severe pain in the elderly: consensus statement of an international expert panel with focus on the six clinically most often used World Health Organization step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone) Pain Pract. 2008;8:287–313. doi: 10.1111/j.1533-2500.2008.00204.x. [DOI] [PubMed] [Google Scholar]
- 52.Benyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Physician. 2008;11:S105–S120. [PubMed] [Google Scholar]
- 53.Prunuske JP, St Hill CA, Hager KD, et al. Opioid prescribing patterns for non-malignant chronic pain for rural versus non-rural US adults: a population-based study using 2010 NAMCS data. BMC Health Serv Res. 2010;14:563. doi: 10.1186/s12913-014-0563-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ringwalt C, Gugelmann H, Garrettson M, et al. Differential prescribing of opioid analgesics according to physician specialty for Medicaid patients with chronic noncancer pain diagnoses. Pain Res Manag. 2014;19:179–185. doi: 10.1155/2014/857952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lembo A, Camilleri M. Chronic constipation. N Engl J Med. 2003;349:1360–1368. doi: 10.1056/NEJMra020995. [DOI] [PubMed] [Google Scholar]
- 56.Clemens KE, Faust M, Jaspers B, et al. Pharmacological treatment of constipation in palliative care. Curr Opin Support Palliat Care. 2013;7:183–191. doi: 10.1097/SPC.0b013e32835f1e17. [DOI] [PubMed] [Google Scholar]
- 57.Vazquez Roque M, Bouras EP. Epidemiology and management of chronic constipation in elderly patients. Clin Interv Aging. 2015;10:919–930. doi: 10.2147/CIA.S54304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hutchison B. Constipation in the elderly. Can Fam Physician. 1978;24:1018–1022. [PMC free article] [PubMed] [Google Scholar]
- 59.Woolery M, Bisanz A, Lyons HF, et al. Putting Evidence into Practice®: evidence-based interventions for the prevention and management of constipation in patients with cancer. Clin J Oncol Nurs. 2008;12:317–337. doi: 10.1188/08.CJON.317-337. [DOI] [PubMed] [Google Scholar]
- 60.Camilleri M. Opioid-induced constipation: challenges and therapeutic opportunities. Am J Gastroenterol. 2011;106:835–842. doi: 10.1038/ajg.2011.30. [DOI] [PubMed] [Google Scholar]
- 61.Holzer P. Opioids and opioid receptors in the enteric nervous system: from a problem in opioid analgesia to a possible new prokinetic therapy in humans. Neurosci Lett. 2004;361:192–195. doi: 10.1016/j.neulet.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 62.Holzer P. Pharmacology of opioids and their effects on gastrointestinal function. Am J Gastroenterol Suppl. 2014;2:9–16. [Google Scholar]
- 63.Kumar L, Barker C, Emmanuel A. Opioid-induced constipation: pathophysiology, clinical consequences, and management. Gastroenterol Res Pract. 2014;2014:141737. doi: 10.1155/2014/141737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sobczak M, Sałaga M, Storr MA, et al. Physiology, signaling, and pharmacology of opioid receptors and their ligands in the gastrointestinal tract: current concepts and future perspectives. J Gastroenterol. 2014;49:24–45. doi: 10.1007/s00535-013-0753-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brock C, Olesen SS, Olesen AE, et al. Opioid-induced bowel dysfunction: pathophysiology and management. Drugs. 2012;72:1847–1865. doi: 10.2165/11634970-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 66.Camilleri M, Drossman DA, Becker G, et al. Emerging treatments in neurogastroenterology: a multidisciplinary working group consensus statement on opioid-induced constipation. Neurogastroenterol Motil. 2014;26:1386–1395. doi: 10.1111/nmo.12417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Coyne KS, LoCasale RJ, Datto CJ, et al. Opioid-induced constipation in patients with chronic noncancer pain in the USA, Canada, Germany, and the UK: descriptive analysis of baseline patient-reported outcomes and retrospective chart review. Clinicoecon Outcomes Res. 2014;6:269–281. doi: 10.2147/CEOR.S61602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ahmedzai SH, Nauck F, Bar-Sela G, et al. A randomized, double-blind, active-controlled, double-dummy, parallel-group study to determine the safety and efficacy of oxycodone/naloxone prolonged-release tablets in patients with moderate/severe, chronic cancer pain. Palliat Med. 2012;26:50–60. doi: 10.1177/0269216311418869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hale ME, Nalamachu SR, Khan A, et al. Effectiveness and gastrointestinal tolerability during conversion and titration with once-daily OROS® hydromorphone extended release in opioid-tolerant patients with chronic low back pain. J Pain Res. 2013;6:319–329. doi: 10.2147/JPR.S39980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Klepstad P, Kaasa S, Skauge M, et al. Pain intensity and side effects during titration of morphine to cancer patients using a fixed schedule dose escalation. Acta Anaesthesiol Scand. 2000;44:656–664. doi: 10.1034/j.1399-6576.2000.440605.x. [DOI] [PubMed] [Google Scholar]
- 71.Epstein RS, Cimen A, Benenson H, et al. Patient preferences for change in symptoms associated with opioid-induced constipation. Adv Ther. 2014;31:1263–1271. doi: 10.1007/s12325-014-0169-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ketwaroo GA, Cheng V, Lembo A. Opioid-induced bowel dysfunction. Curr Gastroenterol Rep. 2013;15:344. doi: 10.1007/s11894-013-0344-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bell T, Annunziata K, Leslie JB. Opioid-induced constipation negatively impacts pain management, productivity, and health-related quality of life: findings from the National Health and Wellness Survey. J Opioid Manag. 2009;5:137–144. doi: 10.5055/jom.2009.0014. [DOI] [PubMed] [Google Scholar]
- 74.Penning-van Beest FJA, van den Haak P, Klok RM, et al. Quality of life in relation to constipation among opioid users. J Med Econ. 2010;13:129–35. [DOI] [PubMed]
- 75.Hjalte F, Berggren AC, Bergendahl H, et al. The direct and indirect costs of opioid-induced constipation. J Pain Symptom Manage. 2010;40:696–703. doi: 10.1016/j.jpainsymman.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 76.LoCasale RJ, Datto CJ, Margolis MK, et al. The impact of opioid-induced constipation among chronic pain patients with sufficient laxative use. Int J Clin Pract. 2015;69:1448–1456. doi: 10.1111/ijcp.12718. [DOI] [PubMed] [Google Scholar]
- 77.Song HJ. Constipation in community-dwelling elders: prevalence and associated factors. J Wound Ostomy Continence Nurs. 2012;39:640–645. doi: 10.1097/WON.0b013e31826a4b70. [DOI] [PubMed] [Google Scholar]
- 78.Chang JY, Locke GR, Schleck CD, et al. Risk factors for chronic constipation and a possible role of analgesics. Neurogastroenterol Motil. 2007;19:905–911. doi: 10.1111/j.1365-2982.2007.00974.x. [DOI] [PubMed] [Google Scholar]
- 79.Catafesta J, Francesconi C. Association between medication use and adverse gastroenterologic events in patients receiving enteral nutrition therapy at a university hospital. Rev Gastroenterol Mex. 2012;77:161–166. doi: 10.1016/j.rgmx.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 80.Gau JT, Walston S, Finamore M, et al. Risk factors associated with stool retention assessed by abdominal radiography for constipation. J Am Med Dir Assoc. 2010;11:572–578. doi: 10.1016/j.jamda.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gau JT, Acharya UH, Khan MS, et al. Risk factors associated with lower defecation frequency in hospitalized older adults: a case control study. BMC Geriatr. 2015;15:44. doi: 10.1186/s12877-015-0041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ueki T, Nagai K, Ooe N, et al. Case-controlled study on risk factors for the development of constipation in hospitalized patients. Yakugaku Zasshi. 2011;131:469–476. doi: 10.1248/yakushi.131.469. [DOI] [PubMed] [Google Scholar]
- 83.Cardin F, Minicuci N, Droghi AT, et al. Constipation in the acutely hospitalized older patients. Arch Gerontol Geriatr. 2010;50:277–281. doi: 10.1016/j.archger.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 84.Robson KM, Kiely DK, Lembo T. Development of constipation in nursing home residents. Dis Colon Rectum. 2000;43:940–943. doi: 10.1007/BF02237354. [DOI] [PubMed] [Google Scholar]
- 85.Hosia-Randell H, Suominen M, Muurinen S, et al. Use of laxatives among older nursing home residents in Helsinki, Finland. Drugs Aging. 2007;24:147–154. doi: 10.2165/00002512-200724020-00006. [DOI] [PubMed] [Google Scholar]
- 86.Holman C, Roberts S, Nicol M. Preventing and treating constipation in later life. Nurs Older People. 2008;20:22–24. doi: 10.7748/nop2008.06.20.5.22.c8223. [DOI] [PubMed] [Google Scholar]
- 87.Kyle G. Risk assessment and management tools for constipation. Br J Community Nurs. 2011;16:224–230. doi: 10.12968/bjcn.2011.16.5.224. [DOI] [PubMed] [Google Scholar]
- 88.Chapman S, Hungerford C. Risk factors for and assessment of constipation. Nurs Older People. 2015;27:16–24. doi: 10.7748/nop.27.3.16.e673. [DOI] [PubMed] [Google Scholar]
- 89.Strassels SA, Maxwell TL, Iyer S. Constipation in persons receiving hospice care. J Pain Symptom Manage. 2010;40:810–820. doi: 10.1016/j.jpainsymman.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 90.Dzierzanowski T, Ciałkowska-Rysz A. Behavioral risk factors of constipation in palliative care patients. Support Care Cancer. 2015;23:1787–1793. doi: 10.1007/s00520-014-2495-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Markland AD, Palsson O, Goode PS, et al. Association of low dietary intake of fiber and liquids with constipation: evidence from the National Health and Nutrition Examination Survey. Am J Gastroenterol. 2013;108:796–803. doi: 10.1038/ajg.2013.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Salmoirago-Blotcher E, Crawford S, Jackson E, et al. Constipation and risk of cardiovascular disease among postmenopausal women. Am J Med. 2011;124:714–723. doi: 10.1016/j.amjmed.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mody R, Guérin A, Fok B, et al. Prevalence and risk of developing comorbid conditions in patients with chronic constipation. Curr Med Res Opin. 2014;30:2505–2513. doi: 10.1185/03007995.2014.964854. [DOI] [PubMed] [Google Scholar]
- 94.Rey E, Barcelo M, Jimenez Cebrián MJ, et al. A nation-wide study of prevalence and risk factors for fecal impaction in nursing homes. PLoS One. 2014;9:e105281. doi: 10.1371/journal.pone.0105281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Levy MH. Management of opioid-induced bowel dysfunction. J Natl Compr Canc Netw. 2003;1(Suppl 3):S-22-6. [PubMed] [Google Scholar]
- 96.Glare P, Lickiss JN. Unrecognized constipation in patients with advanced cancer: a recipe for therapeutic disaster. J Pain Symptom Manage. 1992;7:369–371. doi: 10.1016/0885-3924(92)90092-v. [DOI] [PubMed] [Google Scholar]
- 97.Holzer P, Ahmedzai SH, Niederle N, et al. Opioid-induced bowel dysfunction in cancer-related pain: causes, consequences, and a novel approach for its management. J Opioid Manag. 2009;5:145–151. doi: 10.5055/jom.2009.0015. [DOI] [PubMed] [Google Scholar]
- 98.Thomas J. Opioid-induced bowel dysfunction. J Pain Symptom Manage. 2008;35:103–113. doi: 10.1016/j.jpainsymman.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 99.Obokhare I. Fecal impaction: a cause for concern? Clin Colon Rectal Surg. 2012;25:53–58. doi: 10.1055/s-0032-1301760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Davies A, Webber K. Stercoral perforation of the colon: a potentially fatal complication of opioid-induced constipation. J Pain Symptom Manage. 2015;50:260–262. doi: 10.1016/j.jpainsymman.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 101.Panchal SJ, Müller-Schwefe P, Wurzelmann JI. Opioid-induced bowel dysfunction: prevalence, pathophysiology and burden. Int J Clin Pract. 2007;61:1181–1187. doi: 10.1111/j.1742-1241.2007.01415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113–130. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nour-Eldein H, Salama HM, Abdulmajeed AA, et al. The effect of lifestyle modification on severity of constipation and quality of life of elders in nursing homes at Ismailia city, Egypt. J Family Community Med. 2014;21:100–106. doi: 10.4103/2230-8229.134766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ostaszkiewicz J, Hornby L, Millar L, et al. The effects of conservative treatment for constipation on symptom severity and quality of life in community-dwelling adults. J Wound Ostomy Continence Nurs. 2010;37:193–198. doi: 10.1097/WON.0b013e3181cf7206. [DOI] [PubMed] [Google Scholar]
- 105.Rao SS, Beaty J, Chamberlain M, et al. Effects of acute graded exercise on human colonic motility. Am J Physiol. 1999;276:G1221–G1226. doi: 10.1152/ajpgi.1999.276.5.G1221. [DOI] [PubMed] [Google Scholar]
- 106.De Schryver AM, Keulemans YC, Peters HP, et al. Effects of regular physical activity on defecation pattern in middle-aged patients complaining of chronic constipation. Scand J Gastroenterol. 2005;40:422–429. doi: 10.1080/00365520510011641. [DOI] [PubMed] [Google Scholar]
- 107.Chin A Paw MJ, van Poppel MN, van Mechelen W. Effects of resistance and functional-skills training on habitual activity and constipation among older adults living in long-term care facilities: a randomized controlled trial. BMC Geriatr. 2006;6:9. doi: 10.1186/1471-2318-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Woodward S, Norton C, Chiarelli P. Biofeedback for treatment of chronic idiopathic constipation in adults. Cochrane Database Syst Rev. 2014; 3:CD008486. [DOI] [PMC free article] [PubMed]
- 109.Simmons SF, Schnelle JF. Effects of an exercise and scheduled-toileting intervention on appetite and constipation in nursing home residents. J Nutr Health Aging. 2004;8:116–121. [PubMed] [Google Scholar]
- 110.Fosnes GS, Lydersen S, Farup PG. Effectiveness of laxatives in elderly—a cross sectional study in nursing homes. BMC Geriatr. 2011;11:76. doi: 10.1186/1471-2318-11-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hunold KM, Smith SA, Platts-Mills TF. Constipation prophylaxis is rare for adults prescribed outpatient opioid therapy from US emergency departments. Acad Emerg Med. 2015;22:1118–1121. doi: 10.1111/acem.12745. [DOI] [PubMed] [Google Scholar]
- 112.Nelson AD, Camilleri M. Chronic opioid induced constipation in patients with nonmalignant pain: challenges and opportunities. Therap Adv Gastroenterol. 2015;8:206–220. doi: 10.1177/1756283X15578608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shah BJ, Rughwani N, Rose S. In the Clinic®. Constipation. Ann Intern Med. 2015; 162:ITC1. [DOI] [PubMed]
- 114.Movantik™ (naloxegol) [US prescribing information]. Wilmington (DE): AstraZeneca Pharmaceuticals LP, 2015.
- 115.Relistor® (methylnaltrexone bromide subcutaneous injection) [US prescribing information]. Raleigh (NC): Salix Pharmaceuticals, Inc., 2014.
- 116.Thomas J, Karver S, Cooney GA, et al. Methylnaltrexone for opioid-induced constipation in advanced illness. N Engl J Med. 2008;358:2332–2343. doi: 10.1056/NEJMoa0707377. [DOI] [PubMed] [Google Scholar]
- 117.Bull J, Wellman CV, Israel RJ, et al. Fixed-dose subcutaneous methylnaltrexone in patients with advanced illness and opioid-induced constipation: results of a randomized, placebo-controlled study and open-label extension. J Palliat Med. 2015;18:593–600. doi: 10.1089/jpm.2014.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Michna E, Blonsky ER, Schulman S, et al. Subcutaneous methylnaltrexone for treatment of opioid-induced constipation in patients with chronic, nonmalignant pain: a randomized controlled study. J Pain. 2011;12:554–562. doi: 10.1016/j.jpain.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 119.Rauck RL, Peppin JF, Israel RJ, et al. Oral methylnaltrexone for the treatment of opioid-induced constipation in patients with noncancer pain. Gastroenterology. 2012;142:S160. [Google Scholar]
- 120.Moventig® (naloxegol) [EU prescribing information]. Södertälje (Sweden): AstraZeneca AB, 2014.
- 121.Chey WD, Webster L, Sostek M, et al. Naloxegol for opioid-induced constipation in patients with noncancer pain. N Engl J Med. 2014;370:2387–2396. doi: 10.1056/NEJMoa1310246. [DOI] [PubMed] [Google Scholar]
- 122.Tack J, Lappalainen J, Diva U, et al. Efficacy and safety of naloxegol in patients with opioid-induced constipation and laxative-inadequate response. United European Gastroenterol J. 2015;3:471–480. doi: 10.1177/2050640615604543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tummala R, Diva U, Sostek M. Treatment with naloxegol versus placebo or usual care: safety assessment in patients aged ≥65 years with noncancer pain and opioid-induced constipation. Digestive Disease Week, 16–19 May 2015, Washington, DC.
- 124.Webster L, Chey WD, Tack J, et al. Randomised clinical trial: the long-term safety and tolerability of naloxegol in patients with pain and opioid-induced constipation. Aliment Pharmacol Ther. 2014;40:771–779. doi: 10.1111/apt.12899. [DOI] [PubMed] [Google Scholar]
- 125.Lacy BE, Levy LC. Lubiprostone: a chloride channel activator. J Clin Gastroenterol. 2007;41:345–351. doi: 10.1097/01.mcg.0000225665.68920.df. [DOI] [PubMed] [Google Scholar]
- 126.Amitiza® (lubiprostone capsules) [US prescribing information]. Sucampo Pharma Americas, LLC, Bethesda (MD), and Takeda Pharmaceuticals America, Inc., Deerfield (IL), 2013.
- 127.Cryer B, Katz S, Vallejo R, et al. A randomized study of lubiprostone for opioid-induced constipation in patients with chronic noncancer pain. Pain Med. 2014;15:1825–1834. doi: 10.1111/pme.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jamal MM, Adams AB, Jansen JP, et al. A randomized, placebo-controlled trial of lubiprostone for opioid-induced constipation in chronic noncancer pain. Am J Gastroenterol. 2015;110:725–732. doi: 10.1038/ajg.2015.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Spierings EL, Rauck R, Brewer R, et al. Long-term safety and efficacy of lubiprostone in opioid-induced constipation in patients with chronic noncancer pain. Pain Pract. 2015 doi: 10.1111/papr.12347. [DOI] [PubMed] [Google Scholar]
- 130.Kern DM, Zhou S, Chavoshi S, et al. Treatment patterns, healthcare utilization, and costs of chronic opioid treatment for non-cancer pain in the United States. Am J Manag Care. 2015;21:e222–e234. [PubMed] [Google Scholar]
- 131.Wan Y, Corman S, Gao X, et al. Economic burden of opioid-induced constipation among long-term opioid users with noncancer pain. Am Health Drug Benefits. 2015;8:93–102. [PMC free article] [PubMed] [Google Scholar]
- 132.Argoff CE, Brennan MJ, Camilleri M, et al. Consensus recommendations on initiating prescription therapies for opioid-induced constipation. Pain Med. 2015;16:2324–2337. doi: 10.1111/pme.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920–924. doi: 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- 134.Rentz AM, Yu R, Müller-Lissner S, et al. Validation of the Bowel Function Index to detect clinically meaningful changes in opioid-induced constipation. J Med Econ. 2009;12:371–383. doi: 10.3111/13696990903430481. [DOI] [PubMed] [Google Scholar]
- 135.Bardsley A. Approaches to managing chronic constipation in older people within the community setting. Br J Community Nurs. 2015;20:444–450. doi: 10.12968/bjcn.2015.20.9.444. [DOI] [PubMed] [Google Scholar]
- 136.Brenner DM, Chey WD. An evidence-based review of novel and emerging therapies for constipation in patients taking opioid analgesics. Am J Gastroenterol Suppl. 2014;2:38–46. [Google Scholar]
- 137.Andrews CN, Storr M. The pathophysiology of chronic constipation. Can J Gastroenterol. 2011; 25(Suppl B):16B–21B. [PMC free article] [PubMed]
- 138.McCrea GL, Miaskowski C, Stotts NA, et al. Pathophysiology of constipation in the older adult. World J Gastroenterol. 2008;14:2631–2638. doi: 10.3748/wjg.14.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Alame AM, Bahna H. Evaluation of constipation. Clin Colon Rectal Surg. 2012;25:5–11. doi: 10.1055/s-0032-1301753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Denoya P, Sands DR. Anorectal physiologic evaluation of constipation. Clin Colon Rectal Surg. 2008;21:114–121. doi: 10.1055/s-2008-1075860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Candy B, Jones L, Larkin PJ, et al. Laxatives for the management of constipation in people receiving palliative care. Cochrane Database Syst Rev. 2015; 5:CD003448. [DOI] [PMC free article] [PubMed]
- 142.Castledine G, Grainger M, Wood N, et al. Researching the management of constipation in long-term care: part 1. Br J Nurs. 2007;16:1128–1131. doi: 10.12968/bjon.2007.16.18.27506. [DOI] [PubMed] [Google Scholar]
- 143.Snustad D, Lee V, Abraham I, et al. Dietary fiber in hospitalized geriatric patients: too soft a solution for too hard a problem? J Nutr Elder. 1991;10:49–63. doi: 10.1300/J052v10n02_06. [DOI] [PubMed] [Google Scholar]
- 144.Metamucil®. http://www.metawellness.com. Accessed 10 May 2016.
- 145.MiraLAX® product label. http://www.miralaxmd.com/product-information/miralaxsupsup-label. Accessed 10 May 2016.
- 146.Constulose. http://www.drugs.com/cdi/constulose-solution.html. Accessed 10 May 2016.
- 147.Dulcolax®. https://www.dulcolax.com. Accessed 10 May 2016.
- 148.Senokot® (Senna) product label. http://www.senokot.ca/en/products/tablets. Accessed 10 May 2016.