Abstract
For all sensory organs, the establishment of spatial and temporal cortical resolution is assumed to be initiated by the first sensory experience and a BDNF-dependent increase in intracortical inhibition. To address the potential of cortical BDNF for sound processing, we used mice with a conditional deletion of BDNF in which Cre expression was under the control of the Pax2 or TrkC promoter. BDNF deletion profiles between these mice differ in the organ of Corti (BDNFPax2-KO) versus the auditory cortex and hippocampus (BDNFTrkC-KO). We demonstrate that BDNFPax2-KO but not BDNFTrkC-KO mice exhibit reduced sound-evoked suprathreshold ABR waves at the level of the auditory nerve (wave I) and inferior colliculus (IC) (wave IV), indicating that BDNF in lower brain regions but not in the auditory cortex improves sound sensitivity during hearing onset. Extracellular recording of IC neurons of BDNFPax2 mutant mice revealed that the reduced sensitivity of auditory fibers in these mice went hand in hand with elevated thresholds, reduced dynamic range, prolonged latency, and increased inhibitory strength in IC neurons. Reduced parvalbumin-positive contacts were found in the ascending auditory circuit, including the auditory cortex and hippocampus of BDNFPax2-KO, but not of BDNFTrkC-KO mice. Also, BDNFPax2-WT but not BDNFPax2-KO mice did lose basal inhibitory strength in IC neurons after acoustic trauma. These findings suggest that BDNF in the lower parts of the auditory system drives auditory fidelity along the entire ascending pathway up to the cortex by increasing inhibitory strength in behaviorally relevant frequency regions. Fidelity and inhibitory strength can be lost following auditory nerve injury leading to diminished sensory outcome and increased central noise.
Keywords: BDNF; Central hyperactivity; High-spontaneous rate, low-threshold fibers; Homeostatic plasticity; Inferior colliculus; Sound detection threshold
Introduction
Brain-derived neurotrophic factor (BDNF) was initially identified as a survival factor for peripheral neurons (reviewed in [1, 2]). Increasing evidence is now emerging that BDNF acts locally as a factor that governs dendritogenesis and the spine phenotype [3–6] at certain stages of maturation [7]. Upon sensory experience in the auditory [8, 9], visual [10], somatosensory [11], and olfactory system [12], the dendritic complexity of parvalbumin (PV)-immunoreactive interneurons is particularly enhanced in response to increases in local cortical BDNF [4, 8, 11]. This process is assumed to enhance sensory resolution and acuity, and requires an adequate and timely sensory input [13–15]. The selective role of cortical BDNF for improvement of sensory resolution was questioned in the retina where BDNF was proposed as a mediator of early environmental enrichment effects on visual acuity [16]. Accordingly, blocking retinal BDNF by means of antisense oligonucleotides prior to eye opening was shown to prevent the effects on dendritic segregation of retinal ganglion cells [17] and development of retinal acuity [16] upon exposure to environmental enrichment. As constitutive BDNF knockout (KO) mice [18, 19] die too prematurely to assess the role of BDNF in the mature sensory organs, the role of peripheral versus central BDNF for sensory resolution and acuity is still obscure. Until now, it also remains unclear if and how these crucial effects of BDNF during the development of normal sensory function are linked to the complex changes of BDNF activity in mature systems, which have been described as either adaptive responses to injury [20] or nonadaptive responses in various brain disorders [21].
In rodent auditory systems, BDNF is downregulated in hair cells of the cochlea from birth onwards but remains expressed in the inner border and phalangeal cells that ensheath inner hair cells (IHCs) [22] and is upregulated with hearing onset in spiral ganglion neurons (SGNs) at the level of higher frequency cochlear turns (for a review, see [15]). While loss of BDNF in phalangeal cells does not affect hearing [23], the combined deletion of BDNF in the cochlea, the dorsal cochlear nucleus (DCN), and the inferior colliculus (IC) in BDNFPax2-KO mice [24, 25] leads to reduced exocytosis of otherwise mature IHC synapses and to slightly diminished sound-induced auditory brainstem responses [25]. This finding suggested an unexpected role of BDNF in the lower parts of the auditory pathway for hearing sensitivity upon sensory experience. To obtain more insight into such a role of BDNF on basic sound processing, we compared sound sensitivity and noise vulnerability of BDNFPax2-KO mice with that of mice in which BDNF is deleted more widespread throughout the auditory system including the auditory cortex using a mouse line in which Cre is driven by the TrkC promoter [26]. Strikingly, reduced sound sensitivity occurs only in BDNFPax2-KO mice but not in BDNFTrkC-KO mice. This differential response is observed although both strains exhibit a similar BDNF deletion profile in the SGNs of the cochlea and in the IC, the midbrain integration center of the ascending auditory pathway [27, 28] [present study]. Results from recordings of extracellular sound-evoked responses from IC neurons in BDNFPax2 wild-type (WT) and BDNFPax2-KO mice and measurements of the density of inhibitory marker proteins along the auditory pathway and hippocampus suggest that BDNF in the lower parts of the auditory CNS or within the cochlea triggers basal PV inhibitory circuits to increase auditory fidelity upon sensory experience. However, this early improvement of sensitivity is accompanied by the risk to lose sensitivity and generate elevated noise levels when the BDNF-modified driving force is deteriorated after peripheral auditory nerve injury in the mature auditory system.
Materials and Methods
Animals
BDNFPax2-KO mice were obtained by mating Pax2-Cre [24] with BDNFlox/lox mice [29]. BDNFTrkC-KO mice were generated by mating TrkC-Cre [26] with BDNFlox/lox mice. BDNFPax2-KO mice had a deletion of BDNF in the cochlea, DCN, and IC [25]. In BDNFTrkC-KO mice, BDNF was conditionally inactivated in neurons. TrkC-Cre mice and Pax2-Cre mice were crossed with ROSA26 reporter (ROSA26R) mice as described [25]. For detection of β-galactosidase activity in the Pax2-Cre-ROSA26R or TrkC-Cre-ROSA26R mouse line, adult mice were used. β-Galactosidase activity was analyzed through X-gal staining and immunohistochemistry with anti-β-galactosidase antibody as described below. Deletion of the BDNF gene in distinct brain areas of BDNFPax2-KO and BDNFTrkC-KO mice was verified by Northern and Western blots. Control and knockout mice of either sex were used for the experiments.
The care and use of animals was approved by either the University of Tübingen, Veterinary Care Unit and the Animal Care and Ethics Committee of the regional board of the Federal State Government of Baden-Württemberg, Germany, or by the Ethics Committee of the Institute of Experimental Medicine, Academy of Sciences of the Czech Republic, and followed the guidelines of the EU Directive 2010/63/EU for animal experiments.
Tissue Preparation, X-gal Staining, Immunohistochemistry, and Ribbon Counts
Cochlear and brain tissues were isolated and dissected as previously described [30]. Briefly, cochleae and brains were fixed in 100 mM phosphate-buffered saline (PBS) containing 2 % paraformaldehyde and 125 mM sucrose, pH 7.4, for 2 and 48 h, respectively. Cochleae were decalcified in Rapid Bone Decalcifier (Eurobio, Les Ulis Cedex, France) followed by an overnight incubation in 25 % sucrose in Hanks’ buffered saline (HBS). Cochleae were embedded in O.C.T. compound (Miles Laboratories, Elkhart, IN, USA). Tissue samples were cryosectioned at 10 μm thickness for immunohistochemistry, mounted on Super-Frost*/plus microscope slides, and stored at −20 °C [31]. Brains were embedded after fixation in 4 % agarose, cut at 60 μm thickness with a Vibratome (Leica VT 1000S, Wetzlar, Germany), and stored in PBS at 4 °C. Brain regions were identified in accordance with the mouse atlas of Franklin and Paxinos [32]. Serial sections derived from coronal brain slices between 1.8 and 2.3 mm posterior to bregma were analyzed. For cochlear whole-mount preparations, the temporal bone was dissected on ice and immediately fixed for 2 h using 2 % paraformaldehyde in 100 mM PBS by infusion through the round and oval window. Cochlear turns were dissected and transferred to slides and attached to the surface using Cell-Tak (BD Bioscience, Heidelberg, Germany).
For X-gal staining, brain slices and isolated cochleae, slit from the apex to base, were incubated with the β-galactosidase staining solution containing 0.5 mg/ml X-gal (Sigma), 5 mM K3[Fe(CN)6], and 5 mM K4[Fe(CN)6] in PBS complemented with 20 mM MgCl2, 0.01 % sodium deoxycholate, and 0.02 % Nonidet-P40 overnight at 37 °C. After incubation with β-galactosidase staining solution, whole-mount preparations of the cochleae were performed. In case of a successful deletion of the floxed ROSA26R locus, the presence of β-galactosidase protein is visualized with the enzyme’s substrate (X-gal), resulting in a blue precipitate only in cells expressing Cre recombinase.
Immunohistochemistry was performed as described before [33, 34]. Briefly, after washing and permeabilization, tissue slices were incubated overnight with primary antibody at 4 °C. For double labeling studies, specimens were simultaneously incubated with both antibodies. Primary antibodies were detected with Cy3-conjugated (Jackson ImmunoResearch) and AlexaFluor 488-conjugated secondary antibodies (Life Technologies GmbH, Darmstadt, Germany). Slices were mounted with Vectashield mounting medium containing DAPI (Vector laboratories, Burlingame, CA, USA). Sections and whole-mount preparations were viewed using an Olympus BX61 microscope equipped with epifluorescence illumination.
For ribbon counting, image acquisition and CtBP2/RIBEYE-immunopositive spot counting were carried out as previously described [33]. Briefly, cryosectioned cochleae were imaged over a distance of 8 μm covering the entire IHC nucleus and beyond in an image-stack along the z-axis (z-stack). One z-stack consisted of about 16 layers with a z-increment of 0.49 μm; for each layer, one image per fluorochrome was acquired. To assess spatial protein distribution, z-stacks were three-dimensionally deconvoluted using the cellSens constrained iterative module with the Advanced Maximum Likelihood Estimation (ADMLE) algorithm (OSIS GmbH), Voxel Viewer, and Slice Viewer (cellSens, OSIS GmbH).
Antibodies
The following antibodies were used: mouse anti-β-galactosidase (Promega, Mannheim, Germany), mouse anti-parvalbumin, rabbit anti-SK2 (Sigma-Aldrich, Munich, Germany), rabbit anti-GFAP (Dako, Glostrup, Denmark), rabbit anti-parvalbumin, mouse anti-GAD67 (Merck Millipore, Darmstadt, Germany), rabbit anti-Arc, rabbit anti-VGLUT2, rabbit anti-MAP2 [35] (Synaptic Systems, Göttingen, Germany), rabbit anti-Iba1 (Wako Chemicals GmbH, Neuss, Germany), rabbit anti-BDNF (Santa Cruz Biotechnology, Heidelberg, Germany), rabbit anti-BK (Alomone Labs, Jerusalem, Israel), rabbit anti-CtBP2/RIBEYE (Cell Applications, San Diego, CA, USA), and rabbit anti-KCNQ4 [36].
Image Analysis
The acquired images were analyzed using the free software ImageJ (NIH, Bethesda, MD, USA) to evaluate the expression of parvalbumin and GAD67. In every picture, the background was reduced with the rolling ball algorithm, and therefore, the red, green, and blue channels have been separated. In the green channel (parvalbumin or GAD67), a threshold (with ImageJ standard parameters) has been applied and therefore the ImageJ built-in plugin, Analyze Particles, has been used to count the numbers of parvalbumin or GAD67 puncta.
Northern Blots
Riboprobes were designed as described in [30]. Messenger RNA (mRNA) isolation was performed using the Oligotex mRNA Direct Mini Kit (Qiagen, Germany). mRNA was loaded onto a denaturing 0.8 % agarose formaldehyde gel and transferred onto a nylon membrane (Roche, Germany). The membrane was blocked and hybridized overnight at 65 °C with riboprobes for BDNF, Arg3.1, and cyclophilin. The membrane was incubated with anti-Dig-AP (Roche; 1:20,000). mRNA was detected with CDP-Star ready to use (Roche) and exposed to X-ray films.
Western Blots
Proteins were extracted using the NucleoSpin RNA/protein kit (Macherey-Nagel, Germany) following the manufacturer’s instructions. SDS-PAGE and Western blotting were carried out using the “XCell II SureLock™ Mini-Cell and XCell II Blot Module” (Invitrogen, Germany) as previously described [37]. The blotted proteins were incubated with either rabbit polyclonal or mouse monoclonal antibodies for BDNF (Santa Cruz Biotechnology, Heidelberg, Germany), activity-regulated cytoskeletal protein (Arc) (Synaptic Systems, Göttingen, Germany), parvalbumin (Merck Millipore, Darmstadt, Germany), GAD67 (Merck Millipore, Darmstadt, Germany), or GAPDH (Abcam, Cambridge, UK).
Noise Exposure
For acoustic trauma (AT), animals were anesthetized (75 mg/kg ketamine hydrochloride, Ketavet, Pharmacia, Pfizer, Karlsruhe, Germany; 5 mg/kg xylazine hydrochloride, Rompun 2 %, Bayer Leverkusen, Germany; in injection water to give an application volume of 5 ml/kg body weight) and exposed to intense pure tone noise (10 kHz, 116 dB sound pressure level (SPL) for 40 min) in a reverberating chamber, binaurally in an open field [25]. Control sham-exposed animals underwent the same procedure but with no traumatic sound presented (i.e., the speaker remained turned off).
Hearing Function Evaluation
Hearing thresholds were evaluated from auditory brainstem responses (ABRs). The state of outer hair cells (OHCs) in the organ of Corti was studied by recording otoacoustic emissions. The ABR to click and pure tone stimuli and the cubic 2 × f1 − f2 distortion product of the otoacoustic emission (DPOAE) for f2 = 1.24 × f1 and L2 = L1 − 10 dB were recorded in mice aged 6–9 weeks. All physiological recordings were performed under anesthesia (for details, see above) in a soundproof chamber (IAC, Niederkrüchten, Germany) as previously described [38].
Auditory Brainstem Responses
ABRs, evoked by short-duration sound stimuli, represent the summed activity of neurons in distinct anatomical structures or nuclei along the ascending auditory pathway [39] and are measured by averaging the evoked electrical response recorded via subcutaneous electrodes. Briefly, ABRs were evoked by click (100 μs) or pure tone stimuli (3 ms duration, 1 ms rise/fall times, frequencies 2–45.3 kHz) of gradually increasing sound pressure in 5 dB steps of intensity. The response threshold was determined at each frequency as the minimal sound pressure evoking a noticeable potential peak in the expected time window of the recorded signal. For details, see [38].
Distortion Product Otoacoustic Emissions
We assessed OHC function by the growth function and the maximum response in the distortion product audiogram of the cubic DPOAE as described [34]. Frequency pairs of tones were between f2 = 4 kHz and f2 = 32 kHz.
ABR Wave Form Analysis
ABR waveforms were analyzed for consecutive amplitude deflections (waves), with each wave consisting of a starting negative (n) peak and the following positive (p) peak. Peak amplitudes of ABR waves I and IV were extracted in the present study and defined as wave I: In − Ip (0.9–2 ms); wave IV: IVn − IVp (3.4–5.9 ms). A customized program was used to extract ABR peaks based on these definitions. ABR peak-to-peak (wave amplitude) growth functions were constructed for individual ears based on the extracted peaks for increasing stimulus levels. All ABR wave amplitude growth functions were calculated for increasing stimulus levels with reference to the ABR thresholds (from −20 to a maximum of 75 dB above threshold before noise exposure and from −20 to a maximum of 55 dB above threshold after noise exposure). For illustrative purposes, ABR wave amplitude growth functions were first linearly interpolated to the resolution of 1 data point/dB and then smoothed by a moving zero-phase Gaussian filter with a window length of 9 data points (9 dB). The ABR waveforms shown in the inset of the figures were smoothed by a moving zero-phase Gaussian filter with a window length of 5 data points (0.5 ms).
Extracellular Recording of the Neuronal Activity in the IC
Four groups of animals were tested: control, wild-type (WTc, n = 4), and knockout (KOc, n = 4) mice and mice with auditory trauma, WTat (n = 5) and KOat (n = 5). Mice with auditory trauma were exposed to noise at the age of 9–10 weeks and extracellular recordings were performed at 13–20 weeks. The surgery and extracellular recording in the IC were performed in mice anesthetized with 35 mg/kg ketamine (Narkamon 5 %; Spofa, Prague, Czech Republic) and 6 mg/kg xylazine (Sedazine 2 %; Fort Dodge, Animal Health, Fort Dodge, Iowa) in saline via intraperitoneal injection. Body temperature was maintained with a DC-powered electric temperature-regulated pad. Recordings were carried out in a soundproof anechoic room.
Surgical and Recording Procedures
For access to the IC, an incision was made through the skin of the skull and underlying muscles were retracted to expose the dorsal cranium. A holder was glued to the skull. Small holes were drilled over both IC of the mouse; the animal was transported to the soundproof anechoic room and placed on a heating pad, which maintained a 37–38 °C body temperature. Neuronal activity in the IC was recorded using a 16-channel, single shank probe (NeuroNexus Technologies) with 100 μm between the electrode spots or single parylene-coated tungsten electrode (Bionic Technologies Inc). The signal obtained from the electrode was amplified 10,000 times, band-pass filtered over the range of 300 Hz to 10 kHz, and processed by a TDT System III (Tucker Davis Technologies, Alachua, FL, USA) using an RX5-2 Pentusa Base Station. The recorded data was processed and analyzed with a custom software based on MATLAB.
Frequency-Intensity Mapping
Using frequency-intensity mapping as described in [40], the tuning properties of individual IC neurons were evaluated. To determine the excitatory response area, pure tone bursts (100 ms in duration, 5 ms rise/fall times) with variable frequency (1/8 octave step) and intensity (5 dB step) were presented in random order.
A two-dimensional matrix was thereby obtained, with elements corresponding to the response magnitudes in the respective frequency-intensity points. The discrete point matrix was then converted to a smooth function of two variables, frequency and intensity, a process involving cubic smoothing spline interpolation. This averaging method allowed us to overcome the limited resolution of the frequency-intensity map. The resulting smooth function thereafter served as a basis for the extraction of all the parameters of interest: (i) the excitatory response threshold, the lowest stimulus intensity that excited the neuron, measured in dB SPL; (ii) the characteristic frequency (CF), the frequency with the minimal response threshold, measured in kHz; and (iii) the bandwidth of the excitatory area 10 dB above the excitatory threshold, measured by quality factor Q10. Quality factor Q10 is a common measure of frequency selectivity that is reciprocally related to the excitatory area bandwidth (the higher the Q10, the sharper the response), defined as Q10 = CF/bandwidth.
Two-Tone Stimulation
To detect inhibitory areas, a two-tone stimulation was employed [40]. Two-tone inhibition in the IC consists of two components: intrinsic inhibition and suppression from lower auditory centers. Local inhibition is typically characterized by the effects of off-CF tones on spontaneous activity. Also the two-tone stimulation paradigm is commonly used to evaluate the local inhibition on the level of the cochlear nucleus (CN) [41–43], the IC [44, 45], and the auditory cortex (AC) [46]. Using the two-tone stimulation, the contribution of cochlear suppression cannot be excluded in the current study. However, we conclude that central inhibition on the level of the IC mainly contributes to the neural inhibition, since the shapes of the inhibitory areas in representative neurons stimulated with two-tone stimulation corresponded well with those obtained by inhibition of spontaneous activity. Pure tone at the neuron’s CF fixed 10 dB above the threshold at CF and pure tone bursts of variable frequency and intensity, analogous to those used for the excitatory area mapping, were simultaneously presented. Similar to frequency-intensity mapping, a two-dimensional matrix was obtained. Spike rates of responses in noninhibited areas (outside the excitatory area), inhibited areas, and excited areas (20 dB above threshold) were determined. Inhibitory strength was calculated as the ratio of spike numbers per stimulus in inhibited areas to those in noninhibitory areas. Low- and high-frequency inhibition bands were analyzed separately. Neurons displaying inhibition with a strength of 20 % and higher were considered to be neurons displaying inhibition, and their numbers were compared between groups. In addition to inhibitory strength, the ratio of response magnitude in excitatory to noninhibitory areas was evaluated.
Intensity Coding in the IC
Neuronal responses to broadband noise (BBN) bursts of variable intensity (from 0 to 90 dB SPL, 10 dB steps, 50 repetitions) were used to construct the rate-intensity function (RIF) from which response threshold, maximal response magnitude, dynamic range, and relative initial slope of the RIF were determined [47]. From the RIF, further response parameters such as spontaneous firing rate and first-spike latency were assessed. Spontaneous firing rate of each neuron was determined from poststimulus time histograms to BBN stimulation from 200–300 ms after presentation of the stimulus 10 dB above the threshold. Minimum first-spike latency (mFSL) was evaluated from the poststimulus time histograms to BBN stimuli at 80 dB SPL, as the amount of time between the onset of sound presentation and the appearance of first spikes of neuronal responses.
Statistical Analysis
Data are presented either as the mean ± standard deviation (SD) or standard error of the mean (SEM) for values with normal distributions or the median (Mdn) for values with nonnormal distributions. For statistical analysis, the GraphPad Prism software was used. To assess differences in mean or median values between groups, two-sided Student’s t tests, one-way or two-way ANOVA with Bonferroni’s multiple comparison test, Kruskal-Wallis tests with Dunn’s multiple comparison test, or chi-square tests were employed.
Results
Differential Deletion of BDNF in the Cochlea and Brain in Pax2-Cre Versus TrkC-Cre Mouse Lines
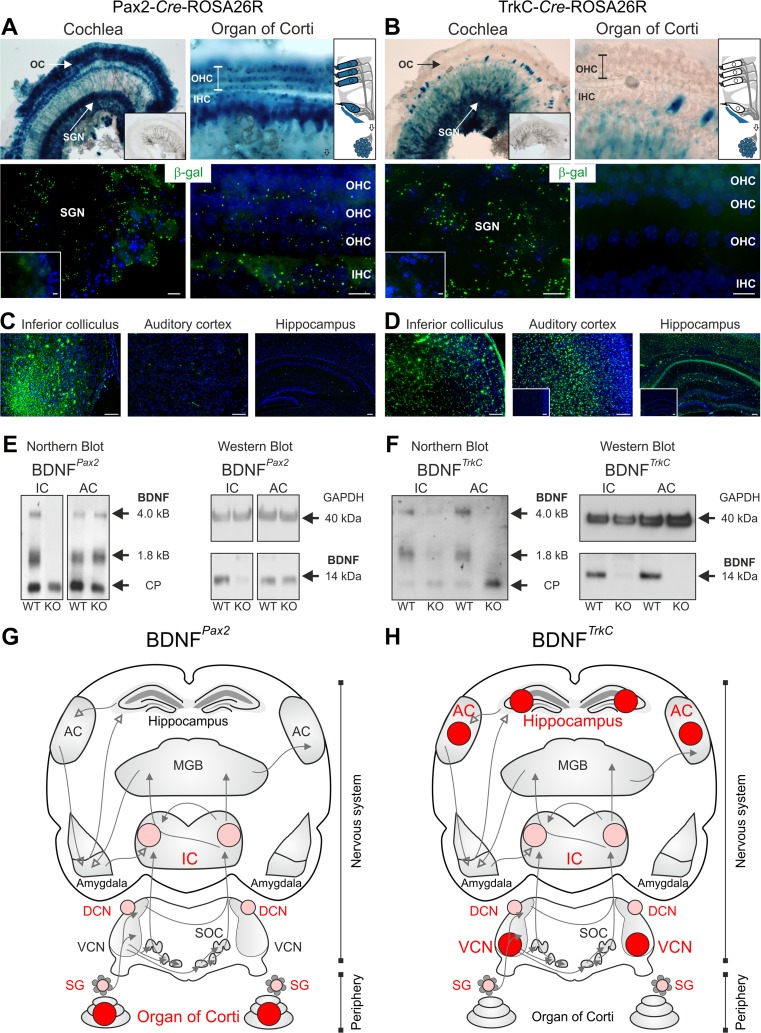
To define the source of BDNF that is responsible for the absence of normal hearing thresholds of BDNFPax2-KO animals [25], we compared the auditory phenotype of BDNF-Pax2-Cre transgenic mice with that of BDNF-TrkC-Cre mice, in which the Cre gene is controlled by the TrkC promoter [26]. A detailed comparative analysis of BDNF deletion patterns in the ascending auditory pathway was performed for both Cre mouse lines, using X-gal staining and anti-β-galactosidase (β-gal) immunolabeling (Fig. 1). X-gal staining confirmed intense labeling of hair cells and SGNs for Pax2-Cre-ROSA26R mice (Fig. 1a, upper panel), whereas for TrkC-Cre-ROSA26R mice, X-gal staining was observed mainly in SGNs but not in hair cells (Fig. 1b, upper panel). A similar result was observed when β-gal immunolabeling was used (Fig. 1a, b, lower panels). Negative controls showed no staining (Fig. 1a, b, inset).
Fig. 1.
Differential BDNF deletion patterns under the Pax2 or the TrkC promoter. a, b X-gal staining and β-gal immunolabeling of mice carrying the Pax2-Cre (a) or the TrkC-Cre transgene (b) on a ROSA26R background. a In the mature cochlea, β-galactosidase activity in Pax2-Cre-ROSA26R mice was detected in inner (IHC) and outer hair cells (OHC) of the organ of Corti (OC) and in spiral ganglion neurons (SGN). b β-Galactosidase activity in TrkC-Cre-ROSA26R mice was detected mainly in SGNs, but not in hair cells, as demonstrated by immunohistochemistry. c Immunohistochemistry for β-gal in the inferior colliculus (IC), auditory cortex (AC), and hippocampus of Pax2-Cre-ROSA26R mice. In the IC, β-gal staining can be detected, whereas no expression is observed in the AC and hippocampus. d Immunohistochemistry for β-gal in the IC, AC, and hippocampus of TrkC-Cre-ROSA26R mice. Clear β-gal staining is observed in the IC, AC, and hippocampus. In Cre-negative mice, no β-gal staining is seen (insets). Scale bars = a, b 10 μm; c, d 100 μm. e Northern and Western blots from IC and AC tissues of wild-type (WT) and BDNFPax2 knockout (KO) mice demonstrating a deletion of BDNF mRNA isoforms (1.8 and 4 kb) and BDNF protein (14 kDa) in the IC but not in the AC of BDNFPax2-KO mice. f Northern and Western blots from IC and AC tissues of WT and BDNFTrkC-KO mice demonstrating a deletion of BDNF mRNA isoforms (1.8 and 4 kb) and protein (14 kDa) in the IC and AC of BDNFTrkC-KO mice. For Northern blots, cyclophilin (CP) was used as a reference (0.8 kb); for Western blots, GAPDH (40 kDa) was used as a loading control. g, h Diagrams of the auditory pathway: the area of BDNF deletion exclusively in either the BDNFPax2-KO or the BDNFTrkC-KO is marked in dark red; the area of BDNF deletion in both BDNF-KO mouse lines is marked in bright red. g BDNF deletion in BDNFPax2-KO mice, h BDNF deletion in BDNFTrkC-KO mice
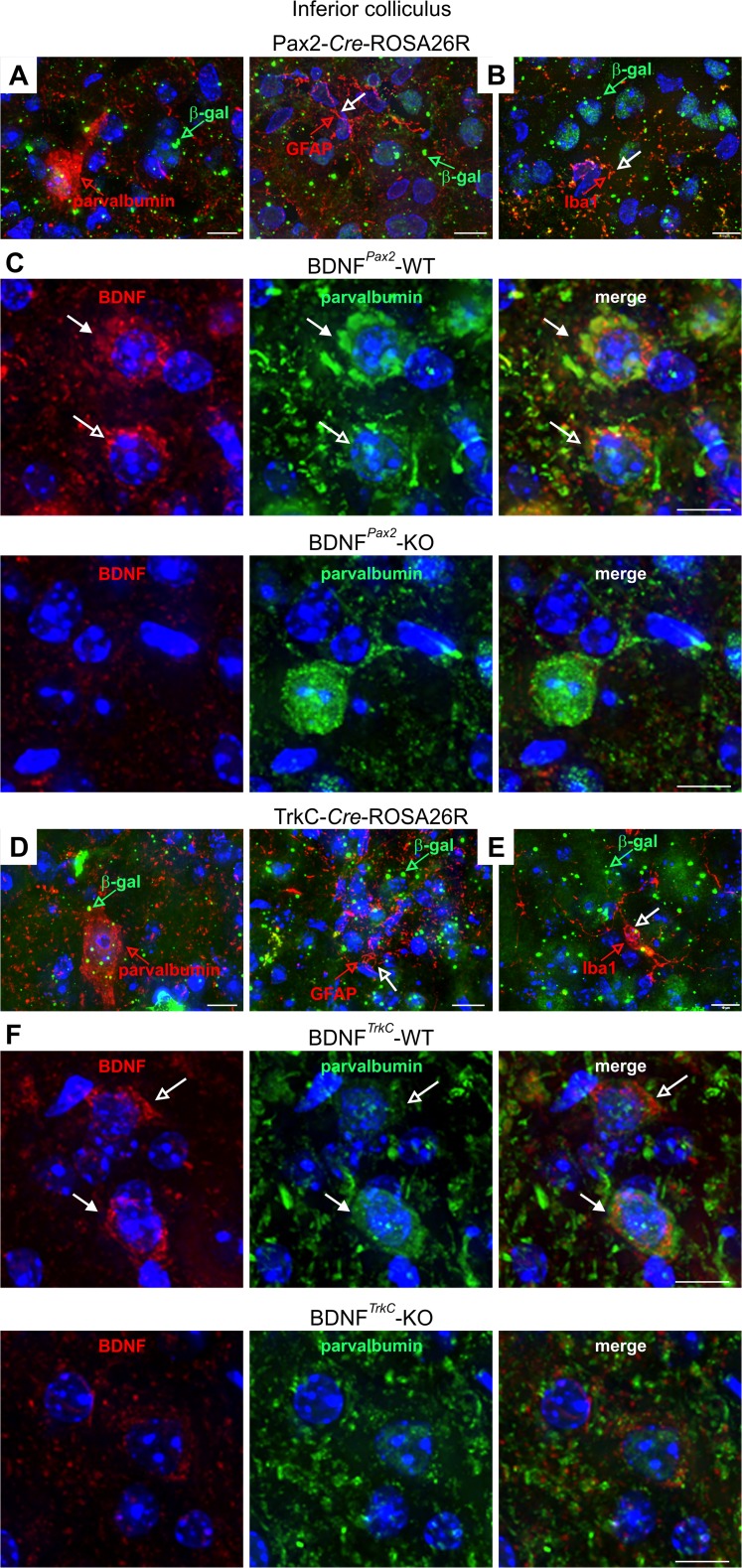
At the level of the CN, β-gal was detected exclusively in the DCN in the presence of the Pax2 promoter [25], whereas with the TrkC promoter, DCN and ventral cochlear nucleus (VCN) neurons were stained (not shown). In both Pax2-Cre-ROSA26R and TrkC-Cre-ROSA26R mice, an intense and widespread staining for β-gal was observed at the level of the IC (Fig. 1c, d). In contrast, in the AC and hippocampus (Fig. 1c, d), TrkC-Cre-ROSA26R but not Pax2-Cre-ROSA26R mice exhibited β-gal staining (Fig. 1c, d). A similar deletion pattern of BDNF in both BDNFPax2-KO and BDNFTrkC-KO mice was confirmed by the almost complete loss of the 4-kB and 1.8-kB BDNF transcripts and the absence of the 14-kDa BDNF polypeptide using Northern and Western blots, respectively (Fig. 1e, f, IC) [25]. In contrast, only in BDNFTrkC-KO but not BDNFPax2-KO mice, BDNF mRNA and protein were absent at the level of the AC (Fig. 1e, f) [25]. The overall deletion pattern is summarized in Fig. 1g, f. In the IC, β-gal (Fig. 2a, d, green) and BDNF immunoreactivity (Fig. 2c, f, red) were found in PV-immunopositive and PV-immunonegative neurons in both BDNFPax2-WT and BDNFTrkC-WT mice. The specificity of BDNF staining in these neurons was confirmed using BDNFPax2-KO and BDNFTrkC-KO animals (Fig. 2c, f). Colabeling with the neuronal microtubule-associated protein MAP2 and the glutamatergic marker protein VGLUT2 indicated that PV-immunoreactive neurons in the IC may correspond to projection neurons (not shown). On the other hand, colabeling of β-gal with either the oligodendrocyte marker GFAP or the microglia marker Iba1 [48] in Pax2-Cre-ROSA26R and TrkC-Cre-ROSA26R mice (Fig. 2b, e) confirmed that β-gal was preferentially localized in the neurons of the IC.
Fig. 2.
Immunohistochemistry of the IC of Pax2-Cre-ROSA26R and TrkC-Cre-ROSA26R mice and BDNFPax2 and BDNFTrkC WT and KO mice. a, d Immunostaining with anti-β-galactosidase (β-gal, green) and anti-parvalbumin (red) showing coexpression of β-galactosidase and parvalbumin in IC sections from both Pax2- and TrkC-Cre-ROSA26R mice. b, e Co-immunostaining with anti-β-galactosidase (β-gal, green) and either the oligodendrocyte marker anti-GFAP (red, open arrow) or the microglia marker anti-Iba1 (red, open arrow) in IC sections from both Pax2- and TrkC-Cre-ROSA26R mice shows no coexpression of β-galactosidase and GFAP or Iba1. Therefore, β-galactosidase is detected in neuronal cells. c, f Immunohistochemistry of IC sections stained with anti-BDNF (red) and anti-parvalbumin (green) antibodies, showing BDNF immunoreactivity in PV-positive and PV-negative neurons in BDNFPax2-WT (c, upper row) and BDNFTrkC-WT (f, upper row) mice. Closed arrows indicate cells positive for BDNF (red) and parvalbumin (green). Open arrows indicate cells expressing only BDNF (red). The specificity of the BDNF antibody is shown by the lack of BDNF immunostaining in BDNFPax2-KO and BDNFTrkC-KO mice (c, f, lower rows). Scale bars = 10 μm
Therefore, BDNFPax2-KO and BDNFTrkC-KO mice exhibit comparable BDNF deletion profiles in the IC, DCN, and SGNs (Fig. 1g, h, green dots) and were expected to develop similar hearing defects.
Deletion of BDNF in BDNFPax2-KO but not BDNFTrkC-KO Mice Constricts Sound-Induced ABR Waves
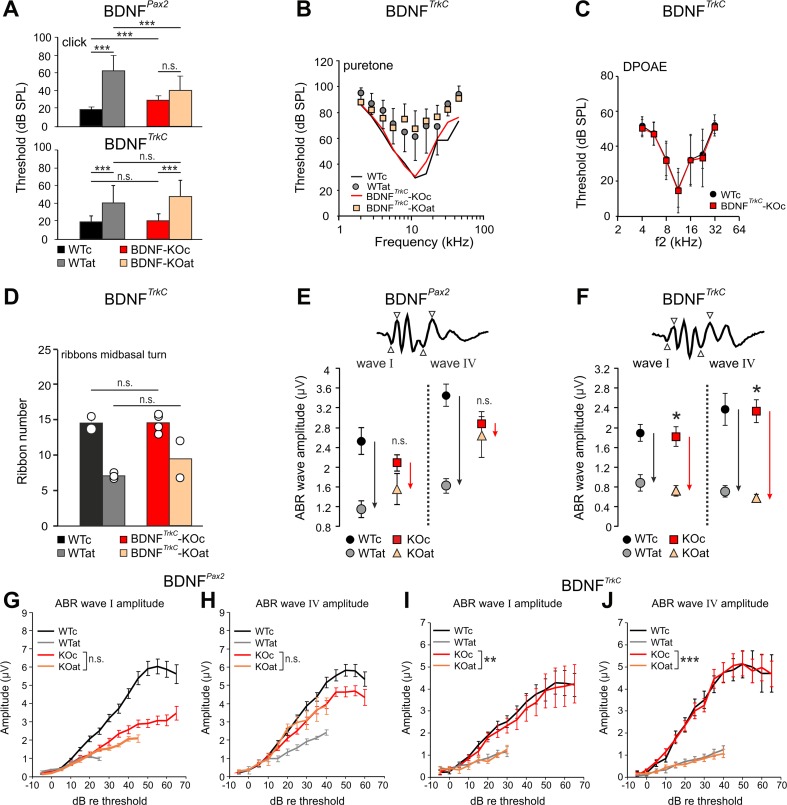
We thus measured hearing function in both BDNF mutant strains as described previously [25] and were surprised to observe significant differences. As shown in Fig. 3a (upper panel), BDNFPax2-KO mice exhibited slightly increased hearing thresholds (WTc, n = 6/12 ears/mice, 18.6 ± 3.92; KOc, n = 6/12 ears/mice, 30.3 ± 5.3; one-way ANOVA p < 0.001; n.s. not significant; ***p < 0.001 Bonferroni’s multiple comparison test), which have been linked with reduced exocytosis and ribbon numbers in otherwise mature IHC synapses in high-frequency cochlear turns [25]. After noise exposure, the loss of hearing thresholds in BDNFPax2-KO animals is, however, significantly less pronounced than in BDNFPax2-WT mice (Fig. 3a, upper panel, WTat, n = 8/16 ears/mice, 64.5 ± 16.64; KOat, n = 8/16 ears/mice, 41.9 ± 16.71; one-way ANOVA p < 0.001; n.s. not significant; ***p < 0.001 Bonferroni’s multiple comparison test) [25]. In contrast, BDNFTrkC-KO mice did not exhibit any differences in hearing thresholds compared to age-matched BDNFTrkC-WT mice, as assessed by click-evoked (Fig. 3a, lower panel, WTc, n = 22/11 ears/mice, 19.6 ± 6.38; KOc, n = 20/10 ears/mice, 20.7 ± 7.52; p = 0.999) and frequency-dependent (Fig. 3b, WTc, n = 22/11 ears/mice; KOc, n = 10/10 ears/mice; p = 0.684) hearing measurements before and after AT (Fig. 3a, WTat, n = 8/8 ears/mice, 40.7 ± 19.54; KOat, n = 7/7 ears/mice, 48.0 ± 18.03; p = 0.543 for click-evoked ABR, p = 0.419 for frequency-dependent ABR). Also, mean numbers of IHC ribbons in BDNFTrkC-KO mice before and after AT were similar to that in BDNFTrkC-WT mice (Fig. 3d). This indicates that the BDNF deletion under the Pax2 but not the TrkC promoter slightly reduced sound sensitivity.
Fig. 3.
Hearing function of BDNFPax2-KO and BDNFTrkC-KO mice before and after acoustic trauma. Auditory thresholds of BDNFPax2-KO (a, upper panel) and BDNFTrkC-KO (a, lower panel) mice analyzed by click (a) and tone-burst-evoked ABR (b) before (KOc) and after acoustic trauma (KOat). Compared to WT mice (WTc, WTat), BDNFPax2-KO are less vulnerable [25]. BDNFTrkC-KO mice exhibited normal hearing thresholds (a, lower panel, WTc, ears/mice: n = 22/11; KOc, n = 20/10; p > 0.999, two-way ANOVA) and show no significant difference to WT mice after acoustic trauma (WTat, ears/mice: n = 8/8; KOat, n = 7/7; p = 0.543, two-way ANOVA). Error bars, SD. c DPOAE thresholds in BDNFTrkC-WT and BDNFTrkC-KO mice were similar (WT, ears/mice: n = 19/10; KOn = 20/10; p = 0.482, two-way ANOVA). Error bars, SD. d IHC ribbon counts of midbasal cochlear turns in BDNFTrkC-WT and BDNFTrkC -KO mice before (WTc, KOc) and after noise exposure (WTat, KOat). The ribbon number of BDNFTrkC-KO mice was not significantly different from that of BDNFTrkC-WT animals. Error bars, SEM, n.s. p > 0.05; WTc, sections/mice: n = 5/2; WTat, n = 6/3; KOc, n = 11/4; KOat, n = 7/2. e, f Comparison of click-evoked ABR wave amplitudes in BDNFPax2-WT and BDNFPax2-KO mice (e) and BDNFTrkC-WT (WTc, WTat) and BDNFTrkC-KO (KOc, KOat) mice (f) before (WTc, KOc) and after noise exposure (WTat, KOat). In BDNFPax2-KO, suprathreshold amplitudes of wave I (auditory nerve) and wave IV (IC) are less reduced after noise exposure than in BDNFPax2-WT mice (compare black and red arrows in e for different reductions in WT and KO, respectively). In BDNFTrkC-KO, the reduction was not different from the reduction in BDNFTrkC-WT mice (compare black and red arrows in f for similar reduction in WT and KO, respectively). Two-way ANOVA with Bonferroni’s post hoc test (e) wave I, n.s. p = 0.254; wave IV, n.s. p = 0.893; WTat, ears/mice: n = 8/4; KOat, n = 7/4; f wave I, *p = 0.04; wave IV, *p = 0.02; WTat, mice/ears: n = 8/16; KOat, n = 8/16; error bars, SEM. g–j Suprathreshold ABR amplitude at the level of the auditory nerve (wave I) and IC (wave IV). Analysis was performed before and after acoustic trauma in BDNFPax2-WT (WTc, n = 16/8 ears/mice; WTat, n = 16/8 ears/mice) and BDNFPax2-KO mice (KOc, n = 16/8 ears/mice; KOat, n = 16/8 ears/mice) (g, h) compared to BDNFTrkC-WT (WTc, n = 17/8 ears/mice; WTat, n = 8/4 ears/mice) and BDNFTrkC-KO mice (KOc, n = 15/8 ears/mice; KOat, n = 7/4 ears/mice) (i, j). Note the near-complete convergence of growth functions of ABR wave I (g, n.s. p = 0.275; i, **p = 0.008) and IV (h, n.s. p = 0.420; j, ***p < 0.001) before and after AT in BDNFPax2-KO but not in BDNFTrkC-KO mice. Two-way ANOVA with Bonferroni’s post hoc test, error bars, SEM
Despite the differences in hearing thresholds, BDNFTrkC-KO and BDNFPax2-KO mice did not differ in OHC function, based on DPOAEs (Fig. 3c, n = 20/10 ears/mice) [25]. Consistent with BDNF acting at the level of the IHC-afferent nerve fiber, suprathreshold amplitudes of spreading auditory nerve activity (wave I) and midbrain activity (lateral lemniscus and IC, wave IV) were significantly reduced before AT but less affected after AT in BDNFPax2-KO compared to BDNFPax2-WT mice (Fig. 3e, WTat, n = 8; KOat, n = 8). This effect was not observed in BDNFTrkC-KO animals (Fig. 3f, wave I: p = 0.885, wave IV: p = 0.814; WTc, n = 17/9 ears/mice; KOc, n = 15/8 ears/mice; control “c” vs. acoustic trauma “at”: WTc, n = 8/4 ears/mice; KOc, n = 8/4 ears/mice; WTat, n = 8/4 ears/mice; KOat, n = 7/4 ears/mice). Likewise, the growth of suprathreshold ABR amplitudes to increasing sound intensities was affected in BDNFPax2-KO but not in BDNFTrkC-KO mice (compare Fig. 3g, h with i, j). Whereas ABR waves I and IV of traumatized BDNFPax2-KO mice were significantly less elevated in comparison to those of BDNFPax2-WT mice, no significant variations in DPOAEs (p = 0.056, n = 8/4 ears/mice) were observed after AT. Also the SPLs that generated significant differences in ABR wave I and IV 20 dB above thresholds in BDNFPax2-WT and BDNFPax2-KO (Fig. 3g, h) were far beyond the stimulus levels (15–30 dB SPL) where variations in the DPOAE response between BDNFPax2-WT and BDNFPax2-KO mice were apparent [25]. Therefore, in the range of the stimulus levels in which the ABR functions were quantified, no difference in DPOAE responses can be found between BDNFPax2-WT and BDNFPax2-KO animals. This indicates that the BDNF deletion under the Pax2 promoter may reduce sound sensitivity independently of OHC functions.
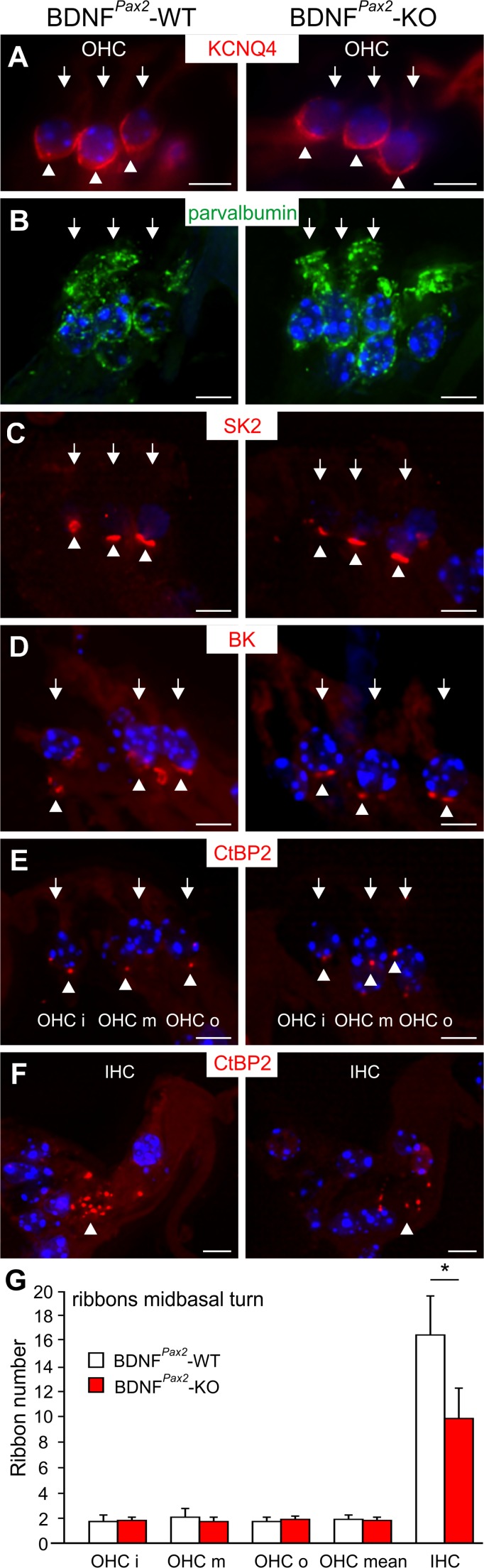
To further strengthen the point that the threshold differences between BDNFPax2-WT and BDNFPax2-KO are not caused by defects in OHC function, the phenotype of OHCs and their efferent and afferent synapses were studied in more detail. As exemplarily shown for midbasal turns, no obvious differences of the morphology of pre- and postsynapses of OHCs were observed between BDNFPax2-WT and BDNFPax2-KO mice. This was judged on the basis of expression of OHC marker proteins such as the outward-rectifying potassium channel KCNQ4 (Fig. 4a) or the Ca2+-binding protein parvalbumin (Fig. 4b). Also markers for pre- and postsynaptic efferent contacts such as SK2 and BK (Fig. 4c, d) [49, 50] and the numbers of CtBP2-stained OHC ribbons were similar between BDNFPax2-WT and BDNFPax2-KO mice (Fig. 4e, g), whereas IHC ribbons in the latter animals were reduced (Fig. 4f, g).
Fig. 4.
Immunohistochemistry of outer hairs cells (OHC, arrows) in BDNFPax2-WT (n = 4, left panels) and BDNFPax2-KO (n = 4, right panels) mice. OHCs are stained for the voltage-gated potassium channel KCNQ4 (a), parvalbumin (b), the small conductance calcium-activated potassium channel SK2 (c), the large-conductance calcium-activated potassium channel BK (d), and CtBP2, a marker of ribbon synapses (e). f In contrast to OHCs, IHCs show reduced number of CtBP2-stained ribbons in BDNFPax2-KO mice. Arrowheads indicate antibody staining. g Quantification of ribbons stained with anti-CtBP2. No differences are observed in any row of the OHCs or in the mean ribbon number of OHCs, although a significantly reduced ribbon number can be seen in IHCs. Two-tailed unpaired Student’s t test with α = 5 (*p < 0.05). Scale bars = 5 μm
These findings suggest that BDNF deletion under the Pax2 but not the TrkC promoter reduced evoked sound amplitudes independently of OHC function. After AT, the weaker reduction of amplitudes in BDNFPax2-KO pointed to a sound sensitivity that cannot be lost after AT, because it has never been enhanced via a sensory experience by BDNF.
The similar deletion profiles of BDNF in BDNFPax2-KO and BDNFTrkC-KO mice shown by immunohistochemistry and Western and Northern blot analyses in the IC suggest that the IC is unlikely to be the BDNF source responsible for the altered sound sensitivity observed in BDNFPax2-KO mice. As BDNF in the VCN or olivary complex is not deleted using the Pax2 promoter [25], the effects of BDNF acting from the superior olivary complex are also unlikely to play a role. Finally, since BDNF released from projection neurons is assumed to drive interneuron stability and not vice versa [51], loss of BDNF in PV-expressing inhibitory interneurons that are suggested to derive from Pax2 precursors [52] is probably not involved in the auditory phenotype of BDNFPax2-KO mice. Therefore, influences from intracortically expressed BDNF acting through descending pathways are very unlikely to be the underlying cause for the auditory phenotype in the BDNFPax2-KO animals, as in these mice BDNF is not deleted in the AC (Fig. 1c). Thus, BDNF in the lower parts of the auditory CNS or within the cochlea has to be considered as the most likely source responsible for altered sound sensitivity before and after AT in BDNFPax2-KO mice.
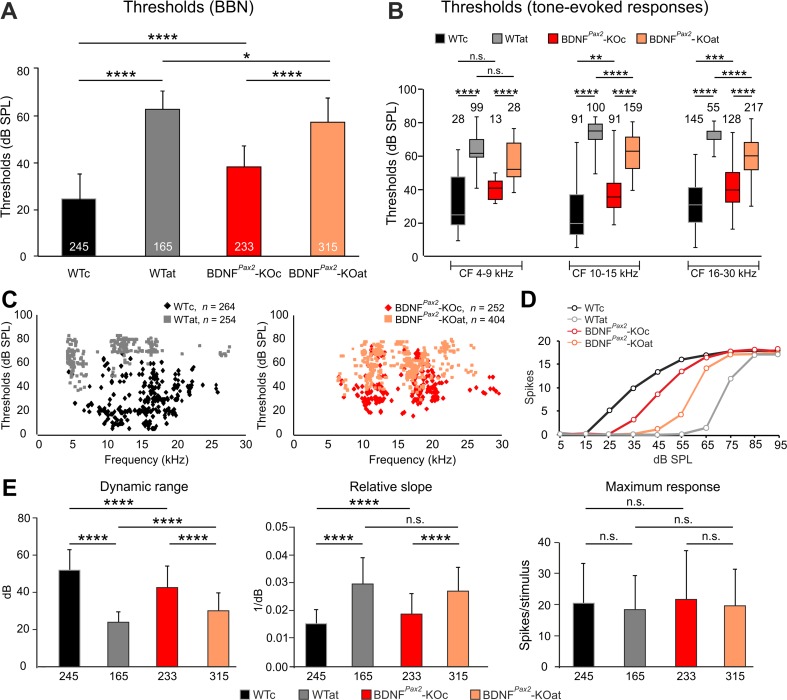
Deletion of BDNF in BDNFPax2-KO Constricts CF Thresholds and Dynamic Range in IC Neurons
To understand the physiology behind the reduced range of suprathreshold ABR responses generated at the level of the IC in BDNFPax2-KO mice (Fig. 3e, h), we analyzed the electrophysiological response behavior of single IC neurons in 3–4-month-old BDNFPax2-WT and BDNFPax2-KO mice, 3–4 weeks after sham or traumatizing acoustic exposure. The responses to BBN and pure tones of a total of 1069 IC units were recorded, comprising 264 units from WTc (n = 4), 232 from KOc (n = 4), 254 from WTat (n = 5), and 404 from KOat (n = 5).
Since AT was produced by exposure to a 10-kHz pure tone, the whole sample of units was divided into three main nonoverlapping frequency bands according to their CF: 4–9 kHz (low), 10–15 kHz (middle), and 16–30 kHz (high). The thresholds of IC neurons to BBN stimulation were significantly higher in BDNFPax2-KO than in BDNFPax2-WT mice (Fig. 5a, BDNFPax2-KOc, 38.62 ± 9.6 dB SPL; BDNFPax2-WTc, 24.78 ± 10.76 dB SPL; ****p < 0.0001). After AT, in KO animals (∼20 dB), these thresholds were significantly less pronounced than in WT mice (∼40 dB; Fig. 5a, b). Also, response thresholds to tone stimulation at neuronal CF 10–15 kHz and 16–30 kHz were significantly higher in BDNFPax2-KO than in BDNFPax2-WT mice (Fig. 5b, middle CF, WTc: Mdn = 19.18; KOc: Mdn = 35.14, **p < 0.01; high CF, WTc: Mdn = 30.28; KOc: Mdn = 39.38, ***p < 0.001) and tended to be smaller after AT in BDNFPax2-KO mice in these CF ranges (Fig. 5b). Low CF thresholds in BDNFPax2-KO mice were not significantly different from WT, even though no units with thresholds below ∼30 dB SPL could be found (Fig. 5b, WTc: Mdn = 24.34; KOc: Mdn = 40.39, n.s. p > 0.05). Thresholds of individual IC neurons as a function of CF are presented in scatter plots for BDNFPax2-WT and BDNFPax2-KO animals (Fig. 5c). Finally, the parameters of the RIFs such as dynamic range (WTc 52.37 ± 11; KOc 43.23 ± 11, ****p < 0.0001) and slope (WTc 0.01424 ± 0.006; KOc 0.01919 ± 0.007, ****p < 0.0001), but not maximum response magnitude (WTc 20.52 ± 12.48; KOc 21.83 ± 15.56, n.s. p > 0.05) of IC neurons, were significantly reduced in BDNFPax2-KO mice and significantly less pronounced after AT in BDNFPax2-KO than in BDNFPax2-WT mice (Fig. 5d, e).
Fig. 5.
Response thresholds of IC neurons to broadband noise (BBN) and tone stimulation for control or sound-exposed BDNFPax2 wild-type (WTc, WTat) and BDNFPax2 knockout (KOc, KOat) animals. a Averaged thresholds of responses to BBN stimulation. b Box and whisker plot for thresholds of tone-evoked responses for neurons of individual CF frequency ranges (4–9, 10–15, 16–30 kHz). c Scatter plots of pure tone thresholds as a function of neuronal CF, shown separately for BDNFPax2-WT (WTc, n = 4; WTat, n = 5) and BDNFPax2-KO (KOc, n = 4; KOat, n = 5) animals. d, e Parameters of the rate-intensity function (RIF) of responses to BBN stimulation for control or sound-exposed BDNFPax2-WT (WTc, WTat) and BDNFPax2-KO (KOc, KOat) animals. d Typical examples of RIFs for all groups of animals. e Average dynamic range, relative slope, and maximum response for all groups of mice. One-way ANOVA with Bonferroni’s post hoc test and Kruskal-Wallis test with Dunn’s multiple comparison test, n.s. p > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Data are presented as mean ± SD or median with interquartile range and extremes. The numbers in the graphs indicate the numbers of neurons
These findings reveal that after the onset of hearing, BDNF in lower brain parts improves sound-evoked ABR amplitudes generated at the level of the IC (ABR wave IV amplitudes) as well as the threshold to which IC neurons can respond to sound intensities above 10 kHz. This sensitivity to sound can be lost after auditory nerve injury in the mature system only when it has been established before with the onset of hearing.
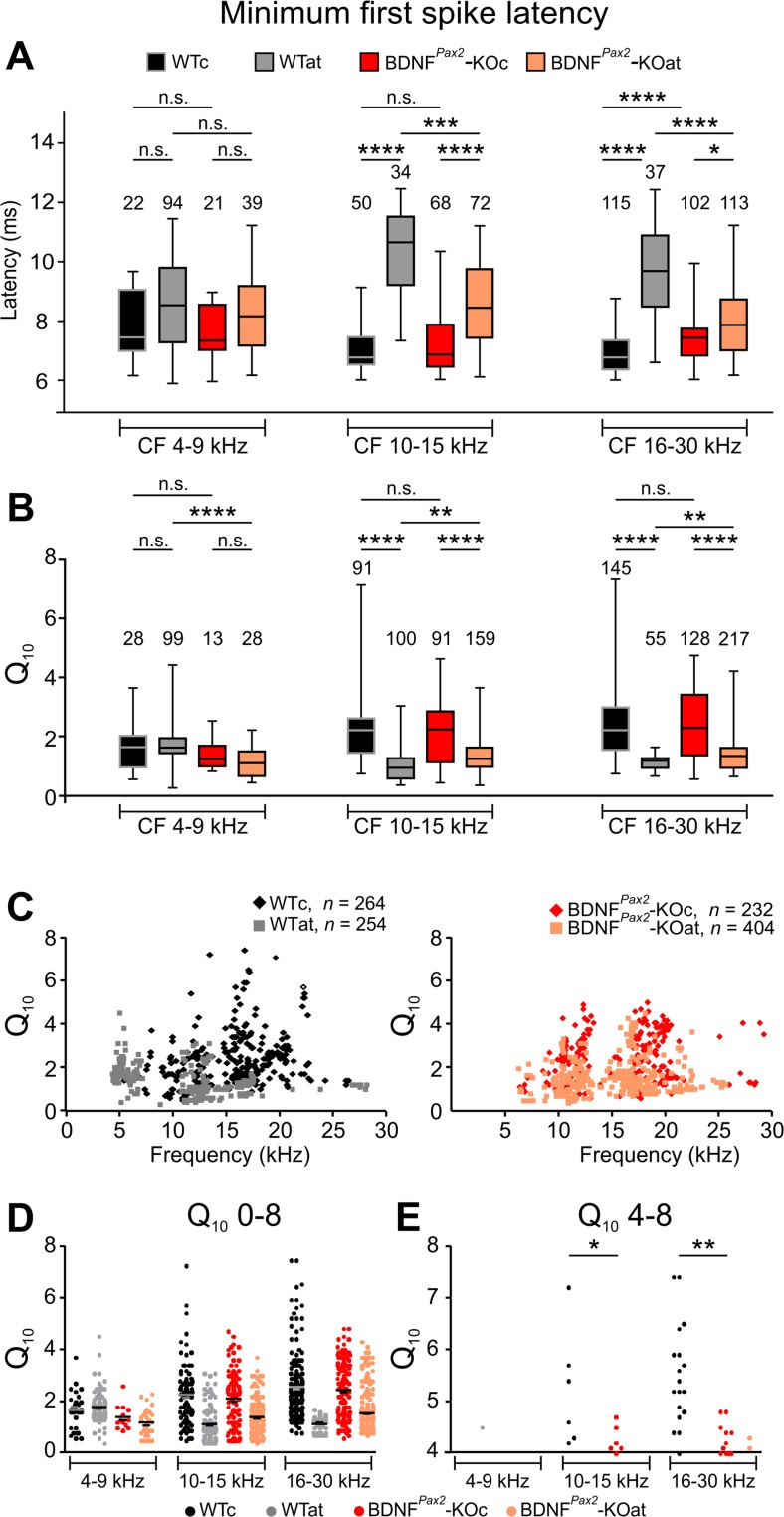
Deletion of BDNF in BDNFPax2-KO Mice Alters Latency and Tuning Characteristic of IC Neurons
CF detection thresholds have been hypothesized to be influenced by onset stimuli [53]. Differences in onset stimuli should be reflected by differences in mFSL, which can be evaluated from poststimulus time histograms. mFSLs for 80 dB SPL stimuli were similar in BDNFPax2-WT and BDNFPax2-KO animals for the low- and middle-frequency bands (Fig. 6a; low CF, 4–9 kHz, WTc: Mdn = 7.28; KOc: Mdn = 7.4, n.s. p > 0.05; middle CF, 10–15 kHz, WTc: Mdn = 6.8; KOc: Mdn = 6.9, n.s. p > 0.05), but were significantly longer for neurons with high CF (16–30 kHz) (Fig. 6a; WTc: Mdn = 6.8; KOc: Mdn = 7.5, ****p < 0.0001). After AT, mFSLs were less enlarged in BDNFPax2-KO mice for neurons with both middle and high CF (Fig. 6a, middle CF, WTat: Mdn = 10.7; KOat: Mdn = 8.5, ***p < 0.001; high CF, WTat: Mdn = 9.8; KOat: Mdn = 7.9, ****p < 0.0001). This indicates that after the onset of hearing, BDNF improves the short latency responses of IC neurons to sound. Only when these responses have been developed, such as in BDNFPax2-WT mice, this faster response behavior can be lost after traumatizing noise in the mature system.
Fig. 6.
Minimum first spike latency (mFSL) and quality factor (Q 10) of IC neurons. a mFSL of responses to 80 dB SPL BBN bursts for control and sound-exposed BDNFPax2-WT (WTc, n = 4; WTat, n = 5) and BDNFPax2-KO (KOc, n = 4; KOat, n = 5) animals. Box and whisker plot for mFSL for neurons of individual CF frequency ranges (4–9, 10–15, 16–30 kHz). Kruskal-Wallis test with Dunn’s post hoc test: n.s. p > 0.05, *p < 0.05, ***p < 0.001, ****p < 0.0001. Data are presented as medians with interquartile ranges and extremes. b–e Q 10 of IC neuron responses to pure tone stimulation for control and sound-exposed BDNFPax2-WT (WTc, n = 4; WTat, n = 5) and BDNFPax2-KO (KOc, n = 4; KOat, n = 5) animals. b Box and whisker plot for Q 10 for neurons of individual CF frequency ranges (4–9, 10–15, 16–30 kHz). c Scatter plots illustrating the Q 10 as a function of neuronal CF, shown separately for BDNFPax2-WT and BDNFPax2-KO animals. d, e Scatter plots for Q 10 with y scale 0–8 (d) and scale 4–8 (e) for the neurons of individual frequency ranges (4–9, 10–15, 16–30 kHz). Note that IC neurons >10 kHz with Q 10 >5 are absent in BDNFPax2-KO mice (e). Kruskal-Wallis test with Dunn’s multiple comparison test: n.s. p > 0.05, **p < 0.01, ****p < 0.0001. Data are presented as medians with interquartile ranges and extremes. The numbers in the graphs indicate the numbers of neurons
The quality factor Q10 of sound responses of IC neurons, which is reciprocally related to the excitatory area bandwidth 10 dB above threshold (the higher the Q10, the sharper the tuning), may help to identify the type of neuron which responds to BDNF in the IC. Q10 did not significantly differ between BDNFPax2-WTc and BDNFPax2-KOc mice over all CF ranges (Fig. 6b). However, after AT, Q10 in neurons with middle and high CF in BDNFPax2-WTat mice was significantly more reduced than in BDNFPax2-KOat animals (Fig. 6b, n.s. p > 0.05, **p < 0.01, ****p < 0.0001). Differences in peak Q10 values in scatter plots categorized for frequency bands in control and KO animals (Fig. 6c, d) prompted us to investigate individual Q10 values. Interestingly, in CF regions >10 kHz, the number of IC neurons with Q10 values >4 was significantly lower in sham-exposed BDNFPax2-KO than in sham-exposed BDNFPax2-WT animals (Fig. 6e, *p < 0.05, **p < 0.01). This indicates that after the onset of hearing, BDNF in lower brain parts sharpens a very narrow tuning characteristic of IC neurons. Only when this sharpening has developed (e.g., in BDNFPax2-WT) it can be lost after traumatizing noise in the mature system.
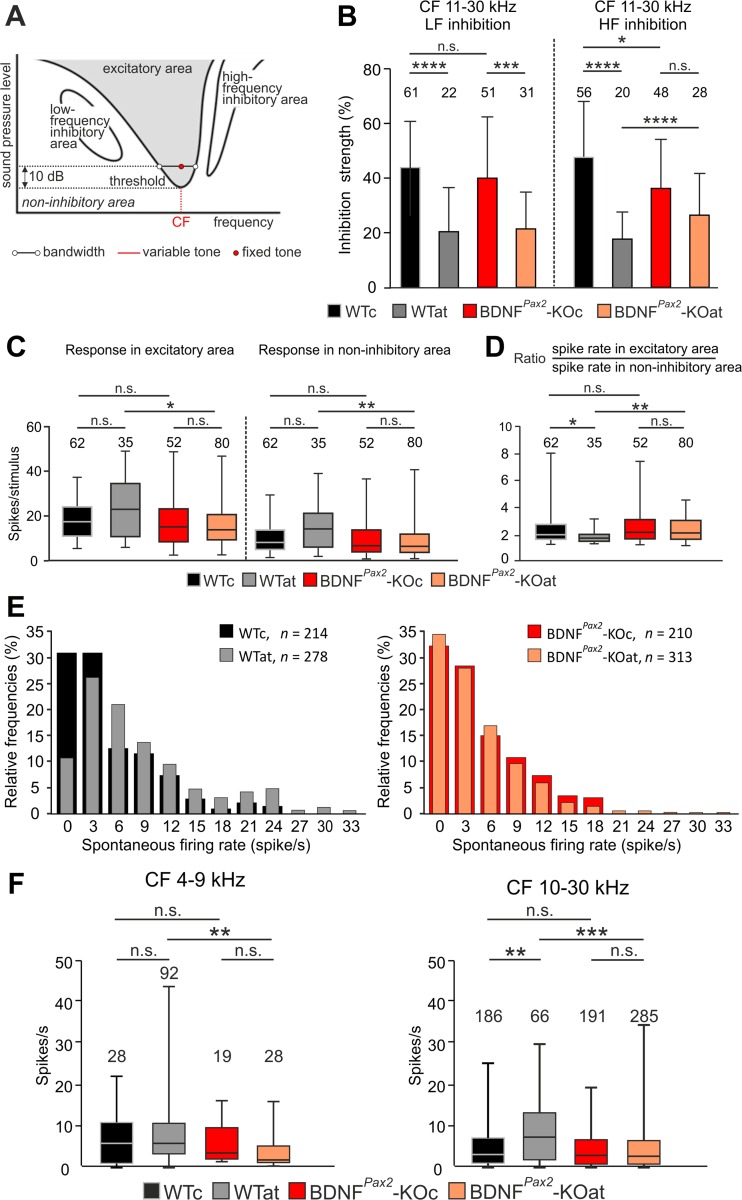
Deletion of BDNF in BDNFPax2-KO Mice Affects the Inhibitory Strength of IC Neurons
To investigate the difference in tuning characteristics of IC neurons in more detail, inhibitory, excitatory, and noninhibitory (Fig. 7) response characteristics of IC neurons of sham-exposed and noise-exposed BDNFPax2-WT and BDNFPax2-KO mice were investigated using two-tone inhibition. In both groups of sham-exposed mice almost 80 % of neurons displayed low-frequency and/or high-frequency lateral inhibition. Likewise, after intense noise exposure, there was a likewise drop in the number of neurons displaying lateral inhibition in both groups of animals (data not shown).
Fig. 7.
Inhibition characteristics in control or sound-exposed BDNFPax2-WT (WTc, n = 4; WTat, n = 5) and BDNFPax2-KO (KOc, n = 4; KOat, n = 5) animals. a Schematic of response map to two-tone stimulation showing excitatory, inhibitory, and noninhibitory areas. b Comparison of low- and high-frequency sideband inhibition strength in middle and high CF (11–30 kHz) IC neurons with inhibitory strength of 1 % and higher. One-way ANOVA with Bonferroni’s post hoc test. c Spike rates of IC neurons with middle and high CF in response to two-tone stimulation, determined 20 dB above threshold in the excitatory area and noninhibitory area. Numbers in the graph indicate number of neurons. d Ratio of spike rates in the excitatory field 20 dB above threshold to spike rates in the noninhibitory area. The numbers of neurons are given above the corresponding boxes. Kruskal-Wallis test with Dunn’s multiple comparison test: n.s. p > 0.05, *p < 0.05, ***p < 0.001, ****p < 0.0001. Data are presented as mean ± SD or median with interquartile range and extremes. e Distribution of IC neurons according to their spontaneous firing rate (spikes/s) for control and sound-exposed BDNFPax2-WT (WTc, n = 4; WTat, n = 5) and BDNFPax2-KO (KOc, n = 4; KOat, n = 5) animals. f Spontaneous firing rates for neurons with CF in the high-frequency band (10–30 kHz) were significantly higher in BDNFPax2-WTat than in BDNFPax2-WTc but were similar in BDNFPax2-KOat and BDNFPax2-KOc. Kruskal-Wallis test with Dunn’s post hoc test demonstrated significant differences between WTc and WTat (**p < 0.01) and WTat and KOat (***p < 0.001) groups of animals. Data are presented as mean ± SD or median with interquartile range and extremes. The numbers in the graphs indicate the numbers of neurons
Next, the strength of firing rate suppression by lateral inhibition was calculated as a percentage of rate suppression separately for low- and high-frequency sideband inhibition for neurons with a CF <10 kHz and CF >10 kHz. Low CF neurons did not exhibit altered low- or high-frequency sideband inhibitory strengths in the absence of BDNF or after AT (Fig. 7b). Likewise, in the neurons with a CF of 11–30 kHz, the low-frequency sideband was similar in BDNFPax2-WT and BDNFPax2-KO animals (Fig. 7b). However, high-frequency sideband inhibition strength was significantly reduced in sham-exposed BDNFPax2-KO mice (Fig. 7b, *p < 0.05). Moreover, the pronounced drop in the strength of high-frequency inhibition after AT observed in BDNFPax2-WT mice was not found in BDNFPax2-KO animals (Fig. 7b, ****p < 0.0001). This indicates that after the onset of hearing, BDNF in lower brain parts increases the inhibitory strength of IC neuron that can be dropped after AT only when it has been increased before.
The stimulus-induced spike rates of IC neurons with middle and high CF in excitatory and noninhibitory regions were significantly elevated after AT in BDNFPax2-WT in comparison to BDNFPax2-KO mice (Fig. 7c). This is likely due to the drop in BDNF-driven inhibitory strength after AT in BDNFPax2-WT but not in BDNFPax2-KO mice (Fig. 7b). Importantly, these higher spike rates in BDNFPax2-WTat animals occur without a corresponding increase in sound-evoked spike output as revealed through a smaller ratio between spikes in excitatory and noninhibitory areas in BDNFPax2-WTat but not BDNFPax2-KOat mice (Fig. 7d). When the spontaneous firing rates of IC neurons from poststimulus histograms for BBN were assessed after AT in BDNFPax2-WT mice (Fig. 7e), there was a significant drop in the proportion of neurons with a spontaneous firing rate close to 0 (0–3 spikes/s) (32 % for WT to 61 % for KO, ***p < 0.001) and a shift of the distribution toward neurons with a higher spontaneous activity compared to BDNFPax2-KOat animals (WT: Mdn = 3.8 to Mdn = 7.13, **p < 0.01; KO: Mdn = 4.13 to Mdn = 2.8, n.s. p > 0.5). These results underscore the loss of suppression of a BDNF-dependent firing rate. When spontaneous firing rates for low, middle, and high CF regions were analyzed separately, a significantly enhanced spontaneous firing rate for a CF >10 kHz after AT in BDNFPax2-WT but not in BDNFPax2-KO mice was observed (Fig. 7f). This finding strengthens the conclusion that BDNF may improve signal-to-noise ratio by increasing the inhibitory strength of neurons under basal conditions.
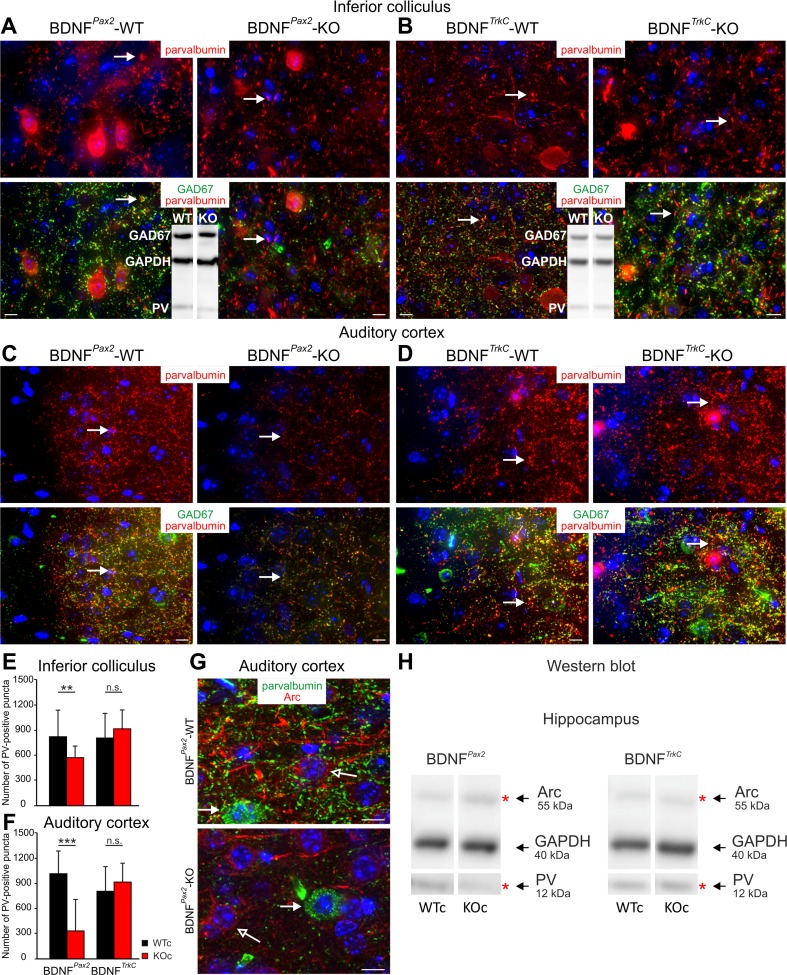
Deletion of BDNF in BDNFPax2-KO but not BDNFTrkC-KO Mice Reduces the Density of PV-Immunopositive Puncta in IC and AC Projecting Neurons
In many projection neurons, changes in inhibitory conductances are assumed to require specialized GABA (γ-aminobutyric acid)A receptors. Particularly, the differentiation of PV basket cells, a subpopulation of GABAergic neurons that regulate a critical period of plasticity in the cortex which depends on BDNF (for a review, see [54]), may play a role during the alteration of inhibitory strength in BDNFPax2-KOs. Likewise, elevated auditory startle amplitude and latencies in PV−/− mice suggested a specific role of PV basket cells for inhibitory circuits in auditory processing [55]. To investigate a role of PV for the observed changes in inhibitory strength in BDNFPax2-KO mice, we used antibodies for PV [56, 57] and antibodies for the 67-kDa isoform of GABAergic interneurons. We found that the intensity and number of PV-immunopositive puncta, but not the number of PV-immunopositive somata or GAD67-immunopositive puncta (not shown), are decreased in the IC and AC of BDNFPax2-KO (Fig. 8a, c) but not in BDNFTrkC-KO mice (Fig. 8b, d). Reduced levels of PV expression could also be confirmed by Western blots (Fig. 8a, b, inset). Quantification of the intensity of labeling revealed a significantly reduced number and intensity of PV-positive puncta (Fig. 8e, 10–20 slices of 3–6 independent experiments of n = 3 animals) in BDNFPax2-KO mice. Also in the hippocampus, PV levels were reduced in BDNFPax2-KO but not in BDNFTrkC-KO mice, as exemplarily shown by Western blots (Fig. 8h). Interestingly, the reduction of PV levels in BDNFPax2-KO mice was associated with elevated levels of the immediate early gene Arc (activity-regulated cytoskeletal protein), as exemplarily shown for the hippocampus (Fig. 8h, n = 3–5). Arc is mobilized in glutamatergic neurons following, e.g., long-term potentiating (LTP)-like activity [58, 59]. As BDNFPax2-KO mice do not lack BDNF in the AC and hippocampus (Fig. 1c, e), future studies are required to investigate a potential developmental downregulation of Arc levels mediated by BDNF-dependent increases in inhibitory strength following onset of sensory function.
Fig. 8.
Immunohistochemistry of the inferior colliculus and auditory cortex of BDNFPax2-KO and BDNFTrkC-KO mice. a–d Immunohistochemistry of the IC and AC of BDNFPax2-KO (a, c) and BDNFTrkC-KO mice (b, d) immunolabeled with anti-parvalbumin (red) and anti-GAD67 (green). A reduction of PV- and GAD67-immunoreactive puncta in both IC (a) and AC (c) in BDNFPax2-KO mice compared to BDNFPax2-WT mice is observed. Arrows point to PV-immunoreactive puncta. No changes in PV and GAD67 expression are observed in the IC (b) and AC (d) of BDNFTrkC-KO mice compared to BDNFTrkC-WT mice. Arrows point to PV-immunoreactive puncta. Reduced PV levels in BDNFPax2-KO but not BDNFTrkC-KO mice in the IC are confirmed by Western blot (a, b insets). e, f Quantification of PV-immunoreactive puncta in the IC (e) of BDNFPax2-WT, BDNFPax2-KO, BDNFTrkC-WT, and BDNFTrkC-KO mice (10–20 slices of 3–6 independent experiments of n = 3 animals) and in the AC (f) of BDNFPax2-WT, BDNFPax2-KO, BDNFTrkC-WT, and BDNFTrkC-KO mice (10–20 slices of 3–6 independent experiments of n = 3 animals). Two-way ANOVA with Bonferroni’s post hoc test (**p < 0.01, ***p < 0.001). Data are presented as mean ± SD. g Immunohistochemistry of AC sections of BDNFPax2-WT (upper image) and BDNFPax2-KO (lower image) mice immunolabeled with anti-Arc (red) and anti-parvalbumin (green) antibodies. In BDNFPax2-KO mice, Arc (red) expression is reduced. Closed arrows point to PV-positive cells. Open arrows indicate PV-immunoreactive puncta surrounding Arc-positive neurons. Scale bars = 10 μm. h Parvalbumin (PV) and Arc levels in the hippocampus of BDNFPax2-WT and BDNFPax2-KO mice and BDNFTrkC-WT and BDNFTrkC-KO mice, respectively, detected by Western blots (exemplarily for n = 3–5 mice). Note that the reduction of PV is associated to an increase of Arc in BDNFPax2-KO but not BDNFTrkC-KO mice
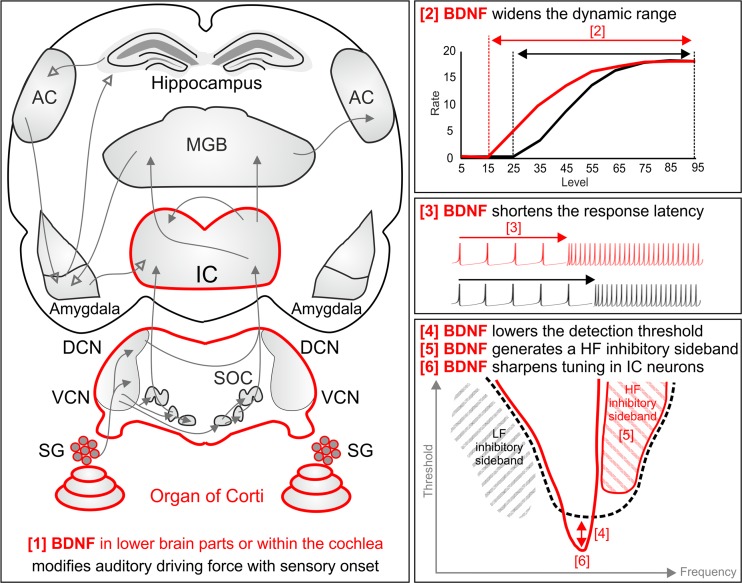
In conclusion and as outlined in Fig. 9, the data in the present study suggest that BDNF in the lower parts of the auditory system or within the cochlea (and not in the upper central auditory system) (Fig. 9 [1]) alters the afferent driving force for sound processing toward a wider dynamic range to which IC neurons can respond to sound intensities (Fig. 9 [2]). This is possibly mediated by generation and maintenance of increased inhibitory PV contacts with the auditory brain network that leads to enhanced spike probability in response to sensory stimulation. As shown for IC neurons, the BDNF-modified afferent driving force shortens response latency (Fig. 9 [3]) and lowers the CF detection threshold in IC neurons (Fig. 9 [4]) through the generation of a high-frequency inhibitory sideband (Fig. 9 [5]) in, e.g., very narrowly tuned IC neurons (Fig. 9 [6]). This suggests that the auditory driving force which depends on BDNF in the lower parts of the auditory CNS or within the cochlea may improve fidelity through enhancing the inhibitory strength within the auditory-specific network. When this driving force is lost after auditory nerve injury, such as following acoustic trauma, as shown here for traumatized BDNFPax2-WT animals, the auditory-specific network may lose its normal signal-to-noise ratio within the affected frequency range.
Fig. 9.
Schematic summary of the results that illustrates our hypothesis. An auditory driving force which is modified with hearing onset and depends on BDNF in the lower parts of the auditory system or within the cochlea [1] widens the dynamic range above which spike rates can be detected [2]. This is achieved upon shortening of the response latency [3], leading to lowering of the detection threshold [4] through generation of a high-frequency inhibitory sideband [5] in, e.g., very narrowly tuned IC neurons [6]. Through these events, the probability to detect a spike above the noise floor may be improved. This BDNF activity on auditory fibers in high-frequency cochlear turns can be lost after acoustic trauma. In this case, spontaneous spike rates in central auditory pathways are elevated (hyperactivity). As a consequence, due to loss of basal inhibitory strength within the ascending circuit of affected frequency regions, the capacity to adapt to sensory deprivation might be reduced. HF high frequency, IC inferior colliculus, IHC inner hair cell, LF low frequency
Discussion
The present study supports the hypothesis that the maturation of auditory nerve activity depends on BDNF in the lower parts of the auditory CNS or within the cochlea and is required to improve the fidelity of sound-induced responses after the onset of hearing. Improved auditory fidelity under control of this BDNF-dependent process is most likely achieved through enhancement of basal inhibitory circuits in the ascending auditory pathway. In the mature auditory organ, this BDNF-dependent driving force can be lost during, e.g., cochlear injury, leading to an elevated spontaneous firing rate, independently of a corresponding increase in sensory-evoked spikes.
Peripheral but not Central BDNF Drives Changes in Sound Processing
We show here that BDNF present in the lower parts of the auditory CNS or the peripheral end organ improves the threshold for sound and the dynamic range of sound responses. In both BDNFPax2-KO [25] and BDNFTrkC-KO animals (present study), BDNF is deleted in SGNs and in the IC, suggesting that alterations of BDNF activities in these compartments are unlikely to be responsible for the differences between sound-induced ABR wave I and IV responses in these mice. As BDNFPax2-KO mice do not have a BDNF deletion in neither the AC, VCN, or olivary complex [present study] [25], descending central feedback loops are also unlikely to be involved. The observed hearing phenotype in BDNFPax2-KO mice may therefore be linked to a Pax2-driven BDNF deletion in the lower parts of the auditory CNS or the cochlea. In the cochlea, BDNF is expressed postnatally in phalangeal, SGN, or satellite cells [22, 25, 50, 60]. Given that (i) BDNF deletion in phalangeal cells does not affect hearing thresholds [23], (ii) BDNF deletion in SGNs and in a minor number of supporting cells as observed in BDNFTrkC-KO mice does not lead to an auditory phenotype, and (iii) BDNF expression in inhibitory interneurons has been excluded [52, 61], the loss of BDNF expression in glial cells may be considered as being responsible for the observed phenotype in BDNFPax2-KO animals.
Peripheral BDNF Improves Amplitudes of Spreading Sound-Induced Activity
In our previous study, reduced exocytosis in otherwise mature IHCs in high-frequency cochlear turns of 2–3-week-old BDNFPax2-KO mice [25] pointed out a critical role of BDNF for pre- and postsynaptic maturation of IHCs. The alterations in ABR waves I and IV found in the present study in BDNFPax2-KO but not BDNFTrkC-KO mice indicate that BDNF activities that are carried out independently of higher auditory brain regions improve sound sensitivity at least up to the level of the IC. We assume that in BDNFPax2-KO mice the decrease of ABR wave I and IV amplitude observed before AT and its reduced decline after AT occurs independently of the electromechanical properties of OHCs for the following reasons: (i) within the range of the stimulus levels in which ABR functions were quantified, no difference in DPOAEs, used as a measurement for mechanical properties of OHCs, was found; (ii) changes of suprathreshold responses after AT are seen in BDNFPax2-KO mice, but no differences are observed in DPOAE amplitudes after AT; and (iii) BDNF is not deleted in the olivocochlear brainstem nuclei that are projection fields of efferent fibers toward OHCs [25]. BDNF is therefore more likely to improve ABR amplitudes through its influence on IHC exocytosis and/or auditory fiber sensitivity for low sound thresholds. In this context, it is important to consider that low-threshold fiber characteristics develop only after hearing onset [62, 63]. Also in the visual system, a role of retinal BDNF for improved retinal and visual acuity has been suggested [64]. Accordingly, an antisense-based inhibition of BDNF in the retina prevented an improvement of retinal stripe segregation induced by environmental enrichment as well as cortical acuity, even prior to eye opening [16, 17, 64]. To date, conditional deletion of retinal BDNF has not been performed, and it is therefore elusive if it improves the baseline of visual sensitivity independently of environmental enrichment, similar to the present findings.
BDNF Drives Increased Inhibitory Strength Along the Auditory Pathway
The current findings suggest that BDNF-dependent effects on auditory nerve activity are responsible not only for improving the ABR amplitude size of high-frequency sound responses spreading along the ascending pathway, but also for expanding the range of CF thresholds in these high-frequency CF regions. The affected CF regions >10 kHz span areas (15–20 kHz) where murine behavioral auditory thresholds are at their lowest level [65]. We thus have to consider the improved CF thresholds, short latencies, and the increased strength of inhibitory sidebands of sharply tuned IC neurons in BDNFPax2-WT versus BDNFPax2-KO animals in the context of an enhanced auditory fidelity in behaviorally relevant frequency regions. The IC neurons that show altered tuning characteristics depending on the presence of BDNF are reminiscent of type II [65] and type I [66] class IC neurons. Both IC cell types predominate in high-frequency regions and are characterized by their short latency, narrow and sharp tuning, low-frequency border with a shallow slope, and high-frequency border with a steep slope [65, 66]. It thus may be considered that these neurons are shaped under the control of BDNF activities at hearing onset.
In BDNFPax2-KO animals, the proportion of neurons with an elevated spontaneous firing rate is slightly, however not yet significantly, increased. After AT, however, a significant drop in neurons with a lower spontaneous firing rate and an increase in neurons with a higher spontaneous firing rate in BDNFPax2-WT mice compared to BDNFPax2-KO animals support a loss of the BDNF-dependent rate of firing suppression after auditory nerve injury. Reminiscent to this finding, a reduced rate of firing suppression that leads to an increase of the spontaneous firing rate without a stimulus-evoked spike output was described after a blockade of tonic inhibition in granule cells of the cerebellar cortex [67]. It is currently assumed that tonic inhibition suppresses spontaneous activity through reduction of the neuronal input resistance and membrane time constants, thereby improving stimulus discrimination above noise [68]. The ability of tonic inhibition to change conductances in many neurons is assumed to require perisynaptic and extrasynaptic δ subunit-containing GABAA receptors, which are likely to be activated through fast-spiking, PV-expressing, and soma-inhibiting interneurons [69]. PV-expressing interneurons play a crucial role for hippocampal microcircuit formation and plasticity changes [70]. The significant loss of density of PV-immunopositive puncta in the IC and AC and the first evidence for declined PV levels associated with elevated Arc levels in the hippocampus of BDNFPax2-KO but not in BDNFTrkC-KO mice may suggest that a BDNF-modified driving force for auditory processing improves the baseline for mature circuit formation and plasticity changes along the entire ascending auditory network. This finding, moreover, suggests that the altered brain network activity shaped under the influence of BDNF in the lower parts of the auditory CNS or within the cochlea is a prerequisite for cortical maturation processes that occur with sensory experience [71–73]. The influence of dendritogenesis of PV-containing interneurons in the olfactory bulb [12], the auditory [8], visual [17, 74], and somatosensory cortex [11] on improved spectral and temporal cortical response properties [71–73] should be reconsidered in the context of these findings.
Peripheral BDNF Improves Sound Fidelity but Carries the Risk to Enhance Central Noise Following Its Loss After Injury
We observed here that suprathreshold ABR wave IV and CF threshold in IC neurons after AT were persistently more elevated in BDNFPax2-WT than in BDNFPax2-KO mice. This may be explained by a loss of auditory fiber characteristics that depend on BDNF and that are essential to maintain tonic inhibitory strength in the ascending pathways. As a result, spontaneous firing rates may be elevated within the affected frequency range without leading to sound-induced output as shown here in BDNFPax2-WT but not in BDNFPax2-KO mice.
A persistent reduction of suprathreshold amplitudes at the level of the IC has also been observed in traumatized rats with behaviorally tested tinnitus [38, 60] indicating that lost auditory nerve fiber activities that are typically maintained by BDNF in the lower parts of the auditory CNS or within the cochlea may be causally related to “phantom” noise. Previous observations that describe elevated intensity discrimination thresholds in tinnitus subjects with normal audiograms [75] may be re-examined in the context of a loss of signal-to-noise ratio in those frequency bands in which the BDNF-dependent driving force is lost. When the BDNF-dependent driving force is lost, the baseline levels required to allow the development of a normal adaptation of central brain responses after cochlear injury may be lost. In view of the current findings, a BDNF-dependent auditory driving force may thus be the prerequisite for adaptive responses and central gain [76] after sensory deprivation [74, 77, 78]. Central maladaptive hyperactivities in various brain disorders including pain, Alzheimer, or epilepsy [79–81] and their enigmatic relationship to BDNF may be revisited in the context of the current findings [20, 21, 80]. For these disorders, a critical loss of the BDNF-dependent driving force should be taken into account.
Acknowledgments
This work was supported by the Marie Curie Research Training Network CavNET MRTN-CT-2006-035367, the Deutsche Forschungsgemeinschaft DFG-Kni-316-4-1, and the Hahn Stiftung (Index AG).
Abbreviations
- ABR
Auditory brainstem response
- AC
Auditory cortex
- Arc
Activity-regulated cytoskeletal protein
- AT
Acoustic trauma
- BBN
Broadband noise
- BDNF
Brain-derived nerve growth factor
- CF
Characteristic frequency
- CN
Cochlear nucleus
- DCN
Dorsal cochlear nucleus
- DPOAE
Distortion product of the otoacoustic emission
- IC
Inferior colliculus
- IHC
Inner hair cell
- KO
Knockout
- mFSL
Minimum first-spike latency
- OHC
Outer hair cells
- PV
Parvalbumin
- RIF
Rate-intensity function
- ROSA26R
ROSA26 reporter mouse
- SGN
Spiral ganglion neuron
- SPL
Sound pressure level
- VCN
Ventral cochlear nucleus
- WT
Wild type
- β-gal
β-Galactosidase
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no competing interests.
Footnotes
Tetyana Chumak and Lukas Rüttiger contributed equally to this work.
References
- 1.Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 2.Barde YA. The nerve growth factor family. Prog Growth Factor Res. 1990;2:237–248. doi: 10.1016/0955-2235(90)90021-B. [DOI] [PubMed] [Google Scholar]
- 3.Rauskolb S, Zagrebelsky M, Dreznjak A, Deogracias R, Matsumoto T, Wiese S, Erne B, Sendtner M, Schaeren-Wiemers N, Korte M, et al. Global deprivation of brain-derived neurotrophic factor in the CNS reveals an area-specific requirement for dendritic growth. J Neurosci. 2010;30:1739–1749. doi: 10.1523/JNEUROSCI.5100-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bramham CR, Panja D. BDNF regulation of synaptic structure, function, and plasticity. Neuropharmacology. 2014;76 Pt C:601–602. doi: 10.1016/j.neuropharm.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Maffei A, Lambo ME, Turrigiano GG. Critical period for inhibitory plasticity in rodent binocular V1. J Neurosci. 2010;30:3304–3309. doi: 10.1523/JNEUROSCI.5340-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maffei A, Turrigiano G. The age of plasticity: developmental regulation of synaptic plasticity in neocortical microcircuits. Prog Brain Res. 2008;169:211–223. doi: 10.1016/S0079-6123(07)00012-X. [DOI] [PubMed] [Google Scholar]
- 7.Kellner Y, Godecke N, Dierkes T, Thieme N, Zagrebelsky M, Korte M. The BDNF effects on dendritic spines of mature hippocampal neurons depend on neuronal activity. Front Synaptic Neurosci. 2014;6:5. doi: 10.3389/fnsyn.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu H, Kotak VC, Sanes DH. Normal hearing is required for the emergence of long-lasting inhibitory potentiation in cortex. J Neurosci. 2010;30:331–341. doi: 10.1523/JNEUROSCI.4554-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kotak VC, Pendola LM, Rodriguez-Contreras A. Spontaneous activity in the developing gerbil auditory cortex in vivo involves GABAergic transmission. Neuroscience. 2012;226:130–144. doi: 10.1016/j.neuroscience.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao M, Maynard KR, Chokshi V, Song L, Jacobs C, Wang H, Tran T, Martinowich K, Lee HK. Rebound potentiation of inhibition in juvenile visual cortex requires vision-induced BDNF expression. J Neurosci. 2014;34:10770–10779. doi: 10.1523/JNEUROSCI.5454-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiao Y, Zhang Z, Zhang C, Wang X, Sakata K, Lu B, Sun QQ. A key mechanism underlying sensory experience-dependent maturation of neocortical GABAergic circuits in vivo. Proc Natl Acad Sci U S A. 2011;108:12131–12136. doi: 10.1073/pnas.1105296108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berghuis P, Agerman K, Dobszay MB, Minichiello L, Harkany T, Ernfors P. Brain-derived neurotrophic factor selectively regulates dendritogenesis of parvalbumin-containing interneurons in the main olfactory bulb through the PLCg pathway. J Neurobiol. 2006;66:1437–1451. doi: 10.1002/neu.20319. [DOI] [PubMed] [Google Scholar]
- 13.Griffen TC, Maffei A. GABAergic synapses: their plasticity and role in sensory cortex. Front Cell Neurosci. 2014;8:91. doi: 10.3389/fncel.2014.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vogels TP, Froemke RC, Doyon N, Gilson M, Haas JS, Liu R, Maffei A, Miller P, Wierenga CJ, Woodin MA, et al. Inhibitory synaptic plasticity: spike timing-dependence and putative network function. Front Neural Circ. 2013;7:119. doi: 10.3389/fncir.2013.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singer W, Panford-Walsh R, Knipper M. The function of BDNF in the adult auditory system. Neuropharmacology. 2014;76 Pt C:719–728. doi: 10.1016/j.neuropharm.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Landi S, Cenni MC, Maffei L, Berardi N. Environmental enrichment effects on development of retinal ganglion cell dendritic stratification require retinal BDNF. PLoS One. 2007;2:e346. doi: 10.1371/journal.pone.0000346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landi S, Sale A, Berardi N, Viegi A, Maffei L, Cenni MC. Retinal functional development is sensitive to environmental enrichment: a role for BDNF. FASEB J. 2007;21:130–139. doi: 10.1096/fj.06-6083com. [DOI] [PubMed] [Google Scholar]
- 18.Ernfors P, Lee KF, Jaenisch R. Mice lacking brain-derived neurotrophic factor develop with sensory deficits. Nature. 1994;368:147–150. doi: 10.1038/368147a0. [DOI] [PubMed] [Google Scholar]
- 19.Jones KR, Farinas I, Backus C, Reichardt LF. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell. 1994;76:989–999. doi: 10.1016/0092-8674(94)90377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu B, Nagappan G, Guan X, Nathan PJ, Wren P. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat Rev Neurosci. 2013;14:401–416. doi: 10.1038/nrn3505. [DOI] [PubMed] [Google Scholar]
- 21.Lu B, Nagappan G, Lu Y. BDNF and synaptic plasticity, cognitive function, and dysfunction. Handb Exp Pharmacol. 2014;220:223–250. doi: 10.1007/978-3-642-45106-5_9. [DOI] [PubMed] [Google Scholar]
- 22.Sobkowicz HM, August BK, Slapnick SM. Influence of neurotrophins on the synaptogenesis of inner hair cells in the deaf Bronx waltzer (bv) mouse organ of Corti in culture. Int J Dev Neurosci. 2002;20:537–554. doi: 10.1016/S0736-5748(02)00084-9. [DOI] [PubMed] [Google Scholar]
- 23.Wan G, Gomez-Casati ME, Gigliello AR, Liberman MC, Corfas G (2014) Neurotrophin-3 regulates ribbon synapse density in the cochlea and induces synapse regeneration after acoustic trauma. Elife 3 [DOI] [PMC free article] [PubMed]
- 24.Ohyama T, Groves AK. Generation of Pax2-Cre mice by modification of a Pax2 bacterial artificial chromosome. Genesis. 2004;38:195–199. doi: 10.1002/gene.20017. [DOI] [PubMed] [Google Scholar]
- 25.Zuccotti A, Kuhn S, Johnson SL, Franz C, Singer W, Hecker D, Geisler HS, Köpschall I, Rohbock K, Gutsche K, et al. Lack of brain-derived neurotrophic factor hampers inner hair cell synapse physiology, but protects against noise-induced hearing loss. J Neurosci. 2012;32:8545–8553. doi: 10.1523/JNEUROSCI.1247-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fünfschilling U, Ng YG, Zang K, Miyazaki J, Reichardt LF, Rice FL. TrkC kinase expression in distinct subsets of cutaneous trigeminal innervation and nonneuronal cells. J Comp Neurol. 2004;480:392–414. doi: 10.1002/cne.20359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Šuta D, Popelář J, Syka J. Coding of communication calls in the subcortical and cortical structures of the auditory system. Physiol Res. 2008;57(Suppl 3):S149–S159. doi: 10.33549/physiolres.931608. [DOI] [PubMed] [Google Scholar]
- 28.Malmierca MS. The structure and physiology of the rat auditory system: an overview. Int Rev Neurobiol. 2003;56:147–211. doi: 10.1016/S0074-7742(03)56005-6. [DOI] [PubMed] [Google Scholar]
- 29.Rios M, Fan G, Fekete C, Kelly J, Bates B, Kuehn R, Lechan RM, Jaenisch R. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol Endocrinol. 2001;15:1748–1757. doi: 10.1210/mend.15.10.0706. [DOI] [PubMed] [Google Scholar]
- 30.Tan J, Rüttiger L, Panford-Walsh R, Singer W, Schulze H, Kilian SB, Hadjab S, Zimmermann U, Köpschall I, Rohbock K, et al. Tinnitus behavior and hearing function correlate with the reciprocal expression patterns of BDNF and Arg3.1/arc in auditory neurons following acoustic trauma. Neuroscience. 2007;145:715–726. doi: 10.1016/j.neuroscience.2006.11.067. [DOI] [PubMed] [Google Scholar]
- 31.Knipper M, Zinn C, Maier H, Praetorius M, Rohbock K, Köpschall I, Zimmermann U. Thyroid hormone deficiency before the onset of hearing causes irreversible damage to peripheral and central auditory systems. J Neurophysiol. 2000;83:3101–3112. doi: 10.1152/jn.2000.83.5.3101. [DOI] [PubMed] [Google Scholar]
- 32.Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. San Diego: Academic; 2008. [Google Scholar]
- 33.Heidrych P, Zimmermann U, Kuhn S, Franz C, Engel J, Duncker SV, Hirt B, Pusch CM, Ruth P, Pfister M, et al. Otoferlin interacts with myosin VI: implications for maintenance of the basolateral synaptic structure of the inner hair cell. Hum Mol Genet. 2009;18:2779–2790. doi: 10.1093/hmg/ddp213. [DOI] [PubMed] [Google Scholar]
- 34.Engel J, Braig C, Rüttiger L, Kuhn S, Zimmermann U, Blin N, Sausbier M, Kalbacher H, Munkner S, Rohbock K, et al. Two classes of outer hair cells along the tonotopic axis of the cochlea. Neuroscience. 2006;143:837–849. doi: 10.1016/j.neuroscience.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 35.Dehmelt L, Halpain S. The MAP2/Tau family of microtubule-associated proteins. Genome Biol. 2005;6:204. doi: 10.1186/gb-2004-6-1-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winter H, Braig C, Zimmermann U, Geisler HS, Franzer JT, Weber T, Ley M, Engel J, Knirsch M, Bauer K, et al. Thyroid hormone receptors TRa1 and TRb differentially regulate gene expression of Kcnq4 and prestin during final differentiation of outer hair cells. J Cell Sci. 2006;119:2975–2984. doi: 10.1242/jcs.03013. [DOI] [PubMed] [Google Scholar]
- 37.Heidrych P, Zimmermann U, Bress A, Pusch CM, Ruth P, Pfister M, Knipper M, Blin N. Rab8b GTPase, a protein transport regulator, is an interacting partner of otoferlin, defective in a human autosomal recessive deafness form. Hum Mol Genet. 2008;17:3814–3821. doi: 10.1093/hmg/ddn279. [DOI] [PubMed] [Google Scholar]
- 38.Rüttiger L, Singer W, Panford-Walsh R, Matsumoto M, Lee SC, Zuccotti A, Zimmermann U, Jaumann M, Rohbock K, Xiong H, et al. The reduced cochlear output and the failure to adapt the central auditory response causes tinnitus in noise exposed rats. PLoS One. 2013;8:e57247. doi: 10.1371/journal.pone.0057247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burkard RF, Don M. The auditory brainstem response. In: Burkard RF, Don M, Eggemond JJ, editors. Auditory evoked potentials: basic principles and clinical application. Baltimore: Lippincott Williams & Wilkins; 2007. pp. 229–250. [Google Scholar]
- 40.Grécová J, Bureš Z, Popelář J, Suta D, Syka J. Brief exposure of juvenile rats to noise impairs the development of the response properties of inferior colliculus neurons. Eur J Neurosci. 2009;29:1921–1930. doi: 10.1111/j.1460-9568.2009.06739.x. [DOI] [PubMed] [Google Scholar]
- 41.Konrad-Martin DL, Rübsamen R, Dörrscheidt GJ, Rubel EW. Development of single- and two-tone responses of anteroventral cochlear nucleus neurons in gerbil. Hear Res. 1998;121:35–52. doi: 10.1016/S0378-5955(98)00063-X. [DOI] [PubMed] [Google Scholar]
- 42.Kopp-Scheinpflug C, Dehmel S, Dörrscheidt GJ, Rübsamen R. Interaction of excitation and inhibition in anteroventral cochlear nucleus neurons that receive large endbulb synaptic endings. J Neurosci. 2002;22:11004–11018. doi: 10.1523/JNEUROSCI.22-24-11004.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spirou GA, Davis KA, Nelken I, Young ED. Spectral integration by type II interneurons in dorsal cochlear nucleus. J Neurophysiol. 1999;82:648–663. doi: 10.1152/jn.1999.82.2.648. [DOI] [PubMed] [Google Scholar]
- 44.Egorova M, Ehret G, Vartanian I, Esser KH. Frequency response areas of neurons in the mouse inferior colliculus. I. Threshold and tuning characteristics. Exp Brain Res. 2001;140:145–161. doi: 10.1007/s002210100786. [DOI] [PubMed] [Google Scholar]
- 45.Lu Y, Jen PH. GABAergic and glycinergic neural inhibition in excitatory frequency tuning of bat inferior collicular neurons. Exp Brain Res. 2001;141:331–339. doi: 10.1007/s002210100885. [DOI] [PubMed] [Google Scholar]
- 46.Sugimoto S, Hosokawa Y, Horikawa J, Nasu M, Taniguchi I. Spatial focusing of neuronal responses induced by asynchronous two-tone stimuli in the guinea pig auditory cortex. Cereb Cortex. 2002;12:506–514. doi: 10.1093/cercor/12.5.506. [DOI] [PubMed] [Google Scholar]
- 47.Bureš Z, Grécová J, Popelář J, Syka J. Noise exposure during early development impairs the processing of sound intensity in adult rats. Eur J Neurosci. 2010;32:155–164. doi: 10.1111/j.1460-9568.2010.07280.x. [DOI] [PubMed] [Google Scholar]
- 48.Babcock AA, Kuziel WA, Rivest S, Owens T. Chemokine expression by glial cells directs leukocytes to sites of axonal injury in the CNS. J Neurosci. 2003;23:7922–7930. doi: 10.1523/JNEUROSCI.23-21-07922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rüttiger L, Sausbier M, Zimmermann U, Winter H, Braig C, Engel J, Knirsch M, Arntz C, Langer P, Hirt B, et al. Deletion of the Ca2+-activated potassium (BK) a-subunit but not the BKb1-subunit leads to progressive hearing loss. Proc Natl Acad Sci U S A. 2004;101:12922–12927. doi: 10.1073/pnas.0402660101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schimmang T, Tan J, Müller M, Zimmermann U, Rohbock K, Köpschall I, Limberger A, Minichiello L, Knipper M. Lack of BDNF and TrkB signalling in the postnatal cochlea leads to a spatial reshaping of innervation along the tonotopic axis and hearing loss. Development. 2003;130:4741–4750. doi: 10.1242/dev.00676. [DOI] [PubMed] [Google Scholar]
- 51.Turrigiano G. Homeostatic signaling: the positive side of negative feedback. Curr Opin Neurobiol. 2007;17:318–324. doi: 10.1016/j.conb.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 52.Leto K, Rolando C, Rossi F. The genesis of cerebellar GABAergic neurons: fate potential and specification mechanisms. Front Neuroanat. 2012;6:6. doi: 10.3389/fnana.2012.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heil P, Neubauer H, Brown M, Irvine DR. Towards a unifying basis of auditory thresholds: distributions of the first-spike latencies of auditory-nerve fibers. Hear Res. 2008;238:25–38. doi: 10.1016/j.heares.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 54.Hu H, Gan J, Jonas P. Interneurons. Fast-spiking, parvalbumin+ GABAergic interneurons: from cellular design to microcircuit function. Science. 2014;345:1255263. doi: 10.1126/science.1255263. [DOI] [PubMed] [Google Scholar]
- 55.Popelář J, Rybalko N, Burianová J, Schwaller B, Syka J. The effect of parvalbumin deficiency on the acoustic startle response and prepulse inhibition in mice. Neurosci Lett. 2013;553:216–220. doi: 10.1016/j.neulet.2013.08.042. [DOI] [PubMed] [Google Scholar]
- 56.Le Magueresse C, Monyer H. GABAergic interneurons shape the functional maturation of the cortex. Neuron. 2013;77:388–405. doi: 10.1016/j.neuron.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 57.Sale A, Berardi N, Spolidoro M, Baroncelli L, Maffei L (2010) GABAergic inhibition in visual cortical plasticity. Front Cell Neurosci 4 [DOI] [PMC free article] [PubMed]
- 58.Bramham CR, Alme MN, Bittins M, Kuipers SD, Nair RR, Pai B, Panja D, Schubert M, Soule J, Tiron A, et al. The Arc of synaptic memory. Exp Brain Res. 2010;200:125–140. doi: 10.1007/s00221-009-1959-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Panja D, Bramham CR. BDNF mechanisms in late LTP formation: a synthesis and breakdown. Neuropharmacology. 2014;76 Pt C:664–676. doi: 10.1016/j.neuropharm.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 60.Singer W, Zuccotti A, Jaumann M, Lee SC, Panford-Walsh R, Xiong H, Zimmermann U, Franz C, Geisler HS, Köpschall I, et al. Noise-induced inner hair cell ribbon loss disturbs central arc mobilization: a novel molecular paradigm for understanding tinnitus. Mol Neurobiol. 2013;47:261–279. doi: 10.1007/s12035-012-8372-8. [DOI] [PubMed] [Google Scholar]
- 61.Heimel JA, van Versendaal D, Levelt CN (2011) The role of GABAergic inhibition in ocular dominance plasticity. Neural Plast 2011:Article ID 391763 [DOI] [PMC free article] [PubMed]
- 62.Grant L, Yi E, Glowatzki E. Two modes of release shape the postsynaptic response at the inner hair cell ribbon synapse. J Neurosci. 2010;30:4210–4220. doi: 10.1523/JNEUROSCI.4439-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Knipper M, Panford-Walsh R, Singer W, Rüttiger L, Zimmermann U. Specific synaptopathies diversify brain responses and hearing disorders: you lose the gain from early life. Cell Tissue Res. 2015;361:77–93. doi: 10.1007/s00441-015-2168-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Landi S, Ciucci F, Maffei L, Berardi N, Cenni MC. Setting the pace for retinal development: environmental enrichment acts through insulin-like growth factor 1 and brain-derived neurotrophic factor. J Neurosci. 2009;29:10809–10819. doi: 10.1523/JNEUROSCI.1857-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hage SR, Ehret G. Mapping responses to frequency sweeps and tones in the inferior colliculus of house mice. Eur J Neurosci. 2003;18:2301–2312. doi: 10.1046/j.1460-9568.2003.02945.x. [DOI] [PubMed] [Google Scholar]
- 66.Hernández O, Espinosa N, Perez-Gonzalez D, Malmierca MS. The inferior colliculus of the rat: a quantitative analysis of monaural frequency response areas. Neuroscience. 2005;132:203–217. doi: 10.1016/j.neuroscience.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 67.Duguid I, Branco T, London M, Chadderton P, Hausser M. Tonic inhibition enhances fidelity of sensory information transmission in the cerebellar cortex. J Neurosci. 2012;32:11132–11143. doi: 10.1523/JNEUROSCI.0460-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Caraiscos VB, Elliott EM, You-Ten KE, Cheng VY, Belelli D, Newell JG, Jackson MF, Lambert JJ, Rosahl TW, Wafford KA, et al. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by a5 subunit-containing g-aminobutyric acid type A receptors. Proc Natl Acad Sci U S A. 2004;101:3662–3667. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ferando I, Mody I. In vitro gamma oscillations following partial and complete ablation of d subunit-containing GABA receptors from parvalbumin interneurons. Neuropharmacology. 2015;88:91–98. doi: 10.1016/j.neuropharm.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jonas P, Buzsaki G. Neural inhibition. Scholarpedia. 2007;2(9):3286. doi: 10.4249/scholarpedia.3286. [DOI] [Google Scholar]
- 71.Carcea I, Froemke RC. Cortical plasticity, excitatory-inhibitory balance, and sensory perception. Prog Brain Res. 2013;207:65–90. doi: 10.1016/B978-0-444-63327-9.00003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Han YK, Köver H, Insanally MN, Semerdjian JH, Bao S. Early experience impairs perceptual discrimination. Nat Neurosci. 2007;10:1191–1197. doi: 10.1038/nn1941. [DOI] [PubMed] [Google Scholar]
- 73.Kilgard MP, Pandya PK, Engineer ND, Moucha R. Cortical network reorganization guided by sensory input features. Biol Cybern. 2002;87:333–343. doi: 10.1007/s00422-002-0352-z. [DOI] [PubMed] [Google Scholar]
- 74.Nahmani M, Turrigiano GG. Adult cortical plasticity following injury: recapitulation of critical period mechanisms? Neuroscience. 2014;283:4–16. doi: 10.1016/j.neuroscience.2014.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Epp B, Hots J, Verhey JL, Schaette R. Increased intensity discrimination thresholds in tinnitus subjects with a normal audiogram. J Acoust Soc Am. 2012;132:EL196–EL201. doi: 10.1121/1.4740462. [DOI] [PubMed] [Google Scholar]
- 76.Knipper M, Van Dijk P, Nunes I, Rüttiger L, Zimmermann U. Advances in the neurobiology of hearing disorders: recent developments regarding the basis of tinnitus and hyperacusis. Prog Neurobiol. 2013;111:17–33. doi: 10.1016/j.pneurobio.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 77.Mandolesi G, Menna E, Harauzov A, von Bartheld CS, Caleo M, Maffei L. A role for retinal brain-derived neurotrophic factor in ocular dominance plasticity. Curr Biol. 2005;15:2119–2124. doi: 10.1016/j.cub.2005.10.045. [DOI] [PubMed] [Google Scholar]
- 78.Anomal R, de Villers-Sidani E, Merzenich MM, Panizzutti R. Manipulation of BDNF signaling modifies the experience-dependent plasticity induced by pure tone exposure during the critical period in the primary auditory cortex. PLoS One. 2013;8:e64208. doi: 10.1371/journal.pone.0064208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Scharfman HE, Chao MV. The entorhinal cortex and neurotrophin signaling in Alzheimer’s disease and other disorders. Cogn Neurosci. 2013;4:123–135. doi: 10.1080/17588928.2013.826184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smith PA. BDNF: no gain without pain? Neuroscience. 2014;283:107–123. doi: 10.1016/j.neuroscience.2014.05.044. [DOI] [PubMed] [Google Scholar]
- 81.Song JH, Yu JT, Tan L. Brain-derived neurotrophic factor in Alzheimer’s disease: risk, mechanisms, and therapy. Mol Neurobiol. 2015;52(3):1477–1493. doi: 10.1007/s12035-014-8958-4. [DOI] [PubMed] [Google Scholar]