Abstract
OBJECTIVE:
To examine rates of recommended of testing and prophylaxis for chlamydia, gonorrhea, and pregnancy in adolescents diagnosed with sexual assault across pediatric emergency departments (EDs) and to determine whether specialized sexual assault pathways and teams are associated with performance of recommended testing and prophylaxis.
METHODS:
In this retrospective study of 12- to 18-year-old adolescents diagnosed with sexual assault at 38 EDs in the Pediatric Hospital Information System database from 2004 to 2013, information regarding routine practice for sexual assault evaluations and presence and year of initiation of specialized ED sexual assault pathways and teams was collected via survey. We examined across-hospital variation and identified patient- and hospital-level factors associated with testing and prophylaxis using logistic regression models, accounting for clustering by hospital.
RESULTS:
Among 12 687 included cases, 93% were female, 79% were <16 years old, 34% were non-Hispanic white, 38% were non-Hispanic black, 21% were Hispanic, and 52% had public insurance. Overall, 44% of adolescents received recommended testing (chlamydia, gonorrhea, pregnancy) and 35% received recommended prophylaxis (chlamydia, gonorrhea, emergency contraception). Across EDs, unadjusted rates of testing ranged from 6% to 89%, and prophylaxis ranged from 0% to 57%. Presence of a specialized sexual assault pathway was associated with increased rates of prophylaxis even after adjusting for case-mix and temporal trends (odds ratio 1.46, 95% confidence interval 1.15 to 1.86).
CONCLUSIONS:
Evaluation and treatment of adolescent sexual assault victims varied widely across pediatric EDs. Adolescents cared for in EDs with specialized sexual assault pathways were more likely to receive recommended prophylaxis.
What’s Known on This Subject:
National guidelines recommend testing and prophylaxis for chlamydia, gonorrhea, and pregnancy for adolescent sexual assault victims. Little is known about rates of testing and prophylaxis in adolescent victims of sexual assault evaluated in pediatric emergency departments.
What This Study Adds:
There is significant variation in testing and prophylaxis practices in the pediatric emergency department evaluation of adolescent victims of sexual assault. Adolescents cared for in emergency departments with clinical pathways are more likely to receive recommended prophylaxis.
Each year, ∼1 in 100 children experiences some form of sexual abuse, resulting in the sexual victimization of 12% to 25% of girls and 8% to 10% of boys by 18 years of age. 1 , 2 Adolescents are particularly vulnerable to sexual assault, defined as sexual contact with or without penetration that occurs because of physical force or psychological coercion or without consent. 3 According to the 2009 Youth Risk Behavior Surveillance Survey, 10.5% of female high school students and 4.5% of male high school students reported being sexually assaulted. 4
Sexual assault victims are at risk for contracting sexually transmitted infections (STIs). Accordingly, the American Academy of Pediatrics (AAP) 3 , 5 – 9 and the Centers for Disease Control and Prevention (CDC) 10 , 11 have published recommendations for testing and prophylaxis of sexually assaulted adolescents. Regardless of time since assault, the AAP and CDC recommend testing for chlamydia and gonorrhea in all adolescent patients and assessment for trichomoniasis in females. 3 , 5 – 11 In addition, the CDC suggests hepatitis B testing in unimmunized victims and consideration of HIV and syphilis testing in populations in which there is a high incidence of infection or when the victim wishes for these tests to be performed. 10 , 11
Because of possible preexisting asymptomatic infection, potential risk of acquisition of new infections from the assault, and substantial risk of pelvic inflammatory disease in this age group, prophylaxis for chlamydia and gonorrhea is recommended by the AAP and CDC for all adolescents evaluated within 72 hours of the assault. 3 , 5 – 11 The CDC also recommends prophylaxis for trichomoniasis. 11 , 12 Deferring antimicrobial treatment pending positive test results is discouraged because compliance with follow-up is poor. 12 , 13
In addition, all postpubertal female victims of sexual assault should be tested for pregnancy. According to the AAP, emergency contraception should be offered to female sexual assault victims evaluated within 120 hours of the assault. Given its excellent safety profile, emergency contraception should be offered even if the adolescent is unsure whether penetration occurred. 3
Although studies have found that patients treated in adult EDs after sexual assault often do not receive the recommended testing or prophylaxis, little is known about these practices in pediatric EDs. 14 – 16 Given the complexities of caring for sexual assault victims, many pediatric EDs have developed clinical pathways and specialized teams to assist with medical and forensic evaluation. Although teams have shown promise in small single-center studies, the impact of sexual assault clinical pathways on care is unknown. 17 – 19
Therefore, we aimed to describe and compare testing and prophylaxis practices among adolescents diagnosed with sexual assault across 38 pediatric EDs. In addition, we examined whether the presence of specialized sexual assault evaluation pathways or teams is associated with increased testing and/or prophylaxis in this population. We hypothesized that the implementation of clinical care pathways and the presence of specialized teams would be associated with an increase in testing and prophylaxis rates. 20 , 21
Methods
Overview
We performed a retrospective study of adolescents diagnosed with sexual assault at 38 EDs in the Pediatric Hospital Information System (PHIS) database to examine the association between recommended testing and prophylaxis performance with pathways and teams. Survey of the EDs and validation of the accuracy of International Classification of Diseases, Revision 9, Clinical Modification (ICD-9-CM) diagnosis codes and billing codes in PHIS to study this population were also conducted.
Data Sources
PHIS
We used the PHIS database, which includes demographic and clinical data from children discharged from 48 children’s hospitals. Included data are deidentified and subjected to rigorous reliability and validity checks. 22 Children 12 to 18 years of age discharged between January 1, 2004, and December 31, 2013, with an ICD-9-CM diagnosis code of 995.53 (child sexual abuse), E.960.1 (rape), or V71.5 (observation after rape) were eligible for inclusion. We focused on adolescents because, although universal testing and prophylactic treatment of postpubertal patients is recommended, more selective criteria are used for prepubertal patients. 7 Thirty-eight hospitals contributed ED administrative data to PHIS and met study inclusion criteria during the study period.
Hospital Survey
We surveyed ED and child abuse physicians from these 38 EDs to determine the presence and year of initiation of specialized sexual assault clinical pathways or teams. The survey included questions regarding ED-specific practices for testing and prophylaxis in adolescents evaluated for sexual assault (Supplemental Appendix 1). The primary investigator (SS) contacted the ED or child abuse team at each hospital, described the survey purpose, and requested to be connected with the most appropriate individual to respond to the survey.
Outcomes
Testing
Laboratory testing was determined by using PHIS-specific Clinical Transaction Classification (CTC) codes (Supplemental Appendix 2). A single dichotomous variable for recommended testing included testing for chlamydia, gonorrhea, and pregnancy if female. Although the AAP and CDC recommend trichomoniasis testing in female adolescent victims of sexual assault, we elected not to include it because the recommendations are not as strong, a gold standard test was unavailable during the study period, and the sensitivity of the trichomoniasis variable was poor in the validation substudy (Supplemental Appendix 3). 23 – 25
Prophylaxis
Medication exposure was determined from pharmacy billing data (Supplemental Appendix 2). A single dichotomous variable for recommended prophylaxis included chlamydia prophylaxis, gonorrhea prophylaxis, and emergency contraception if female. Prophylaxis against HIV was not included because although it may be offered after an acute assault, additional patient and alleged perpetrator information is considered in clinical decision-making. 8 – 11
Primary Independent Variables
The primary independent variables were presence of pathway or team, as determined by the ED survey, and could change over time depending on year of initiation.
Covariates
Patient-level covariates included age in years, sex, race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, other), insurance (private, public, uninsured, other), and discharge year. Hospital region (midwest, northeast, south, west) was included as a hospital-level covariate.
Validation Substudy
We retrospectively reviewed medical records of a random sample of children from 4 of the participating PHIS hospitals for the following information: diagnosis; time since assault; testing for chlamydia, gonorrhea, trichomoniasis, and pregnancy; treatment of chlamydia, gonorrhea, and trichomoniasis; and emergency contraception. The hospitals included in the substudy were geographically distinct and demonstrated a range of performance in the primary outcomes of interest.
Statistical Methods
Unadjusted rates of testing and prophylaxis were calculated for each hospital. Descriptive statistics were used to summarize patient-level and hospital-level characteristics as well as the primary predictor variables (pathway, team). Next, we performed logistic regression to examine the association of pathway and team with testing. Hospital region and the following patient-level covariates were included in the model: age, sex, race/ethnicity, insurance, and discharge year. Age was included as a categorical variable, with ages 16, 17, and 18 combined into a single category because of smaller numbers of patients of these ages. We repeated the analysis using prophylaxis as the outcome. Robust variance estimators were used to account for clustering of patients within hospitals. 26 Results are presented as odds ratios (ORs) and marginal probabilities calculated from the multivariable models. Because varying time cutoffs were used for the prophylaxis outcomes, a sensitivity analysis was performed to examine the association of pathway and team with chlamydia prophylaxis, gonorrhea prophylaxis, and emergency contraception.
For the validation substudy, positive predictive value was calculated for the ICD-9-CM diagnosis codes. Hospital-specific and overall sensitivity, specificity, positive predictive values, and negative predictive values were calculated for each testing and medication variable. We used frequency weights such that the overall sample represented all subjects from the 4 included hospitals. Time since assault and the survey results were summarized using descriptive statistics. All analyses were conducted using Stata 13.0 (Stata Corp, College Station, TX). The Children’s Hospital of Philadelphia Committee for the Protection of Human Subjects and Institutional Review Board approved this study.
Results
Study Population
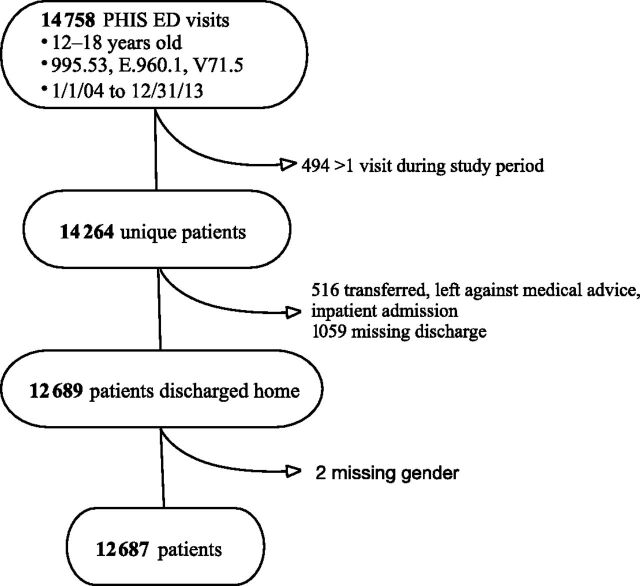
Of 14 758 visits across 38 hospitals meeting eligibility criteria, only the first visit per patient was included (n = 14 264) because our research question targeted care provided at the initial visit after assault. We also excluded 412 subjects who were transferred, 95 who left against medical advice, 8 who were admitted, 1 who died, 1059 with missing discharge status, and 2 with missing gender (Fig 1).
FIGURE 1.
Selection of study population.
Subject Characteristics
Among the 12 687 included cases, 93% were female, 52% had public insurance, 34% were non-Hispanic white, 38% were non-Hispanic black, and 21% were Hispanic (Tables 1 and 2). Overall, 30% presented to institutions with a clinical pathway and 64% to institutions with a specialized sexual assault evaluation team. Twenty-seven percent of patients presented to hospitals with both a pathway and a team (n = 3450), 3% to hospitals with a pathway only (n = 410), 37% to hospitals with a team only (n = 4680), and 33% to hospitals with neither a pathway nor a team (n = 4147).
TABLE 1.
Characteristics of Study Population (n = 12 687)
| Factor | n (%) |
|---|---|
| Hospital level | |
| Pathway | |
| Yes | 3860 (30) |
| No | 8827 (70) |
| Team | |
| Yes | 8130 (64) |
| No | 4557 (36) |
| Region | |
| Midwest | 3531 (28) |
| Northeast | 1173 (9) |
| South | 6966 (55) |
| West | 1017 (8) |
| Patient level | |
| Age, y | |
| 12 | 2077 (16) |
| 13 | 2735 (21) |
| 14 | 2870 (23) |
| 15 | 2371 (19) |
| 16–18 | 2634 (21) |
| Gender | |
| Female | 11 862 (93) |
| Male | 825 (7) |
| Race/ethnicity | |
| Non-Hispanic white | 4331 (34) |
| Non-Hispanic black | 4851 (38) |
| Hispanic | 2691 (21) |
| Other | 814 (7) |
| Insurance | |
| Public | 6616 (52) |
| Private | 3955 (31) |
| Uninsured | 1193 (10) |
| Other | 923 (7) |
TABLE 2.
Characteristics of Patient Population by Performance of Testing and Prophylaxis (n = 12 687)
| Factor | n (%) | |||
|---|---|---|---|---|
| Appropriate Testing | Appropriate Prophylaxis | |||
| Yes | No | Yes | No | |
| Hospital level | ||||
| Pathway | ||||
| Yes | 1893 (49) | 1967 (51) | 1653 (43) | 2207 (57) |
| No | 3712 (42) | 5115 (58) | 2734 (31) | 6093 (69) |
| Team | ||||
| Yes | 3777 (46) | 4353 (54) | 2828 (35) | 5302 (65) |
| No | 1828 (40) | 2729 (60) | 1559 (34) | 2998 (66) |
| Region | ||||
| Midwest | 1842 (52) | 1689 (48) | 1414 (40) | 2117 (60) |
| Northeast | 568 (48) | 605 (52) | 493 (42) | 680 (58) |
| South | 2774 (40) | 4192 (60) | 2193 (31) | 4773 (69) |
| West | 421 (41) | 596 (59) | 287 (28) | 730 (72) |
| Patient level | ||||
| Age, y | ||||
| 12 | 759 (37) | 1318 (63) | 501 (24) | 1576 (76) |
| 13 | 1164 (43) | 1571 (57) | 869 (32) | 1866 (68) |
| 14 | 1283 (45) | 1587 (55) | 1009 (35) | 1861 (65) |
| 15 | 1167 (49) | 1204 (51) | 994 (42) | 1377 (58) |
| 16–18 | 1232 (47) | 1402 (53) | 1014 (39) | 1620 (61) |
| Gender | ||||
| Female | 5266 (44) | 6596 (56) | 4212 (36) | 7650 (64) |
| Male | 339 (41) | 486 (59) | 175 (21) | 650 (79) |
| Race/ethnicity | ||||
| Non-Hispanic white | 1953 (45) | 2378 (55) | 1578 (36) | 2753 (64) |
| Non-Hispanic black | 2281 (47) | 2570 (53) | 1798 (37) | 3053 (63) |
| Hispanic | 1009 (38) | 1682 (62) | 695 (26) | 1996 (74) |
| Other | 362 (44) | 452 (56) | 316 (39) | 498 (61) |
| Insurance | ||||
| Private | 1737 (44) | 2218 (56) | 1333 (34) | 2622 (66) |
| Public | 2923 (44) | 3693 (56) | 2330 (35) | 4286 (65) |
| Uninsured | 568 (48) | 625 (52) | 448 (38) | 745 (62) |
| Other | 377 (41) | 546 (59) | 276 (30) | 647 (70) |
Reported System of Care
From our survey of 38 EDs, the majority (76%) reported that STI testing in adolescents was conducted regardless of time from assault (Table 3). Most EDs (63%) did not report withholding STI testing in consideration of future criminal proceedings, but 26% reported withholding STI testing at least some of the time.
TABLE 3.
Reported Time Cutoff Used for Testing and Prophylaxis
| Time, h | STI Testing | STI Prophylaxis | Emergency Contraception |
|---|---|---|---|
| ≤48 | 0 | 0 | 2 (5) |
| ≤72 | 3 (8) | 13 (34) | 10 (26) |
| ≤96 | 0 | 1 (3) | 2 (5) |
| ≤120 | 2 (5) | 5 (13) | 16 (42) |
| No cutoff | 29 (76) | 14 (37) | 4 (11) |
| No response | 4 (11) | 5 (13) | 4 (11) |
Values are expressed as n (%). Survey data from 38 EDs regarding time cutoff used for gonorrhea/chlamydia testing and prophylaxis and emergency contraception for adolescents evaluated in the ED for sexual assault.
Approximately one-third of hospitals reported using 72 hours as a cutoff for chlamydia/gonorrhea prophylaxis (Table 3). For emergency contraception, 42% used a cutoff of 120 hours. Most EDs (68% to 71%) reported administering prophylaxis and emergency contraception during the ED visit rather than providing a prescription for these medications (Table 4).
TABLE 4.
Reported Medication Prescribing and Administration Practices
| Indication | Administered During Visit | Provided Prescription | Administered During Visit and Provided Prescription | No Response |
|---|---|---|---|---|
| Chlamydia | 26 (68) | 0 | 5 (13) | 7 (18) |
| Gonorrhea | 27 (71) | 0 | 4 (11) | 7 (18) |
| Emergency contraception | 27 (71) | 3 (8) | 1 (3) | 7 (18) |
Values are expressed as n (%). Survey data from 38 EDs regarding prescribing and administration practices for gonorrhea/chlamydia prophylaxis and emergency contraception for adolescents evaluated in the ED for sexual assault.
Testing
In the unadjusted analysis, 44% (5605) of adolescents received recommended testing (chlamydia, gonorrhea, pregnancy). Performance of all recommended testing ranged from 6% to 89% across hospitals (Fig 2). Rates of chlamydia testing (14% to 92%) and gonorrhea testing (8% to 92%) ranged widely across hospitals. Pregnancy testing among females ranged from 15% to 95%. In the adjusted model, there were no associations between testing and any hospital-level factors including pathway or team presence (Table 5).
FIGURE 2.
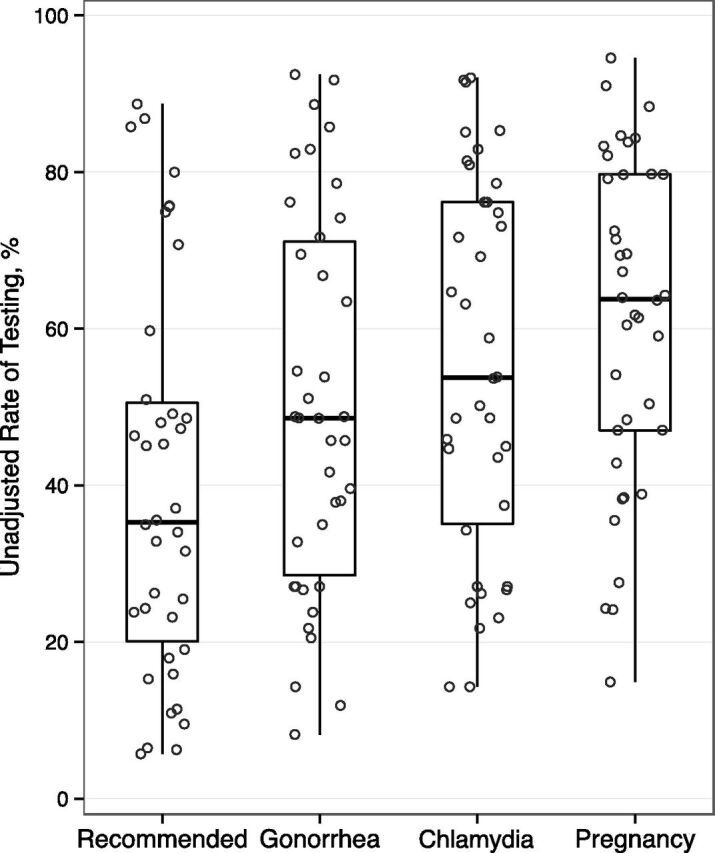
Variation in percentage of adolescents receiving testing across hospitals. Circles represent the rate of testing at each hospital. Box plots summarize the distribution across hospitals: median, interquartile range (25th and 75th percentiles), and range (minimum and maximum). Recommended testing included testing for gonorrhea and chlamydia in all patients and testing for pregnancy in female patients.
TABLE 5.
Association of Hospital-Level and Patient-Level Factors With Testing and Prophylaxis
| Factor | Testing | Prophylaxis | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Hospital level | ||||
| Pathway | 0.88 | 0.46–1.68 | 1.46 | 1.15–1.86 |
| Team | 1.30 | 0.63–2.69 | 0.83 | 0.61–1.12 |
| Region | ||||
| Midwest | Referent | Referent | Referent | Referent |
| Northeast | 0.76 | 0.25–2.35 | 1.04 | 0.69–1.56 |
| South | 0.61 | 0.29–1.26 | 0.77 | 0.55–1.08 |
| West | 0.71 | 0.23–2.18 | 0.69 | 0.43–1.10 |
| Patient level | ||||
| Age, y | ||||
| 12 | Referent | Referent | Referent | Referent |
| 13 | 1.28 | 1.15–1.43 | 1.45 | 1.32–1.60 |
| 14 | 1.39 | 1.20–1.61 | 1.67 | 1.39–2.00 |
| 15 | 1.62 | 1.34–1.97 | 2.12 | 1.63–2.76 |
| 16–18 | 1.47 | 1.18–1.83 | 1.86 | 1.45–2.40 |
| Gender | ||||
| Male | Referent | Referent | Referent | Referent |
| Female | 1.08 | 0.86–1.37 | 2.01 | 1.56–2.60 |
| Race/ethnicity | ||||
| Non-Hispanic white | Referent | Referent | Referent | Referent |
| Non-Hispanic black | 1.23 | 0.96–1.58 | 1.09 | 0.93–1.29 |
| Hispanic | 0.82 | 0.59–1.15 | 0.71 | 0.47–1.08 |
| Other | 0.96 | 0.72–1.28 | 1.11 | 0.92–1.35 |
| Insurance | ||||
| Private | Referent | Referent | Referent | Referent |
| Public | 0.92 | 0.72–1.18 | 0.95 | 0.82–1.09 |
| Uninsured | 1.19 | 0.85–1.67 | 1.10 | 0.76–1.59 |
| Other | 0.94 | 0.70–1.26 | 0.87 | 0.58–1.31 |
| Discharge year | 1.10 | 0.97–1.24 | 1.10 | 1.03–1.16 |
Results generated from logistic regression models accounting for clustering by hospital. Testing included testing for chlamydia, gonorrhea, and pregnancy if female. Prophylaxis included treatment of chlamydia and gonorrhea and emergency contraception if female. The year the patient was discharged from the ED (2004 to 2013) was included as a continuous variable.
Prophylaxis
In the unadjusted analysis, 35% (4387) of patients received recommended prophylaxis (chlamydia, gonorrhea, emergency contraception). Across EDs, unadjusted rates of recommended prophylaxis ranged from 0% to 57% (Fig 3). Chlamydia prophylaxis ranged from 0% to 74%, gonorrhea prophylaxis ranged from 0% to 70%, and emergency contraception among females ranged from 0% to 65%.
FIGURE 3.
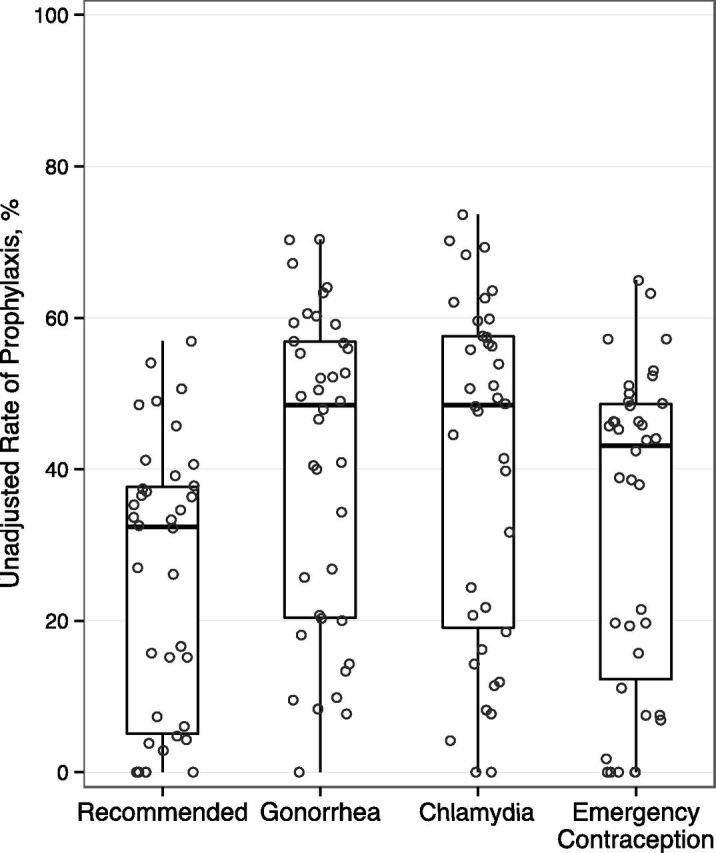
Variation in percentage of adolescents receiving prophylaxis across hospitals. Circles represent the rate of prophylaxis at each hospital. Box plots summarize the distribution across hospital: median, interquartile range (25th and 75th percentiles), and range (minimum and maximum). Recommended prophylaxis included treatment of gonorrhea and chlamydia in all patients and emergency contraception in female patients.
In the adjusted model, hospitals with pathways were almost 50% more likely to provide prophylaxis than hospitals without pathways (OR 1.46, 95% confidence interval [CI] 1.15 to 1.86), but specialized teams were not associated with prophylaxis rates (0.83, 0.61 to 1.12) (Table 5). After adjusting for patient and hospital characteristics, 40% (95% CI 36% to 45%) of patients received prophylaxis when a pathway was present compared with 32% (27% to 37%) of patients with no pathway. In the sensitivity analysis, the presence of a pathway was associated with increased odds of provision of each of the individual prophylaxis components (all P < .011) (Supplemental Appendix 4). The presence of a team was associated with decreased odds of gonorrhea prophylaxis, but this association was not present for other prophylaxis components.
Validation Substudy
The positive predictive value for the sexual assault ICD-9-CM codes by hospital was 93.7%, 90.0%, 90.0%, and 94.0%; for all hospitals combined it was 92.2%. The sensitivity and specificity of the CTC codes for testing were as follows: chlamydia 98.0% and 97.4%, gonorrhea 98.6% and 93.7%, pregnancy 90.4% and 92.0%. The sensitivity and specificity of the CTC codes for prophylaxis were as follows: chlamydia 99.1% and 91.2%, gonorrhea 89.8% and 91.9%, emergency contraception 96.0% and 86.1% (Supplemental Appendix 3). The majority (74%) of patients were evaluated within 72 hours of assault (Table 6).
TABLE 6.
Time Since Assault to ED Evaluation
| Time, h | Hospital 1 | Hospital 2 | Hospital 3 | Hospital 4 | Total |
|---|---|---|---|---|---|
| n | 239 | 50 | 50 | 50 | 389 |
| <72 h | 176 (74) | 29 (58) | 39 (78) | 42 (84) | 286 (74) |
| <120 h | 189 (79) | 34 (68) | 41(82) | 42 (84) | 306 (79) |
| ≥120 h | 44 (18) | 10 (20) | 5 (10) | 4 (8) | 63 (16) |
| Not available | 6 (3) | 6 (12) | 4 (8) | 4 (8) | 20 (5) |
Values are expressed as n (%). Patients were randomly selected from 4 hospitals.
Discussion
In this large, multicenter study of adolescents evaluated in pediatric EDs for sexual assault, we found substantial variation in rates of performance of recommended testing and prophylaxis across hospitals. Pathways were associated with higher rates of prophylaxis, but not testing. Teams were not associated with outcome performance. Our survey demonstrated variation in reported practice, although not to the degree observed in the patient-level data.
As reported in prior studies, 16 we found that STI prophylaxis was more common in females. Perhaps because boys are more likely than girls to make delayed disclosures, boys may have been more likely to present for care outside of the recommended timeframe (72 hours) for prophylaxis. 27 It is also possible that the younger boys in the study were more likely to be prepubertal compared with the younger girls, and therefore clinicians may have opted not to treat them based on the adolescent guidelines. However, there could also be a difference in the clinician’s perception of risk of acquiring STIs among girls and boys. Rates of testing and prophylaxis were lower in younger patients, which may be because they were more likely to be prepubertal. We chose 12 for the lower age limit for our study, consistent with previous studies, 15 , 16 because on average, girls begin puberty at ages 10–11 and boys at 11–12. 28
Our results showed that clinical pathway presence was associated with modest improvement in hospital performance of recommended prophylaxis, even after adjusting for case mix and temporal trends. This finding is expected, as the goals of clinical pathways are to standardize care, improve outcomes, and reduce cost; pathways have proven successful in this regard for multiple pediatric conditions. 29 – 31 We were unable to review the clinical pathways directly, however, and pathway content and clinician uptake may have varied across centers. Future research could explore factors associated with pathway adherence such as clinical decision support embedded into electronic medical records or designated pathway champions.
The lack of association of care with specialized sexual assault teams may be because the teams captured in our data do not represent a standardized model of care but are heterogeneous in nature. 17 – 19 For instance, some but not all specialized teams require Sexual Assault Nurse Examiner certification. 20 Others require initial certification and additional ongoing training. At some hospitals, a small number of examiners see a large volume of cases; at others, many examiners each see a few cases per year. Some institutions practice a rigorous peer review process using the expertise of board-certified child abuse pediatricians, and others do not. Additional investigation into the impact of such specialized teams on quality of care for sexual assault victims will need to include more detail regarding team composition and training.
The observed variation in rates of testing and prophylaxis across hospitals suggests underlying differences in the clinical approach to the care of this population. Our survey found that most EDs did not use a time cutoff for STI testing in adolescents, yet only 44% of the adolescents in the study sample actually received the recommended testing. In spite of laws in all 50 states that limit the evidentiary use of a victim’s previous sexual history to protect the credibility of the victim’s testimony, 11 26% of the EDs endorsed not performing STI testing during the acute evaluation at least some of the time due to this concern. Most experts, including the AAP and CDC, favor universal screening of consenting adolescent victims of sexual assault to detect preexisting and new infections. Perhaps some clinicians reason that if prophylaxis is administered for chlamydia or gonorrhea, obtaining the test does not influence medical care and thus is not cost-effective. This reasoning ignores the importance of reporting communicable diseases and the opportunity for the sexually active adolescent to inform his or her partner of a positive test and refer them for treatment as well. Furthermore, if this practice were occurring, we would expect the overall rate of prophylaxis to be higher than the overall rate of testing, which was not the case (35% vs 44%).
Some EDs might provide outpatient prescriptions for prophylaxis rather than administering the medication during the visit, and this would not be captured in our data. However, almost 70% of the EDs reported administering all prophylaxis during the ED encounter and therefore cannot account for the overall prophylaxis rate of 35%.
Thus, whereas our survey provides some insight into practice variation in the medical evaluation of adolescent sexual assault victims, the reported differences are not fully explained by the observed differences. This suggests a knowledge-to-practice gap, and that clinician education and dissemination of existing guidelines could potentially lead to improvements in care. Future research may be helpful in understanding barriers to guideline implementation and interventions to improve adherence.
There are limitations to our study design. First, our data were limited to the ED setting, so we cannot be certain of any testing or prophylaxis provision before or after the ED visit. We tried to address this by including only patients discharged from the hospital; however, it is possible that some patients were discharged to child advocacy centers to complete the evaluation or received testing or treatment at outpatient follow-up visits, although this practice is not recommended. 12 , 13 In addition, we excluded 1059 patients because of missing discharge status. However, there was no evidence that these patients differed systematically from those included.
Second, unmeasured differences could exist in history and physical exam findings that influenced the decision to obtain testing or provide prophylaxis. In particular, time since assault is critical for decisions regarding prophylaxis administration, and some adolescents being evaluated in EDs for sexual assault may not require prophylaxis. However, our validation substudy suggests that this is not the case for the majority, as 74% of included adolescents presented within 72 hours and 79% presented within 120 hours. Other historical factors such as alleged perpetrator characteristics or the nature of the sexual contact were unavailable and might have influenced medical care. However, such historical characteristics are not considered in the AAP and CDC recommendations for testing or prophylaxis practices for adolescents after sexual assault. Misclassifications in the administrative data are possible because of miscoded or inaccurate ICD-9-CM diagnosis or billing codes. For this reason, we validated the diagnosis codes and laboratory and pharmacy CTC codes at 4 centers using chart review and demonstrated excellent sensitivity and specificity for the variables in this study. Finally, it is possible that our study was underpowered to detect differences in testing and prophylaxis treatment by presence of pathways or teams.
Conclusions
The evaluation and treatment of adolescent sexual assault victims varies significantly across pediatric EDs. Such variation raises concern over the quality of care for adolescents with sexual assault and highlights the importance of dissemination of guidelines and standardization of medical care for this vulnerable population. Our findings suggest that sexual assault pathways show promise in improving adherence to recommended treatments for this population, but further research is needed to better understand the role of pathways in improving quality of care.
Supplementary Material
Acknowledgments
We thank the following study collaborators who participated in acquisition of data: Hui-Fai Fong, MD, MSHP, Boston Children’s Hospital (Boston, MA); Daniel Lindberg, MD, Children’s Hospital Colorado (Aurora, CO); Benjamin Murphy, MD, Children’s Hospital Colorado (Aurora, CO); and Jonathan D. Thackeray, MD, Nationwide Children’s Hospital (Columbus, OH).
Glossary
- AAP
American Academy of Pediatrics
- CDC
Centers for Disease Control and Prevention
- CI
confidence interval
- CTC
Clinical Transaction Classification
- ED
emergency department
- ICD-9-CM
International Classification of Diseases, Revision 9, Clinical Modification
- OR
odds ratio
- PHIS
Pediatric Hospital Information System
- STI
sexually transmitted infection
Footnotes
Dr Schilling drafted the initial manuscript; Drs Samuels-Kalow, Gerber, Scribano, French, and Wood reviewed and revised the manuscript; and all authors contributed to conceptualization, design, and analysis, approved the final manuscript as submitted, and agree to be accountable for all aspects of the work.
FUNDING: Dr Wood received salary funding from the National Institute of Child Health and Human Development (grant 1K23HD071967).
COMPANION PAPER: A companion to this article can be found on page e1600, online at www.pediatrics.org/cgi/doi/10.1542/peds.2015-3346.
References
- 1.U.S. Department of Health and Human Services. Child Maltreatment 2012. Washington, DC: Administration on Children, Youth and Families, Children’s Bureau. Available at: www.acf.hhs.gov/sites/default/files/cb/cm2013.pdf. Accessed September 21, 2015
- 2. Finkelhor D . Current information on the scope and nature of child sexual abuse. Future Child. 1994;4(2):31–53 [PubMed] [Google Scholar]
- 3. Kaplan DW , Feinstein RA , Fisher MM , et al. ; Committee on Adolescence . Care of the adolescent sexual assault victim. Pediatrics. 2001;107(6). Available at: www.pediatrics.org/cgi/content/full/107/6/e1476 [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Youth risk behavior surveillance–United States 2009. Available at: www.cdc.gov/mmwr/preview/mmwrhtml/ss5905a1.htm. Accessed September 21, 2015
- 5. Kaufman M ; American Academy of Pediatrics Committee on Adolescence . Care of the adolescent sexual assault victim. Pediatrics. 2008;122(2). Available at: www.pediatrics.org/cgi/content/full/122/2/e462 [DOI] [PubMed] [Google Scholar]
- 6. American Academy of Pediatrics Committee on Child Abuse and Neglect . Guidelines for the evaluation of sexual abuse of children: subject review. Pediatrics. 1999;103(1). Available at: www.pediatrics.org/cgi/content/full/103/1/e186 [DOI] [PubMed] [Google Scholar]
- 7. Kellogg N ; American Academy of Pediatrics Committee on Child Abuse and Neglect . The evaluation of sexual abuse in children. Pediatrics. 2005;116(2). Available at: www.pediatrics.org/cgi/content/full/116/2/e506 [DOI] [PubMed] [Google Scholar]
- 8. American Academy of Pediatrics . Sexually transmitted diseases. In: Pickering LK , ed. Red Book: 2003 Report of the Committee of Infectious Diseases, 29th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2012:181–185 [Google Scholar]
- 9. American Academy of Pediatrics . Sexually transmitted infections. In: Pickering LK , ed. Red Book: 2012 Report of the Committee of Infectious Diseases, 26th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2003:157–167 [Google Scholar]
- 10. Centers for Disease Control and Prevention . 1998 guidelines for treatment of sexually transmitted diseases. MMWR Recomm Rep. 1998;47(RR1):1–111 [PubMed] [Google Scholar]
- 11. Centers for Diseases Control and Prevention . Sexually transmitted disease treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR12):90–93 [Google Scholar]
- 12. Ackerman DR , Sugar NF , Fine DN , Eckert LO . Sexual assault victims: factors associated with follow-up care. Am J Obstet Gynecol. 2006;194(6):1653–1659 [DOI] [PubMed] [Google Scholar]
- 13. Parekh V , Brown CB . Follow up of patients who have been recently sexually assaulted. Sex Transm Infect. 2003;79(4):349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Straight JD , Heaton PC . Emergency department care for victims of sexual offense. Am J Health Syst Pharm. 2007;64(17):1845–1850 [DOI] [PubMed] [Google Scholar]
- 15. Rovi S , Shimoni N . Prophylaxis provided to sexual assault victims seen at US emergency departments. J Am Med Womens Assoc. 2002;57(4):204–207 [PubMed] [Google Scholar]
- 16. Merchant RC , Kelly ET , Mayer KH , Becker BM , Duffy SJ , Pugatch DL . Compliance in Rhode Island emergency departments with American Academy of Pediatrics recommendations for adolescent sexual assaults. Pediatrics. 2008;121(6). Available at: www.pediatrics.org/cgi/content/full/121/6/e1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bechtel K , Ryan E , Gallagher D . Impact of sexual assault nurse examiners on the evaluation of sexual assault in a pediatric emergency department. Pediatr Emerg Care. 2008;24(7):442–447 [DOI] [PubMed] [Google Scholar]
- 18. Sampsel K , Szobota L , Joyce D , Graham K , Pickett W . The impact of a sexual assault/domestic violence program on ED care. J Emerg Nurs. 2009;35(4):282–289 [DOI] [PubMed] [Google Scholar]
- 19. Goyal MK , Mollen CJ , Hayes KL , et al. Enhancing the emergency department approach to pediatric sexual assault care: implementation of a pediatric sexual assault response team program. Pediatr Emerg Care. 2013;29(9):969–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sexual assault nurse examiner development and operation guide. Washington, DC: US Department of Justice, Office for Victims of Crime. Available at: www.ncjrs.gov/ovc_archives/reports/saneguide.pdf. Accessed September 21, 2015
- 21. Bergman DA . Evidence-based guidelines and critical pathways for quality improvement. Pediatrics. 1999;103(1 suppl E):225–232 [PubMed] [Google Scholar]
- 22. Fletcher DM . Achieving data quality. How data from a pediatric health information system earns the trust of its users. J AHIMA. 2004;75(10):22–26 [PubMed] [Google Scholar]
- 23. Hammerschlag MR , Gaydos CA . Guidelines for the use of molecular biological methods to detect sexually transmitted pathogens in cases of suspected sexual abuse in children. Methods Mol Biol. 2012;903:307–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gallion HR , Dupree LJ , Scott TA , Arnold DH . Diagnosis of Trichomonas vaginalis in female children and adolescents evaluated for possible sexual abuse: a comparison of the InPouch TV culture method and wet mount microscopy. J Pediatr Adolesc Gynecol. 2009;22(5):300–305 [DOI] [PubMed] [Google Scholar]
- 25. Adams JA , Kellogg ND , Farst KJ , et al. Updated guidelines for the medical assessment and care of children who may have been sexually abused [published online ahead of print February 11, 2015]. J Pediatr Adolesc Gynecol. 10.1016/j.jpag.2015.01.007 [DOI] [PubMed] [Google Scholar]
- 26. French B , Heagerty PJ . Analysis of longitudinal data to evaluate a policy change. Stat Med. 2008;27(24):5005–5025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. O’Leary PJ , Barber J . Gender differences in silencing following childhood sexual abuse. J Child Sex Abuse. 2008;17(2):133–143 [DOI] [PubMed] [Google Scholar]
- 28. Euling SY , Herman-Giddens ME , Lee PA , et al. Examination of US puberty-timing data from 1940 to 1994 for secular trends: panel findings. Pediatrics. 2008;121(suppl 3):S172–S191 [DOI] [PubMed] [Google Scholar]
- 29. Todd J , Bertoch D , Dolan S . Use of a large national database for comparative evaluation of the effect of a bronchiolitis/viral pneumonia clinical care guideline on patient outcome and resource utilization. Arch Pediatr Adolesc Med. 2002;156(11):1086–1090 [DOI] [PubMed] [Google Scholar]
- 30. Norton SP , Pusic MV , Taha F , Heathcote S , Carleton BC . Effect of a clinical pathway on the hospitalisation rates of children with asthma: a prospective study. Arch Dis Child. 2007;92(1):60–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Newman K , Ponsky T , Kittle K , et al. Appendicitis 2000: variability in practice, outcomes, and resource utilization at thirty pediatric hospitals. J Pediatr Surg. 2003;38(3):372–379, discussion 372–379 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.