A lactonase, named GcL, from G. caldoxylosilyticus has been isolated, purified, characterized and crystallized. Here, it is shown that GcL is a thermostable enzyme that is extremely proficient at degrading lactones. Its structure is expected to provide insights regarding the catalytic mechanism and to highlight the structural determinants involved in the high proficiency of this enzyme.
Keywords: quorum sensing, lactonase, thermophile, quorum quenching
Abstract
Lactonases are enzymes that are capable of hydrolyzing various lactones such as aliphatic lactones or acyl-homoserine lactones (AHLs), with the latter being used as chemical signaling molecules by numerous Gram-negative bacteria. Lactonases therefore have the ability to quench the chemical communication, also known as quorum sensing, of numerous bacteria, and in particular to inhibit behaviors that are regulated by this system, such as the expression of virulence factors or the production of biofilms. A novel representative from the metallo-β-lactamase superfamily, dubbed GcL, was isolated from the thermophilic bacterium Geobacillus caldoxylosilyticus. Because of its thermophilic origin, GcL may constitute an interesting candidate for the development of biocontrol agents. Here, we show that GcL is a thermostable enzyme with a half-life at 75°C of 152.5 ± 10 min. Remarkably, it is also shown that GcL is among the most active lactonases characterized to date, with catalytic efficiencies (k cat/K m) against AHLs of greater than 106 M −1 s−1. The structure of GcL is expected to shed light on the catalytic mechanism of the enzyme and the molecular determinants for the substrate specificity in this class of lactonases. Here, the expression, purification, characterization, crystallization and X-ray diffraction data collection to 1.6 Å resolution of GcL are reported.
1. Introduction
Quorum sensing (QS) is a strategy that is widely used by microbes to coordinate behavior such as bioluminescence, biofilm formation, expression of virulence factors, swarming motility and many others (Bassler, 1999 ▸; Miller & Bassler, 2001 ▸). This chemical communication is mediated by small diffusible molecules that can vary significantly in terms of chemical structure and properties. A well studied bacterial communication system uses acyl-homoserine lactones (AHLs) as signaling molecules (Miller & Bassler, 2001 ▸). These molecules are composed of an (S)-α-amino-γ-butyrolactone ring that is linked to an alkyl chain by an amide bond. The alkyl chain can vary in length (typically between four and 14 C atoms) or can include some decoration [for example, oxidation at carbon 3 (Lade et al., 2014 ▸) or a p-coumaroyl substituent (Schaefer et al., 2008 ▸)] and determines the specificity of the signal. AHLs are primarily produced by Gram-negative bacteria, and have mainly been described in intra-species communication molecules (Miller & Bassler, 2001 ▸; Fuqua et al., 2001 ▸); however, examples of inter-species communication have recently been documented (Schaefer et al., 2008 ▸; Soares & Ahmer, 2011 ▸; Galloway et al., 2010 ▸). The chemical signal is quenched by enzymes that can catalyse opening of the lactone ring or cleavage of the N-acylamide bond (Bokhove et al., 2010 ▸; Hiblot, Gotthard, Elias et al., 2013 ▸).
AHL-degrading enzymes have been isolated from numerous organisms, including bacteria, archaea, plants, fungi and mammals (LaSarre & Federle, 2013 ▸; Elias & Tawfik, 2012 ▸). These enzymes are of two main types: AHL acylases that cleave the N-acylamide bond (Liu et al., 2005 ▸) and AHL lactonases that hydrolyze the lactone ring (Elias et al., 2008 ▸). Quorum-quenching lactonases (QQLs) exhibit different specificities with respect to the acyl-chain lengths and substitutions of the AHLs. QQLs were found to effectively inhibit QS-regulated bacterial behavior in vitro and in vivo (LaSarre & Federle, 2013 ▸; Hraiech et al., 2014 ▸; Dong et al., 2001 ▸).
To date, three main families of lactonases have been identified (Elias & Tawfik, 2012 ▸). The paraoxonases, which are found in bacteria, mammals and a few other vertebrates, are calcium-dependant lactonases that exhibit a six-bladed β-propeller fold (Ben-David et al., 2013 ▸, 2015 ▸). Paraoxonases have been reported to degrade δ-lactones, γ-lactones and AHLs (Bar-Rogovsky et al., 2013 ▸; Khersonsky & Tawfik, 2005 ▸). A second family of lactonases are the phosphotriesterase-like lactonases (PLLs). They belong to the amidohydrolase superfamily and adopt a (β/α)8 fold (Afriat et al., 2006 ▸; Elias et al., 2008 ▸; Del Vecchio et al., 2009 ▸). PLLs have been isolated from numerous sources (Xiang et al., 2009 ▸; Hawwa et al., 2009 ▸), including extremophilic archaea (Hiblot et al., 2012 ▸, 2015 ▸; Bzdrenga et al., 2014 ▸; Hiblot, Gotthard, Elias et al., 2013 ▸). PLLs have been subdivided into two classes: PLL-As are proficient against δ-lactones, γ-lactones and AHLs, whereas PLL-Bs prefer δ-lactones and γ-lactones (Hiblot et al., 2015 ▸).
A third family of lactonases are the metallo-β-lactamase-like lactonases (MLLs). The first discovered QQL, the autoinducer inactivator A (AiiA), was isolated from Bacillus thuringiensis. Its crystal structure has been solved and its catalytic mechanism has been investigated (Liu et al., 2005 ▸). AiiA contains a bimetallic active site ligated by five histidine and two aspartate residues. As for PLLs, the lactone substrate binds to the metals via the two O atoms of the lactone ring (Liu et al., 2005 ▸; Fig. 1 ▸). The N-acyl chain is accommodated in a hydrophobic crevice. A water molecule bridging the two metal cations is hypothesized to serve as the nucleophile that attacks the carbonyl C atom of the lactone ring, forming a tetrahedral transition state. While this high-energy intermediate breaks down into a carboxylate and an alkoxide group, Asp108, a metal-ligating residue, may serve as a base to protonate the alkoxide leaving group (Liu et al., 2008 ▸; Momb et al., 2008 ▸).
Figure 1.
Putative catalytic mechanism of lactonases in the metallo-β-lactamase family. Adapted from Liu et al. (2008 ▸) and Momb et al. (2008 ▸).
GcL (WP_017434252.1) is a recently identified enzyme that was isolated from the thermophilic bacterium Geobacillus caldoxylosilyticus. Interestingly, this enzyme is a rare thermophilic representative of the MLLs. GcL shares 28.5% sequence identity with AiiA and 44.3% sequence identity with the closest known crystallized lactonase (AiiB). Previous work, possibly on the exact same protein (only identified by N-terminal sequencing), identified a thermophilic enzyme from G. caldoxylosilyticus and demonstrated the catalytic activity of this enzyme against a few lactone substrates (Morohoshi et al., 2015 ▸; Seo et al., 2011 ▸). Here, we report the protein production, purification, crystallization and preliminary X-ray diffraction data of this new thermophilic representative of the MLL family.
2. Cloning, expression and purification of GcL
The gene encoding GcL, isolated from the organism G. caldoxylosilyticus (WP_017434252), was synthesized and optimized for heterologous expression in Escherichia coli by GenScript (Piscataway, New Jersey, USA). The synthetic gene included an N-terminal Strep-tag (WSHPQFEK) for affinity chromatography purification followed by a TEV cleavage site (ENLYFQS) to remove the tag. This construct leaves an N-terminal serine residue after cleavage with TEV protease. The gene was cloned in pET-22b(+) (Novagen) using NdeI and XhoI restriction sites.
The protein was overproduced in E. coli strain BL21 (DE3)-pGro7/GroEL (Takara) using a similar protocol to previous studies of PLLs (Hiblot, Gotthard, Champion et al., 2013 ▸; Hiblot et al., 2012 ▸, 2015 ▸; Gotthard et al., 2011 ▸). Briefly, the expression of the protein was performed in 2 l ZYP autoinduction medium (100 µg ml−1 ampicillin and 34 µg ml−1 chloramphenicol) inoculated with 10 ml overnight preculture. The culture was grown at 309 K until the cells reached the exponential growth phase (OD600 nm). Culture flasks were then transferred to 290 K overnight and 0.2 mM CoCl2 was added to the cultures. Induction of protein expression is caused by consumption of the lactose from the ZYP medium. The cells were pelleted by centrifugation (4400g, 272 K, 15 min) and resuspended in lysis buffer consisting of 50 mM HEPES pH 8, 150 mM NaCl, 0.2 mM CoCl2, 0.25 mg ml−1 lysozyme, 0.1 mM phenylmethylsulfonyl fluoride (PMSF). The suspended cells were lysed by three sonication steps of 30 s at an amplitude of 45 (1 s pulse on, 1 s pulse off; Q700 Sonicator, Qsonica, USA). Cell debris was removed by centrifugation (5000g, 276 K, 20 min). The cell lysate was filtered at 0.45 µm (VWR, USA) and subsequently loaded onto a Strep-Tag column (StrepTrap HP, GE Healthcare) at a flow rate of 1 ml min−1. Elution of protein was performed using a buffer composed of 100 mM Tris–HCl pH 8, 150 mM NaCl, 0.2 mM CoCl2, 2.5 mM desthiobiotin. Pure eluted protein fractions were pooled and cleaved by TEV protease overnight at 4°C. To remove the TEV protease, some of which precipitated overnight, the samples were filtered at 0.2 µm (VWR, USA), concentrated and loaded onto a size-exclusion column (HiLoad 16/600, Superdex 200 pg, GE Healthcare) in a buffer consisting of 50 mM HEPES, 150 mM NaCl and 0.2 mM CoCl2. Fractions containing the pure protein were pooled and concentrated to 11.66 mg ml−1 using a centrifugation device (Vivaspin 15R, Sartorius, Germany). The yield of production was approximately 5 mg pure protein per litre of culture. The purity of the produced protein was assessed by Coomassie-stained SDS–PAGE (Fig. 2 ▸). This gel reveals a dominant band at ∼32 kDa that corresponds to a monomer of GcL.
Figure 2.
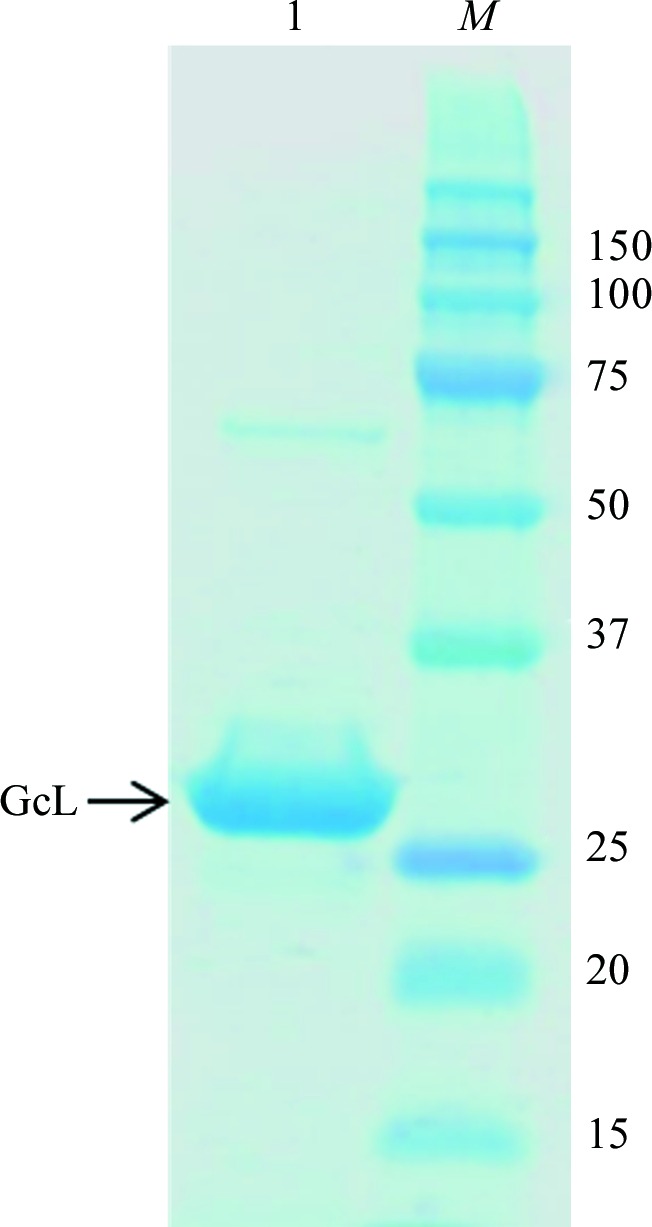
12% SDS–PAGE of the GcL protein. Lane M contains molecular-weight markers (Precision Plus Protein Kaleidoscope Prestained Protein Standards, Bio-Rad; labelled in kDa). Lane 1 contains 5 µl GcL protein at 1 mg ml−1.
3. Kinetic characterization
Experiments were performed in triplicate at 25°C using a microplate reader (Synergy HTX, BioTek, USA) and Gen5.1 software. We used a 200 µl reaction volume in 96-well plates with a path length of 5.8 mm. The catalytic parameters were calculated by fitting the kinetic data to the Michaelis–Menten equation using GraphPad Prism. Kinetic parameters are given in Table 1 ▸.
Table 1. Kinetic parameters of GcL at 25°C.
| k cat (s−1) | K m (µM) | k cat/K m (s−1 M −1) | |
|---|---|---|---|
| C4-AHL | 19.06 ± 1.51 | 229 ± 57 | (8.31 ± 2.19) × 104 |
| C6-AHL | 8.95 ± 0.48 | 7.97 ± 1.89 | (1.12 ± 0.27) × 106 |
| C10-AHL | 5.48 ± 0.37 | 1.45 ± 0.47 | (3.77 ± 1.27) × 106 |
| 3-Oxo-C8-AHL | 9.48 ± 0.35 | 2.19 ± 0.37 | (4.31 ± 0.76) × 106 |
The lactonase hydrolysis experiment was performed using a previously established assay with cresol purple as a pH indicator (Hiblot, Gotthard, Elias et al., 2013 ▸). Indeed, the opening of the lactone ring generates a proton that acidifies the solution and can be monitored. The time course of the hydrolysis of lactones was recorded at 577 nm in a buffer consisting of 2.5 mM Bicine pH 8.3, 150 mM NaCl, 0.2 mM CoCl2, 0.2 mM cresol purple, 0.5% DMSO over a range of substrate concentrations (0–2 mM). The extinction coefficient (∊577 nm = 2923 M −1 cm−1) of the pH indicator cresol purple (pK a = 8.3 at 25°C) was evaluated by measuring the absorbance of the buffer at different concentrations of acetic acid (0–0.35 mM).
4. Thermal stability assays
Purified GcL enzyme (1 mg ml−1) in a buffer consisting of 50 mM HEPES, 150 mM NaCl, 0.2 mM CoCl2 was used for this assay. Protein aliquots were incubated at 70 and 75°C and were assayed at various time points (30, 60, 90, 120, 180 and 240 min; Fig. 3 ▸). The lactonase activity against C6-AHL was monitored as explained above. Lactone hydrolysis, in µM min−1, was normalized to the enzyme activity measured at room temperature, and measurements were made in triplicate. The half-life of the enzyme (t 1/2), defined here as the incubation time for the enzyme at which 50% of the initial activity is retained, was determined by fitting the normalized activities to the following equation at different tested temperatures,
where X, Y and h represent the incubation time, the enzyme lactonase activity and the slope coefficient, respectively.
Figure 3.
The half-life of GcL incubated at 70 and 75°C. The enzyme was incubated for different times at two different temperatures and the lactonase activity against C6-AHL is reported. The inferred half life (t 1/2) of the protein is 152.5 ± 10 and 109.1 ± 7 min at 70 and 75°C, respectively.
5. Protein crystallization
Concentrated protein samples were submitted to crystallization trials. Assays were performed using the sitting-drop vapor-diffusion method set up in a 96-well plate. Initial screening was performed with the commercial kit JCSG+ using different protein:precipitant ratios (1:1, 1:2 and 1:3) and the plate was incubated at 292 K. The best condition identified consisted of 1.25 M ammonium sulfate, 0.1 M sodium acetate pH 5.5. This condition was further refined for pH (in the range 4.0–5.5) and ammonium sulfate concentration (1–2.25 M). Diffraction-quality crystals appeared after 1 d at 292 K (Fig. 4 ▸).
Figure 4.

Crystals of the GcL protein appeared after 1 d at 20°C.
6. Data collection
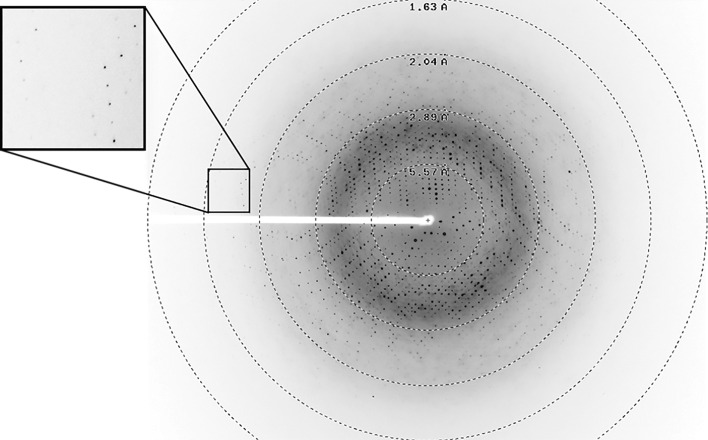
For cryoprotection, crystals were transferred into a drop composed of the mother liquor supplemented with 25%(v/v) glycerol for 1 min. The crystals were mounted on a CryoLoop (Hampton Research) and flash-cooled at 100 K in liquid nitrogen. X-ray diffraction data were collected on the 23-ID-B beamline at the Advanced Photon Source (APS), Argonne, Illinois, USA using a wavelength of 1.03323 Å and a MAR 300 CCD detector with 0.2 s exposures. Individual frames consisted of 0.5° steps over a range of 400° (Fig. 5 ▸). Data-collection statistics are given in Table 2 ▸.
Figure 5.
A diffraction frame from a crystal of GcL. The edge of the frame is at 1.40 Å resolution.
Table 2. Data-collection statistics.
Values in parentheses are for the highest resolution shell.
| Beamline | 23-ID-B, APS |
| Wavelength (Å) | 1.03323 |
| Detector | MAR 300 CCD |
| Oscillation (°) | 0.5 |
| No. of frames | 400 |
| Resolution (Å) | 1.6 (1.7–1.6) |
| Space group | C2 |
| Unit-cell parameters (Å, °) | a = 145.42, b = 108.68, c = 78.74, β = 115.845 |
| No. of observed reflections | 602221 (99530) |
| No. of unique reflections | 144425 (23974) |
| Completeness (%) | 99.6 (99.7) |
| R meas (%) | 3.3 (64.5) |
| CC1/2 | 100 (92.4) |
| 〈I/σ(I)〉 | 26.50 (3.1) |
| Multiplicity | 4.17 (4.15) |
7. Results and conclusions
In this study, we show that GcL is a thermostable enzyme, with t 1/2 values of 152.5 ± 10 and 109.1 ± 7 min at 70 and 75°C, respectively (Fig. 3 ▸). Its intrinsic stability makes GcL an interesting candidate for further studies, with the aim of functionalizing the enzyme and developing antimicrobial solutions. Moreover, our kinetic characterization data shows that GcL is a very proficient enzyme and is among the most active lactonases characterized thus far. For instance, at 25°C it is about two orders of magnitude more active than the well characterized AiiA against C6-HSL (Liu et al., 2008 ▸). Catalytic efficiencies suggest that GcL exhibits a subtle preference for long aliphatic chains, as previously observed (Seo et al., 2011 ▸). We expect that the solution of the structure will shed light on the structural determinants explaining its high proficiency.
We have therefore crystallized and collected diffraction data from GcL crystals. The X-ray diffraction data were indexed, integrated and scaled using the XDS software package (Kabsch, 2010 ▸). The GcL crystals belonged to space group C2, with unit-cell parameters a = 145.42, b = 108.68, c = 78.74 Å, α = γ = 90, β = 115.845°. As GcL is a 32 kDa protein, Matthews coefficient calculation suggested between one and six monomers in the asymmetric unit (V M = 8.05 and 1.34 Å3 Da−1, corresponding to 84.72 and 8.34% solvent content, respectively). The most likely value is three monomers (V M = 2.68 Å3 Da−1, corresponding to 54.17% solvent content). Molecular replacement was performed using MOLREP (Vagin & Teplyakov, 2010 ▸) with the structure of AiiB (PDB entry 2r2d; Liu et al., 2007 ▸) as a starting model. Three molecules were placed in the asymmetric unit (R = 34.54%, R free = 39.77%). The initial solution was submitted to Buccaneer for automated model reconstruction (Cowtan, 2006 ▸). After five cycles of model building the R factor was 32.77% and R free was 36.07%. Cycles of manual building and structure refinement using REFMAC (Murshudov et al., 2011 ▸) allowed improvement of the model and the R and R free values decreased to 15.55% and 21.01%, respectively. Manual model improvement is currently in progress using Coot (Emsley et al., 2010 ▸). With the exceptions of the solvent, some rotamers and loop conformations, inspection of the electron-density maps suggest that the model is near-final. The asymmetric unit contains one homodimer of GcL and another molecule. Interpretation of the structure is in progress.
Acknowledgments
We are grateful to the Nano Crystallization Facility and the Kahlert Structural Biology Laboratory, and in particular to Carrie Wilmot and Ke Shi for assistance in setting up crystallization screens and to Ed Hoeffner for assistance in using the in-house X-ray diffraction setup.
References
- Afriat, L., Roodveldt, C., Manco, G. & Tawfik, D. S. (2006). Biochemistry, 45, 13677–13686. [DOI] [PubMed]
- Bar-Rogovsky, H., Hugenmatter, A. & Tawfik, D. S. (2013). J. Biol. Chem. 288, 23914–23927. [DOI] [PMC free article] [PubMed]
- Bassler, B. L. (1999). Curr. Opin. Microbiol. 2, 582–587. [DOI] [PubMed]
- Ben-David, M., Sussman, J. L., Maxwell, C. I., Szeler, K., Kamerlin, S. C. & Tawfik, D. S. (2015). J. Mol. Biol. 427, 1359–1374. [DOI] [PubMed]
- Ben-David, M., Wieczorek, G., Elias, M., Silman, I., Sussman, J. L. & Tawfik, D. S. (2013). J. Mol. Biol. 425, 1028–1038. [DOI] [PubMed]
- Bokhove, M., Nadal Jimenez, P., Quax, W. J. & Dijkstra, B. W. (2010). Proc. Natl Acad. Sci. USA, 107, 686–691. [DOI] [PMC free article] [PubMed]
- Bzdrenga, J., Hiblot, J., Gotthard, G., Champion, C., Elias, M. & Chabriere, E. (2014). BMC Res. Notes, 7, 333. [DOI] [PMC free article] [PubMed]
- Cowtan, K. (2006). Acta Cryst. D62, 1002–1011. [DOI] [PubMed]
- Del Vecchio, P., Elias, M., Merone, L., Graziano, G., Dupuy, J., Mandrich, L., Carullo, P., Fournier, B., Rochu, D., Rossi, M., Masson, P., Chabriere, E. & Manco, G. (2009). Extremophiles, 13, 461–470. [DOI] [PubMed]
- Dong, Y.-H., Wang, L.-H., Xu, J.-L., Zhang, H.-B., Zhang, X.-F. & Zhang, L.-H. (2001). Nature (London), 411, 813–817. [DOI] [PubMed]
- Elias, M., Dupuy, J., Merone, L., Mandrich, L., Porzio, E., Moniot, S., Rochu, D., Lecomte, C., Rossi, M., Masson, P., Manco, G. & Chabriere, E. (2008). J. Mol. Biol. 379, 1017–1028. [DOI] [PubMed]
- Elias, M. & Tawfik, D. S. (2012). J. Biol. Chem. 287, 11–20. [DOI] [PMC free article] [PubMed]
- Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. (2010). Acta Cryst. D66, 486–501. [DOI] [PMC free article] [PubMed]
- Fuqua, C., Parsek, M. R. & Greenberg, E. P. (2001). Annu. Rev. Genet. 35, 439–468. [DOI] [PubMed]
- Galloway, W. R., Hodgkinson, J. T., Bowden, S. D., Welch, M. & Spring, D. R. (2010). Chem. Rev. 111, 28–67. [DOI] [PubMed]
- Gotthard, G., Hiblot, J., Elias, M. & Chabrière, E. (2011). Acta Cryst. F67, 354–357. [DOI] [PMC free article] [PubMed]
- Hawwa, R., Larsen, S. D., Ratia, K. & Mesecar, A. D. (2009). J. Mol. Biol. 393, 36–57. [DOI] [PubMed]
- Hiblot, J., Bzdrenga, J., Champion, C., Chabriere, E. & Elias, M. (2015). Sci. Rep. 5, 8372. [DOI] [PMC free article] [PubMed]
- Hiblot, J., Gotthard, G., Chabriere, E. & Elias, M. (2012). PLoS One, 7, e47028. [DOI] [PMC free article] [PubMed]
- Hiblot, J., Gotthard, G., Champion, C., Chabriere, E. & Elias, M. (2013). Acta Cryst. F69, 1235–1238. [DOI] [PMC free article] [PubMed]
- Hiblot, J., Gotthard, G., Elias, M. & Chabriere, E. (2013). PLoS One, 8, e75272. [DOI] [PMC free article] [PubMed]
- Hraiech, S., Hiblot, J., Lafleur, J., Lepidi, H., Papazian, L., Rolain, J.-M., Raoult, D., Elias, M., Silby, M. W., Bzdrenga, J., Bregeon, F. & Chabriere, E. (2014). PLoS One, 9, e107125. [DOI] [PMC free article] [PubMed]
- Kabsch, W. (2010). Acta Cryst. D66, 125–132. [DOI] [PMC free article] [PubMed]
- Khersonsky, O. & Tawfik, D. S. (2005). Biochemistry, 44, 6371–6382. [DOI] [PubMed]
- Lade, H., Paul, D. & Kweon, J. H. (2014). Biomed. Res. Int. 2014, 162584. [DOI] [PMC free article] [PubMed]
- LaSarre, B. & Federle, M. J. (2013). Microbiol. Mol. Biol. Rev. 77, 73–111. [DOI] [PMC free article] [PubMed]
- Liu, D., Lepore, B. W., Petsko, G. A., Thomas, P. W., Stone, E. M., Fast, W. & Ringe, D. (2005). Proc. Natl Acad. Sci. USA, 102, 11882–11887. [DOI] [PMC free article] [PubMed]
- Liu, D., Momb, J., Thomas, P. W., Moulin, A., Petsko, G. A., Fast, W. & Ringe, D. (2008). Biochemistry, 47, 7706–7714. [DOI] [PMC free article] [PubMed]
- Liu, D., Thomas, P. W., Momb, J., Hoang, Q. Q., Petsko, G. A., Ringe, D. & Fast, W. (2007). Biochemistry, 46, 11789–11799. [DOI] [PubMed]
- Miller, M. B. & Bassler, B. L. (2001). Annu. Rev. Microbiol. 55, 165–199. [DOI] [PubMed]
- Momb, J., Wang, C., Liu, D., Thomas, P. W., Petsko, G. A., Guo, H., Ringe, D. & Fast, W. (2008). Biochemistry, 47, 7715–7725. [DOI] [PMC free article] [PubMed]
- Morohoshi, T., Tominaga, Y., Someya, N. & Ikeda, T. (2015). J. Biosci. Bioeng. 120, 1–5. [DOI] [PubMed]
- Murshudov, G. N., Skubák, P., Lebedev, A. A., Pannu, N. S., Steiner, R. A., Nicholls, R. A., Winn, M. D., Long, F. & Vagin, A. A. (2011). Acta Cryst. D67, 355–367. [DOI] [PMC free article] [PubMed]
- Schaefer, A. L., Greenberg, E., Oliver, C. M., Oda, Y., Huang, J. J., Bittan-Banin, G., Peres, C. M., Schmidt, S., Juhaszova, K., Sufrin, J. R. & Harwood, C. S. (2008). Nature (London), 454, 595–599. [DOI] [PubMed]
- Seo, M.-J., Lee, B.-S., Pyun, Y.-R. & Park, H. (2011). Biosci. Biotechnol. Biochem. 75, 1789–1795. [DOI] [PubMed]
- Soares, J. A. & Ahmer, B. M. (2011). Curr. Opin. Microbiol. 14, 188–193. [DOI] [PMC free article] [PubMed]
- Vagin, A. & Teplyakov, A. (2010). Acta Cryst. D66, 22–25. [DOI] [PubMed]
- Xiang, D. F., Kolb, P., Fedorov, A. A., Meier, M. M., Fedorov, L. V., Nguyen, T. T., Sterner, R., Almo, S. C., Shoichet, B. K. & Raushel, F. M. (2009). Biochemistry, 48, 2237–2247. [DOI] [PMC free article] [PubMed]