Abstract
Previous research supports a relationship between psychological stress and chronic disease in Puerto Rican adults living in the Boston, Massachusetts area. Stress may affect health by influencing dietary and physical activity patterns. Therefore, perceived stress and two hypothesized mediators of stress-related food intake, insulin and cortisol, were examined for possible associations with dietary and activity patterns in >1300 Puerto Ricans (aged 45–75 years; 70% women) living in the Boston, Massachusetts area. Data were analyzed using multiple linear regression and ANCOVA. Greater perceived stress was associated with lower fruit, vegetable, and protein intake, greater consumption of salty snacks, and lower participation in physical activity. Stress was associated with higher intake of sweets, particularly in those with type 2 diabetes. Cortisol and stress were positively associated in those without diabetes. Cortisol was associated with higher intake of saturated fat and, in those with diabetes, sweet foods. Independent of diabetes, perceived stress was associated with higher circulating insulin and BMI. Our findings support a link between stress, cortisol, and dietary and activity patterns in this population. For high-sugar foods, this relationship may be particularly important in those with type 2 diabetes. Longitudinal research to determine causal pathways for these identified associations is warranted.
Keywords: Psychological stress, Cortisol, Type 2 diabetes, Dietary patterns, Physical activity, Hispanic, Puerto Rican
Introduction
Puerto Rican elders in Massachusetts have been shown to be more likely than non-Hispanic white elders living in the same neighborhoods to present with depression, cognitive impairment, diabetes, and other chronic health problems (Tucker, Bermudez, & Castaneda, 2000a; Tucker, Falcon, Bianchi, Cacho, & Bermudez, 2000b). Therefore, factors beside physical and neighborhood location may explain these apparent health disparities. Differential exposure and response to psychological stress, which is a perception of an actual or anticipated threat to well-being, may contribute to the development of these health disparities through the accumulation of allostatic load, particularly if stress exposure and its behavioral and physiological sequelae (e.g., cortisol) persist over days to months (McEwen, 2004). Cultural and ethnic minorities face more stressors, quantitatively and qualitatively, than the majority population (Cervantes, Padilla, & de Snyder, 1991) and, therefore, Puerto Ricans, like many minority and underprivileged populations, may be especially vulnerable to societal stress and its effects on health and well being (Cervantes et al., 1991; Kubzansky, Kawachi, & Sparrow, 1999; National Institutes of Health, 2001). Chronic psychological stress has been linked to poor physical and mental health (Adler et al., 1994; Chrousos & Gold, 1992; Epel, 2009; McEwen, 2004; Sapolsky, 1992), but responses to stress and strategies for coping with stress vary across individuals, ethnic and cultural groups, and populations. If we are to create better intervention strategies aimed at decreasing health disparities, it is important to identify the extent to which different individuals and groups of people respond to and cope with stress. Further, associations between life stress and nutritional behavior may interact in ways that increase risk for developing chronic health problems.
Chronic psychological stress has profound effects on human health and well being, and it is generally accepted that psychological stress is a burgeoning public health problem in modern day life. While stress may directly influence processes that lead to or exacerbate metabolic (e.g., diabetes) and psychiatric (e.g., depression) disease, the effects of stress on health may also act indirectly through its effects on dietary and physical activity patterns. Stress is a risk factor for dietary relapse (Foreyt & Poston, 1998) and it can trigger binge eating and disinhibition in restrained eaters (see Adam & Epel, 2007). Experimentally induced stress has been shown to increase intake of highly palatable, energy-dense foods and to inhibit intake of high-fiber, low fat foods in humans (Epel, Lapidus, McEwen, & Brownell, 2001; Oliver, Wardle, & Gibson, 2000; Wallis & Hetherington, 2004, 2009; Zellner et al., 2006; Zellner, Saito, & Gonzalez, 2007) and in animals (Dallman et al., 2003; Pecoraro, Reyes, Gomez, Bhargava, & Dallman, 2004; Wilson et al., 2008). Experimental stress has also been shown to increase snacking (Roemmich, Wright, & Epstein, 2002) and, when evaluated under free living conditions, daily hassles were positively associated with snacking (Newman, O'Connor, & Conner, 2007). Other indices of increased stress, such as student exams and workload have been associated with greater intakes of fat (McCann, Warnick, & Knopp, 1990), high fat-sugar foods (Wardle, Steptoe, Oliver, & Lipsey, 2000), energy (Michaud et al., 1990), or less healthy foods (Weidner, Kohlmann, Dotzauer, & Burns, 1996). However, dietary response to stress varies and, while some individuals may be classified as stress-eaters, others are characterized by decreasing energy intake in response to psychological stress (Adam & Epel, 2007). The physiological basis of stress-eating and individual variation in stress-related food intake are unknown, but may be related to differences in the state of, and interplay between, glucocorticoid and insulin signaling (e.g., diabetes) (see (Dallman et al., 2003; Dallman, Warne, Foster, & Pecoraro, 2007)).
In addition to eating behavior, there is also evidence that links psychological stress and physical activity. Although certain forms of exercise can ameliorate negative feelings associated with stress or stimulate mood in some people (Brown & Lawton, 1986; Hoffman & Hoffman, 2008; Norris, Carroll, & Cochrane, 1992), this varies from person to person and with level of exercise experience (Hoffman & Hoffman, 2008). Moreover, stress may also reduce the motivational salience of physical activity. For example, children in one study who were exposed to experimental stress more frequently chose to play video games and watch television than to exercise (Roemmich, Gurgol, & Epstein, 2003). Stress was shown to reduce adherence to physical activity in adults (Griffin, Friend, Eitel, & Lobel, 1993; King, 1997; Krause, Goldenhar, Liang, Jay, & Maeda, 1993; Oman & King, 2000; Steptoe, Wardle, Pollard, Canaan, & Davies, 1996; Stetson, Rahn, Dubbert, Wilner, & Mercury, 1997) and adolescents (Kornitzer & Kittel, 1986). In focus groups designed to identify barriers and facilitators of healthy nutritional practices in low-income overweight mothers, Chang, Nitzke, Guilford, Adair, and Hazard (2008) found that stressful experiences were significant barriers to engaging in physical activity.
Therefore, psychological stress may present a significant barrier to adopting and adhering to dietary and activity patterns that promote health. To begin to understand the potential relationships between stress and nutrition in Puerto Rican adults, we examined associations between life stress and dietary and activity patterns in a cross-section of more than 1300 Puerto Ricans living in Boston, Massachusetts. Although this cross sectional assessment does not allow inferences of direction or causality between stress and nutritional behavior, or their associations with chronic disease, this work may help to highlight potentially important relationships between psychological stress and nutrition in this population.
Subjects and methods
Subjects
This analysis used data from a cohort of volunteers participating in an ongoing longitudinal investigation, The Boston Puerto Rican Health Study (BPRHS). BPRHS aims to understand relationships between psychological stress, nutrition, and chronic health conditions in Puerto Rican older adults living in Boston, Massachusetts (Tucker, 2005). Briefly, using year 2000 census data to identify areas of high Hispanic density, community approaches and door-to-door enumeration were enlisted to recruit participants from census blocks in the greater Boston area. Eligible persons were of self-identified Puerto Rican decent, able to answer questions in English or Spanish, ages 45–75 years, and living in Boston, MA or surrounding areas at the time of the study. Individuals were disqualified from participating in the study if they were unable to answer questions due to serious health conditions, did not plan to be living in the area within 2 years, or if they scored ≤10 on the Mini Mental State Examination (MMSE). Following the initial screening contact, eligible participants were notified in writing and phoned 1–2 days in advance of the interview date. Five scheduling attempts were made, after which individuals were considered de-facto refusals. All procedures were in accordance with the guidelines established by the Institutional Review Board at Tufts Medical Center. A total of 1336 Puerto Rican adults aged 45–75 years were included in the following analyses.
General health status, anthropometrics, and health behaviors
After obtaining informed consent, participants were interviewed in their homes by trained bilingual research staff via questionnaires to assess age, education level, household income, employment history, family size, health history, socioeconomic status, acculturation, cognitive functioning, and food security. Questionnaires were based on those used in the NHANES III (Dreon, John, DiCiccio, & Whittemore, 1993), the Hispanic Health and Nutrition Examination Survey (Delgado, Johnson, Roy, & Trevino, 1990), and the National Health Interview Survey Supplement on Aging (Block & Subar, 1992). Participants were asked to specify whether they had been diagnosed with any of a specific list of chronic conditions. Health insurance information was also provided by the participants, and participants self-rated their health status. Smoking and alcohol type, frequency, and history were also assessed. Anthropometrics, blood pressure, and physical performance measures were also conducted during the interview. Body mass index was estimated using weight (kg) divided by height (m) squared. Physical activity was determined from a modified Paffenbarger questionnaire of the Harvard Alumni Activity Survey (Paffenbarger et al., 1993), and the activity score was calculated as the sum of hours spent on typical 24-h activities (heavy, moderate, light, or sedentary activity, and sleeping) multiplied by weighing factors consistent with the oxygen consumption rate associated with each activity. Participant's dietary intake, reflecting a time period over the past 12 months, was assessed with a 126-item semiquantitative food frequency questionnaire adapted and validated for this population (Tucker, Bianchi, Maras, & Bermudez, 1998). Nutrient intakes were calculated from a database developed using nutrient information from the Nutrition Data System for Research software version 2007, developed by the Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN. Those with energy intakes <600 or >4800 kilocalories and/or >10 questions blank on the FFQ were excluded from dietary analyses.
Life stress measures and depression symptomology
Life stress was measured using the Spanish version of the Perceived Stress Scale (PSS) (Cohen, Kamarck, & Mermelstein, 1983). Life events, emotional support, and depression symptomology were assessed, respectively, by the Life Events Inventory (Norbeck, 1984), Norbeck Social Support Questionnaire (Norbeck, Lindsey, & Carrieri, 1981), and Center for Epidemiology Studies Depression (CESD) Scale (Mackinnon, McCallum, Andrews, & Anderson, 1998).
Blood and urine samples
On a day following the interview and in the participant's home, a study phlebotomist collected blood, saliva, and urine. Blood and urine were collected in the morning after a 12 h overnight fast. Blood was collected in a serum separator tube and allowed to clot for 20 min and immediately centrifuged onsite. The samples were kept cold and brought back to the Human Nutrition Research Center on Aging for further processing and analysis at the Nutrition Evaluation Laboratory. Sample aliquots were stored at −80 °C. Serum insulin was measured using the Immulite 1000 Insulin Kit (LKIN1) on the Immulite 1000 (Seimens Medical Solutions Diagnostics, Los Angeles, CA). A 12-h urine sample was collected beginning on the evening after the home interview to the next morning. Urinary cortisol was determined by EIA (Alpco Diagnostics, Windham, NH), and urine was processed and stored according to the manufacturer's instructions. Cortisol was standardized by multiplying by total urine volume and dividing by urinary creatinine excretion.
Statistical analysis
Data were analyzed using SAS 9.1.3. covariate analysis of variance (ANCOVA) and multiple linear regression were used to examine relationships between perceived stress, food intake and intake patterns, physical activity, urinary cortisol and serum insulin, health status (including diabetes), and BMI. Diabetes was included as a covariate because (1) dietary patterns, particularly sugar intake, can be significantly influenced by this condition and (2) diabetes has been shown to influence stress responsiveness. Analyses of intake patterns were conducted on information derived from a food frequency questionnaire previously developed and validated for use in Hispanic persons living in the Boston, MA area (Tucker et al., 1998). Food groups derived from this food frequency questionnaire in this subject population (previously described by Noel, Newby, Ordovas, & Tucker, 2009) were also examined for their associations with stress variables. Participants were divided into four groups based on the quartile ranking, from lowest to highest, of perceived stress, cortisol, and insulin. The ANCOVA and regression models were adjusted for education, sex, age, income to poverty ratio and type 2 diabetes. Income to poverty ratio was calculated as the total household income divided by the poverty threshold (using poverty guidelines 2004–2007). Where appropriate, to adjust for effects of BMI and physical activity, these variables were also included as covariates in the model. For ANCOVA, all dietary variables were adjusted for energy intake prior to entry into the model. In cases where there was a significant interaction (P ≤ 0.05) between diabetes and either perceived stress, urinary cortisol, or insulin, we conducted separate analyses for participants with and without diabetes. Multiple linear regression analysis was also used to compliment the ANCOVA. For multiple linear regression, associations between nutritional variables and perceived stress, urinary cortisol, and insulin were tested. Sex, age, education, total energy intake, BMI, activity, income to poverty ratio, and diabetes were also included in the regression model as independent variables. (P ≤ 0.05) was considered to be statistically significant and, to determine whether each of the three highest quartiles differed statistically from the lowest quartile, a Dunnett's test of the least squared means was conducted.
Results
Perceived stress
As expected, we found significant associations between perceived stress and indices of chronic stress exposure (Table 1). Individuals who reported higher frequencies of bad events in their lives showed higher levels of perceived stress, and reported level of emotional support was inversely associated with perceived stress. Compared to the lowest quartile of perceived stress, the highest quartile of perceived stress was associated with significantly fewer life events considered by participants to affect their lives in a good way. General health status was poorer across perceived stress quartiles and this was, at least in part, a function of significantly higher depression scores, depressive symptomaltology, and measures of anxiety in persons reporting a higher degree of perceived stress. Perceived stress was also associated with elevated BMI, but this was specific to the middle highest, and not highest, level of stress. Compared to participants in the lowest perceived stress quartile, there were a greater number of cigarettes smoked per day by participants in the middle-lowest and highest quartile.
Table 1.
Health status and stress.a
| Variable | Quartile of perceived stress |
P stress | |||
|---|---|---|---|---|---|
| Lowest | Middle lowest | Middle highest | Highest | ||
| Perceived stress | 11.1 ± 0.1 | 21.2 ± 0.1* | 27.4 ± 0.1* | 35.1 ± 0.1* | 0.000 |
| Self-rated general healthb | 3.3 ± 0.04 | 3.5 ± 0.04* | 3.8 ± 0.04* | 4.0 ± 0.04* | 0.000 |
| Self-reported clinical depression | 0.24 ± 0.02 | 0.49 ± 0.02* | 0.65 ± 0.02* | 0.80 ± 0.02* | 0.000 |
| CES-D scorec | 8.5 ± 0.5 | 17.1 ± 0.5* | 22.5 ± 0.5* | 32.7 ± 0.5* | 0.000 |
| Self-reported clinical anxiety | 0.16 ± 0.02 | 0.31 ± 0.02* | 0.48 ± 0.02* | 0.63 ± 0.02* | 0.000 |
| Bad life eventsd | 4.6 ± 0.4 | 7.6 ± 0.4* | 8.2 ± 0.4* | 1.21 ± 0.4* | 0.000 |
| Good life eventsd | 7.5 ± 0.3 | 8.2 ± 0.3 | 7.5 ± 0.3 | 6.1 ± 0.4* | 0.003 |
| Emotional supporte | 87.1 ± 2.6 | 78.9 ± 2.5 | 7.61 ± 2.6* | 67.6 ± 2.7* | 0.000 |
| Food securityf | 1.2 ± 0.04 | 1.4 ± 0.04* | 15 ± 0.04* | 18 ± 0.04* | 0.000 |
| Smoking status (# cigarettes/day) | 8.1 ± 1.0 | 11.6 ± 1.0* | 11.3 ± 1.0 | 12.7 ± 0.9* | 0.012 |
| BMI | 31.5 ± 0.3 | 31.7 ± 0.3 | 32.8 ± 0.3* | 31.3 ± 0.3 | 0.023 |
Values are 1s mean ± S.E. and adjusted for sex, age, education, BMI, income to poverty ratio, and diabetes.
A higher score reflects poorer health.
Center for Epidemiologic Studies Depression Score, range 0–60.
Reported number of bad and good life events over the past year measured through the Life Events Inventory Questionnaire.
Reported emotional support was measured through the Norbeck Social Support Questionnaire.
A higher score indicates a greater frequency of not having enough food to eat in the past 12 months.
Statistically different from the lowest quartile (P ≤ 0.05).
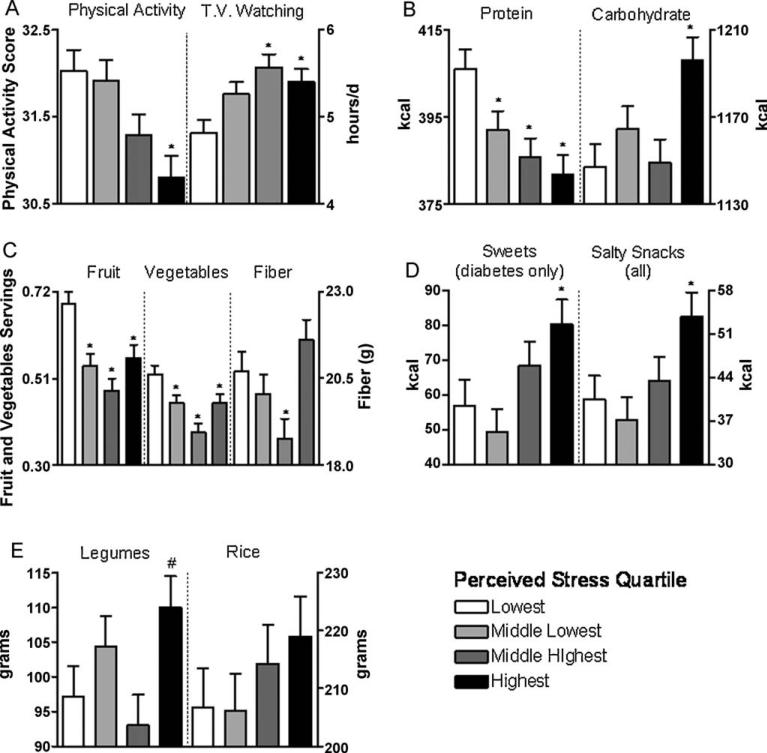
Physical activity was lower in the highest quartile of perceived stress, while time watching television was greater in the two highest quartiles (Fig. 1 and Tables 2 and 3). Independent of energy intake, BMI and physical activity, perceived stress was associated with lower intake of protein, fruit, vegetables, fiber, and omega-3 fatty acids and higher intake of carbohydrate and food groups characterized by salty snacks, sweets, and high glycemic index (Fig. 1 and Tables 2 and 3). For the sweets food group, only in individuals with diabetes was relative level of perceived stress associated with significantly higher intake of sweets. The energy adjusted intake of vitamins B-6 and K, iron, magnesium, potassium, and essential amino acids were all lower across quartiles of perceived stress (Table 4). We did not find significant associations between stress and total energy intake or other nutrients in the database.
Fig. 1.
Higher levels of perceived stress were associated with lower physical activity and greater amount of time watching television (A).Higher levels of stress were also associated with lower intake of protein, greater intake of carbohydrate (B) and lower intakes of fruit, vegetables, and fiber (C), although there was a U-shaped pattern of dietary fiber intake across increasing levels of perceived stress.Eating more salty snacks was associated with higher levels of perceived stress; an association between sweets and stress was found only in those with diabetes (D).Consumption of legumes, but not rice, tended to be associated with stress, with the highest quartile tending to have higher intakes relative to the lowest quartile (E). Values are least square mean ± S.E. and adjusted for age, sex, education, energy intake, BMI, physical activity, income to poverty ratio, and diabetes.Dietary intakes are daily values. *Statistically different from the lowest quartile (P ≤ 0.05). #Indicates a tendency (P = 0.08) to be statistically different from the lowest quartile.
Table 2.
Nutrient intakes associated with relative level of perceived stress.a
| Quartile of perceived stress |
P stress | ||||
|---|---|---|---|---|---|
| Lowest | Middle lowest | Middle highest | Highest | ||
| Variable | |||||
| Energy (kcal/day) | 2317 ± 67 | 2261 ± 63 | 2300 ± 67 | 2416 ± 67 | ns |
| Carbohydrate (g/day) | 284 ± 2 | 289 ± 2 | 285 ± 2 | 297 ± 2* | 0.010 |
| Protein (g/day) | 100.5 ± 1.1 | 966 ± 1.1* | 95.9 ± 1.1* | 94.6 ± 1.1* | 0.003 |
| Fat (g/day) | 83.9 ± 0.9 | 84.5 ± 09 | 83.3 ± 0.9 | 82.0 ± 0.9 | ns |
| n3 fatty acids (g/day) | |||||
| EPA | 0.08 ± 0.004 | 0.06 ± 0.004* | 0.06 ± 0.004* | 0.06 ± 0.004* | 0.010 |
| DHA | 0.16 ± 0.007 | 0.14 ± 0.006 | 0.13 ± 0.007* | 0.13 ± 0.007* | 0.005 |
| DPA | 0.03 ± 0.001 | 0.02 ± 0.001* | 0.02 ± 0.001* | 0.02 ± 0.001* | 0.000 |
| Fruit servings/day | 0.69 ± 0.03 | 0.55 ± 0.02* | 0.47 ± 0.03* | 0.56 ± 0.03* | 0.000 |
| Vegetable servings/day | 0.52 ± 0.02 | 0.46 ± 0.02 | 0.37 ± 0.02* | 0.44 ± 0.02* | 0.000 |
| Total fiber (g/day) | 206 ± 0.3 | 20.4 ± 03 | 189 ± 03* | 207 ± 03 | 0.000 |
| Sweets (kcal/day) | 63.2 ± 77 | 55.5 ± 6.9 | 74.0 ± 7.2 | 86.1 ± 7.2* | 0.020 |
| Diabetes | |||||
| Sweets (kcal/day) | 999 ± 69 | 996 ± 68 | 1052 ± 72 | 102.3 ± 7.1 | ns |
| Non-diabetes | |||||
| Salty snacks (kcal/day) | 39.1 ± 3.7 | 36.1 ± 3.5 | 39.6 ± 3.7 | 51.0 ± 3.6* | 0.048 |
| Beans/legumes (kcal/day) | 95.7 ± 4.6 | 102.9 ± 4.3 | 93.3 ± 4.6 | 106.7 ± 4.6* | ns# |
| Rice (kcal/day) | 206.2 ± 7.0 | 205.5 ± 6.7 | 214.7 ± 7.0 | 218.9 ± 7.0 | ns |
| Glycemic index (g/day) | 55.9 ± 0.2 | 56.5 ± 0.2* | 57.0 ± 0.2* | 57.0 ± 0.2* | ns# |
| Activity score | 32.0 ± 0.2 | 31.8 ± 0.2 | 31.2 ± 0.2 | 30.8 ± 0.2* | 0.008 |
| t.v. watching (h/day) | 43 ± 0.1 | 47 ± 0.1 | 5.1 ± 0.1* | 4.9 ± 0.1* | 0.010 |
Values are 1s mean ± S.E. and adjusted for energy intake, BMI, physical activity score, sex, age, education, income to poverty ratio, and diabetes. For sweets, analyses were stratified by diabetes due to a stress × diabetes interaction. ns, not statistically significant (P > 0.05).
Indicates a statistical difference (P ≤ 0.05) from the lowest quartile within the same row.
Indicates a tendency (P ≤ 0.01) to be statistically different.
Table 3.
Associations between stress measures and selected dietary and physical activity variables.a
| Variable | Parameter (β) estimates | P stress | P cortisol | P insulin | |
|---|---|---|---|---|---|
| Stressb | Cortisolc | Insulind | |||
| Energy (kcal/day) | – | – | ns | ns | ns |
| Carbohydrate (g/day) | 0.31 ± 0.16 | – | 0.050 | ns | ns |
| Protein (g/day) | −0.20 ± 0.06 | – | 0.001 | ns | ns |
| Fat (g/day) | – | 0.04 ± 0.01 | ns | ns | ns |
| Saturated fat (g/day) | – | 0.02 ± 0.01 | ns | 0.025 | ns |
| High-fat dairy (kcal/day) | – | 0.40 ± 0.15 | ns | 0.008 | ns |
| n3 fatty acids (g/day) | |||||
| EPA | −0.001 ± 0.002 | – | 0.020 | ns | ns |
| DHA | −0.001 ± 0.001 | – | 0.024 | ns | ns |
| DPA | −0.0002 ± 0.001 | – | 0.000 | ns | ns |
| Fruit servings/day | −0.01 ± 0001 | – | 0.000 | ns | ns |
| Vegetable servings/day | −0.004 ± 0.001 | – | 0.000 | ns | ns |
| Beans/legumes (kcal/day) | – | – | ns | ns | ns |
| Rice (kcal/day) | 0.75 ± 0.39 | – | 0.050 | ns | ns |
| Sweets (kcal/day) | 0.93 ± 0.41 | – | 0.023 | ns | ns |
| Diabetes | |||||
| Sweets (kcal/day) | – | – | ns | ns | ns |
| Non-diabetes | |||||
| Salty snacks (g/day) | 0.48 ± 0.21 | – | 0024 | ns | ns |
| Glycemic index (g/day) | 0.03 ± 0.01 | – | 0017 | ns | ns |
| White bread (kcal/day) | – | 0.39 ± 0.14 | ns | 0.006 | ns |
| Potatoes (kcal/day) | – | 0.14 ± 0.05 | ns | 0.013 | ns |
| Mexican/pizza (kcal/day) | – | 0.19 ± 0.09 | ns | 0.040 | ns |
| Activity score | −004 ± 0.01 | – | 0.003 | ns | ns |
| t.v. watching (h/day) | 0.02 ± 0.01 | 0.01 ± 0.004 | 0.005 | ns | 0.008 |
Stress, cortisol, and insulin independent effects on dietary intake and activity were tested in linear regression models that also included the independent variables sex, age, education, energy intake, BMI, physical activity score, income to poverty ratio, and diabetes For analyses of activity and time spent watching t.v. physical activity, t v watching, and energy intake were removed as independent variables.
Multiple linear regression parameter estimates When statistically significant (P ≤ 0.05), regression slope (β) estimates ± S.E. for stress, cortisol, and insulin are shown, ns, not statistically significant (P > 0.05).
Stress was measured through the Perceived Stress Scale, range 0–56.
Urinary cortisol (μg/g creatinine; range 0.45–339).
Serum insulin (μIU/ml; range 1.3–419).
Table 4.
Micro nutrient intakes associated with relative level of perceived stress.a
| Variable | Quartile of perceived stress | P stress | |||
|---|---|---|---|---|---|
| Lowest* | Middle lowest | Middle highest | Highest | ||
| Vitamin A (IU/day) | 9113 ± 320 | 8696 ± 302 | 7901 ± 318* | 8601 ± 318 | ns# |
| Vitamin B-6 (mg/day) | 2.71 ± 0.04 | 2.57 ± 0.04* | 2.49 ± 0.04* | 2.60 ± 0.04 | 0.006 |
| Total folate (μg/day) | 558 ± 10 | 536 ± 9 | 509 ± 10* | 553 ± 10 | 0.003 |
| Natural folate (μg/day) | 336 ± 5 | 331 ± 5 | 311 ± 5* | 340 ± 5 | 0.003 |
| Vitamin B-12 (mg/day) | 10.9 ± 0.4 | 10.0 ± 0.3 | 9.6 ± 0.4 | 9.7 ± 0.4 | ns |
| Vitamin C (mg/day) | 156 ± 4 | 145 ± 4 | 139 ± 4* | 150 ± 4 | ns# |
| Vitamin K (μg/day) | 91 ± 2 | 82 ± 2* | 77 ± 2* | 86 ± 2 | 0.001 |
| Beta-carotene (μg/day) | 3556 ± 167 | 3371 ± 157 | 3000 ± 166 | 3308 ± 166 | ns |
| Beta-cryptoxanthan (μg/day) | 238 ± 13 | 192 ± 12* | 196 ± 13* | 200 ± 13 | 0.050 |
| Lutem + zeaxanthin (μg/day) | 1646 ± 55 | 1405 ± 52* | 1368 ± 54* | 1482 ± 54* | 0.001 |
| Ca (mg/day) | 1112 ± 26 | 1062 ± 25 | 1074 ± 26 | 1092 ± 26 | ns |
| Fe (mg/day) | 21 ± 0.3 | 20 ± 0.3* | 20 ± 0.3* | 21 ± 0.3 | 0.030 |
| Mg (mg/day) | 377 ± 5 | 363 ± 5 | 347 ± 5* | 369 ± 5 | 0.001 |
| K (mg/day) | 3535 ± 39 | 3371 ± 37* | 3298 ± 39* | 3450 ± 38 | 0.000 |
Values are 1s mean ± S.E. and adjusted for energy intake, BMI, physical activity, sex, age, education, income to poverty ratio and diabetes.
Indicates a statistical difference (P ≤ 0.5) from the lowest quartile of perceived stress within the same row.
Indicates a tendency (P ≤ 0.1) to be statistically different.
We also found that the associations between perceived stress and intake of fruit, vegetables, and fiber were not linear (Fig. 1 and Table 2). Individuals displaying the highest level of perceived stress had fiber intakes that were not different than individuals displaying the lowest degree of stress, and this pattern reflected the relationship between BMI and perceived stress level, with significantly elevated BMI only in the middle highest group. In contrast, there was higher intake of legumes, carbohydrate, sweets, and salty snacks only in persons reporting the highest level of perceived stress (Table 2). Although mean rice intake did not differ significantly across stress quartiles (Fig. 1), linear regression analysis showed a positive association between stress and rice consumption (Table 3).
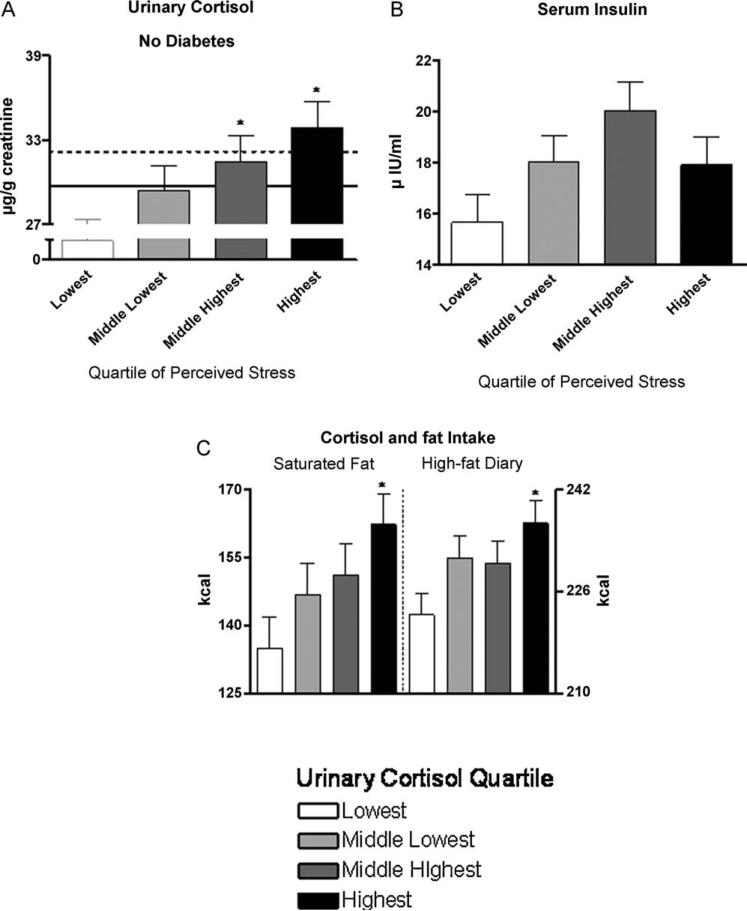
Perceived stress was associated with higher urinary cortisol, but only in those without diabetes (there was a significant stress × diabetes interaction; P = 0.01, Fig. 2). After adjusting for diabetes, there was a positive association between perceived stress and circulating insulin (Fig. 2), but only after removing the income to poverty ratio variable from the ANCOVA model. As expected, urinary cortisol tended (P = 0.11) to be higher in those with diabetes compared to those without diabetes, and this became significant after adjusting for insulin.
Fig. 2.
Independent of age, sex, education, BMI, and income to poverty ratio, perceived stress was associated with higher urinary cortisol in those without diabetes (A), but not in those with this condition.Independent of stress and not unexpectedly, diabetes tended (P = 0.11) to be associated with higher urinary cortisol (compare the dashed (diabetes) with the solid (without diabetes) lines, which represent average concentrations across stress quartiles).Similar to the pattern observed for BMI and independent of age, sex, education, income to poverty ratio, and diabetes, perceived stress tended to be associated with higher plasma insulin concentration, and this was due to a higher concentration in the middle highest stress quartile (B).When income to poverty ratio was removed from the model, the effect of stress on insulin was significant.After adjusting for sex, age, education, energy intake, BMI, physical activity, income to poverty ratio, and diabetes, urinary cortisol was associated with higher intakes of saturated fat and high-fat dairy foods (C). Values are least square mean ± S.E.Dietary intakes are daily values. *Statistically different from the lowest quartile (P ≤ 0.05).
Cortisol
When examined as quartiles and adjusted for energy intake, BMI, physical activity, education, sex, age, income to poverty ratio, and type 2 diabetes, urinary cortisol was associated with higher intakes of high-fat foods and foods with a high glycemic index (Fig. 2 and Table 3). More specifically, only in the highest quartile of cortisol were there significantly higher intakes of total saturated fatty acids, high-fat dairy foods, potatoes, and white bread. For the food group defined by sweets, there was a significant cortisol × diabetes interaction that was due to a directional difference in the association between cortisol and sweets intake. When separating the analysis by diabetes, we found a tendency (P = 0.08) for urinary cortisol to be associated with higher intake and, in those without diabetes, lower intake of sweet foods (P = 0.11). Although we did not find a relationship between cortisol and total energy intake, we found a positive and significant association between cortisol and intake of sweets, not adjusted for energy intake, only in those with diabetes. Individuals with diabetes and in the middle highest and highest quartiles of cortisol had significantly higher intake of sweets (middle highest, 71 ± 7 kcal; highest 72 ± 87) compared to those in the lowest quartile (44 ± 8 kcal). We did not find a relationship between cortisol and physical activity.
Insulin
Insulin concentrations were positively associated with television watching (Table 3), but we did not find any significant relationship between insulin and physical activity score or with any other dietary variable examined, after adjusting for energy intake, BMI, physical activity, sex, age, education, income to poverty ratio, and diabetes.
Discussion
Previous research has pointed to nutrition as a factor that may contribute to the high prevalence of chronic disease in Puerto Ricans living in the Boston, MA region (Lin, Bermudez, & Tucker, 2003; Tucker, 2005). For the first time in a large sample of this minority population, we investigated relationships between psychological stress and variables related to the physiology and behavior of eating and activity patterns. Overall, our findings suggest that psychological stress, which is also associated with the development of chronic mental and physical disease (Adler & Snibbe, 2003; Chrousos & Gold, 1992; McEwen, 2004; Sapolsky, 1992), is linked to dietary and activity patterns in this population. These findings, together with previous work that demonstrates effects of stress on food choice and exercise, suggest that stress may influence dietary and activity patterns in Puerto Rican older adults living in the Boston, Massachusetts area. Although we cannot determine the extent to which these relationships are causally linked, this cross sectional study is a key first step toward identifying stress as a potentially important barrier to healthy dietary and activity patterns in this minority group, particularly in those with type 2 diabetes attempting to adopt and adhere to a treatment strategy that includes exercise and limited intake of high-sugar foods to manage their disease.
Measures of life stress
The Perceived Stress Scale (PSS) is a validated instrument for measuring psychological stress (Cohen et al., 1983). Nonetheless, as with any self reported measure, the perceived stress score may not be an exact reflection of stress, particularly when subjects are asked to recall their experiences over past weeks. However, as might be expected, perceived stress scores in the current study were higher in participants with the poorest health (including more depression and anxiety), lowest social support, and highest ratio of bad to good life experiences. Furthermore, urinary cortisol, which is a well described physiological marker of psychological stress, was higher in participants reporting more stress. It is interesting that this relationship was true only in participants without type 2 diabetes. Although this may seem counterintuitive, uncontrolled diabetes has been associated with basal elevations in circulating glucocorticoids (Chiodini et al., 2007; Dallman et al., 1994; Schwartz et al., 1997; Schwartz, Strack, & Dallman, 1997; Sipols, Baskin, & Schwartz, 1995) and hypo-responsiveness of the HPA axis to psychological stress (Chan, Inouye, Vranic, & Matthews, 2002) and other stimuli, such as waking (Bruehl, Wolf, & Convit, 2009). Consistent with these studies, we found a tendency for average cortisol to be elevated in those with diabetes, but cortisol did not significantly differ across stress quartiles in these individuals. The lack of association between cortisol and perceived stress in those with diabetes may be due to a diabetes-induced decline in the expression of the neuropeptide corticotropin releasing factor (CRF), believed to mediate responsiveness of the HPA axis to psychological stress (Dallman et al., 2002) and to waking (Chang & Opp, 2001). Insulin concentrations and/or signaling may also play a role in this lack of association between perceived stress and cortisol in individuals with diabetes. We found that insulin was significantly higher with diabetes, but also with higher stress, independent of diabetes. Moreover, we found a negative association between insulin and cortisol only in those with diabetes. Therefore, it is not surprising that stress failed to vary with cortisol in participants with type 2 diabetes. Together, the relative magnitude of perceived stress reported by participants in the current study is consistent with other behavioral and physiological outcomes expected to contribute to and/or reflect psychological stress.
Dietary patterns
There is some evidence in humans, and considerable evidence in animal models, suggesting that stress can decrease intake of foods typically considered to promote good health. For example, in one study of the effect of a laboratory administered stressor on food choice, consumption of healthy, nutrient-rich foods (e.g., fruit) was reduced following the stressful task (Zellner et al., 2006). Rodent studies have consistently shown that, when either provided with only typical low-fat, low-sugar rodent chow or given a choice between the typical low-fat, low-sugar rodent chow and high fat, sweet foods, the animals reduce their intake of the low-fat, low-sugar diet in the presence of stressors (see Dallman et al., 2002). There is a physiological basis for this effect of stress; central corticotropin-releasing-factor has been shown to mediate, at least in part, this effect in rodents (Krahn, Gosnell, Grace, & Levine, 1986; Smagin, Howell, Redmann, Ryan, & Harris, 1999). Together with these previous findings, our results support the notion that stress may reduce motivation to eat recommended foods like fruit, vegetables, and protein. However, given that this was a cross sectional study, it is important to point out that we cannot draw conclusions about causality. For example, while stress may determine eating and activity patterns, it is also possible that certain eating and physical activity patterns lead to greater perceived stress in some persons. Although we did attempt to statistically account for factors like relative income, we also appreciate the possibility that other unmeasured confounding factors may influence dietary and activity patterns. Regardless of these potential issues of causality, our results highlight an important link between perceived stress and dietary patterns in this population, suggesting that stress is inconsistent with the intake of many health promoting foods and nutrients, including certain health-promoting fatty acids (e.g., omega-3, see Yashodhara et al., 2009), vitamin B-6, antioxidant vitamins A and C, minerals, and essential amino acids.
Stress has been shown to promote intake of highly palatable, typically nutrient-poor foods (e.g., “comfort foods”) and snacking (see Epel et al., 2001; Pecoraro et al., 2004). This potentially important effect of stress may be particularly relevant in modern societies where easy access to inexpensive and highly palatable foods is pervasive, and where overconsumption of these food types may increase risk for obesity, diabetes, and cardiovascular disease (Appel et al., 1997; Ludwig, 2002; Sacks et al., 2001). In light of previous reports showing increased intake of palatable snack foods after experimentally induced stress, our findings suggest that, in the Puerto Rican adults participating in this study, stress may increase the intake of these salty snacks and sweets. Moreover, those with type 2 diabetes, possibly due to basally higher insulin concentrations (Warne et al., 2009), may be particularly susceptible to these apparent effects of stress on the intake of high sugar foods (i.e., “sweets”). Alternatively, it is possible that consuming these highly palatable foods may stimulate stressfulness, particularly in persons attempting to limit their intake (e.g., restrained eaters). Furthermore, other factors that influence both perceived stress and food intake are also possible explanations for the observed relationships. Longitudinal data are needed to determine the causal pathways.
Stress-related eating results, in part, from the actions of cortisol and insulin on the brain (Dallman et al., 2007), and the concentrations of these hormones, relative to each other, play a primary role in mediating the actions of chronic stress to alter the composition of dietary intake and the partitioning of energy stores (Dallman, Akana, Strack, Hanson, & Sebastian, 1995; Dallman et al., 2003; Dallman et al., 2006; Laugero, Gomez, Manalo, & Dallman, 2002). In humans, elevated glucocorticoids clearly increase hunger and food intake (Aron, 2001; Tataranni et al., 1996), but it remains uncertain whether this effect is specific to any particular food types. Rodent studies suggest that glucocorticoids act in the brain to amplify motivational salience of high-fat and high-sugar, “comfort foods” (see Bhatnagar et al., 2000; Dallman et al., 2006; Laugero et al., 2002). Consistent with this effect of glucocorticoids, we found that urinary cortisol was associated with intakes of foods high in saturated fat, which may partly explain the link between this steroid hormone and the development of chronic disease. Increased consumption of saturated fat (see Feldeisen & Tucker, 2007; Riserus, Willett, & Hu, 2009; Woodside, McKinley, & Young, 2008) and higher cortisol concentrations (Chrousos, 2000; Peeke & Chrousos, 1995) have been linked to cardiovascular disease, diabetes, and other chronic diseases. Therefore, elevated cortisol, which was highly prevalent in this population, may make it more difficult for Puerto Ricans, and other groups, to adopt and adhere to nutritional guidelines that typically limit saturated fat. Alternatively, increasing high-fat foods may lead to elevations in cortisol. One study in rodents showed that consumption of a high-fat diet was associated with increases in circulating corticosterone (Tannenbaum et al., 1997). However, in that study, rats were not allowed a choice between the high-fat diet and a typical low-fat, low-sweet rodent chow. Studies have since shown that, when provided a choice between low-fat, low-sweet chow and high-fat or high-sweet diets, consumption of the palatable diet fails to alter basal corticosterone and actually blunts the corticosterone response to psychological stress (la Fleur, Houshyar, Roy, & Dallman, 2005). Therefore, the relationship between cortisol and fat intake is complex and may depend on factors that include food availability and access.
Not surprisingly, diabetes was associated with lower overall intakes of sweets. However, higher perceived stress and cortisol concentration were related to elevated consumption of sweets only in those with diabetes. Compared to non-Hispanic whites, Puerto Ricans living in the U.S. were more likely to have diabetes (Tucker, Bermudez et al., 2000). Therefore, recurrent exposure to psychological stressors may have particularly important implications in this Hispanic community. Because lifestyle modifications, such as the improvement of diet and physical activity can help with diabetes management, our results, together with previous reports (Bradshaw et al., 2007; Chiodini et al., 2007; Delahanty, Conroy, & Nathan, 2006), suggest that stress and/or elevated cortisol, along with rising insulin, may make it particularly challenging for those with diabetes trying to improve diet.
Physical activity
Clearly, being physically active can positively affect both mental and physical health. Furthermore, and not surprisingly, sedentary behaviors such as watching television have been shown to be associated with poor health (see Owen, Bauman, & Brown, 2009). Similar to eating behavior, regular participation in physical activity depends on several factors, including genetics, health status, and the environment (e.g., geographic proximity) (see Schutzer & Graves, 2004). However, growing evidence also supports the importance of psychosocial factors as potential barriers to physical activity. Prospective and cross sectional research suggest that stress may depress desire to engage in physical activity and increase motivation for sedentary behaviors, particularly those that are highly rewarding (see Epstein, Roemmich, Saad, & Handley, 2004; Roemmich et al., 2003). Stress-related psychological conditions such as depression may also increase risk for decline in physical activity (Roshanaei-Moghaddam, Katon, & Russo, 2009). On the other hand, physical activity may reduce feelings of stress and has long been known to reduce risk for developing stress-related psychiatric diseases such as depression. Given these previous reports, our findings support a possible link between higher perceived stress, less physical activity, and more television watching in our sample of Puerto Rican older adults.
Relatively little is known about the physiological mechanisms that might link stress and physical activity or inactivity. We measured two stress responsive hormones, cortisol and insulin, but the inverse relationship between perceived stress and physical activity remained after statistically adjusting for the status of these hormones. However, given the cross sectional nature of our study, we cannot discount the possible discordance between self reported stress and physiological responses to stress (Rachman & Hodgson, 1974). Independent from stress and cortisol we did find that increases in circulating insulin were associated with more time watching television, which is consistent with previous reports (see Ford et al., 2010) and may be due to increased snacking that frequently occurs while watching television.
BMI
Psychological stress can cause body weight gain or loss (see Gold & Chrousos, 1999; Kivimaki et al., 2006), and this may depend on changes in appetite and patterns of food intake and physical activity. We found that the association between stress and BMI in Puerto Rican elders may be determined by dietary patterns as well as other variables that include smoking, insulin, and overall health status. When covariate analysis of stress quartiles was applied, the patterns of fruit, vegetable, and fiber intake were not always linear across increasing self-reports of stress. Individuals in the middle-highest quartile of stress consistently had the lowest intakes of these recommended foods. For fiber, similar intakes were observed in the highest and lowest quartile of stress, which may reflect the increased intake of legumes by persons in the highest stress quartile. Although intakes of fruit and vegetables were still lower in the highest quartile of stress compared to the lowest quartile, average intakes of these foods were greater in the highest stress quartile compared to the middle-highest quartile. Together, this pattern of eating across levels of perceived stress may explain why the mean BMI of persons in the highest quartile of perceived stress was similar to the mean BMI observed in the lowest stress quartile. Given that this was a cross sectional analysis, we can only speculate on this apparent U-shaped pattern of BMI and intakes of these foods, but we did find some differences unique to the highest quartile of stress that might help explain this pattern.
The highest relative level of perceived stress could be uniquely characterized as having the poorest health (including more depression and anxiety), lowest social support, highest ratio of bad to good life experiences, and the greatest amount of smoking. Moreover, relative to total energy, intakes of sweets, salty snacks, and carbohydrates were elevated only in persons reporting the highest level of stress. However, legume intake was also higher in this group, possibly because rice and beans may be considered to be a form of “comfort” food in this culture, and the high fiber content of legumes may contribute to lower BMI and obesity (Newby, Muller, Hallfrisch, Andres, & Tucker, 2004; Newby et al., 2003); we found a negative correlation between intake of legumes and BMI. Beans have traditionally been a dietary staple of Puerto Ricans (e.g., Samolsky, Dunker, & Hynak-Hankinson, 1990). However, increasing exposure to the available foods of the “American” diet can alter the traditional dietary patterns in this population (Bermudez, Falcon, & Tucker, 2000). Smoking is also known to be negatively correlated with body weight (e.g., Akbartabartoori, Lean, & Hankey, 2005), and this may also explain why BMI was similar in the lowest and the highest quartiles of stress. Lastly, as with major depression, psychological stress can cause body weight gain or loss (see Gold & Chrousos, 1999; Kivimaki et al., 2006), and this may depend on changes in appetite and pattern of food intake. Therefore, although energy intake did not significantly differ across stress quartiles, it is possible that individuals reporting the highest degree of stress have lower weight as a consequence of the catabolic effects of stress in combination with their unique dietary patterns and tendency to eat less.
Perspective
Puerto Rican older adults in Massachusetts have been shown to be more likely to present with depression, cognitive impairment, diabetes, and other chronic health problems than non-Hispanic whites (Tucker, Falcon et al., 2000). Psychological stress is linked with chronic disease, and it has been hypothesized that this may be particularly important to Puerto Ricans and other stress-vulnerable populations and individuals. Given the important link between exercise, diet, body weight, and chronic disease, and the paucity of information describing the relationship between stress and nutritional behavior in this minority population, our results (1) provide new information about the association patterns between stress, food intake, and physical activity in Puerto Rican adults and (2) highlight the importance of further understanding the possible impact of psychological stress as a barrier to adopting and maintaining healthy eating and activity practices in this population, particularly in those with type 2 diabetes. Longitudinal research to determine causal pathways for these identified associations is warranted.
Footnotes
Supported by NIH 5P01-AG023394 and USDA-Agricultural Research Service CRIS# 5306-51530-019-00D and USDA-Agricultural Research Service agreement 58-1950-7-707.
The authors’ responsibilities were as follows – KLT and LMF: study concept and design; acquisition of data; KDL, LMF, and KLT: analysis and interpretation of data; KDL: draft of the manuscript; KDL, LMF, and KLT: critical revision of the manuscript for important intellectual content; KDL, LMF, and KLT: statistical analysis; LMF and KLT: obtained funding for study; KLT: study supervision. No conflicts of interest reported.
References
- Adam TC, Epel ES. Stress, eating and the reward system. Physiology & Behavior. 2007;91(4):449–458. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Adler NE, Boyce T, Chesney MA, Cohen S, Folkman S, Kahn RL, et al. Socioeconomic status and health. The challenge of the gradient. American Psychologist. 1994;49:15–24. doi: 10.1037//0003-066x.49.1.15. [DOI] [PubMed] [Google Scholar]
- Adler NE, Snibbe AC. The role of psychosocial processes in explaining the gradient between socioeconomic status and health. Current Directions in Psychological Science. 2003;12(4):119–123. [Google Scholar]
- Akbartabartoori M, Lean ME, Hankey CR. Relationships between cigarette smoking, body size and body shape. International Journal of Obesity (London) 2005;29(2):236–243. doi: 10.1038/sj.ijo.0802827. [DOI] [PubMed] [Google Scholar]
- Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. The New England Journal of Medicine. 1997;336(16):1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- Aron DC. Glucocorticoids and Adrenal Androgens. Vol. 6. Lange Medical Books/McGraw-Hill; New York: 2001. Basic and Clinical Endocrinology. pp. 334–376. [Google Scholar]
- Bermudez OI, Falcon LM, Tucker KL. Intake and food sources of macronutrients among older Hispanic adults. Association with ethnicity, acculturation, and length of residence in the United States. Journal of the American Dietetic Association. 2000;100(6):665–673. doi: 10.1016/s0002-8223(00)00195-4. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Bell ME, Liang J, Soriano L, Nagy TR, Dallman MF. Corticosterone facilitates saccharin intake in adrenalectomized rats. Does corticosterone increase stimulus salience? Journal of Neuroendocrinology. 2000;12(5):453–460. doi: 10.1046/j.1365-2826.2000.00487.x. [DOI] [PubMed] [Google Scholar]
- Block G, Subar AF. Estimates of nutrient intake from a food frequency questionnaire. The 1987 National Health Interview Survey. Journal of the American Dietetic Association. 1992;92(8):969–977. [PubMed] [Google Scholar]
- Bradshaw BG, Richardson GE, Kumpfer K, Carlson J, Stanchfield J, Overall J, et al. Determining the efficacy of a resiliency training approach in adults with type 2 diabetes. 2007;33:650–659. doi: 10.1177/0145721707303809. [DOI] [PubMed] [Google Scholar]
- Brown JD, Lawton M. Stress and well-being in adolescence. The moderating role of physical exercise. Journal of Human Stress. 1986;12(3):125–131. doi: 10.1080/0097840X.1986.9936777. [DOI] [PubMed] [Google Scholar]
- Bruehl H, Wolf OT, Convit A. A blunted cortisol awakening response and hippocampal atrophy in type 2 diabetes mellitus. Psychoneuroendocrinology. 2009 doi: 10.1016/j.psyneuen.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes RC, Padilla AM, de Snyder NS. The hispanic stress inventory. A culturally relevant approach to psychosocial. Assessment Psychological, Assessment: A Journal of Consulting and Clinical Psychology: American Psychological Association. 1991;3(3):438–447. [Google Scholar]
- Chan O, Inouye K, Vranic M, Matthews SG. Hyperactivation of the hypothalamo-pituitary-adrenocortical axis in streptozotocin-diabetes is associated with reduced stress responsiveness and decreased pituitary and adrenal sensitivity. Endocrinology. 2002;143(5):1761–1768. doi: 10.1210/endo.143.5.8809. [DOI] [PubMed] [Google Scholar]
- Chang FC, Opp MR. Corticotropin-releasing hormone (CRH) as a regulator of waking. Neuroscience and Biobehavioral Reviews. 2001;25(5):445–453. doi: 10.1016/s0149-7634(01)00024-0. [DOI] [PubMed] [Google Scholar]
- Chang MW, Nitzke S, Guilford E, Adair CH, Hazard DL. Motivators and barriers to healthful eating and physical activity among low-income over-weight and obese mothers. Journal of the American Dietetic Association. 2008;108(6):1023–1028. doi: 10.1016/j.jada.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Chiodini I, Adda G, Scillitani A, Coletti F, Morelli V, Di Lembo S, et al. Cortisol secretion in patients with type 2 diabetes. Relationship with chronic complications. 2007;30:83–88. doi: 10.2337/dc06-1267. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. The role of stress and the hypothalamic-pituitary-adrenal axis in the pathogenesis of the metabolic syndrome. Neuro-endocrine and target tissue-related causes. International Journal of Obesity and Related Metabolic Disorders. 2000;24(Suppl. 2):S50–S55. doi: 10.1038/sj.ijo.0801278. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. Journal of the American Medical Association. 1992;267(9):1244–1252. [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24(4):385–396. [PubMed] [Google Scholar]
- Dallman M, Pecoraro N, Akana S, la Fleur S, Gomez F, Houshyar H, et al. Chronic stress and obesity. A new view of “comfort food”. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Bradbury MJ, Strack AM, Hanson ES, Scribner KA. Regulation of the hypothalamo-pituitary-adrenal axis during stress. Feedback, facilitation and feeding. Seminars in Neuroscience. 1994;6(4):205–213. [Google Scholar]
- Dallman MF, Akana SF, Strack AM, Hanson ES, Sebastian RJ. The neural network that regulates energy balance is responsive to glucocorticoids and insulin and also regulates HPA axis responsivity at a site proximal to CRF neurons. Annals of the New York Academy of Sciences. 1995;771:730–742. doi: 10.1111/j.1749-6632.1995.tb44724.x. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro NC, La Fleur SE, Warne JP, Ginsberg AB, Akana SF, et al. Progress in Brain Research. Vol. 153. Elsevier; 2006. Glucocorticoids, chronic stress, and obesity. pp. 75–105. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Viau V, Bhatnagar S, Gomez F, Laugero KD, Bell MF. Corticotropin-releasing factor, corticosteroids, stress, and sugar. Energy balance, the brain, and behavior. In: Pfaff DW, editor. Hormones, brain, and behavior. Academic Press; San Diego, CA, USA: 2002. pp. 571–631. [Google Scholar]
- Dallman MF, Warne JP, Foster MT, Pecoraro NC. Glucocorticoids and insulin both modulate caloric intake through actions on the brain. The Journal of Physiology. 2007;583(Pt 2):431–436. doi: 10.1113/jphysiol.2007.136051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delahanty LM, Conroy MB, Nathan DM. psychological predictors of physical activity in the diabetes prevention program. Journal of the American Dietetic Association. 2006;106(5):698–705. doi: 10.1016/j.jada.2006.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado JL, Johnson CL, Roy I, Trevino FM. Hispanic health and nutrition examination survey. Methodological considerations. American Journal of Public Health. 1990;80(Suppl.):6–10. doi: 10.2105/ajph.80.suppl.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreon DM, John EM, DiCiccio Y, Whittemore AS. Use of NHANES data to assign nutrient densities to food groups in a multiethnic diet history questionnaire. Nutrition and Cancer. 1993;20(3):223–230. doi: 10.1080/01635589309514290. [DOI] [PubMed] [Google Scholar]
- Epel E, Lapidus R, McEwen B, Brownell K. Stress may add bite to appetite in women. A laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology. 2001;26(1):37–49. doi: 10.1016/s0306-4530(00)00035-4. [DOI] [PubMed] [Google Scholar]
- Epel ES. Psychological and metabolic stress. A recipe for accelerated cellular aging? Hormones (Athens) 2009;8(1):7–22. doi: 10.14310/horm.2002.1217. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Roemmich JN, Saad FG, Handley EA. The value of sedentary alternatives influences child physical activity choice. International Journal of Behavioral Medicine. 2004;11(4):236–242. doi: 10.1207/s15327558ijbm1104_7. [DOI] [PubMed] [Google Scholar]
- Feldeisen SE, Tucker KL. Nutritional strategies in the prevention and treatment of metabolic syndrome. Applied Physiology, Nutrition, and Metabolism. 2007;32(1):46–60. doi: 10.1139/h06-101. [DOI] [PubMed] [Google Scholar]
- Ford ES, Li C, Zhao G, Pearson WS, Tsai J, Churilla JR. Sedentary behavior, physical activity, and concentrations of insulin among US adults. Metabolism: Clinical and Experimental. 2010 doi: 10.1016/j.metabol.2009.11.020. [DOI] [PubMed] [Google Scholar]
- Foreyt JP, Poston WS., 2nd The role of the behavioral counselor in obesity treatment. Journal of the American Dietetic Association. 1998;98(10 Suppl. 2):S27–S30. doi: 10.1016/s0002-8223(98)00707-x. [DOI] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP. The endocrinology of melancholic and atypical depression. Relation to neurocircuitry and somatic consequences. Proceedings of the Association of American Physicians. 1999;111(1):22–34. doi: 10.1046/j.1525-1381.1999.09423.x. [DOI] [PubMed] [Google Scholar]
- Griffin KW, Friend R, Eitel P, Lobel M. Effects of environmental demands, stress, and mood on health practices. Journal of Behavioral Medicine. 1993;16(6):643–661. doi: 10.1007/BF00844724. [DOI] [PubMed] [Google Scholar]
- Hoffman MD, Hoffman DR. Exercisers achieve greater acute exercise-induced mood enhancement than nonexercisers. Archives of Physical Medicine and Rehabilitation. 2008;89(2):358–363. doi: 10.1016/j.apmr.2007.09.026. [DOI] [PubMed] [Google Scholar]
- King AC. Intervention strategies and determinants of physical activity and exercise behavior in adult and older adult men and women. World Review of Nutrition and Dietetics. 1997;82:148–158. doi: 10.1159/000059626. [DOI] [PubMed] [Google Scholar]
- Kivimaki M, Head J, Ferrie JE, Shipley MJ, Brunner E, Vahtera J, et al. Work stress, weight gain and weight loss. Evidence for bidirectional effects of job strain on body mass index in the Whitehall II study. International Journal of Obesity (London) 2006;30(6):982–987. doi: 10.1038/sj.ijo.0803229. [DOI] [PubMed] [Google Scholar]
- Kornitzer M, Kittel F. How does stress exert its effects. Smoking, diet and obesity, physical activity? Postgraduate Medical Journal. 1986;62(729):695–696. doi: 10.1136/pgmj.62.729.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahn DD, Gosnell BA, Grace M, Levine AS. CRF antagonist partially reverses CRF- and stress-induced effects on feeding. Brain Research Bulletin. 1986;17(3):285–289. doi: 10.1016/0361-9230(86)90233-9. [DOI] [PubMed] [Google Scholar]
- Krause N, Goldenhar L, Liang J, Jay G, Maeda D. Stress and exercise among the Japanese elderly. Social Science & Medicine. 1993;36(11):1429–1441. doi: 10.1016/0277-9536(93)90385-h. [DOI] [PubMed] [Google Scholar]
- Kubzansky LD, Kawachi I, Sparrow D. Socioeconomic status, hostility, and risk factor clustering in the Normative Aging Study. Any help from the concept of allostatic load? Annals of Behavioral Medicine. 1999;21(4):330–338. doi: 10.1007/BF02895966. [DOI] [PubMed] [Google Scholar]
- la Fleur SE, Houshyar H, Roy M, Dallman MF. Choice of lard, but not total lard calories, damps adrenocorticotropin responses to restraint. Endo: revue francaise d'endodontie: publication officielle de la Societe francaise d'endodontie. 2005;146(5):2193–2199. doi: 10.1210/en.2004-1603. [DOI] [PubMed] [Google Scholar]
- Laugero KD, Gomez F, Manalo S, Dallman MF. Corticosterone infused intracerebroventricularly inhibits energy storage and stimulates the hypothalamo-pituitary axis in adrenalectomized rats drinking sucrose. Endocrinology. 2002;143(12):4552–4562. doi: 10.1210/en.2002-220613. [DOI] [PubMed] [Google Scholar]
- Lin H, Bermudez OI, Tucker KL. Dietary patterns of Hispanic elders are associated with acculturation and obesity. The Journal of Nutrition. 2003;133(11):3651–3657. doi: 10.1093/jn/133.11.3651. [DOI] [PubMed] [Google Scholar]
- Ludwig DS. The glycemic index. Physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. The Journal of the American Medical Association. 2002;287(18):2414–2423. doi: 10.1001/jama.287.18.2414. [DOI] [PubMed] [Google Scholar]
- Mackinnon A, McCallum J, Andrews G, Anderson I. The center for epidemiological studies depression scale in older community samples in Indonesia, North Korea, Myanmar, Sri Lanka, and Thailand. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences. 1998;53(6):P343–P352. doi: 10.1093/geronb/53b.6.p343. [DOI] [PubMed] [Google Scholar]
- McCann BS, Warnick GR, Knopp RH. Changes in plasma lipids and dietary intake accompanying shifts in perceived workload and stress. Psychosomatic Medicine. 1990;52(1):97–108. doi: 10.1097/00006842-199001000-00008. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Annals of the New York Academy of Sciences. Vol. 1032. Blackwell Publishing; 2004. Protection and damage from acute and chronic stress. Allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. pp. 1–7. [DOI] [PubMed] [Google Scholar]
- Michaud CI, Kahn JP, Musse N, Burlet C, Nicolas JP, Mejean L. Relationships between a critical life event and eating behaviour in high school students. Stress Medicine. 1990;6:57–64. [Google Scholar]
- National Institutes of Health, N Five-year strategic plan for reducing health disparities. 2001 Retrieved from: http://www.nimh.nih.gov/strategic/healthdisparitites.pdf.
- Newby PK, Muller D, Hallfrisch J, Andres R, Tucker KL. Food patterns measured by factor analysis and anthropometric changes in adults. The American Journal of Clinical Nutrition. 2004;80(2):504–513. doi: 10.1093/ajcn/80.2.504. [DOI] [PubMed] [Google Scholar]
- Newby PK, Muller D, Hallfrisch J, Qiao N, Andres R, Tucker KL. Dietary patterns and changes in body mass index and waist circumference in adults. The American Journal of Clinical Nutrition. 2003;77(6):1417–1425. doi: 10.1093/ajcn/77.6.1417. [DOI] [PubMed] [Google Scholar]
- Newman E, O'Connor DB, Conner M. Daily hassles and eating behaviour. The role of cortisol reactivity status. Psychoneuroendocrinology. 2007;32(2):125–132. doi: 10.1016/j.psyneuen.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Noel SE, Newby PK, Ordovas JM, Tucker KL. A traditional rice and beans pattern is associated with metabolic syndrome in Puerto Rican older adults. Journal of Nutrition. 2009;139(7):1360–1367. doi: 10.3945/jn.109.105874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norbeck JS. Modification of life event questionnaires for use with female respondents. Research in Nursing & Health. 1984;7(1):61–71. doi: 10.1002/nur.4770070110. [DOI] [PubMed] [Google Scholar]
- Norbeck JS, Lindsey AM, Carrieri VL. The development of an instrument to measure social support. Nursing Research. 1981;30(5):264–269. [PubMed] [Google Scholar]
- Norris R, Carroll D, Cochrane R. The effects of physical activity and exercise training on psychological stress and well-being in an adolescent population. Journal of Psychosomatic Research. 1992;36(1):55–65. doi: 10.1016/0022-3999(92)90114-h. [DOI] [PubMed] [Google Scholar]
- Oliver G, Wardle J, Gibson EL. Stress and food choice. A laboratory study. Psychosomatic Medicine. 2000;62(6):853–865. doi: 10.1097/00006842-200011000-00016. [DOI] [PubMed] [Google Scholar]
- Oman RF, King AC. The effect of life events and exercise program format on the adoption and maintenance of exercise behavior. Health Psychology. 2000;19(6):605–612. doi: 10.1037//0278-6133.19.6.605. [DOI] [PubMed] [Google Scholar]
- Owen N, Bauman A, Brown W. Too much sitting. A novel and important predictor of chronic disease risk? British Journal of Sports Medicine. 2009;43(2):81–83. doi: 10.1136/bjsm.2008.055269. [DOI] [PubMed] [Google Scholar]
- Paffenbarger RS, Hyde RT, Wing AL, Lee IM, Jung DL, Kampert JB. The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. 1993;328:538–545. doi: 10.1056/NEJM199302253280804. [DOI] [PubMed] [Google Scholar]
- Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress. Feedforward and feedback effects of chronic stress. Endo: revue francaise d'endodontie: publication officielle de la Societe francaise d'endodontie. 2004;145(8):3754–3762. doi: 10.1210/en.2004-0305. [DOI] [PubMed] [Google Scholar]
- Peeke PM, Chrousos GP. Hypercortisolism and obesity. Annals of the New York Academy of Sciences. 1995;771:665–676. doi: 10.1111/j.1749-6632.1995.tb44719.x. [DOI] [PubMed] [Google Scholar]
- Rachman S, Hodgson RI. Synchrony and desynchrony in fear and avoidance. Behaviour Research and Therapy. 1974;12(4):311–318. doi: 10.1016/0005-7967(74)90005-9. [DOI] [PubMed] [Google Scholar]
- Riserus U, Willett WC, Hu FB. Dietary fats and prevention of type 2 diabetes. Progress in Lipid Research. 2009;48(1):44–51. doi: 10.1016/j.plipres.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roemmich JN, Gurgol CM, Epstein LH. Influence of an interpersonal laboratory stressor on youths’ choice to be physically active. Obesity Research. 2003;11(9):1080–1087. doi: 10.1038/oby.2003.148. [DOI] [PubMed] [Google Scholar]
- Roemmich JN, Wright SM, Epstein LH. Dietary restraint and stress-induced snacking in youth. Obesity Research. 2002;10(11):1120–1126. doi: 10.1038/oby.2002.152. [DOI] [PubMed] [Google Scholar]
- Roshanaei-Moghaddam B, Katon WJ, Russo J. The longitudinal effects of depression on physical activity. General Hospital Psychiatry. 2009;31(4):306–315. doi: 10.1016/j.genhosppsych.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, et al. DASH-Sodium Collaborative Research Group Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. The New England Journal of Medicine. 2001;344(1):3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- Samolsky S, Dunker K, Hynak-Hankinson MT. Feeding the Hispanic hospital patient. Cultural considerations. Journal of the American Dietetic Association. 1990;90(12):1707–1710. [PubMed] [Google Scholar]
- Sapolsky RM. Stress, the aging brain, and the mechanisms of neuron death. MIT Press; Cambridge, Massachusetts: 1992. [Google Scholar]
- Schutzer KA, Graves BS. Barriers and motivations to exercise in older adults. Preventive Medicine. 2004;39(5):1056–1061. doi: 10.1016/j.ypmed.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Strack AM, Dallman MF. Evidence that elevated plasma corticosterone levels are the cause of reduced hypothalamic corticotrophin-releasing hormone gene expression in diabetes. Regulatory Peptides. 1997;72(2–3):105–112. doi: 10.1016/s0167-0115(97)01043-4. [DOI] [PubMed] [Google Scholar]
- Sipols AJ, Baskin DG, Schwartz MW. Effect of intracerebroventricular insulin infusion on diabetic hyperphagia and hypothalamic neuropeptide gene expression. Diabetes. 1995;44(2):147–151. doi: 10.2337/diab.44.2.147. [DOI] [PubMed] [Google Scholar]
- Smagin GN, Howell LA, Redmann S, Jr., Ryan DH, Harris RB. Prevention of stress-induced weight loss by third ventricle CRF receptor antagonist. The American Journal of Physiology. 1999;276(5 Pt 2):R1461–R1468. doi: 10.1152/ajpregu.1999.276.5.R1461. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Wardle J, Pollard TM, Canaan L, Davies GJ. Stress, social support and health-related behavior. A study of smoking, alcohol consumption and physical exercise. Journal of Psychosomatic Research. 1996;41(2):171–180. doi: 10.1016/0022-3999(96)00095-5. [DOI] [PubMed] [Google Scholar]
- Stetson BA, Rahn JM, Dubbert PM, Wilner BI, Mercury MG. Prospective evaluation of the effects of stress on exercise adherence in community-residing women. Health Psychology. 1997;16(6):515–520. doi: 10.1037//0278-6133.16.6.515. [DOI] [PubMed] [Google Scholar]
- Tannenbaum BM, Brindley DN, Tannenbaum GS, Dallman MF, McArthur MD, Meaney MJ. High-fat feeding alters both basal and stress-induced hypothalamic-pituitary-adrenal activity in the rat. The American Journal of Physiology. 1997;273(6 Pt 1):E1168–E1177. doi: 10.1152/ajpendo.1997.273.6.E1168. [DOI] [PubMed] [Google Scholar]
- Tataranni PA, Larson DE, Snitker S, Young JB, Flatt JP, Ravussin E. Effects of glucocorticoids on energy metabolism and food intake in humans. American Journal of Physiology. Endocrinology and Metabolism. 1996;271(2):E317–E325. doi: 10.1152/ajpendo.1996.271.2.E317. [DOI] [PubMed] [Google Scholar]
- Tucker KL. Stress and nutrition in relation to excess development of chronic disease in Puerto Rican adults living in the Northeastern USA. The Journal of Medical Investigation. 2005;52(Suppl):252–258. doi: 10.2152/jmi.52.252. [DOI] [PubMed] [Google Scholar]
- Tucker KL, Bermudez OI, Castaneda C. Type 2 diabetes is prevalent and poorly controlled among Hispanic elders of Caribbean origin. American Journal of Public Health. 2000;90(8):1288–1293. doi: 10.2105/ajph.90.8.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker KL, Bianchi LA, Maras J, Bermudez OI. Adaptation of a food frequency questionnaire to assess diets of Puerto Rican and non-Hispanic adults. American Journal of Epidemiology. 1998;148(5):507–518. doi: 10.1093/oxfordjournals.aje.a009676. [DOI] [PubMed] [Google Scholar]
- Tucker KL, Falcon LM, Bianchi LA, Cacho E, Bermudez OI. Self-reported prevalence and health correlates of functional limitation among Massachusetts elderly Puerto Ricans, Dominicans, and non-Hispanic white neighborhood comparison group. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2000;55(2):M90–97. doi: 10.1093/gerona/55.2.m90. [DOI] [PubMed] [Google Scholar]
- Wallis DJ, Hetherington MM. Stress and eating. The effects of ego-threat and cognitive demand on food intake in restrained and emotional eaters. Appetite. 2004;43(1):39–46. doi: 10.1016/j.appet.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Wallis DJ, Hetherington MM. Emotions and eating. Self-reported and experimentally induced changes in food intake under stress. Appetite. 2009;52(2):355–362. doi: 10.1016/j.appet.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Wardle J, Steptoe A, Oliver G, Lipsey Z. Stress, dietary restraint and food intake. Journal of Psychosomatic Research. 2000;48(2):195–202. doi: 10.1016/s0022-3999(00)00076-3. [DOI] [PubMed] [Google Scholar]
- Warne JP, Akana SF, Ginsberg AB, Horneman HF, Pecoraro NC, Dallman MF. Disengaging insulin from corticosterone. Roles of each on energy intake and disposition. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2009;296(5):R1366–1375. doi: 10.1152/ajpregu.91016.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner G, Kohlmann C-W, Dotzauer E, Burns LR. Anxiety, stress, and coping. Vol. 9. Routledge; 1996. The effects of academic stress on health behaviors in young adults. pp. 123–133. [Google Scholar]
- Wilson ME, Fisher J, Fischer A, Lee V, Harris RB, Bartness TJ. Quantifying food intake in socially housed monkeys. Social status effects on caloric consumption. Physiology & Behavior. 2008;94(4):586–594. doi: 10.1016/j.physbeh.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodside JV, McKinley MC, Young IS. Saturated and trans fatty acids and coronary heart disease. Current Atherosclerosis Reports. 2008;10(6):460–466. doi: 10.1007/s11883-008-0072-5. [DOI] [PubMed] [Google Scholar]
- Yashodhara BM, Umakanth S, Pappachan JM, Bhat SK, Kamath R, Choo BH. Omega-3 fatty acids. A comprehensive review of their role in health and disease. Postgraduate Medical Journal. 2009;85(1000):84–90. doi: 10.1136/pgmj.2008.073338. [DOI] [PubMed] [Google Scholar]
- Zellner DA, Loaiza S, Gonzalez Z, Pita J, Morales J, Pecora D, et al. Food selection changes under stress. Physiology & Behavior. 2006;87(4):789–793. doi: 10.1016/j.physbeh.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Zellner DA, Saito S, Gonzalez J. The effect of stress on men's food selection. Appetite. 2007;49(3):696–699. doi: 10.1016/j.appet.2007.06.013. [DOI] [PubMed] [Google Scholar]