Abstract
Introduction
Kidney transplantation is the preferred treatment for patients with end-stage renal disease, as it substantially increases a patient’s survival and is cost-saving compared to a lifetime of dialysis. However, transplantation is not universally chosen by patients with renal failure, and limited knowledge about the survival benefit of transplantation versus dialysis may play a role. We created a mobile application clinical decision aid called iChoose Kidney to improve access to individualized prognosis information comparing dialysis and transplantation outcomes.
Methods
We describe the iChoose Kidney study, a randomized controlled trial designed to test the clinical efficacy of a mobile health decision aid among end-stage renal disease patients referred for kidney transplantation at 3 large, diverse transplant centers across the United States. Approximately 450 patients will be randomized to receive either (i) standard of care or “usual” transplantation education, or (ii) standard of care plus iChoose Kidney.
Results
The primary outcome is change in knowledge about the survival benefit of kidney transplantation versus dialysis from baseline to immediate follow-up; secondary outcomes include change in treatment preferences, improved decisional conflict, and increased access to kidney transplantation. Analyses are also planned to examine effectiveness across subgroups of race, socioeconomic status, health literacy, and health numeracy.
Discussion
Engaging patients in health care choices can increase patient empowerment and improve knowledge and understanding of treatment choices. If the effectiveness of iChoose Kidney has a greater impact on patients with low health literacy, lower socioeconomic status, and minority race, this decision aid could help reduce disparities in access to kidney transplantation.
Keywords: education, kidney transplantation, mobile health, randomized trial, staff
For the majority of the more than 600,000 patients in the United States with end-stage renal disease (ESRD),1 kidney transplantation is the preferred treatment, providing longer survival, better quality of life, lower hospitalization rates, and substantial cost savings compared to dialysis.2, 3
Patients who receive dialysis have an expected remaining lifetime of 5.9 years versus 16.4 years for transplant recipients, yet a large number of patients have not actively pursued wait-listing for a transplant.1 However, the relative risk of death varies substantially depending on individual characteristics, including age, race, or comorbidities.4 Prior studies suggest that most patients want information about treatment options and want to participate in the selection of treatment.5, 6 However, current literature suggests that ESRD patients have very limited knowledge about their mortality rate on dialysis versus transplant, and that not all patients are aware of the survival benefit of transplantation.7, 8, 9 In dialysis facilities, only 18% of centers reported having detailed discussions about the risks and benefits of living- and deceased-donor transplant.10 In addition, dialysis facility transplant educators have been found to need improved education on the benefits and process of renal transplantation.11 While transplant education ideally should start prior to ESRD and referral to a transplant center, providing information about the survival benefit of transplant versus dialysis, and in particular living- versus deceased-donor transplant, could help to increase patients’ knowledge and preferences to get a transplant.
We previously developed and validated a novel, shared patient–provider clinical decision aid called iChoose Kidney (iPad, iPhone, and website: www.ichoosekidney.emory.edu). iChoose Kidney is an electronic application that compares mortality on dialysis versus kidney transplantation to translate medical evidence into terms understandable to patients. Models of mortality for patients receiving dialysis versus deceased-donor (DD) or living-donor (LD) kidney transplantation were developed using a cohort of more than 700,000 patients in the nationally representative United States Renal Data System (2000–2011 data).12 The intention of this clinical decision aid is to help facilitate patient–provider discussions about the risks and benefits of transplantation versus dialysis and to support informed decision making among patients with ESRD.
While mobile health application production has increased substantially in the last several years,13 with more than 100,000 iOS and Android health-related applications14, 15 currently in the marketplace, few methodologically rigorous studies have been conducted to confirm the efficacy or effectiveness of mobile health applications in improving access and outcomes of health. To our knowledge, no studies have been conducted to examine whether a mobile health application, designed to influence the shared clinical decision-making process between patient and provider to choose a treatment option, influences knowledge about treatment options and access to kidney transplantation.
The purpose of this paper is to describe the study protocol used to design the iChoose Kidney randomized controlled trial to test the clinical efficacy of a clinical decision aid in improving knowledge about the survival benefit of transplantation versus dialysis. We will also examine secondary end points of decreased decisional conflict in choosing treatment options for ESRD, changing treatment preference from dialysis to transplant, and access to kidney transplantation. Planned subgroup analyses will also examine whether the efficacy of iChoose Kidney varies by health literacy, numeracy, and race. Finally, this study will seek to evaluate the usability of the iChoose Kidney tool among clinical providers (transplant nephrologists and surgeons).
Materials and Methods
Study Overview
The iChoose Kidney Study is a 2-arm randomized trial to test the efficacy of a mobile health clinical decision aid on improving patient knowledge about the survival benefit of transplantation versus dialysis. Prior to initiation of study activities, the iChoose Kidney Study was registered on ClinicalTrials.gov (protocol NCT02235571). This study was approved by Institutional Review Boards at Emory University, Columbia University, and Northwestern University. All patients will be consented for participation in the study before study involvement.
Target Population, Setting, and Inclusion and Exclusion Criteria
The study will be conducted in 3 US kidney transplant centers with a total target enrollment of 450 (150 at each site): Emory Transplant Center in Atlanta, Georgia, Columbia University Medical Center in New York, New York, and Northwestern University Transplant Center in Chicago, Illinois.
ESRD patients will be recruited into the study during transplant medical evaluation if they meet inclusion criteria: (i) 18 to 70 years of age; (ii) no previous solid organ or multiorgan transplant; (iii) English speaking; and (iv) no severe cognitive or visual impairment.
Study Arms
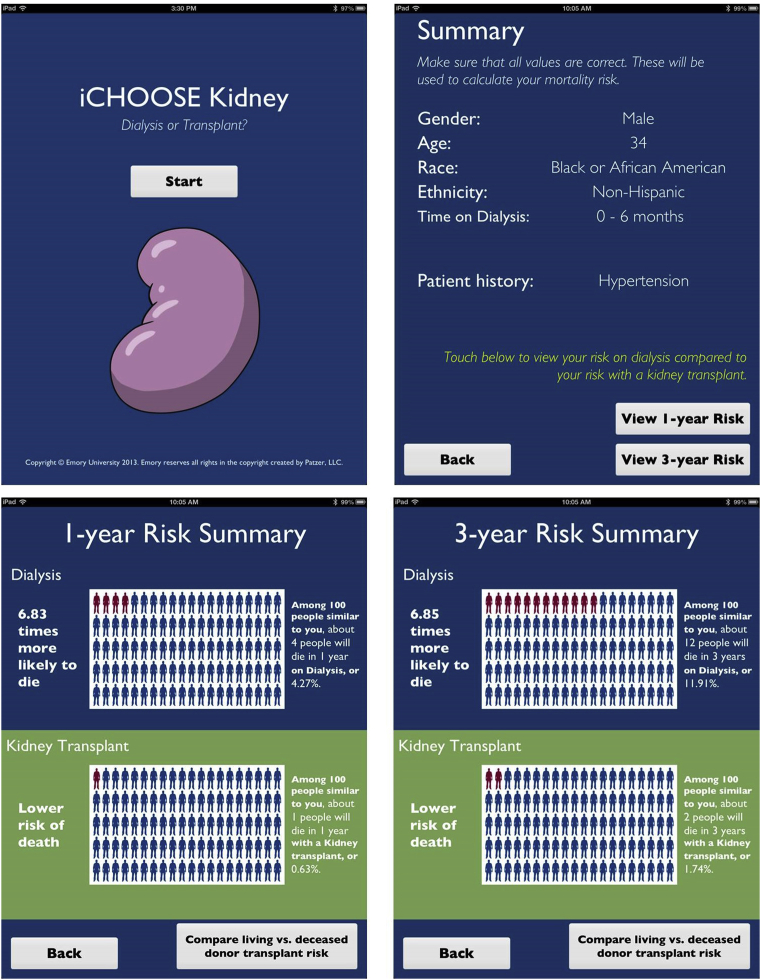
At kidney transplant evaluation, the control group will receive the usual center-specific standard-of-care education about renal transplantation, without the iChoose Kidney decision aid. The intervention group will also receive the usual center-specific standard-of-care education. However, it will be supplemented by the use of the iChoose Kidney decision aid (either iPad or iPhone version). Among patients randomized to the intervention study arm, a nephrologist or transplant surgeon will use iChoose Kidney to provide individualized risk estimates of mortality or survival by treatment (dialysis vs. transplant; LD vs. DD transplant) based on a patient’s demographic and clinical characteristics. Using visual displays, the iChoose Kidney decision aid communicates both absolute and relative risk estimates in several messaging frames to increase patient and provider understanding of treatment benefit (Figure 1). The provider will have the option of which format to display for the patient when discussing the risks and benefits of each treatment option.
Figure 1.
Screenshots of the iChoose Kidney decision aid (iPad version), which communicate both absolute and relative risk estimates in several messaging frames. By entering a patient’s clinical information (sex, age, race, ethnicity, time on dialysis, and several comorbidities), the risk prediction calculator generates individualized 1- and 3-year mortality and survival risk estimates for (i) dialysis versus kidney transplant and (ii) deceased- versus living-donor transplant.
Study Procedures
At each site, research assistants will identify patients who meet inclusion criteria at the time of the patient’s evaluation visit. Patients who agree to participate will be provided with detailed study information, and informed consent with written documentation will be obtained from the research participant or appropriate representative prior to initiation in the iChoose Kidney Study. Research assistants will then generate study identification numbers for consented participants and randomize patients to 1 of 2 study arms (the intervention arm or the control arm) with a random number generator application via iPad or iPhone. Patients will be surveyed on the same day before (baseline) and after (follow-up) meeting with the provider, completing a total of 2 surveys. Providers will take a baseline survey prior to the start of the study, a follow-up survey directly after meeting with each study patient, and a final poststudy completion survey (Figure 2). All patient and provider surveys will be administered through SurveyMonkey, an electronic surveying tool (or paper surveys later entered into SurveyMonkey if Internet access is unavailable). Patients will be offered a $10 gift card for their participation.
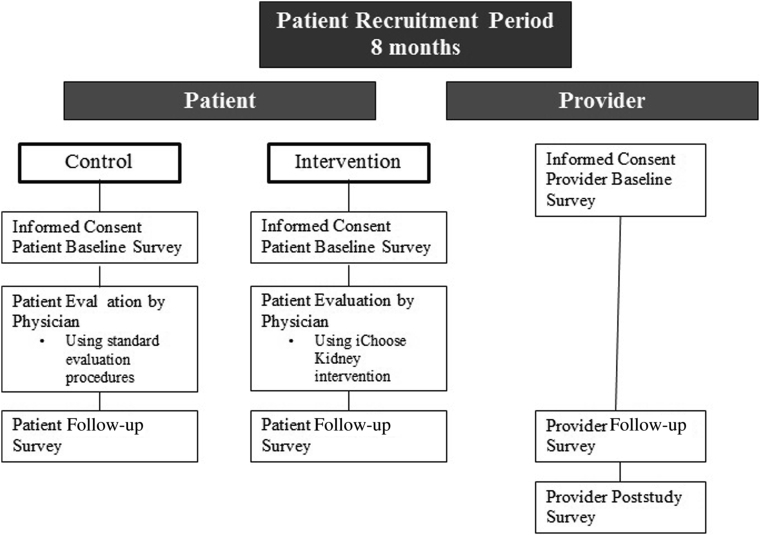
Figure 2.
iChoose Kidney Study schema shows the study process and points of data collection for both control and intervention patients, and clinical providers (i.e., nephrologists or surgeons). All patients will receive informed consent and a baseline survey before being evaluated by a transplant provider. Patients will complete a postconsultation survey after the provider consultation. Providers will receive a baseline survey prior to patient recruitment. After consulting with each patient, providers will take a postconsultation survey. At study completion, providers will complete a poststudy survey.
In the 2 months prior to initiation of the study, we will conduct pilot testing among the 3 sites to finalize study design and inform a power calculation. Pilot testing of the study will enable us to modify any issues with study design and data capture, and to ensure that all sites are conducting and recording data uniformly.
Surveys
Provider Baseline Survey
Prior to using the iChoose tool in the RCT, providers will be surveyed regarding their professional background and experience with educational tools (mobile or other). Surveys will also assess the amount of time providers spend with patients during evaluation appointments, how often they discuss survival benefit of transplant versus dialysis, and how they communicate mortality risk with patients during the kidney pretransplant evaluation appointment.
Patient Baseline Survey
On the day of evaluation, research assistants will administer baseline surveys to patients prior to the commencement of their nephrology or transplant surgery consultation. Baseline surveys include questions about patient demographics, exposure to transplant education, transplant knowledge, preferences for treatment, and decisional conflict.
Provider Follow-up Survey
Directly following each nephrology or surgery transplant evaluation, research assistants will administer a short survey to providers to assess satisfaction with and usefulness of the tool. Providers will report whether or not they discussed the survival benefit of transplant versus dialysis and report other conversation topics. Providers who use the tool with intervention patients will be asked whether they used mortality or survival estimates. They will also report whether they perceived that the patient gained transplant knowledge through the use of the iChoose Kidney tool. Providers who do not use the tool (if the patient was a control, or for some other reason) will be asked whether they felt their conversation with the patient could have benefited from using iChoose Kidney.
Patient Follow-up Survey
Following the provider consultation, patients will be given a follow-up survey, which consists of questions similar to the baseline survey, including transplant knowledge, preferences of treatment, decisional conflict, and health literacy and numeracy.
Provider Poststudy Survey
Within 1 month of study completion, providers will be surveyed again on their conversations with patients during the pretransplant evaluation and opinions on the iChoose Kidney application. Specifically, they will be asked about the average time spent during an evaluation visit, how often they discuss survival benefit of transplant versus dialysis, words used to communicate mortality risk, and perceived barriers to using mobile technology. They will also be asked their opinions of iChoose Kidney: its effect on patient knowledge and uncertainty regarding treatment, challenges to using the tool, how it could be improved, how it would best be used in practice (e.g., the time point when it should be used, who should administer it, which patients would benefit most), and whether they intend to use it in the future.
Outcome Measures
Transplant Knowledge
The primary outcome of the iChoose Kidney Study is change in patient knowledge about the survival benefits of transplantation. Improvement in knowledge will be measured using 8 survey questions in the pre- and postassessment. First, patients will be asked 2 knowledge questions in the baseline and follow-up survey: “On average, dialysis patients live: 1) longer than transplant patients, 2) about the same time as transplant patients, 3) a shorter time than transplant patients, or 4) unsure”; and “On average, living donor transplant patients live 1) a longer time than deceased donor transplant patients, 2) about the same time as deceased donor transplant patients, 3) a shorter time than deceased donor transplant patients, or 4) unsure.” Patients in both study arms will also be asked to estimate their absolute chance of 3-year survival on dialysis, transplant overall, deceased-donor transplant, and living-donor transplant (4 items) both pre- and postassessment. Lastly, patients will be asked to estimate their relative risk of mortality with dialysis versus kidney transplant (on a scale from 1 to 9) and whether they are more, less, or equally likely to die with dialysis compared to with a kidney transplant (2 items) both pre- and postassessment.
Decisional Conflict
Decisional conflict was measured in both the pre- and postsurvey by a validated scale of 10 items that assess personal perceptions of uncertainty in choosing options, modifiable factors contributing to uncertainty, and effective decision making (Table 1).16 This particular version of the decisional conflict scale was selected for the study given its recommendation to be used on individuals with “limited reading or response skills.” The subscales for decisional conflict include uncertainty (patient feels certain about choice), informed status (patient informed of treatment options), values clarity (patient clear regarding personal values), and support (patient feels supported in decision making). We used the scale to determine whether or not patients had lower decisional conflict after using iChoose Kidney during the evaluation appointment.
Table 1.
Description of scales and tests administered to patients, pre– and post–provider consultation
| Item | Description of purpose | How measured | Number of items |
|---|---|---|---|
| Knowledge of survival benefit | To assess patient knowledge of survival benefit of treatment options (dialysis, deceased-donor transplant, and living-donor transplant), patient-reported survival estimates of each treatment, and relative risk of mortality with dialysis versus transplant | Patient baseline and follow-up survey; scored on a scale by assessing whether survival benefit questions were correct or incorrect | 8 |
| Decisional conflict scale16 | To assess personal perceptions of uncertainty in choosing options, modifiable factors contributing to uncertainty, and effective decision making | Patient baseline and follow-up surveys, and scored on a scale from 1 to 100 | 10 |
| Patient treatment preferences | To assess treatment preferences for end-stage renal disease | Patient baseline and follow-up surveys; patients asked what type of treatment they prefer and whether they want a kidney transplant (yes or no) | 2 |
| Newest Vital Signs17 | To assess general literacy and numeracy skills as applied to health information, yielding an overall estimate of health literacy | Patient follow-up survey; patients given a nutrition label and asked a series of free-response questions; responses scored and categorized into low, medium, or high literacy | 6 |
| Lipkus numeracy test18 | To assess numeracy, or the ability to understand and use numeric information | Patient baseline survey; scored on a scale from 1 to 11 and categorized into low, medium, or high | 11 |
Patient Treatment Preferences
To assess whether the tool affected treatment preferences (from dialysis to kidney transplantation), patients will be asked in both the pre- and postsurvey what type of treatment they prefer for their kidney disease (hemodialysis, peritoneal dialysis, transplant, or unsure). Participants will also be asked whether they want a kidney transplant (yes or no) (Table 1).
Provider Opinions
We will use qualitative and quantitative methods to assess provider preferences, opinions, and satisfaction in order to evaluate usability of the iChoose Kidney tool among providers. We will also compare providers’ intent to use mobile technology and the length of time providers report discussing the survival benefit of transplant versus dialysis pre- versus poststudy.
Access to Transplant
Transplant access is a combined end point measured as completion of the transplant evaluation, number of living-donor inquiries, and wait-listing or transplant receipt. We will collect information on these measures from the patient medical record through data extraction at 6 months and 1 year from the patient’s evaluation appointment. Variables collected will include dates relevant to the transplant process (evaluation start and end date, wait-listing date, and transplant date) and whether the patient received a living-donor inquiry since evaluation (Table 2).
Table 2.
Variables collected for iChoose Kidney randomized control trial
| Variable name | Mode of collection |
||
|---|---|---|---|
| Patient baseline survey | Research assistant observation | EMR data abstraction or extraction | |
| Patient demographic and socioeconomic factors | |||
| Age | x | ||
| Race | x | x | x |
| Hispanic ethnicity | x | x | x |
| Sex | x | x | x |
| Income | x | ||
| Marital status | x | ||
| Education level | x | ||
| Health insurance | x | ||
| Employment status | x | ||
| Self-rated health | x | ||
| Internet access | x | ||
| Social support | x | x | |
| Health literacy | x | ||
| Health numeracy | x | ||
| Prior exposure to transplant | x | ||
| Time point first educated about transplant | x | ||
| Patient clinical factors | |||
| Body mass index | x | ||
| History of hypertension | x | ||
| History of diabetes | x | ||
| History of cardiovascular disease | x | ||
| Low albumin levels | x | ||
| Date of dialysis start | x | ||
| Time on dialysis | x | x | |
| Dialysis modality | x | x | |
| Outcome measures | |||
| Knowledge about transplant | x | ||
| Decisional conflict | x | ||
| Treatment preferences | x | ||
| Access to transplant measures | |||
| Date of transplant evaluation | x | ||
| Transplant evaluation end date | x | ||
| Date of wait-listing | x | ||
| Date of transplant | x | ||
| Number of living-donor inquiries | x | ||
All variables collected were measures at the time of kidney transplant evaluation.
EMR, electronic medical record.
Other Covariates
Patient Factors
We will collect the following demographic, socioeconomic, and clinical variables from patient surveys: age, race, sex, income, marital status, education level, health insurance, time on dialysis, dialysis type, self-rated health, and Internet access. A clinical data warehouse for all 3 transplant centers will be used to collect data on patient race, Hispanic ethnicity, age, body mass index, history of comorbidities (diabetes, hypertension, cardiovascular disease), low albumin level (<3.5 g/dl), and dialysis start date. Research assistants will also track whether patients had a social support member with them at the evaluation appointment (Table 2).
Prior Exposure to Transplant
Patients will be asked to check a list of several ways that they may have been exposed to information about transplant (e.g., through brochures, websites, by attending kidney support groups, etc.). They will also be asked when they were first educated about transplant as a treatment option (Table 2).
Health Literacy and Numeracy
Health literacy will be measured using the Newest Vital Signs assessment17 (6 items), and numeracy will be assessed using the Lipkus test18 (11 items) (Table 1). To administer Newest Vital Signs, patients will be given a nutrition label and asked a series of free-response questions. Responses will then be scored and categorized into low, medium, or high literacy. The Lipkus test assesses the patient’s ability to understand and use numeric information, and includes questions on risks and percentages. Numeracy will be scored continuously on a scale from 1 to 11.
Provider Factors
The following provider factors will be collected: time practicing medicine or surgery, use of patient educational tools, perceived barriers to using mobile technology, average time spent during an evaluation visit, how often the survival benefit of transplant versus dialysis is discussed, and specific reasons why the provider might not discuss the survival benefit of transplant versus dialysis.
Data Management
Research assistants will track study participants uniformly across sites, using a worksheet they will later transcribe into a Microsoft Excel spreadsheet. Patient demographics, social support, and any deviations from the study protocol will be recorded in this spreadsheet (e.g., if the provider performed the intervention with a control patient). At each site, patient demographics (race, age, sex, ethnicity), dialysis start date, and comorbidities included in the iChoose Kidney application will be abstracted or extracted from patient electronic medical records. To ensure data accuracy, self-reported patient survey data will be compared to data collected from electronic medical records.
The limited de-identified electronic medical record–collected data from all 3 sites, as well the de-identified SurveyMonkey patient and provider survey data, will be merged, cleaned, and operationalized at Emory University. Microsoft Excel and SAS 9.4 (SAS Institute, Cary, NC) will be used to prepare and merge study data.
Statistical Analyses
Descriptive Analyses
All analyses will be conducted using an intention-to-treat approach where patients will remain assigned to the treatment condition they were randomized to regardless of whether they receive the intervention (e.g., patients randomized to the intervention arm but who did not receive the iChoose Kidney clinical decision aid will still be considered as intervention participants). Descriptive analyses of transplant center–level baseline variables (demographic and clinical characteristics and transplant access measures) will be compared. To evaluate the differences between study arms at baseline, Pearson’s χ2 tests and t-tests, or their non-parametric equivalents, will be performed for categorical and continuous variables, respectively. Statistically significant differences in baseline characteristics will be adjusted when assessing the overall intervention effect as described above.
Change in Knowledge
To assess change in knowledge from pre- to postassessment, the change in score from the knowledge questions will be calculated for each patient. A paired t-test will be used to determine whether this difference in proportions is significant in intervention versus control patients.
Change in Decisional Conflict
Each of 10 items in the decisional conflict scale will be given a value of 0, 2, or 4 for responses “yes,” “no,” or “unsure,” respectively. The items will then be summed, divided by 10, and multiplied by 25 to determine the total score of the decisional conflict variable. Decisional conflict will then be scored on a scale from 0 (no decisional conflict) to 100 (high decisional conflict) (Table 1). The change in decisional conflict will be calculated for each patient from pre– to post–provider consultation to determine whether the change is significantly higher in intervention patients versus control patients using a χ2 test or multivariable logistic regression.
Change in Treatment Preferences
We will calculate the proportion of patients who initially preferred dialysis to transplantation but changed to transplantation during the postsurvey. A χ2 test will be used to determine whether this difference is significant in intervention versus control patients.
Access to Transplant
We will create a composite measure of living-donor inquiries, placement on the wait list, and receipt of transplant to determine long-term outcomes at 1 year after inclusion in the study. We will use χ2 tests or multivariable logistic regression to determine whether positive outcomes are significantly different among intervention patients versus control patients.
Planned Subgroup Analyses
To determine whether the effect of iChoose Kidney varies by health literacy, numeracy, and race, we will conduct similar analyses for knowledge and decisional conflict but across different subgroups of patients.
Provider Attitudes
To evaluate whether providers believe the tool impacted study patients, we will use descriptive analyses to assess variables collected directly after the patient appointment. We will use χ2 tests to assess whether there was a difference in the number of times providers had the conversation about the survival benefit of transplant versus dialysis, and of living- versus deceased-donor transplants with intervention versus control patients. We will also compare differences between provider responses at baseline and poststudy regarding use of mobile decision aids in general and intention to use iChoose Kidney using χ2 tests.
Power and Sample Size Calculations
Sample size calculations will be based on our primary aim to improve patient knowledge about the survival benefit of transplantation versus dialysis. To test the primary null hypothesis of no difference in referral proportions between the control and intervention groups, a sample size of 450 patients (225 patients per study arm) achieves 80% power to detect a moderate knowledge difference of 13% between the 2 groups at 5% significance level.
Discussion
Critically important treatment decisions are often made without evidence-based information about a patient’s prognosis. Typically, average, population-based, non-tailored prognosis estimates of mortality on dialysis versus transplant are the only types of estimates communicated to individual ESRD patients, if these estimates are communicated at all.19 Despite overwhelming evidence to support transplantation for certain ESRD patients,20 patient-specific prognostic information is rarely used to calculate a patient's individualized prognosis.21 The iChoose Kidney clinical decision aid12 is a tool that clinical providers, including nephrologists, surgeons, and other ESRD educators, could potentially use with patients to explain their chance of mortality or survival on dialysis versus transplant, ultimately helping to facilitate more informed decision making among ESRD patients about their treatment options. However, prior to dissemination for use in a clinical setting, the efficacy of the clinical decision aid in improving patient knowledge about the survival benefit of transplantation is needed.
While transplantation is the preferred treatment for most ESRD patients, significant barriers exist in access to multiple steps of the kidney transplantation process. Patients who are minorities, of lower socioeconomic status,22 and of lower health literacy23 are less likely to receive a kidney transplant. Education about transplantation as a treatment option may also play a role in disparities in access to kidney transplantation. According to the US Agency for Healthcare Research and Quality, disparities in health outcomes are due, in part, to differences in access to health care, provider biases, poor patient–provider communication, and poor health literacy.24 Mobile health technology can be a useful way to deliver interventions and may have a high potential for reducing health disparities. Effective risk communication strategies must consider patients with varying degrees of health literacy, numeracy, and education levels to ensure that the information provided by the tool is relevant and understandable to patients from diverse backgrounds. The iChoose Kidney decision aid communicates risks of mortality on dialysis versus transplant in both absolute and relative terms to meet best practices in conveying health risks.25 Evidence-based research supports presenting absolute risk in visuals to emphasize the clinical significance and size of risks,26 which iChoose Kidney utilizes.
Upon completion of this research, we will have assessed the effect of a decision aid in improving knowledge about the survival benefits of kidney transplantation versus dialysis. We will also have assessed providers’ attitudes toward clinical decision tools and their impact on the patient–provider interaction. If found to be effective in improving knowledge about the survival benefit of transplant, the clinical decision aid could be a useful tool for improving access to transplantation and improving decisional conflict with regard to treatment options.
A strength of this randomized controlled trial is the conduct of the research among a diverse, multicenter population of ESRD patients, as results may be broadly generalizable to other ESRD patients who have been referred for transplantation to the more than 250 transplant centers across the United States. Further, with additional testing in a population of incident ESRD patients, the iChoose Kidney decision aid may also be applicable for use across the more than 5000 dialysis facilities or chronic kidney disease clinics in the United States. Future studies could also assess the applicability of the use of such a tool outside of the United States.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This trial is registered as NCT02235571 in ClinicalTrials.gov. We thank the Norman S. Coplon Satellite Healthcare Foundation for providing funding for this clinical trial. This work is also supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award number KL2TR000455. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.US Renal Data System. 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. 2013. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
- 2.Danovitch G.M. Handbook of Kidney Transplantation. 2nd ed. Lippincott Williams & Wilkins; Philadelphia, PA: 2005. Options for patients with kidney failure. [Google Scholar]
- 3.Tonelli M., Wiebe N., Knoll G. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant. 2011;11:2093–2109. doi: 10.1111/j.1600-6143.2011.03686.x. [DOI] [PubMed] [Google Scholar]
- 4.Wolfe R.A., Ashby V.B., Milford E.L. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–1730. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 5.Flynn K.E., Smith M.A., Vanness D. A typology of preferences for participation in healthcare decision making. Soc Sci Med. 2006;63:1158–1169. doi: 10.1016/j.socscimed.2006.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee A., Gudex C., Povlsen J.V. Patients' views regarding choice of dialysis modality. Nephrol Dial Transplant. 2008;23:3953–3959. doi: 10.1093/ndt/gfn365. [DOI] [PubMed] [Google Scholar]
- 7.Finkelstein F.O., Story K., Firanek C. Perceived knowledge among patients cared for by nephrologists about chronic kidney disease and end-stage renal disease therapies. Kidney Int. 2006;74:1178–1184. doi: 10.1038/ki.2008.376. [DOI] [PubMed] [Google Scholar]
- 8.Boulware L.E., Hill-Briggs F., Kraus E.S. Effectiveness of educational and behavioral interventions to improve pursuit of pre-emptive living-related kidney transplantation: the Talking About Living Kidney Donation (TALK) study. Am J Transplant. 2011;2(suppl S2):28–211. [Google Scholar]
- 9.Boulware L.E., Hill-Briggs F., Kraus E.S. Protocol of a randomized controlled trial of culturally sensitive interventions to improve African Americans' and non-African Americans' early, shared, and informed consideration of live kidney transplantation: the Talking About Live Kidney Donation (TALK) study. BMC Nephrol. 2011;12:34. doi: 10.1186/1471-2369-12-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waterman A.D., Peipert J.D., Goalby C.J. Assessing transplant education practices in dialysis centers: comparing educator reported and Medicare data. Clin J Am Soc Nephrol. 2015;10:1617–1625. doi: 10.2215/CJN.09851014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waterman A.D., Goalby C., Hyland S.S. Transplant education practices and attitudes in dialysis centers: dialysis leadership weighs in. J Nephrol Ther. 2012;S4:007. [Google Scholar]
- 12.Patzer R.E., Basu M., Larsen C.P. iChoose Kidney: a clinical decision aid for kidney transplantation vs dialysis treatment. Transplantation. 2016;100:630–639. doi: 10.1097/TP.0000000000001019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox S, Duggan M. Half of smartphone owners use their devices to get health information and one-fifth of smartphone owners have health apps. Mobile Health 2012. 2012. Available at: http://www.pewinternet.org/files/old-media//Files/Reports/2012/PIP_MobileHealth2012_FINAL.pdf.
- 14.Rock Health. Digital Health Funding 2015: Midyear Review. 2015. Available at: https://rockhealth.com/reports/digital-health-2015-midyear/.
- 15.research2guidance. mHealth App Developer Economics 2014: The State of the Art of mHealth App Publishing. 2014. Available at: http://research2guidance.com/r2g/research2guidance-mHealth-App-Developer-Economics-2014.pdf. Accessed August 21, 2015.
- 16.O'Connor A.M. Validation of a decisional conflict scale. Med Decis Making. 1995;15:25–30. doi: 10.1177/0272989X9501500105. [DOI] [PubMed] [Google Scholar]
- 17.Weiss B.D., M.M., Martz W. Quick assessment of literacy in primary care: the Newest Vital Sign. Ann Fam Med. 2005;3:514–522. doi: 10.1370/afm.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lipkus I.M., Samsa G., Rimer B.K. General performance on a numeracy scale among highly educated samples. Med Decis Making. 2001;21:37–44. doi: 10.1177/0272989X0102100105. [DOI] [PubMed] [Google Scholar]
- 19.US Renal Data System. 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. 2010. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
- 20.Verdalles U., Abad S., Aragoncillo I. Factors predicting mortality in elderly patients on dialysis. Nephron Clin Pract. 2010;115:c28–c34. doi: 10.1159/000286347. [DOI] [PubMed] [Google Scholar]
- 21.Moore J., He X., Shabir S. Mortality prediction after kidney transplantation: comparative clinical use of 7 comorbidity indices. Exp Clin Transplant. 2011;9:32–41. [PubMed] [Google Scholar]
- 22.Patzer R.E., McClellan W.M. Influence of race, ethnicity and socioeconomic status on kidney disease. Nat Rev Nephrol. 2012;8:533–541. doi: 10.1038/nrneph.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grubbs V., Gregorich S.E., Perez-Stable E.J. Health literacy and access to kidney transplantation. Clin J Am Soc Nephrol. 2009;4:195–200. doi: 10.2215/CJN.03290708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.2010 National Healthcare Disparities Report. Agency for Healthcare Research and Quality; 2011. Rockville, MD: US Department of Health and Human Services.
- 25.Lipkus I.M. Numeric, verbal, and visual formats of conveying health risks: suggested best practices and future recommendations. Med Decis Making. 2007;27:696–713. doi: 10.1177/0272989X07307271. [DOI] [PubMed] [Google Scholar]
- 26.Paling J. Strategies to help patients understand risks. BMJ. 2003;327:745–748. doi: 10.1136/bmj.327.7417.745. [DOI] [PMC free article] [PubMed] [Google Scholar]