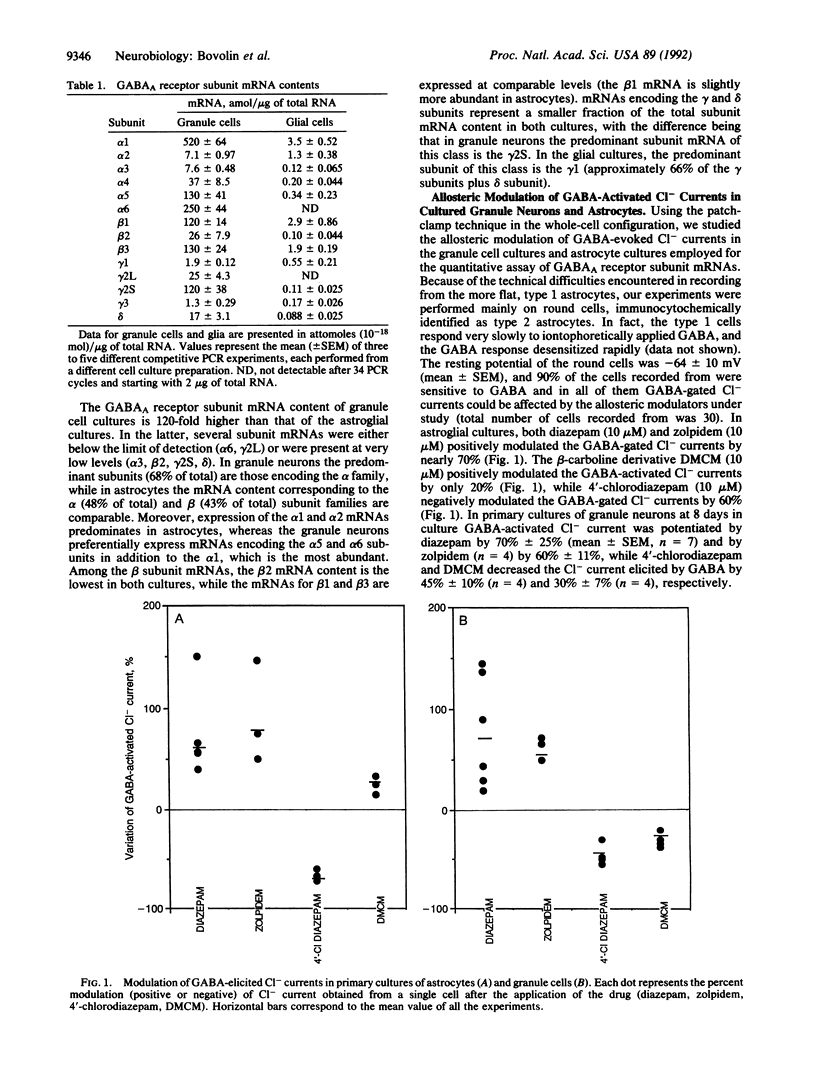
Abstract
Using a competitive polymerase chain reaction assay, we have quantitated the absolute amounts of mRNA encoding 14 distinct subunits of the gamma-aminobutyric acid type A (GABAA) receptor in primary cultures of rat cerebellar granule neurons and cerebellar astrocytes. We found that the total amount of GABAA receptor subunit mRNA in astrocytes was 2 orders of magnitude lower than in neuronal cells. Furthermore, granule cell cultures expressed all 14 different GABAA subunit mRNAs, while the astroglial cultures contained detectable amounts of all the subunits expressed by granule cells except the alpha 6 and the gamma 2L subunits. Of the alpha subunit family members, the alpha 1, alpha 5, and alpha 6 mRNAs were prominent in granule cells, while the alpha 1 and alpha 2 mRNAs were abundant in astrocytes. Of the beta receptor subunit mRNAs, the beta 1 and beta 3 mRNAs were abundantly expressed in both cultures. The gamma 2S and gamma 2L mRNAs constituted the great majority of gamma subunit mRNAs in neurons, while the gamma 1 subunit mRNA was the most abundant gamma subunit mRNA in astrocytes. When various allosteric modulators of GABAA receptors were tested electrophysiologically, methyl 6,7-dimethoxy-4-ethyl-beta-carboline- 3-carboxylate (DMCM) was the only one to modulate chloride currents elicited by GABA in a significantly different manner in granule cells (negative modulation) compared with astrocytes (positive modulation). The latter effect was previously observed in transiently expressed recombinant GABAA receptors containing a gamma 1 instead of a gamma 2 subunit. Our quantitative mRNA results suggest that an important molecular determinant responsible for the DMCM-positive modulatory effect on astroglial native GABAA receptors is the presence of the gamma 1 subunit in the receptor assembly.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bormann J., Ferrero P., Guidotti A., Costa E. Neuropeptide modulation of GABA receptor C1- channels. Regul Pept Suppl. 1985;4:33–38. doi: 10.1016/0167-0115(85)90215-0. [DOI] [PubMed] [Google Scholar]
- Bormann J., Kettenmann H. Patch-clamp study of gamma-aminobutyric acid receptor Cl- channels in cultured astrocytes. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9336–9340. doi: 10.1073/pnas.85.23.9336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovolin P., Santi M. R., Memo M., Costa E., Grayson D. R. Distinct developmental patterns of expression of rat alpha 1, alpha 5, gamma 2S, and gamma 2L gamma-aminobutyric acidA receptor subunit mRNAs in vivo and in vitro. J Neurochem. 1992 Jul;59(1):62–72. doi: 10.1111/j.1471-4159.1992.tb08876.x. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Duggan M. J., Stephenson F. A. Biochemical evidence for the existence of gamma-aminobutyrateA receptor iso-oligomers. J Biol Chem. 1990 Mar 5;265(7):3831–3835. [PubMed] [Google Scholar]
- Estin C., Vernadakis A. Primary glial cells and brain fibroblasts: interactions in culture. Brain Res Bull. 1986 May;16(5):723–731. doi: 10.1016/0361-9230(86)90144-9. [DOI] [PubMed] [Google Scholar]
- Gallo V., Ciotti M. T., Coletti A., Aloisi F., Levi G. Selective release of glutamate from cerebellar granule cells differentiating in culture. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7919–7923. doi: 10.1073/pnas.79.24.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V., Wise B. C., Vaccarino F., Guidotti A. gamma-Aminobutyric acid- and benzodiazepine-induced modulation of [35S]-t-butylbicyclophosphorothionate binding to cerebellar granule cells. J Neurosci. 1985 Sep;5(9):2432–2438. doi: 10.1523/JNEUROSCI.05-09-02432.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambarana C., Pittman R., Siegel R. E. Developmental expression of the GABAA receptor alpha 1 subunit mRNA in the rat brain. J Neurobiol. 1990 Dec;21(8):1169–1179. doi: 10.1002/neu.480210803. [DOI] [PubMed] [Google Scholar]
- Gilliland G., Perrin S., Blanchard K., Bunn H. F. Analysis of cytokine mRNA and DNA: detection and quantitation by competitive polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2725–2729. doi: 10.1073/pnas.87.7.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Herb A., Wisden W., Lüddens H., Puia G., Vicini S., Seeburg P. H. The third gamma subunit of the gamma-aminobutyric acid type A receptor family. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1433–1437. doi: 10.1073/pnas.89.4.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989 Apr 15;77(1):51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Kettenmann H., Backus K. H., Schachner M. gamma-Aminobutyric acid opens Cl-channels in cultured astrocytes. Brain Res. 1987 Feb 24;404(1-2):1–9. doi: 10.1016/0006-8993(87)91349-7. [DOI] [PubMed] [Google Scholar]
- Laurie D. J., Seeburg P. H., Wisden W. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. II. Olfactory bulb and cerebellum. J Neurosci. 1992 Mar;12(3):1063–1076. doi: 10.1523/JNEUROSCI.12-03-01063.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi G., Aloisi F., Ciotti M. T., Gallo V. Autoradiographic localization and depolarization-induced release of acidic amino acids in differentiating cerebellar granule cell cultures. Brain Res. 1984 Jan 2;290(1):77–86. doi: 10.1016/0006-8993(84)90737-6. [DOI] [PubMed] [Google Scholar]
- MacLennan A. J., Brecha N., Khrestchatisky M., Sternini C., Tillakaratne N. J., Chiang M. Y., Anderson K., Lai M., Tobin A. J. Independent cellular and ontogenetic expression of mRNAs encoding three alpha polypeptides of the rat GABAA receptor. Neuroscience. 1991;43(2-3):369–380. doi: 10.1016/0306-4522(91)90301-4. [DOI] [PubMed] [Google Scholar]
- McKernan R. M., Quirk K., Prince R., Cox P. A., Gillard N. P., Ragan C. I., Whiting P. GABAA receptor subtypes immunopurified from rat brain with alpha subunit-specific antibodies have unique pharmacological properties. Neuron. 1991 Oct;7(4):667–676. doi: 10.1016/0896-6273(91)90379-e. [DOI] [PubMed] [Google Scholar]
- Memo M., Bovolin P., Costa E., Grayson D. R. Regulation of gamma-aminobutyric acidA receptor subunit expression by activation of N-methyl-D-aspartate-selective glutamate receptors. Mol Pharmacol. 1991 May;39(5):599–603. [PubMed] [Google Scholar]
- Olsen R. W., Tobin A. J. Molecular biology of GABAA receptors. FASEB J. 1990 Mar;4(5):1469–1480. doi: 10.1096/fasebj.4.5.2155149. [DOI] [PubMed] [Google Scholar]
- Pritchett D. B., Sontheimer H., Shivers B. D., Ymer S., Kettenmann H., Schofield P. R., Seeburg P. H. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature. 1989 Apr 13;338(6216):582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- Puia G., Santi M. R., Vicini S., Pritchett D. B., Seeburg P. H., Costa E. Differences in the negative allosteric modulation of gamma-aminobutyric acid receptors elicited by 4'-chlorodiazepam and by a beta-carboline-3-carboxylate ester: a study with natural and reconstituted receptors. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7275–7279. doi: 10.1073/pnas.86.18.7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puia G., Vicini S., Seeburg P. H., Costa E. Influence of recombinant gamma-aminobutyric acid-A receptor subunit composition on the action of allosteric modulators of gamma-aminobutyric acid-gated Cl- currents. Mol Pharmacol. 1991 Jun;39(6):691–696. [PubMed] [Google Scholar]
- Vicini S. Pharmacologic significance of the structural heterogeneity of the GABAA receptor-chloride ion channel complex. Neuropsychopharmacology. 1991 Jan;4(1):9–15. [PubMed] [Google Scholar]
- Vicini S., Wroblewski J. T., Costa E. Pharmacological modulation of GABAergic transmission in cultured cerebellar neurons. Neuropharmacology. 1986 Feb;25(2):207–211. doi: 10.1016/0028-3908(86)90043-2. [DOI] [PubMed] [Google Scholar]
- Wang A. M., Doyle M. V., Mark D. F. Quantitation of mRNA by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9717–9721. doi: 10.1073/pnas.86.24.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisden W., Laurie D. J., Monyer H., Seeburg P. H. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992 Mar;12(3):1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. H., Sato M., Tohyama M. Different postnatal development profiles of neurons containing distinct GABAA receptor beta subunit mRNAs (beta 1, beta 2, and beta 3) in the rat forebrain. J Comp Neurol. 1991 Jun 22;308(4):586–613. doi: 10.1002/cne.903080407. [DOI] [PubMed] [Google Scholar]



