Abstract
The recently-proposed ACTTION-APS Pain Taxonomy provides an evidence-based, multidimensional, chronic pain classification system. Psychosocial factors play a crucial role within several dimensions of the Taxonomy. In this paper, we discuss the evaluation of psychosocial factors that influence the diagnosis and trajectory of chronic pain disorders. We review studies in individuals with a variety of persistent pain conditions, and describe evidence that psychosocial variables play key roles in conferring risk for the development of pain, in shaping long-term pain-related adjustment, and in modulating pain treatment outcomes. We consider both “general” psychosocial variables such as negative affect, childhood trauma, and social support, as well as “pain-specific” psychosocial variables that include pain-related catastrophizing, self-efficacy for managing pain, and pain-related coping. Collectively, the complexity and profound variability in chronic pain highlights the need to better understand the multidimensional array of interacting forces that determine the trajectory of chronic pain conditions.
Keywords: Biopsychosocial, Phenotype, Chronic Pain, Affect, Fear-Avoidance
Introduction
Persistent pain is a significant therapeutic challenge and a public health epidemic placing burdens on those experiencing pain as well as society more broadly. A survey of 10 developed and 7 developing countries suggests that the point prevalence of chronic pain among adults is 41% and 37% respectively227, figures which encompass a wide array of diverse conditions. In the United States, chronic pain is estimated to affect over 100 million adults at any given time, is among the leading causes of reduced quality of life, and carries direct and indirect costs of over $600 billion dollars annually in the U.S. alone72. Moreover, the experience of persistent pain starts early; as many as 38% of children and adolescents in community samples report the presence of chronic pain122. Despite the widely-recognized impact of chronic pain on global health, however, pain science continues to lack a precise, evidence-based taxonomy of chronic pain conditions, which would facilitate improvements in diagnosis and treatment.65
The Analgesic, Anesthetic, and Addiction Clinical Trial Translations Innovations Opportunities and Networks (ACTTION) public-private partnership with the U.S. Food and Drug Administration, and the American Pain Society (APS) have joined together to develop an evidence-based chronic pain classification system termed the ACTTION-APS Pain Taxonomy (AAPT). As noted in the initial description of the taxonomy, the field stands to benefit substantially from the development of an empirically-based classification system that can serve to illuminate individual differences in the pain experience, inform policy, clarify prognosis, and guide treatment decisions.65 The structuring of the AAPT was based on a consensus decision that the dimension along which pain disorders would be categorized is organ system/anatomical structure, which includes: peripheral and central neuropathic pain, musculoskeletal pain, pelvic/urogenital, visceral pain and disease-related pains not classified elsewhere (e.g. pain associated with active cancer, sickle cell disease, and Parkinson’s disease). Some of the most important characteristics of the taxonomy are that: 1) it be based on the best available evidence rather than based solely on consensus or expert opinion, 2) the diagnostic criteria for specific chronic pain disorders should be determined using existing mechanistic and diagnostic evidence, rather than historical precedent or theoretical biases, 3) it reflects the multidimensional and biopsychosocial nature of chronic pain, and 4) it emphasizes the inclusion of existing information regarding mechanistic features and risk factors for pain conditions, including not only neurobiological but also psychosocial processes. 65
In addition to establishing Core Diagnostic Criteria for numerous chronic pain conditions (Dimension 1), the AAPT provides dimensions on which to categorize common features and comorbidities of the conditions (Dimensions 2 and 3), as well as detailing the consequences (Dimension 4), and contributory mechanisms (Dimension 5) of persistent pain disorders. A number of these identified features, consequences, and mechanisms are psychosocial in nature. Indeed, processes such as mood, affect (negative and positive), coping, and social support are included in the taxonomy as specific examples of risk factors, protective factors, or comorbidities that impact the experience of chronic pain and its presentation.65 The purpose of this article is to highlight the contributory role of psychosocial factors (e.g., their function as risk factors, protective factors, or moderators) in the context of the AAPT classification system. The present review is one of a series of foundational supporting articles intended to highlight and describe crucial areas that are common to many or all of the conditions within the AAPT taxonomy. The process of psychosocial assessment (e.g., conceptualization of psychosocial domains, evaluation and selection of assessment instruments) is addressed in a complementary supporting article in this Supplement (see Turk, Ohrbach, & Fillingim); in order to avoid redundancy, in this article we focus specifically on the understanding of the role of psychosocial processes in shaping the development and trajectory of pain conditions. It is also important to note that although the AAPT taxonomy is in many ways a typical category-based diagnostic taxonomy, most of the psychosocial processes described here, which have important roles in shaping the development and trajectory of chronic pain conditions, are best considered as continuous, dimensional variables rather than as categorical designations.
One additional important consideration: though AAPT describes “psychosocial mechanisms” as part of Dimension 5, we have endeavored to limit the use of the term “mechanism” because of some well-known limitations of the existing literature.18;104;113;126 In particular, for a process to function as a causal mechanism, it must be fully distinct from its effect and must clearly precede that effect in time. The literature in this area, while rich in suggestive associations between psychosocial constructs and pain-related outcomes, is impeded by substantial conceptual and theoretical overlap of constructs, as well as overlap in the methods by which they are measured (i.e., usually by self-report on numerically scaled questionnaires), and from a relative dearth of clear prospective studies. That is, many of the findings that identify putative psychosocial mechanisms are based on respondent recall of past events or states, or on mediational analysis of cross-sectional data, the limitations of which we elaborate on later in the article.
The Biopsychosocial Model of Pain
Prior to the 1960s, most people viewed chronic pain conditions as primarily medical issues with clear pathophysiological bases that required physical treatments such as surgery or medication.104 Subsequently, though, a biopsychosocial understanding has come to dominate the professional scientific community’s characterization of chronic pain. Collectively, the biopsychosocial approach describes pain and disability as a multidimensional, dynamic interaction among physiological, psychological, and social factors that reciprocally influence one another, resulting in chronic and complex pain syndromes.73;104 The overlap between affective disturbance and chronic pain has been widely recognized for many decades.74;231 Pain is defined as both a sensory and affective experience, and reviews of pain assessment invariably emphasize that pain unpleasantness, or affective responses to pain, should be assessed along with pain intensity and other “sensory” features.65 Reviews of the biopsychosocial model of pain cite its substantial history, including Engel’s call for a new “medical model”64, Fordyce’s seminal work on the contribution of communication and contextual factors69, and Loeser’s synthesis of biopsychosocial principles143, while emphasizing its nearly universal acceptance in principle if not practice148;169. A good deal of empirical evidence underpins the biopsychosocial model, and few in the field would likely argue that psychological constructs and processes are irrelevant to the experience of pain. In practice, however, psychosocial factors are often assigned secondary status and viewed largely as reactions to pain. As we will describe below, longitudinal, observational research supports a strong bidirectional link between mood disorders and persistent pain; the development of an enduring pain condition confers a substantially increased risk for the subsequent diagnosis of an affective disorder, while psychosocial variables such as depression, anxiety, and distress are among the most potent and robust predictors of the transition from acute to chronic pain.10;12;59;140;174;258
Across numerous studies, individuals with a variety of chronically painful conditions generally have a several-fold increase in the risk of experiencing clinically significant mood or anxiety symptoms10;12;59;162, and instruments assessing depression, anxiety, and distress have been recommended for use as outcome measures in randomized controlled trials of pain treatments.58;228–230 Given the synergy between pain and negative affect, this makes good logical sense: efficacious analgesic treatments that reduce the frequency and intensity of pain should have a beneficial effect on patients’ affective states and appropriate treatment of emotional distress should have a positive influence on the experience of pain9;12;128;137. Indeed, several randomized controlled trials in primary care settings have shown that antidepressant treatment in patients with comorbid chronic pain and mood disorders produces fairly rapid analgesic benefits that are anticipated by, and robustly correlated with, improvements in indices of psychosocial distress127;193.
We should note that it is important not to confuse psychological constructs and processes with psychiatric illness. Although psychiatric conditions certainly co-occur with chronic pain, and chronic pain samples do show elevations in rates of psychiatric disorders10;12;59;162, elevated levels of negative affect and diminished cognition at sub-threshold levels for a psychiatric disorder also play an influential role in shaping pain responses and pain-related outcomes. For example, evidence suggests that elevations in negative affect and pain-specific distress are associated with reduced benefit from a variety of potentially pain-reducing treatments.59;247;248 Wasan and colleagues reported that back pain patients with high levels of negative affect experienced a roughly 50% reduction in oral opioid analgesia relative to patients with low negative affect 249. Recently, a number of relevant prospective studies in this area have involved studies of inter-patient variability in pain outcomes following surgery. Longitudinal studies evaluating variability in pain-related outcomes after joint replacement surgery highlight the importance of assessing mental health, or psychosocial functioning, pre-operatively56;84;238, as patients with higher baseline levels of anxiety and depression report less benefit, more complications, increased pain and analgesic use, and poorer function for years after total knee or total hip replacement.56;84;238
Overall, recent reviews and meta-analyses support the importance of a broad array of psychosocial factors in shaping pain-related experiences and outcomes across numerous pain conditions.174;183;238 A good deal of this work has involved studies of back pain. For example, Taylor and colleagues noted that variables such as job dissatisfaction, low job control, minimal social support, depression, and interpersonal conflict were strongly associated with incident low back pain (LBP).218 In another systematic review and meta-analysis, similar processes such as distress, low self-efficacy, and pain-related fear were identified as crucially important in both back and neck pain.132 Reviews of other conditions such as post-operative pain203, chronic pelvic pain196, fibromyalgia (FM)44, and neuropathic pain45 have reached similar conclusions. Some of these factors are discussed in further detail below; here we simply note that these variables appear to serve as robust risk factors that confer vulnerability for the development of persistent pain. We should also emphasize that characterizing domains of variables as “psychological” or “psychosocial” refers principally to the method of assessment rather than the presumed underlying pathophysiologic mechanism that drives pain-related outcomes.54 That is, although they may be assessed via patient self-report of cognitive and emotional processes, constructs such as somatic awareness, anxiety, and pain-related catastrophizing likely reflect altered peripheral and central nervous system processing of sensory stimuli. For example, these “psychological” features of patients are often significantly correlated with measures of somatosensory amplification or central sensitization.54;59;104 Indeed, recent reviews of vulnerability and resilience factors in chronic pain emphasize the association of psychological processes with neurobiological pathways such as epigenetic processes, cellular priming, and alterations in brain networks concerned with reward, motivation and learning, and descending modulatory control (see Figure 1).51 Thus, neuropathic processes of sensitization at the peripheral, spinal cord, and/or brain levels are likely to be heightened by psychosocial factors, present concurrently, longitudinally, or antecedent in relation to the painful condition.
Figure 1.
Interactive pathways shaping risk for chronic pain (Denk et al., 2014)51.
Despite its widespread support, there has also been some criticism of the limitations of the biopsychosocial model of pain. The model is rather vague about the specific pathways by which its elements interact and there are often no clear boundaries between categories of processes and constructs. Moreover, many of the explanations invoked by the biopsychosocial model to account for inter-individual variability in pain-related outcomes are so multifactorial as to be unfalsifiable by empirical research.73–75;250 Additional criticisms include the observation that most “biopsychosocial” studies do not routinely measure variables within each of the 3 domains (biological, psychological, and social)184; indeed, it has been suggested that the biopsychosocial model may over-weight psychosocial factors, especially in the absence of clear anatomic pathology, which risks reverting to a dualistic perspective of mind vs. body.250 Other researchers have noted that the biopsychosocial model, as commonly described, may be too restrictive as it fails to capture important elements of quality of life, such as spirituality and religion, which has led to the development of a biopsychosocial-spiritual model of chronic pain that was recently applied to patients with sickle cell disease.219 Still other approaches have emphasized behavioral aspects of pain, with a biopsychomotor model highlighting the critical roles of communicative pain behaviors, protective pain behaviors, and social response behaviors.216 Despite these criticisms, however, the biopsychosocial model of pain has been enormously valuable in shaping our understanding of individual differences in pain, and in guiding the development of efficacious and effective psychosocial and behavioral interventions to reduce the suffering and sequelae associated with persistent pain.73;104;258 The AAPT taxonomy is designed to give balance to the sets of biological, psychological, and social variables and importantly their interactions across chronic pain disorders. Each of these constituent domains should be considered in classifying all chronic pain disorders, hence the multidimensional system outlined.48
Mechanism-Based Pain “Models”
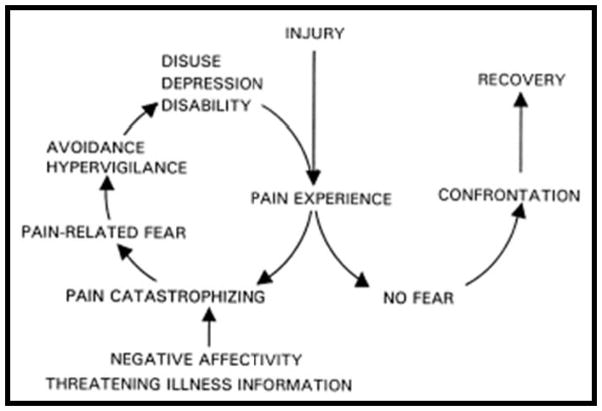
Under the broad umbrella of the biopsychosocial approach to understanding chronic pain, a number of more specific, mechanism-oriented models have been developed to describe the pathways by which particular processes can influence pain-related outcomes. Perhaps the most widely-known and influential of these models is the Fear Avoidance Model (FAM), which was advanced nearly 20 years ago to explain the development and persistence of disabling LBP in a subgroup of patients.239 The model proposes that pain-related disability is caused by an interacting, cyclical sequence of fear-related cognitive, affective, and behavioral processes (see Figure 2). The basic concept underpinning the model is that fear of pain leads to a cascade of deleterious consequences (see Figure 2). ‘Confrontation’ and ‘avoidance’ are postulated as the 2 categories of behavioral responses to fear about pain; the former leads to the eventual reduction of fear over time. Avoidance, in contrast, leads to the maintenance or amplification of fear, which in turn results in disuse and disability.47;242 Since its introduction, the FAM has inspired productive research and has grown to be the leading paradigm for understanding disability associated with a wide range of musculoskeletal pain conditions. Over the past decade, the original FAM has been extended to include learning, motivation, and self-regulation theory.47 In several recent systematic reviews of the elements of the FAM, including thousands of patients in clinical studies, the authors concluded that within cohorts of patients with LBP for less than 6 months, there was high-quality prospective evidence that fear avoidance behaviors were associated with more pain and functional disability, poorer treatment outcomes overall, and reduced probabilities of return to work, while decreased fear avoidance was associated with improved clinical outcomes.251;252 Collectively, over ¾ of published studies found that baseline levels of fear avoidance significantly influenced treatment outcomes, with participants high in fear avoidance reporting more pain and disability, and demonstrating lower levels of return to work following treatment 251;252. In addition, treatment-related reductions in fear avoidance beliefs were important mediators of treatment benefits such as reduced pain and return to work.
Figure 2.
The Fear Avoidance Model of Pain (Vlaeyen and Linton, 2000 & 2012)240;241
A key prediction of the original FAM is the specific, prospective, sequential interrelationships between catastrophizing, fear, depression, and pain-related disability, and the role of fear as a common barrier to recovery. Many of the initial studies used cross-sectional analyses to test aspects of the FAM; these provided firm evidence of associations between the constructs of interest (e.g., pain-related fear was positively associated with measures of disability), but longitudinal designs are needed to confirm the sequence and direction of relationships proposed in Figure 2.254 Interestingly, several recent studies using a 3-panel, prospective design (in which earlier changes in one variable, from time 1 to time 2, are investigated as predictors of later changes in an outcome variable, from time 2 to time 3) have failed to show that changes in pain catastrophizing precede changes in pain-related fear or that changes in fear precede changes in depression.19;253 These findings highlight a common conceptual problem in research that purports to examine causal psychosocial mechanisms: the hypothesized associations between variables of interest are often strong, but the postulated temporal pathways (variable X impacts variable Y, which in turn affects outcome Z) are rarely specific. That is, although these factors inter-relate across time, there is minimal evidence that the sequence of influence unfolds uniquely in the manner specified by the model. Thus, while the extant literature provides valuable information on psychosocial constructs and processes that are related to the experience of pain, and in some cases supports the concept of specific variables as prospective risk factors, or protective factors, there is a general lack of definitive evidence for causal mechanistic influences.
While the FAM emphasizes cyclical relationships between its hypothesized risk factors (catastrophizing, fear, depression), recent findings support the central importance of cumulative interactions among overlapping factors, with cumulative risk load being a key contributor to pain outcomes, and potentially serving as the optimal target of treatment.254 For example, patients with elevated scores on a greater number of risk factors (i.e., those with a higher cumulative risk load) are more likely to develop prolonged pain and disability.257 The clinical value of this approach has been highlighted by findings that a single measure of cumulative risk load shows greater predictive strength and scope than combined severity measures of catastrophizing, fear, and depression.255;257 Additionally, stratifying clinical interventions based on cumulative prognostic risk has been shown to lead to improved outcome and reduced treatment cost.254;257 Although the cyclical relationships of the FAM offer appealingly specific clinical implications (e.g., treating catastrophizing before fear), model-relevant interventions fail to meet this level of specificity and increasing evidence suggests that cumulative, global risk indices may be a more important target of treatment than particular psychosocial constructs.254;257 Given the complexity of chronic pain73;104, it should perhaps not be surprising that the FAM fails to identify a universal pathway leading to pain-related disability. Moreover, the unique associations between FAM measures (such as fear of pain and catastrophizing) and outcomes such as longitudinal changes in pain intensity or return to work after a painful injury are generally modest, with these measures often explaining 10–15% of the variance in outcomes in the context of multidimensional predictive models254;257. For example, a given patient may indeed experience intense fear during an acute pain episode, which leads to later avoidance behavior and disability, but another may have a pre-existing mood disorder that subsequently amplifies negative pain-related cognitions such as fear and catastrophizing. Despite the limitations described, this mechanism-based model has collectively produced enormous heuristic value in facilitating our understanding and testing of some of the pathways by which psychosocial processes can shape long-term pain-related outcomes.
A number of other explanatory biopsychosocial models have been proposed, and it would require more space than we have available to adequately describe them. We briefly mention one other model- the avoidance-endurance model (AEM)85;86, which has some overlap with the FAM, though it emphasizes the importance of particular behaviorally-defined subgroups.87 The AEM hypothesizes that while some patients experiencing persistent pain will become fearful and activity-avoidant, others will show an “endurance response”, characterized by maintained activity and task persistence. The type of endurance response is determined by affective and cognitive factors, as patients with high levels of anxiety and thought suppression will show a maladaptive “distress endurance response pattern” while others with high levels of positive affect and a tendency to minimize the threat value of pain will show a more adaptive “eustress endurance response pattern”. Thus, the AEM highlights the interplay of psychosocial processes with behavior, and defines patient subgroups on that basis (i.e., the adaptiveness of showing “endurance” in the face of pain depends on the psychosocial correlates of that behavior). Overall, these various models postulate that patterns of affect, cognition, and behavior interact with an array of neurobiological pathways to shape long-term pain outcomes such as disability.87;198 For examples of other disease-related biopsychosocial models, the reader is referred to publications on multiple sclerosis pain 165, sickle cell pain 219, cancer pain 176, and HIV-related neuropathic pain.154 A comprehensive assessment of the overlap and the unique features of these models would be well beyond the scope of the present article, but we note that many influential psychosocial factors are common to most of these disease-related models.
Evaluating Psychosocial Contributions to Chronic Pain Outcomes
Below we discuss 2 broad categories of “mechanistic” studies. In the first, psychosocial processes either exist within an individual as pre-existing “vulnerability” factors (e.g., childhood trauma51) or potentially “protective” factors (e.g., social support103;214), or emerge in response to the experience of pain (e.g., fear-avoidance behavior47, self-efficacy212;261). See Figure 3. These psychosocial forces then shape individual variability in pain-related outcomes over time. For example, specific psychosocial characteristics place individuals at elevated or reduced risk for the transition from an acute to a persistent pain state, or for the development of pain-related disability in the context of a persisting pain condition.140;184 In the second type of mechanistic study, which involves an intervention within a group of patients with chronic pain, the treatment is hypothesized to directly affect a psychological factor, a change that is then associated with a subsequent change in one or more outcome variables of interest. That is, the psychological factor acts as a mediator (though not necessarily a causal mechanism) through which a treatment confers its benefits. This type of study parallels, in its structure, many biological and pharmacological studies in which a medication acts on a specific receptor, a TENS unit reduces the transmission of pain-related information in the distribution of a nerve, or a physical therapy regimen strengthens a particular group of muscles. In general, if a psychosocial process contributes causally to treatment-related changes in outcomes, then several conditions should be met.35;112 First, a change in the psychosocial factor should precede any changes in the outcome. Second, changes in that factor should be statistically related to subsequent changes in the outcome. Third, the temporal relationship should not be reversible; that is, changes in the outcome should not produce subsequent changes in the psychosocial factor (although meeting this criterion is complicated by the dynamic, bidirectional interactions between pain and psychosocial processes). A study meeting these conditions would provide substantive, though not definitive, evidence that the identified psychosocial process is on a causal pathway linking a treatment with an outcome. However, rarely do studies in this area meet such rigorous standards.
Figure 3.
Illustration of the impact of psychosocial constructs and processes on pain-related outcomes.
Often, mediation analysis is used to test the hypothesized effects. Mediation analysis offers a method of testing theories regarding the causal links between a predictor and an outcome.132;152 Mediators, also known as intermediate variables, or indirect effects, are variables that are on a causal pathway between predictor and outcome, and “explain” the effect of the former on the latter. Mediation analysis, which is increasingly applied to study psychosocial contributions to the experience of pain, tests whether the influence of a predictor or treatment on an outcome occurs via change in a particular intermediate variable, the mediator. Mediation analysis can be applied to data from various types of study designs, from cross-sectional surveys to randomized controlled trials, though different study designs impose different limitations on the interpretation of mediational effects. Unfortunately, mediation analysis has been most frequently applied to cross-sectional data, often resulting in inappropriate causal conclusions. Cross-sectional mediation analysis, which uses patterns of between-subject variation to substitute for within-subject temporal variation, cannot provide the basis for causal assertions. Longitudinal designs are an essential prerequisite for drawing any type of conclusions about causal associations between variables. In addition, while many mediational studies evaluate a single mediator of the association between predictor and outcome, it is much more likely, in the context of a complex condition such as chronic pain, that a number of mediators contribute to any observed relationships.132;152
To assess mediation in pain studies, linear regression, bootstrapping approaches, or structural equation modeling (SEM) techniques are most often applied.132;152 Linear regression is relatively simple to use and widely familiar to researchers but makes strong assumptions about the data that are not always met (e.g., the assumption of no measurement error). In an influential publication, Baron and Kenny 14 laid out several requirements that must be met to form a mediation relationship. In short, the independent (predictor) variable must be significantly associated with both the dependent (outcome) and mediator variable in univariate regression analyses, and in a regression in which both the mediator and independent (predictor) variable are entered together, the mediator must remain significantly associated with the dependent (outcome) variable, while the previously significant path between the independent (predictor) and dependent (outcome) variable must be reduced in magnitude, or rendered non-significant. Increasingly, mediation studies in the field have utilized bootstrapping, which is a general approach to statistical inference based on random re-sampling from the observed data. In general, the traditional Baron and Kenny 14 regression-based mediation analyses has fairly limited power; bootstrapping has been widely recommended as it improves power, even in relatively small samples, and obviates concerns over violating normality assumptions17;124.
Structural Equation Modeling (SEM) can also be used to test mediation effects, and is being increasingly employed by psychosocial pain researchers.3;11;215 SEM is a combination of regression analysis and factor analysis and, while making many of the same assumptions about the data, it handles the inclusion of several mediating factors more readily, it can include latent (unobserved) factors, and it can account for measurement error. SEM also provides goodness-of-fit statistics that allow comparisons between tested models. This technique does, however, require substantially larger sample sizes than traditional regression analysis. It is important to emphasize that studies employing mediation analysis (including those that utilize SEM), no matter how sophisticated their statistical approaches, are limited by their design, and that cross-sectional studies cannot provide evidence for causal, mechanistic relationships between variables.
Psychosocial Factors Influencing Pain-Related Outcomes
In the following sections we evaluate evidence for a number of psychosocial factors that have been studied as contributors to the development, long-term consequences, and sequelae of persistent pain, as well as treatment-related outcomes. In general, these variables do not appear to be condition-specific; most have been studied across multiple AAPT diagnostic categories with similar results. Conceptually, we organize these factors as “general” psychosocial constructs and processes, which are not unique to individuals experiencing pain, and “pain-specific” psychosocial factors, which are defined and measured with reference to individuals’ pain experience. This review is not exhaustive; rather we highlight some of the most influential and commonly-studied factors (see Figure 3). Specific assessment instruments to measure psychosocial constructs are described in a complementary article in this supplement (Turk, Ohrbach, & Fillingim). It is important to keep in mind that many of the constructs discussed below overlap to at least a moderate degree. For example, catastrophizing is often significantly associated with indices of depression, anxiety, and fear of pain, with correlation coefficients that are frequently in the range of .4 to .6, revealing a substantial degree of shared variance among these constructs. 59;95;253;256
General Psychosocial Factors
Distress
Depression, anxiety, and general indices of emotional distress are probably the most commonly-assessed psychological factors in patients with persistent pain, and as a cluster of negative emotions, thoughts, and behaviors also termed, “negative affect”. Recent systematic reviews indicate that chronic pain patients show elevations, relative to pain-free controls, in all of these indices of self-reported negative affect.28;96 Although psychological symptomatology is often interpreted as a consequence of chronic pain, prospective studies suggest that pre-morbid psychological dysfunction represents a risk factor for the future development of numerous chronic pain conditions.54;66;140 Moreover, similar psychosocial constructs and processes predict the likelihood of transition from acute to chronic musculoskeletal pain (i.e., higher distress levels are prospectively related to an increased probability of transitioning to chronic pain).54;184 Overall, there is a wealth of evidence that symptoms of depression, anxiety, and emotional distress contribute strongly (more strongly than pain intensity, in many studies) to key long-term outcomes of persistent pain such as physical disability82;98;197, work disability119, healthcare costs16, mortality108;210, and suicide.88;99 In general, these studies establish the association of pain with the deleterious outcomes of interest, and then show that some or all of that association can be statistically accounted for by indices of depression, anxiety, or distress. For example, in a recent study of lumbar fusion for degenerative spondylolisthesis, patients were followed post-surgically for 2 years to determine the predictors of functional outcomes.181 In multivariate analysis, high pre-operative symptoms of depression remained the only significant predictor of failure to return to work after surgery, even in multivariate models with pre- and post-operative pain intensity included, fully mediating the prospective association between pain intensity and occupational disability. Patients in the upper half of the distribution of pre-operative depression scores were approximately less likely to return to work and, among those who did return, took nearly twice as long post-operatively to begin working again. 181
Childhood Traumatic Experiences and Post-Traumatic Stress Disorder (PTSD)
Strong prospective links have been observed between early traumatic experiences and the subsequent development of chronic pain (see2;25;107). We should note that many of these traumatic experiences are social and interpersonal in nature. Childhood physical, sexual, and psychological abuse are reported to be risk factors for the adult development of pain conditions such as FM, irritable bowel syndrome, chronic pelvic pain, and temporomandibular joint disorders.2;167 Many of these effects are substantial in magnitude; a recent meta-analysis reported that the presence of past trauma was associated with a 2 to 3-fold increase in the subsequent development of chronic widespread pain in multivariate models across dozens of studies; report of abuse in childhood conferred a 97% increase in risk (i.e., Odds Ratio= 1.97) for having a painful somatic syndrome (e.g., FM) in adulthood 2. To date, it is not clear whether the association between trauma and later chronic pain is a direct result of exposure to the trauma, is driven predominantly by individual affective, cognitive, and behavioral responses to the traumatic event (e.g., intense fear, avoidance behavior), or is primarily a retrospective attempt at explaining clusters of diverse symptoms for which there may be no immediately apparent etiology.25
Not all individuals exposed to trauma go on to experience pain-related consequences, of course, but such exposures appear to substantially enhance those risks. A recent meta-analysis revealed that individuals who reported exposure to psychological trauma were nearly 3 times more likely (than those with no trauma exposure) to have persistent pain, regardless of the type of trauma.2 Moreover, it is not only abuse that confers risk for the subsequent development of persistent pain. In a prospective, longitudinal study of over 7,500 children surveyed at age 7 and again at age 45, those with childhood reports of distressing events such as hospitalizations, familial financial crises, and the death of a parent showed an approximately doubled risk for the adult development of chronic widespread pain.107 These associations remained even after adjusting for potential confounding variables such as psychological distress and socioeconomic status. Not surprisingly, the links between trauma and pain may summate cumulatively across the lifespan. Based on retrospective reporting, several studies have shown that military veterans with combat exposure and PTSD symptomatology may be more likely to have experienced previous childhood or adulthood traumas as well.117;118 Thus, it is possible that the large association of adult-experienced trauma (e.g., combat exposure) with deleterious pain-related outcomes may reflect the cumulative effect of multiple historical traumas as well.
Some categories of traumatic stress appear more likely to serve as pain-relevant risk factors than others. For example, 1 longitudinal study noted that while some childhood stressful medical events such as prolonged hospitalization were associated with chronic pain in adulthood, other events such as childhood surgery conferred no additional risk.107 Overall, combat exposure and PTSD in adulthood are reported to have the strongest statistical association with chronic pain.2;25 PTSD is a psychiatric condition that results from exposure to a traumatic event, and involves an array of negative cognitive and behavioral response to the trauma, including emotional hyperarousal, avoidance behavior, and re-experiencing of the traumatic event. PTSD has been identified as a risk factor for chronic pain100;101, for the transition from acute to chronic pain123 and for elevated severity of pain and disability in abuse victims.262;263 A number of studies have evaluated PTSD symptoms as a statistical mediator of the association between trauma and various pain-related outcomes. In a cross-sectional primary care survey study, patient recall of child abuse was linked with the report of pain and pain-related limitations in adulthood, and current levels of PTSD symptomatology fully mediated those associations.188 Similarly, in a sample of veterans, PTSD symptoms mediated the association between childhood maltreatment (physical and emotional abuse) and physical health outcomes, including the presence and intensity of persistent pain.131 Finally, though a 30-year prospective study failed to find that PTSD symptoms formally mediated the longitudinal relationship between childhood abuse and chronic pain in adulthood, there was an interactive, synergistic effect of these variables, with the presence of PTSD symptoms amplifying the predictive effect of childhood abuse on later-life pain outcomes.192 Collectively, there is strong evidence from a number of studies that abuse and trauma are linked with the subsequent development and impact of pain, with PTSD symptomatology in adulthood making a substantial contribution to those associations.
Social and Interpersonal Processes
Social forces shape a variety of health-related outcomes, and pain is no exception. As noted in a review of the factors affecting adjustment to chronic pain in individuals with disabilities, most studies have focused on either perceived global social support or solicitous social responses (e.g., offering to take over tasks or encouragement to become less active).103 Of more than a dozen studies of perceived social support as a contributor to pain-related functioning, most reported that more perceived social support was associated with better outcomes in persons with conditions such as spinal cord injury, multiple sclerosis, and acquired amputation103, whereas a higher degree of solicitousness in the social environment predicted increased pain-related disability.103 The social environment may be particularly important for persons with acquired amputation during the first few months after the amputation, as several studies found that patients reporting positive general social support were less likely to develop persistent phantom limb pain post-amputation.83 The immediate social environment in the form of parents (for children experiencing pain) and spouses (for married adult patients with pain) exerts a powerful influence on pain-related outcomes. In the case of children with persistent pain, parents’ cognitive and behavioral functioning and responses in reaction to children’s pain have also been shown to play an important determining role in children’s pain responses.178–180;194 In particular, parental pain catastrophizing is strongly related to the development of children’s persistent pain following major surgery175, and is significantly related to the child’s disability.90 Parental attention to pain and solicitousness behaviors that encourage children to avoid regular activities may provide specific pathways by which parental catastrophizing amplifies a child’s pain experience and behavior.43;90;235
It is clear that the interactions between patients with chronic pain and their significant others can either facilitate or impair adjustment to chronic pain.30;36;136 Studies across painful conditions illustrate the important role of significant others. For example, among couples, high levels of spousal depressive symptoms predict worsening patient disability and disease activity in patients with rheumatoid arthritis over a 1-year period.130 In cancer pain, social support and interpersonal effectiveness seem to play an important role in shaping pain report and general health.176 Patients with partners showing avoidant attachment styles and anxious attachment styles are more likely to report increased pain and decreased well-being.76;186;187 Patients’ attachment styles are also important predictors of pain-related outcomes among both children and adults; individuals with anxious or insecure attachment styles are at elevated risk for poorer mental and physical health129, for reduced engagement in physical activity5, and for less treatment-related improvement in affective outcomes among patients participating in a multidisciplinary treatment program125. It is also important to understand the nature (e.g., supportive, solicitous, adversarial), of other important social interactions, such as relationships at work; lack of social support at work and dissatisfaction with co-workers; and interactions with the disability compensation system are among the most potent predictors of work disability related to pain.91;163;164 For example, Li and colleagues studied workplace support among arthritis patients; those who reported low workplace support were much more likely to develop depressive symptoms and work-related disability 18 months later.139 Moreover, differing social and occupational structures across countries appear to contribute to cross-national differences in rates of return to work and occupational disability in the context of painful work injury. Anema and colleagues6 compared sustainable return-to-work rates between 6 different countries and found that differences in job characteristics and social disability systems were more important than medical interventions, patient, and injury-related factors in explaining the large between-country differences in rates of return to work following painful occupational injuries.
Of course, the social environment can also be harnessed for adaptive purposes. Keefe and colleagues have added spouse-assisted coping skills training to standard CBT and multidisciplinary pain management programs, hypothesizing that the supportive and reinforcing effects of a spouse will facilitate improved pain-related coping and enhance self-efficacy for managing pain-related symptoms.114;115 Such interventions generally involve dyadic sessions that teach couples communication skills and use mutual goal setting to assist chronic pain patients in acquiring, maintaining, and effectively deploying pain-coping skills. In the most recent randomized controlled trial, in patients with LBP and their spouses1, the spouse-assisted intervention produced larger decreases in fear of pain and catastrophizing than the standard multidisciplinary intervention.
Other individual social and interpersonal relationships are also important influences on pain-related outcomes. In particular, the results from many studies of psychotherapy process and outcome confirm that 2 interpersonal factors: (1) stimulating patient expectations that treatment will help; and (2) establishing a sound therapeutic relationship between patient and therapist, are crucial foundations upon which successful interventions are built.157 A handful of studies suggest that an index of the therapeutic relationship (i.e., the working alliance) statistically mediates the positive effects of rehabilitative treatments among people with musculoskeletal pain.32;80;81 Indeed, creating and sustaining an effective therapeutic alliance appears to be a necessary and sufficient condition for promoting the pain-improving effects of diverse interventions.34;35 It is also the case that psychosocial processes such as depression can negatively impact patient-provider relationships. For example, a recent study revealed that depression was associated with patient-physician discordance in estimates of disease severity15 (i.e., depressed patients estimated their disease severity as much worse, on average, than their physicians did). Such discordance is likely to be common, especially in light of the “invisible” nature of pain, and it can have deleterious effects on patient satisfaction and adherence to treatment regimens.89
Pain-Specific Psychosocial Constructs
Catastrophizing
Catastrophizing is a pain-specific psychosocial construct comprised of negative cognitive and emotional processes such as helplessness, pessimism, rumination about pain-related symptoms, and magnification of pain reports.59 While catastrophizing positively correlates with general measures of negative affect such as depressive symptoms and anxiety, it also shows a unique and specific influence on pain-related outcomes.59;121;185 Overall, higher catastrophizing has been shown to be a risk factor for the development of long-term pain, and for negative sequelae of pain such as worsening physical disability, higher healthcare costs, and the amplification of pain sensitivity among patients with LBP and joint pain.37;61;63 Retrospective survey studies in patients with musculoskeletal pain have indicated that catastrophizing often emerges as one of the most important pre-treatment variables predicting surgical outcomes138;203, and a risk factor that impairs the effectiveness of pain-relieving interventions.93;110 Longitudinal studies show associations of catastrophizing with worsening pain and reduced treatment benefit among, for example, arthritis patients recovering from knee surgery.60;70;195;238 Multiple randomized controlled trials have shown that pain patients with high pre-treatment catastrophizing scores report less benefit from topical analgesics151, cortisone150, oral analgesics200, pain-relieving surgeries238, and psychosocial treatments such as CBT.53;232
A recent study of patients with persistent orofacial pain, randomized to 6 weeks of either standard care or CBT and followed for 12 months, confirmed the treatment-mediating effects of catastrophizing.141 Patients with high levels of pre-treatment catastrophizing, and those whose catastrophizing scores did not change after treatment, were significantly more likely to be non-responders at 1 year follow-up. Indeed, baseline levels of catastrophizing in the non-responder group were over 1 standard deviation higher than baseline catastrophizing scores for the responder groups.141 It is interesting to note that catastrophizing may have its most influential mechanistic effects in the context of active, rather than placebo treatments. In a recent trial of transcutaneous electrical nerve stimulation (TENS) for post-operative pain191, patients undergoing joint replacement surgery were randomized to receive TENS, placebo TENS, or standard care (no TENS) for 6 weeks. Those in the TENS group with high baseline catastrophizing scores showed less pain reduction and reduced functional outcomes (e.g., lower range of motion) at 6 weeks. In contrast, there was no association of catastrophizing with pain-related outcomes in the other groups (i.e., those receiving placebo or standard care treatment).
Furthermore, the benefits of many diverse analgesic therapies appear to be explained partly by their effects on cognitive-emotional processes such as catastrophizing. This is certainly true for CBT and similar psychosocial treatments. Longitudinal process analyses indicate that changes in catastrophizing and negative affect precede changes in clinical pain33;142;221;223, that CBT can produce substantial reductions in catastrophizing even among patients whose chronic pain has persisted for decades168;172, and CBT’s catastrophizing-reducing effects may last for months or years.233 Multiple studies that employ cross-lagged panel analyses, or similar statistical approaches, have shown that substantial portions of the variability in end-of-treatment outcomes for CBT and multidisciplinary treatment can be accounted for by early-treatment changes in catastrophizing.29;31;33 Interestingly, as recent reviews point out102;221, we know relatively little about the mechanisms underlying CBT and other non-pharmacologic pain treatment approaches, and it may be that disparate treatments operate in part via common mechanisms. For example, changes in catastrophizing statistically mediate the benefits of CBT31;33;105;233, and multidisciplinary treatment programs50, as well as exercise- and activity-based physical therapy interventions that do not explicitly target catastrophizing.134;209 Smeets and colleagues209 compared CBT, active physical treatment (i.e., aerobic and strength training), and their combination, and found that the 3 active treatments did not differ significantly on pre- to post-treatment change in pain catastrophizing, but that pre- to post-treatment changes in pain catastrophizing predicted pre to post changes in most outcomes across the intervention groups (i.e., catastrophizing diminished just as much in the active physical treatment group as in the CBT group, and reductions in catastrophizing were equivalently influential predictors of improvements in pain across groups). Across the active treatments, catastrophizing reduction accounted for 35–40% of the benefit of treatment in terms of reduced pain and disability. Collectively, these results suggest that reduction in catastrophizing among chronic pain patients may account for some of the beneficial effects of many behavioral pain treatments.
Catastrophizing clearly overlaps with numerous other psychosocial processes, showing positive associations with indices of depression, anxiety, distress, and fear of pain, and inverse associations with self-efficacy, optimism, and other positive factors 59;95;170. However, even when controlling for some of these related factors, catastrophizing often retains significant unique, predictive influence (though it is true that no published studies, to our knowledge, control for every other variable listed here). For example, after statistically adjusting for indices of depression and anxiety, catastrophizing remained significantly associated with such diverse outcomes as return to work 77;217, pain-related physical disability 94, risk for prescription opioid misuse 155;156, brain responses to a noxious stimulus79;144, pain intensity8, pain tolerance 226, and suicidal ideation 62. Moreover, catastrophizing likely interacts with other processes such as social support 26. It is not just the patient’s degree of catastrophizing that has been shown to influence important pain-related outcomes; spousal levels of catastrophizing and patient catastrophizing are modestly correlated with one another and often both emerge as unique influential predictors 135;182. Similar findings obtain when evaluating the influence of parental catastrophizing on children’s reports of pain, particularly post-operative pain 175;177;190.
Self-efficacy
Self-efficacy is a broad concept that refers to an individual’s belief in his or her own ability to perform a certain behavior to achieve a desired outcome.116;212 According to Bandura’s social cognitive theory, self-efficacy is a major determinant of individuals’ thoughts, feelings, and behaviors in stressful situations, and affects individuals’ ability to cope successfully when confronted with difficult challenges. Pain-related self-efficacy is often measured using self-report scales such as the general chronic pain self-efficacy questionnaire4, or disease-specific measures such as the arthritis self-efficacy scale (for patients with arthritis pain)147, which assess patients’ perceived ability to control pain symptoms and to function in spite of pain (See Turk, Ohrbach, & Fillingim in this supplement). Self-efficacy has been characterized as a protective psychological resource in patients with persistent pain, and a resiliency factor associated with improved functional outcomes among children, adolescents, and adults with chronic pain214. A number of prospective studies have assessed self-efficacy as an influential contributor to functional outcomes in a variety of painful conditions. For example, a longitudinal study in patients with chronic LBP showed that self-efficacy partially mediated the association between pain and disability at multiple study time points.46 Moreover, changes in self-efficacy (but not changes in pain-related fear) over the 1-year course of the study partially mediated the association between changes in pain and changes in disability.46 Very similar findings (e.g., high self-efficacy is associated with better functional outcomes, and variability in self-efficacy mediates the association between pain intensity and disability) have been observed in other pain conditions such as arthritis, headache109, FM166 and pediatric pain conditions.92 To illustrate, in a prospective treatment study, self-efficacy was among the most potent mediators of CBT-related improvements in pain and disability among patients with persistent orofacial pain.232 Overall, high levels of self-efficacy are associated with lower reported intensity and unpleasantness of pain, and with less physical disability. As noted in recent reviews 116;212, the persistent and pervasive nature of chronic pain requires patients to make constant adjustments to learn to live with their disease. Thus, many non-pharmacologic treatments target self-efficacy as an important process variable.
“Positive” factors
Most of the frequently-studied psychological facets of the biopsychosocial model could be broadly classified as having a negative valence (e.g., negative affect, distress, trauma, catastrophizing). Indeed, some past reviewers of this literature have called for more attention to positive factors that may confer protection from and resilience against chronic pain and related suffering.22;23;120;224 Such resiliency research focuses on how individuals successfully adapt to adverse stimuli or situations, such as prolonged and persistent pain, and its impact on multiple areas of physical, emotional, and social functioning.39;109 While resiliency factors have been linked to outcomes in the FAM, previous research suggests that risk and resilience factors do not represent opposite ends of a spectrum because individuals can be concurrently high or low in both types of factors.254 This research suggests that consideration of both risk and resiliency factors may help explain how individuals can live with chronic pain without concurrently experiencing disability.254
A number of studies have indicated that improving active pain coping is an important component of many non-pharmacological treatments.34;35;50;142;225 Such “active” coping generally includes engaging in positive thinking, making encouraging self-statements, distracting one’s attention from pain, undertaking as much physical activity as possible within pacing guidelines, or using physical pain-reducing techniques such as relaxation exercises and stretching. For example, a recent prospective study of multidisciplinary treatment revealed that patients who entered treatment with stronger personal beliefs in their ability to control pain, and those who increased their use of positive self-statements and cognitive reinterpretation of pain, showed the most substantial decreases in pain-related interference at 6 months and 18 months post-treatment.50 As the authors note, this highlights a process-oriented role for active cognitive coping cognitions in shaping the outcomes of multidisciplinary treatment. That is, facilitation and encouragement of adaptive active pain-coping efforts may be one pathway by which such treatments exert their beneficial effects. It is important to mention, however, that negative cognitive-emotional processes were assessed in this study as well, and treatment-related changes in negative affective and cognitive states such as pain-related catastrophizing were stronger predictors of outcomes than “positive” factors.
With the fairly recent advent of interventions such as acceptance and commitment therapy (ACT) and mindfulness meditation for chronic pain, a good deal of interest has arisen in what psychosocial processes may underlie the observed benefits of these and related treatments.120,121 ACT is an empirically-based, process-focused psychological intervention that de-emphasizes active efforts to control pain, and encourages acceptance, psychological flexibility, and values-based action as the most productive response to persistent pain.159;161 Numerous cross-sectional studies have demonstrated the important potential contributions of acceptance-related processes to the physical functioning of chronic pain patients160;161;243–246, with higher levels of patient acceptance often buffering the effect of high pain severity on pain-related disability. Some interventional studies have also found acceptance to act as a mediator in interventional research. For example, in a recent study of several hundred chronic pain patients completing multidisciplinary treatment, Akerblom and colleagues3 identified acceptance as the single most important mediator of treatment outcomes such as pain interference. Mindfulness has also been studied in a similar role: Schmidt et al.201 compared mindfulness-based stress reduction (MBSR) to a pain education control condition, and found that significant pre-post changes in mindfulness were equivalent across MBSR and placebo (i.e., pain education), and that changes in mindfulness were strongly associated with a host of functional outcomes about equally across the 2 treatments. Other studies of acceptance-oriented interventions have arrived at similar conclusions regarding the importance of acceptance and psychological flexibility as process variables that may serve as treatment targets.161;243;246 Such findings suggest that, much like catastrophizing, shifts in “positive” psychosocial factors can be associated with, or potentially predictive of, individual differences in the outcomes of a variety of treatments, whether those treatments specifically target those factors (e.g., MBSR) or not (e.g., pain education). Indeed, some recent work has suggested that mindfulness-based interventions may have greater effects on pain-related catastrophizing, especially in the context of high levels of pain intensity, than traditional CBT approaches. 49;55 Such findings suggest the possibility of tailoring individual psychosocial interventions on the basis of important patient characteristics, though we do not yet have firm evidence from large randomized, controlled trials on which to base such recommendations. For example, given the importance of the social environment in shaping pain responses, individuals with a supportive significant other might benefit most from couples-bases coping skills training, which has been shown to outperform standard multidisciplinary treatments for some patients 1.
Finally, there has been some exciting recent work in the area of cognitive-behaviorally-oriented educational interventions that aim to increase knowledge of pain-related biology.148;169 Such Explaining Pain (EP) treatments include a range of educational techniques designed to change patients’ understanding of the biological processes that underlie pain; these changes in the conceptualization of pain are hypothesized to serve as a mechanism to reduce pain itself. EP interventions are grounded in educational psychology and current theories of pain biology148;169. The core objective of the EP approach to treatment is to help patients shift from the understanding of pain as a marker of tissue damage or pathology, to the understanding that pain is a marker of danger, and the perceived need to protect body tissue. Recent reviews reveal that EP interventions increase accurate knowledge of pain-related biology, decrease catastrophizing and pain-related negative affect, and reduce the intensity of patients’ pain133;148;169;220.
Other Factors
Numerous other process variables have also been evaluated in mediational studies of treatment outcomes. Pain-related fear has already been discussed with respect to the FAM, and it is helpful to keep in mind that indices of fear and catastrophizing are strongly inter-correlated such that their unique influence can be difficult to identify statistically when they are measured together.27;257 Additional key process variables such as pain-related expectations likely overlap with these factors as well. Expectations are a crucial component of placebo responses, they can strongly influence the outcomes of active treatments, from surgery71;264, to opioid analgesics20, to acupuncture.260 A recent analysis of multiple large acupuncture trials revealed that both patient and provider expectations for treatment success were potent predictors of response259;260, with better pre-treatment expectations prospectively predicting improved patient outcomes following treatment. Finally, measures of somatization, somatic focus, or somatic awareness assess important psychosocial characteristics as well, particularly in the setting of chronic widespread pain conditions such as FM or its comorbid conditions.54;66;67 These measures have rarely been studied as targets of change in interventional studies, but a good deal of evidence exists for their role as key risk factors predicting the development and course (including the transition from acute to chronic pain) of numerous pain conditions such as temporomandibular joint disorders66, and neuropathic pain conditions like post-herpetic neuralgia57;111 or burning mouth syndrome.199
“Downstream” Pathways
Psychosocial processes likely impact a number of specific pathways that convey some of their effects (whether beneficial or deleterious) on pain outcomes. Below we touch on several such pathways:
Maladaptive Health Behaviors
General and pain-specific negative cognitions appear to reduce the likelihood of exercise and other health-promoting behaviors among patients with chronic pain, which may contribute to their impact on long-term pain outcomes such as functional disability. Features of pain-related catastrophizing have been shown to correlate with less effective medication use173, less positive health behavior such as exercise38, and a lower likelihood of attending scheduled treatment visits;141;207 these are plausible pathways by which psychosocial distress could enhance disease, amplify pain, and promote mortality. In samples of obese patients with knee osteoarthritis, catastrophizing was associated with poorer weight management, with more frequent binge eating, with reduced physical capacity, and with reduced weight-related quality of life.211 In prospective studies in patients with acute LBP, those with high levels of negative affect and catastrophizing are most likely to engage in extended periods of bed rest, least likely to exercise, and most likely to become physically de-conditioned over time.24;237 In contrast, protective and resilience factors such as social support are associated with greater engagement in physical activity and exercise.214
Information Processing Biases and Increased Attention to Pain
High levels of depression, distress, and catastrophizing, and low levels of self-efficacy for managing pain may produce attentional and information-processing biases that lead individuals to attend selectively and intensely to pain-related stimuli.48;189;202;234 Catastrophizers experience more difficulty controlling or suppressing pain-related thoughts than do non-catastrophizers, they ruminate more about their pain sensations, and their cognitive and physical task performance is more disrupted by anticipation of pain (see189). Similarly, in samples of patients with painful rheumatic disease, individuals with clinical depression demonstrate a word-recall bias for disability-related and pain-related words52, as well as a tendency to ruminate about pain-related word meaning.208 Collectively, the results of these studies suggest that patients with particular psychosocial characteristics conveying risk for negative long-term pain outcomes (i.e., high levels of distress and catastrophizing, and low levels of self-efficacy) are most likely to anticipate pain, to interpret ambiguous signals as being related to pain, to attend to pain-related visual cues, and to experience interference of pain with other cognitive activities.
Central Nervous System Pathways
Progress in brain imaging has been exponential in recent years, producing evidence of alterations in both brain structure and function among patients with chronic pain.51;68;149 Functional magnetic resonance imaging (fMRI), positron emission tomography, and electroencephalography are commonly used to study the neural bases of pain. Other magnetic resonance-based measures (e.g., diffusion tensor imaging, spectroscopy) are also being used to assess pain-related changes in the brain’s interconnections, chemistry, and structure in order to gain further insights into the neurobiology of chronic pain. Recent reviews nicely summarize the literature comparing patients with a variety of persistent pain conditions to pain-free controls13;149;153, identifying a number of cortical regions that are considered to be important for the perception of pain.51;68 These include the primary and secondary somatosensory cortices, insular and anterior cingulate cortices, the prefrontal cortices, and many subcortical areas such as the periaqueductal gray, amygdala, and cerebellum). Many of these areas have been found to have altered gray matter density in patients with persistent pain7;158, and they also show changes in the brain’s default mode network and other resting state networks146;171, suggesting long-lasting functional brain changes related to the presence of chronic pain. Non-neural components of the central nervous system also appear to be affected; patients with chronic back pain show enhanced microglial activation relative to pain-free controls.145
Although a thorough treatment of the role of cognition and emotion in shaping the brain’s processing of sensory information is well beyond the score of this article, we note that many of the psychosocial factors mentioned previously have been shown to modulate the perception of pain and the neural consequences of chronic pain. For example, past studies of grey matter loss in FM have indicated that atrophy of specific brain regions is more strongly related to the presence of symptoms of anxiety and depression than to chronic pain.97 That is, psychosocial distress may directly contribute to amplification of the central nervous system consequences of living with a long-term pain condition. Pain-specific cognitive and affective processes also have important associations with functional brain responses to pain. In several studies, higher levels of catastrophizing were related to enhanced fMRI responses to calibrated noxious stimuli in areas such as anterior insular cortex among both FM patients78 and pain-free adults.204 Structurally, elevations in catastrophizing among patients with chronic abdominal pain were associated with thinning of the dorsolateral prefrontal cortex, a key pain-modulatory site.21 Several psychophysical studies have also shown that catastrophizing, anxiety, and other negative affective processes are related to reduced effectiveness in descending pain-inhibitory systems (see 236). In addition, we recently reported that impaired activity in regions of the prefrontal cortices mediated the association between catastrophizing and hyperalgesia in patients with FM.144 This effect was observed even after statistically controlling for patients’ degree of depressive symptomatology, which is significantly positively correlated with catastrophizing scores. Finally, psychosocial and behavioral interventions that target cognitive processes have been shown to reverse these functional and structural brain changes over the course of just months.205;206 Collectively, these findings substantiate the possibility that interventions that reduce catastrophizing and negative affect may produce long-lasting, adaptive shifts in brain processing of pain.
Conclusions
The AAPT provides an empirically-based, multidimensional, chronic pain classification system, within which psychosocial factors play key roles. As the tenets of the biopsychosocial model suggest, a number of these variables act as risk or resilience factors, influencing the probability of developing a chronic pain condition, the severity of pain-related consequences such as disability, and the success or failure of various pain treatments (see Figure 3). Studies in patients with a variety of pain diagnoses reveal that both “general” and “pain-specific” psychosocial variables exert substantive influences on pain outcomes. These psychosocial variables have anatomical and neurophysiological counterparts. That is, psychosocial processes do not exclude involvement of neurophysiological processes, but provide a useful, alternative perspective on these processes. At times, pain physiologists, pain psychologists, and philosophers have assumed that psychological concepts and processes used to explain pain would be gradually replaced by physiological concepts and processes. For example, in the 1970’s and 1980’s many philosophers would have predicted that the statement “my c-fibers are firing” would replace the statement, “I am in pain” as a description of an individual’s experience (see 40–42). This has not occurred. In general, we doubt that the replacement of psychological terms with physiological terms will occur anytime soon, despite impressive advances in functional neuroimaging.
Future studies in this area may benefit from the measurement of larger sets of these process variables, and from additional theoretical work on their inter-relationships, in order to further illuminate their interactions. At this point, additional cross-sectional mediational analyses are unlikely to make meaningful contributions to the nature of these biopsychosocial interactions, though longitudinal mediational studies may shed light on the temporal dynamics of such associations. An additional complication is that since some of the specifically-postulated causal and temporal associations (e.g., the sequential cyclical relationships hypothesized in the FAM) have not withstood empirical scrutiny, there has been little consistency in how researchers organize the psychosocial variables being investigated. For example, in recent studies, depressive symptoms, catastrophizing, self-efficacy, and mindfulness have each been examined statistically as predictors, mediators, and outcome variables. This reflects the overlap among these constructs, but makes planning future process studies a challenge.
Such considerations are especially important in light of recent evidence that the theoretically-based techniques comprising particular treatment approaches may be less important than whether the techniques impact key factors that underlie changes in cognition, emotion, and behavior.31;33–35 Treatment-related improvements in cognitive content variables such as pain catastrophizing106;213;222, self-efficacy, and perceived pain control105;106;213;232 appear to be influential across modalities of intervention and across pain-related outcomes, though the hierarchical and temporal relationships among these factors are presently not well understood. Collectively, the complexity and profound variability in chronic pain highlights the need to better understand the interacting forces that determine the trajectory of chronic pain conditions, and we recommend that future studies employing the AAPT classification system consider assessing the psychosocial factors identified here. It is important to acknowledge that psychological and social factors are not solely secondary reactions to persistent pain; rather, they are intricately involved in an amalgam of biopsychosocial processes that characterizes chronic pain. Across biological diagnoses, a diverse array of psychological, social and contextual factors need to be considered in their roles as potential risk factors, protective factors, and process variables within the dynamic system of forces that constitutes a chronic pain condition. Moreover, their broad applicability suggests that such factors should be considered in classifying patients within all domains of chronic pain disorders, regardless of presumed etiology (e.g., neuropathic, musculoskeletal, inflammatory). The AAPT taxonomy represents an important, evidence-based step toward that goal.
Perspective.
The ACTTION-APS Pain Taxonomy is an evidence-based chronic pain classification system in which psychosocial concepts and processes are essential in understanding the development of chronic pain and its impact. In this manuscript we review psychosocial processes that influence the onset, exacerbation, and maintenance of chronic pain disorders.
Acknowledgments
We thank Mina Lazaridou, Ph.D. for her valuable assistance in organizing the manuscript.
The views expressed in this article are those of the authors and no official endorsement by the U.S. Food and Drug Administration (FDA) or the pharmaceutical companies that have provided unrestricted grants to support the activities of the Analgesic, Anesthetic, and Addiction Clinical Trial Translations, Innovations, Opportunities, and Networks (ACTTION) public-private partnership with the FDA should be inferred.
Footnotes
Disclosures: The views expressed in this article are those of the authors, none of whom has a financial conflict of interest related to the specific issues discussed in this manuscript. All of the authors attended the consensus meeting on which this article is based. In addition, the consensus meeting was attended by employees of Pfizer, one of the companies that provided unrestricted grants to ACTTION to support its activities. However, these individuals did not contribute to the content of this manuscript. The consensus meeting on which this article is based was funded by ACTTION, which has received research grants or other revenue from the FDA, various pharmaceutical companies, and other sources.
None of the authors have a conflict of interest related to this work. This manuscript is the work of the authors, and no official endorsement by the FDA, US National Institutes of Health, or the pharmaceutical and device companies that have provided unrestricted grants to support the activities of ACTTION should be inferred.
Reference List
- 1.Abbasi M, Dehghani M, Keefe FJ, Jafari H, Behtash H, Shams J. Spouse-assisted training in pain coping skills and the outcome of multidisciplinary pain management for chronic low back pain treatment: a 1-year randomized controlled trial. Eur J Pain. 2012;16:1033–1043. doi: 10.1002/j.1532-2149.2011.00097.x. [DOI] [PubMed] [Google Scholar]
- 2.Afari N, Ahumada SM, Wright LJ, Mostoufi S, Golnari G, Reis V, Cuneo JG. Psychological trauma and functional somatic syndromes: a systematic review and meta-analysis. Psychosom Med. 2014;76:2–11. doi: 10.1097/PSY.0000000000000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akerblom S, Perrin S, Rivano FM, McCracken LM. The Mediating Role of Acceptance in Multidisciplinary Cognitive-Behavioral Therapy for Chronic Pain. J Pain. 2015;16(7):606–15. doi: 10.1016/j.jpain.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Anderson KO, Dowds BN, Pelletz RE, Edwards WT, Peeters-Asdourian C. Development and initial validation of a scale to measure self-efficacy beliefs in patients with chronic pain. Pain. 1995;63:77–84. doi: 10.1016/0304-3959(95)00021-J. [DOI] [PubMed] [Google Scholar]
- 5.Andrews NE, Meredith PJ, Strong J, Donohue GF. Adult attachment and approaches to activity engagement in chronic pain. Pain Res Manag. 2014;19:317–327. doi: 10.1155/2014/838954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anema JR, Schellart AJ, Cassidy JD, Loisel P, Veerman TJ, van der Beek AJ. Can cross country differences in return-to-work after chronic occupational back pain be explained? An exploratory analysis on disability policies in a six country cohort study. J Occup Rehabil. 2009;19:419–426. doi: 10.1007/s10926-009-9202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apkarian AV, Hashmi JA, Baliki MN. Pain and the brain: specificity and plasticity of the brain in clinical chronic pain. Pain. 2011;152:S49–S64. doi: 10.1016/j.pain.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Archer KR, Abraham CM, Song Y, Obremskey WT. Cognitive-behavioral determinants of pain and disability two years after traumatic injury: A cross-sectional survey study. J Trauma Acute Care Surg. 2012;72:473–479. doi: 10.1097/TA.0b013e3182245ece. [DOI] [PubMed] [Google Scholar]
- 9.Arola HM, Nicholls E, Mallen C, Thomas E. Self-reported pain interference and symptoms of anxiety and depression in community-dwelling older adults: Can a temporal relationship be determined? Eur J Pain. 2010;14(9):966–71. doi: 10.1016/j.ejpain.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Asmundson GJ, Katz J. Understanding the co-occurrence of anxiety disorders and chronic pain: state-of-the-art. Depress Anxiety. 2009;26:888–901. doi: 10.1002/da.20600. [DOI] [PubMed] [Google Scholar]
- 11.Asmundson GJ, Parkerson HA, Petter M, Noel M. What is the role of fear and escape/avoidance in chronic pain? Models, structural analysis and future directions. Pain Manag. 2012;2:295–303. doi: 10.2217/pmt.12.15. [DOI] [PubMed] [Google Scholar]
- 12.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163:2433–2445. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- 13.Baliki MN, Mansour AR, Baria AT, Apkarian V. Functional reorganization of the default mode network across chronic pain conditions. PLoS One. 2014;9:e106133. doi: 10.1371/journal.pone.0106133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baron R, Kenny D. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Personal Soc Psychol. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 15.Barton JL, Imboden J, Graf J, Glidden D, Yelin EH, Schillinger D. Patient-physician discordance in assessments of global disease severity in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2010;62:857–864. doi: 10.1002/acr.20132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baumeister H, Knecht A, Hutter N. Direct and indirect costs in persons with chronic back pain and comorbid mental disorders--a systematic review. J Psychosom Res. 2012;73:79–85. doi: 10.1016/j.jpsychores.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Beasley TM. Tests of Mediation: Paradoxical Decline in Statistical Power as a Function of Mediator Collinearity. J Exp Educ. 2014;82:283–306. doi: 10.1080/00220973.2013.813360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bechtel W. The challenge of characterizing operations in the mechanisms underlying behavior. J Exp Anal Behav. 2005;84:313–325. doi: 10.1901/jeab.2005.103-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bergbom S, Boersma K, Linton SJ. Both early and late changes in psychological variables relate to treatment outcome for musculoskeletal pain patients at risk for disability. Behav Res Ther. 2012;50:726–734. doi: 10.1016/j.brat.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Bingel U, Wanigasekera V, Wiech K, Ni Mhuircheartaigh R, Lee MC, Ploner M, Tracey I. The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Sci Transl Med. 2011;3:70ra14. doi: 10.1126/scitranslmed.3001244. [DOI] [PubMed] [Google Scholar]
- 21.Blankstein U, Chen J, Diamant NE, Davis KD. Altered brain structure in irritable bowel syndrome: potential contributions of pre-existing and disease-driven factors. Gastroenterology. 2010;138:1783–1789. doi: 10.1053/j.gastro.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 22.Bonanno GA. Loss, trauma, and human resilience: have we underestimated the human capacity to thrive after extremely aversive events? Am Psychol. 2004;59:20–28. doi: 10.1037/0003-066X.59.1.20. [DOI] [PubMed] [Google Scholar]
- 23.Bonanno GA, Mancini AD. The human capacity to thrive in the face of potential trauma. Pediatrics. 2008;121:369–375. doi: 10.1542/peds.2007-1648. [DOI] [PubMed] [Google Scholar]
- 24.Bousema EJ, Verbunt JA, Seelen HA, Vlaeyen JW, Knottnerus JA. Disuse and physical deconditioning in the first year after the onset of back pain. Pain. 2007;130:279–286. doi: 10.1016/j.pain.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 25.Brennstuhl MJ, Tarquinio C, Montel S. Chronic Pain and PTSD: Evolving Views on Their Comorbidity. Perspect Psychiatr Care. 2014;51(4):295–304. doi: 10.1111/ppc.12093. [DOI] [PubMed] [Google Scholar]
- 26.Buenaver LF, Edwards RR, Haythornthwaite JA. Pain-related catastrophizing and perceived social responses: Inter-relationships in the context of chronic pain. Pain. 2007;127:234–242. doi: 10.1016/j.pain.2006.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buer N, Linton SJ. Fear-avoidance beliefs and catastrophizing: occurrence and risk factor in back pain and ADL in the general population. Pain. 2002;99:485–491. doi: 10.1016/S0304-3959(02)00265-8. [DOI] [PubMed] [Google Scholar]
- 28.Burke AL, Mathias JL, Denson LA. Psychological functioning of people living with chronic pain: A meta-analytic review. Br J Clin Psychol. 2015;54(3):345–6. doi: 10.1111/bjc.12078. [DOI] [PubMed] [Google Scholar]
- 29.Burns JW, Day MA, Thorn BE. Is reduction in pain catastrophizing a therapeutic mechanism specific to cognitive-behavioral therapy for chronic pain? Transl Behav Med. 2012;2:22–29. doi: 10.1007/s13142-011-0086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burns JW, Gerhart JI, Bruehl S, Post KM, Smith DA, Porter LS, Schuster E, Buvanendran A, Fras AM, Keefe FJ. Anger arousal and behavioral anger regulation in everyday life among people with chronic low back pain: Relationships with spouse responses and negative affect. Health Psychol. 2016;35:29–40. doi: 10.1037/hea0000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burns JW, Glenn B, Bruehl S, Harden RN, Lofland K. Cognitive factors influence outcome following multidisciplinary chronic pain treatment: a replication and extension of a cross-lagged panel analysis. Behav Res Ther. 2003;41:1163–1182. doi: 10.1016/s0005-7967(03)00029-9. [DOI] [PubMed] [Google Scholar]
- 32.Burns JW, Higdon LJ, Mullen JT, Lansky D, Wei JMl. Relationships among patient hostility, anger expression, depression, and the working alliance in a work hardening program. Ann Behav Med. 1999;21:77–82. doi: 10.1007/BF02895037. [DOI] [PubMed] [Google Scholar]
- 33.Burns JW, Kubilus A, Bruehl S, Harden RN, Lofland K. Do changes in cognitive factors influence outcome following multidisciplinary treatment for chronic pain? A cross-lagged panel analysis. J Consult Clin Psychol. 2003;71:81–91. doi: 10.1037//0022-006x.71.1.81. [DOI] [PubMed] [Google Scholar]
- 34.Burns JW, Nielson WR, Jensen MP, Heapy A, Czlapinski R, Kerns RD. Does Change Occur for the Reasons We Think It Does? A Test of Specific Therapeutic Operations During Cognitive-Behavioral Treatment of Chronic Pain. Clin J Pain. 2015;31:603–611. doi: 10.1097/AJP.0000000000000141. [DOI] [PubMed] [Google Scholar]
- 35.Burns JW, Nielson WR, Jensen MP, Heapy A, Czlapinski R, Kerns RD. Specific and general therapeutic mechanisms in cognitive behavioral treatment of chronic pain. J Consult Clin Psychol. 2015;83:1–11. doi: 10.1037/a0037208. [DOI] [PubMed] [Google Scholar]
- 36.Burns JW, Peterson KM, Smith DA, Keefe FJ, Porter LS, Schuster E, Kinner E. Temporal associations between spouse criticism/hostility and pain among patients with chronic pain: a within-couple daily diary study. Pain. 2013;154:2715–2721. doi: 10.1016/j.pain.2013.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campbell CM, kronfli T, Buenaver LF, Smith MT, Berna C, Haythornthwaite JA, Edwards RR. Situational versus dispositional measurement of catastrophizing: associations with pain responses in multiple samples. J Pain. 2010;11:443–453. doi: 10.1016/j.jpain.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Castaneda DM, Bigatti S, Cronan TA. Gender and exercise behavior among women and men with osteoarthritis. Women Health. 1998;27:33–53. doi: 10.1300/J013v27n04_03. [DOI] [PubMed] [Google Scholar]
- 39.Chen E, Miller GE, Lachman ME, Gruenewald TL, Seeman TE. Protective factors for adults from low-childhood socioeconomic circumstances: the benefits of shift-and-persist for allostatic load. Psychosom Med. 2012;74:178–186. doi: 10.1097/PSY.0b013e31824206fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Churchland PS. Mind-brain reduction: new light from the philosophy of science. Neuroscience. 1982;7:1041–1047. doi: 10.1016/0306-4522(82)91117-4. [DOI] [PubMed] [Google Scholar]
- 41.Churchland PS. Neurophilosophy: the early years and new directions. Funct Neurol. 2007;22:185–195. [PubMed] [Google Scholar]
- 42.Churchland PS, Phil B. The significance of neuroscience for philosophy. Funct Neurol. 2008;23:175–178. [PubMed] [Google Scholar]
- 43.Claar RL, Simons LE, Logan DE. Parental response to children’s pain: the moderating impact of children’s emotional distress on symptoms and disability. Pain. 2008;138:172–179. doi: 10.1016/j.pain.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 44.Clauw DJ. Fibromyalgia and Related Conditions. Mayo Clin Proc. 2015;90:680–692. doi: 10.1016/j.mayocp.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 45.Cohen SP, Mao J. Neuropathic pain: mechanisms and their clinical implications. BMJ. 2014;348:f7656. doi: 10.1136/bmj.f7656. [DOI] [PubMed] [Google Scholar]
- 46.Costa LC, Maher CG, McAuley JH, Hancock MJ, Smeets RJ. Self-efficacy is more important than fear of movement in mediating the relationship between pain and disability in chronic low back pain. Eur J Pain. 2011;15:213–219. doi: 10.1016/j.ejpain.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 47.Crombez G, Eccleston C, Van Damme S, Vlaeyen JW, Karoly P. Fear-avoidance model of chronic pain: the next generation. Clin J Pain. 2012;28:475–483. doi: 10.1097/AJP.0b013e3182385392. [DOI] [PubMed] [Google Scholar]
- 48.Crombez G, Van Ryckeghem DM, Eccleston C, Van Damme S. Attentional bias to pain-related information: a meta-analysis. Pain. 2013;154:497–510. doi: 10.1016/j.pain.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 49.Davis MC, Zautra AJ, Wolf LD, Tennan H, Yeung EW. Mindfulness and cognitive-behavioral interventions for chronic pain: differential effects on daily pain reactivity and stress reactivity. J Consult Clin Psychol. 2015;83:24–35. doi: 10.1037/a0038200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Rooij A, de Boer MR, van der LM, Roorda LD, Steultjens MP, Dekker J. Cognitive mechanisms of change in multidisciplinary treatment of patients with chronic widespread pain: a prospective cohort study. J Rehabil Med. 2014;46:173–180. doi: 10.2340/16501977-1252. [DOI] [PubMed] [Google Scholar]
- 51.Denk F, McMahon SB, Tracey I. Pain vulnerability: a neurobiological perspective. Nat Neurosci. 2014;17:192–200. doi: 10.1038/nn.3628. [DOI] [PubMed] [Google Scholar]
- 52.Denton FJ, Sharpe L, Schrieber L. Cognitive bias in systemic lupus erythematosus. Eur J Pain. 2005;9:5–14. doi: 10.1016/j.ejpain.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 53.Desrochers G, Bergeron S, Khalife S, Dupuis MJ, Jodoin M. Provoked vestibulodynia: psychological predictors of topical and cognitive-behavioral treatment outcome. Behav Res Ther. 2010;48:106–115. doi: 10.1016/j.brat.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 54.Diatchenko L, Fillingim RB, Smith SB, Maixner W. The phenotypic and genetic signatures of common musculoskeletal pain conditions. Nat Rev Rheumatol. 2013;9:340–350. doi: 10.1038/nrrheum.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dowd H, Hogan MJ, McGuire BE, Davis MC, Sarma KM, Fish RA, Zautra AJ. Comparison of an Online Mindfulness-based Cognitive Therapy Intervention With Online Pain Management Psychoeducation: A Randomized Controlled Study. Clin J Pain. 2015;31:517–527. doi: 10.1097/AJP.0000000000000201. [DOI] [PubMed] [Google Scholar]
- 56.Duivenvoorden T, Vissers MM, Verhaar JA, Busschbach JJ, Gosens T, Bloem RM, Bierma-Zeinstra SM, Reijman M. Anxiety and depressive symptoms before and after total hip and knee arthroplasty: a prospective multicentre study. Osteoarthritis Cartilage. 2013;21:1834–1840. doi: 10.1016/j.joca.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 57.Dworkin RH, Hartstein G, Rosner HL, Walther RR, Sweeney EW, Brand L. A high-risk method for studying psychosocial antecedents of chronic pain: the prospective investigation of herpes zoster. J Abnorm Psychol. 1992;101:200–205. doi: 10.1037//0021-843x.101.1.200. [DOI] [PubMed] [Google Scholar]
- 58.Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 59.Edwards RR, Cahalan C, Mensing G, Smith M, Haythornthwaite JA. Pain, catastrophizing, and depression in the rheumatic diseases. Nat Rev Rheumatol. 2011;7:216–224. doi: 10.1038/nrrheum.2011.2. [DOI] [PubMed] [Google Scholar]
- 60.Edwards RR, Haythornthwaite JA, Smith MT, Klick B, Katz JN. Catastrophizing and depressive symptoms as prospective predictors of outcomes following total knee replacement. Pain Res Manag. 2009;14:307–311. doi: 10.1155/2009/273783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Edwards RR, Mensing G, Cahalan C. Alteration in Pain Modulation in Women With Persistent Pain After Lumpectomy: Influence of Catastrophizing. J Pain Symptom Manage. 2013;46(1):30–42. doi: 10.1016/j.jpainsymman.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Edwards RR, Smith MT, Kudel I. Pain-related catastrophizing as a risk factor for suicidal ideation in chronic pain. Pain. 2006;126:272–279. doi: 10.1016/j.pain.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 63.Edwards RR, Wasan AD, Michna E, Greenbaum S, Ross E, Jamison RN. Elevated pain sensitivity in chronic pain patients at risk for opioid misuse. J Pain. 2011;12:953–963. doi: 10.1016/j.jpain.2011.02.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Engel GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196:129–136. doi: 10.1126/science.847460. [DOI] [PubMed] [Google Scholar]
- 65.Fillingim RB, Bruehl S, Dworkin RH, Dworkin SF, Loeser JD, Turk DC, Widerstrom-Noga E, Arnold L, Bennett R, Edwards RR, Freeman R, Gewandter J, Hertz S, Hochberg M, Krane E, Mantyh PW, Markman J, Neogi T, Ohrbach R, Paice JA, Porreca F, Rappaport BA, Smith SM, Smith TJ, Sullivan MD, Verne GN, Wasan AD, Wesselmann U. The ACTTION-American Pain Society Pain Taxonomy (AAPT): an evidence-based and multidimensional approach to classifying chronic pain conditions. J Pain. 2014;15:241–249. doi: 10.1016/j.jpain.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fillingim RB, Ohrbach R, Greenspan JD, Knott C, Diatchenko L, Dubner R, Bair E, Baraian C, Mack N, Slade GD, Maixner W. Psychological factors associated with development of TMD: the OPPERA prospective cohort study. J Pain. 2013;14:T75–T90. doi: 10.1016/j.jpain.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fillingim RB, Slade GD, Diatchenko L, Dubner R, Greenspan JD, Knott C, Ohrbach R, Maixner W. Summary of findings from the OPPERA baseline case-control study: implications and future directions. J Pain. 2011;12:T102–T107. doi: 10.1016/j.jpain.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Flor H. Psychological pain interventions and neurophysiology: implications for a mechanism-based approach. Am Psychol. 2014;69:188–196. doi: 10.1037/a0035254. [DOI] [PubMed] [Google Scholar]
- 69.Fordyce W. Pain and suffering: A reappraisal. Am Psychol. 1988;43:276–283. doi: 10.1037//0003-066x.43.4.276. [DOI] [PubMed] [Google Scholar]
- 70.Forsythe ME, Dunbar MJ, Hennigar AW, Sullivan MJ, Gross M. Prospective relation between catastrophizing and residual pain following knee arthroplasty: two-year follow-up. Pain Res Manag. 2008;13:335–341. doi: 10.1155/2008/730951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gandhi R, Davey JR, Mahomed N. Patient expectations predict greater pain relief with joint arthroplasty. J Arthroplasty. 2009;24:716–721. doi: 10.1016/j.arth.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 72.Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13:715–724. doi: 10.1016/j.jpain.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 73.Gatchel RJ, McGeary DD, McGeary CA, Lippe B. Interdisciplinary chronic pain management: past, present, and future. Am Psychol. 2014;69:119–130. doi: 10.1037/a0035514. [DOI] [PubMed] [Google Scholar]
- 74.Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull. 2007;133:581–624. doi: 10.1037/0033-2909.133.4.581. [DOI] [PubMed] [Google Scholar]
- 75.Gatchel RJ, Turk DC. Criticisms of the biopsychosocial model in spine care: creating and then attacking a straw person. Spine (Phila Pa 1976) 2008;33:2831–2836. doi: 10.1097/BRS.0b013e31817d24ad. [DOI] [PubMed] [Google Scholar]
- 76.Gauthier LR, Rodin G, Zimmermann C, Warr D, Librach SL, Moore M, Shepherd FA, Gagliese L. The communal coping model and cancer pain: the roles of catastrophizing and attachment style. J Pain. 2012;13:1258–1268. doi: 10.1016/j.jpain.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 77.Gauthier N, Sullivan MJ, Adams H, Standish WD, Thibault P. Investigating risk factors for chronicity: the importance of distinguishing between return-to-work status and self-report measures of disability. J Occup Environ Med. 2006;48:312–318. doi: 10.1097/01.jom.0000184870.81120.49. [DOI] [PubMed] [Google Scholar]
- 78.Gracely RH, Geisser ME, Giesecke T, Grant MA, Petzke F, Williams DA, Clauw DJ. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain. 2004;127:835–843. doi: 10.1093/brain/awh098. [DOI] [PubMed] [Google Scholar]
- 79.Gracely RH, Geisser ME, Giesecke T, Grant MA, Petzke F, Williams DA, Clauw DJ. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain. 2004;127:835–843. doi: 10.1093/brain/awh098. [DOI] [PubMed] [Google Scholar]
- 80.Hall AM, Ferreira ML, Clemson L, Ferreira P, Latimer J, Maher CG. Assessment of the therapeutic alliance in physical rehabilitation: a RASCH analysis. Disabil Rehabil. 2012;34:257–266. doi: 10.3109/09638288.2011.606344. [DOI] [PubMed] [Google Scholar]
- 81.Hall AM, Ferreira PH, Maher CG, Latimer J, Ferreira ML. The influence of the therapist-patient relationship on treatment outcome in physical rehabilitation: a systematic review. Phys Ther. 2010;90:1099–1110. doi: 10.2522/ptj.20090245. [DOI] [PubMed] [Google Scholar]
- 82.Hall AM, Kamper SJ, Maher CG, Latimer J, Ferreira ML, Nicholas MK. Symptoms of depression and stress mediate the effect of pain on disability. Pain. 2011;152:1044–1051. doi: 10.1016/j.pain.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 83.Hanley MA, Jensen MP, Ehde DM, Hoffman AJ, Patterson DR, Robinson LR. Psychosocial predictors of long-term adjustment to lower-limb amputation and phantom limb pain. Disabil Rehabil. 2004;26:882–893. doi: 10.1080/09638280410001708896. [DOI] [PubMed] [Google Scholar]
- 84.Hanusch BC, O’Connor DB, Ions P, Scott A, Gregg PJ. Effects of psychological distress and perceptions of illness on recovery from total knee replacement. Bone Joint J. 2014;96-B:210–216. doi: 10.1302/0301-620X.96B2.31136. [DOI] [PubMed] [Google Scholar]
- 85.Hasenbring MI, Chehadi O, Titze C, Kreddig N. Fear and anxiety in the transition from acute to chronic pain: there is evidence for endurance besides avoidance. Pain Manag. 2014;4:363–374. doi: 10.2217/pmt.14.36. [DOI] [PubMed] [Google Scholar]
- 86.Hasenbring MI, Hallner D, Klasen B, Streitlein-Böhme I, Willburger R, Rusche H. Pain-related avoidance versus endurance in primary care patients with subacute back pain: psychological characteristics and outcome at a 6-month follow-up. Pain. 2012;153:211–217. doi: 10.1016/j.pain.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 87.Hasenbring MI, Verbunt JA. Fear-avoidance and endurance-related responses to pain: new models of behavior and their consequences for clinical practice. Clin J Pain. 2010;26:747–753. doi: 10.1097/AJP.0b013e3181e104f2. [DOI] [PubMed] [Google Scholar]
- 88.Hassett AL, Aquino JK, Ilgen MA. The risk of suicide mortality in chronic pain patients. Curr Pain Headache Rep. 2014;18:436. doi: 10.1007/s11916-014-0436-1. [DOI] [PubMed] [Google Scholar]
- 89.Haugli L, Strand E, Finset A. How do patients with rheumatic disease experience their relationship with their doctors? A qualitative study of experiences of stress and support in the doctor-patient relationship. Patient Educ Couns. 2004;52:169–174. doi: 10.1016/s0738-3991(03)00023-5. [DOI] [PubMed] [Google Scholar]
- 90.Hechler T, Vervoort T, Hamann M, Tietze AL, Vocks S, Goubert L, Hermann C, Wager J, Blankenburg M, Schroeder S, Zernikow B. Parental catastrophizing about their child’s chronic pain: are mothers and fathers different? Eur J Pain. 2011;15:515–519. doi: 10.1016/j.ejpain.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 91.Helmhout PH, Staal JB, Heymans MW, Harts CC, Hendriks EJ, de Bie RA. Prognostic factors for perceived recovery or functional improvement in non-specific low back pain: secondary analyses of three randomized clinical trials. Eur Spine J. 2010;19:650–659. doi: 10.1007/s00586-009-1254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hermann C. Psychological interventions for chronic pediatric pain: state of the art, current developments and open questions. Pain Manag. 2011;1:473–483. doi: 10.2217/pmt.11.48. [DOI] [PubMed] [Google Scholar]
- 93.Hill JC, Lewis M, Sim J, Hay EM, Dziedzic K. Predictors of poor outcome in patients with neck pain treated by physical therapy. Clin J Pain. 2007;23:683–690. doi: 10.1097/AJP.0b013e3181468e67. [DOI] [PubMed] [Google Scholar]
- 94.Hirsh AT, Bockow TB, Jensen MP. Catastrophizing, pain, and pain interference in individuals with disabilities. Am J Phys Med Rehabil. 2011;90:713–722. doi: 10.1097/PHM.0b013e31822409b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hirsh AT, Kupper AE, Carter GT, Jensen MP. Psychosocial factors and adjustment to pain in individuals with postpolio syndrome. Am J Phys Med Rehabil. 2010;89:213–224. doi: 10.1097/PHM.0b013e3181c9f9a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Howe CQ, Robinson JP, Sullivan MD. Psychiatric and Psychological Perspectives on Chronic Pain. Phys Med Rehabil Clin N Am. 2015;26:283–300. doi: 10.1016/j.pmr.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 97.Hsu MC, Harris RE, Sundgren PC, Welsh RC, Fernandes CR, Clauw DJ, Williams DA. No consistent difference in gray matter volume between individuals with fibromyalgia and age-matched healthy subjects when controlling for affective disorder. Pain. 2009;143:262–267. doi: 10.1016/j.pain.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hung CI, Liu CY, Fu TS. Depression: An important factor associated with disability among patients with chronic low back pain. Int J Psychiatry Med. 2015;49:187–198. doi: 10.1177/0091217415573937. [DOI] [PubMed] [Google Scholar]
- 99.Ilgen MA, Kleinberg F, Ignacio RV, Bohnert AS, Valenstein M, McCarthy JF, Blow FC, Katz IR. Noncancer pain conditions and risk of suicide. JAMA Psychiatry. 2013;70:692–697. doi: 10.1001/jamapsychiatry.2013.908. [DOI] [PubMed] [Google Scholar]
- 100.Jenewein J, Moergeli H, Wittmann L, Buchi S, Kraemer B, Schnyder U. Development of chronic pain following severe accidental injury. Results of a 3-year follow-up study. J Psychosom Res. 2009;66:119–126. doi: 10.1016/j.jpsychores.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 101.Jenewein J, Wittmann L, Moergeli H, Creutzig J, Schnyder U. Mutual influence of posttraumatic stress disorder symptoms and chronic pain among injured accident survivors: a longitudinal study. J Trauma Stress. 2009;22:540–548. doi: 10.1002/jts.20453. [DOI] [PubMed] [Google Scholar]
- 102.Jensen MP. Psychosocial approaches to pain management: an organizational framework. Pain. 2011;152:717–725. doi: 10.1016/j.pain.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 103.Jensen MP, Moore MR, Bockow TB, Ehde DM, Engel JM. Psychosocial factors and adjustment to chronic pain in persons with physical disabilities: a systematic review. Arch Phys Med Rehabil. 2011;92:146–160. doi: 10.1016/j.apmr.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jensen MP, Turk DC. Contributions of psychology to the understanding and treatment of people with chronic pain: why it matters to ALL psychologists. Am Psychol. 2014;69:105–118. doi: 10.1037/a0035641. [DOI] [PubMed] [Google Scholar]
- 105.Jensen MP, Turner JA, Romano JM. Changes in beliefs, catastrophizing, and coping are associated with improvement in multidisciplinary pain treatment. J Consult Clin Psychol. 2001;69:655–662. doi: 10.1037//0022-006x.69.4.655. [DOI] [PubMed] [Google Scholar]
- 106.Jensen MP, Turner JA, Romano JM. Changes after multidisciplinary pain treatment in patient pain beliefs and coping are associated with concurrent changes in patient functioning. Pain. 2007;131:38–47. doi: 10.1016/j.pain.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jones GT, Power C, Macfarlane GJ. Adverse events in childhood and chronic widespread pain in adult life: Results from the 1958 British Birth Cohort Study. Pain. 2009;143:92–96. doi: 10.1016/j.pain.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 108.Kadam UT, Thomas E, Croft PR. Is chronic widespread pain a predictor of all-cause morbidity? A 3 year prospective population based study in family practice. J Rheumatol. 2005;32:1341–1348. [PubMed] [Google Scholar]
- 109.Kalapurakkel S, Carpino EA, Lebel A, Simons LE. “Pain Can’t Stop Me”: Examining Pain Self-Efficacy and Acceptance as Resilience Processes Among Youth With Chronic Headache. J Pediatr Psychol. 2015;40(9):926–3. doi: 10.1093/jpepsy/jsu091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Karels CH, Bierma-Zeinstra SM, Burdorf A, Verhagen AP, Nauta AP, Koes BW. Social and psychological factors influenced the course of arm, neck and shoulder complaints. J Clin Epidemiol. 2007;60:839–848. doi: 10.1016/j.jclinepi.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 111.Katz J, McDermott MP, Cooper EM, Walther RR, Sweeney EW, Dworkin RH. Psychosocial risk factors for postherpetic neuralgia: a prospective study of patients with herpes zoster. J Pain. 2005;6:782–790. doi: 10.1016/j.jpain.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 112.Kazdin AE. Mediators and mechanisms of change in psychotherapy research. Annu Rev Clin Psychol. 2007;3:1–27. doi: 10.1146/annurev.clinpsy.3.022806.091432. [DOI] [PubMed] [Google Scholar]
- 113.Kazdin AE, Kraemer HC, Kessler RC, Kupfer DJ, Offord DR. Contributions of risk-factor research to developmental psychopathology. Clin Psychol Rev. 1997;17:375–406. doi: 10.1016/s0272-7358(97)00012-3. [DOI] [PubMed] [Google Scholar]
- 114.Keefe FJ, Caldwell DS, Baucom D, Salley A, Robinson E, Timmons K, Beaupre P, Weisberg J, Helms M. Spouse-assisted coping skills training in the management of osteoarthritic knee pain. Arthritis Care & Research. 1996;9:279–291. doi: 10.1002/1529-0131(199608)9:4<279::aid-anr1790090413>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 115.Keefe FJ, Caldwell DS, Baucom D, Salley A, Robinson E, Timmons K, Beaupre P, Weisberg J, Helms M. Spouse-assisted coping skills training in the management of knee pain in osteoarthritis: long-term followup results. Arthritis Care Res. 1999;12:101–111. doi: 10.1002/1529-0131(199904)12:2<101::aid-art5>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 116.Keefe FJ, Somers TJ. Psychological approaches to understanding and treating arthritis pain. Nat Rev Rheumatol. 2010;6:210–216. doi: 10.1038/nrrheum.2010.22. [DOI] [PubMed] [Google Scholar]
- 117.Kelsall H, Sim M, McKenzie D, Forbes A, Leder K, Glass D, Ikin J, McFarlane A. Medically evaluated psychological and physical health of Australian Gulf War veterans with chronic fatigue. J Psychosom Res. 2006;60:575–584. doi: 10.1016/j.jpsychores.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 118.Kelsall HL, McKenzie DP, Forbes AB, Roberts MH, Urquhart DM, Sim MR. Pain-related musculoskeletal disorders, psychological comorbidity, and the relationship with physical and mental well-being in Gulf War veterans. Pain. 2014;155:685–692. doi: 10.1016/j.pain.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 119.Kenardy J, Heron-Delaney M, Warren J, Brown EA. Effect of mental health on long-term disability after a road traffic crash: results from the UQ SuPPORT study. Arch Phys Med Rehabil. 2015;96:410–417. doi: 10.1016/j.apmr.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 120.Kerns RD, Sellinger J, Goodin BR. Psychological treatment of chronic pain. Annu Rev Clin Psychol. 2011;7:411–434. doi: 10.1146/annurev-clinpsy-090310-120430. [DOI] [PubMed] [Google Scholar]
- 121.Khan RS, Ahmed K, Blakeway E, Skapinakis P, Nihoyannopoulos L, Macleod K, Sevdalis N, Ashrafian H, Platt M, Darzi A, Athanasiou T. Catastrophizing: a predictive factor for postoperative pain. Am J Surg. 2011;201:122–131. doi: 10.1016/j.amjsurg.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 122.King S, Chambers CT, Huguet A, MacNevin RC, McGrath PJ, Parker L, MacDonald AJ. The epidemiology of chronic pain in children and adolescents revisited: a systematic review. Pain. 2011;152:2729–2738. doi: 10.1016/j.pain.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 123.Kongsted A, Bendix T, Qerama E, Kasch H, Bach FW, Korsholm L, Jensen TS. Acute stress response and recovery after whiplash injuries A one-year prospective study. Eur J Pain. 2008;12:455–463. doi: 10.1016/j.ejpain.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 124.Koopman J, Howe M, Hollenbeck JR, Sin HP. Small sample mediation testing: misplaced confidence in bootstrapped confidence intervals. J Appl Psychol. 2015;100:194–202. doi: 10.1037/a0036635. [DOI] [PubMed] [Google Scholar]
- 125.Kowal J, McWilliams LA, Péloquin K, Wilson KG, Henderson PR, Fergusson DA. Attachment insecurity predicts responses to an interdisciplinary chronic pain rehabilitation program. J Behav Med. 2015;38:518–526. doi: 10.1007/s10865-015-9623-8. [DOI] [PubMed] [Google Scholar]
- 126.Kraemer HC, Kazdin AE, Offord DR, Kessler RC, Jensen PS, Kupfer DJ. Coming to terms with the terms of risk. Arch Gen Psychiatry. 1997;54:337–343. doi: 10.1001/archpsyc.1997.01830160065009. [DOI] [PubMed] [Google Scholar]
- 127.Kroenke K, Bair MJ, Damush TM, Wu J, Hoke S, Sutherland J, Tu W. Optimized antidepressant therapy and pain self-management in primary care patients with depression and musculoskeletal pain: a randomized controlled trial. JAMA. 2009;301:2099–2110. doi: 10.1001/jama.2009.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kroenke K, Wu J, Bair MJ, Krebs EE, Damush TM, Tu W. Reciprocal relationship between pain and depression: a 12-month longitudinal analysis in primary care. J Pain. 2011;12:964–973. doi: 10.1016/j.jpain.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Laird KT, Preacher KJ, Walker LS. Attachment and adjustment in adolescents and young adults with a history of pediatric functional abdominal pain. Clin J Pain. 2015;31:152–158. doi: 10.1097/AJP.0000000000000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lam M, Lehman AJ, Puterman E, Delongis A. Spouse depression and disease course among persons with rheumatoid arthritis. Arthritis Rheum. 2009;61:1011–1017. doi: 10.1002/art.24510. [DOI] [PubMed] [Google Scholar]
- 131.Lang AJ, Laffaye C, Satz LE, McQuaid JR, Malcarne VL, Dresselhaus TR, Stein MB. Relationships among childhood maltreatment, PTSD, and health in female veterans in primary care. Child Abuse Negl. 2006;30:1281–1292. doi: 10.1016/j.chiabu.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 132.Lee H, Hübscher M, Moseley GL, Kamper SJ, Traeger AC, Mansell G, McAuley JH. How does pain lead to disability? A systematic review and meta-analysis of mediation studies in people with back and neck pain. Pain. 2015;156:988–997. doi: 10.1097/j.pain.0000000000000146. [DOI] [PubMed] [Google Scholar]
- 133.Lee H, Moseley GL, Hübscher M, Kamper SJ, Traeger AC, Skinner IW, McAuley JH. Understanding how pain education causes changes in pain and disability: protocol for a causal mediation analysis of the PREVENT trial. J Physiother. 2015;61:156. doi: 10.1016/j.jphys.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 134.Leeuw M, Goossens ME, van Breukelen GJ, de Jong JR, Heuts PH, Smeets RJ, Köke AJ, Vlaeyen JW. Exposure in vivo versus operant graded activity in chronic low back pain patients: results of a randomized controlled trial. Pain. 2008;138:192–207. doi: 10.1016/j.pain.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 135.Lemieux AJ, Bergeron S, Steben M, Lambert B. Do romantic partners’ responses to entry dyspareunia affect women’s experience of pain? The roles of catastrophizing and self-efficacy. J Sex Med. 2013;10:2274–2284. doi: 10.1111/jsm.12252. [DOI] [PubMed] [Google Scholar]
- 136.Leonard MT, Cano A, Johansen AB. Chronic pain in a couples context: a review and integration of theoretical models and empirical evidence. J Pain. 2006;7:377–390. doi: 10.1016/j.jpain.2006.01.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lewandowski Holley A, Law EF, Zhou C, Murphy L, Clarke G, Palermo TM. Reciprocal longitudinal associations between pain and depressive symptoms in adolescents. Eur J Pain. 2013;17:1058–1067. doi: 10.1002/j.1532-2149.2012.00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lewis GN, Rice DA, McNair PJ, Kluger M. Predictors of persistent pain after total knee arthroplasty: a systematic review and meta-analysis. Br J Anaesth. 2015;114:551–561. doi: 10.1093/bja/aeu441. [DOI] [PubMed] [Google Scholar]
- 139.Li X, Gignac MA, Anis AH. Workplace, psychosocial factors, and depressive symptoms among working people with arthritis: a longitudinal study. J Rheumatol. 2006;33:1849–1855. [PubMed] [Google Scholar]
- 140.Linton SJ, Nicholas MK, MacDonald S, Boersma K, Bergbom S, Maher C, Refshauge K. The role of depression and catastrophizing in musculoskeletal pain. Eur J Pain. 2011;15(4):416–22. doi: 10.1016/j.ejpain.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 141.Litt MD, Porto FB. Determinants of pain treatment response and nonresponse: identification of TMD patient subgroups. J Pain. 2013;14:1502–1513. doi: 10.1016/j.jpain.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Litt MD, Shafer DM, Ibanez CR, Kreutzer DL, Tawfik-Yonkers Z. Momentary pain and coping in temporomandibular disorder pain: exploring mechanisms of cognitive behavioral treatment for chronic pain. Pain. 2009;145:160–168. doi: 10.1016/j.pain.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Loeser JD. Pain and suffering. Clin J Pain. 2000;16:S2–S6. doi: 10.1097/00002508-200006001-00002. [DOI] [PubMed] [Google Scholar]
- 144.Loggia ML, Berna C, Kim J, Cahalan CM, Martel MO, Gollub RL, Wasan AD, Napadow V, Edwards RR. The Lateral Prefrontal Cortex Mediates the Hyperalgesic Effects of Negative Cognitions in Chronic Pain Patients. J Pain. 2015;16(8):692–9. doi: 10.1016/j.jpain.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Loggia ML, Chonde DB, Akeju O, Arabasz G, Catana C, Edwards RR, Hill E, Hsu S, Izquierdo-Garcia D, Ji RR, Riley M, Wasan AD, Zürcher NR, Albrecht DS, Vangel MG, Rosen BR, Napadow V, Hooker JM. Evidence for brain glial activation in chronic pain patients. Brain. 2015;138:604–615. doi: 10.1093/brain/awu377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Loggia ML, Kim J, Gollub RL, Vangel MG, Kirsch I, Kong J, Wasan AD, Napadow V. Default mode network connectivity encodes clinical pain: an arterial spin labeling study. Pain. 2013;154:24–33. doi: 10.1016/j.pain.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lorig K, Chastain RL, Ung E, Shoor S, Holman HR. Development and evaluation of a scale to measure perceived self-efficacy in people with arthritis. Arthritis Rheum. 1989;32:37–44. doi: 10.1002/anr.1780320107. [DOI] [PubMed] [Google Scholar]
- 148.Lotze M, Moseley GL. Theoretical Considerations for Chronic Pain Rehabilitation. Phys Ther. 2015;95(9):1316–2. doi: 10.2522/ptj.20140581. [DOI] [PubMed] [Google Scholar]
- 149.Mackey SC. Central neuroimaging of pain. J Pain. 2013;14:328–331. doi: 10.1016/j.jpain.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 150.Makarawung DJ, Becker SJ, Bekkers S, Ring D. Disability and pain after cortisone versus placebo injection for trapeziometacarpal arthrosis and de Quervain syndrome. Hand (NY) 2013;8:375–381. doi: 10.1007/s11552-013-9529-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mankovsky T, Lynch M, Clark A, Sawynok J, Sullivan MJ. Pain catastrophizing predicts poor response to topical analgesics in patients with neuropathic pain. Pain Res Manag. 2012;17:10–14. doi: 10.1155/2012/970423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mansell G, Hill JC, Kamper SJ, Kent P, Main C, van der Windt DA. How can we design low back pain intervention studies to better explain the effects of treatment? Spine (Phila Pa 1976) 2014;39:E305–E310. doi: 10.1097/BRS.0000000000000144. [DOI] [PubMed] [Google Scholar]
- 153.Mansour AR, Farmer MA, Baliki MN, Apkarian AV. Chronic pain: the role of learning and brain plasticity. Restor Neurol Neurosci. 2014;32:129–139. doi: 10.3233/RNN-139003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Marcus KS, Kerns RD, Rosenfeld B, Breitbart W. HIV/AIDS-related pain as a chronic pain condition: implications of a biopsychosocial model for comprehensive assessment and effective management. Pain Med. 2000;1:260–273. doi: 10.1046/j.1526-4637.2000.00033.x. [DOI] [PubMed] [Google Scholar]
- 155.Martel MO, Jamison RN, Wasan AD, Edwards RR. The association between catastrophizing and craving in patients with chronic pain prescribed opioid therapy: a preliminary analysis. Pain Med. 2014;15:1757–1764. doi: 10.1111/pme.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Martel MO, Wasan AD, Jamison RN, Edwards RR. Catastrophic thinking and increased risk for prescription opioid misuse in patients with chronic pain. Drug Alcohol Depend. 2013;132:335–341. doi: 10.1016/j.drugalcdep.2013.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Martin DJ, Garske JP, Davis MK. Relation of the therapeutic alliance with outcome and other variables: a meta-analytic review. J Consult Clin Psychol. 2000;68:438–450. [PubMed] [Google Scholar]
- 158.May A. Structure equals function: cortical correlates of pain. Pain. 2012;153:1551–1552. doi: 10.1016/j.pain.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 159.McCracken LM, Carson JW, Eccleston C, Keefe FJ. Acceptance and change in the context of chronic pain. Pain. 2004;109:4–7. doi: 10.1016/j.pain.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 160.McCracken LM, Vowles KE. Acceptance of chronic pain. Curr Pain Headache Rep. 2006;10:90–94. doi: 10.1007/s11916-006-0018-y. [DOI] [PubMed] [Google Scholar]
- 161.McCracken LM, Vowles KE. Acceptance and commitment therapy and mindfulness for chronic pain: model, process, and progress. Am Psychol. 2014;69:178–187. doi: 10.1037/a0035623. [DOI] [PubMed] [Google Scholar]
- 162.McWilliams LA, Goodwin RD, Cox BJ. Depression and anxiety associated with three pain conditions: results from a nationally representative sample. Pain. 2004;111:77–83. doi: 10.1016/j.pain.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 163.Melloh M, Elfering A, Chapple CM, Käser A, Rolli Salathé C, Barz T, Röder C, Theis JC. Prognostic occupational factors for persistent low back pain in primary care. Int Arch Occup Environ Health. 2013;86:261–269. doi: 10.1007/s00420-012-0761-9. [DOI] [PubMed] [Google Scholar]
- 164.Melloh M, Elfering A, Stanton TR, Käser A, Salathé CR, Barz T, Röder C, Theis JC. Who is likely to develop persistent low back pain? A longitudinal analysis of prognostic occupational factors. Work. 2013;46:297–311. doi: 10.3233/WOR-131672. [DOI] [PubMed] [Google Scholar]
- 165.Michalski D, Liebig S, Thomae E, Hinz A, Bergh FT. Pain in patients with multiple sclerosis: a complex assessment including quantitative and qualitative measurements provides for a disease-related biopsychosocial pain model. J Pain Res. 2011;4:219–225. doi: 10.2147/JPR.S20309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Miró E, Martínez MP, Sánchez AI, Prados G, Medina A. When is pain related to emotional distress and daily functioning in fibromyalgia syndrome? The mediating roles of self-efficacy and sleep quality. Br J Health Psychol. 2011;16:799–814. doi: 10.1111/j.2044-8287.2011.02016.x. [DOI] [PubMed] [Google Scholar]
- 167.Moeller-Bertram T, Keltner J, Strigo IA. Pain and post traumatic stress disorder - review of clinical and experimental evidence. Neuropharmacology. 2012;62:586–597. doi: 10.1016/j.neuropharm.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 168.Morley S, Williams A, Hussain S. Estimating the clinical effectiveness of cognitive behavioural therapy in the clinic: evaluation of a CBT informed pain management programme. Pain. 2008;137:670–680. doi: 10.1016/j.pain.2008.02.025. [DOI] [PubMed] [Google Scholar]
- 169.Moseley GL, Butler DS. Fifteen Years of Explaining Pain: The Past, Present, and Future. J Pain. 2015;16(9):807–13. doi: 10.1016/j.jpain.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 170.Mounce C, Keogh E, Eccleston C. A principal components analysis of negative affect-related constructs relevant to pain: evidence for a three component structure. J Pain. 2010;11:710–717. doi: 10.1016/j.jpain.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 171.Napadow V, Kim J, Clauw DJ, Harris RE. Decreased intrinsic brain connectivity is associated with reduced clinical pain in fibromyalgia. Arthritis Rheum. 2012;64(7):2398–403. doi: 10.1002/art.34412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Naylor MR, Krauthamer GM, Naud S, Keefe FL, Helzer JE. Predictive relationships between chronic pain and negative emotions: a 4-month daily process study using Therapeutic Interactive Voice Response (TIVR) Compr Psychiatry. 2011;52:731–736. doi: 10.1016/j.comppsych.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Neame R, Hammond A. Beliefs about medications: a questionnaire survey of people with rheumatoid arthritis. Rheumatology (Oxford) 2005;44(6):762–7. doi: 10.1093/rheumatology/keh587. [DOI] [PubMed] [Google Scholar]
- 174.Nicholas MK, Linton SJ, Watson PJ, Main CJ. Early identification and management of psychological risk factors (“yellow flags”) in patients with low back pain: a reappraisal. Phys Ther. 2011;91:737–753. doi: 10.2522/ptj.20100224. [DOI] [PubMed] [Google Scholar]
- 175.Noel M, Rabbitts JA, Tai GG, Palermo TM. Remembering pain after surgery: a longitudinal examination of the role of pain catastrophizing in children’s and parents’ recall. Pain. 2015;156:800–808. doi: 10.1097/j.pain.0000000000000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Novy DM, Aigner CJ. The biopsychosocial model in cancer pain. Curr Opin Support Palliat Care. 2014;8:117–123. doi: 10.1097/SPC.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 177.Page MG, Campbell F, Isaac L, Stinson J, Katz J. Parental risk factors for the development of pediatric acute and chronic postsurgical pain: a longitudinal study. J Pain Res. 2013;6:727–741. doi: 10.2147/JPR.S51055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Palermo TM. Impact of recurrent and chronic pain on child and family daily functioning: a critical review of the literature. J Dev Behav Pediatr. 2000;21:58–69. doi: 10.1097/00004703-200002000-00011. [DOI] [PubMed] [Google Scholar]
- 179.Palermo TM, Holley AL. The importance of the family environment in pediatric chronic pain. JAMA Pediatr. 2013;167:93–94. doi: 10.1001/jamapediatrics.2013.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Palermo TM, Valrie CR, Karlson CW. Family and parent influences on pediatric chronic pain: a developmental perspective. Am Psychol. 2014;69:142–152. doi: 10.1037/a0035216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Parker SL, Godil SS, Zuckerman SL, Mendenhall SK, Devin CJ, McGirt MJ. Extent of preoperative depression is associated with return to work after lumbar fusion for spondylolisthesis. World Neurosurg. 2015;83:608–613. doi: 10.1016/j.wneu.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 182.Pence L, Cano A, Thorn B, Ward LC. Perceived spouse responses to pain: the level of agreement in couple dyads and the role of catastrophizing, marital satisfaction, and depression. J Behav Med. 2006;29:511–522. doi: 10.1007/s10865-006-9073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Pincus T, Burton AK, Vogel S, Field AP. A systematic review of psychological factors as predictors of chronicity/disability in prospective cohorts of low back pain. Spine. 2002;27:E109–E120. doi: 10.1097/00007632-200203010-00017. [DOI] [PubMed] [Google Scholar]
- 184.Pincus T, Kent P, Bronfort G, Loisel P, Pransky G, Hartvigsen J. Twenty-five years with the biopsychosocial model of low back pain-is it time to celebrate? A report from the twelfth international forum for primary care research on low back pain. Spine (Phila Pa 1976) 2013;38:2118–2123. doi: 10.1097/BRS.0b013e3182a8c5d6. [DOI] [PubMed] [Google Scholar]
- 185.Pinto PR, McIntyre T, Almeida A, Araújo-Soares V. The mediating role of pain catastrophizing in the relationship between presurgical anxiety and acute postsurgical pain after hysterectomy. Pain. 2012;153:218–226. doi: 10.1016/j.pain.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 186.Porter LS, Davis D, Keefe FJ. Attachment and pain: recent findings and future directions. Pain. 2007;128:195–198. doi: 10.1016/j.pain.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Porter LS, Keefe FJ, Davis D, Rumble M, Scipio C, Garst J. Attachment styles in patients with lung cancer and their spouses: associations with patient and spouse adjustment. Support Care Cancer. 2012;20:2459–2466. doi: 10.1007/s00520-011-1367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Powers A, Fani N, Pallos A, Stevens J, Ressler KJ, Bradley B. Childhood abuse and the experience of pain in adulthood: the mediating effects of PTSD and emotion dysregulation on pain levels and pain-related functional impairment. Psychosomatics. 2014;55:491–499. doi: 10.1016/j.psym.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Quartana PJ, Campbell CM, Edwards RR. Pain catastrophizing: a critical review. Expert Rev Neurother. 2009;9:745–758. doi: 10.1586/ERN.09.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Rabbitts JA, Groenewald CB, Tai GG, Palermo TM. Presurgical psychosocial predictors of acute postsurgical pain and quality of life in children undergoing major surgery. J Pain. 2015;16:226–234. doi: 10.1016/j.jpain.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Rakel BA, Zimmerman MB, Geasland K, Embree J, Clark CR, Noiseux NO, Callaghan JJ, Herr K, Walsh D, Sluka KA. Transcutaneous electrical nerve stimulation for the control of pain during rehabilitation after total knee arthroplasty: A randomized, blinded, placebo-controlled trial. Pain. 2014;155:2599–2611. doi: 10.1016/j.pain.2014.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Raphael KG, Widom CS. Post-traumatic stress disorder moderates the relation between documented childhood victimization and pain 30 years later. Pain. 2011;152:163–169. doi: 10.1016/j.pain.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Rej S, Dew MA, Karp JF. Treating concurrent chronic low back pain and depression with low-dose venlafaxine: an initial identification of “easy-to-use” clinical predictors of early response. Pain Med. 2014;15:1154–1162. doi: 10.1111/pme.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Rhee H. Physical symptoms in children and adolescents. Annu Rev Nurs Res. 2003;21:95–121. [PubMed] [Google Scholar]
- 195.Riddle DL, Wade JB, Jiranek WA, Kong X. Preoperative pain catastrophizing predicts pain outcome after knee arthroplasty. Clin Orthop Relat Res. 2010;468:798–806. doi: 10.1007/s11999-009-0963-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Riegel B, Bruenahl CA, Ahyai S, Bingel U, Fisch M, Löwe B. Assessing psychological factors, social aspects and psychiatric co-morbidity associated with Chronic Prostatitis/Chronic Pelvic Pain Syndrome (CP/CPPS) in men -- a systematic review. J Psychosom Res. 2014;77:333–350. doi: 10.1016/j.jpsychores.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 197.Ross C, Juraskova I, Lee H, Parkitny L, Stanton TR, Moseley GL, McAuley JH. Psychological distress mediates the relationship between pain and disability in hand or wrist fractures. J Pain. 2015;16(9):836–43. doi: 10.1016/j.jpain.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 198.Rusu AC, Boersma K, Turk DC. Subgroups of pain patients - the potential of customizing treatments. In: Hasenbring M, Rusu AC, Turk DC, editors. From acute to chronic back pain: Risk factors, mechanisms, and clinical implications. London: Oxford University Press; 2012. pp. 485–511. [Google Scholar]
- 199.Schiavone V, Adamo D, Ventrella G, Morlino M, De Notaris EB, Ravel MG, Kusmann F, Piantadosi M, Pollio A, Fortuna G, Mignogna MD. Anxiety, depression, and pain in burning mouth syndrome: first chicken or egg? Headache. 2012;52:1019–1025. doi: 10.1111/j.1526-4610.2012.02171.x. [DOI] [PubMed] [Google Scholar]
- 200.Schiphorst Preuper HR, Geertzen JH, van Wijhe M, Boonstra AM, Molmans BH, Dijkstra PU, Reneman MF. Do analgesics improve functioning in patients with chronic low back pain? An explorative triple-blinded RCT. Eur Spine J. 2014;23(4):800–6. doi: 10.1007/s00586-014-3229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Schmidt S, Grossman P, Schwarzer B, Jena S, Naumann J, Walach H. Treating fibromyalgia with mindfulness-based stress reduction: results from a 3-armed randomized controlled trial. Pain. 2011;152:361–369. doi: 10.1016/j.pain.2010.10.043. [DOI] [PubMed] [Google Scholar]
- 202.Schoth DE, Nunes VD, Liossi C. Attentional bias towards pain-related information in chronic pain; a meta-analysis of visual-probe investigations. Clin Psychol Rev. 2012;32:13–25. doi: 10.1016/j.cpr.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 203.Schreiber KL, Kehlet H, Belfer I, Edwards RR. Predicting, preventing and managing persistent pain after breast cancer surgery: the importance of psychosocial factors. Pain Manag. 2014;4:445–459. doi: 10.2217/pmt.14.33. [DOI] [PubMed] [Google Scholar]
- 204.Seminowicz DA, Davis KD. Cortical responses to pain in healthy individuals depends on pain catastrophizing. Pain. 2006;120:297–306. doi: 10.1016/j.pain.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 205.Seminowicz DA, Shpaner M, Keaser ML, Krauthamer GM, Mantegna J, Dumas JA, Newhouse PA, Filippi CG, Keefe FJ, Naylor MR. Cognitive-behavioral therapy increases prefrontal cortex gray matter in patients with chronic pain. J Pain. 2013;14:1573–1584. doi: 10.1016/j.jpain.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Seminowicz DA, Wideman TH, Naso L, Hatami-Khoroushahi Z, Fallatah S, Ware MA, Jarzem P, Bushnell MC, Shir Y, Ouellet JA, Stone LS. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J Neurosci. 2011;31:7540–7550. doi: 10.1523/JNEUROSCI.5280-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Shelby RA, Scipio CD, Somers TJ, Soo MS, Weinfurt KP, Keefe FJ. Prospective study of factors predicting adherence to surveillance mammography in women treated for breast cancer. J Clin Oncol. 2012;30:813–819. doi: 10.1200/JCO.2010.34.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Sitges C, García-Herrera M, Pericás M, Collado D, Truyols M, Montoya P. Abnormal brain processing of affective and sensory pain descriptors in chronic pain patients. J Affect Disord. 2007;104:73–82. doi: 10.1016/j.jad.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 209.Smeets RJ, Vlaeyen JW, Kester AD, Knottnerus JA. Reduction of pain catastrophizing mediates the outcome of both physical and cognitive-behavioral treatment in chronic low back pain. J Pain. 2006;7:261–271. doi: 10.1016/j.jpain.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 210.Smith D, Wilkie R, Uthman O, Jordan JL, McBeth J. Chronic pain and mortality: a systematic review. PLoS One. 2014;9:e99048. doi: 10.1371/journal.pone.0099048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Somers TJ, Keefe FJ, Godiwala N, Hoyler GH. Psychosocial factors and the pain experience of osteoarthritis patients: new findings and new directions. Curr Opin Rheumatol. 2009;21:501–506. doi: 10.1097/BOR.0b013e32832ed704. [DOI] [PubMed] [Google Scholar]
- 212.Somers TJ, Wren AA, Shelby RA. The context of pain in arthritis: self-efficacy for managing pain and other symptoms. Curr Pain Headache Rep. 2012;16:502–508. doi: 10.1007/s11916-012-0298-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Spinhoven P, Ter Kuile M, Kole-Snijders AM, Hutten Mansfeld M, Den Ouden DJ, Vlaeyen JW. Catastrophizing and internal pain control as mediators of outcome in the multidisciplinary treatment of chronic low back pain. Eur J Pain. 2004;8:211–219. doi: 10.1016/j.ejpain.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 214.Stewart DE, Yuen T. A systematic review of resilience in the physically ill. Psychosomatics. 2011;52:199–209. doi: 10.1016/j.psym.2011.01.036. [DOI] [PubMed] [Google Scholar]
- 215.Sturgeon JA, Zautra AJ, Arewasikporn A. A multilevel structural equation modeling analysis of vulnerabilities and resilience resources influencing affective adaptation to chronic pain. Pain. 2014;155:292–298. doi: 10.1016/j.pain.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sullivan MJ. Toward a biopsychomotor conceptualization of pain: implications for research and intervention. Clin J Pain. 2008;24:281–290. doi: 10.1097/AJP.0b013e318164bb15. [DOI] [PubMed] [Google Scholar]
- 217.Sullivan MJ, Ward LC, Tripp D, French DJ, Adams H, Stanish WD. Secondary prevention of work disability: community-based psychosocial intervention for musculoskeletal disorders. J Occup Rehabil. 2005;15:377–392. doi: 10.1007/s10926-005-5944-7. [DOI] [PubMed] [Google Scholar]
- 218.Taylor JB, Goode AP, George SZ, Cooke CE. Incidence and risk factors for first-time incident low back pain: a systematic review and meta-analysis. Spine J. 2014;14:2299–2319. doi: 10.1016/j.spinee.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 219.Taylor LE, Stotts NA, Humphreys J, Treadwell MJ, Miaskowski C. A biopsychosocial-spiritual model of chronic pain in adults with sickle cell disease. Pain Manag Nurs. 2013;14:287–301. doi: 10.1016/j.pmn.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Thacker MA, Moseley GL. First-person neuroscience and the understanding of pain. Med J Aust. 2012;196:410–411. doi: 10.5694/mja12.10468. [DOI] [PubMed] [Google Scholar]
- 221.Thorn BE, Burns JW. Common and specific treatment mechanisms in psychosocial pain interventions: the need for a new research agenda. Pain. 2011;152:705–706. doi: 10.1016/j.pain.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 222.Thorn BE, Day MA, Burns J, Kuhajda MC, Gaskins SW, Sweeney K, McConley R, Ward LC, Cabbil C. Randomized trial of group cognitive behavioral therapy compared with a pain education control for low-literacy rural people with chronic pain. Pain. 2011;152:2710–2720. doi: 10.1016/j.pain.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Thorn BE, Pence LB, Ward LC, Kilgo G, Clements KL, Cross TH, Davis AM, Tsui PW. A randomized clinical trial of targeted cognitive behavioral treatment to reduce catastrophizing in chronic headache sufferers. J Pain. 2007;8:938–949. doi: 10.1016/j.jpain.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 224.Thorn BE, Ward LC, Sullivan MJ, Boothby JL. Communal coping model of catastrophizing: conceptual model building. Pain. 2003;106:1–2. doi: 10.1016/s0304-3959(03)00228-8. [DOI] [PubMed] [Google Scholar]
- 225.Treharne GJ, Lyons AC, Booth DA, Kitas GD. Psychological well-being across 1 year with rheumatoid arthritis: coping resources as buffers of perceived stress. Br J Health Psychol. 2007;12:323–345. doi: 10.1348/135910706X109288. [DOI] [PubMed] [Google Scholar]
- 226.Trost Z, Strachan E, Sullivan M, Vervoort T, Avery AR, Afari N. Heritability of pain catastrophizing and associations with experimental pain outcomes: a twin study. Pain. 2015;156:514–520. doi: 10.1097/01.j.pain.0000460326.02891.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Tsang A, Von Korff M, Lee S, Alonso J, Karam E, Angermeyer MC, Borges GL, Bromet EJ, Demytteneare K, de Girolamo G, de Graaf R, Gureje O, Lepine JP, Haro JM, Levinson D, Oakley Browne MA, Posada-Villa J, Seedat S, Watanabe M. Common Chronic Pain Conditions in Developed and Developing Countries: Gender and Age Differences and Comorbidity With Depression-Anxiety Disorders. J Pain. 2008;9(10):883–91. doi: 10.1016/j.jpain.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 228.Turk DC, Dworkin RH. What should be the core outcomes in chronic pain clinical trials? Arthritis Res Ther. 2004;6:151–154. doi: 10.1186/ar1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Turk DC, Dworkin RH, Allen RR, Bellamy N, Brandenburg N, Carr DB, Cleeland C, Dionne R, Farrar JT, Galer BS, Hewitt DJ, Jadad AR, Katz NP, Kramer LD, Manning DC, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robinson JP, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Witter J. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain. 2003;106:337–345. doi: 10.1016/j.pain.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 230.Turk DC, Dworkin RH, Revicki D, Harding G, Burke LB, Cella D, Cleeland CS, Cowan P, Farrar JT, Hertz S, Max MB, Rappaport BA. Identifying important outcome domains for chronic pain clinical trials: an IMMPACT survey of people with pain. Pain. 2008;137:276–285. doi: 10.1016/j.pain.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 231.Turk DC, Okifuji A. Psychological factors in chronic pain: evolution and revolution. J Consult Clin Psychol. 2002;70:678–690. doi: 10.1037//0022-006x.70.3.678. [DOI] [PubMed] [Google Scholar]
- 232.Turner JA, Holtzman S, Mancl L. Mediators, moderators, and predictors of therapeutic change in cognitive-behavioral therapy for chronic pain. Pain. 2007;127:276–286. doi: 10.1016/j.pain.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 233.Turner JA, Mancl L, Aaron LA. Short- and long-term efficacy of brief cognitive-behavioral therapy for patients with chronic temporomandibular disorder pain: a randomized, controlled trial. Pain. 2006;121:181–194. doi: 10.1016/j.pain.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 234.Van Damme S, Crombez G, Eccleston C. Disengagement from pain: the role of catastrophic thinking about pain. Pain. 2004;107:70–76. doi: 10.1016/j.pain.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 235.van Tilburg MA, Claar RL, Romano JM, Langer SL, Walker LS, Whitehead WE, Abdullah B, Christie DL, Levy RL. The Role of Coping With Symptoms in Depression and Disability: Comparison Between Inflammatory Bowel Disease and Abdominal Pain. J Pediatr Gastroenterol Nutr. 2015;61(4):431–6. doi: 10.1097/MPG.0000000000000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.van Wijk G, Veldhuijzen DS. Perspective on diffuse noxious inhibitory controls as a model of endogenous pain modulation in clinical pain syndromes. J Pain. 2010;11:408–419. doi: 10.1016/j.jpain.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 237.Verbunt JA, Sieben J, Vlaeyen JW, Portegijs P, André Knottnerus J. A new episode of low back pain: who relies on bed rest? Eur J Pain. 2008;12:508–516. doi: 10.1016/j.ejpain.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 238.Vissers MM, Bussmann JB, Verhaar JA, Busschbach JJ, Bierma-Zeinstra SM, Reijman M. Psychological factors affecting the outcome of total hip and knee arthroplasty: a systematic review. Semin Arthritis Rheum. 2012;41:576–588. doi: 10.1016/j.semarthrit.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 239.Vlaeyen JW, Kole-Snijders Am, Boeren RG, Van Eek H. Fear of movement/(re)injury in chronic low back pain and its relation to behavioral performance. Pain. 1995;62:363–372. doi: 10.1016/0304-3959(94)00279-N. [DOI] [PubMed] [Google Scholar]
- 240.Vlaeyen JW, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain. 2000;85:317–332. doi: 10.1016/S0304-3959(99)00242-0. [DOI] [PubMed] [Google Scholar]
- 241.Vlaeyen JW, Linton SJ. Fear-avoidance model of chronic musculoskeletal pain: 12 years on. Pain. 2012;153:1144–1147. doi: 10.1016/j.pain.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 242.Volders S, Boddez Y, De Peuter S, Meulders A, Vlaeyen JW. Avoidance behavior in chronic pain research: A cold case revisited. Behav Res Ther. 2015;64:31–37. doi: 10.1016/j.brat.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 243.Vowles KE, McCracken LM, Eccleston C. Processes of change in treatment for chronic pain: the contributions of pain, acceptance, and catastrophizing. Eur J Pain. 2007;11:779–787. doi: 10.1016/j.ejpain.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 244.Vowles KE, McCracken LM, O’Brien JZ. Acceptance and values-based action in chronic pain: a three-year follow-up analysis of treatment effectiveness and process. Behav Res Ther. 2011;49:748–755. doi: 10.1016/j.brat.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 245.Vowles KE, Sowden G, Ashworth J. A comprehensive examination of the model underlying acceptance and commitment therapy for chronic pain. Behav Ther. 2014;45:390–401. doi: 10.1016/j.beth.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 246.Vowles KE, Witkiewitz K, Sowden G, Ashworth J. Acceptance and commitment therapy for chronic pain: evidence of mediation and clinically significant change following an abbreviated interdisciplinary program of rehabilitation. J Pain. 2014;15:101–113. doi: 10.1016/j.jpain.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 247.Wasan AD, Davar G, Jamison R. The association between negative affect and opioid analgesia in patients with discogenic low back pain. Pain. 2005;117:450–461. doi: 10.1016/j.pain.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 248.Wasan AD, Jamison RN, Pham L, Tipirneni N, Nedeljkovic SS, Katz JN. Psychopathology predicts the outcome of medial branch blocks with corticosteroid for chronic axial low back or cervical pain: a prospective cohort study. BMC Musculoskelet Disord. 2009;10:22. doi: 10.1186/1471-2474-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Wasan AD, Michna E, Edwards RR, Katz JN, Nedeljkovic SS, Dolman AJ, Janfaza D, Isaac Z, Jamison RN. Psychiatric Comorbidity Is Associated Prospectively with Diminished Opioid Analgesia and Increased Opioid Misuse in Patients with Chronic Low Back Pain. Anesthesiology. 2015;123:861–872. doi: 10.1097/ALN.0000000000000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Weiner BK. Spine update: the biopsychosocial model and spine care. Spine (Phila Pa 1976) 2008;33:219–223. doi: 10.1097/BRS.0b013e3181604572. [DOI] [PubMed] [Google Scholar]
- 251.Wertli MM, Rasmussen-Barr E, Held U, Weiser S, Bachmann LM, Brunner F. Fear-avoidance beliefs-a moderator of treatment efficacy in patients with low back pain: a systematic review. Spine J. 2014;14:2658–2678. doi: 10.1016/j.spinee.2014.02.033. [DOI] [PubMed] [Google Scholar]
- 252.Wertli MM, Rasmussen-Barr E, Weiser S, Bachmann LM, Brunner F. The role of fear avoidance beliefs as a prognostic factor for outcome in patients with nonspecific low back pain: a systematic review. Spine J. 2014;14:816–836. doi: 10.1016/j.spinee.2013.09.036. [DOI] [PubMed] [Google Scholar]
- 253.Wideman TH, Adams H, Sullivan MJ. A prospective sequential analysis of the fear-avoidance model of pain. Pain. 2009;145:45–51. doi: 10.1016/j.pain.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 254.Wideman TH, Asmundson GG, Smeets RJ, Zautra AJ, Simmonds MJ, Sullivan MJ, Haythornthwaite JA, Edwards RR. Rethinking the fear avoidance model: toward a multidimensional framework of pain-related disability. Pain. 2013;154:2262–2265. doi: 10.1016/j.pain.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Wideman TH, Hill JC, Main CJ, Lewis M, Sullivan MJ, Hay EM. Comparing the responsiveness of a brief, multidimensional risk screening tool for back pain to its unidimensional reference standards: the whole is greater than the sum of its parts. Pain. 2012;153:2182–2191. doi: 10.1016/j.pain.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 256.Wideman TH, Sullivan MJ. Differential predictors of the long-term levels of pain intensity, work disability, healthcare use, and medication use in a sample of workers’ compensation claimants. Pain. 2011;152:376–383. doi: 10.1016/j.pain.2010.10.044. [DOI] [PubMed] [Google Scholar]
- 257.Wideman TH, Sullivan MJ. Development of a cumulative psychosocial factor index for problematic recovery following work-related musculoskeletal injuries. Phys Ther. 2012;92:58–68. doi: 10.2522/ptj.20110071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Williams DA. The importance of psychological assessment in chronic pain. Curr Opin Urol. 2013;23:554–559. doi: 10.1097/MOU.0b013e3283652af1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Witt CM, Martins F, Willich SN, Schutzler Can I help you? Physicians’ expectations as predictor for treatment outcome. Eur J Pain. 2012;16:1455–1466. doi: 10.1002/j.1532-2149.2012.00152.x. [DOI] [PubMed] [Google Scholar]
- 260.Witt CM, Schutzler L, Ludtke R, Wegscheider K, Willich SN. Patient characteristics and variation in treatment outcomes: which patients benefit most from acupuncture for chronic pain? Clin J Pain. 2011;27:550–555. doi: 10.1097/AJP.0b013e31820dfbf5. [DOI] [PubMed] [Google Scholar]
- 261.Wright LJ, Zautra AJ, Going S. Adaptation to early knee osteoarthritis: the role of risk, resilience, and disease severity on pain and physical functioning. Ann Behav Med. 2008;36:70–80. doi: 10.1007/s12160-008-9048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Wuest J, Ford-Gilboe M, Merritt-Gray M, Varcoe C, Lent B, Wilk P, Campbell J. Abuse-related injury and symptoms of posttraumatic stress disorder as mechanisms of chronic pain in survivors of intimate partner violence. Pain Med. 2009;10:739–747. doi: 10.1111/j.1526-4637.2009.00624.x. [DOI] [PubMed] [Google Scholar]
- 263.Wuest J, Ford-Gilboe M, Merritt-Gray M. Pathways of chronic pain in survivors of intimate partner violence. J Womens Health (Larchmt) 2010;19:1665–1674. doi: 10.1089/jwh.2009.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Zywiel MG, Mahomed A, Gandhi R, Perruccio AV, Mahomed NN. Measuring expectations in orthopaedic surgery: a systematic review. Clin Orthop Relat Res. 2013;471:3446–3456. doi: 10.1007/s11999-013-3013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]