Abstract
Optimal management of a lesion yielding radial scar (RS) without epithelial atypia on breast biopsy is controversial. In this single-institution study spanning 17 years, 53 patients with this biopsy diagnosis were evaluated in terms of clinical, radiologic, and pathologic features and outcomes. RSs were categorized as either “incidental” or as the “targeted” lesion according to defined criteria. Of 48 patients who underwent surgical excision after a diagnosis of RS on biopsy, only 1 had an “upgrade” diagnosis of malignancy (2%). No “incidental” RS was associated with the presence of malignancy on surgical excision. Meta-analysis of 20 RS excision studies demonstrated an overall upgrade rate of 10.4%, with a higher rate in patients with a diagnosis of RS with atypia (26%). The upgrade rate for RS without atypia was 7.5% overall. The lower rate of upgrade to malignancy in this study (2%) is likely related to the thorough radiologic-pathologic review undertaken. In the setting of multidisciplinary agreement and careful radiologic-pathologic correlation, it may be appropriate for patients with a biopsy diagnosis of RS without atypia to forego surgical excision in favor of imaging follow-up.
Keywords: radial scar, high-risk breast lesions, breast carcinoma
The optimal management of a lesion yielding radial scar (RS) without epithelial atypia on breast needle core biopsy (NCB) is controversial. From a radiologic point of view RS is diagnostically challenging, as its mammographic appearance overlaps with that of invasive carcinoma.1–4 Historically, a lesion with radiologic features of RS was surgically excised without prior biopsy, because of a perceived significant risk of underestimation of malignancy on NCB.5,6 However, thanks to the improved tissue retrieval techniques and radiologic imaging modalities that have become available over the last decade, most patients now undergo diagnostic NCB before excision. Surgical excision of RS constitutes current standard practice, but some authors have suggested that radiologic follow-up without excision may be appropriate in these cases, if RS is not associated with epithelial atypia. 7,8 No prospective studies on the optimal management of biopsy-proven RS have been conducted, and most published series have been retrospective, involved small cohorts of patients, and lacked detailed radiologic-pathologic correlation.
In this study, we evaluated patients with NCB diagnosis of RS without epithelial atypia who were treated at our institution over a 17-year period and correlated clinical, radiologic, and pathologic features and outcomes. We also conducted a meta-analysis of prior studies of RS with and without associated epithelial atypia diagnosed on NCB to assess the overall upgrade rate of RS to carcinoma.
MATERIALS AND METHODS
After securing IRB approval, we undertook a search of institutional radiologic and pathologic databases using the terms “radial scar,” “radial sclerosing lesion,” and “complex sclerosing lesion,” to identify all diagnoses of RS on NCB rendered at our institution over a 17-year period (1996 to 2013). The initial search yielded 223 NCB cases. Selection criteria for our study included a concordant diagnosis of RS on review of the NCB slides by the 3 study pathologists (A.D.C., C.D., N.C.) and the absence of any coexistent high-risk lesion warranting surgical excision. Furthermore, only cases in which both the index NCB and imaging studies had been performed at our center were included in the study. A total of 53 cases met with all inclusion criteria.
Two dedicated breast radiologists (J.B.K./L.L.) reviewed all index images and recorded the characteristics of the target lesion (size, calcifications [Ca++], architectural distortion, mass, magnetic resonance imaging [MRI] enhancement, or a combination thereof). Further radiologic features recorded are detailed in Table 1.
TABLE 1.
Radiologic Features of Whole Study Cohort, Targeted RS, and Incident RS
| Radiologic Features | Total Cohort (N=53) | Targeted RS (N=35) | Incidental RS (N=18) |
|---|---|---|---|
| Target lesion (n [%]) | |||
| Mass | 18 (34) | 13 (37) | 5 (28) |
| Calcifications | 15 (28) | 6 (17) | 9 (50) |
| Mass with calcifications | 3 (6) | 3 (9) | 0 |
| Architectural distortion | 9 (17) | 8 (23) | 1 (5) |
| MRI enhancement | 8 (15) | 5 (14) | 3 (17) |
| Median no. cores (range) | 6 (3–18) | 5 (3–18) | 12 (3–17) |
| Median target lesion size (range) (cm) | 0.8 (0.3–5.3) | 0.75 (0.3–2.5) | 0.9 (0.3–5.3) |
| Imaging modalities | |||
| Mammogram only | 14 | 6 | 8 |
| Mammogram and ultrasound | 28 | 22 | 6 |
| Mammogram and MRI | 6 | 3 | 3 |
| Mammogram, ultrasound, and MRI | 4 | 4 | 0 |
| MRI only | 1 | 0 | 1 |
| Biopsy needle gauge (G) | |||
| 14 | 26 | 21 | 5 |
| 11 | 18 | 7 | 11 |
| 9 | 7 | 5 | 2 |
| Other | 2 | 2 | |
| Biopsy-guidance modality | |||
| Ultrasound | 27 | 22 | 5 |
| Stereotactic | 18 | 8 | 10 |
| MRI | 8 | 5 | 3 |
All available NCB hematoxylin and eosin (H&E)-stained slides were rereviewed, and the size of each RS (defined as the greatest dimension of RS on examined slides) and the presence of associated Ca++ were recorded. In excision specimens, the presence of invasive carcinoma, ductal carcinoma in situ (DCIS), lobular carcinoma in situ, and the various forms of epithelial atypia (including atypical ductal hyperplasia [ADH], atypical lobular hyperplasia, columnar cell change with atypia [CCCWA], and atypical apocrine adenosis) was assessed, as was the presence of residual RS. CCCWA is synonymous with flat epithelial atypia. An upgrade was defined as the presence of carcinoma (DCIS or invasive carcinoma) in the excision specimen.
Two pathologists (C.D./A.D.C.) and 2 radiologists (J.B.K./L.L.) compared the pathologic and radiologic data to determine whether the RS was incidental or constituted the target lesion of the NCB. If the radiologic target was Ca++, an RS containing ≤10% of the total Ca++ seen on the histologic slide was defined as incidental, providing that the overall volume of Ca++ in the NCB specimen correlated with the imaging findings. If MRI enhancement, a mass, or architectural distortion was the target, the RS was deemed incidental if its size was a third or less of the size of that target lesion, and other benign histologic findings in the NCB specimen accounted for the radiologic target.
Finally, clinical details such as demographic information, patient personal and family history of breast carcinoma, length of follow-up, and subsequent diagnosis of breast malignancy were extracted from the electronic medical record.
RESULTS
Patient Population
The study cohort consisted of 53 patients with an NCB diagnosis of RS. The median patient age at index diagnosis was 51 years, with a range from 31 to 74 years. One patient had a synchronous ipsilateral diagnosis of DCIS, which was biopsied at the same time as the index RS NCB. This was the only case with an upgrade to malignancy on surgical excision of the RS site. Six patients (11%) had a remote personal history of breast carcinoma (1 to 18 y previously; median 3.5 y), 3 had prior contralateral invasive breast carcinoma, and 3 had prior contralateral DCIS. None of these 6 patients had an upgrade on excision of the index RS. Eleven patients (20%) had a positive family history of breast carcinoma in a first-degree relative. This positive family history pertained to the index patient’s mother in 6/11 cases (age of mother at breast cancer diagnosis 59 to 72 y) and the index patient’s sister in 5/11 cases (age of sister at breast cancer diagnosis 32 to 50 y). No patient with a family history of breast malignancy had a diagnosis of malignancy on surgical excision of the RS lesion.
Forty-eight patients underwent surgical excision of the radiologic target. The remaining 5 patients were followed up clinically and radiologically for >40 months (median follow-up 84 mo, range 41 to 132 mo). All NCB H&E slides were reviewed. The original H&E slides of the excision specimens were available for review in 47 of 48 cases. Biopsy site changes were identified in 46 of 47 excisions; in the remaining case, the specimen contained a marker clip on gross pathologic examination, the specimen was entirely submitted, but no biopsy site was identified.
Radiologic and Pathologic Findings
On combined radiologic and pathologic review, the RS was deemed to be the target lesion in 35 of 53 (66%) cases and an incidental lesion in 18 cases (34%). The overall median RS size on NCB was 0.3 cm, with a range of 0.1 to 0.7 cm. In 24 (45%) cases, microscopic Ca++ was present in the RS, whereas in 36 cases (65%) microscopic Ca++ was noted in surrounding tissues. Residual RS was identified in 32 of 47 excisions (68%). The mean residual RS maximum dimension of these cases was 0.5 cm. The radiologic features of the index lesions classified as target RS and incidental RS are detailed in Table 1.
Targeted RS
The median microscopic size of targeted RS lesions on NCB was 0.3 cm. The most frequently targeted radiologic lesion was a mass (46% of cases), whereas calcifications were the sole target in 17% of cases. All patients had mammography; 74% of patients also had an ultrasound examination, and 20% also had an MRI. The needle gauge was 14G in 60% of cases, 11G in 20%, and 9G in 14%. Further details are listed in Table 1.
DCIS was diagnosed in 1 patient (3%) aged 65 years. The radiologic target consisted of pleomorphic and clustered Ca++ spanning 0.5 cm. An 11G vacuum-assisted stereotactic core biopsy needle was used for the procedure, and 9 tissue cores were taken. In the corresponding surgical excision, there was a single 0.2 cm focus of low-grade DCIS arising on a background of ADH. DCIS was located >0.5 cm away from the RS biopsy site. The patient had a synchronous ipsilateral DCIS, which was excised during the same surgery, in a separate lumpectomy specimen.
Epithelial atypia was identified in 8 of 35 excisions of a targeted RS. ADH was present in 4 cases, CCCWA in 5 cases, atypical lobular hyperplasia in 2 cases, and lobular carcinoma in situ and atypical apocrine adenosis in a single case each.
Incidental RS
The median microscopic size of incidental RS on NCB was 0.2 cm. The radiologic target lesion consisted of Ca++ in 50% of cases and a mass in 28%. Ninety-four percent of patients had a mammogram, whereas 33% had an ultrasound examination, and 22% had an MRI. The needle gauge used in 61% of cases was 11 G. A 14G needle was used in 28% of cases, and a 9G needle in 11%. Epithelial atypia was identified in 22% (4 of 18) excisions. No incidental RS was upgraded to malignancy.
Clinical Follow-up Information
The 48 patients who underwent excision after index NCB had no subsequent diagnosis of carcinoma after a median follow-up of 48 months (range, 6 to 146mo). The 5 patients with RS lesions without subsequent excision had a median follow-up of 84 months (range, 41 to 132 mo). One of these 5 patients developed an ipsilateral high-grade invasive ductal carcinoma 78 months after the diagnosis of RS on NCB. Radiologic correlation demonstrated that the invasive carcinoma occurred in an area of the breast that was anterior and medial to the biopsy site.
DISCUSSION
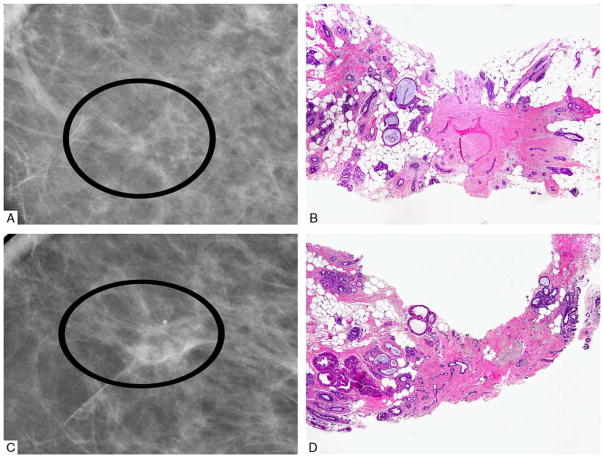
The optimal management of a radiologic target lesion yielding an RS without epithelial atypia on NCB is debated. The radiologic definition of RS formulated by Tabar and Dean9 requires at least 3 of the following imaging features: translucent or small dense center, elongated thin radiating spicules, varying appearance in different projections, and absence of a palpable lesion or skin change. The pathologic term “radial scar” was introduced in 1980 by Linell,10 although a number of pathologic pseudonyms had been used before his description to describe the histologic features of this entity.11 Histologically, RS is characterized by a central elastotic “nidus” containing entrapped tubules, associated with a corona of ducts radiating from the center of the scar and frequently demonstrating extensive proliferation. Cystic changes are present at the periphery of the lesion (Fig. 1). In the modern era of breast screening, the increased clinical recognition of RS has been attributed to the more frequent radiologic detection of small asymptomatic lesions using increasingly sensitive and sophisticated modalities.
FIGURE 1.
Radiologic (A and C) and corresponding pathologic (B and D) RS showing classic radiologic features of architectural distortion with central lucency, and pathologic features of central elastotic nidus with entrapped tubules and associated glandular proliferation.
The true extent of the relationship between RS and malignancy is difficult to assess. Autopsy studies have demonstrated that RSs occur incidentally in a significant proportion of women (8% to 16%).12,13 Higher rates are seen in more extensively sampled breasts and in the contralateral breast of patients with a history of invasive ductal carcinoma (42%).14 RSs are frequently bilateral and multiple.12,13 These 3 studies12–14 give an overall rate of malignancy of 8.6% (32/374 cases) in RS detected in autopsy studies.
The results of surgical excision studies of histologically confirmed RS in the context of mammographically detected stellate lesions are summarized in Table 2.8,15–22 These studies predate the era of routine presurgical NCB and provide an estimate of the underlying rate of malignancy in radiologically detected RS. In these studies, the malignancy rate ranged from 10% to 41%, with a mean malignancy rate on lesion excision of 20%. However, many of these studies did not undertake a detailed radiologic-pathologic assessment of whether the RS was the targeted lesion or whether it was an incidental finding near a stellate carcinoma. In the small subset of series that has addressed this, the malignancy rate on excision of “targeted” RS is substantially higher than for “incidental” RS (Table 2). Ultimately, however, the underlying rate of malignancy in association with RS does not address the clinical question of how to manage NCB-confirmed RS. A more pertinent question is whether the diagnostic sensitivity of NCB for RS-associated malignancy is sufficiently high to allow patients to forego surgical excision when their biopsy shows RS without epithelial atypia.
TABLE 2.
Meta-analysis of Surgical Excision Studies of RS-associated Malignancy (Pre-NCB Era)
| n (%)
|
Cancer Type | |||
|---|---|---|---|---|
| Malignancy Rate | Targeted Lesions | Incidental RS | ||
| Sloane et al15 | 17/126 (13.5) | 13/30 (43.3) | 1/82 (1.2) | 16 DCIS, 2 TC, 2 IDC |
| Frouge et al16 | 8/27 (30) | 8/27 (30) | NA | 7 TC, 1 IDC |
| Alleva et al17 | 9/22 (41) | 9/22 (41) | NA | 2 TC, 4 IDC, 1 ILC, 2 DCIS |
| King et al18 | 1/16 (6) | 1/10 (10) | 0/6 | (0) 1 DCIS |
| Patterson et al19 | 35/175 (20) | NA | NA | Not documented |
| Farshid et al20 | 9/94 (10) | NA | NA | 6 DCIS, 3 invasive carcinomas |
| Fasih et al21 | 20/124 (16) | NA | NA | Not documented |
| Doyle et al22 | 31/125 (25) | 31/125 (25) | NA | 3 TC, 5 IDC, 3 ILC, 5 mixed carcinomas, 15 DCIS |
| Manfrin et al8 | 37/117 (32) | 37/117 (32) | NA | 14 DCIS, 6 LCIS, 2 mixed CIS, 8 TC, 2 IDC, 5 ILC |
| Total | 167/826 (20) | 99/331 (30) | 1/88 (1) | |
CIS indicates carcinoma in situ; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; LCIS, lobular carcinoma in situ; NA, not applicable; TC, tubular carcinoma.
In the last decade, the implementation of NCB protocols using larger bore (9 to 14G) vacuum-assisted needles has permitted more extensive target lesion sampling at biopsy. This has been associated with a progressive decline in the rate of underestimation ofmalignancy associated with the presence of RS alone on NCB.7,23–25 This trend has led many to suggest that an adequately sampled lesion showing RS without epithelial atypia may not require any further excision.7,26,27 Table 36,7,23–25,28–41 demonstrates a meta-analysis of 20 reviews (including the present study) of the rate of upgrade to malignancy of NCB-confirmed RS on excision. Although many of the studies involved limited numbers of patients, together they demonstrate an overall upgrade rate of 10.5%. In particular, they illustrate the differing risks for malignancy at excision of RS with epithelial atypia (27%) versus without atypia (7.5%). The meta-analysis demonstrates that DCIS constituted the majority of RS-associated malignancy in the studies reviewed, and in cases where invasive carcinoma was identified, it tended to be low grade, with a high frequency of tubular carcinomas.39
TABLE 3.
Meta-analysis of Malignant Upgrade Rate of NCB-confirmed RS at Surgical Excision
| Needle Gauge (G) | No. Cores (Mean) | Total Cases (N) | Upgrade Rate of RS Without Atypia on CNB (n [%]) | Carcinoma Type in Upgraded Cases | Upgrade Rate of CNB RS With Atypia | Carcinoma Type in Upgraded Cases | Overall Upgrade Rate of RS on CNB (n [%]) | |
|---|---|---|---|---|---|---|---|---|
| Jackman et al6 | 14 | 10 | 5 | 2/5 (40) | 1 DCIS, 1 IDC | NA | NA | 2/5 (40) |
| Kirwan et al28 | 14 | 9.5 | 34 | 0/34 (0) | NIL | NA | NA | 0/34 (0) |
| Philpotts et al23 | 14, 11 | 9 | 8 | 0/7 (0) | NIL | 0/1 (0) | NIL | 0/8 (0) |
| Brenner et al29 | 14, 12, 11 | NS | 103 | 5/74 (7) | 3 DCIS, 2 IDC | 8/29 (28) | 5 DCIS, 3 IDC | 13/103 (13) |
| Brodie et al30 | 14 | 4 | 16 | 2/16 (13) | 2 DCIS | NA | NA | 2/16 (13) |
| Cawson et al31 | 14 | 6 | 54 | 0/27 (0) | NIL | 3/27 (11) | 3 DCIS | 3/54 (6) |
| Lee et al32 | NS | NS | 32 | 1/23 (4) | 1 DCIS | 4/9 (44) | 3 DCIS, 1 IDC | 5/32 (16) |
| Dillon et al33 | 16, 14, 11 | NS | 63 | 2/41 (5) | 2 DCIS | 7/22 (32) | 6 DCIS, 1 IDC | 9/63 (14) |
| Lopez-Medina et al34 | 14 | 6.4 | 43 | 6/38 (16) | 1 DCIS, 3 TC, 2 IDC | 2/5 (40) | 1 DCIS, 1 TC | 8/43 (19) |
| Lieske et al35 | 14 | 5 | 43 | 4/43 (9) | NS | NA | NA | 4/43 (9) |
| Hayes et al36 | NS | NS | 57 | 4/42 (10) | 4 DCIS | 3/15 (20) | 3 DCIS | 7/57 (12) |
| El-Sayed et al37 | NS | NS | 153 | 12/132 (9) | 6 DCIS, 6 IC | 5/21 (24) | 1 DCIS, 4 IC | 17/153 (11) |
| Resetkova et al7 | 11, 9 | 10 | 19 | 0/10 (0) | NIL | 0/9 (0) | NIL | 0/19 (0) |
| Rajan et al38 | 14 | 6 | 25 | 1/22 (5) | 1 DCIS | 0/3 (0) | NIL | 1/25 (4) |
| Linda et al24 | 14, 11 | 7 | 65 | 5/62 (8) | 3 DCIS, 1 IDC, 1 ILC | 0/3 (0) | NIL | 5/65 (8) |
| Rakha et al39 | NS | NS | 329 | 25/278 (9) | 14 DCIS, 5 TC, 2 ILC, 4 IC | 20/51 (39) | 12 DCIS, 8 IC | 45/329 (13.7) |
| Rakha et al40 | NS | NS | 42 | 1/39 (3) | 1 IDC | 0/3 (0) | NIL | 1/42 (24) |
| Bianchi et al41* | 14 | 4 | 49 | 4/49 (8) | 3 DCIS, 1 ILC | NA | NA | 4/49 (8) |
| Andacoglu et al25 | 11, 9 | 7.6 | 67 | 4/67 (6) | 4 DCIS | NA | NA | 4/67 (6) |
| This study | 11, 9 | 8 | 48 | 1/48 (2) | 1 DCIS | NA | NA | 1/48 (2) |
| Total | 7 | 1255 | 79/1057 (7.5) | 46 DCIS, 7 IDC, 8 TC, 4 ILC, 10 IC, 4 N/S | 52/198 (26) | 34 DCIS, 5 IDC, 1 TC, 12 IC | 131/1255 (10.4) |
This paper excluded all cases with synchronous ipsilateral DCIS or invasive carcinoma.
IC indicates invasive carcinoma, not otherwise specified; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; NA, not applicable; NS, not specified; TC, tubular carcinoma.
Our results demonstrate a lower upgrade rate (2%) for RS without epithelial atypia than the overall upgrade rate in the meta-analysis in Table 3 (7.5%). This lower rate is likely attributable to the detailed radiologic-pathologic review undertaken in this study, which led to the exclusion of 2 cases due to their spatial proximity to known, synchronous DCIS. In the absence of this detailed radiologic-pathologic review, these cases would have been recorded as an upgrade of an RS lesion to DCIS on surgical excision. Interestingly, the largest series on upgrade rates in NCB-detected RS39 was based on pathology reports rather than a radiologic-pathologic review, and therefore it is possible that the higher rate of upgrade to RS in this study may be related to this fact.
Because of the very low rate of upgrade in our study, we were unable to assess the impact of features such as target lesion size and extent of tissue sampling on the risk for malignancy at excision of NCB-proven RS. Rare studies have suggested that features such as lesion size8 may be statistically related to higher risk for malignancy on excision. However, no consistent statistically significant correlation has been previously demonstrated between malignancy at excision of RS and factors such as patient age, parity, menopausal status, and family history or personal history of breast carcinoma. In this study, none of the patients with remote personal history or family history of breast carcinoma had a diagnosis of malignancy on excision of the RS.
Data on the effect of RS on patient outcomes and survival are limited, as few studies include survival data on patients who underwent surgery. Patterson et al19 reported 1 invasive breast carcinoma in a cohort of 175 patients with a median follow-up of 5 years. Sanders et al42 demonstrated that the risk of developing invasive carcinoma after RS diagnosis is 7% in the first 10 years, compared with 5.5% of all patients without a history of breast cancer who had a benign breast biopsy during the time period studied. On multivariate analysis, there was no additional risk associated with the presence of RS when the data were adjusted for the presence of either proliferative disease or atypical hyperplasia. Most of the cases in this study were described as “incidental RS.” Jacobs et al43 demonstrates a relative risk (RR) of subsequent breast carcinoma of 1.8 in patients with RS, whereas Berg et al44 showed an RR of 1.38 for RS overall, with an RR of 1.88 for RS with proliferation and RR of 2.81 for RS with atypia. Among our 50 patients who underwent excision, no carcinomas developed at a median follow-up of 48 months.
Examination of the reported outcomes of patients who did not undergo excision for their biopsy-diagnosed RS raises interesting questions about the optimal management of these patients. Resetkova et al7 presented follow-up data on 46 patients who did not have their RS excised and found no subsequent carcinomas at a median follow-up of 29 months. Brenner et al29 reported no carcinomas in 55 patients who did not undergo excision with a follow-up of at least 48 months, whereas Sohn et al26 detected no carcinomas in 10 patients with a mean follow-up of 47 months. In our series of 5 patients who did not undergo excision, 1 patient developed an ipsilateral invasive carcinoma 6.5 years later. Careful radiologic-pathologic correlation demonstrated that this invasive high-grade carcinoma did not occur at the site of prior biopsy. Most carcinomas associated with RS are low grade (both in situ and invasive) and therefore may be unlikely to progress significantly within the 2.5 to 4 years of follow-up reported in most studies. It is possible that a small proportion of these patients may eventually develop a radiologically apparent carcinoma at the site of the RS lesion. However, in the context of a patient who is willing to forego surgery and who will follow clinical advice on further screening, it may be acceptable to hold off on surgical excision and closely monitor the patient for radiologic evidence of disease progression. This view has been endorsed by other authors.7,27 Krishnamurthy et al45 reported that a protocol of imaging follow-up, risk assessment, and counseling on breast cancer risk reduction options for carefully selected cases of high-risk breast lesions, including RS, has been adopted at their center. No prospective data are available on the outcome of this protocol as yet.
In summary, we have demonstrated a rate of upgrade to malignancy of 2% in patients with an initial biopsy diagnosis of RS without epithelial atypia, a finding that is consistent with prior RS NCB studies. The assessment of these lesions requires thorough radiologic-pathologic correlation to determine whether the RS accounts for the radiologic finding of, for example, CA++ or a mass, or whether the RS is an incidental finding. On the basis of the results of this study and our meta-analysis, we believe that in the context of multidisciplinary agreement and careful radiologic-pathologic correlation, it is appropriate for patients with an NCB diagnosis of RS without epithelial atypia, and without synchronous malignancy, to undergo imaging follow-up in place of surgical excision. As radiologic modalities continue to refine their ability to detect smaller and smaller lesions in asymptomatic women at breast screening, the clinical dilemma of how to manage these lesions is likely to arise more frequently, and it behooves the medical community to strongly consider whether conservative management of these lesions is appropriate, in light of the associated low risk of development of a life-threatening malignancy.
Acknowledgments
The thorough radiologic-pathology correlation of our cases would never have materialized without the wisdom and guidance of Dr Laura Liberman. To her, we express our heartfelt thanks.
Footnotes
Conflicts of Interest and Source of Funding: The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
References
- 1.Meyer JE, Christian RL, Lester SC, et al. Evaluation of nonpalpable solid breast masses with stereotaxic large-needle core biopsy using a dedicated unit. Am J Roentgenol. 1996;167:179–182. doi: 10.2214/ajr.167.1.8659367. [DOI] [PubMed] [Google Scholar]
- 2.Ciatto S, Morrone D, Catarzi S, et al. Radial scars of the breast: review of 38 consecutive mammographic diagnoses. Radiology. 1993;187:757–760. doi: 10.1148/radiology.187.3.8388568. [DOI] [PubMed] [Google Scholar]
- 3.Orel SG, Evers K, Yeh IT, et al. Radial scar with micro-calcifications: radiologic-pathologic correlation. Radiology. 1992;183:479–482. doi: 10.1148/radiology.183.2.1561353. [DOI] [PubMed] [Google Scholar]
- 4.Mitnick JS, Vazquez MF, Harris MN. Differentiation of radial scar from scirrhous carcinoma of the breast: mammographic-pathologic correlation. Radiology. 1989;173:697–700. doi: 10.1148/radiology.173.3.2554361. [DOI] [PubMed] [Google Scholar]
- 5.Zuiani C, Londero V, Bestagno A, et al. Proliferative high-risk lesions of the breast: contribution and limits of US-guided core biopsy. Radiol Med. 2005;110:589–602. [PubMed] [Google Scholar]
- 6.Jackman RJ, Nowels KW, Rodriguez-Soto J, et al. Stereotactic, automated, large-core needle biopsy of nonpalpable breast lesions: false-negative and histologic underestimation rates after long-term follow-up. Radiology. 1999;210:799–805. doi: 10.1148/radiology.210.3.r99mr19799. [DOI] [PubMed] [Google Scholar]
- 7.Resetkova E, Edelweiss M, Albarracin CT, et al. Management of radial sclerosing lesions of the breast diagnosed using percutaneous vacuum-assisted core needle biopsy: recommendations for excision based on seven years’ of experience at a single institution. Breast Cancer Res Treat. 2011;127:335–343. doi: 10.1007/s10549-008-0119-x. [DOI] [PubMed] [Google Scholar]
- 8.Manfrin E, Remo A, Falsirollo F, et al. Risk of neoplastic transformation in asymptomatic radial scar: analysis of 117 cases. Breast Cancer Res Treat. 2008;107:371–377. doi: 10.1007/s10549-007-9569-9. [DOI] [PubMed] [Google Scholar]
- 9.Tabar L, Dean PB. Teaching Atlas of Mammography. New York: Thieme Verlag; 1983. [PubMed] [Google Scholar]
- 10.Linell F. Breast carcinoma. Aspect, stages of early progression and related problems. Acta Pathol Microbiol Scand. 1980;272(suppl):1–233. [PubMed] [Google Scholar]
- 11.Rosen PP. Rosen’s Breast Pathology. Philadelphia: Lippincott-Raven; 2009. [Google Scholar]
- 12.Nielsen M, Jensen J, Andersen JA. An autopsy study of radial scar in the female breast. Histopathology. 1985;9:287–295. doi: 10.1111/j.1365-2559.1985.tb02446.x. [DOI] [PubMed] [Google Scholar]
- 13.Bhatal PS, Brown RW, Lesueur GC, et al. Frequency of benign and malignant breast lesions in 207 consecutive autopsies in Australian women. Br J Cancer. 1985;51:271–278. doi: 10.1038/bjc.1985.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nielsen M, Christensen L, Andersen J. Radial scars in women with breast cancer. Cancer. 1987;59:1019–1025. doi: 10.1002/1097-0142(19870301)59:5<1019::aid-cncr2820590528>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 15.Sloane JP, Mayers MM. Carcinoma and atypical hyperplasia in radial scars and complex sclerosing lesions: importance of lesion size and patient age. Histopathology. 1993;23:225–231. doi: 10.1111/j.1365-2559.1993.tb01194.x. [DOI] [PubMed] [Google Scholar]
- 16.Frouge C, Tristant H, Guinebretiere JM, et al. Mammographic lesions suggestive of radial scars: microscopic findings in 40 cases. Radiology. 1995;195:623–625. doi: 10.1148/radiology.195.3.7753984. [DOI] [PubMed] [Google Scholar]
- 17.Alleva DQ, Smetherman DH, Farr GH, Jr, et al. Radial scar of the breast: radiologic-pathologic correlation in 22 cases. Radiographics. 1999;19:S27–S37. doi: 10.1148/radiographics.19.suppl_1.g99oc05s27. [DOI] [PubMed] [Google Scholar]
- 18.King TA, Scharfenberg JC, Smetherman DH, et al. A better understanding of the term radial scar. Am J Surg. 2000;180:428–433. doi: 10.1016/s0002-9610(00)00506-7. [DOI] [PubMed] [Google Scholar]
- 19.Patterson JA, Scott M, Anderson N, et al. Radial scar, complex sclerosing lesion and risk of breast cancer. Analysis of 175 cases in Northern Ireland. Eur J Surg Oncol. 2004;30:1065–1068. doi: 10.1016/j.ejso.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Farshid G, Rush G. Assessment of 142 stellate lesions with imaging features suggestive of radial scar discovered during population-based screening for breast cancer. Am J Surg Pathol. 2004;28:1626–1631. doi: 10.1097/00000478-200412000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Fasih T, Jain M, Shrimankar J, et al. All radial scars/complex sclerosing lesions seen on breast screening mammograms should be excised. Eur J Surg Oncol. 2005;31:1125–1128. doi: 10.1016/j.ejso.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Doyle EM, Banville N, Quinn CM, et al. Radial scars/complex sclerosing lesions and malignancy in a screening programme: incidence and histological features revisited. Histopathology. 2007;50:607–614. doi: 10.1111/j.1365-2559.2007.02660.x. [DOI] [PubMed] [Google Scholar]
- 23.Philpotts LE, Shaheen NA, Jain KS, et al. Uncommon high-risk lesions of the breast diagnosed at stereotactic core-needle biopsy: clinical importance. Radiology. 2000;216:831–837. doi: 10.1148/radiology.216.3.r00se31831. [DOI] [PubMed] [Google Scholar]
- 24.Linda A, Zuiani C, Furlan A, et al. Radial scars without atypia diagnosed at imaging-guided needle biopsy: how often is associated malignancy found at subsequent surgical excision, and do mammography and sonography predict which lesions are malignant? Am J Roentgenol. 2010;194:1146–1151. doi: 10.2214/AJR.09.2326. [DOI] [PubMed] [Google Scholar]
- 25.Andacoglu O, Kanbour-Shakir A, Teh YC, et al. Rationale of excisional biopsy after the diagnosis of benign radial scar on core biopsy: a single institutional outcome analysis. Am J Clin Oncol. 2013;36:7–11. doi: 10.1097/COC.0b013e3182354a3f. [DOI] [PubMed] [Google Scholar]
- 26.Sohn VY, Causey MW, Steele SR, et al. The treatment of radial scars in the modern era: surgical excision is not required. Am Surg. 2010;76:522–525. [PubMed] [Google Scholar]
- 27.Johnson NB, Collins LC. Update on percutaneous needle biopsy of nonmalignant breast lesions. Adv Anat Pathol. 2009;16:183–195. doi: 10.1097/PAP.0b013e3181a9d33e. [DOI] [PubMed] [Google Scholar]
- 28.Kirwan SE, Denton ER, Nash RM, et al. Multiple 14G stereotactic core biopsies in the diagnosis of mammographically detected stellate lesions of the breast. Clin Radiol. 2000;55:763–766. doi: 10.1053/crad.2000.0513. [DOI] [PubMed] [Google Scholar]
- 29.Brenner RJ, Jackman RJ, Parker SH, et al. Percutaneous core needle biopsy of radial scars of the breast: when is excision necessary? Am J Roentgenol. 2002;179:1179–1184. doi: 10.2214/ajr.179.5.1791179. [DOI] [PubMed] [Google Scholar]
- 30.Brodie C, O’Doherty A, Quinn C. Fourteen-gauge needle core biopsy of mammographically evident radial scars. Cancer. 2004;100:652–653. doi: 10.1002/cncr.11951. [DOI] [PubMed] [Google Scholar]
- 31.Cawson JN, Malara F, Kavanagh A, et al. Fourteen-gauge needle core biopsy of mammographically evident radial scars: is excision necessary? Cancer. 2003;97:345–351. doi: 10.1002/cncr.11070. [DOI] [PubMed] [Google Scholar]
- 32.Lee AHS, Denley HE, Pinder SE, et al. Excision biopsy findings of patients with breast needle core biopsies reported as suspicious of malignancy (B4) or lesion of uncertain malignant potential (B3) Histopathology. 2003;42:331–336. doi: 10.1046/j.1365-2559.2003.01582.x. [DOI] [PubMed] [Google Scholar]
- 33.Dillon MF, McDermott EW, Hill A, et al. Predictive value of breast lesions of “uncertain malignant potential” and “suspicious for malignancy” determined by needle core biopsy. Ann Surg Oncol. 2006;14:704–711. doi: 10.1245/s10434-006-9212-8. [DOI] [PubMed] [Google Scholar]
- 34.Lopez-Medina A, Cintora E, Mugica B, et al. Radial scars diagnosed at stereotactic core-needle biopsy: surgical biopsy findings. Eur Radiol. 2006;16:1803–1810. doi: 10.1007/s00330-006-0196-3. [DOI] [PubMed] [Google Scholar]
- 35.Lieske B, Ravichandran D, Alvi A, et al. Screen-detected breast lesions with an indeterminate (B3) core needle biopsy should be excised. Eur J Surg Oncol. 2008;34:1293–1298. doi: 10.1016/j.ejso.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Hayes BD, O’Doherty A, Quinn CM. Correlation of needle core biopsy with excision histology in screen-detected B3 lesions: the Merrion Breast Screening Unit experience. J Clin Pathol. 2009;62:1136–1140. doi: 10.1136/jcp.2009.067280. [DOI] [PubMed] [Google Scholar]
- 37.El-Sayed ME, Rakha EA, Reed J, et al. Predictive value of needle core biopsy diagnoses of lesion of uncertain malignant potential (B3) in abnormalities detected by mammographic screening. Histopathology. 2008;53:650–657. doi: 10.1111/j.1365-2559.2008.03158.x. [DOI] [PubMed] [Google Scholar]
- 38.Rajan S, Wason AM, Carder PJ. Conservative management of screen-detected radial scars: role of mammotome excision. J Clin Pathol. 2011;64:65–68. doi: 10.1136/jcp.2010.083485. [DOI] [PubMed] [Google Scholar]
- 39.Rakha EA, Lee AHS, Jenkins JA, et al. Characterization and outcome of breast needle core biopsy diagnoses of lesions of uncertain malignant potential (B3) in abnormalities detected by mammographic screening. Int J Cancer. 2011;129:1417–1424. doi: 10.1002/ijc.25801. [DOI] [PubMed] [Google Scholar]
- 40.Rakha EA, Ho BC, Naik V, et al. Outcome of breast lesions diagnosed as lesion of uncertain malignant potential (B3) or suspicious of malignancy (B4) on needle core biopsy, including detailed review of epithelial atypia. Histopathology. 2011;58:626–632. doi: 10.1111/j.1365-2559.2011.03786.x. [DOI] [PubMed] [Google Scholar]
- 41.Bianchi S, Giannotti E, Vanzi E, et al. Radial scar without associated atypical epithelial proliferation on image-guided 14-gauge needle core biopsy: analysis of 49 cases from a single centre and review of the literature. Breast. 2012;21:159–164. doi: 10.1016/j.breast.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 42.Sanders ME, Page DL, Simpson JF, et al. Interdependence of radial scar and proliferative disease with respect to invasive breast carcinoma risk in patients with benign breast biopsies. Cancer. 2006;106:1453–1461. doi: 10.1002/cncr.21730. [DOI] [PubMed] [Google Scholar]
- 43.Jacobs TW, Byrne C, Colditz G, et al. Radial scars in benign breast-biopsy specimens and the risk of breast cancer. N Engl J Med. 1999;340:430–436. doi: 10.1056/NEJM199902113400604. [DOI] [PubMed] [Google Scholar]
- 44.Berg JC, Visscher DW, Vierkant VS, et al. Breast cancer risk in women with radial scars in benign breast biopsies. Breast Cancer Res Treat. 2008;108:167–174. doi: 10.1007/s10549-007-9605-9. [DOI] [PubMed] [Google Scholar]
- 45.Krishnamurthy S, Bevers T, Kuerer H, et al. Multidisciplinary considerations in the management of high-risk breast lesions. Am J Roentgenol. 2012;198:W132–W140. doi: 10.2214/AJR.11.7799. [DOI] [PubMed] [Google Scholar]