Abstract
Background
Through ventricular interdependence, pulmonary hypertension (PH) induces left ventricular (LV) dysfunction. We hypothesized that pediatric PH patients have LV diastolic dysfunction, related to adverse pulmonary hemodynamics, leftward septal shift, and prolonged right ventricular (RV) systole.
Methods and Results
Echocardiography was prospectively performed at two institutions in 54 pediatric PH patients during cardiac catheterization, and in 54 matched controls. Diastolic LV measures including myocardial deformation were assessed by echocardiography. PH patients had evidence of LV diastolic dysfunction, most consistent with impaired LV relaxation, though some features of reduced ventricular compliance were present. PH patients demonstrated the following: reduced mitral E velocity and inflow duration, mitral E’ and E’/A’, septal E’ and A’, pulmonary vein S and D wave velocities, and LV basal global early diastolic circumferential strain rate; and increased mitral E deceleration time, LV isovolumic relaxation time, mitral E/E’, and pulmonary vein A wave duration. PH patients demonstrated leftward septal shift and prolonged RV systole, both known to affect LV diastole. These changes were exacerbated in severe PH. There were no statistically significant differences in diastolic measures between patients with and without a shunt, and minimal differences between patients with and without congenital heart disease. Multiple echocardiographic LV diastolic parameters demonstrated weak to moderate correlations with invasively-determined PH severity, leftward septal shift, and prolonged RV systole.
Conclusions
Pediatric PH patients exhibit LV diastolic dysfunction most consistent with impaired relaxation and reduced myocardial deformation, related to invasive hemodynamics, leftward septal shift, and prolonged RV systole.
Keywords: echocardiography, pediatric, hypertension, pulmonary, ventricular diastolic dysfunction
Pulmonary hypertension (PH) is a progressive disease with substantial mortality.1, 2 While right ventricular (RV) systolic dysfunction is a central cause of morbidity and death in PH,1-4 through ventricular interdependence, the left ventricle (LV) is significantly impacted. LV systolic dysfunction has been highlighted as a key risk factor for adverse outcomes in adult PH.5 Moreover, we recently documented impaired LV systolic mechanics in pediatric PH.6 However, LV diastolic function, an important component of cardiac (dys)function, remains under-investigated in PH and is consequently not accounted for in its management.
Increased RV pressure and systolic duration can cause leftward septal shift during LV relaxation, potentially affecting LV diastole. Adult PH studies reveal decreased LV filling rates and size, and abnormal pulmonary vein and mitral valve flow patterns.5, 7-14 However, the hemodynamic impact of PH on LV diastolic function (including myocardial performance) has not been fully evaluated as few studies have directly related the two; fewer yet have done so simultaneously. Furthermore, little is known about how congenital heart disease (CHD) and intra-/extra-cardiac shunting – commonly associated with pediatric PH – affect cardiopulmonary hemodynamics and LV diastolic function in PH.
Accordingly, this study aimed to investigate LV diastolic function in pediatric PH and the relation to invasive pulmonary hemodynamics, using simultaneous echocardiography and cardiac catheterization. We hypothesized that children and young adults with PH have LV diastolic dysfunction, related to adverse pulmonary hemodynamics, leftward septal shift, and prolonged RV systole.
Methods
Study Population
Between November 1, 2008 and October 1, 2013 at Children's Hospital Colorado (CHCO) and the Hospital for Sick Children (SickKids) in Toronto, we prospectively performed simultaneous transthoracic echocardiography in children and adolescents during clinically-indicated right-heart catheterization for initial evaluation of suspected PH or routine follow-up of previously documented pre-capillary PH (resting mean pulmonary artery pressure ≥25 mmHg and pulmonary capillary wedge pressure [PCWP] ≤ 15 mmHg at catheterization).15 We previously published systolic strain results in this patient cohort.6 The study was approved by the Institutional Review Board at both institutions. Informed consent was obtained for all patients.
We excluded those with conditions that might directly impact LV function aside from PH, including single ventricle physiology, active pacing, cardiomyopathies, heart transplant, (branch) pulmonary artery stenosis, uncontrolled systemic hypertension, ventricular septal defect occlusion devices, any left-sided obstructive lesion, PCWP >15 mmHg, or lack of raw echocardiography data preventing analysis. Ten patients were excluded, leaving 54 in our final cohort (37 from CHCO, 17 from SickKids); one patient was found to not have PH on initial diagnostic catheterization and thus was excluded from comparisons of PH patients versus controls, but included in correlative data.
Right-Heart Catheterization
Under general anesthesia, a right-heart catheterization was performed by individuals blinded to echocardiographic measures. Cardiac index was measured by thermodilution (if no shunting) or calculated using the modified Fick equation (if shunt present); pulmonary (Qp) and systemic (Qs) blood flow were documented. We measured pressures in the right atrium, RV, pulmonary artery, left atrium (and/or PCWP), and femoral (systemic) artery. We calculated the transpulmonary gradient (PCWP served as a surrogate when left atrial pressure not obtained), systemic and indexed pulmonary vascular resistances (PVRi).
Echocardiography
During the baseline condition of cardiac catheterization, we used transthoracic echocardiography to obtain images from apical four- and two-chamber views and parasternal short-axis views at the LV base (mitral valve), mid (papillary muscles) and apex (apical to the papillary muscles), using a General Electric (GE) Vivid 7 or E9 system (GE Healthcare, USA). Results of 3-5 cardiac cycles were averaged to account for beat-to-beat variability. Measurements were made by individuals blinded to clinical and catheterization data (not present during cardiac catheterization).
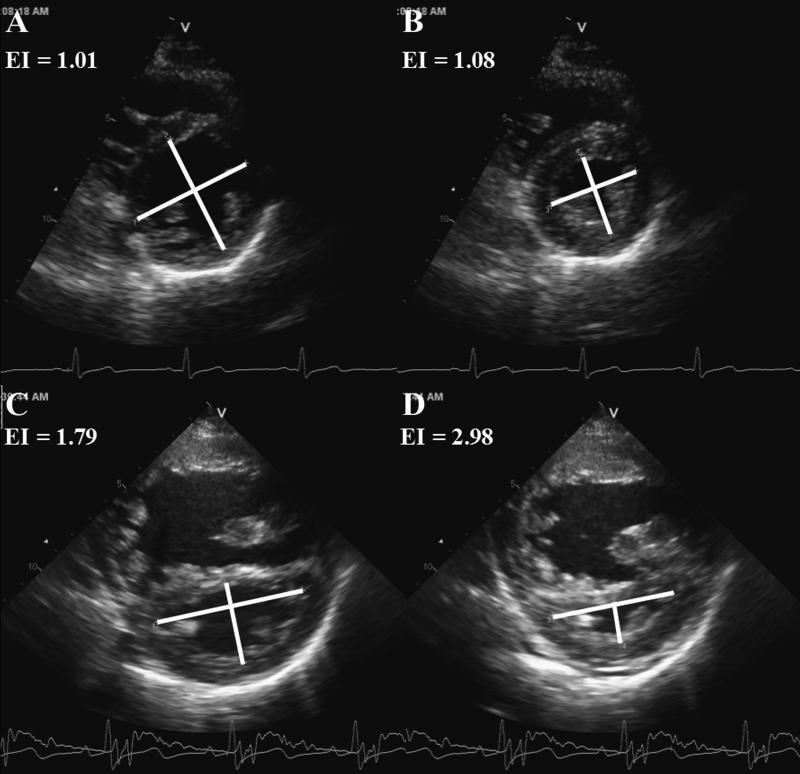
Simpson's biplane LV volumes and ejection fraction (EF) were calculated.16 LV eccentricity index was calculated from mid parasternal short-axis images at end-diastole and end-systole (Figure 1).17
Figure 1.
Normal septal geometry in a control patient is quantified with end-diastolic (A) and endsystolic (B) eccentricity index. Leftward septal shift in a pulmonary hypertension patient quantified with end-diastolic (C) and end-systolic (D) eccentricity index. EI indicates eccentricity index.
From the apical four-chamber, we recorded the following: pulmonary vein systolic (S), diastolic (D), and atrial contraction flow-reversal (A) wave velocities, and A wave duration; mitral and tricuspid peak early (E) and late (A) diastolic velocities; mitral E deceleration time; mitral inflow duration (mitral valve opening to closure); and duration of aortic valve ejection.
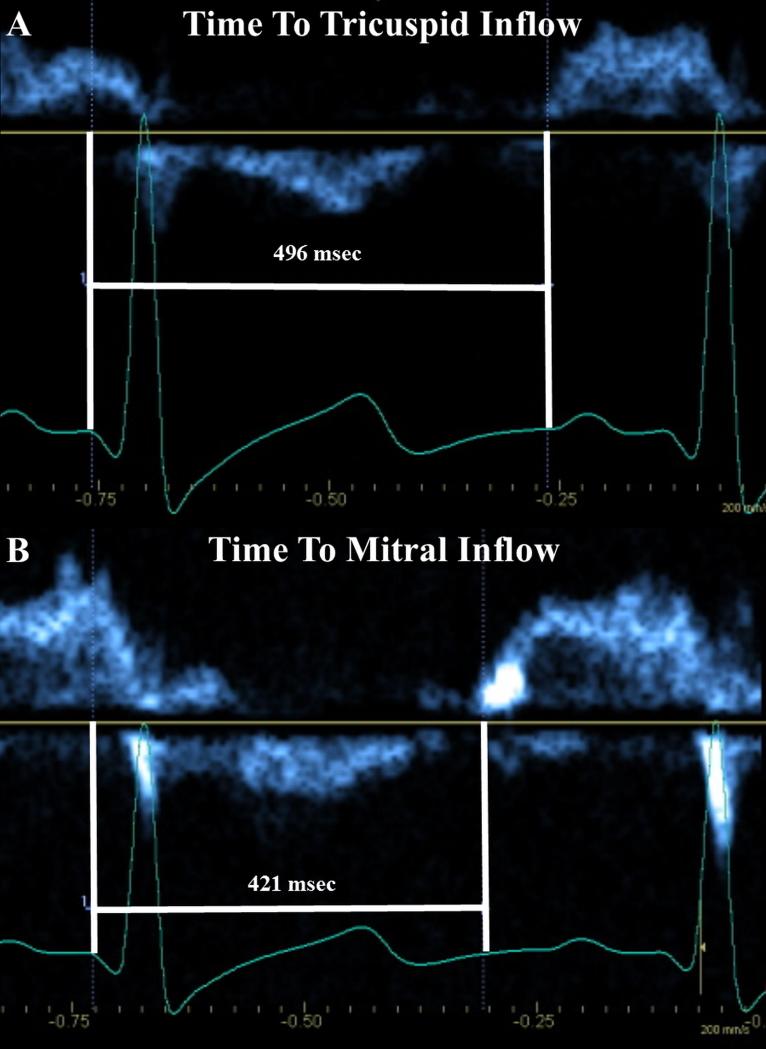
To assess if RV systole is prolonged beyond the LV, and the potential impact on LV filling, the time from QRS onset to initiation of tricuspid and mitral valve inflow (Figure 2) was recorded (heart rates within 5 bpm). This surrogate measure of ventricular systolic duration includes isovolumic relaxation time – a period where RV pressure can still be elevated over LV pressure. The ratio of time to tricuspid inflow (RV systole) divided by time to mitral inflow (LV systole) quantified RV systolic prolongation: a value of 1 = simultaneous completion of RV and LV systole; >1 = prolonged RV systole.
Figure 2.
Timing from onset of QRS to the onset of tricuspid (A) and mitral (B) inflow, as a surrogate measure of right (A) and left (B) ventricular systole. The ratio of right ventricular systolic duration divided by left ventricular systolic duration yields a quantifiable measure of the degree of prolongation of right ventricular systole. Here, the ratio measures 1.18.
From the apical four-chamber view, pulsed-wave tissue Doppler imaging (TDI) was performed at the lateral and septal mitral annulus, and the lateral tricuspid annulus; peak early (E’) and late (A’) diastolic myocardial annular velocities and isovolumic relaxation time (by TDI of the lateral mitral annulus) were recorded.
Speckle-Tracking Echocardiography
Two-dimensional images were used for off-line speckle-tracking echocardiography analysis (EchoPAC version 113, GE Healthcare, USA). The region of interest was set to myocardial wall thickness. Tracking was visually assessed throughout the cardiac cycle and the region of interest adjusted as needed to ensure accurate tracking.18 If ≥4 segments tracked well by visual and EchoPAC assessment, the curve was accepted; segments that tracked poorly were excluded. Aortic valve closure, by pulsed-wave Doppler (heart rate within 5 bpm to images used for strain), defined LV end-systole. LV (IVS included as part of the LV) peak global early diastolic longitudinal strain rate (DLSR) was assessed from the apical four-chamber view. LV peak global early diastolic circumferential strain rate (DCSR) was assessed from the base, mid and apical parasternal short-axis views.
Peak diastolic apical and basal rotation rates (recoil) were recorded to determine peak diastolic untwist rate (apical recoil – basal recoil), which was indexed to LV length.
Control Population
Comparable measurements were obtained from the echocardiograms of 54 age-, gender-, and institution-matched healthy children (37 at CHCO, 17 at SickKids); no controls underwent catheterization. Subjects were healthy volunteers or children undergoing evaluation for murmur, chest pain, palpitations, syncope, or family history of CHD, with a normal echocardiogram.15 We excluded those with documented arrhythmias, chemotherapy exposure, family history of cardiomyopathy or bicuspid aortic valve,19 systemic illness, or documented/suspected genetic abnormality. The systolic strain values of this control cohort was previously published.6
Statistical Analysis
Data were not normally distributed by the Shapiro-Wilk test. Thus, continuous data are presented as median with interquartile range unless otherwise noted; categorical data are presented as frequencies (%). Comparative analysis was performed using Wilcoxon Mann-Whitney and Chi-square testing as appropriate. Matched data was analyzed for differences using Wilcoxon signed rank testing. Exact methods were utilized as necessary. ANOVA testing was used to define the presence of differences between subgroups; post-hoc analysis was performed with Student-Newman-Keuls. Spearman correlation coefficients were used to assess relationships between echocardiographic LV filling parameters, invasive hemodynamics, septal shift, and prolonged RV systole. Reproducibility was assessed for diastolic strain measures. To assess interobserver variability, SickKids PH echocardiograms (31.5% of patients with PH) were reanalyzed by a second observer (C.S.), also blinded to clinical and catheterization data. To assess intraobserver variability, 10% of echocardiograms were reanalyzed at least 8 weeks apart. Inter-rater and intra-rater reliability were calculated using intraclass correlation coefficients with 95% confidence interval (CI). A P value of <0.05 was considered statistically significant. All analyses were performed using SAS software, version 9.3 (SAS Corporation, Cary, NC).
Results
Patient Characteristics
Patient characteristics are presented in Table 1, and hemodynamic data in Table 2. In total, 54 PH patients and 54 controls were analyzed. One study patient did not have PH on initial catheterization; he and his matched control were omitted from comparisons between PH and controls. Most PH patients were female (34/53, 64.2%), and born with CHD (33/53, 62.3%), defined as any structural defect (including a patent foramen ovale); some with CHD had multiple defects (7/33, 21.2%). Common abnormalities were atrial and ventricular septation defects, and a patent ductus arteriosus; “other” forms of CHD included atrioventricular septal defect (2), transposition of the great arteries (1), and anomalous pulmonary venous drainage due to a posterior/leftward deviated atrial septum, repaired without pulmonary vein manipulation/obstruction (2).
Table 1.
Patient Characteristics
| PH Patients (n = 53) | Control Subjects (n = 53) | P Value | |
|---|---|---|---|
| Age, yr (range) | 8 (0 - 23) | 8 (0 - 23) | 0.99 |
| Female, n (%) | 34 (64.2%) | 34 (64.2%) | 1.00 |
| Weight, kg | 32.5 (14.7 - 51.4) | 30.6 (16.3 - 54.8) | 0.20 |
| Height, cm | 133.0 (98.0 - 161.0) | 136.0 (104.5 - 162.0) | 0.11 |
| Body Surface Area, m2 | 1.12 (0.63 - 1.52) | 1.10 (0.69 - 1.57) | 0.06 |
| Heart Rate, bpm | 81 (70 - 100) | 79 (65 - 93) | 0.09 |
| Congenital Heart Disease, n (%) | 33 (62.3%) | ... | ... |
| Atrial Septal Defect / Patent Foramen Ovale | 24 (45.3%) | ... | ... |
| Ventricular Septal Defect | 5 (9.4%) | ... | ... |
| Patent Ductus Arteriosus | 11 (20.8%) | ... | ... |
| Other | 5 (9.4%) | ... | ... |
| Shunt Present at Catheterization, n (%) | 21 (39.6%) | ... | ... |
| Idiopathic Pulmonary Arterial Hypertension | 14 (26.4%) | ... | ... |
| World Health Organization Class, n (%) | |||
| Not Available | 15 (28.3%) | ... | ... |
| I / II | 15 (28.3%) / 16 (30.2%) | ... | ... |
| III / IV | 6 (11.3%) / 1 (1.9%) | ... | ... |
Presented as n (%) or median (interquartile range), unless otherwise noted. PH indicates pulmonary hypertension.
Table 2.
Hemodynamic Characteristics
| PH Patients (n = 53) | Severe PH (n = 13) | CHD (n = 33) | No CHD (n = 20) | CHD P value | Shunt (n = 21) | No Shunt (n = 32) | Shunt P value | |
|---|---|---|---|---|---|---|---|---|
| MPAP, mm Hg | 33.0 (25.0 - 43.0) | 60.0 (46.0 - 61.0) | 40.5 (28.0 - 52.0) | 27.0 (23.0 - 31.0) | 0.0074* | 41.5 (32.0 - 54.0) | 28.5 (20.5 - 38.5) | 0.0082* |
| MAP, mm Hg | 58.0 (55.0 - 63.0) | 58.0 (52.0 - 60.0) | 59.0 (53.0 - 63.0) | 57.0 (55.0 - 63.0) | 0.77 | 57.5 (52.5 - 61.0) | 59.5 (55.0 - 63.0) | 0.26 |
| MPAP/MAP | 0.59 (0.43 - 0.79) | 1.00 (0.81 - 1.04) | 0.66 (0.42 - 0.94) | 0.44 (0.39 - 0.56) | 0.0086* | 0.71 (0.59 - 0.97) | 0.46 (0.35 - 0.61) | 0.003* |
| SPAP, mm Hg | 52.0 (38.0 - 63.0) | 84.0 (63.0 - 90.0) | 58.0 (39.0 - 67.0) | 41.0 (35.0 - 52.0) | 0.0134* | 60.5 (52.0 - 73.0) | 43.0 (34.0 - 56.0) | 0.0068* |
| DPAP, mm Hg | 23.0 (15.0 - 32.0) | 42.0 (33.0 - 46.0) | 27.0 (14.0 - 35.0) | 19.0 (15.0 - 22.0) | 0.035* | 29.5 (22.0 - 36.0) | 19.5 (14.0 - 27.0) | 0.0186* |
| TPG, mm Hg | 23.0 (16.0 - 35.0) | 51.0 (38.0 - 54.0) | 30.0 (19.0 - 43.0) | 17.0 (15.0 - 22.0) | 0.0029* | 33.0 (22.0 - 48.0) | 18.5 (13.5 - 29.0) | 0.0035* |
| DTPG, mm Hg | 13.0 (6.0 - 26.0) | 35.0 (26.0 - 39.0) | 16.5 (5.0 - 27.0) | 10.0 (6.0 - 12.0) | 0.0087* | 21.5 (13.0 - 28.0) | 11.0 (5.0 - 16.0) | 0.0069* |
| MLAP, mm Hg | 8.5 (7.0 - 10.0) | 7.0 (6.0 - 8.0) | 8.0 (6.0 - 10.0) | 8.8 (7.3 - 10.5) | 0.29 | 7.5 (6.0 - 9.0) | 9.0 (7.0 - 10.0) | 0.17 |
| MRAP, mm Hg | 7.0 (5.0 - 8.0) | 7.0 (5.0 - 9.0) | 7.0 (5.0 - 8.0) | 5.5 (5.0 - 7.5) | 0.35 | 7.0 (5.0 - 7.0) | 6.0 (5.0 - 8.0) | 0.96 |
| RVEDP, mm Hg | 8.0 (6.0 - 10.0) | 9.0 (6.0 - 11.0) | 8.6 (7.0 - 10.2) | 7.0 (5.1 - 8.6) | 0.05 | 9.0 (7.0 - 9.9) | 7.0 (6.0 - 9.1) | 0.18 |
| CI, L/min/m2 | 3.38 (2.98 - 3.82) | 3.52 (3.23 - 3.98) | 3.23 (2.94 - 3.98) | 3.42 (3.00 - 3.68) | 0.93 | 3.23 (3.03 - 3.63) | 3.38 (2.84 - 3.82) | 0.95 |
| Qp/Qs | 1.00 (1.00 - 1.00) | 1.00 (0.93 - 1.00) | 1.00 (1.00 - 1.19) | 1.00 (1.00 - 1.00) | 0.09 | 1.16 (1.00 - 1.55)† | 1.00 (1.00 - 1.00) | 0.0043* |
| PVRi, WU·m2 | 6.79 (4.10 - 10.89) | 15.8 (11.7 - 19.1) | 7.64 (4.81 - 13.89) | 5.33 (3.48 - 6.96) | 0.07 | 7.67 (5.57 - 14.09) | 5.60 (3.21 - 8.70) | 0.08 |
| PVR/SVR | 0.37 (0.25 - 0.60) | 0.85 (0.77 - 1.12) | 0.49 (0.23 - 0.69) | 0.33 (0.25 - 0.38) | 0.22 | 0.49 (0.26 - 0.77) | 0.34 (0.25 - 0.53) | 0.29 |
Presented as n (%) or median (interquartile range). PH indicates pulmonary hypertension; CHD, congenital heart disease; MPAP; mean pulmonary arterial pressure; MAP, mean (systemic) arterial pressure; SPAP, systolic pulmonary arterial pressure; DPAP, diastolic pulmonary arterial pressure; MLAP, mean left atrial pressure or pulmonary capillary wedge pressure; MRAP, mean right atrial pressure; CI, cardiac index; Qp/Qs, ratio of pulmonary-to-systemic blood flow; PVRi, indexed pulmonary vascular resistance; SVR, systemic vascular resistance.
P<0.05.
Four patients had a net right-to-left shunt (Qp:Qs of 0.94, 0.86, 0.89, 0.93). CHD and Shunt P values represent comparison between those without/with CHD and a shunt, respectively.
Of those born with CHD, 36.4% (12/33) underwent previous repair. Thus, 63.6 % (21/33) of those with CHD, and 39.6% (21/53) of the entire PH cohort, had a potential intra-/extra-cardiac shunt at the time of catheterization; this group had a median pulmonary-to-systemic blood flow ratio (Qp:Qs) of 1.16 (1.00–1.55). Cardiac index was preserved across the entire PH cohort and did not correlate with mean pulmonary artery pressure (r=0.06, P=0.67) or PVRi (r=−0.27, P=0.07). All patients had systemic saturations >90%.
Idiopathic PH was present in 26.4% (14/53) of patients. “Other” etiologies included CHD, lung disease/hypoxia, hematologic disorders, chronic thromboembolism, or a combination of etiologies. Sixteen patients had ≥1 PH-related hospital admission (24 admissions total). There was 1 death and 1 transplant (lung) during the study period.
Echocardiographic Measures
LV echocardiographic measures are presented in Table 3. LV eccentricity indices were increased in PH, consistent with increased leftward septal shift. Indexed end-systolic volume was larger in PH patients, even after controlling for shunting. Although at the lower limits of normal, LV EF was significantly lower in PH compared to controls.
Table 3.
Left Ventricular Echocardiographic Variables
| Control Subjects (n = 53) | PH Patients (n = 53) | P Value | Severe PH (n = 13) | P value | |
|---|---|---|---|---|---|
| 2-Dimensional Measures | |||||
| End-Diastolic Eccentricity Index | 1.02 (0.96 - 1.06) | 1.18 (1.09 - 1.28) | <0.0001* | 1.43 (1.15 - 1.76) | 0.043* |
| End-Systolic Eccentricity Index | 0.99 (0.96 - 1.03) | 1.27 (1.11 - 1.50) | <0.0001* | 1.82 (1.30 - 2.17) | 0.005* |
| End-Diastolic Volume, ml | 49.0 (29.0 - 79.5) | 52.0 (26.0 - 90.0) | 0.25 | 77.0 (39.0 - 105.0) | 0.75 |
| Indexed End-Diastolic Volume, ml/m2 | 37.9 (32.8 - 48.4) | 44.3 (37.2 - 59.8) | 0.07 | 61.5 (45.6 - 82.4) | 0.61 |
| End-Systolic Volume, ml | 16.5 (9.5 - 27.5) | 19.0 (12.0 - 46.0) | 0.004* | 33.0 (16.0 - 47.0) | 0.46 |
| Indexed End-Systolic Volume, ml/m2 | 12.9 (9.8 - 16.5) | 20.3 (14.2 - 28.1) | 0.0006* | 23.5 (21.4 - 33.1) | 0.24 |
| Biplane Ejection Fraction, % | 66.0 (62.0 - 69.0) | 56.0 (50.0 - 63.0) | <0.0001* | 58.0 (50.0 - 66.0) | 0.015* |
| Doppler Measures | |||||
| Pulmonary Vein | |||||
| S, m/s | 0.46 (0.38 - 0.49) | 0.31 (0.27 - 0.37) | <0.0001* | 0.28 (0.20 - 0.34) | 0.0063* |
| D, m/s | 0.60 (0.53 - 0.67) | 0.46 (0.40 - 0.55) | <0.0001* | 0.42 (0.40 - 0.47) | 0.045* |
| S/D Ratio | 0.75 (0.65 - 0.86) | 0.71 (0.56 - 0.83) | 0.22 | 0.62 (0.57 - 0.83) | 0.25 |
| A, m/s | 0.16 (0.13 - 0.18) | 0.15 (0.12 - 0.19) | 0.84 | 0.16 (0.13 - 0.19) | 0.89 |
| A Duration, msec | 68.0 (59.0 - 83.0) | 85.0 (69.0 - 107.0) | <0.0001* | 91.0 (70.0 - 107.0) | 0.12 |
| Mitral Valve | |||||
| E, m/s | 0.96 (0.85 - 1.10) | 0.86 (0.73 - 0.93) | 0.011* | 0.74 (0.71 - 0.82) | 0.035* |
| A, m/s | 0.47 (0.39 - 0.57) | 0.49 (0.34 - 0.72) | 0.12 | 0.49 (0.39 - 0.62) | 0.14 |
| E/A | 1.99 (1.66 - 2.54) | 1.80 (1.16 - 2.87) | 0.53 | 1.54 (1.08 - 2.04) | 0.12 |
| E Deceleration Time, msec | 133.0 (121.0 - 170.0) | 179.0 (137.0 - 210.0) | 0.0002* | 222.5 (153.5 - 279.5) | 0.032* |
| Inflow Duration, msec | 400.5 (306.5 - 579.0) | 363.0 (263.0 - 497.5) | 0.013* | 416.0 (304.5 - 497.5) | 0.37 |
| PVAD/MVAD | 0.65 (0.57 - 0.79) | 0.72 (0.62 - 0.84) | 0.017* | 0.70 (0.67 - 0.78) | 0.18 |
| PVAD - MVAD | −38.0 (−48.0 - −20.0) | −29.5 (−45.0 - −14.0) | 0.09 | −30.0 (−45.0 - −20.0) | 0.31 |
| RV/LV Systolic Duration | 1.01 (0.98 - 1.032) | 1.12 (1.06 - 1.18) | <0.0001* | 1.19 (1.14 - 1.30) | 0.0005* |
| Tissue Doppler Imaging | |||||
| Lateral Mitral E′, cm/s | 18.8 (16.0 - 20.2) | 12.0 (9.0 - 15.0) | <0.0001* | 10.0 (8.5 - 13.0) | 0.027* |
| Lateral Mitral A′, cm/s | 6.4 (5.4 - 7.9) | 6.0 (4.0 - 7.0) | 0.15 | 6.0 (4.0 - 7.0) | 0.03* |
| Lateral Mitral E′/A′ | 2.9 (2.3 - 3.3) | 2.1 (1.7 - 3.0) | 0.001* | 1.9 (1.7 - 2.5) | 0.16 |
| Septal E′, cm/s | 14.5 (13.0 - 16.0) | 9.0 (7.0 - 11.0) | <0.0001* | 7.0 (6.0 - 9.0) | 0.0003* |
| Septal A′, cm/s | 6.3 (5.9 - 7.0) | 5.0 (4.0 - 6.0) | 0.0002* | 5.0 (3.5 - 6.0) | 0.0303* |
| Isovolumic Relaxation Time, msec | 47.0 (38.0 - 52.0) | 58.0 (48.0 - 72.0) | 0.0003* | 76.5 (56.5 - 98.5) | 0.009* |
| Mitral Valve E/E′ | 5.2 (4.5 - 6.0) | 7.2 (6.0 - 9.5) | <0.0001* | 7.3 (6.0 - 8.9) | 0.033* |
| Speckle-Tracking Echocardiography | |||||
| Global DLSR, 1/s | 2.17 (1.84 - 2.68) | 2.08 (1.53 - 2.45) | 0.05 | 1.55 (1.26 - 2.08) | 0.05 |
| Basal Global DCSR, 1/s | 2.05 (1.54 - 2.78) | 1.44 (1.20 - 2.48) | 0.019* | 1.19 (1.13 - 1.68) | 0.023* |
| Mid Global DCSR, 1/s | 1.78 (1.42 - 2.10) | 1.68 (1.23 - 1.99) | 0.06 | 1.22 (1.09 - 1.74) | 0.17 |
| Apical Global DCSR, 1/s | 2.48 (1.73 - 2.79) | 2.34 (1.67 - 2.85) | 0.88 | 1.85 (1.29 - 2.52) | 0.44 |
| Apical Recoil, °/s | −49.8 (−18.9 - −75.5) | −80.9 (−49.2 - −107.2) | 0.0009* | −72.6 (−52.5 - −95.5) | 0.0008* |
| Basal Recoil, °/s | 39.4 (18.1 - 54.1) | 16.4 (7.3 - 41.2) | 0.70 | 15.9 (−3.3 - 38.3) | 0.17 |
| Indexed Diastolic Untwist Rate, °/s/cm | −11.3 (−7.8 - −16.4) | −15.4 (−11.5 - −22.4) | 0.003* | −14.9 (−8.3 - −15.4) | 0.0162* |
Presented as median (interquartile range). PH indicates pulmonary hypertension; S, systolic pulmonary vein flow; D, diastolic pulmonary vein flow; A, pulmonary vein flow during atrial contraction; E, early transmitral flow velocity; A, late transmitral flow velocity; PVAD; pulmonary vein A duration; MVAD, mitral valve A duration; RV, right ventricle; LV, left ventricle; E′, peak early diastolic myocardial annular velocity; A′, peak late diastolic myocardial annular velocity; DLSR, diastolic longitudinal strain rate; DCSR, diastolic circumferential strain rate. Severe PH patients are compared to their matched controls (not shown).
P<0.05.
1) LV Diastolic Measures
Patients with PH demonstrated the following: reduced pulmonary venous S and D velocities and increased A wave duration; reduced mitral E and inflow duration, and increased E deceleration time; reduced E’ in the septum and free-wall and A’ in the septum; increased mitral E/E’; increased isovolumic relaxation time; decreased basal DCSR and a trend towards reduced LV DLSR and mid DCSR. Apical recoil was increased (more negative) in PH, resulting in increased indexed diastolic untwist rate, despite no statistically significant difference in basal recoil. RV systole was prolonged.
Severe Pulmonary Hypertension
To further assess the impact of pulmonary hemodynamics on LV diastolic function, PH patients were separated into quartiles of severity, determined by the ratio of pulmonary-to-systemic mean arterial pressures (MPAP/MAP). Though cardiac index was not significantly different statistically between quartiles (P=0.59), median MPAP/MAP and PVRi for the most severe quartile were higher than each of the other three quartiles (P<0.0001 for both). Echocardiographic measures for this quartile are compared to their matched controls in Table 3. These patients demonstrated worsened diastolic dysfunction: further reduced pulmonary vein S and D, mitral E, E’, and septal E’; and further increased mitral E deceleration time, isovolumic relaxation time, eccentricity indices, and RV systolic prolongation. Apical recoil remained increased (more negative), as did indexed diastolic untwist rate.
Congenital Heart Disease
We further analyzed PH patients by presence of CHD. Those with CHD demonstrated increased pulmonary vein A velocity (0.17 [0.14-0.21] vs. 0.14 [0.10-0.18] m/s, P=0.0382) and decreased septal E’ (8.0 [7.0-10.0] vs. 10.0 [8.0-13.0] cm/s, P=0.0344), compared to those without CHD (respectively). There were no other statistically significant differences in diastolic measures or RV systolic duration (P>0.05 for all).
Shunting
We further analyzed PH patients by presence of a shunt. There were no statistically significant differences in LV diastolic measures or RV systolic duration between those with and those without a shunt (P>0.05 for all).
2) Relationships With LV Diastolic Measures
Invasive Hemodynamics
Invasive hemodynamics are related to LV echocardiographic diastolic measures in Table 4. Positive moderate correlations were seen with LV isovolumic relaxation time, and weak correlations with E deceleration time. Negative moderate and weak correlations were seen with mitral E, E/A, E’/A’, septal E’, DLSR, mid and apical DCSR. Pulmonary venous measures did not correlate with invasive hemodynamics (P>0.05 for all). LV E/E’ did not correlate with MPAP/MAP, PVRi, or left atrial pressure/PCWP (r=0.13, P=0.36). Similarly, invasively-measured left atrial pressure/PCWP did not correlate with predicted PCWP using Nagueh's original regression formula, when using either the lateral mitral E’ alone (r=0.09, P=0.51), or a combined average of lateral mitral E’ and septal E’ in the equation (r=0.2, P=0.15).20
Table 4.
Correlations Between Left Ventricular Diastolic Measures and Invasive Hemodynamics
| Left Ventricular Diastolic Variable | MPAP/MAP | PVRi | DPAP | TPG | DTPG | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | P Value | r | P Value | r | P Value | r | P Value | r | P Value | |
| Mitral Valve | ||||||||||
| E | −0.35 | 0.0125* | −0.52 | <0.0001* | −0.36 | 0.0098* | −0.42 | 0.0017* | −0.39 | 0.0046* |
| A | 0.26 | 0.08 | 0.10 | 0.50 | 0.22 | 0.12 | 0.22 | 0.12 | 0.25 | 0.08 |
| E/A | −0.37 | 0.0115* | −0.35 | 0.0138* | −0.36 | 0.0129* | −0.40 | 0.0037* | −0.41 | 0.0036* |
| E Deceleration Time | 0.14 | 0.34 | 0.30 | 0.0284* | 0.26 | 0.07 | 0.20 | 0.16 | 0.24 | 0.10 |
| Inflow Duration | −0.09 | 0.56 | 0.07 | 0.64 | −0.03 | 0.86 | −0.04 | 0.77 | −0.06 | 0.68 |
| Tissue Doppler Imaging | ||||||||||
| Lateral Mitral E′ | −0.16 | 0.29 | −0.18 | 0.22 | −0.11 | 0.45 | −0.14 | 0.34 | −0.09 | 0.54 |
| Lateral Mitral A′ | 0.19 | 0.19 | 0.17 | 0.25 | 0.29 | 0.0394* | 0.22 | 0.12 | 0.30 | 0.0354* |
| Lateral Mitral E′/A′ | −0.31 | 0.0314* | −0.27 | 0.056 | −0.29 | 0.0429* | −0.27 | 0.056 | −0.29 | 0.0431* |
| Septal E′ | −0.49 | 0.0002* | −0.50 | 0.0001* | −0.47 | 0.0004* | −0.49 | 0.0002* | −0.47 | 0.0004* |
| Septal A′ | 0.04 | 0.81 | 0.02 | 0.90 | 0.08 | 0.59 | 0.05 | 0.71 | 0.11 | 0.44 |
| Isovolumic Relaxation Time | 0.45 | 0.0013* | 0.58 | <0.0001* | 0.46 | 0.0007* | 0.47 | 0.0003* | 0.48 | 0.0004* |
| Mitral Valve E/E′ | −0.01 | 0.95 | −0.10 | 0.47 | −0.07 | 0.64 | −0.08 | 0.57 | −0.10 | 0.51 |
| Speckle-Tracking Echocardiography | ||||||||||
| Global DLSR | −0.37 | 0.0097* | −0.50 | 0.0002* | −0.42 | 0.0024* | −0.40 | 0.0034* | −0.44 | 0.0016* |
| Basal Global DCSR | −0.22 | 0.16 | −0.27 | 0.07 | −0.21 | 0.16 | −0.23 | 0.13 | −0.18 | 0.26 |
| Mid Global DCSR | −0.21 | 0.15 | −0.36 | 0.0107* | −0.24 | 0.10 | −0.26 | 0.07 | −0.26 | 0.08 |
| Apical Global DCSR | −0.23 | 0.14 | −0.38 | 0.0066* | −0.25 | 0.09 | −0.31 | 0.0332* | −0.27 | 0.07 |
| Apical Recoil | 0.08 | 0.61 | 0.11 | 0.50 | 0.08 | 0.64 | 0.03 | 0.83 | 0.07 | 0.66 |
| Basal Recoil | −0.05 | 0.77 | 0.04 | 0.82 | 0.04 | 0.83 | −0.03 | 0.84 | 0.003 | 0.98 |
| Indexed Diastolic Untwist Rate | 0.16 | 0.16 | 0.22 | 0.16 | 0.14 | 0.40 | 0.14 | 0.39 | 0.15 | 0.37 |
MPAP/MAP indicates mean pulmonary-to-systemic arterial pressure ratio; PVRi, indexed pulmonary vascular resistance; DPAP, diastolic pulmonary artery pressure; TPG, mean transpulmonary gradient; DTPG, diastolic transpulmonary gradient; E, early transmitral flow velocity; A, late transmittal flow velocity; E′, peak early diastolic myocardial annular velocity; A′, peak late diastolic myocardial annular velocity; DLSR, diastolic longitudinal strain rate; DCSR, diastolic circumferential strain rate.
P<0.05.
Septal Shift
Eccentricity index is related to LV echocardiographic diastolic measures in Table 5. Systolic and diastolic eccentricity index demonstrated moderate and weak positive correlations with pulmonary A duration, E deceleration time, isovolumic relaxation time, and E/E’; and moderate and weak negative correlations with pulmonary vein S and D, mitral E’, septal E’, A’, DLSR, basal and mid DCSR, and apical recoil. Additionally, weak negative correlations were seen between systolic eccentricity index with mitral E and E’/A’, and diastolic eccentricity index with mitral A’.
Table 5.
Correlations Between Left Ventricular Diastolic Measures, Septal Shift, and Prolonged Right Ventricular Systole
| Left Ventricular Diastolic Variable | End-Systolic Eccentricity Index |
End-Diastolic Eccentricity Index |
Prolonged Right Ventricular Systole |
|||
|---|---|---|---|---|---|---|
| r | P Value | r | P Value | r | P Value | |
| Pulmonary Vein | ||||||
| S | −0.46 | <0.0001* | −0.49 | <0.0001* | −0.47 | <0.0001* |
| D | −0.37 | 0.0004* | −0.30 | 0.0056* | −0.33 | 0.0012* |
| S/D Ratio | −0.09 | 0.42 | −0.14 | 0.20 | −0.10 | 0.37 |
| A | 0.06 | 0.59 | 0.03 | 0.77 | 0.10 | 0.37 |
| A Duration | 0.40 | 0.0004* | 0.33 | 0.0042* | 0.32 | 0.0053* |
| Mitral Valve | ||||||
| E | −0.24 | 0.0266* | −0.17 | 0.13 | −0.36 | 0.0002* |
| A | 0.09 | 0.43 | −0.04 | 0.70 | 0.13 | 0.20 |
| E/A | −0.15 | 0.17 | 0.001 | 0.99 | −0.23 | 0.0308* |
| E Deceleration Time | 0.34 | 0.0014* | 0.38 | 0.0002* | 0.32 | 0.0018* |
| Tissue Doppler Imaging | ||||||
| Lateral Mitral E′ | −0.45 | <0.0001* | −0.35 | 0.0010* | −0.42 | <0.0001* |
| Lateral Mitral A′ | −0.18 | 0.10 | −0.25 | 0.0229* | −0.05 | 0.67 |
| Lateral Mitral E′/A′ | −0.22 | 0.0494* | −0.12 | 0.29 | −0.37 | 0.0002* |
| Septal E′ | −0.58 | <0.0001* | −0.45 | <0.0001* | −0.55 | <0.0001* |
| Septal A′ | −0.30 | 0.0061* | −0.31 | 0.0039* | −0.18 | 0.07 |
| Isovolumic Relaxation Time | 0.44 | <0.0001* | 0.35 | 0.0008* | 0.36 | 0.0008* |
| Mitral Valve E/E′ | 0.34 | 0.0017* | 0.29 | 0.0080* | 0.27 | 0.0086* |
| Speckle-Tracking Echocardiography | ||||||
| Global DLSR | −0.32 | 0.0027* | −0.30 | 0.0052* | −0.14 | 0.24 |
| Basal Global DCSR | −0.35 | 0.0011* | −0.43 | <0.0001* | −0.20 | 0.08 |
| Mid Global DCSR | −0.22 | 0.0369* | −0.23 | 0.0314* | −0.07 | 0.53 |
| Apical Global DCSR | −011 | 0.34 | −0.16 | 0.13 | −0.13 | 0.26 |
| Apical Recoil | −0.24 | 0.0327* | −0.21 | 0.07 | −0.33 | 0.0056* |
| Basal Recoil | −0.18 | 0.11 | −0.10 | 0.40 | −0.12 | 0.32 |
| Indexed Diastolic Untwist Rate | −0.13 | 0.27 | −0.14 | 0.22 | −0.29 | 0.0133* |
S indicates systolic pulmonary vein flow; D, diastolic pulmonary vein flow; A, pulmonary vein flow during atrial contraction; E, early transmitral flow velocity; A, late transmitral flow velocity; E′, peak early diastolic myocardial annular velocity; A′, peak late diastolic myocardial annular velocity; DLSR, diastolic longitudinal strain rate; DCSR, diastolic circumferential strain rate.
P<0.05.
Prolonged RV Systole
Prolonged RV systole, which was strongly associated with MPAP/MAP (r=0.71, P<0.0001) and PVRi (r=0.70, P<0.0001), is related to echocardiographic LV diastolic measures in Table 5. Prolonged RV systole demonstrated moderate and weak negative correlations with pulmonary vein S and D, mitral E, E/A, E’, E’/A’, and septal E’; and weak positive correlations with pulmonary vein A duration, E deceleration time and isovolumic relaxation.
Reproducibility
Intraobserver intraclass correlation coefficients (95% confidence interval) were LV DLSR=0.88 (0.73-0.95), basal DCSR=0.73 (0.44-0.88), mid DCSR=0.86 (0.70-0.94), and apical DCSR=0.92 (0.81-0.97). Interobserver intraclass correlation coefficients (95% confidence interval) were LV DLSR=0.92 (0.82-0.97), basal DCSR=0.69 (0.44-0.84), mid DCSR=0.78 (0.59-0.89), and apical DCSR=0.68 (0.42-0.84).
Discussion
Using simultaneous echocardiography and catheterization, we evaluated LV diastolic function in pediatric PH and the relation to invasive hemodynamics, septal shift, and prolonged RV systole. The main findings of this study are the following: (1) children with PH have LV early diastolic dysfunction in a pattern most consistent with impaired relaxation, though features of reduced ventricular compliance are also present; (2) tissue Doppler and strain imaging data suggest myocardial dysfunction beyond changes in LV filling patterns; (3) LV diastolic dysfunction is associated with the severity of invasive hemodynamics, leftward septal shift, and prolonged RV systole. While we cannot directly relate our findings to mortality due to low event rate, the findings are important for a number of reasons. First, whereas the overwhelming majority of studies emphasize RV systolic dysfunction in PH, this is one of the first to highlight LV diastolic dysfunction in pediatric PH – certainly with simultaneous cardiopulmonary hemodynamics. Second, as invasive hemodynamics determine outcomes, the correlation with LV diastolic parameters is likely important. Third, in many cardiovascular conditions, diastolic dysfunction is a significant determinant of outcomes.21 Lastly, biventricular dysfunction likely confers worse outcomes.22
1) LV Diastolic Measures
Although systolic measures like LV EF and cardiac index were within normal ranges (even in severe PH), pediatric PH patients demonstrate LV diastolic dysfunction by multiple indices. The pattern is most consistent with impaired relaxation, with progressively reduced early diastolic filling and prolonged isovolumic relaxation and E deceleration times as PH worsens – a pattern also seen in adult PH.7-9, 23-27
Abnormal LV filling in PH is thought to arise predominantly from two etiologies: 1) diminished RV output due to increased afterload, resulting in reduced LV preload (series effect); 2) leftward septal shift directly interfering with LV filling (direct effect). Baker et al. elegantly demonstrated the two are interrelated and both responsible for impaired LV filling.28 Prolonged RV systolic duration, which causes leftward septal shift, inefficient RV contraction, and reduced RV filling time, likely worsens both mechanisms.
Though early filling and relaxation abnormalities predominated, increased pulmonary venous A duration in relation to mitral A duration and mitral E/E’ suggest decreased LV compliance. We intentionally selected patients with normal left-sided filling pressures to avoid confounding LV adaptations in PH, so changes consistent with reduced compliance may precede an occult rise in end-diastolic pressure. LV fibrosis seen in animals with RV hypertension,29 and possibly in adults with PH,30 likely affects ventricular relaxation and compliance. Thus, as in pediatric cardiomyopathy, our data suggest that there can be a mixed picture of early diastolic dysfunction/impaired relaxation and decreased compliance in pediatric PH.31 Notably, similar to tricuspid E/E’ and RV end-diastolic pressure, mitral E/E’ may not accurately reflect LV filling pressures in pediatric PH.32
PH patients demonstrated diastolic myocardial dysfunction, beyond altered filling patterns alone, with free-wall and septal tissue Doppler changes and reduced early diastolic strain rate. Progressively increased isovolumic relaxation time in PH corroborates impaired LV relaxation prior to mitral valve opening. Similar diastolic myocardial alterations have been found in adult PH by echocardiography and recently by cardiac MRI, with some relation to outcomes.33, 34
Although our PH patients were younger with less chronic/severe PH than published adult populations, numerous parameters of LV diastolic dysfunction were already present. It is unclear if such changes are reversible or improve with improving right ventricular function during therapy, and thus warrant further investigation. Notably, no patient demonstrated systemic hypoxemia, which alone can impair ventricular function.
Temporal disparities between RV and LV contraction and relaxation also impact diastolic function in PH. We previously found that RV systolic duration increases at the expense of diastole with increasing PH severity – quantified by the systolic-to-diastolic ratio.35 Here, RV systole was prolonged in PH, by a measure which included isovolumic relaxation for a few reasons. First, there may be post-systolic myocardial shortening after pulmonary valve closure that does not contribute to RV ejection, but may disrupt LV early diastolic function.11 Second, in PH patients, RV pressure during isovolumic relaxation may be higher than LV pressure (which may be in isovolumic relaxation or mitral inflow), and may still exert septal shift and direct diastolic interaction. MRI studies demonstrate prolonged RV systole in PH, corresponding with leftward septal shift and impaired LV filling,11 and that peak LV filling rate occurs after maximal septal bowing.7 That mitral inflow may begin while the RV is still in isovolumic relaxation with elevated pressures, or even prior to the completion of RV systole, highlights the confusion in easily defining systole and diastole in PH, which may occur at different times for each ventricle. This likely contributes to adverse ventricular-ventricular interactions.
Not all changes observed were consistent with reduced diastolic function. Apical recoil was increased in PH, enough to overcome reduced basal recoil and yield an increase in the diastolic untwist rate. Apical diastolic myocardial mechanics may potentially compensate for reduced basal mechanics or altered diastolic filling in PH.
CHD and Shunts
The presence of CHD or a shunt did not greatly alter echocardiographic LV diastolic measures. In contrast, we previously found different RV diastolic perturbations based on the presence of a shunt.32 In any event, the presence of a shunt in PH remains clinically relevant.36-38
2) Relationships with LV Diastolic Measures
Numerous, albeit modest, correlations between invasive hemodynamics and echocardiographic LV diastolic measures support progressive diastolic dysfunction with worsening PH hemodynamics, and highlight the relationship between pulmonary impedance and LV filling. Similarly, leftward septal shift and prolonged RV systole were related to most measures of impaired LV relaxation, demonstrating pathologic early diastolic ventricular-ventricular interactions. We found similar modest correlations between RV diastolic measures and cardiopulmonary hemodynamics,32 where diastolic dysfunction has clear clinical implications.2-4 Collectively, these suggest that worsening PH hemodynamics, with increased leftward septal shift and prolonged RV systole, all contribute to LV early diastolic dysfunction, which may ultimately impact outcomes.
Limitations
The cross-sectional nature of this study and the low number of deaths precluded analysis of the prognostic significance of the diastolic parameters investigated. The heterogeneity of our study population (PH etiology, CHD, active shunts) may limit our ability to detect consistent diastolic changes, but accurately reflects the pediatric PH population. Selection bias could have been introduced because some controls presented to a cardiologist for evaluation, or that PH patients presented to a tertiary care center, though hemodynamic data suggests a mixture of well- and poorly-controlled PH. We chose to stratify PH severity by MPAP/MAP, given this reflects the degree of PH in pediatrics better than other pressure measures alone. However, other invasive measures could have been used to stratify patients as well. Due to the large number of comparisons and correlations performed on a modest number of patients, the possibility of type I error exists.
The altered measures presented likely reflect pathologic ventricular interdependence, related in part to geometric changes, but may not represent intrinsic LV dysfunction. However, as it would have incurred unnecessary risk to patients, the LV was not accessed during the clinically-indicated right heart catheterization and so the relatively load-independent measure tau, and invasive measures of LV compliance, were not assessed. Over-/underwedged PCWP can affect estimation of left atrial pressure, though PCWP is standard of care to estimate mean left atrial pressure during right heart catheterization, and we preferentially used left atrial pressures when obtained. We attempted to have consistent catheterization protocols between both sites, and although fluid-filled catheters were used at both locations, SickKids used Millar catheters for RV pressure assessment. Oxygen consumption was measured by mass spectrometry at SickKids and estimated by the LaFarge table at CHCO, though this does not affect pressure measurements. We used institutionally-matched controls given altitude differences between Denver and Toronto, which can affect PVRi and MPAP. PH patients were all under general anesthesia, though we did not dictate an anesthesia regimen or duration, or the timing of catheterization during anesthesia. Subtle differences in anesthetics and ventilation could affect hemodynamics and thus potentially diastolic measures, possibly limiting comparisons with unsedated controls; it would not limit comparisons between diastolic measures and hemodynamics.
Conclusion
Pediatric PH patients exhibit LV diastolic dysfunction most consistent with impaired relaxation with reduced myocardial deformation, related to invasive hemodynamics, leftward septal shift, and prolonged RV systole. Though focus is placed on RV systolic dysfunction in PH, our results highlight that pediatric PH involves biventricular systolic and diastolic dysfunction, which should stimulate further research into the importance of adverse ventricular interdependence and LV diastolic dysfunction in pediatric PH.
Clinical Perspective.
We evaluated left ventricular diastolic function in children and young adults with pulmonary hypertension by echocardiography, performed simultaneously with cardiac catheterization to allow direct comparisons between invasive hemodynamics and echocardiographic measures. We included unique, previously understudied pulmonary hypertension populations, including pediatric patients, those with congenital heart disease, and those with intra-/extracardiac shunts. We documented left ventricular diastolic dysfunction in a pattern most consistent with impaired relaxation, though some aspects of reduced ventricular compliance were also present. Changes were more pronounced in those with severe pulmonary hypertension. Beyond changes in left ventricular filling patterns, tissue Doppler and diastolic strain imaging data suggest left ventricular myocardial dysfunction in pulmonary hypertension. There were minimal differences in diastolic measures between those with and without congenital heart disease, or between those with and without a shunt. Echocardiographic measures of diastolic function were associated with invasive hemodynamics, as well as leftward septal shift and prolonged right ventricular systole, which likely play an important role in left ventricular diastolic dysfunction in pulmonary hypertension. Though much focus is placed on right ventricular systolic dysfunction in pulmonary hypertension, our results show that through ventricular interdependence, pediatric pulmonary hypertension patients have evidence of left ventricular diastolic dysfunction. Future research is needed to determine the clinical importance of left ventricular diastolic dysfunction in this population, and the reversibility of such changes.
Acknowledgments
Sources of Funding
This research was supported in part by The Frederick and Margaret L Weyerhaeuser Foundation, The Jayden de Luca Foundation, the Canadian Institutes of Health Research, and NIH grants R01HL114753, U01HL121518, and UL1TR000154.
Footnotes
Disclosures
None.
References
- 1.D'Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT, Levy PS, Pietra GG, Reid LM, Reeves JT, Rich S, Vreim CE, Williams GW, Wu M. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115:343–9. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 2.Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, Dupuis J, Long CS, Rubin LJ, Smart FW, Suzuki YJ, Gladwin M, Denholm EM, Gail DB, National Heart L, Blood Institute Working Group on C Molecular Mechanisms of Right Heart F. Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation. 2006;114:1883–91. doi: 10.1161/CIRCULATIONAHA.106.632208. [DOI] [PubMed] [Google Scholar]
- 3.Chin KM, Kim NH, Rubin LJ. The right ventricle in pulmonary hypertension. Coron Artery Dis. 2005;16:13–8. doi: 10.1097/00019501-200502000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Bogaard HJ, Abe K, Vonk Noordegraaf A, Voelkel NF. The right ventricle under pressure: cellular and molecular mechanisms of right-heart failure in pulmonary hypertension. Chest. 2009;135:794–804. doi: 10.1378/chest.08-0492. [DOI] [PubMed] [Google Scholar]
- 5.Hardegree EL, Sachdev A, Fenstad ER, Villarraga HR, Frantz RP, McGoon MD, Oh JK, Ammash NM, Connolly HM, Eidem BW, Pellikka PA, Kane GC. Impaired left ventricular mechanics in pulmonary arterial hypertension: identification of a cohort at high risk. Circ Heart Fail. 2013;6:748–55. doi: 10.1161/CIRCHEARTFAILURE.112.000098. [DOI] [PubMed] [Google Scholar]
- 6.Burkett DA, Slorach C, Patel SS, Redington AN, Ivy DD, Mertens L, Younoszai AK, Friedberg MK. Left Ventricular Myocardial Function in Children With Pulmonary Hypertension: Relation to Right Ventricular Performance and Hemodynamics. Circ Cardiovasc Imaging. 2015;8:e003260. doi: 10.1161/CIRCIMAGING.115.003260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gan C, Lankhaar JW, Marcus JT, Westerhof N, Marques KM, Bronzwaer JG, Boonstra A, Postmus PE, Vonk-Noordegraaf A. Impaired left ventricular filling due to right-to-left ventricular interaction in patients with pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol. 2006;290:H1528–33. doi: 10.1152/ajpheart.01031.2005. [DOI] [PubMed] [Google Scholar]
- 8.Louie EK, Rich S, Brundage BH. Doppler echocardiographic assessment of impaired left ventricular filling in patients with right ventricular pressure overload due to primary pulmonary hypertension. J Am Coll Cardiol. 1986;8:1298–306. doi: 10.1016/s0735-1097(86)80300-x. [DOI] [PubMed] [Google Scholar]
- 9.Moustapha A, Kaushik V, Diaz S, Kang SH, Barasch E. Echocardiographic evaluation of left- ventricular diastolic function in patients with chronic pulmonary hypertension. Cardiology. 2001;95:96–100. doi: 10.1159/000047353. [DOI] [PubMed] [Google Scholar]
- 10.Tonelli AR, Plana JC, Heresi GA, Dweik RA. Prevalence and prognostic value of left ventricular diastolic dysfunction in idiopathic and heritable pulmonary arterial hypertension. Chest. 2012;141:1457–65. doi: 10.1378/chest.11-1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marcus JT, Gan CT, Zwanenburg JJ, Boonstra A, Allaart CP, Gotte MJ, Vonk-Noordegraaf A. Interventricular mechanical asynchrony in pulmonary arterial hypertension: left-to-right delay in peak shortening is related to right ventricular overload and left ventricular underfilling. J Am Coll Cardiol. 2008;51:750–7. doi: 10.1016/j.jacc.2007.10.041. [DOI] [PubMed] [Google Scholar]
- 12.Vonk-Noordegraaf A, Marcus JT, Gan CT, Boonstra A, Postmus PE. Interventricular mechanical asynchrony due to right ventricular pressure overload in pulmonary hypertension plays an important role in impaired left ventricular filling. Chest. 2005;128:628S–630S. doi: 10.1378/chest.128.6_suppl.628S. [DOI] [PubMed] [Google Scholar]
- 13.Marcus JT, Vonk Noordegraaf A, Roeleveld RJ, Postmus PE, Heethaar RM, Van Rossum AC, Boonstra A. Impaired left ventricular filling due to right ventricular pressure overload in primary pulmonary hypertension: noninvasive monitoring using MRI. Chest. 2001;119:1761–5. doi: 10.1378/chest.119.6.1761. [DOI] [PubMed] [Google Scholar]
- 14.Stojnic BB, Brecker SJ, Xiao HB, Helmy SM, Mbaissouroum M, Gibson DG. Left ventricular filling characteristics in pulmonary hypertension: a new mode of ventricular interaction. Br Heart J. 1992;68:16–20. doi: 10.1136/hrt.68.7.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS, Gomez-Sanchez MA, Jondeau G, Klepetko W, Opitz C, Peacock A, Rubin L, Zellweger M, Simonneau G. Guidelines ESCCfP. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J. 2009;30:2493–537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 16.Lai WW, Geva T, Shirali GS, Frommelt PC, Humes RA, Brook MM, Pignatelli RH, Rychik J, Task Force of the Pediatric Council of the American Society of E, Pediatric Council of the American Society of E. Guidelines and standards for performance of a pediatric echocardiogram: a report from the Task Force of the Pediatric Council of the American Society of Echocardiography. J Am Soc Echocardiogr. 2006;19:1413–30. doi: 10.1016/j.echo.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Ryan T, Petrovic O, Dillon JC, Feigenbaum H, Conley MJ, Armstrong WF. An echocardiographic index for separation of right ventricular volume and pressure overload. J Am Coll Cardiol. 1985;5:918–27. doi: 10.1016/s0735-1097(85)80433-2. [DOI] [PubMed] [Google Scholar]
- 18.Voigt JU, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R, Pedri S, Ito Y, Abe Y, Metz S, Song JH, Hamilton J, Sengupta PP, Kolias TJ, d'Hooge J, Aurigemma GP, Thomas JD, Badano LP. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. 2015;16:1–11. doi: 10.1093/ehjci/jeu184. [DOI] [PubMed] [Google Scholar]
- 19.Biner S, Rafique AM, Ray I, Cuk O, Siegel RJ, Tolstrup K. Aortopathy is prevalent in relatives of bicuspid aortic valve patients. J Am Coll Cardiol. 2009;53:2288–95. doi: 10.1016/j.jacc.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quinones MA. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30:1527–33. doi: 10.1016/s0735-1097(97)00344-6. [DOI] [PubMed] [Google Scholar]
- 21.McMahon CJ, Nagueh SF, Pignatelli RH, Denfield SW, Dreyer WJ, Price JF, Clunie S, Bezold LI, Hays AL, Towbin JA, Eidem BW. Characterization of left ventricular diastolic function by tissue Doppler imaging and clinical status in children with hypertrophic cardiomyopathy. Circulation. 2004;109:1756–62. doi: 10.1161/01.CIR.0000124723.16433.31. [DOI] [PubMed] [Google Scholar]
- 22.Le Tourneau T, Deswarte G, Lamblin N, Foucher-Hossein C, Fayad G, Richardson M, Polge AS, Vannesson C, Topilsky Y, Juthier F, Trochu JN, Enriquez-Sarano M, Bauters C. Right ventricular systolic function in organic mitral regurgitation: impact of biventricular impairment. Circulation. 2013;127:1597–608. doi: 10.1161/CIRCULATIONAHA.112.000999. [DOI] [PubMed] [Google Scholar]
- 23.Mahmud E, Raisinghani A, Hassankhani A, Sadeghi HM, Strachan GM, Auger W, DeMaria AN, Blanchard DG. Correlation of left ventricular diastolic filling characteristics with right ventricular overload and pulmonary artery pressure in chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol. 2002;40:318–24. doi: 10.1016/s0735-1097(02)01959-9. [DOI] [PubMed] [Google Scholar]
- 24.Gurudevan SV, Malouf PJ, Auger WR, Waltman TJ, Madani M, Raisinghani AB, DeMaria AN, Blanchard DG. Abnormal left ventricular diastolic filling in chronic thromboembolic pulmonary hypertension: true diastolic dysfunction or left ventricular underfilling? J Am Coll Cardiol. 2007;49:1334–9. doi: 10.1016/j.jacc.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 25.Dittrich HC, Chow LC, Nicod PH. Early improvement in left ventricular diastolic function after relief of chronic right ventricular pressure overload. Circulation. 1989;80:823–30. doi: 10.1161/01.cir.80.4.823. [DOI] [PubMed] [Google Scholar]
- 26.Menzel T, Wagner S, Kramm T, Mohr-Kahaly S, Mayer E, Braeuninger S, Meyer J. Pathophysiology of impaired right and left ventricular function in chronic embolic pulmonary hypertension: changes after pulmonary thromboendarterectomy. Chest. 2000;118:897–903. doi: 10.1378/chest.118.4.897. [DOI] [PubMed] [Google Scholar]
- 27.Boussuges A, Pinet C, Molenat F, Burnet H, Ambrosi P, Badier M, Sainty JM, Orehek J. Left atrial and ventricular filling in chronic obstructive pulmonary disease. An echocardiographic and Doppler study. Am J Respir Crit Care Med. 2000;162:670–5. doi: 10.1164/ajrccm.162.2.9908056. [DOI] [PubMed] [Google Scholar]
- 28.Baker AE, Dani R, Smith ER, Tyberg JV, Belenkie I. Quantitative assessment of independent contributions of pericardium and septum to direct ventricular interaction. Am J Physiol. 1998;275:H476–83. doi: 10.1152/ajpheart.1998.275.2.H476. [DOI] [PubMed] [Google Scholar]
- 29.Friedberg MK, Cho MY, Li J, Assad RS, Sun M, Rohailla S, Honjo O, Apitz C, Redington AN. Adverse biventricular remodeling in isolated right ventricular hypertension is mediated by increased transforming growth factor-beta1 signaling and is abrogated by angiotensin receptor blockade. Am J Respir Cell Mol Biol. 2013;49:1019–28. doi: 10.1165/rcmb.2013-0149OC. [DOI] [PubMed] [Google Scholar]
- 30.Manders E, Bogaard HJ, Handoko ML, van de Veerdonk MC, Keogh A, Westerhof N, Stienen GJ, Dos Remedios CG, Humbert M, Dorfmuller P, Fadel E, Guignabert C, van der Velden J, Vonk-Noordegraaf A, de Man FS, Ottenheijm CA. Contractile dysfunction of left ventricular cardiomyocytes in patients with pulmonary arterial hypertension. J Am Coll Cardiol. 2014;64:28–37. doi: 10.1016/j.jacc.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 31.Dragulescu A, Mertens L, Friedberg MK. Interpretation of left ventricular diastolic dysfunction in children with cardiomyopathy by echocardiography: problems and limitations. Circ Cardiovasc Imaging. 2013;6:254–61. doi: 10.1161/CIRCIMAGING.112.000175. [DOI] [PubMed] [Google Scholar]
- 32.Okumura K, Slorach C, Mroczek D, Dragulescu A, Mertens L, Redington AN, Friedberg MK. Right ventricular diastolic performance in children with pulmonary arterial hypertension associated with congenital heart disease: correlation of echocardiographic parameters with invasive reference standards by high-fidelity micromanometer catheter. Circ Cardiovasc Imaging. 2014;7:491–501. doi: 10.1161/CIRCIMAGING.113.001071. [DOI] [PubMed] [Google Scholar]
- 33.Huez S, Vachiery JL, Unger P, Brimioulle S, Naeije R. Tissue Doppler imaging evaluation of cardiac adaptation to severe pulmonary hypertension. Am J Cardiol. 2007;100:1473–8. doi: 10.1016/j.amjcard.2007.06.047. [DOI] [PubMed] [Google Scholar]
- 34.Knight DS, Steeden JA, Moledina S, Jones A, Coghlan JG, Muthurangu V. Left ventricular diastolic dysfunction in pulmonary hypertension predicts functional capacity and clinical worsening: a tissue phase mapping study. J Cardiovasc Magn Reson. 2015;17:116. doi: 10.1186/s12968-015-0220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alkon J, Humpl T, Manlhiot C, McCrindle BW, Reyes JT, Friedberg MK. Usefulness of the right ventricular systolic to diastolic duration ratio to predict functional capacity and survival in children with pulmonary arterial hypertension. Am J Cardiol. 2010;106:430–6. doi: 10.1016/j.amjcard.2010.03.048. [DOI] [PubMed] [Google Scholar]
- 36.Barst RJ, Ivy DD, Foreman AJ, McGoon MD, Rosenzweig EB. Four- and seven-year outcomes of patients with congenital heart disease-associated pulmonary arterial hypertension (from the REVEAL Registry). Am J Cardiol. 2014;113:147–55. doi: 10.1016/j.amjcard.2013.09.032. [DOI] [PubMed] [Google Scholar]
- 37.Bui MT, Grollmus O, Ly M, Mandache A, Fadel E, Decante B, Serraf A. Surgical palliation of primary pulmonary arterial hypertension by a unidirectional valved Potts anastomosis in an animal model. J Thorac Cardiovasc Surg. 2011;142:1223–8. doi: 10.1016/j.jtcvs.2010.10.060. [DOI] [PubMed] [Google Scholar]
- 38.Baruteau AE, Serraf A, Levy M, Petit J, Bonnet D, Jais X, Vouhe P, Simonneau G, Belli E, Humbert M. Potts shunt in children with idiopathic pulmonary arterial hypertension: long-term results. Ann Thorac Surg. 2012;94:817–24. doi: 10.1016/j.athoracsur.2012.03.099. [DOI] [PubMed] [Google Scholar]