Summary
Although remote ischemic pre-conditioning (RIPC) reduced infarct size in animal experiments and proof-of-concept clinical trials, recent phase III trials failed to confirm cardioprotection during cardiac surgery. Here, we characterized the kinetic properties of humoral factors that are released after RIPC, as well as the signal transduction pathways that were responsible for cardioprotection in an ex vivo model of global ischemia reperfusion injury. Venous blood from 20 healthy volunteers was collected at baseline and 5 min, 30 min, 1 h, 6 h, and daily from 1 to 7 days after RIPC (3 × 5/5 min upper-limb ischemia/reperfusion). Plasma-dialysates (cut-off: 12 to 14 kDa; dilution: 1:20) were infused into Langendorff-perfused mouse hearts subjected to 20/120 min global ischemia/reperfusion. Infarct size and phosphorylation of signal transducer and activator of transcription (STAT)3, STAT5, extracellular-regulated kinase 1/2 and protein kinase B were determined. In a subgroup of plasma-dialysates, an inhibitor of STAT3 (Stattic) was used in mouse hearts. Perfusion with baseline-dialysate resulted in an infarct size of 39% of ventricular mass (interquartile range: 36% to 42%). Perfusion with dialysates obtained 5 min to 6 days after RIPC significantly reduced infarct size by ∼50% and increased STAT3 phosphorylation beyond that with baseline-dialysate. Inhibition of STAT3 abrogated these effects. These results suggest that RIPC induces the release of cardioprotective, dialyzable factor(s) within 5 min, and that circulate for up to 6 days. STAT3 is activated in murine myocardium by RIPC-induced human humoral factors and is causally involved in cardioprotection.
Key Words: cardioprotection, human, humoral factor, kinetics, remote ischemic pre-conditioning, signaling
Abbreviations and Acronyms: AKT, protein kinase B; ERK, extracellular-regulated kinase; IQR, interquartile range; LV+RV, left and right ventricular; LVDP, left ventricular developed pressure; RIC, remote ischemic conditioning; RIPC, remote ischemic pre-conditioning; SAFE, survival activating factor enhancement; STAT, signal transducer and activator of transcription; TTC, 2,3,5-triphenyltetrazolium chloride
Visual Abstract
Highlights
-
•
Pre-clinical and early phase clinical studies with remote ischemic preconditioning (RIPC) appeared promising; however, RIPC was not effective in phase III clinical trials.
-
•
To improve the translation of RIPC into clinical practice, the kinetic properties and functional effects of humoral factors released after RIPC in humans were characterized ex vivo.
-
•
Venous blood from 20 healthy volunteers was collected at baseline and 5 min, 30 min, 1 h, 6 h and daily from 1 to 7 days after RIPC. Plasma dialysates were infused into Langendorff-perfused mouse hearts subjected to 20/120 min global ischemia/reperfusion.
-
•
Perfusion with dialysates obtained 5 min to 6 days after RIPC significantly reduced infarct size by ∼50% when compared to perfusion with dialysates obtained at baseline prior to RIPC, and increased STAT3 phosphorylation beyond values obtained with baseline-dialysate.
Remote ischemic conditioning (RIC) with transient limb ischemia/reperfusion is a noninvasive method to protect the myocardium and other parenchymal organs from ischemia/reperfusion injury. Cardioprotection is achieved by RIC before (pre-conditioning; RIPC), during (per-conditioning) or after myocardial ischemia (post-conditioning) (1). RIC has been demonstrated in many experimental studies and also attenuates myocardial ischemia/reperfusion injury in patients undergoing elective interventional (2) or surgical coronary revascularization 3, 4, 5 as well as in patients with acute myocardial infarction 6, 7, 8, 9, 10. The efficacy of RIC was evidenced by reduced cardiac biomarker release 2, 3, 4, 5, 9 and reduced infarct size on magnetic resonance imaging 6, 8, 10; some smaller studies also reported improved short- 5, 8 and long-term clinical outcome 2, 4, 7. However, the recent large-scaled, randomized ERICCA (Effect of Remote Ischaemic Preconditioning on Clinical Outcomes in CABG Surgery) trial and RIPHeart (Remote Ischaemic Preconditioning for Heart Surgery) study in patients undergoing cardiac surgery and ischemic cardioplegic arrest failed to confirm reduced biomarker release and improved clinical outcome with RIPC 11, 12. Reasons for the failure of these trials to confirm protection by RIPC have been discussed in detail and related to confounding variables 13, 14, 15, notably the use of propofol in the majority of all patients in both trials, which might have abrogated the cardioprotective effect (16).
For more successful translation of experimental animal studies and smaller proof-of-concept trials to clinical reality a better understanding of RIC’s signal transduction is mandatory. In particular, the transfer signal from the remote peripheral organ where the RIC maneuver is performed to the heart is still enigmatic. Both neuronal and humoral transfer as well as a neurohumoral interaction have been proposed. Humoral transfer by nitrite (17), stromal cell–derived factor-1α (18), and microRNA-144 (19) has been reported. More systematic proteome analyses of plasma taken after RIPC have not yet identified a specific protein 20, 21.
We therefore used another strategy to identify the potential humoral transfer factor(s) by characterizing their properties in kinetic terms and by the signal activation which they elicit in the heart to effect cardioprotection. Prior studies have reported a time delay between the RIPC stimulus and the injurious event from 5 to 10 min 22, 23 to more than 24 h 24, 25, 26, 27, suggesting that RIPC is a fast acting as well as a long-lasting phenomenon. Supporting the notion of a long-lasting effect, flow-mediated forearm vasodilation in patients with reperfused acute myocardial infarction was improved by RIPC for 1 week (28). However, this particular study could not distinguish whether there was a long-lasting circulation of transfer factor(s) or a long-lasting effect in the target organ. We have therefore obtained plasma from healthy volunteers at different time points before and after a RIPC maneuver and tested its cardioprotective effect in a Langendorff-perfused mouse heart used as bioassay. Cardioprotection relies on a complex signal transduction of triggers, mediators, and effectors, which also vary between different species (29). The cytosolic signal transduction within the cardiomyocyte includes the reperfusion injury salvage kinase (SAFE) and the survival activating factor enhancement systems (30), and both these systems are also recruited for cardioprotection in mice 31, 32, 33, 34. We therefore went on to identify the signal transduction, which was activated by the transfer of a human plasma-dialysate to achieve infarct size reduction in the bioassay mouse heart.
Methods
Volunteer recruitment and blood sampling
The study was approved by the Institutional Review Board (No.14-5995-BO) and conforms to the principles of the Declaration of Helsinki. Written informed consent was obtained from all volunteers. Twenty nonsmoking healthy volunteers (10 men/10 women) were recruited between October 2014 and July 2015. They had no history of disease or any medication other than oral contraceptives in women.
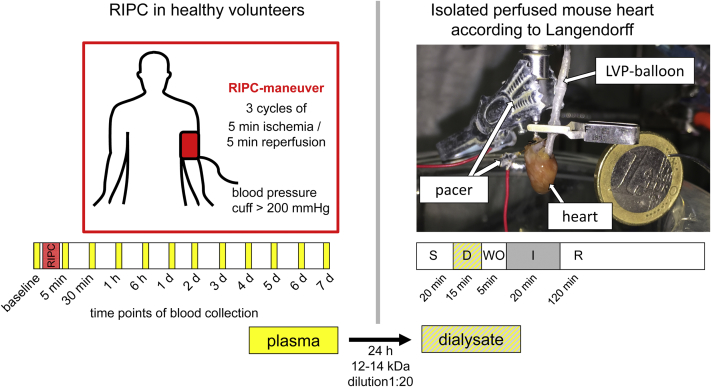
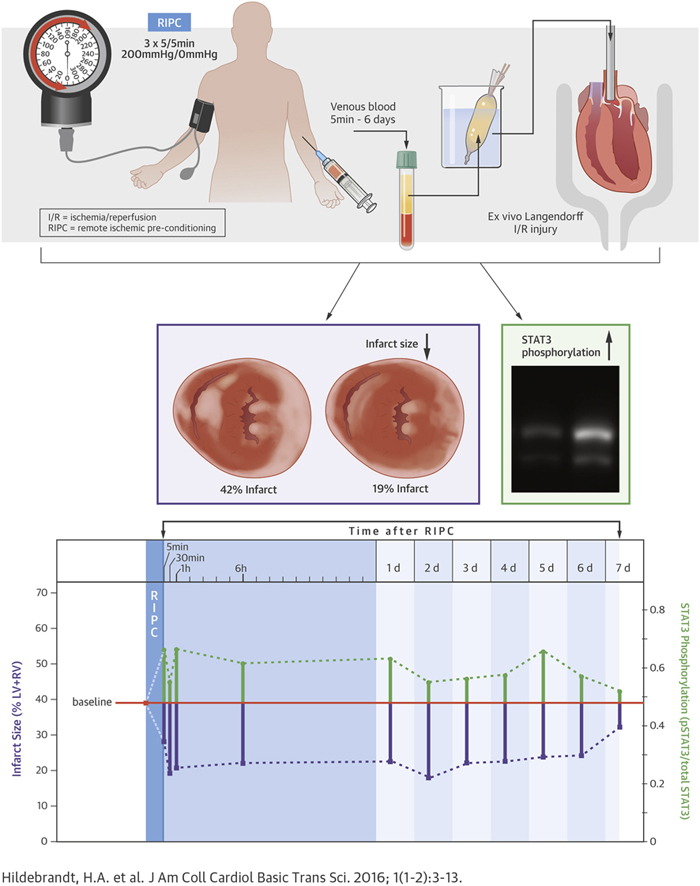
RIPC was performed between 8 and 10 ante meridiem independently from the day of the week. The RIPC-maneuver consisted of 3 cycles of 5 min upper-limb ischemia/inflation of a blood pressure cuff to 200 mm Hg and reperfusion/deflation for 5 min. Venous blood samples from the contralateral arm were obtained before RIPC (baseline) and at 5 min, 30 min, 1 h, 6 h, 1 day, and daily thereafter until 7 days at the same time of day (Figure 1). The blood was collected in heparinized tubes (S-Monovette 9 ml LH, SARSTEDT AG & Co., Nümbrecht, Germany) and immediately centrifuged (Multifuge 3SR, Heraeus, Hanau, Germany) at 800 g for 20 min at 4°C. The plasma was separated and stored at –80°C until use.
Figure 1.
Experimental Setup for the Identification of Humoral Transfer Factor(s) From Healthy Volunteers Undergoing RIPC to Isolated Langendorff-Perfused Mouse Hearts
Plasma from 20 volunteers was obtained at 12 pre-defined time points and dialyzed with a dilution of 1:20 for 24 h, using a cutoff of 12 to 14 kDa. After 20 min of stabilization, isolated mouse hearts were perfused with dialysates of human plasma for 15 min, followed by 5 min of wash-out by perfusion with Krebs-Henseleit buffer, before undergoing 20 min of global ischemia. After 120 min of reperfusion, infarct size was determined with 2,3,5-triphenyltetrazolium chloride (TTC) staining, and activation of proteins was analyzed with Western blot analysis. D = dialysate; d = day; I = ischemia; LVP = left ventricular pressure; R = reperfusion; RIPC = remote ischemic pre-conditioning; S = stabilization; WO = wash-out.
Isolated mouse heart perfusion
To assess the cardioprotective effect of humoral factor(s) released after RIPC from humans, a modified protocol of isolated mouse heart perfusion was used (17). Mice (C57Bl6/J; age: 8 to 12 weeks; weight: 20 to 30 g) were purchased from Harlan Laboratories, Inc. (Horst, the Netherlands) and from Charles River Laboratories (Sulzfeld, Germany). The protocol was approved by the governmental Animal Care and Use Committee and conforms to the “Position of the American Heart Association on Research Animal Use”, adopted by the American Heart Association on November 11, 1984. After cervical dislocation, 200 IU of heparin (Heparin-Natrium-25000-ratiopharm, Ratiopharm GmbH, Ulm, Germany) was intraperitoneally injected, and hearts were excised. Within 2 min hearts were cannulated (mouse heart cannula, Hugo Sachs Elektronik, March-Hugstetten, Germany) under a stereomicroscope (LS 6000IC, Beckman Coulter, Krefeld, Germany) through the aorta in cold 0.9% NaCl solution and mounted on a Langendorff apparatus. At a constant pressure of 100 mm Hg, hearts were perfused with modified Krebs-Henseleit buffer, containing (in mmol/l) 118 NaCl (AppliChem GmbH, Darmstadt, Germany), 4.7 KCl, 1.64 MgSO4 × 7 H2O (Merck, Darmstadt, Germany), 1.18 KH2PO4 (Merck, Darmstadt, Germany), 2.00 Na-pyruvate, 5.55 glucose, 24.88 NaHCO3 (AppliChem GmbH, Darmstadt, Germany), and 2.5 mmol/l CaCl2. The perfusate was filtered through a 0.45 μm filter (Filter Type HWAP, Merck, Darmstadt, Germany), oxygenated and equilibrated to 37°C before use. A water-filled balloon made out of saran wrap (Toppits, Cofresco Frischhalteprodukte GmbH & Co. KG, Minden, Germany) was inserted through the mitral valve into the left ventricular cavity and connected to a pressure transducer (DPT-6000, Pvb Codan, Forstinning, Germany) to allow continuous monitoring of left ventricular pressure. The left ventricular end-diastolic pressure was initially set to 5 to 15 mm Hg, and hearts were paced at a constant rate of 500 beats/min (DPT-6000, Pvb Codan, Forstinning, Germany) through a metallic clamp connected to the cannula and a metallic needle inserted into the right auricle (Figure 1).
Plasma (4 ml) was defrozen and centrifuged at 4.500 g for 10 min at 4°C. The supernatant was placed in a 12 to 14 kDa dialysis tubing (SpectraPor, Spectrum Europe B.V., Breda, the Netherlands) and dialyzed for 24 h at 4°C against a 20-fold volume of modified Krebs-Henseleit buffer. Dialysates were adjusted to 2.5 mmol/l CaCl2 and 24.88 mmol/l NaHCO3, filtered (5 μm Chromafil Xtra PES-500/25, Macherey-Nagel GmbH & Co. KG, Düren, Germany), oxygenated and equilibrated to 37°C before use (Figure 1).
After a stabilization period of 20 min, hearts were perfused with dialysate for 15 min, followed by 5 min of wash-out with Krebs-Henseleit buffer to avoid adherence of proteins from a stagnant dialysate to the Langendorff apparatus or the coronary circulation during its occlusion. The hearts were subjected to 20 min global zero-flow ischemia and 120 min reperfusion with Krebs-Henseleit buffer. Peak and end-diastolic left ventricular pressure and coronary flow (TS410 Ultraschall Flowmeter, Transonic Systems Inc., Ithaca, New York) were continuously recorded (LabChart 8, LabChart, ADInstruments Pty Ltd, Oxford, United Kingdom). Hearts with a left ventricular developed pressure (LVDP) <50 mm Hg and hearts with a coronary flow <1 ml/min or >5 ml/min after the stabilization period were discarded. The median LVDP and coronary flow of all hearts perfused with dialysates of human plasma obtained at the same time point after the RIPC maneuver were calculated for every minute. LVDP and coronary flow of hearts perfused with baseline-dialysates and of hearts perfused with post-RIPC-dialysates were compared at the end of stabilization (20 min), during perfusion with dialysate (35 min), just before ischemia (40 min), at the end of ischemia (60 min), at 15 min and 30 min of reperfusion (75 min and 90 min of the protocol, respectively) (Supplemental Figures 1 and 2).
Infarct staining
After 120 min reperfusion, hearts were frozen at –20°C for 30 min and cut into 1 mm thick slices (approximately 5 slices per heart). The slices were immersed in 2,3,5-triphenyltetrazolium chloride (TTC) solution (1% (w/V) dissolved in phosphate buffer, consisting of 77.4% (V/V) 0.1 mol/l Na2HPO4 and 22.6% (V/V) 0.1 mol/l NaH2PO4), and incubated in a water bath at 37°C for 5 min. After photographing, the slices were quickly frozen in liquid nitrogen and stored at –80°C for later analysis by Western blot. Areas of viable tissue (red) and necrotic tissue (white) were measured by computerized planimetry (ImageJ 1.48v, National Institutes of Health, Bethesda, Maryland). Infarct size was calculated as percentage of left and right ventricular (LV+RV) mass (% of LV+RV).
Investigators performing infarct size quantification were blinded for Western blot analyses and vice versa, and all were blinded for the experimental protocols.
Western blot analysis
Proteins of murine myocardium were solubilized; protein lysates were electrophoretically separated on pre-casted sodium dodecyl sulfate-polyacrylamide electrophoresis gels (BioRad, Munich, Germany) and transferred to polyvinylidene fluoride membranes. We used 30 μg total protein for the detection of signal transducer and activator of transcription 3 (STAT3) and extracellular-regulated kinase (ERK) 1/2 and 50 μg for the detection of STAT5 and protein kinase B (AKT). After blocking, membranes were incubated with antibodies—pSTAT3tyrosine705 (mouse, monoclonal), pSTAT5tyrosine694 (rabbit, monoclonal), pAKTserine473, pERK1/2threonine202/tyrosine204 (rabbit, polyclonal)—all from Cell Signaling Technology, Inc. (Danvers, Massachusetts) directed against the phosphorylated forms of STAT3, STAT5, AKT, and ERK1/2. After incubation with the respective secondary antibodies—antimouse (horse, polyclonal) and antirabbit (goat, polyclonal)—both from Cell Signaling Technology, Inc. (Danvers, Massachusetts), immunoreactive signals were detected by chemiluminescence (SuperSignal West Femto, Thermo Fisher Scientific, Rockford, Illinois) using a charge-coupled device camera (ChemoCam INTAS, Göttingen, Germany) and quantified with LabImage1D (INTAS, Göttingen, Germany). Membranes were reprobed for detection of the respective total form of each protein—STAT3 (rabbit, monoclonal), STAT5, AKT, ERK1/2 (rabbit, polyclonal)—all from Cell Signaling Technology, Inc. (Danvers, Massachusetts). Immunoreactivities of phosphorylated proteins were normalized to those of the total form of the respective protein.
In vitro STAT3 inhibition
Further investigation of the SAFE pathway was performed by inhibition of STAT3 using Stattic (Stattic, Tocris bioscience, Bristol, England) 35, 36. Plasma from 7 volunteers taken at 3 time points was tested for this in vitro inhibition of the SAFE pathway: baseline, 30 min and 6 days after RIPC. Stattic was dissolved in dimethylsulfoxide and added to the Krebs-Henseleit buffer at a final concentration of 10 μmol/l and a final dilution of dimethylsulfoxide of 1:10.000. After stabilization for 20 min, mouse hearts were pre-treated with Stattic-containing Krebs-Henseleit buffer for 20 min, followed by perfusion with human dialysate. During wash-out and reperfusion, plain Krebs-Henseleit buffer was used again. After TTC staining the slices of the hearts were immunoblotted to verify successful STAT3 inhibition.
Statistical analysis
Most Western blot data failed normality and equal variance test, therefore all continuous variables are presented as median and interquartile range (IQR) and categorical variables as frequencies and percentages. Comparisons were made between hearts perfused with dialysates of plasma obtained at baseline and hearts perfused with dialysates of plasma obtained at the pre-defined time points after RIPC. Statistical analysis was performed using Friedman repeated measures analysis of variance on ranks with post hoc Dunn’s method. A p value <0.05 was considered statistically significant. All statistical analyses were performed using SPSS software (SigmaStat 3.5, SPSS, Chicago, Illinois).
Results
Volunteer demographics are presented in Supplemental Table 1. There were no protocol deviations or adverse events.
Infarct size reduction and improvement in LVDP
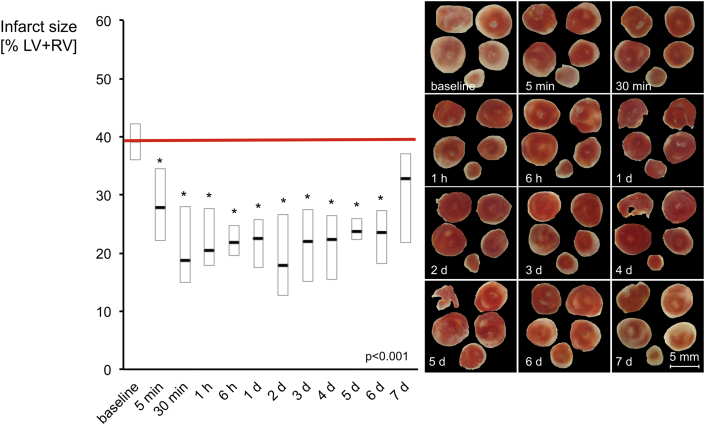
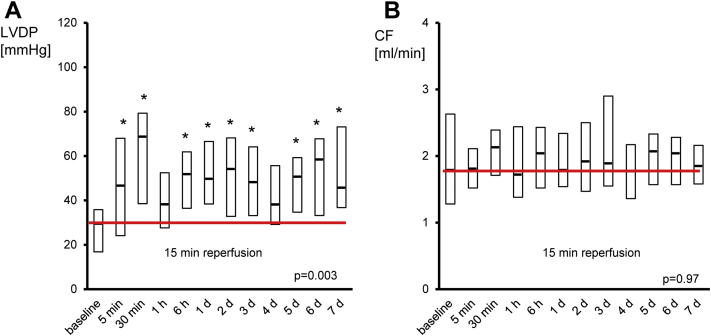
Perfusion with dialysates of human plasma obtained at baseline resulted in an infarct size of 39% of LV+RV (IQR: 36% to 42%). Perfusion with dialysates of human plasma obtained within the pre-specified time points between 5 min and 6 days after RIPC reduced infarct size in comparison to that with baseline dialysate (Figure 2). Dialysates of plasma obtained 5 min after RIPC reduced infarct size to 28% of LV+RV (IQR: 22% to 35%), and minimal infarct size was observed with dialysates of plasma obtained 2 days after RIPC (i.e., 18% of LV+RV [IQR: 13% to 27%]. While dialysates of human plasma obtained 6 days after the RIPC maneuver still reduced infarct size to 24% of LV+RV (IQR: 18% to 27%), dialysates of plasma obtained 7 days after the RIPC maneuver did not (i.e., infarct size was 32% of LV+RV [IQR: 22% to 37%]) (Figure 2). LVDP was significantly improved at 15 min reperfusion by post-RIPC-dialysates compared to baseline-dialysate, while coronary flow was not different at any time point (Figure 3, Supplemental Figures 1 and 2).
Figure 2.
Kinetics of Humoral Transfer Factor(s) as Evidenced by Reduction in Infarct Size
In isolated mouse hearts, dialysates of human plasma obtained between 5 min and 6 days after RIPC induced a reduction of infarct size, as shown in the examples. Data are presented as median with interquartile ranges of n = 20 at each time point; *p < 0.05 versus baseline. d = day; LV+RV = left and right ventricle.
Figure 3.
LVDP and Coronary Flow at 15 Min Reperfusion of Isolated Mouse Hearts
(A) Improvement of left ventricular developed pressure (LVDP) by perfusion with dialysates of human plasma obtained after RIPC compared to dialysates of human plasma obtained at baseline. (B) Coronary flow was not affected. Data are median with interquartile ranges of n = 20 at each time point; *p < 0.05 versus baseline. d = day; CF = coronary flow.
Signal activation
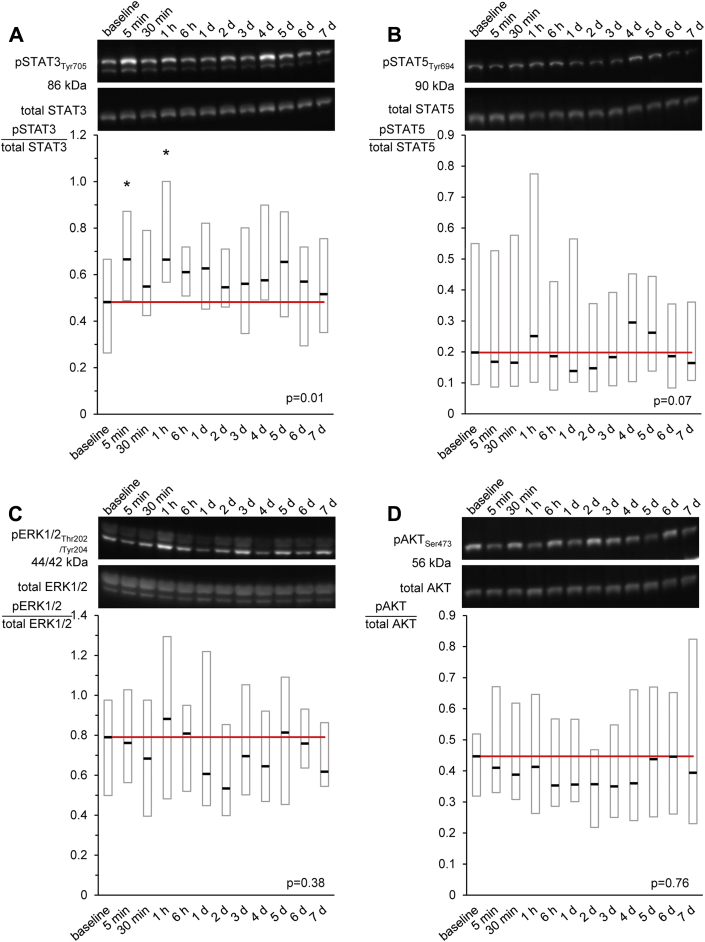
In murine myocardium dialysates of plasma obtained after RIPC induced an increase of STAT3 phosphorylation over that with baseline-dialysate (p = 0.01) (Figure 4A). The phosphorylation of STAT5 (p = 0.07) showed a similar tendency, while phosphorylation of AKT (p = 0.76) and ERK1/2 (p = 0.38) was not affected (Figures 4B to 4D). Examples of Western blot membranes and the respective chemiluminescence signals are displayed in the supplemental appendix for each protein (Supplemental Figure 3).
Figure 4.
STAT3 Phosphorylation in Murine Myocardium After Infusion of Human Plasma Dialysates Obtained Before and After RIPC
Time courses of phosphorylation of (A) signal transducer and activator of transcription 3 (STAT3), (B) STAT5, (C) protein kinase B (AKT), and (D) extracellular-regulated kinase 1/2 (ERK1/2) in murine myocardium perfused with human plasma-dialysates obtained at baseline (before) as well as at different time points after RIPC and exemplary Western blots, respectively. Data are presented as median with interquartile ranges of n = 20 at each time point; *p < 0.05 versus baseline. d = day; RIPC = remote ischemic pre-conditioning; Ser = serine; Thr = threonine; Tyr = tyrosine.
STAT3 inhibition
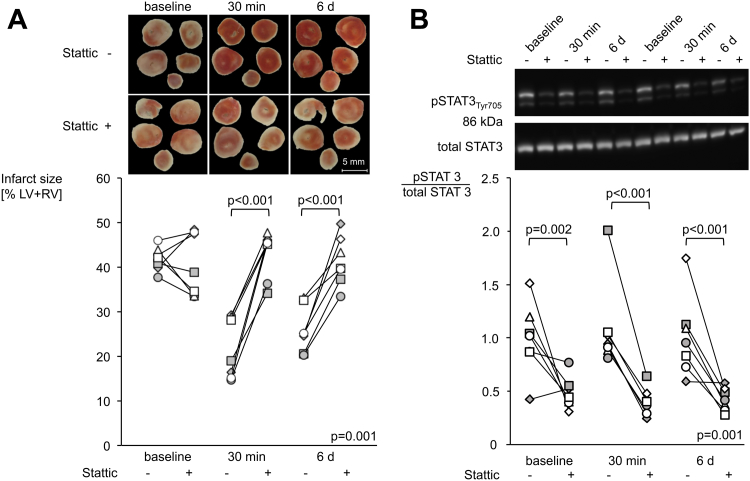
Infarct size reduction and STAT3 activation were abolished by Stattic (Figure 5). Infarct sizes of hearts perfused with dialysates of plasma obtained 30 min after RIPC were increased by pre-treatment with Stattic (19% of LV+RV [IQR: 16% to 29%] to 45% of LV+RV [IQR: 41% to 46%], p < 0.05) (Figure 5A). The same was true for hearts perfused with dialysates of plasma obtained 6 days after RIPC (25% of LV+RV [IQR: 23% to 29%] to 40% of LV+RV [IQR: 38% to 45%], p < 0.05) (Figure 5A). Stattic had no influence on infarct size per se (42% of LV+RV [IQR: 40% to 43%] vs. 39% of LV+RV [IQR: 34% to 48%], p = NS) (Figure 5A). Stattic did not change LVDP and coronary flow (Supplemental Figure 4). The efficacy of STAT3 inhibition by Stattic was verified by the reduced STAT3 phosphorylation at baseline, 30 min, and 6 days after RIPC (pSTAT3/total STAT3: 1.02 [IQR: 0.87 to 1.12] to 0.45 [IQR: 0.40 to 0.54], 0.94 [IQR: 0.90 to 1.04] to 0.37 [IQR: 0.31 to 0.44], and 0.95 [IQR: 0.78 to 1.11] to 0.42 [IQR: 0.35 to 0.51], respectively) (Figure 5B). Original Western blot results and the respective chemiluminescence signals are displayed in the supplemental appendix (Supplemental Figure 5).
Figure 5.
STAT3 Inhibition With Stattic Abolished the Protection by Dialysates of Human Plasma Obtained After RIPC in Murine Myocardium
(A) Effect of Stattic (signal transducer and activator of transcription 3 [STAT3] inhibitor) on infarct size and (B) STAT3 phosphorylation in murine myocardium perfused with dialysates of human plasma obtained before (baseline) as well as 30 min and 6 days after RIPC for n = 7 each; *p < 0.05 versus without Stattic. d = day; LV+RV = left and right ventricle; RIPC = remote ischemic pre-conditioning; Tyr = tyrosine.
Discussion
The present study characterized the kinetics and identified a signal transduction property of humoral factor(s) after a single RIPC-maneuver in healthy volunteers. The dialysate of human plasma obtained at 5 min and thereafter until at least 6 days after the RIPC-maneuver was transferred to a Langendorff-perfused mouse heart where it reduced infarct size from global ischemia/reperfusion through STAT3 activation.
Kinetics of humoral factor(s)
The release of a circulating factor with a rapid onset of cardioprotection in the bioassay heart of the present study is consistent with prior experimental and clinical studies, which revealed evidence for protection with an interval of only 5 to 10 min between the RIPC stimulus and the injurious event 22, 23, 37, 38. The continuous presence of a circulating factor that provides cardioprotection in the bioassay mouse heart in the present study is again consistent with prior experimental and clinical studies, which revealed evidence of long-lasting protection 24, 26, 27, 39 for up to 1 week after the RIPC stimulus (28). In contrast to our present study, however, these studies could not distinguish between a long-lasting presence of a circulating factor or a long-lasting protective effect in the target organ. Given the dilution of the circulating factor that we had to use for technical reasons to perfuse the mouse heart with a dialysate, the appearance of a circulating factor which initiates protection may even be faster and last longer than we observed. However, we cannot distinguish whether we are dealing with the rapid and continuous release of a factor with a short half-life or a rapid release of a factor with a long half-life. Also, we have no information whether we are dealing with a single factor or a combination of factors.
In the present study, we have not investigated potential candidate molecules—nitrite (17), stromal cell–derived factor-1α (18), and microRNA-144 (19)—in the dialysate. However, with a pore size of ≤5 nm of the dialysis tubing, exosomes that also contribute to protection by RIPC (40) are excluded, unless they have released their protective molecules during the dialysis procedure.
Signal activation in murine myocardium
In murine myocardium cardioprotection by local ischemic conditioning is associated with the activation of AKT, STAT3, and STAT5 31, 32, 35, 41; RIPC in mice has been shown to be associated with the activation of ERK1/2, AKT, and STAT3 42, 43. Apparently, in our present study the humoral factor(s) obtained from healthy volunteers after a single RIPC maneuver activated only STAT3 significantly whereas AKT and ERK1/2 were not activated and STAT5 only nonsignificantly. The STAT3 activation was causal for infarct size reduction since Stattic abrogated the protection. Although not significant, the trend for an activation of STAT5 is noteworthy because STAT5 is the only signaling protein, which has so far been demonstrated to be activated by RIPC in human tissue (44). The activation of STAT3 points to members of the cytokine and/or the growth hormone family as potential circulating RIPC transfer factor(s), as both cytokines and growth hormones classically activate the STAT3 and also the STAT5 pathway 29, 45, 46. We did not investigate the signal transduction downstream of STAT3 activation. However, the causal role of STAT3 in the rapid infarct size reduction (within 30 min) precludes a role of STAT3 as a transcription factor, which in turn may interact with other cardioprotective transcription factors such as hypoxia-inducible factors 47, 48, for the present study. The causal role of STAT3 is rather attributable to better mitochondrial function, as we have shown before in protocols of ischemic post-conditioning 35, 36.
Study limitations
We used a saline-perfused Langendorff heart preparation with all its limitations, including the development of edema. The human factor(s) in the present study were obtained from healthy volunteers but protection and its signal transduction were only identified after inter-species transfer in the isolated mouse heart. The humoral factor(s) may be different in kinetics and properties in patients with cardiovascular disease, confounding co-morbidities and comedications. Also, the signal transduction and the protective effect may be quantitatively and qualitatively different in the diseased human than the healthy mouse heart (13).
Conclusions
A single RIPC maneuver in healthy volunteers induces release of 1 or more cardioprotective, dialyzable, humoral factors that circulate already after 5 min and for up to 6 days. These factors reduce infarct size through STAT3 activation in the mouse heart.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: RIPC protects against myocardial ischemia/reperfusion injury in animal studies and clinical proof-of-concept trials. Recent large-scaled, randomized trials did not confirm protection by RIPC in patients undergoing cardiac surgery, possibly because of confounding comorbidities and medications. The definition of basic characteristics of RIPC-induced cardioprotection in healthy volunteers may help to design future clinical trials.
TRANSLATIONAL OUTLOOK 1: The kinetics and signaling of cardioprotection by RIPC must be better defined.
TRANSLATIONAL OUTLOOK 2: Identification of the transfer factor(s) is mandatory for its potential use as a therapeutic agent.
Acknowledgment
Part of the data was obtained by V. Kreienkamp and will be presented in his MD thesis.
Footnotes
Supported by: German Research Foundation (He 1320/18-3; SFB 1116 B8); IFORES 10+2 (D/107-40700), Medical Faculty of the University Duisburg-Essen to HH, Essen, Germany. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Appendix
References
- 1.Heusch G., Bøtker H.E., Przyklenk K., Redington A., Yellon D. Remote ischemic conditioning. J Am Coll Cardiol. 2015;65:177–195. doi: 10.1016/j.jacc.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies W.R., Brown A.J., Watson W. Remote ischemic preconditioning improves outcome at 6 years after elective percutaneous coronary intervention: the CRISP stent trial long-term follow-up. Circ Cardiovasc Interv. 2013;6:246–251. doi: 10.1161/CIRCINTERVENTIONS.112.000184. [DOI] [PubMed] [Google Scholar]
- 3.Hausenloy D.J., Mwamure P.K., Venugopal V. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomised controlled trial. Lancet. 2007;370:575–579. doi: 10.1016/S0140-6736(07)61296-3. [DOI] [PubMed] [Google Scholar]
- 4.Thielmann M., Kottenberg E., Kleinbongard P. Cardioprotective and prognostic effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: a single-centre randomised, double-blind, controlled trial. Lancet. 2013;382:597–604. doi: 10.1016/S0140-6736(13)61450-6. [DOI] [PubMed] [Google Scholar]
- 5.Candilio L., Malik A., Ariti C. Effect of remote ischaemic preconditioning on clinical outcomes in patients undergoing cardiac bypass surgery: a randomised controlled clinical trial. Heart. 2015;101:185–192. doi: 10.1136/heartjnl-2014-306178. [DOI] [PubMed] [Google Scholar]
- 6.Bøtker H.E., Kharbanda R., Schmidt M.R. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet. 2010;375:727–734. doi: 10.1016/S0140-6736(09)62001-8. [DOI] [PubMed] [Google Scholar]
- 7.Sloth A.D., Schmidt M.R., Munk K. Improved long-term clinical outcomes in patients with ST-elevation myocardial infarction undergoing remote ischaemic conditioning as an adjunct to primary percutaneous coronary intervention. Eur Heart J. 2014;35:168–175. doi: 10.1093/eurheartj/eht369. [DOI] [PubMed] [Google Scholar]
- 8.Eitel I., Stiermaier T., Rommel K.P. Cardioprotection by combined intrahospital remote ischaemic perconditioning and postconditioning in ST-elevation myocardial infarction: the randomized LIPSIA conditioning trial. Eur Heart J. 2015;36:3049–3057. doi: 10.1093/eurheartj/ehv463. [DOI] [PubMed] [Google Scholar]
- 9.Yellon D.M., Ackbarkhan A.K., Balgobin V. Remote ischemic conditioning reduces myocardial infarct size in STEMI patients treated by thrombolysis. J Am Coll Cardiol. 2015;65:2764–2765. doi: 10.1016/j.jacc.2015.02.082. [DOI] [PubMed] [Google Scholar]
- 10.White S.K., Frohlich G.M., Sado D.M. Remote ischemic conditioning reduces myocardial infarct size and edema in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol Intv. 2015;8:178–188. doi: 10.1016/j.jcin.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 11.Hausenloy D.J., Candilio L., Evans R. Remote ischemic preconditioning and outcomes of cardiac surgery. N Engl J Med. 2015;373:1408–1417. doi: 10.1056/NEJMoa1413534. [DOI] [PubMed] [Google Scholar]
- 12.Meybohm P., Bein B., Brosteanu O. A multicenter trial of remote ischemic preconditioning for heart surgery. N Engl J Med. 2015;373:1397–1407. doi: 10.1056/NEJMoa1413579. [DOI] [PubMed] [Google Scholar]
- 13.Ferdinandy P., Hausenloy D.J., Heusch G., Baxter G.F., Schulz R. Interaction of risk factors, comorbidities, and comedications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacol Rev. 2014;66:1142–1174. doi: 10.1124/pr.113.008300. [DOI] [PubMed] [Google Scholar]
- 14.Heusch G., Gersh B.J. ERICCA and RIPHeart: two nails in the coffin for cardioprotection by remote ischemic conditioning? Probably not. Eur Heart J. 2016;37:200–202. doi: 10.1093/eurheartj/ehv606. [DOI] [PubMed] [Google Scholar]
- 15.Kleinbongard P., Neuhäuser M., Thielmann M. Confounders of cardioprotection by remote ischemic preconditioning in patients undergoing coronary artery bypass grafting. Cardiology. 2016;133:128–133. doi: 10.1159/000441216. [DOI] [PubMed] [Google Scholar]
- 16.Kottenberg E., Thielmann M., Bergmann L. Protection by remote ischemic preconditioning during coronary artery bypass graft surgery with isoflurane but not propofol - a clinical trial. Acta Anaesthesiol Scand. 2012;56:30–38. doi: 10.1111/j.1399-6576.2011.02585.x. [DOI] [PubMed] [Google Scholar]
- 17.Rassaf T., Totzeck M., Hendgen-Cotta U.B., Shiva S., Heusch G., Kelm M. Circulating nitrite contributes to cardioprotection by remote ischemic preconditioning. Circ Res. 2014;114:1601–1610. doi: 10.1161/CIRCRESAHA.114.303822. [DOI] [PubMed] [Google Scholar]
- 18.Davidson S.M., Selvaraj P., He D. Remote ischaemic preconditioning involves signalling through the SDF-1α/CXCR4 signalling axis. Basic Res Cardiol. 2013;108:377. doi: 10.1007/s00395-013-0377-6. [DOI] [PubMed] [Google Scholar]
- 19.Li J., Rohailla S., Gelber N. MicroRNA-144 is a circulating effector of remote ischemic preconditioning. Basic Res Cardiol. 2014;109:423. doi: 10.1007/s00395-014-0423-z. [DOI] [PubMed] [Google Scholar]
- 20.Hepponstall M., Ignjatovic V., Binos S. Remote ischemic preconditioning (RIPC) modifies plasma proteome in humans. PLoS One. 2012;7:e48284. doi: 10.1371/journal.pone.0048284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helgeland E., Breivik L.E., Vaudel M. Exploring the human plasma proteome for humoral mediators of remote ischemic preconditioning-a word of caution. PLoS One. 2014;9:e109279. doi: 10.1371/journal.pone.0109279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheung M.M., Kharbanda R.K., Konstantinov I.E. Randomized controlled trial of the effects of remote ischemic preconditioning on children undergoing cardiac surgery: first clinical application in humans. J Am Coll Cardiol. 2006;47:2277–2282. doi: 10.1016/j.jacc.2006.01.066. [DOI] [PubMed] [Google Scholar]
- 23.Heinen N.M., Pütz V.E., Görgens J.I. Cardioprotection by remote ischemic preconditioning exhibits a signaling pattern different from local ischemic preconditioning. Shock. 2011;36:45–53. doi: 10.1097/SHK.0b013e31821d8e77. [DOI] [PubMed] [Google Scholar]
- 24.Li G., Labruto F., Sirsjo A., Chen F., Vaage J., Valen G. Myocardial protection by remote preconditioning: the role of nuclear factor kappa-B p105 and inducible nitric oxide synthase. Eur J Cardiothorac Surg. 2004;26:968–973. doi: 10.1016/j.ejcts.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 25.Zhou W., Zeng D., Chen R. Limb ischemic preconditioning reduces heart and lung injury after an open heart operation in infants. Pediatr Cardiol. 2010;31:22–29. doi: 10.1007/s00246-009-9536-9. [DOI] [PubMed] [Google Scholar]
- 26.Wagner R., Piler P., Bedanova H., Adamek P., Grodecka L., Freiberger T. Myocardial injury is decreased by late remote ischaemic preconditioning and aggravated by tramadol in patients undergoing cardiac surgery: a randomised controlled trial. Interact Cardiovasc Thorac Surg. 2010;11:758–762. doi: 10.1510/icvts.2010.243600. [DOI] [PubMed] [Google Scholar]
- 27.Liu Z., Wang Y.L., Hua Q., Chu Y.Y., Ji X.M. Late remote ischemic preconditioning provides benefit to patients undergoing elective percutaneous coronary intervention. Cell Biochem Biophys. 2014;70:437–442. doi: 10.1007/s12013-014-9936-1. [DOI] [PubMed] [Google Scholar]
- 28.Manchurov V., Ryazankina N., Khmara T. Remote ischemic preconditioning and endothelial function in patients with acute myocardial infarction and primary PCI. Am J Med. 2014;127:670–673. doi: 10.1016/j.amjmed.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 29.Heusch G. Molecular basis of cardioprotection: signal transduction in ischemic pre-, post-, and remote conditioning. Circ Res. 2015;116:674–699. doi: 10.1161/CIRCRESAHA.116.305348. [DOI] [PubMed] [Google Scholar]
- 30.Skyschally A., Gent S., Amanakis G., Schulte C., Kleinbongard P., Heusch G. Across-species transfer of protection by remote ischemic preconditioning with species-specific myocardial signal transduction by reperfusion injury salvage kinase and survival activating factor enhancement pathways. Circ Res. 2015;117:279–288. doi: 10.1161/CIRCRESAHA.117.306878. [DOI] [PubMed] [Google Scholar]
- 31.Yamaura G., Turoczi T., Yamamoto F., Siddqui M.A., Maulik N., Das D.K. STAT signaling in ischemic heart: a role of STAT5A in ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H476–H482. doi: 10.1152/ajpheart.00079.2003. [DOI] [PubMed] [Google Scholar]
- 32.Suleman N., Somers S., Smith R., Opie L.H., Lecour S.C. Dual activation of STAT-3 and Akt is required during the trigger phase of ischaemic preconditioning. Cardiovasc Res. 2008;79:127–133. doi: 10.1093/cvr/cvn067. [DOI] [PubMed] [Google Scholar]
- 33.Lacerda L., Somers S., Opie L.H., Lecour S. Ischaemic postconditioning protects against reperfusion injury via the SAFE pathway. Cardiovasc Res. 2009;84:201–208. doi: 10.1093/cvr/cvp274. [DOI] [PubMed] [Google Scholar]
- 34.Somers S.J., Frias M., Lacerda L., Opie L.H., Lecour S. Interplay between SAFE and RISK pathways in sphingosine-1-phosphate-induced cardioprotection. Cardiovasc Drugs Ther. 2012;26:227–237. doi: 10.1007/s10557-012-6376-2. [DOI] [PubMed] [Google Scholar]
- 35.Boengler K., Hilfiker-Kleiner D., Heusch G., Schulz R. Inhibition of permeability transition pore opening by mitochondrial STAT3 and its role in myocardial ischemia/reperfusion. Basic Res Cardiol. 2010;105:771–785. doi: 10.1007/s00395-010-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heusch G., Musiolik J., Gedik N., Skyschally A. Mitochondrial STAT3 activation and cardioprotection by ischemic postconditioning in pigs with regional myocardial ischemia/reperfusion. Circ Res. 2011;109:1302–1308. doi: 10.1161/CIRCRESAHA.111.255604. [DOI] [PubMed] [Google Scholar]
- 37.Konstantinov I.E., Arab S., Kharbanda R.K. The remote ischemic preconditioning stimulus modifies inflammatory gene expression in humans. Physiol Genomics. 2004;19:143–150. doi: 10.1152/physiolgenomics.00046.2004. [DOI] [PubMed] [Google Scholar]
- 38.Zitta K., Meybohm P., Bein B. Serum from patients undergoing remote ischemic preconditioning protects cultured human intestinal cells from hypoxia-induced damage: involvement of matrixmetalloproteinase-2 and -9. Mol Med. 2012;18:29–37. doi: 10.2119/molmed.2011.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gonzalez N.R., Hamilton R., Bilgin-Freiert A. Cerebral hemodynamic and metabolic effects of remote ischemic preconditioning in patients with subarachnoid hemorrhage. Acta Neurochir Suppl. 2013;115:193–198. doi: 10.1007/978-3-7091-1192-5_36. [DOI] [PubMed] [Google Scholar]
- 40.Giricz Z., Varga Z.V., Baranyai T. Cardioprotection by remote ischemic preconditioning of the rat heart is mediated by extracellular vesicles. J Mol Cell Cardiol. 2014;68:75–78. doi: 10.1016/j.yjmcc.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Smith R.M., Suleman N., Lacerda L. Genetic depletion of cardiac myocyte STAT-3 abolishes classical preconditioning. Cardiovasc Res. 2004;63:611–616. doi: 10.1016/j.cardiores.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 42.Cai Z.P., Parajuli N., Zheng X., Becker L. Remote ischemic preconditioning confers late protection against myocardial ischemia-reperfusion injury in mice by upregulating interleukin-10. Basic Res Cardiol. 2012;107:277. doi: 10.1007/s00395-012-0277-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oba T., Yasukawa H., Nagata T. Renal nerve-mediated erythropoietin release confers cardioprotection during remote ischemic preconditioning. Circ J. 2015;79:1557–1567. doi: 10.1253/circj.CJ-14-1171. [DOI] [PubMed] [Google Scholar]
- 44.Heusch G., Musiolik J., Kottenberg E., Peters J., Jakob H., Thielmann M. STAT5 activation and cardioprotection by remote ischemic preconditioning in humans: short communication. Circ Res. 2012;110:111–115. doi: 10.1161/CIRCRESAHA.111.259556. [DOI] [PubMed] [Google Scholar]
- 45.Boengler K., Hilfiker-Kleiner D., Drexler H., Heusch G., Schulz R. The myocardial JAK/STAT pathway: from protection to failure. Pharmacol Ther. 2008;120:172–185. doi: 10.1016/j.pharmthera.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 46.Haghikia A., Stapel B., Hoch M., Hilfiker-Kleiner D. STAT3 and cardiac remodeling. Heart Fail Rev. 2011;16:35–47. doi: 10.1007/s10741-010-9170-x. [DOI] [PubMed] [Google Scholar]
- 47.Cai Z., Luo W., Zhan H., Semenza G.L. Hypoxia-inducible factor 1 is required for remote ischemic preconditioning of the heart. Proc Natl Acad Sci U S A. 2013;110:17462–17467. doi: 10.1073/pnas.1317158110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nechemia-Arbely Y., Khamaisi M., Rosenberger C. In vivo evidence suggesting reciprocal renal hypoxia-inducible factor-1 upregulation and signal transducer and activator of transcription 3 activation in response to hypoxic and non-hypoxic stimuli. Clin Exp Pharmacol Physiol. 2013;40:262–272. doi: 10.1111/1440-1681.12064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.