Abstract
Alternative splicing is the highly regulated process of variation in the removal of introns from premessenger-RNA transcripts. The consequences of alternative splicing on the phenotype are well documented, but the impact of the environment on alternative splicing is not yet clear. We studied variation in alternative splicing among four different temperatures, 13, 18, 23, and 29°, in two Drosophila melanogaster genotypes. We show plasticity of alternative splicing with up to 10% of the expressed genes being differentially spliced between the most extreme temperatures for a given genotype. Comparing the two genotypes at different temperatures, we found <1% of the genes being differentially spliced at 18°. At extreme temperatures, however, we detected substantial differences in alternative splicing—with almost 10% of the genes having differential splicing between the genotypes: a magnitude similar to between species differences. Genes with differential alternative splicing between genotypes frequently exhibit dominant inheritance. Remarkably, the pattern of surplus of differences in alternative splicing at extreme temperatures resembled the pattern seen for gene expression intensity. Since different sets of genes were involved for the two phenotypes, we propose that purifying selection results in the reduction of differences at benign temperatures. Relaxed purifying selection at temperature extremes, on the other hand, may cause the divergence in gene expression and alternative splicing between the two strains in rarely encountered environments.
Keywords: alternative splicing, temperature, plasticity, dominance
SPLICING, the removal of introns from precursor messenger RNAs (mRNAs) together with the subsequent ligation of exons, is an integral part of gene expression regulation. Alternative splicing is the combination of different exons from the same precursor mRNA and provides the basis for the impressive diversity of gene products originating from a substantially smaller set of genes (Pan et al. 2008; Nilsen and Graveley 2010; Brown et al. 2014). There are several types of alternative splicing; such as the exclusion of exons, sometimes mutually exclusive; or the retention of intronic sequence in the mature transcript. Furthermore, the alternative selection of 5′ or 3′ splice sites, a special form of exon skipping (Koren et al. 2007), has been shown to make an important contribution to transcript diversification.
Splicing, in particular alternative splicing, is a highly regulated process that depends on cis-regulatory sequences (splicing enhancers and suppressors) and trans-regulatory splicing factors, such as heterogeneous nuclear ribonucleoproteins and SR and SR-related proteins (Nilsen and Graveley 2010). The repertoire of isoforms, different mature mRNAs originating from a single gene, differs widely among tissues, developmental stages, and environmental conditions (Barberan-Soler and Zahler 2008; Gan et al. 2010; Barbosa-Morais et al. 2012; Bartok et al. 2013; Leviatan et al. 2013; Long et al. 2013; Reyes et al. 2013; Telonis-Scott et al. 2013; Brown et al. 2014; Chang et al. 2014; Vitulo et al. 2014). It could, therefore, be considered as a prototype for phenotypic plasticity on the molecular level (Mastrangelo et al. 2012; Chen et al. 2015b).
Phenotypic plasticity describes the ability of a given genotype to display a range of phenotypes as a response to environmental heterogeneity. On the organismal level, phenotypic plasticity has been of key interest to evolutionary biologists as it provides the opportunity to respond quickly to environmental changes. On the cellular level, phenotypic plasticity is the impressive manifestation of cellular differentiation of multicellular organisms; a property favored by natural selection.
While the selective advantage of both the presence or absence of phenotypic plasticity is conceptually appealing, their relative importance is not yet clear. Traditionally, plasticity has been studied using high-order phenotypes, such as morphology and life history traits, which integrate the effects of many genes. Nevertheless, the advances in molecular biology have opened the possibility to expand these studies to lower-level phenotypes such as gene expression and alternative splicing. Over the past years, an impressive amount of data has been collected demonstrating plasticity of gene expression and alternative splicing in different tissues and developmental stages (Jin et al. 2001; Wang et al. 2008; Graveley et al. 2011; Zhou et al. 2012; Smith et al. 2013; Brown et al. 2014; Etges et al. 2015). Much less is known about the influence of environmental conditions on this plasticity (Levine et al. 2011; Yampolsky et al. 2012; Telonis-Scott et al. 2013; Brown et al. 2014; Chang et al. 2014; Sikkink et al. 2014; Vitulo et al. 2014; Yampolsky et al. 2014; Chen et al. 2015a; Zhao et al. 2015), and the conservation of these patterns across genetically diverged organisms (Barberan-Soler and Zahler 2008; Etges et al. 2015; Chen et al. 2015a; Zhao et al. 2015).
Temperature is one of the key environmental parameters, in particular for ectotherms such as Drosophila. A broad range of morphological, behavioral, and physiological responses to temperature has been described, but few studies attempted to compare the patterns of gene expression plasticity across temperatures. Most of these studies compared the pattern of gene expression at two temperatures (Sikkink et al. 2014; Zhao et al. 2015) and found a large number of genes significantly affected by temperature. Recently, Chen et al. (2015a) attempted a more refined characterization of the temperature effect on gene expression by describing the reaction norm of gene expression across a broad temperature range (13–29°). Remarkably, they found that the reaction norm did not only cluster genes according to function, but also explained some of the underlying regulatory architecture (Chen et al. 2015b).
Extending the plasticity analysis to diverged genotypes often found significant differences in the reaction norm between genotypes. In studies that compared gene expression between differentially evolved genotypes, differences in gene expression plasticity were good indicators for direct or indirect selection targets (Telonis-Scott et al. 2009; Yampolsky et al. 2012). An interesting pattern was found when Chen et al. (2015a) contrasted the pattern of gene expression between two genotypes at different temperatures. At 18° the authors observed almost no differences in gene expression intensity between two inbred Drosophila laboratory strains, but at more extreme temperatures the expression divergence increased. This pattern was interpreted as evidence for canalized gene expression at 18°, which becomes lost when flies are exposed to more extreme environments (decanalization) (Chen et al. 2015a).
Despite its well-characterized influence on the phenotype, alternative splicing plasticity has been studied only in the context of exposure to acute stress conditions (Mastrangelo et al. 2012; Long et al. 2013; Telonis-Scott et al. 2013; Vitulo et al. 2014). Very little is known, however, about how long-term exposure to typically encountered environments modulates alternative splicing.
Here, we have used the data from the gene expression study by Chen et al. (2015a), studied the influence of temperature on the pattern of alternative splicing in Drosophila melanogaster, and compared this response between two genotypes. We contrasted the patterns of alternative splicing to those of gene expression intensities from a study by Chen et al. (2015a). Like for gene expression intensities, we found that temperature has a strong effect on alternative splicing, resulting in up to 568 (10.4%) genes being differentially spliced between the two most extreme temperatures for a given genotype. Even more surprising was the consistency of the pattern of increasing differences between the genotypes on both levels of the phenotype at extreme temperatures: at 18° only very few genes were differentially spliced between the two genotypes, whereas at extreme temperatures we detected the largest number of genes with differential splicing. Despite the similarity of this pattern, the involved genes did not overlap more than expected by chance.
Materials and Methods
Females (f) from Oregon-R (O) and Samarkand (S) laboratory strains were crossed with males (m) from both strains (Of × Om, Of × Sm, Sf × Sm, Sf × Om) in three replicates. After 2 days of egg laying at 23°, the eggs were transferred to one of the four assaying temperatures (13, 18, 23, and 29°). Virgin females were used for extraction and sequencing of mRNA. Further details on fly rearing can be found in Chen et al. (2015a) and Supplemental Material, File S1. Library preparation and sequencing are described in Chen et al. (2015a). Raw sequence reads (National Center for Biotechnology Information accession number SRP041398 and SRP041395) were trimmed based on sequencing quality using PoPoolation2 (Kofler et al. 2011) and mapped to the D. melanogaster reference genome (Flybase assembly 5) using the genomic short-read nucleotide alignment program (GSNAP) (Wu and Nacu 2010). All mapped RNA sequencing (RNA-seq) reads were randomly downsampled to the same coverage and counted with a DEXSeq counter. Differential exon usage analysis was conducted using the DEXSeq R package (Anders et al. 2012). Due to 3′ gene-transcript coverage bias in some samples, we restricted some analyses by using only the reads mapping to the 3′ side of the transcript (Figure A, File S1). Inheritance assignment followed the procedures described in McManus et al. (2010) and Chen et al. (2015a) and is described in detail in File S1. Splice types were assigned based on the D. melanogaster annotation (see File S1). Gene ontology (GO) analysis was performed using Gowinda (Kofler and Schlötterer 2012) and accounted for different splicing opportunities (i.e., intron numbers) among GO categories. Gene set overlaps were assessed using receiver operating characteristic (ROC)-like curves, which indicate if the overlap between two sets of ranked data are higher or lower than expected by chance (curve above and below the diagonal). Further details about the methods used are described in File S1.
Data availability
All raw sequence data used in this study is deposited in the National Center for Biotechnology Information Sequence Read Archive with accession numbers SRP041398 (Oregon-R and Samarkand) and SRP041395 (F1). All unfiltered read counts, custom scripts, and protocols will be available at DataDryad.org.
Results
We used 100-bp paired-end RNA-seq reads from two D. melanogaster genotypes, Samarkand (S) and Oregon-R (O), which were exposed to four different developmental temperatures ranging from 13 to 29° (Chen et al. 2015a). Each genotype–temperature combination was analyzed in three replicates. We measured alternative splicing by means of exon usage (Anders et al. 2012), using only those multi-exon genes with an average of at least 50 reads across all samples in the analysis (Table C, File S1).
Temperature-mediated plasticity of splicing
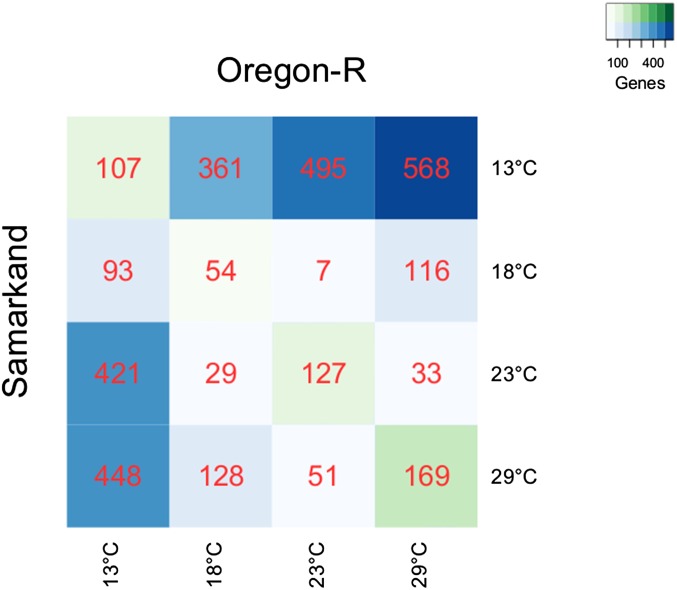
Pairwise comparisons of alternative splicing revealed a substantial effect of temperature, with up to 10.4% (568 out of 5463) of the multi-exon genes showing differential splicing between two temperatures for a given genotype. The highest plasticity of splicing was seen between the two extreme temperatures, but as few as seven genes differed in splicing between 18 and 23° in Oregon-R. Overall both D. melanogaster strains showed the same pattern of differential splicing with exons being more commonly retained at 13° and spliced out at 29° in both strains (Figure G, File S1). Oregon-R was more plastic than Samarkand (Figure 1).
Figure 1.
Temperature-dependent differential splicing between temperatures and between genotypes. Differentially spliced genes for pairwise temperature comparisons (blue) are shown for each of the genotypes, above the diagonal for Oregon-R and below the diagonal for Samarkand. Differential splicing between genotypes at a given temperature is shown on the diagonal (green). The heat map of pairwise temperature comparisons of differential splicing shows strong plasticity in both strains when the most extreme temperatures (13 and 29°) are compared. Alternative splicing between the more benign temperatures, 18 and 23°, however, exhibited only a weak plastic response to temperature in both genotypes. The data are based on reads mapping to the 3′ side of the transcript (see File S1 for more details).
The splicing differences between the two most extreme temperatures (13 and 29°) within genotypes were mostly caused by exon skipping (O = 67%, S = 65%) followed by both alternative 3′ (O = 13%, S = 17%) and 5′ (O = 14%, S = 11%) splice site selection, and with least changes caused by intron retention (O = 4%, S = 5%; Figure 2).
Figure 2.
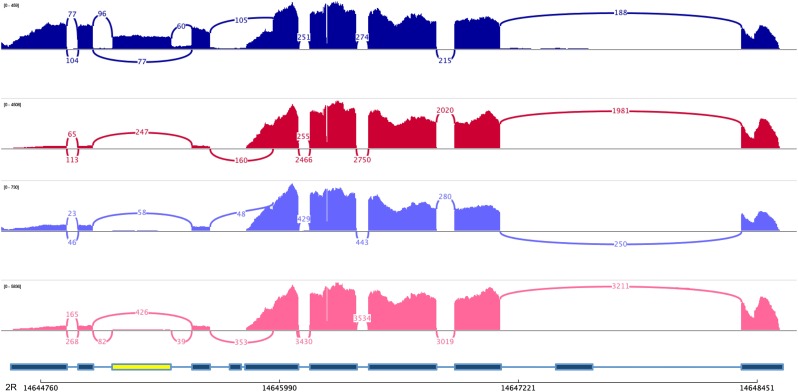
Distribution of splice types for genes with differential splicing between 13 and 29° for each of the two genotypes. The most prevalent splice type is exon skipping (red), followed by alternative 3′ splice site usage (yellow), 5′ splice site usage (green), and intron retention (blue). Overall, our statistically inferred splicing differences are also reflected by Sashimi plots based on reads covering exon junctions (Figure 3).
Temperature-dependent differences in alternative splicing between genotypes
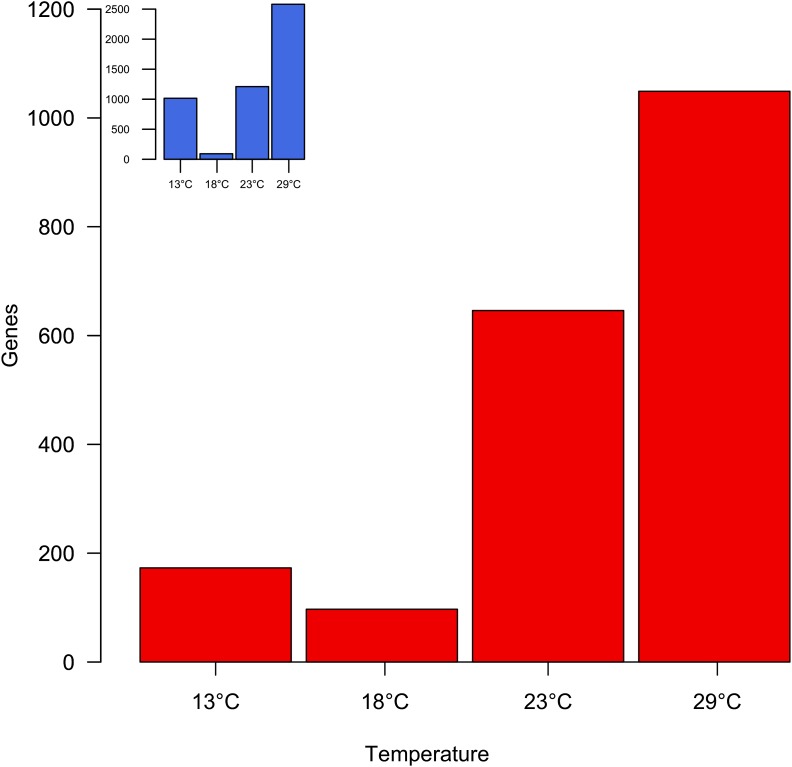
Despite the overall similarity of the two strains in splicing patterns across temperatures, we systematically tested for differential splicing between the two genotypes (Oregon-R and Samarkand) at each of the four developmental temperatures (Figure 3 and Figure 4). The highest similarity in splicing between the two strains for a given temperature was observed at 18°, with 1.21% of all tested multi-exon genes (97 out of 8021) showing significantly different splicing patterns. However, at the other three temperatures, 13, 23 and 29°, splicing differed between the two genotypes for 1.95% (173 out of 8858), 7.99% (646 out of 8090) and 12.81% (1049 out of 8186) genes, respectively; (Figure 4) suggesting that difference in alternative splicing between strains is strongly dependent on the assaying temperature. This pattern was previously observed for gene expression intensities (Figure 4 inset). The difference in alternative splicing between the two genotypes at 13° becomes clearer after adjusting for variance in the 3′ gene-body read coverage across replicates (see File S1).
Figure 3.
Genotype- and temperature-dependent alternative splicing. Sashimi plots for exon skipping of the third exon (marked in yellow) of the gene CG42351. Dark blue, Oregon-R 13°; dark red, Oregon-R 29°; light blue, Samarkand 13°; pink, Samarkand 29°.
Figure 4.
Genotype-specific alternative splicing. At 18° only a few genes differ in splicing between Oregon-R and Samarkand, while at more extreme temperatures the splicing patterns become increasingly different. This pattern resembles the one seen for gene expression intensity (blue inset; redrawn from Chen et al. 2015a).
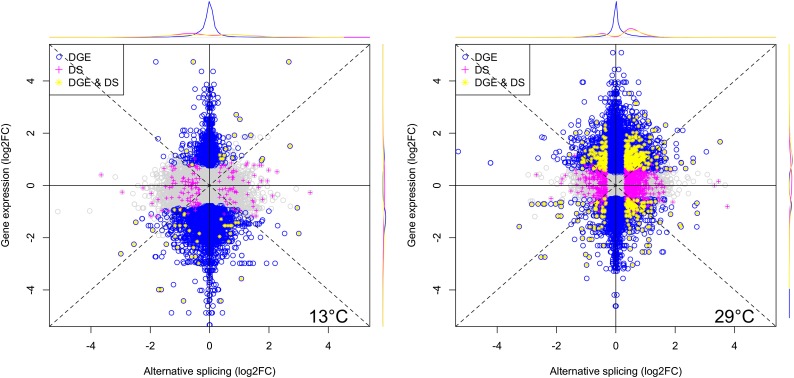
With temperature stress resulting in increasing differences in the splicing pattern between the two strains, we were interested to understand this better. Since different reaction norms of alternative splicing between the two strains may have caused the differences between genotypes at a certain temperature, we related these patterns to the intrastrain plasticity between different temperatures. Plotting the fold change in exon expression between genotypes within a temperature for each exon (corrected for overall gene expression) and fold changes of exons with splicing plasticity (differences between temperatures, within a strain) against each other, clearly indicated that the two are not congruent. Hence, we conclude that differences between strains are not a consequence of different reaction norms for alternative splicing of the two genotypes (Figure 5). Further support for this lack of congruence comes from ROC for exon expression intensities as well as a difference in GO term enrichment for genes with significant genetic differences and plasticity (Figure H, File S1).
Figure 5.
Genetically vs. environmentally induced differences in alternative splicing. Log2-fold changes between Oregon-R and Samarkand at (A and B) 13° and (C and D) 29° are plotted against log2-fold changes between 13 and 29° in (A and C) Oregon-R and in (B and D) Samarkand. Exons that are significantly differentially spliced between genotypes (GD, green) mostly do not overlap with the exons that are plastic (ED) in Oregon-R (red) or Samarkand (blue). Density plots on the top and on the right show the distribution of plotted points with corresponding colors. ED, environmental differences; GD, genetic differences.
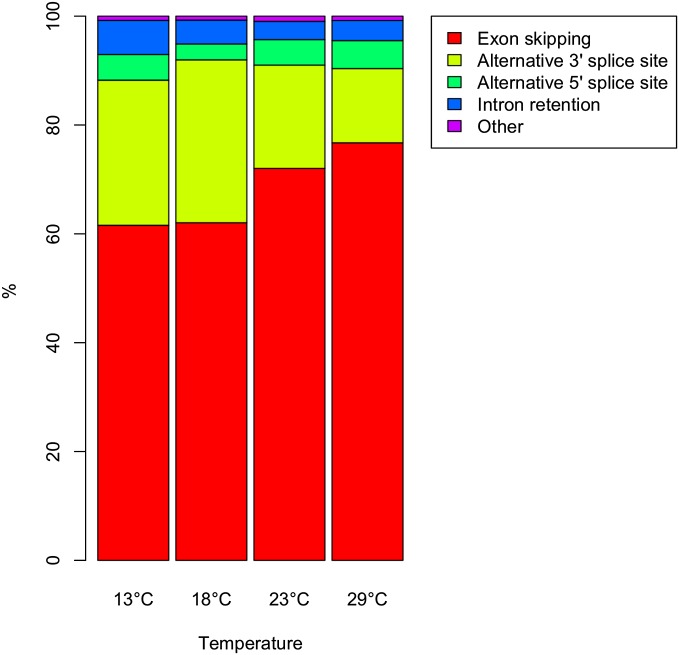
Out of all splicing events that differed between the strains for a given temperature, exon skipping was the most frequent one (76%), followed by 3′ alternative splicing site usage (14–30%), and 5′ alternative splice site usage (3–5%). The least frequent event was intron retention (1%). This pattern was very similar across the entire temperature range, with a trend toward more exon skipping at higher temperatures (Figure 6). Importantly, a similar distribution of alternative splicing events has been described previously (McManus et al. 2014).
Figure 6.
Contribution of splice types to differential splicing contrasting Oregon-R and Samarkand at each of the four temperatures. Exon skipping is the most frequent splice type among genes with differential splicing between genotypes and with increasing temperatures this pattern becomes even more pronounced.
Candidate genes for differential exon skipping
Previously, Chen et al. (2015a) showed enrichment in the GO categories “spliceosome” and “mRNA splicing, via spliceosome”, indicating that the expression differences in the core splicing machinery could result in the differences in alternative splicing between the genotypes. Dominant inheritance of alternative splicing between the genotypes also suggested that alternative splicing regulation is guided mostly by trans-acting factors. To test this hypothesis further, we took advantage of trans-acting factors with genome-wide influence on alternative splicing. The exon junction complex serves a central role in splicing (Tange et al. 2004). Knockdown of two members of the exon junction complex, mago nashi and eIF4AIII, increases the rate of exon skipping (Tange et al. 2004; Ashton-Beaucage et al. 2010; Wang et al. 2014). The three core exon junction complex genes that can be found in the nucleus and can, therefore, have the ability to interact with the splicing process, show a consistent expression pattern across temperatures. At 13° they are more expressed in Oregon-R, while at 29° Samarkand has the higher expression level (Table 1). If the exon junction complex is involved in the splicing differences between the two strains, we expect to find more exon skipping in Oregon-R at 13°, while Samarkand would have more exon skipping at higher temperatures. In support of this hypothesis, in Samarkand flies we find on average downregulation of differentially spliced exons at 13°, and the opposite pattern at higher temperatures (Table 1). While these results strongly suggest a substantial influence of the exon junction complex on the alternative splicing, the observation of different genes being alternatively spliced across temperatures indicates that other splicing factors may also shape the plasticity of alternative splicing. Brooks et al. (2015) recently reported 56 splicing factors and their target genes. We used this set of splicing factors to further test our hypothesis. In our data, 49 of the factors reported by Brooks et al. were expressed (on average at least 20 mapped reads across all samples). A total of 31 (63%) of the splicing factors showed a similar pattern as the exon junction complex genes: they were upregulated in one strain at 13° and upregulated in the other strain at 29°. Genes with differential splicing between the two genotypes were enriched with genes regulated for 19 splicing factors (Table D, File S1). Five splicing factors, snRNP-U1-70K (FBgn0016978), RpS3 (FBgn0002622), SC35 (FBgn0265298), RnpS1 (FBgn0037707), and Hrb27C (FBgn0004838) showed the same concordance of expression level and exon skipping as core exon junction complex genes (Table D, File S1). The protein components of the spliceosome, snRNP-U1-70K and SC35, are strong candidates for regulating differential splicing between the two genotypes. The auxiliary protein component of the exon junction complex RnpS1 provides further support for the importance of the exon junction complex for the alternative splicing patterns seen in this study.
Table 1. Expression of nuclear core exon junction complex negatively correlates with exon skipping at extreme temperatures.
| 13° | 29° | |||
|---|---|---|---|---|
| FDR | log2FC | FDR | log2FC | |
| mago nashi | 0.077 | 0.915 | 0.006 | −0.923 |
| tsunagi | 0.769 | 0.136 | 0.001 | −1.024 |
| eIF4AIII | 0.035 | 0.914 | 0.024 | −0.659 |
| Mean log2FC expression of exons differentially spliced between Oregon-R and Samarkand | −0.26 | 0.3 | ||
FDR, false discovery rate; FC, fold change.
Similar patterns of temperature-dependent differences among strains for gene expression and alternative splicing
Interestingly, the striking temperature dependence of differential splicing between Oregon-R and Samarkand is mirrored for gene expression intensity (Figure 4) (Chen et al. 2015a). While at 18° the differences in both splicing and gene expression intensity between genotypes are very small, at extreme temperatures the differences increase. Given these parallel patterns, we were interested in whether the same genes were affected and compared the expression intensity differences of the entire gene against the expression differences in each exon (Figure 7; Figure I, File S1). Independent of the developmental temperature, genes with significant differences in gene expression intensity have only limited overlap with genes with differential splicing (Figure 7). These results suggest that despite the overall similarity in temperature dependence of differential splicing and gene expression intensities, both processes are regulated by different mechanisms. This conclusion is further substantiated in a comparison of GO categories that are enriched for genes with significant differential splicing or gene expression intensities at 23 and 29°. Despite both categories harboring a significant enrichment for some genes, there is very little similarity in the enrichment patterns (GO categories) between differential splicing and gene expression (Figure I, File S1). A similar pattern has been observed by Brooks et al. (2015) who found that expression levels of splicing factors that regulate alternative splicing of thousands of genes do not influence their expression intensities (Brooks et al. 2015).
Figure 7.
Limited overlap between genes with differential splicing and those that differ in expression intensity. Differential splicing between Oregon-R and Samarkand is plotted against differential gene expression between the two strains. Significant differences are indicated in color (blue, differential gene expression only; magenta, differential splicing only; yellow, significant for both categories). Density plots on top and on the right of the figure indicate nonoverlapping distribution of genes with significant differences for gene expression and alternative splicing.
Dominance prevails for differential splicing
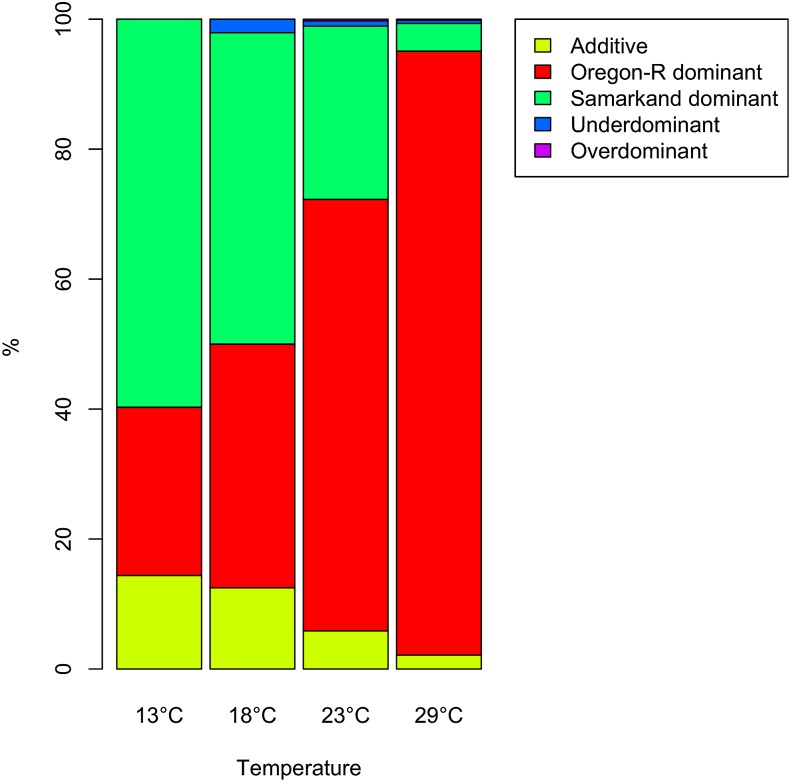
The mode of inheritance of alternative splicing can be studied by contrasting two parental genotypes to offspring of a cross between them. Between 92 and 99% of the genes did not differ significantly from the splicing pattern of both parents. Splicing of most (83–96%) remaining genes matched one of parents (i.e., were dominant; Table 2). Unexpectedly, this dominance was not evenly distributed between the two parental genotypes and differed strikingly among temperatures (Figure 8). This pattern was most extreme at 13 and 29°. While at 13° the splicing pattern of Samarkand was dominant for the majority of genes (58%) and made up for 70% of all genes with dominant splicing inheritance, at 29° splicing of most genes in F1 individuals matched Oregon-R (92%) and corresponded to 96% of all dominant genes. At 18°, no such imbalance of dominance was found (44% Oregon-R dominant vs. 56% Samarkand dominant). To test to what extent allele-specific gene expression may have affected our inference of dominance, we evaluated if genes with dominant splicing also have imbalanced allele-specific expression favoring the allele coming from the dominant parent. On average, 21.75% of the dominant genes have imbalanced allele-specific expression favoring the dominant allele (Figure J, File S1). Nevertheless, even if only genes with no allele specific differences are considered, we still find the same temperature-dependent dominance pattern (Figure J, File S1).
Table 2. Inheritance modes of alternative splicing.
| 13° | 18° | 23° | 29° | |
|---|---|---|---|---|
| Testable genes Oregon-R:F1 | 8982 | 8320 | 8236 | 8182 |
| Testable genes Samarkand:F1 | 8789 | 8352 | 8372 | 8340 |
| Conserved Oregon-R | 8768 | 8267 | 7864 | 7573 |
| Conserved Samarkand | 8672 | 8307 | 8201 | 8243 |
| Conserved both | 8551 | 8204 | 7792 | 7505 |
| Additive | 20 | 6 | 22 | 13 |
| Dominant Oregon-R | 36 | 18 | 249 | 567 |
| Dominant Samarkand | 83 | 23 | 100 | 26 |
| Underdominant | 0 | 1 | 3 | 3 |
| Overdominant | 0 | 0 | 1 | 1 |
Figure 8.
Temperature-dependent dominance. At 13° most of the differences in alternative splicing showed Samarkand dominance (green), while at 29° the pattern was reversed with Oregon-R dominance (red) for most genes.
Similar patterns of swapping dominance for gene expression and alternative splicing
This change in the direction of dominance is not restricted to the patterns of alternative splicing but it can be also be found for gene expression intensities (Chen et al. 2015a). Particularly remarkable is that for gene expression intensity and alternative splicing, more genes in the F1 resemble the Samarkand parent, while at 29° the pattern of the Oregon-R parent is dominant. Despite this overall similarity, we did not find an overlap between the genes showing swapping dominance for alternative splicing and gene expression intensity; suggesting different regulatory mechanisms.
Discussion
This study evaluates the interplay of temperature and genotype on the patterns of alternative splicing. We show that both temperature and genotype have a significant effect on the splicing patterns and that the interaction of both causes a highly complex splicing signature. We identified the exon junction complex as a strong candidate for regulation of temperature-dependent alternative splicing.
Temperature has a very strong effect on alternative splicing. Ranging from only a few genes having differential splicing at 18° to about the same fraction of genes with differentially spliced exons as found in interspecific comparisons (McManus et al. 2014). The same pattern has been observed for gene expression (Chen et al. 2015a). In comparing the differences in alternative splicing to gene expression between the two genotypes across a range of temperatures, we found that even though these two phenotypic levels behave in a similar manner, they operate on clearly distinct groups of genes. To shed light on this phenomenon it is necessary to consider different factors that may influence it, such as the underlying regulation of the two phenotypic levels and selection forces that might shape the differences between the two.
Mode of inheritance
In this study we report, for the first time, an inheritance mode of alternative splicing and its temperature dependence. Our analyses of alternative splicing revealed a striking pattern of prevailing dominant inheritance. Furthermore, dominance of alternative splicing showed temperature dependence by preferably using alternative splicing patterns of Oregon-R at warm temperatures and of Samarkand at cold temperatures. This suggests that temperature-specific alternative splicing might stem from the usage of splicing factors carrying alleles or interacting with alleles that enable them to perform better at a certain temperature. Using the identical data set, Chen et al. (2015a) found the same pattern for gene expression intensities, despite different genes being affected. Interestingly, this prevalence of dominance was also found for other inter- and intraspecific gene expression studies in Drosophila and Cirsium (Gibson et al. 2004; McManus et al. 2010; Bell et al. 2013; Suvorov et al. 2013; Meiklejohn et al. 2014; Chen et al. 2015a). Strong departure from additivity was seen in oysters, with the highest proportion of differentially expressed genes being overdominant (Hedgecock et al. 2007). Nevertheless, the majority of studies in different organisms reported prevalent additive effects with only a minor portion of genes showing dominant or other types of nonadditive inheritance for gene expression (Vuylsteke et al. 2005; Cui et al. 2006; Stupar et al. 2008). This study supports the prevalence of dominant inheritance in Drosophila by extending the study of inheritance to alternative splicing, and suggests that trans-acting factors may be important regulators for alternative splicing as was shown for gene expression (Lemos et al. 2008; Suvorov et al. 2013; Meiklejohn et al. 2014; Chen et al. 2015a).
Selection for genotype × temperature interaction
By analyzing alternative splicing in adult D. melanogaster females from two different genotypes developing at four different temperatures, we observed a complex pattern of plasticity: alternative splicing showed pronounced genotype–temperature interactions. Interestingly, the genotypic differences in splicing were most pronounced at extreme temperatures, while at 18° almost no differences in alternative splicing could be recognized.
Strong differences between genotypes at extreme temperatures were previously described for gene expression intensity (Chen et al. 2015a). The authors argued that this pattern suggests temperature–stress-mediated decanalization of gene expression, reasoning that 18° represents the most benign temperature for D. melanogaster.
What evolutionary forces caused this striking pattern at both phenotypic levels? Unfortunately, the impact of gene expression and alternative splicing differences on organismal fitness is not yet understood. For the sake of argument, we will distinguish extreme scenarios and discuss their consequences.
Hypothesis 1: Gene expression and alternative-splicing differences between strains are adaptive:
Contrasting expression patterns of strains/populations from different habitats in a common garden setting is common practice to identify differentially expressed genes, which serve as candidates for local adaptation (Telonis-Scott et al. 2009; Yampolsky et al. 2012). Along these lines, the differences at extreme temperatures seen in our experiment may be viewed as the signature of adaptation of the two strains used in this study to different environments. On the other hand, if the environments of the two strains are not as different, differences in their expression and splicing patterns may stem from convergent adaptation.
Hypothesis 2: Gene expression and alternative splicing differences between strains are maladaptive:
If selection favors similar gene expression between different genotypes, mechanisms will evolve that result in a pattern of genetic canalization in a specific environment. One classic example for canalization is the Hsp90 gene, which has been shown to suppress phenotypic differences between diverged genotypes. Once the function of Hsp90 is compromised, through mutations or environmental stress, the genetic differences usually manifest in deleterious phenotypic differences (Rutherford and Lindquist 1998). Since these differences are typically sheltered by the action of a buffering system consisting of putative canalization factors such as Hsp90, they will independently accumulate between strains. Thus, once the buffering system is broken, these independently accumulated variants result in differences in gene expression and alternative splicing.
Alternatively, instead of buffering systems, purifying selection could cause the pattern of low phenotypic divergence in a given environment by removing variants causing differences in gene expression and alternative splicing. Reasoning that purifying selection is most effective in the environment in which an organism spends most time, at 18° (the favorite temperature of D. melanogaster larvae) (Kwon et al. 2008; Shen et al. 2011) the fewest deleterious variants are expected. Extreme temperatures, such as 13 and 29°, are avoided by D. melanogaster. Genes that are expressed at these temperatures can accumulate mutations that are inaccessible to purifying selection if they do not affect the gene expression patterns at 18°. In this way, variation resulting in differences in gene expression and alternative splicing could accumulate and will only be detected in environments that are rarely encountered. Some support for this hypothesis is provided by a recent study (Richardson et al. 2013). Comparing the phenotypic variation in mutation-accumulation lines with and without HTZ1, a gene implicated in mutational robustness, they found no difference. Since this observation contrasted results comparing phenotypic variation with and without a gene conferring robustness, they concluded that natural selection must have purged those variants that cannot be buffered (Richardson et al. 2013). We follow this reasoning to explain the phenomenon seen in our data.
Several lines of evidence support the accumulation of deleterious alleles in rarely encountered environments as an explanation for the differences observed between the two strains. First, the differences in alternative splicing and gene expression are affecting different gene sets, suggesting that the two processes are independent from each other. Second, assuming that the recessive allele is the deleterious variant, the concordance of the dominance patterns for alternative splicing and gene expression intensity suggest that Oregon-R has acquired deleterious mutations in warm environments that are expressed at low-assaying temperatures. Samarkand, on the other hand, did the same in cold environments and accumulated deleterious mutations that are uncovered at high temperatures.
This leads to a clear prediction for flies from different temperature environments. While flies originating from hot environments are more likely to accumulate mutations that are deleterious in cold environments, the opposite is true for flies originating in warm environments. This phenomenon can eventually lead to an increase in the gene expression and alternative-splicing variance between populations, which creates a pattern of high-phenotypic differentiation in extreme environments. Future work using flies evolved in extreme environments may yield new evidence in support of this hypothesis.
Acknowledgments
We thank Jun Chen, Alexander Kalinka, François Mallard, and Ray Tobler for helpful discussions. This study was supported by the Austrian Science Fund (FWF P22834 and FWF W1225) and the European Research Council (ArchAdapt).
Footnotes
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.116.192310/-/DC1.
Communicating editor: M. W. Hahn
Literature Cited
- Anders S., Reyes A., Huber W., 2012. Detecting differential usage of exons from RNA-seq data. Genome Res. 22: 2008–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton-Beaucage D., Udell C. M., Lavoie H., Baril C., Lefrançois M., et al. , 2010. The exon junction complex controls the splicing of MAPK and other long intron-containing transcripts in Drosophila. Cell 143: 251–262. [DOI] [PubMed] [Google Scholar]
- Barberan-Soler S., Zahler A. M., 2008. Alternative splicing regulation during C. elegans development: splicing factors as regulated targets. PLoS Genet. 4: e1000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa-Morais N. L., Irimia M., Pan Q., Xiong H. Y., Gueroussov S., et al. , 2012. The evolutionary landscape of alternative splicing in vertebrate species. Science 338: 1587–1593. [DOI] [PubMed] [Google Scholar]
- Bartok O., Kyriacou C. P., Levine J., Sehgal A., Kadener S., 2013. Adaptation of molecular circadian clockwork to environmental changes: a role for alternative splicing and miRNAs. Proc. Biol. Sci. 280: 20130011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. D. M., Kane N. C., Rieseberg L. H., Adams K. L., 2013. RNA-seq analysis of allele-specific expression, hybrid effects, and regulatory divergence in hybrids compared with their parents from natural populations. Genome Biol. Evol. 5: 1309–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks A. N., Duff M. O., May G., Yang L., Bolisetty M., et al. , 2015. Regulation of alternative splicing in Drosophila by 56 RNA binding proteins. Genome Res. 25: 1771–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. B., Boley N., Eisman R., May G. E., Stoiber M. H., et al. , 2014. Diversity and dynamics of the Drosophila transcriptome. Nature 512: 393–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.-Y., Lin W.-D., Tu S.-L., 2014. Genome-Wide Analysis of Heat-Sensitive Alternative Splicing in Physcomitrella patens. Plant Physiol. 165: 826–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J., V. Nolte, and C. Schlötterer, 2015a Temperature Stress Mediates Decanalization and Dominance of Gene Expression in Drosophila melanogaster. PLoS Genet. 11: e1004883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Nolte V., Schlötterer C., 2015b Temperature-Related Reaction Norms of Gene Expression: Regulatory Architecture and Functional Implications. Mol. Biol. Evol. 32: 2393–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X., Affourtit J., Shockley K. R., Woo Y., Churchill G. A., 2006. Inheritance patterns of transcript levels in F1 hybrid mice. Genetics 174: 627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etges W. J., Trotter M. V., de Oliveira C. C., Rajpurohit S., Gibbs A. G., et al. , 2015. Deciphering life history transcriptomes in different environments. Mol. Ecol. 24: 151–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan Q., Chepelev I., Wei G., Tarayrah L., Cui K., et al. , 2010. Dynamic regulation of alternative splicing and chromatin structure in Drosophila gonads revealed by RNA-seq. Cell Res. 20: 763–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G., Riley-Berger R., Harshman L., Kopp A., Vacha S., et al. , 2004. Extensive sex-specific nonadditivity of gene expression in Drosophila melanogaster. Genetics 167: 1791–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graveley B. R., Brooks A. N., Carlson J. W., Duff M. O., Landolin J. M., et al. , 2011. The developmental transcriptome of Drosophila melanogaster. Nature 471: 473–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedgecock D., Lin J. Z., DeCola S., Haudenschild C. D., Meyer E., et al. , 2007. Transcriptomic analysis of growth heterosis in larval Pacific oysters (Crassostrea gigas). Proc. Natl. Acad. Sci. USA 104: 2313–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W., Riley R. M., Wolfinger R. D., White K. P., Passador-Gurgel G., et al. , 2001. The contributions of sex, genotype and age to transcriptional variance in Drosophila melanogaster. Nat. Genet. 29: 389–395. [DOI] [PubMed] [Google Scholar]
- Kofler R., Pandey R. V., Schlötterer C., 2011. PoPoolation2: identifying differentiation between populations using sequencing of pooled DNA samples (Pool-Seq). Bioinformatics 27: 3435–3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofler R., Schlötterer C., 2012. Gowinda: unbiased analysis of gene set enrichment for genome-wide association studies. Bioinformatics 28: 2084–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren, E., G. Lev-Maor, and G. Ast, 2007 The Emergence of Alternative 3′ and 5′ Splice Site Exons from Constitutive Exons. PLoS Comput. Biol. 3: e95. [DOI] [PMC free article] [PubMed]
- Kwon Y., Shim H. S., Wang X., Montell C., 2008. Control of thermotactic behavior via coupling of a TRP channel to a phospholipase C signaling cascade. Nat. Neurosci. 11: 871–873. [DOI] [PubMed] [Google Scholar]
- Lemos, B., L. O. Araripe, P. Fontanillas, and D. L. Hartl, 2008 Dominance and the evolutionary accumulation of cis- and trans-effects on gene expression. Proc. Natl. Acad. Sci. USA 105: 14471–14476. [DOI] [PMC free article] [PubMed]
- Leviatan N., Alkan N., Leshkowitz D., Fluhr R., 2013. Genome-wide survey of cold stress regulated alternative splicing in Arabidopsis thaliana with tiling microarray. PLoS One 8: e66511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. T., Eckert M. L., Begun D. J., 2011. Whole-genome expression plasticity across tropical and temperate Drosophila melanogaster populations from Eastern Australia. Mol. Biol. Evol. 28: 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Y., Song G., Yan J., He X., Li Q., et al. , 2013. Transcriptomic characterization of cold acclimation in larval zebrafish. BMC Genomics 14: 612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrangelo, A. M., D. Marone, G. Laidò, A. M. De Leonardis, and P. De Vita, 2012 Alternative splicing: enhancing ability to cope with stress via transcriptome plasticity. Plant Sci. 185–186: 40–49. [DOI] [PubMed]
- McManus C. J., Coolon J. D., Duff M. O., Eipper-Mains J., Graveley B. R., et al. , 2010. Regulatory divergence in Drosophila revealed by mRNA-seq. Genome Res. 20: 816–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus C. J., Coolon J. D., Eipper-Mains J., Wittkopp P. J., Graveley B. R., 2014. Evolution of splicing regulatory networks in Drosophila. Genome Res. 24: 786–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiklejohn C. D., Coolon J. D., Hartl D. L., Wittkopp P. J., 2014. The roles of cis- and trans-regulation in the evolution of regulatory incompatibilities and sexually dimorphic gene expression. Genome Res. 24: 84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen T. W., Graveley B. R., 2010. Expansion of the eukaryotic proteome by alternative splicing. Nature 463: 457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Q., Shai O., Lee L. J., Frey B. J., Blencowe B. J., 2008. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat. Genet. 40: 1413–1415. [DOI] [PubMed] [Google Scholar]
- Reyes A., Anders S., Weatheritt R. J., Gibson T. J., Steinmetz L. M., et al. , 2013. Drift and conservation of differential exon usage across tissues in primate species. Proc. Natl. Acad. Sci. USA 110: 15377–15382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson J. B., Uppendahl L. D., Traficante M. K., Levy S. F., Siegal M. L., 2013. Histone variant HTZ1 shows extensive epistasis with, but does not increase robustness to, new mutations. PLoS Genet. 9: e1003733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford S. L., Lindquist S., 1998. Hsp90 as a capacitor for morphological evolution. Nature 396: 336–342. [DOI] [PubMed] [Google Scholar]
- Shen W. L., Kwon Y., Adegbola A. A., Luo J., Chess A., et al. , 2011. Function of rhodopsin in temperature discrimination in Drosophila. Science 331: 1333–1336. [DOI] [PubMed] [Google Scholar]
- Sikkink K. L., Reynolds R. M., Ituarte C. M., Cresko W. A., Phillips P. C., 2014. Rapid evolution of phenotypic plasticity and shifting thresholds of genetic assimilation in the nematode Caenorhabditis remanei. G3 (Bethesda) 4: 1103–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G., Fang Y., Liu X., Kenny J., Cossins A. R., et al. , 2013. Transcriptome-wide expression variation associated with environmental plasticity and mating success in cactophilic Drosophila mojavensis. Evolution 67: 1950–1963. [DOI] [PubMed] [Google Scholar]
- Stupar R. M., Gardiner J. M., Oldre A. G., Haun W. J., Chandler V. L., et al. , 2008. Gene expression analyses in maize inbreds and hybrids with varying levels of heterosis. BMC Plant Biol. 8: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvorov A., Nolte V., Pandey R. V., Franssen S. U., Futschik A., et al. , 2013. Intra-specific regulatory variation in Drosophila pseudoobscura. PLoS One 8: e83547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tange T. Ø., Nott A., Moore M. J., 2004. The ever-increasing complexities of the exon junction complex. Curr. Opin. Cell Biol. 16: 279–284. [DOI] [PubMed] [Google Scholar]
- Telonis-Scott M., Hallas R., McKechnie S. W., Wee C. W., Hoffmann A. A., 2009. Selection for cold resistance alters gene transcript levels in Drosophila melanogaster. J. Insect Physiol. 55(6): 549–555. [DOI] [PubMed] [Google Scholar]
- Telonis-Scott M., van Heerwaarden B., Johnson T. K., Hoffmann A. A., Sgrò C. M., 2013. New levels of transcriptome complexity at upper thermal limits in wild Drosophila revealed by exon expression analysis. Genetics 195: 809–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitulo N., Forcato C., Carpinelli E. C., Telatin A., Campagna D., et al. , 2014. A deep survey of alternative splicing in grape reveals changes in the splicing machinery related to tissue, stress condition and genotype. BMC Plant Biol. 14: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuylsteke M., van Eeuwijk F., Van Hummelen P., Kuiper M., Zabeau M., 2005. Genetic analysis of variation in gene expression in Arabidopsis thaliana. Genetics 171: 1267–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E. T., Sandberg R., Luo S., Khrebtukova I., Zhang L., et al. , 2008. Alternative isoform regulation in human tissue transcriptomes. Nature 456: 470–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Murigneux V., Le Hir H., 2014. Transcriptome-wide modulation of splicing by the exon junction complex. Genome Biol. 15: 551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T. D., Nacu S., 2010. Fast and SNP-tolerant detection of complex variants and splicing in short reads. Bioinformatics 26: 873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yampolsky L. Y., Glazko G. V., Fry J. D., 2012. Evolution of gene expression and expression plasticity in long-term experimental populations of Drosophila melanogaster maintained under constant and variable ethanol stress. Mol. Ecol. 21: 4287–4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yampolsky L. Y., Zeng E., Lopez J., Williams P. J., Dick K. B., et al. , 2014. Functional genomics of acclimation and adaptation in response to thermal stress in Daphnia. BMC Genomics 15: 859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, L., J. Wit, N. Svetec, and D. J. Begun, 2015 Parallel Gene Expression Differences between Low and High Latitude Populations of Drosophila melanogaster and D. simulans. PLOS Genet. 11: e1005184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Campbell T. G., Stone E. A., Mackay T. F., Anholt R. R., 2012. Phenotypic plasticity of the Drosophila transcriptome. PLoS Genet. 8: e1002593. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All raw sequence data used in this study is deposited in the National Center for Biotechnology Information Sequence Read Archive with accession numbers SRP041398 (Oregon-R and Samarkand) and SRP041395 (F1). All unfiltered read counts, custom scripts, and protocols will be available at DataDryad.org.