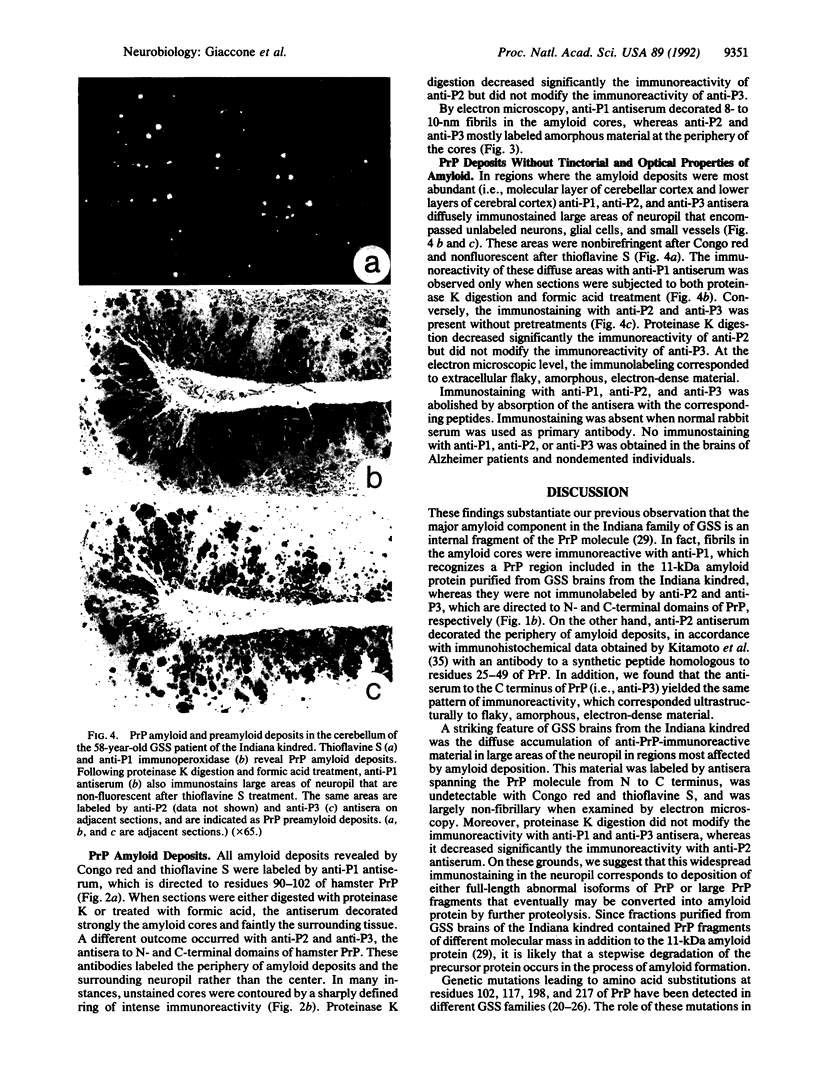
Abstract
Gerstmann-Sträussler-Scheinker disease (GSS) is a familial neurological disorder pathologically characterized by amyloid deposition in the cerebrum and cerebellum. In GSS, the amyloid is immunoreactive to antisera raised against the prion protein (PrP) 27-30, a proteinase K-resistant peptide of 27-30 kDa that is derived by limited proteolysis from an abnormal isoform of a neuronal sialoglycoprotein of 33-35 kDa designated PrPSc. Polyclonal antibodies raised against synthetic peptides homologous to residues 15-40 (P2), 90-102 (P1), and 220-232 (P3) of the amino acid sequence deduced from hamster PrP cDNA were used to investigate immunohistochemically the distribution of PrP and PrP fragments in the brains of two patients from the Indiana kindred of GSS. Two types of anti-PrP-immunoreactive deposits were found: (i) amyloid deposits, which were exclusively labeled by anti-P1 antiserum to residues 90-102 of PrP, and (ii) preamyloid deposits, which were labeled by all anti-PrP antisera but did not exhibit the tinctorial and optical properties of amyloid. The latter appeared as diffuse immunostaining of the neuropil that targeted to areas in which amyloid deposits were most abundant. They were partially resistant to proteinase K digestion and consisted ultrastructurally of amorphous, flaky, electron-dense material. These findings substantiate our previous observation that the major amyloid component in the GSS Indiana kindred is an internal fragment of PrP and indicate that full-length abnormal isoforms of PrP and/or large PrP fragments accumulate in brain regions most affected by amyloid deposition. These findings support the view that in the GSS Indiana kindred a stepwise degradation of PrP occurs in situ in the process of amyloid fibril formation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry R. A., Vincent M. T., Kent S. B., Hood L. E., Prusiner S. B. Characterization of prion proteins with monospecific antisera to synthetic peptides. J Immunol. 1988 Feb 15;140(4):1188–1193. [PubMed] [Google Scholar]
- Basler K., Oesch B., Scott M., Westaway D., Wälchli M., Groth D. F., McKinley M. P., Prusiner S. B., Weissmann C. Scrapie and cellular PrP isoforms are encoded by the same chromosomal gene. Cell. 1986 Aug 1;46(3):417–428. doi: 10.1016/0092-8674(86)90662-8. [DOI] [PubMed] [Google Scholar]
- Bolton D. C., McKinley M. P., Prusiner S. B. Identification of a protein that purifies with the scrapie prion. Science. 1982 Dec 24;218(4579):1309–1311. doi: 10.1126/science.6815801. [DOI] [PubMed] [Google Scholar]
- Brown P., Coker-Vann M., Pomeroy K., Franko M., Asher D. M., Gibbs C. J., Jr, Gajdusek D. C. Diagnosis of Creutzfeldt-Jakob disease by Western blot identification of marker protein in human brain tissue. N Engl J Med. 1986 Feb 27;314(9):547–551. doi: 10.1056/NEJM198602273140904. [DOI] [PubMed] [Google Scholar]
- Brown P., Goldfarb L. G., Brown W. T., Goldgaber D., Rubenstein R., Kascsak R. J., Guiroy D. C., Piccardo P., Boellaard J. W., Gajdusek D. C. Clinical and molecular genetic study of a large German kindred with Gerstmann-Sträussler-Scheinker syndrome. Neurology. 1991 Mar;41(3):375–379. doi: 10.1212/wnl.41.3.375. [DOI] [PubMed] [Google Scholar]
- Bruce M. E., McBride P. A., Farquhar C. F. Precise targeting of the pathology of the sialoglycoprotein, PrP, and vacuolar degeneration in mouse scrapie. Neurosci Lett. 1989 Jul 17;102(1):1–6. doi: 10.1016/0304-3940(89)90298-x. [DOI] [PubMed] [Google Scholar]
- Bugiani O., Giaccone G., Frangione B., Ghetti B., Tagliavini F. Alzheimer patients: preamyloid deposits are more widely distributed than senile plaques throughout the central nervous system. Neurosci Lett. 1989 Sep 11;103(3):263–268. doi: 10.1016/0304-3940(89)90110-9. [DOI] [PubMed] [Google Scholar]
- DeArmond S. J., Mobley W. C., DeMott D. L., Barry R. A., Beckstead J. H., Prusiner S. B. Changes in the localization of brain prion proteins during scrapie infection. Neurology. 1987 Aug;37(8):1271–1280. doi: 10.1212/wnl.37.8.1271. [DOI] [PubMed] [Google Scholar]
- Diedrich J. F., Bendheim P. E., Kim Y. S., Carp R. I., Haase A. T. Scrapie-associated prion protein accumulates in astrocytes during scrapie infection. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):375–379. doi: 10.1073/pnas.88.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diringer H., Gelderblom H., Hilmert H., Ozel M., Edelbluth C., Kimberlin R. H. Scrapie infectivity, fibrils and low molecular weight protein. Nature. 1983 Dec 1;306(5942):476–478. doi: 10.1038/306476a0. [DOI] [PubMed] [Google Scholar]
- Dlouhy S. R., Hsiao K., Farlow M. R., Foroud T., Conneally P. M., Johnson P., Prusiner S. B., Hodes M. E., Ghetti B. Linkage of the Indiana kindred of Gerstmann-Sträussler-Scheinker disease to the prion protein gene. Nat Genet. 1992 Apr;1(1):64–67. doi: 10.1038/ng0492-64. [DOI] [PubMed] [Google Scholar]
- Doh-ura K., Tateishi J., Sasaki H., Kitamoto T., Sakaki Y. Pro----leu change at position 102 of prion protein is the most common but not the sole mutation related to Gerstmann-Sträussler syndrome. Biochem Biophys Res Commun. 1989 Sep 15;163(2):974–979. doi: 10.1016/0006-291x(89)92317-6. [DOI] [PubMed] [Google Scholar]
- Ghetti B., Tagliavini F., Masters C. L., Beyreuther K., Giaccone G., Verga L., Farlow M. R., Conneally P. M., Dlouhy S. R., Azzarelli B. Gerstmann-Sträussler-Scheinker disease. II. Neurofibrillary tangles and plaques with PrP-amyloid coexist in an affected family. Neurology. 1989 Nov;39(11):1453–1461. doi: 10.1212/wnl.39.11.1453. [DOI] [PubMed] [Google Scholar]
- Giaccone G., Tagliavini F., Linoli G., Bouras C., Frigerio L., Frangione B., Bugiani O. Down patients: extracellular preamyloid deposits precede neuritic degeneration and senile plaques. Neurosci Lett. 1989 Feb 13;97(1-2):232–238. doi: 10.1016/0304-3940(89)90169-9. [DOI] [PubMed] [Google Scholar]
- Giaccone G., Tagliavini F., Verga L., Frangione B., Farlow M. R., Bugiani O., Ghetti B. Neurofibrillary tangles of the Indiana kindred of Gerstmann-Sträussler-Scheinker disease share antigenic determinants with those of Alzheimer disease. Brain Res. 1990 Oct 22;530(2):325–329. doi: 10.1016/0006-8993(90)91304-y. [DOI] [PubMed] [Google Scholar]
- Hsiao K., Baker H. F., Crow T. J., Poulter M., Owen F., Terwilliger J. D., Westaway D., Ott J., Prusiner S. B. Linkage of a prion protein missense variant to Gerstmann-Sträussler syndrome. Nature. 1989 Mar 23;338(6213):342–345. doi: 10.1038/338342a0. [DOI] [PubMed] [Google Scholar]
- Hsiao K., Dlouhy S. R., Farlow M. R., Cass C., Da Costa M., Conneally P. M., Hodes M. E., Ghetti B., Prusiner S. B. Mutant prion proteins in Gerstmann-Sträussler-Scheinker disease with neurofibrillary tangles. Nat Genet. 1992 Apr;1(1):68–71. doi: 10.1038/ng0492-68. [DOI] [PubMed] [Google Scholar]
- Kitamoto T., Muramoto T., Hilbich C., Beyreuther K., Tateishi J. N-terminal sequence of prion protein is also integrated into kuru plaques in patients with Gerstmann-Sträussler syndrome. Brain Res. 1991 Apr 5;545(1-2):319–321. doi: 10.1016/0006-8993(91)91306-l. [DOI] [PubMed] [Google Scholar]
- Kitamoto T., Tateishi J., Sato Y. Immunohistochemical verification of senile and kuru plaques in Creutzfeldt-Jakob disease and the allied disease. Ann Neurol. 1988 Oct;24(4):537–542. doi: 10.1002/ana.410240410. [DOI] [PubMed] [Google Scholar]
- Kitamoto T., Tateishi J., Tashima T., Takeshita I., Barry R. A., DeArmond S. J., Prusiner S. B. Amyloid plaques in Creutzfeldt-Jakob disease stain with prion protein antibodies. Ann Neurol. 1986 Aug;20(2):204–208. doi: 10.1002/ana.410200205. [DOI] [PubMed] [Google Scholar]
- Kretzschmar H. A., Honold G., Seitelberger F., Feucht M., Wessely P., Mehraein P., Budka H. Prion protein mutation in family first reported by Gerstmann, Sträussler, and Scheinker. Lancet. 1991 May 11;337(8750):1160–1160. doi: 10.1016/0140-6736(91)92826-n. [DOI] [PubMed] [Google Scholar]
- Kretzschmar H. A., Stowring L. E., Westaway D., Stubblebine W. H., Prusiner S. B., Dearmond S. J. Molecular cloning of a human prion protein cDNA. DNA. 1986 Aug;5(4):315–324. doi: 10.1089/dna.1986.5.315. [DOI] [PubMed] [Google Scholar]
- Masters C. L., Gajdusek D. C., Gibbs C. J., Jr Creutzfeldt-Jakob disease virus isolations from the Gerstmann-Sträussler syndrome with an analysis of the various forms of amyloid plaque deposition in the virus-induced spongiform encephalopathies. Brain. 1981 Sep;104(3):559–588. doi: 10.1093/brain/104.3.559. [DOI] [PubMed] [Google Scholar]
- McKinley M. P., Bolton D. C., Prusiner S. B. A protease-resistant protein is a structural component of the scrapie prion. Cell. 1983 Nov;35(1):57–62. doi: 10.1016/0092-8674(83)90207-6. [DOI] [PubMed] [Google Scholar]
- Oesch B., Westaway D., Wälchli M., McKinley M. P., Kent S. B., Aebersold R., Barry R. A., Tempst P., Teplow D. B., Hood L. E. A cellular gene encodes scrapie PrP 27-30 protein. Cell. 1985 Apr;40(4):735–746. doi: 10.1016/0092-8674(85)90333-2. [DOI] [PubMed] [Google Scholar]
- Picken M. M., Frangione B., Barlogie B., Luna M., Gallo G. Light chain deposition disease derived from the kappa I light chain subgroup. Biochemical characterization. Am J Pathol. 1989 Apr;134(4):749–754. [PMC free article] [PubMed] [Google Scholar]
- Prusiner S. B., Bolton D. C., Groth D. F., Bowman K. A., Cochran S. P., McKinley M. P. Further purification and characterization of scrapie prions. Biochemistry. 1982 Dec 21;21(26):6942–6950. doi: 10.1021/bi00269a050. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Groth D. F., Bolton D. C., Kent S. B., Hood L. E. Purification and structural studies of a major scrapie prion protein. Cell. 1984 Aug;38(1):127–134. doi: 10.1016/0092-8674(84)90533-6. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., McKinley M. P., Bowman K. A., Bolton D. C., Bendheim P. E., Groth D. F., Glenner G. G. Scrapie prions aggregate to form amyloid-like birefringent rods. Cell. 1983 Dec;35(2 Pt 1):349–358. doi: 10.1016/0092-8674(83)90168-x. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Scott M., Foster D., Pan K. M., Groth D., Mirenda C., Torchia M., Yang S. L., Serban D., Carlson G. A. Transgenetic studies implicate interactions between homologous PrP isoforms in scrapie prion replication. Cell. 1990 Nov 16;63(4):673–686. doi: 10.1016/0092-8674(90)90134-z. [DOI] [PubMed] [Google Scholar]
- Stahl N., Borchelt D. R., Hsiao K., Prusiner S. B. Scrapie prion protein contains a phosphatidylinositol glycolipid. Cell. 1987 Oct 23;51(2):229–240. doi: 10.1016/0092-8674(87)90150-4. [DOI] [PubMed] [Google Scholar]
- Tagliavini F., Giaccone G., Frangione B., Bugiani O. Preamyloid deposits in the cerebral cortex of patients with Alzheimer's disease and nondemented individuals. Neurosci Lett. 1988 Nov 11;93(2-3):191–196. doi: 10.1016/0304-3940(88)90080-8. [DOI] [PubMed] [Google Scholar]
- Tagliavini F., Prelli F., Ghiso J., Bugiani O., Serban D., Prusiner S. B., Farlow M. R., Ghetti B., Frangione B. Amyloid protein of Gerstmann-Sträussler-Scheinker disease (Indiana kindred) is an 11 kd fragment of prion protein with an N-terminal glycine at codon 58. EMBO J. 1991 Mar;10(3):513–519. doi: 10.1002/j.1460-2075.1991.tb07977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateishi J., Kitamoto T., Doh-ura K., Sakaki Y., Steinmetz G., Tranchant C., Warter J. M., Heldt N. Immunochemical, molecular genetic, and transmission studies on a case of Gerstmann-Sträussler-Scheinker syndrome. Neurology. 1990 Oct;40(10):1578–1581. doi: 10.1212/wnl.40.10.1578. [DOI] [PubMed] [Google Scholar]
- Tranchant C., Doh-Ura K., Steinmetz G., Chevalier Y., Kitamoto T., Tateishi J., Warter J. M. Mutation du codon 117 du gène du prion dans une maladie de Gerstmann-Sträussler-Scheinker. Rev Neurol (Paris) 1991;147(4):274–278. [PubMed] [Google Scholar]
- Verga L., Frangione B., Tagliavini F., Giaccone G., Migheli A., Bugiani O. Alzheimer patients and Down patients: cerebral preamyloid deposits differ ultrastructurally and histochemically from the amyloid of senile plaques. Neurosci Lett. 1989 Nov 6;105(3):294–299. doi: 10.1016/0304-3940(89)90636-8. [DOI] [PubMed] [Google Scholar]
- Yang G. C., Gallo G. R. Protein A-gold immunoelectron microscopic study of amyloid fibrils, granular deposits, and fibrillar luminal aggregates in renal amyloidosis. Am J Pathol. 1990 Nov;137(5):1223–1231. [PMC free article] [PubMed] [Google Scholar]