Abstract
Dosage compensation mechanisms equalize the level of X chromosome expression between sexes. Yet the X chromosome is often enriched for genes exhibiting sex-biased, i.e., imbalanced expression. The relationship between X chromosome dosage compensation and sex-biased gene expression remains largely unexplored. Most studies determine sex-biased gene expression without distinguishing between contributions from X chromosome copy number (dose) and the animal’s sex. Here, we uncoupled X chromosome dose from sex-specific gene regulation in Caenorhabditis elegans to determine the effect of each on X expression. In early embryogenesis, when dosage compensation is not yet fully active, X chromosome dose drives the hermaphrodite-biased expression of many X-linked genes, including several genes that were shown to be responsible for hermaphrodite fate. A similar effect is seen in the C. elegans germline, where X chromosome dose contributes to higher hermaphrodite X expression, suggesting that lack of dosage compensation in the germline may have a role in supporting higher expression of X chromosomal genes with female-biased functions in the gonad. In the soma, dosage compensation effectively balances X expression between the sexes. As a result, somatic sex-biased expression is almost entirely due to sex-specific gene regulation. These results suggest that lack of dosage compensation in different tissues and developmental stages allow X chromosome copy number to contribute to sex-biased gene expression and function.
Keywords: dosage compensation, C. elegans, RNA-seq, sex, sex-biased gene expression, chromatin, X chromosome, transcription, gene regulation, germline, XO, genetics of sex
MALES and females exhibit many phenotypic differences, termed sexual dimorphisms. With the exception of the gene-poor Y chromosome, the two sexes share identical genomes. Thus, sexual dimorphisms stem from differences in gene expression between sexes. Genes that are expressed differently between sexes include those expressed exclusively (sex specific) or at a higher level in one sex (sex biased). Sex-biased expression allows a gene to be more active in the sex that it benefits without a potential cost to the opposite sex. Sex-biased gene expression is common throughout metazoans. Previous studies indicate up to 60% of all genes show sex-biased expression [in mice (Khil et al. 2004; Yang et al. 2006; Reinius et al. 2012), flies (Parisi et al. 2003; Ranz et al. 2003; Allen et al. 2013; Kaiser and Bachtrog 2014), and nematodes (Reinke et al. 2004; Thomas et al. 2012; Albritton et al. 2014)].
Previous genome-wide studies found that compared to autosomes, the X chromosome generally harbors more genes with female-biased expression and fewer genes with male-biased expression [in mice (Khil et al. 2004; Yang et al. 2006; Reinius et al. 2012), flies (Parisi et al. 2003; Ranz et al. 2003), and nematodes (Reinke et al. 2004; Thomas et al. 2012; Albritton et al. 2014)]. However, this observed enrichment of female bias and depletion of male bias from the X is a generalization that may not apply universally to every tissue type or developmental stage. For instance, while genes expressed during later spermatogenesis are depleted from the X, genes expressed during early spermatogenesis are enriched on the mouse X chromosome compared to autosomes (Khil et al. 2004). This switch from enrichment to depletion has been linked to the onset of meiotic sex chromosome inactivation (MSCI), which acts to silence the single male X during spermatogenesis, driving male-biased genes to the autosomes. Germ-cell-specific genes located on the X are silenced by MSCI, during male meiosis, while autosomal genes are largely unaffected (Wang et al. 2005). However, a subset of germ-cell-specific X-linked genes is reactivated later in spermatids (Mueller et al. 2008). Indeed, newly acquired genes on human X chromosomes show specific expression in testis (Bellott et al. 2010; Mueller et al. 2013). In flies, MSCI may not be the sole force driving depletion of male-biased genes from the X chromosome; male-biased genes that are expressed in somatic tissues are also depleted from the X (Parisi et al. 2003; Sturgill et al. 2007). In nematodes, genes exhibiting high male-biased expression in the germline are depleted from the X chromosome, while genes that show lower levels of male-biased expression are enriched on the X (Albritton et al. 2014). Thus, sex-biased gene expression on the X chromosomes is shaped by the mechanisms that regulate X chromosome expression in different tissues and species.
While some X chromosomal genes show sex-biased expression, others are expressed equally between sexes due to X chromosome dosage compensation mechanisms. The strategies of dosage compensation differ between different species. The three best-studied cases of dosage compensation are mammalian female X inactivation, male-specific X upregulation in Drosophila melanogaster, and hermaphrodite-specific X downregulation in Caenorhabditis elegans (reviewed in Ercan 2015). In each of these cases, a multisubunit protein complex specifically binds and regulates transcription of the X chromosome in one of the two sexes. In mammals, Xist RNA coats one of the two female X chromosomes, resulting in transcriptional silencing. In Drosophila, the MSL complex binds to the single X chromosome in males and upregulates transcription approximately twofold. In C. elegans, the dosage compensation complex (DCC) binds to both X chromosomes in hermaphrodites and represses transcription of each approximately twofold.
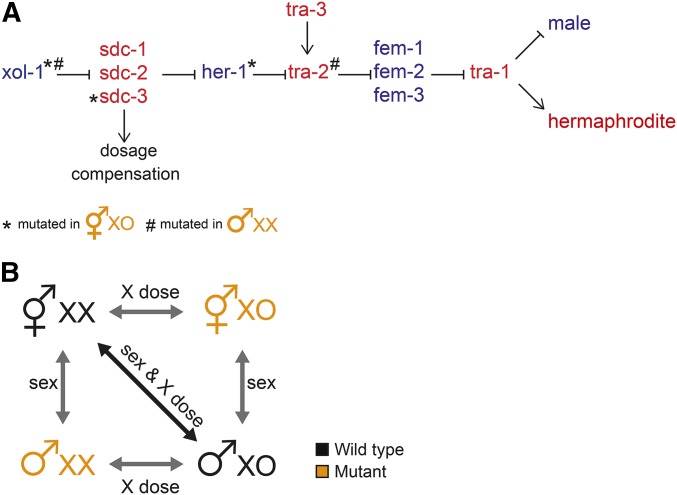
In C. elegans, hermaphrodites have two X chromosomes and males have a single X (C. elegans males do not have a Y chromosome). Sex is determined by the ratio of X chromosomes to autosomes (Madl and Herman 1979). Hermaphrodites have an X:A ratio of 2:2 and males have a ratio of 1:2. This difference leads to differential expression of xol-1, which triggers the male sex determination pathway (Figure 1A) (Miller et al. 1988; Rhind et al. 1995). Lack of xol-1 expression steers the pathway toward hermaphrodite development (Miller et al. 1988). xol-1 represses sdc-1, sdc-2, and sdc-3, named for their role in sex determination and dosage compensation (Rhind et al. 1995). SDC-1, SDC-2, and SDC-3 regulate dosage compensation by forming part of the DCC and regulate sex determination by repressing the autosomal male-promoting gene her-1 (Villeneuve and Meyer 1987; Nusbaum and Meyer 1989; DeLong et al. 1993; Klein and Meyer 1993; Dawes et al. 1999; Chu et al. 2002). In hermaphrodites, the lack of her-1 expression activates transmembrane receptor TRA-2A (Hunter and Wood 1990; Perry et al. 1993). This represses three fem (feminizing when mutated) genes: fem-1, fem-2, and fem-3, which inhibit tra-1 (Hodgkin 1986). As a consequence, tra-1 is active in hermaphrodites and inactive in males (Figure 1A). tra-1 regulates expression of genes that create sexually dimorphic cells and tissues in hermaphrodites by repressing genes that promote male differentiation (Hodgkin 1987; Villeneuve and Meyer 1990; Berkseth et al. 2013).
Figure 1.
Uncoupling X chromosome number from sex in C. elegans. (A) When comparing wild-type hermaphrodites and males, both sex and X chromosome dose differ. Previous studies used sex determination pathway (adapted from Zarkower 2006) mutations that uncouple sex from X dose by creating XX males and XO hermaphrodites (for details on the mutations, refer to the text). Briefly, in C. elegans, X chromosome dose regulates the xol-1 gene, which controls both sex determination and dosage compensation. In males, xol-1 represses the DCC and activates the male-determining gene her-1. In hermaphrodites, xol-1 is repressed, thus the DCC is activated and her-1 is repressed, activating the hermaphrodite pathway. The downstream pathway leads to tra-1, which encodes a transcription factor that represses male development. Mutant genes that create XX males and XO hermaphrodites are indicated. (B) Comparing mutant strains to wild-type males or hermaphrodites allows isolation of sex and X dose effect.
Sex-specific gene regulation is accomplished by transcription factors that control sex-biased expression of their target genes. Sex-specific gene regulation that creates sex-biased gene expression on the X chromosome is directly countered by dosage compensation working to balance X expression between the sexes. It is possible that these processes evolved independently and that the effect of one mechanism is superimposed onto the other, such that the net effect is additive. Alternatively, it may be that the two mechanisms interact. For instance, a mammalian gene escaping X inactivation would show twofold higher expression in females (i.e., female-biased expression). In both C. elegans and D. melanogaster, dosage compensation machinery “fine tunes” transcription by reducing or increasing X-linked gene expression twofold, respectively. Any imprecision could lead to sex-biased gene expression. For example, a lack of repression by the C. elegans DCC would result in hermaphrodite-biased expression.
Comparing gene expression between sexes cannot distinguish between the effects of sex-specific gene regulation and dosage compensation on the establishment of sex-biased gene expression. As a result, it is not clear to what extent X chromosome number contributes to chromosomal distribution of sex-biased genes, which is important to understand the evolution of X chromosomes (Mank et al. 2011; Dean and Mank 2014). Here, we addressed this question by uncoupling sex from X chromosome number using well-characterized sex determination pathway mutants in C. elegans. We performed messenger RNA sequencing (mRNA-seq) analysis of wild-type XX hermaphrodites, wild-type XO males, and mutants that produce XO hermaphrodites and XX males. To study potential differences between tissues, we performed the analyses in L3 larvae and in young adults, before and after sexually dimorphic organs developed, respectively. Our data untangle the contribution of sex-specific gene regulation and X dose toward the establishment of sex-biased gene expression of the X chromosome. Moreover, we reveal differences between developmental stages and tissues. We find that sex-biased gene expression is more prevalent in young adults than larvae and X chromosome number contributes to sex-biased expression among early zygotic and gonadal genes.
Material and Methods
Worm strains and growth
XX hermaphrodites and XO males were from the N2 Bristol strain. XO hermaphrodites were collected from strain TY2205 her-1(e1520) sdc-3(y126) V; xol-1(y9) X (Chuang et al. 1996; Yonker and Meyer 2003), and XX males were collected from CB5362 tra-2(ar221) II; xol-1 (y9) X (Hodgkin 2002). Unless otherwise noted, strains were maintained at 20° on NGM agar plates using standard C. elegans growth methods. Larvae were collected after growing hatched L1s for 24 hr (L3 larvae) on NGM plates at room temperature. Young adults were synchronized by growing hatched L1s for 44 hr at 25° and collected prior to the accumulation of fertilized embryos in the gonad. Developmental stages were assessed by observing DAPI-stained nuclei, assessing gonad, male tail, and vulva morphology.
mRNA-seq
A summary of all the data sets including replicates, read numbers, mapping percentages and correlations between replicates is provided in Supplemental Material, File S1. In short, three to four biological replicates were collected for each condition and independent sequencing libraries created. The replicates were well correlated, as measured by Spearman’s rank correlation coefficients, between 0.90 and 0.99. Raw data files and RNA-seq fragments per kilobase of exon per million fragments mapped (FPKM) values are provided at Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/geo/) under accession nos. GSE67650 and GSE77794. For RNA preparation, samples in TRIzol were frozen and thawed three to five times to freeze-crack worms, and the total RNA was cleaned up using the Qiagen RNeasy kit. mRNA was purified using Sera-Mag oligo(dT) beads (Thermo Scientific) from 0.5 to 10 μg of total RNA. Stranded mRNA-seq libraries were prepared based on incorporation of deoxyuridine triphosphates (dUTPs) during complementary DNA (cDNA) synthesis using a previously described protocol (Parkhomchuk et al. 2009). Single-end 50-bp sequencing was performed using the Illumina HiSeq-2000. Reads were mapped to the C. elegans genome version WS220 with TopHat version 1.4.1 (Trapnell et al. 2009) using default parameters. Gene expression was quantified using Cufflinks version 2.0.2 (Trapnell et al. 2012) with default parameters and supplying WS220 gene annotations. Gene expression values for each gene were calculated from the mean of at least three biological replicates, and are provided in File S2. Differential expression analysis was performed using DESeq2 version 1.0.17 (Love et al. 2014) in R version 3.0.2, and the results are given in File S3. RNA-seq data clustering was performed by K-means clustering using the Hartigan–Wong method in R version 3.02. Gene ontology (GO) term analysis was performed using GOrilla (Eden et al. 2009). The list of genes in identified clusters and the details of the GO terms are provided in File S4.
Analysis of sex-biased expression
Biased gene expression was defined based on our previously published methods (Albritton et al. 2014). We first identified those genes that were differentially expressed between two conditions (DESeq2, P-adj < 0.05). Genes with FPKM > 1 in at least one condition were considered “expressed” (FPKM, as calculated by Cufflinks). The “biased” genes had FPKM > 1 in one condition and FPKM > 0 in the other. The magnitude of bias was calculated as the log2 ratio of expression values between the two sexes (DESeq2). Genes that have FPKM > 1 in one sex and FPKM = 0 in the other were categorized as “specific” genes. The “nonbiased” genes were not called differentially expressed by DESeq2 (P-adj > 0.05), had FPKM > 1 in both sexes, and showed less than twofold expression difference between the sexes. We categorized genes with high and low sex-biased expression based on a log2 expression ratio cutoff of 3. The cutoff was previously selected based on a breakpoint in the distribution of sex-biased expression ratios (Albritton et al. 2014). Sex-biased expression greater than a log2 expression ratio of 3 is largely driven by gonadal expression. Approximately 1% of expressed genes showed more than twofold difference between sexes, but were not called significant by DESeq2 (File S4). Due to the uncertain nature of the bias in these genes and their low frequency (0.2–3.2%), we chose to exclude them from our analyses.
Strain and data availability
Strains are available from the Caenorhabditis Genetics Center. File S1 contains descriptions of all the data and their GEO submission nos. File S2 contains mean expression values for each data set. File S3 contains the results of the differential expression analyses. File S4 contains gene classifications and GO analyses. Gene expression data are available at GEO with the accession no. GSE77794.
Results
Uncoupling X chromosome number from sex determination
Because SDC proteins control both sex determination and dosage compensation, the effects of those two processes on gene regulation are coupled. To separate the effects of sex-specific regulation from those of X chromosome dose, we compared gene expression in wild-type worms (XX hermaphrodites and XO males) to mutant strains that produce XO hermaphrodites and XX males (Figure 1B). The XO hermaphrodite strain TY2205 bears three mutations: her-1(e1520) and xol-1(y9) mutations result in hermaphrodite sexual fate; sdc-3(y126) mutation disables dosage compensation. TY2205 larvae are viable phenotypic hermaphrodites that do not activate dosage compensation (Csankovszki et al. 2009; Pferdehirt et al. 2011). The XX male strain CB5362 contains tra-2(ar221) that results in male sexual fate and xol-1 (y9) that enhances masculinization (Hodgkin 2002). Because tra-2 is downstream of DCC activation, dosage compensation is still activated in these XX males (Hodgkin 2002; Chandler et al. 2009; Kalis et al. 2010). To determine expression differences due to sex-specific gene regulation, we compared gene expression between XX hermaphrodites and XX males and between XO hermaphrodites and XO males (Figure 1B). To determine expression differences due to X chromosome dose, we compared expression between XX and XO hermaphrodites and between XX and XO males.
X chromosome expression is balanced between the sexes in L3 larvae
We first analyzed sex-biased gene expression in L3 larvae. At this stage, sexually dimorphic organs, such as the gonad and the male tail are not yet fully developed (Kimble and Hirsh 1979; Hubbard and Greenstein 2005; Wolff and Zarkower 2008). In adults, germline cells outnumber somatic cells 2:1, but in L3 larvae, most cells are somatic. Previous work demonstrated that X chromosome dosage compensation is fully active by the L3 stage (Kramer et al. 2015). We made L3 collections of XX hermaphrodites, XO hermaphrodites, and XX males. Because the male tail is not fully developed by L3, we could not distinguish wild-type XO males and XX hermaphrodites. As a proxy for wild-type XO males, we used data from mixed populations of XO males and XX hermaphrodites (Kramer et al. 2015). Mixed sex larvae were isolated by crossing males and hermaphrodites of obligate an outcrossing strain [fog-2(oz40)] to ensure a 50% male population. As a result, the magnitude of sex-biased expression will be reduced by ∼50% in this comparison. We analyzed sex-biased expression of genes that were deemed expressed, by using FPKM > 1 cutoff. Similar results were observed using expression cutoffs of 0.1 FPKMs and 10 FPKMs to determine expressed genes (Figure S1).
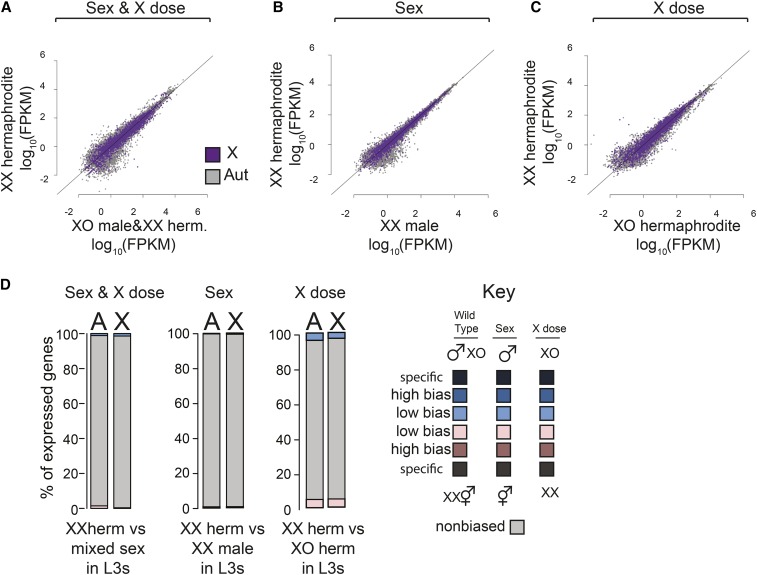
Compared to adults (Thomas et al. 2012; Albritton et al. 2014), there were fewer genes showing sex-biased expression in L3 larvae (Figure 2A). This result is expected, as most sexually dimorphic tissues develop after the L3 stage (Kimble and Hirsh 1979). Comparing wild-type XX hermaphrodites to mixed-sex populations, only 59 and 221 genes showed hermaphrodite and male-biased expression, respectively. Similarly, comparison between XX hermaphrodites and XX males also revealed few genes with sex-biased expression (Figure 2B). The contribution of X chromosome dose to sex-biased expression was also small (Figure 2C). We grouped genes into sex-specific, high sex-biased, low-sex biased, and nonbiased expression categories with the same cutoffs from our previous work (Albritton et al. 2014; see Materials and Methods). Consistent with the observation that the X chromosome is dosage compensated extensively in L3 larvae (Kramer et al. 2015), genes with sex-biased expression were distributed similarly between the X and autosomes (Figure 2D). Because the genes with sex-biased expression are found at similar proportions on the X and autosomes (9.0% of X genes vs. 8.1% of autosomal genes), the differences are likely caused by non-X specific effects such as strain or developmental differences. These results indicate that X chromosome dose and sex-specific gene regulation create little sex-biased expression at the L3 stage.
Figure 2.
Contribution of sex and X chromosome copy number (X dose) to sex-biased gene expression in L3 larvae. (A) Scatter plot compares expression of X chromosomal (purple) and autosomal (gray) genes in XX hermaphrodites and mixed sex (XO males/XX hermaphrodites). Expression values were calculated as FPKM and are the mean of at least three biological replicates. Diagonal line represents equal expression. (B) As in A, but comparison measures the effect of sex alone: XX hermaphrodites vs. XX males. (C) As in A, but comparison measures the effects of X dose alone: XX vs. XO hermaphrodites. (D) Genes with high and low sex-biased expression, sex-specific expression, and nonbiased expression were identified from each comparison that analyzed the effect of sex and X dose (wild type), sex only, or X dose only. Bar plots show the proportion of each class on the X and autosomes. Blue indicates male or XO bias. Pink indicates hermaphrodite or XX bias. Darker colors indicate stronger bias. In L3s, sexually dimorphic organs are not yet developed, thus there is less sex-biased expression compared to adults. In addition, X chromosome number did not significantly increase the distribution of hermaphrodite-biased genes on the X compared to autosomes, suggesting that dosage compensation is effective in L3s.
We noticed that the comparison between wild-type XX and the mutant XO hermaphrodites showed a higher number of differentially expressed genes (Figure 2D). However, similar distribution of these genes on X and autosomes indicated that the differential expression is not driven by X chromosome dose. We reason that the observed differential expression reflects subtle developmental timing differences between the wild-type XX and mutant XO hermaphrodites. Indeed, genes differentially expressed between XX and XO hermaphrodites were enriched for GO term functions related to growth on both the X and autosomes (File S4). These included cuticle genes such as col-146 and col-58, which are upregulated prior to molting. The absence of an X-specific XX-biased expression suggests that in L3s, X chromosome dosage compensation effectively represses X expression in XX worms by twofold to equalize expression to that of XO worms.
Correlation between DCC regulation and sex-biased gene expression in larvae
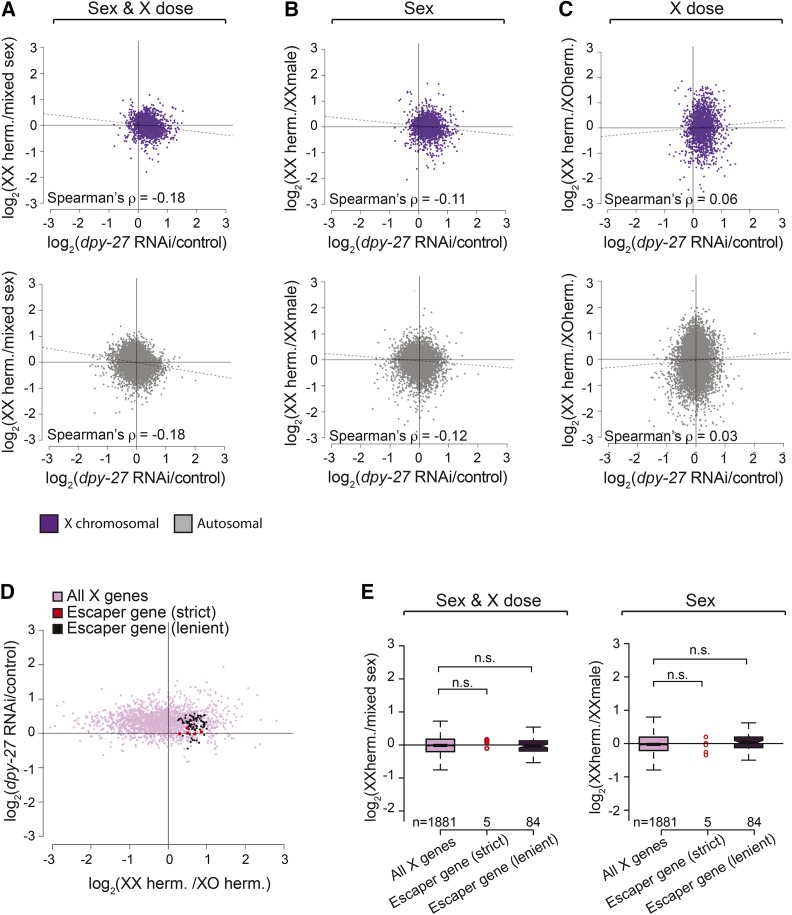
We next asked if the DCC has a role in creating any sex-biased expression in L3 larvae. We reasoned that if the DCC contributes to sex-biased gene expression, then X-linked genes that are not repressed by the DCC would be hermaphrodite biased. Similarly, genes that are over-repressed (more than twofold) by the DCC would be male biased. This scenario should result in a negative correlation between XX-biased expression and change in expression upon DCC knockdown on the X chromosome. To measure DCC regulation, we looked at expression changes caused by RNA interference (RNAi) depletion of dpy-27 in L3 XX hermaphrodites (Kramer et al. 2015). We separately calculated correlations for biased expression observed in three pairwise comparisons that separate sex and X dose (XX hermaphrodites vs. XO males; XX hermaphrodites vs. XX males; and XX hermaphrodites vs. XO hermaphrodites). For both the X and the autosomes, we found a weak negative correlation between DCC-mediated repression and XX-biased expression due to sex, and no correlation due to X dose (Figure 3A, Spearman’s rank correlation). These weak correlations that are not X chromosome specific suggest that the potential role of the DCC in sex-biased expression must be subtle or applies to few genes that escape X chromosome dosage compensation.
Figure 3.
Little escape from X chromosome dosage compensation in L3 larvae. (A–C) The correlation between the level of repression by the DCC (dpy-27/control RNAi) and the level of sex-biased expression due to sex and X dose (A, XXh vs. XOm), sex alone (B, XXh vs. XXm), and dose alone (C, XXh vs. XOh). A negative correlation is expected for each of the comparisons if escape from DCC repression creates XX-biased expression. Spearman’s rank correlation values were similar on the X and autosomes, suggesting that the DCC does not specifically regulate sex-biased expression of X chromosomal genes. (D) Genes that escape dosage compensation in L3 larvae were identified using either strict (5 genes in red) or lenient (84 genes in dark purple) criteria to find X chromosomal genes that are sensitive to X dose and not repressed by the DCC (see Materials and Methods). The scatter plot shows the level of repression by the DCC (y-axis) to XX biased expression due to X chromosome number (x-axis) for all X genes and escapees. (E) Genes that escape dosage compensation showed similar levels of sex-biased expression compared to other X genes in both the wild-type (left panel) or sex-only comparisons (right panel) (Wilcoxon rank-sum test, P > 0.05).
Escape from dosage compensation is rare in L3 larvae
To determine if escape from DCC-mediated repression is responsible for the XX-biased expression of the X chromosomal genes in L3s, we looked for individual genes that escape dosage compensation. To be considered an escaper, a gene must be located on the X chromosome, sensitive to X chromosome dose, and not transcriptionally repressed by the DCC. A previous study used similar criteria to identify 293 genes that escape dosage compensation in mixed-stage embryos (Jans et al. 2009). However, dosage compensation is incomplete until after the comma stage of embryogenesis (Kramer et al. 2015), thus the large number of previously identified escaper genes is inflated. Since the DCC is fully active by the L3 stage, our escaper analysis is not susceptible to this inflation.
Genes sensitive to X chromosome dose were identified as being upregulated in XX hermaphrodites compared to XO hermaphrodites (DESeq2 P-adj < 0.05). We then found genes whose expression does not change upon dpy-27 RNAi. While most methods identify genes that are differentially expressed between RNAi and control conditions, a lack of differential expression does not necessarily mean that genes are statistically similarly expressed. We identified genes that are statistically similarly expressed between dpy-27 RNAi and control conditions by constructing a 95% confidence interval around each gene’s fold change upon dpy-27 RNAi and taking genes that fell entirely below a 1.3-fold difference in expression. Using these strict criteria, we found five genes that escape dosage compensation in L3 larvae: F09B9.4, csq-1, gpd-3, dhs-30, and mec-7 (Figure 3B). mec-7 and dhs-30 were previously found to escape dosage compensation in embryos (Jans et al. 2009). These genes’ expression was not significantly more sex biased compared to other genes expressed from the X chromosome (Figure 4B, Wilcoxon rank-sum test, P = 0.3729) and were not enriched for any functional GO term annotations.We next used more lenient criteria to find genes that escape dosage compensation, this time requiring the genes to have <1.5-fold change upon dpy-27 RNAi, which is a reasonable cutoff as 94% of autosomal genes (9,926 of 10,554 expressed) showed a <1.5-fold change upon dpy-27 RNAi. Using this lenient approach, 4.5% of expressed X chromosomal genes escape dosage compensation (84 of 1881 genes, Figure 3B). A total of 21 of these genes were previously found to escape dosage compensation in embryos, an overlap slightly more than expected by chance (Fisher’s exact test, P = 0.01). Among the 84 genes that escape dosage compensation, hrg-1 is categorized as hermaphrodite biased. A total of 82 of the escaper genes are nonbiased and one, F09C8.1 is male biased. Therefore, similar to the 5 escaper genes identified by the stricter criteria, these 84 genes did not show significantly more sex-biased expression (Figure 3C, Wilcoxon rank-sum test, P = 0.37) and were enriched for one functional annotation (oxidoreductase activity, P = 8.4 × 10−4). Lack of a clear hermaphrodite bias for the identified dosage compensation escapees (Figure 3E) suggest that some of these genes were identified due to strain effect between XX and XO hermaphrodite comparison. From these results, we conclude that escape from dosage compensation is rare in L3 larvae and does not significantly contribute to sex-biased gene expression.
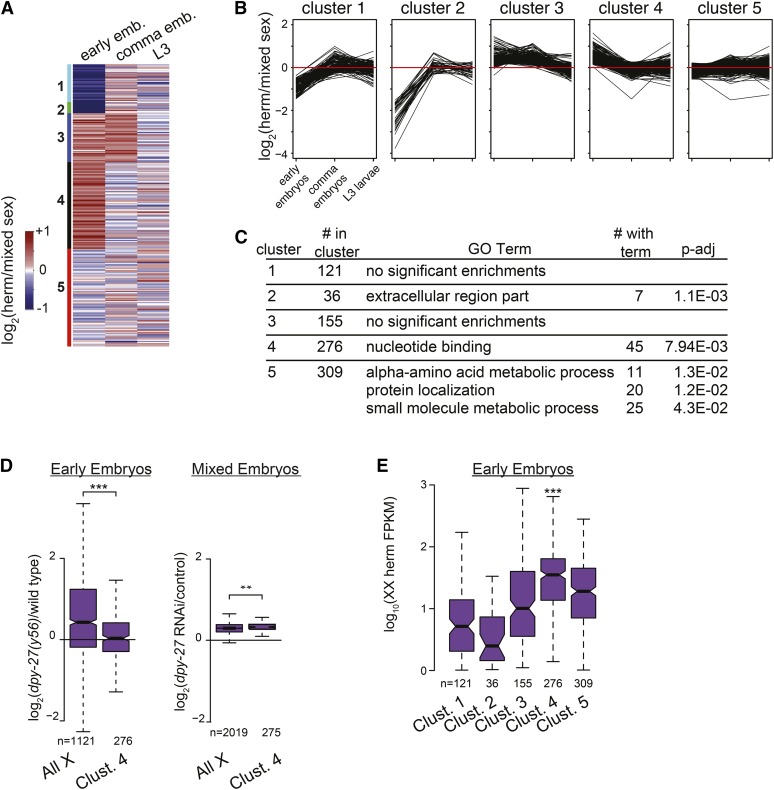
Figure 4.
Function of genes that are similarly dosage compensated during development. (A) To determine groups of genes that are similarly dosage compensated during development, we used K-means clustering of expression ratios between sexes for genes on the X chromosome in early embryos, comma embryos, and L3 larvae. Genes are sorted into five clusters. (B) Line plots indicate the level of hermaphrodite-biased expression (y-axis) at each time point for individual genes, separated by clusters determined in A. Red line shows equal expression between the sexes. (C) GO term analysis of enrichments within each cluster from A. (D) Cluster 4 consists of genes that are hermaphrodite biased in early embryos and become dosage compensated in comma stage embryos. Compared to other X chromosomal genes, genes in cluster 4 show less sensitivity to dpy-27 mutation in early embryos and slightly higher sensitivity to dpy-27 RNAi in older embryos (Wilcoxon rank-sum test, P-value = 4.7 × 10−12 and 2.8 × 10−5, respectively). (E) Genes in cluster 4 show higher expression compared to other clusters in hermaphrodite embryos before the DCC is fully active (Wilcoxon rank sum test, P-value < 1.0 × 10−7). This suggests that cluster 4 contains sex-determination genes that are expressed before DCC activation, thus escaping dosage compensation in early embryos when their hermaphrodite-biased expression is important for sex determination.
Function of dosage compensated genes during development
A large number of genes on the X chromosome show hermaphrodite-biased expression prior to the L3 stage (Kramer et al. 2015). Here, without using any cutoff for dosage compensation, we identified groups of genes that are expressed similarly between sexes across different developmental times. We used published expression data from hermaphrodite and mixed-sex populations at three stages of development: early embryos (<40 cell), comma embryos, and L3 larvae (Kramer et al. 2015). We performed K-means clustering of expression ratio between sexes for each X-linked gene (Figure 4A) and grouped genes in five clusters (Figure 4B). Clusters 1 and 2 consist of genes that show male bias in early embryos but have unbiased expression in comma-stage embryos and in larvae. Cluster 3 consists of genes with hermaphrodite-biased expression in early and comma embryos but unbiased expression in L3 larvae. Cluster 4 is made up of genes with initial hermaphrodite-biased expression in early embryos that become unbiased in comma embryos and L3 larvae. Cluster 5 is made up of genes with unbiased expression between sexes throughout development. GO term analysis of the genes in cluster 5 showed functions associated with basic cellular processes including “membrane organization,” “single-organism metabolic process,” and “protein transport” (Figure 4C). These genes included nmy-1 (nonmuscle myosin heavy chain), hpl-1 (HP1 heterochromatin protein homolog), and his-24 (H1 linker histone). Overall, these dosage-compensated genes are involved with basic cellular processes that may be similar between the two sexes.
Delayed dosage compensation in early embryos contributes to sex-biased expression and function
Cluster 4 genes show hermaphrodite bias only in the early embryo. To determine if lack of repression by the DCC contributes to this observed early bias, we examined previously published dpy-27 mutant data in early embryos and dpy-27 RNAi data from older, mixed-stage embryos (Kramer et al. 2015). If lack of DCC-mediated repression is responsible for the observed bias, we expect that genes in cluster 4 should be unaffected by DCC mutation in early embryos. Consistent with this hypothesis, in early embryos, cluster 4 genes were significantly less derepressed in dpy-27 mutant compared to all expressed genes on the X (Figure 4D, median 1.3-fold change vs. median 1.34-fold change for all expressed X genes, Wilcoxon rank-sum test, P-value = 4.6 × 10−12). In mixed-stage embryos, DCC disruption affected cluster 4 genes similarly to all expressed X genes (Figure 4D, median 1.25-fold change vs. median 1.24-fold change for all expressed X genes, Wilcoxon rank-sum test, P-value = 0.0014), suggesting that cluster 4 genes escape DCC repression early, but are repressed later. The mechanism of escape is likely to be expression of cluster 4 genes before the DCC is recruited to bind to the X chromosomes. Indeed, cluster 4 genes are expressed significantly higher in early embryos (<40 cell) compared to genes in other clusters (Figure 4E, median expression 35.2 FPKM vs. 2.5-19.0 FPKMs for other clusters, Wilcoxon rank sum test, P-value < 1.0 × 10−7). Additionally, analysis of independently published data from 15- to 20-cell hermaphrodite embryos (Hashimshony et al. 2015) confirmed that a higher proportion of genes in cluster 4 (77%) were highly expressed (FPKM > 10) compared to other clusters (19–60%). These results indicate that genes in cluster 4 are expressed earlier than other genes on the X chromosome and thus escape dosage compensation.
Interestingly, cluster 4 includes X signal elements (XSEs) ceh-39, sex-1, and fox-1, known to be dosage compensated later in development after the critical time period for their function has passed (Gladden et al. 2007). Cluster 4 is enriched for genes involved in regulating developmental processes (Figure 4C), including utx-1 (H3K27 demethylase); lin-15A and lin15B (Ras-signaling regulators); spat-3 (required for PAR protein-dependent cell-polarity and sex-specific characteristics); the DCC subunits sdc-1 and sdc-2; sex-determination genes ceh-39, sex-1, and fox-1; genes that regulate migrating sex myoblasts sax-3 and ksr-1, which regulate migrating sex myoblasts; and pnk-4, which is required later in development for hermaphrodite genitalia formation. The functional composition of cluster 4 suggests that the delay in dosage compensation contributes to early hermaphrodite-biased expression and functions.
Sex-biased gene expression in young adults
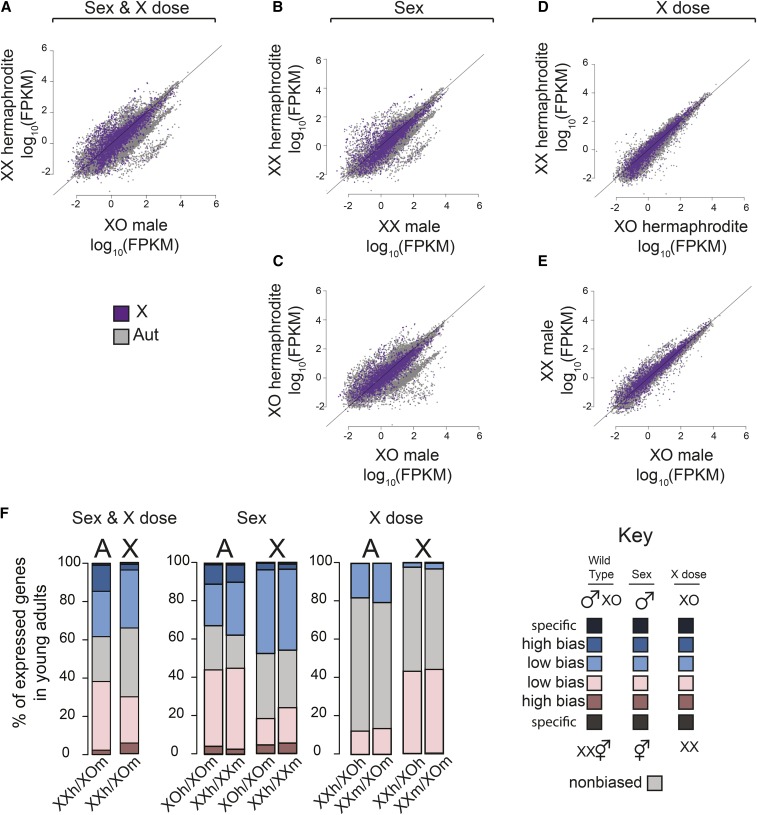
We next measured the contributions of sex-specific gene regulation and X chromosome dose to sex-biased gene expression in young adults, where sexual dimorphism is extensive. Here, sex-biased expression was calculated for expressed genes as defined by those that show FPKM > 1 in one or more of the samples. Similar results were achieved using expression cutoffs of 0.1 FPKMs and 10 FPKMs (Figure S2). The level of sex-biased gene expression between wild-type XX hermaphrodites and XO males was high (Figure 5A). Sex-biased gene expression due to sex alone showed a similar distribution of biased genes in XX (Figure 5B) and XO (Figure 5C) hermaphrodite–male comparisons. In contrast, X dose caused fewer sex-biased genes and lower levels of bias, as measured between XX and XO hermaphrodites (Figure 5D) and XX and XO males (Figure 5E). Overall, high sex-biased expression was mainly due to sex alone, which suggests that sex-specific gene regulation in sexually dimorphic tissues account for the majority of sex-biased expression seen in adults.
Figure 5.
Contribution of sex and X chromosome number (X dose) to sex-biased gene expression in young adults (A) Scatter plot compares expression of X chromosomal (purple) and autosomal (gray) gene expression in XX hermaphrodites and mixed sex (XO males/XX hermaphrodites). Expression values were calculated as FPKM and are the mean of at least three biological replicates. Diagonal line represents equal expression. (B and C) As in A, but comparison measures the effect of sex alone: (B) XX hermaphrodites vs. XX males and (C) XO hermaphrodites vs. XO males. (D and E) As in A, but comparison measures the effects of X dose alone: (D) XX vs. XO hermaphrodites and (E) XX vs. XO males. (F) Genes with high and low sex-biased expression, sex-specific expression, and nonbiased expression were identified from each comparison that analyzed the effect of sex and X dose (wild type), sex only, or X dose only. Bar plots show the proportion of each class on the X and autosomes. Blue indicates male or XO bias. Pink indicates hermaphrodite or XX bias. Darker colors indicate stronger bias. Note that the proportion of genes with low hermaphrodite-biased expression on the X is lower in sex-alone comparison and higher in X-dose comparison. Wild-type proportion of genes with low hermaphrodite-biased expression appears to be an average of the two, suggesting that X chromosome dose contributes to hermaphrodite-biased expression in wild-type adults. Genes that show low male-biased expression on the X is higher in sex-alone comparison than X-dose comparison. In wild-type young adults the distribution of sex-biased genes is a combination of contributions from sex and X dose.
Function of genes with sex-biased expression
In L3 larvae, we found 59 genes with low hermaphrodite-biased expression and 221 genes with low male-biased expression by comparing wild-type XX hermaphrodite to mixed-sex expression. To determine if these genes with sex-biased expression have shared functions, we performed GO term analysis (File S4). Among genes with low hermaphrodite-biased expression, endocytosis function was enriched. These genes included pro-2, known to regulate germline growth and development (Voutev et al. 2006), and mrps-18B, which plays a role in vulva formation (Ceron et al. 2007). Among genes with low male-biased expression, lysosome-associated genes were enriched, including rab-7, a Rab GTPase required for lysosome trafficking. We also looked at the expression of genes with known sex-biased functions in larvae or adults (Table 1). For example, the male-tail transcription factors dmd-3, mab-5, and egl-5 showed male-biased expression. Among the genes with higher hermaphrodite expression, we found nos-1, which functions in germline specification and maintenance and is homologous to the Drosophila Nanos; dpy-30, a member of the DCC and COMPASS complexes; and tra-2, a transmembrane receptor required for hermaphrodite sex determination. These genes with known sex-biased functions showed significantly biased expression in young adults (DESeq2, P-adj < 0.05) and were biased but fell below the significance test in L3 larvae, presumably due to being expressed in a small number of sex-specific cells in the larvae.
Table 1. Expression of genes with known sex-biased function.
| L3 | Young adult | |||||
|---|---|---|---|---|---|---|
| Gene | XX herm. FPKM | Mixed sex FPKM | P-adj | XX herm. FPKM | XO male FPKM | P-adj |
| Male function | ||||||
| dmd-3 | 1.5 | 11.0 | 0.13 | 0.2 | 5.3 | 1.40E-14 |
| mab-5 | 11.8 | 15.2 | 0.59 | 2.2 | 11.2 | 3.80E-05 |
| egl-5 | 8.2 | 19.1 | NA | 2.0 | 18.1 | 2.60E-16 |
| Hermaphrodite function | ||||||
| nos-1 | 2.3 | 2.1 | 0.98 | 20.9 | 4.4 | 2.70E-25 |
| tra-1 | 15.9 | 13.8 | 0.32 | 25.2 | 11.5 | 1.20E-12 |
| dpy-30 | 247 | 235.4 | 0.94 | 270.8 | 176.2 | 3.10E-10 |
In young adults, we identified many more genes with sex-specific and sex-biased expression, reflecting the full development of sexually dimorphic tissues. Overall, we found 4606 genes with hermaphrodite-biased expression and 4681 genes with male-biased expression. Compared to all expressed genes, genes with hermaphrodite-biased expression were enriched for reproductive and developmental functions, including “embryo development,” “anatomical structure development,” and “reproduction” (Table 2 and File S4). Genes with male-biased expression were enriched for “signal transduction,” “G-protein coupled receptor signaling pathway,” and “transmembrane transport.” Notably, the genes that show male-biased expression were also enriched for several neuron-associated functions such as “synaptic signaling” and “neuron part.” This suggests that a large portion of male-biased gene function is to create the nervous system specialization in males.
Table 2. Gene ontology analysis of genes that show sex-specific or sex-biased expression in young adults.
| Bias | No. of genes in group | GO term | No. of genes in GO term | P-adj |
|---|---|---|---|---|
| Hermaphrodite | 4606 | Embryo development | 1645 | 2.39E-177 |
| Anatomical structure development | 1939 | 1.17E-173 | ||
| Reproduction | 1230 | 2.23E-135 | ||
| Male | 4681 | Signal transduction | 408 | 1.27E-26 |
| G-protein-coupled receptor signaling | 124 | 1.43E-26 | ||
| Neuron part | 161 | 2.08E-21 | ||
| Synaptic signaling | 76 | 3.65E-18 |
Contribution of sex-specific gene regulation and X chromosome number to sex-biased gene expression in young adults
The expected contribution of X chromosome number to hermaphrodite-biased expression is up to twofold; thus it can mainly contribute to low-hermaphrodite biased gene expression. Therefore we analyzed high and low sex-biased expression separately (Figure 5F). When comparing number and distribution of genes with high sex-biased expression, we find that sex alone accounts for the enrichment of genes with high hermaphrodite-biased expression and depletion of genes with high male-biased expression on the X compared to autosomes (Figure 5F, compare middle plot to wild type, Fisher’s exact tests, P > 0.05). Interestingly, sex alone does not account for the enrichment of genes with low hermaphrodite-biased expression on the X. There are fewer genes that show low hermaphrodite-biased expression in the sex-only comparisons compared to wild type. Instead, X chromosome dose contributes to the presence of many more genes with low hermaphrodite-biased expression on the X (303 low hermaphrodite-biased genes in wild type vs. 633 and 706 in X-dose comparisons, Fisher’s exact test, P < 10−20). X chromosome dose contributes to hermaphrodite-biased expression in wild-type animals as 75.2% of X chromosomal genes with low hermaphrodite-bias in the wild-type comparison were found to have hermaphrodite-biased expression in X-dose-only comparison. In addition to analyzing the number of genes with sex-biased expression, we analyzed the level of sex-biased expression per gene, based on sex alone, X dose alone, and both (wild type) separately on the X and autosomes (Figure 6A). X chromosome dose created low hermaphrodite-biased expression on the X chromosome but not on autosomes. Collectively, our analyses in adults indicate that while sex-specific gene regulation is the major contributor to high sex-biased expression, X chromosome copy number contributes to low hermaphrodite-biased expression in adults.
Figure 6.
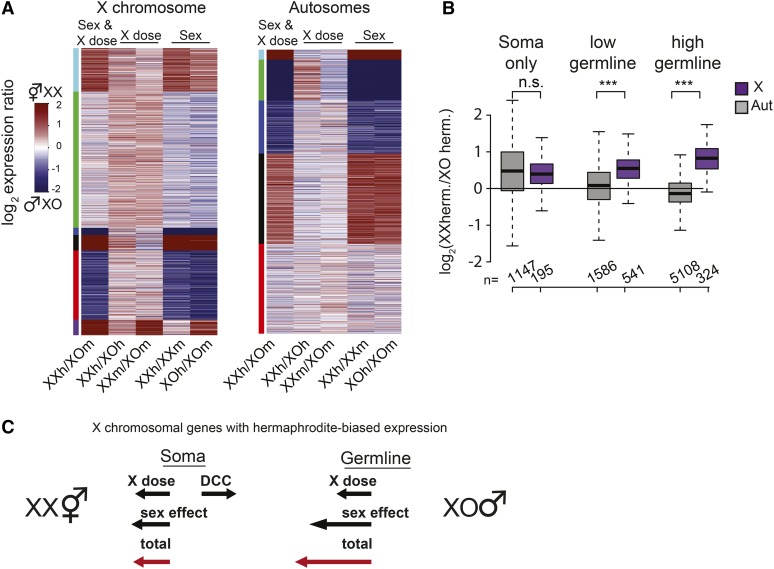
The X chromosome number contributes to XX hermaphrodite-biased expression in the C. elegans hermaphrodite germline. (A) K-means clustering of expression ratios based on sex and X dose (wild type), sex only, and X dose only comparisons in young adults. Clustering was performed separately for genes on the X chromosome (left) and on autosomes (right). X chromosome clustering showed similar patterns of high sex-biased expression between wild-type and sex-only comparisons, suggesting that sex-specific regulation determines most of high sex-biased expression. For the green cluster on the X, the low level of hermaphrodite bias in wild-type comparison is due to X chromosome number. (B) The level of XX-biased expression for genes expressed only in the soma, and genes that are expressed lowly and highly in hermaphrodite gonad (also referred to here as the germline genes). X chromosomal genes with germline expression showed significantly higher sensitivity to X dose compared to autosomal genes, suggesting that X chromosome number contributes to higher expression of X genes in the germline (Wilcoxon rank-sum test, P < 0.05). (C) Contribution of sex and X dose to hermaphrodite-biased expression of the X chromosome in the soma and germline. Total number of hermaphrodite-biased genes is promoted (arrow toward left, XX) or hindered (arrow toward right, XO) by the X dose, sex, and the DCC. In the soma, dosage compensation balances the effect of X chromosome dose. In the germline, lack of dosage compensation allows X dose to contribute to hermaphrodite bias, increasing the total bias in the germline compared to the soma.
X chromosome copy number contributes to hermaphrodite-biased expression in the germline
X chromosome number may influence germline expression more than that of soma, as the DCC is not expressed in the meiotic germline (Lieb et al. 1996). To test if X chromosome number contributes to hermaphrodite-biased expression specifically in the germline, we used germline and soma expression categories defined in a recent study that measured gene expression in dissected hermaphrodite oogenic gonads (Ortiz et al. 2014). We grouped genes with no, low, and high germline expression using cutoffs FPKM < 1, 1 < FPKM < 10, and FPKM > 10, respectively. Here we focused on our XX vs. XO hermaphrodite comparison where any expression differences should be due to X dose. We found significantly higher XX bias on the X chromosome compared to autosomes for both low- and high-expressed germline genes (Figure 6B, middle and right comparisons, Wilcoxon rank-sum test, P < 0.001). X-linked genes with high germline expression showed the greatest XX bias, with a median fold change of 1.77-fold, slightly less than the 2-fold expected from a complete lack of dosage compensation. In contrast, genes expressed only in the soma did not show a difference in XX bias between X and autosomes (Figure 6B, left comparison). These results suggest that in the absence of dosage compensation, X dose increases hermaphrodite-biased expression of the X chromosome in the XX hermaphrodite germline.
Discussion
Role of X chromosome copy number in sex-biased gene expression
Sex-biased gene regulation results in sexual dimorphisms that are critical for many animals. Since X chromosome dose differs between males and females, we wondered if it contributes to sex-biased expression. Knowing that dosage compensation mechanisms exist to balance X expression between males and females, we reasoned that escape from dosage compensation might contribute to sex-biased expression on the X chromosome. Escape from dosage compensation has been best studied in mammals, where one of the two X chromosomes is silenced in females. As a result, cells express only one allele of any X-linked gene, thus biallelic expression indicates escape from X inactivation. The proportion of X chromosomal genes that escape inactivation is 15–25% in humans and 4–8% in mice (Carrel and Willard 2005; Yang et al. 2010; Zhang et al. 2013; Berletch et al. 2015). Although some genes may escape inactivation due to having an ortholog on the Y chromosome (Carrel and Willard 2005; Berletch et al. 2015), others may escape due to potential female-biased function. For instance, Kdm6a escapes X inactivation (Greenfield et al. 1998) and activates the expression of Rhox6 and Rhox9, hox genes expressed in reproductive tissues (Berletch et al. 2013). Interestingly, we found that the C. elegans homolog of Kdm6a, a putative histone H3K27 demethylase utx-1, is also located on the X and escapes dosage compensation in early embryos. Female-biased expression due to X chromosome copy number is important, as human females with a single X chromosome (XO, Turner syndrome), exhibit several developmental phenotypes perhaps due to insufficient expression of genes that escape X inactivation.
Genes that escape mammalian dosage compensation lack the typical Xist RNA binding and H3K27me3 associated with X inactivation and instead are enriched for H3K4me3 (Khalil and Driscoll 2007; Yang et al. 2010; Pinter et al. 2012; Wu et al. 2014). In humans, escaper genes are clustered and more often found in evolutionarily young regions of the X chromosome, often far from the X inactivation center (Carrel and Willard 2005). Mouse escaper genes are less clustered and are found in regions of open chromatin marked by RNA Polymerase II accessibility, DNase I hypersensitive sites, and association with CTCF (Berletch et al. 2015). In C. elegans L3 larvae, which is composed mostly of somatic cells, we found few genes that escape dosage compensation, consistent with previous results that DCC has a repressive effect widely across the X chromosome (Kramer et al. 2015).
Reducing the effect of X chromosome copy number on gene expression
In the soma, the DCC efficiently reduces the effect of X chromosome copy number on expression difference between sexes. In the germline where the DCC is not expressed, we found that the X chromosome copy number contributed to ∼1.8-fold higher X expression. This is less than the expected 2-fold higher expression based on copy number difference between sexes. Similarly, in C. elegans, embryos mutating the DCC resulted in ∼1.4- to 1.7-fold increase in X expression (Jans et al. 2009; Kruesi et al. 2013; Kramer et al. 2015). In D. melanogaster, the MSL complex accounts for an ∼1.4-fold increase in male X expression, also less than the 2-fold increase needed for complete dosage compensation (Zhang et al. 2010; Larschan et al. 2011). While a specific mechanism is yet undiscovered, a general buffering in genetic networks may mitigate the effect of X dose differences as well as other aneuploidies (Zhang et al. 2010; Philip and Stenberg 2013; Chen and Oliver 2015). Another way to mitigate the effect of X chromosome number difference between sexes is to reduce the need to dosage compensate. Indeed, C. elegans X chromosome is depleted of predicted dosage-sensitive genes, such as the haploinsufficient genes and genes that encode for subunits of protein complexes (Albritton et al. 2014; Kramer et al. 2015).
X chromosome copy number contributes to sex-biased gene expression in the germline and in early embryogenesis
We found that in young adults, X chromosome copy number difference between the sexes increases the number of genes that show low hermaphrodite-biased expression and reduces the number of genes that show low male-biased expression on the X (Figure 5F). Contribution of X dose to hermaphrodite-biased expression in the absence of the DCC is seen in the germline (Figure 6B). It is unclear if X dose-mediated hermaphrodite-biased expression is functional or adaptive, but there are some X chromosomal genes with low hermaphrodite-biased expression and hermaphrodite germline functions. For example, mes-6 and cgh-1 are both required in the germline and are expressed slightly more in hermaphrodites. mes-6 is a member of the Polycomb-like chromatin repressive complex (Xu et al. 2001) and cgh-1 inhibits apoptosis in oocytes (Navarro et al. 2001). Consistent with the idea that sex-biased expression correlates with sex-biased roles, we find that genes with sex-biased expression are enriched for reproductive and neuronal functions (Table 2), while genes that are dosage compensated are known to be important in both sexes (Figure 4C). Thus, sex-biased expression patterns reflect sex-biased functions that underlie the development and function of sexually dimorphic tissues.
In C. elegans early embryos, X chromosome dose directly controls sex determination. As a result, we could not uncouple sex and X chromosome copy number effects at this stage. However, we did find a group of genes on the X chromosome that showed hermaphrodite-biased expression that correlates with a delay in DCC-mediated repression compared to other genes in early embryos (Figure 4). Among these genes were ceh-39, sex-1, and fox-1, whose XX-biased expression determines sex. The fact that genes with clear sex-biased functions show delayed DCC repression during embryogenesis suggests that temporal regulation of DCC activity is important for hermaphrodite-biased expression and function. Similar to our results, in four species of Drosophila studied, there was higher female-biased expression from the X chromosome prior to activation of canonical dosage compensation (Paris et al. 2015). Such conservation suggests that early embryonic X chromosomal genes have female-biased functions.
In summary, our results show that sex-specific gene regulation and X chromosome copy number together shape sex-biased expression of the X chromosome in C. elegans. In particular, X chromosome copy number contributes to hermaphrodite-biased gene expression in early embryos when the DCC is not fully active and in the germline where the DCC is not expressed. Functional significance of X dose-mediated hermaphrodite-biased expression is clear during early C. elegans embryogenesis, as genes important for sex determination are expressed prior to DCC activation, creating hermaphrodite-biased expression at the time when their sex-biased expression is critical for their function. While the function of X dose-mediated sex-biased expression in the C. elegans germline remains to be tested, in humans, reproductive sterility is one of the phenotypes associated with XO Turner syndrome, suggesting that the X chromosome copy number contributes to female-biased function (Monk and McLaren 1981; Baarends et al. 2005; Turner et al. 2005; Turner 2007). We speculate that the conserved lack of dosage compensation in the gonad contributes to female-biased expression of X-linked genes with female gonadal functions. In summary, our work suggests that in different tissues and developmental stages, X chromosome copy number contributes to functionally relevant sex-biased gene expression and function.
Acknowledgments
We thank Sarah Albritton for critical reading and suggestions on the manuscript and Anna-Lena Kranz for advice and contributions to data analysis. This work was supported by the National Institute of General Medical Sciences, grant R01-GM107293. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Some strains were provided by the Caenorhabditis Genetics Center, which is funded by National Institutes of Health Office of Research Infrastructure Programs (P40-OD010440).
Footnotes
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.116.190298/-/DC1.
Communicating editor: V. Reinke
Literature Cited
- Albritton S. E., Kranz A. L., Rao P., Kramer M., Dieterich C., et al. , 2014. Sex-biased gene expression and evolution of the X chromosome in nematodes. Genetics 197: 865–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen S. L., Bonduriansky R., Chenoweth S. F., 2013. The genomic distribution of sex-biased genes in drosophila serrata: X chromosome demasculinization, feminization, and hyperexpression in both sexes. Genome Biol. Evol. 5: 1986–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baarends W. M., Wassenaar E., van der Laan R., Hoogerbrugge J., Sleddens-Linkels E., et al. , 2005. Silencing of unpaired chromatin and histone H2A ubiquitination in mammalian meiosis. Mol. Cell. Biol. 25: 1041–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellott D. W., Skaletsky H., Pyntikova T., Mardis E. R., Graves T., et al. , 2010. Convergent evolution of chicken Z and human X chromosomes by expansion and gene acquisition. Nature 466: 612–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkseth M., Ikegami K., Arur S., Lieb J. D., Zarkower D., 2013. TRA-1 ChIP-seq reveals regulators of sexual differentiation and multilevel feedback in nematode sex determination. Proc. Natl. Acad. Sci. USA 110: 16033–16038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berletch J. B., Deng X., Nguyen D. K., Disteche C. M., 2013. Female bias in Rhox6 and 9 regulation by the histone demethylase KDM6A. PLoS Genet. 9: e1003489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berletch J. B., Ma W., Yang F., Shendure J., Noble W. S., et al. , 2015. Escape from X inactivation varies in mouse tissues. PLoS Genet. 11: e1005079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrel L., Willard H. F., 2005. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature 434: 400–404. [DOI] [PubMed] [Google Scholar]
- Ceron J., Rual J. F., Chandra A., Dupuy D., Vidal M., et al. , 2007. Large-scale RNAi screens identify novel genes that interact with the C. elegans retinoblastoma pathway as well as splicing-related components with synMuv B activity. BMC Dev. Biol. 7: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler C. H., Phillips P. C., Janzen F. J., 2009. The evolution of sex-determining mechanisms: lessons from temperature-sensitive mutations in sex determination genes in Caenorhabditis elegans. J. Evol. Biol. 22: 192–200. [DOI] [PubMed] [Google Scholar]
- Chen Z. X., Oliver B., 2015. X chromosome and autosome dosage responses in Drosophila melanogaster heads. G3 (Bethesda) 5: 1057–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D. S., Dawes H. E., Lieb J. D., Chan R. C., Kuo A. F., et al. , 2002. A molecular link between gene-specific and chromosome-wide transcriptional repression. Genes Dev. 16: 796–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang P. T., Lieb J. D., Meyer B. J., 1996. Sex-specific assembly of a dosage compensation complex on the nematode X chromosome. Science 274: 1736–1739. [DOI] [PubMed] [Google Scholar]
- Csankovszki G., Collette K., Spahl K., Carey J., Snyder M., et al. , 2009. Three distinct condensin complexes control C. elegans chromosome dynamics. Curr. Biol. 19: 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes H. E., Berlin D. S., Lapidus D. M., Nusbaum C., Davis T. L., et al. , 1999. Dosage compensation proteins targeted to X chromosomes by a determinant of hermaphrodite fate. Science 284: 1800–1804. [DOI] [PubMed] [Google Scholar]
- Dean R., Mank J. E., 2014. The role of sex chromosomes in sexual dimorphism: discordance between molecular and phenotypic data. J. Evol. Biol. 27: 1443–1453. [DOI] [PubMed] [Google Scholar]
- DeLong L., Plenefisch J. D., Klein R. D., Meyer B. J., 1993. Feedback control of sex determination by dosage compensation revealed through Caenorhabditis elegans sdc-3 mutations. Genetics 133: 875–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden E., Navon R., Steinfeld I., Lipson D., Yakhini Z., 2009. GOrilla: a tool for discovery and visualization of enriched GO terms in ranked gene lists. BMC Bioinformatics 10: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercan S., 2015. Mechanisms of X chromosome dosage compensation. J Genomics 3: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladden J. M., Farboud B., Meyer B. J., 2007. Revisiting the X:A signal that specifies Caenorhabditis elegans sexual fate. Genetics 177: 1639–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield A., Carrel L., Pennisi D., Philippe C., Quaderi N., et al. , 1998. The UTX gene escapes X inactivation in mice and humans. Hum. Mol. Genet. 7: 737–742. [DOI] [PubMed] [Google Scholar]
- Hashimshony T., Feder M., Levin M., Hall B. K., Yanai I., 2015. Spatiotemporal transcriptomics reveals the evolutionary history of the endoderm germ layer. Nature 519: 219–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin J., 1986. Sex determination in the nematode C. elegans: analysis of tra-3 suppressors and characterization of fem genes. Genetics 114: 15–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin J., 1987. A genetic analysis of the sex-determining gene, tra-1, in the nematode Caenorhabditis elegans. Genes Dev. 1: 731–745. [DOI] [PubMed] [Google Scholar]
- Hodgkin J., 2002. Exploring the envelope. Systematic alteration in the sex-determination system of the nematode Caenorhabditis elegans. Genetics 162: 767–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard E. J., Greenstein D., 2005. Introduction to the germ line. WormBook 1: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter C. P., Wood W. B., 1990. The tra-1 gene determines sexual phenotype cell-autonomously in C. elegans. Cell 63: 1193–1204. [DOI] [PubMed] [Google Scholar]
- Jans J., Gladden J. M., Ralston E. J., Pickle C. S., Michel A. H., et al. , 2009. A condensin-like dosage compensation complex acts at a distance to control expression throughout the genome. Genes Dev. 23: 602–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser V. B., Bachtrog D., 2014. De novo transcriptome assembly reveals sex-specific selection acting on evolving neo-sex chromosomes in Drosophila miranda. BMC Genomics 15: 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalis A. K., Kroetz M. B., Larson K. M., Zarkower D., 2010. Functional genomic identification of genes required for male gonadal differentiation in Caenorhabditis elegans. Genetics 185: 523–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil A. M., Driscoll D. J., 2007. Trimethylation of histone H3 lysine 4 is an epigenetic mark at regions escaping mammalian X inactivation. Epigenetics 2: 114–118. [DOI] [PubMed] [Google Scholar]
- Khil P. P., Smirnova N. A., Romanienko P. J., Camerini-Otero R. D., 2004. The mouse X chromosome is enriched for sex-biased genes not subject to selection by meiotic sex chromosome inactivation. Nat. Genet. 36: 642–646. [DOI] [PubMed] [Google Scholar]
- Kimble J., Hirsh D., 1979. The postembryonic cell lineages of the hermaphrodite and male gonads in Caenorhabditis elegans. Dev. Biol. 70: 396–417. [DOI] [PubMed] [Google Scholar]
- Klein R. D., Meyer B. J., 1993. Independent domains of the Sdc-3 protein control sex determination and dosage compensation in C. elegans. Cell 72: 349–364. [DOI] [PubMed] [Google Scholar]
- Kramer M., Kranz A. L., Su A., Winterkorn L. H., Albritton S. E., et al. , 2015. Developmental dynamics of X–chromosome dosage compensation by the DCC and H4K20me1 in C. elegans. PLoS Genet. 11: e1005698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruesi W. S., Core L. J., Waters C. T., Lis J. T., Meyer B. J., 2013. Condensin controls recruitment of RNA polymerase II to achieve nematode X-chromosome dosage compensation. eLife 2: e00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larschan E., Bishop E. P., Kharchenko P. V., Core L. J., Lis J. T., et al. , 2011. X chromosome dosage compensation via enhanced transcriptional elongation in Drosophila. Nature 471: 115–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieb J. D., Capowski E. E., Meneely P., Meyer B. J., 1996. DPY-26, a link between dosage compensation and meiotic chromosome segregation in the nematode. Science 274: 1732–1736. [DOI] [PubMed] [Google Scholar]
- Love M. I., Huber W., Anders S., 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15: 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madl J. E., Herman R. K., 1979. Polyploids and sex determination in Caenorhabditis elegans. Genetics 93: 393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mank J. E., Hosken D. J., Wedell N., 2011. Some inconvenient truths about sex chromosome dosage compensation and the potential role of sexual conflict. Evolution 65: 2133–2144. [DOI] [PubMed] [Google Scholar]
- Miller L. M., Plenefisch J. D., Casson L. P., Meyer B. J., 1988. xol-1: a gene that controls the male modes of both sex determination and X chromosome dosage compensation in C. elegans. Cell 55: 167–183. [DOI] [PubMed] [Google Scholar]
- Monk M., McLaren A., 1981. X-chromosome activity in foetal germ cells of the mouse. J. Embryol. Exp. Morphol. 63: 75–84. [PubMed] [Google Scholar]
- Mueller J. L., Mahadevaiah S. K., Park P. J., Warburton P. E., Page D. C., et al. , 2008. The mouse X chromosome is enriched for multicopy testis genes showing postmeiotic expression. Nat. Genet. 40: 794–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller J. L., Skaletsky H., Brown L. G., Zaghlul S., Rock S., et al. , 2013. Independent specialization of the human and mouse X chromosomes for the male germ line. Nat. Genet. 45: 1083–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro R. E., Shim E. Y., Kohara Y., Singson A., Blackwell T. K., 2001. cgh-1, a conserved predicted RNA helicase required for gametogenesis and protection from physiological germline apoptosis in C. elegans. Development 128: 3221–3232. [DOI] [PubMed] [Google Scholar]
- Nusbaum C., Meyer B. J., 1989. The Caenorhabditis elegans gene sdc-2 controls sex determination and dosage compensation in XX animals. Genetics 122: 579–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz M. A., Noble D., Sorokin E. P., Kimble J., 2014. A new dataset of spermatogenic vs. oogenic transcriptomes in the nematode Caenorhabditis elegans. G3 (Bethesda) 4: 1765–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris M., Villalta J. E., Eisen M. B., Lott S. E., 2015. Sex bias and maternal contribution to gene expression divergence in Drosophila blastoderm embryos. PLoS Genet. 11: e1005592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi M., Nuttall R., Naiman D., Bouffard G., Malley J., et al. , 2003. Paucity of genes on the Drosophila X chromosome showing male-biased expression. Science 299: 697–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhomchuk D., Borodina T., Amstislavskiy V., Banaru M., Hallen L., et al. , 2009. Transcriptome analysis by strand-specific sequencing of complementary DNA. Nucleic Acids Res. 37: e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry M. D., Li W., Trent C., Robertson B., Fire A., et al. , 1993. Molecular characterization of the her-1 gene suggests a direct role in cell signaling during Caenorhabditis elegans sex determination. Genes Dev. 7: 216–228. [DOI] [PubMed] [Google Scholar]
- Pferdehirt R. R., Kruesi W. S., Meyer B. J., 2011. An MLL/COMPASS subunit functions in the C. elegans dosage compensation complex to target X chromosomes for transcriptional regulation of gene expression. Genes Dev. 25: 499–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip P., Stenberg P., 2013. Male X-linked genes in Drosophila melanogaster are compensated independently of the Male-Specific Lethal complex. Epigenetics Chromatin 6: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter S. F., Sadreyev R. I., Yildirim E., Jeon Y., Ohsumi T. K., et al. , 2012. Spreading of X chromosome inactivation via a hierarchy of defined Polycomb stations. Genome Res. 22: 1864–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranz J. M., Castillo-Davis C. I., Meiklejohn C. D., Hartl D. L., 2003. Sex-dependent gene expression and evolution of the Drosophila transcriptome. Science 300: 1742–1745. [DOI] [PubMed] [Google Scholar]
- Reinius B., Johansson M. M., Radomska K. J., Morrow E. H., Pandey G. K., et al. , 2012. Abundance of female-biased and paucity of male-biased somatically expressed genes on the mouse X-chromosome. BMC Genomics 13: 607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke V., Gil I. S., Ward S., Kazmer K., 2004. Genome-wide germline-enriched and sex-biased expression profiles in Caenorhabditis elegans. Development 131: 311–323. [DOI] [PubMed] [Google Scholar]
- Rhind N. R., Miller L. M., Kopczynski J. B., Meyer B. J., 1995. xol-1 acts as an early switch in the C. elegans male/hermaphrodite decision. Cell 80: 71–82. [DOI] [PubMed] [Google Scholar]
- Sturgill D., Zhang Y., Parisi M., Oliver B., 2007. Demasculinization of X chromosomes in the Drosophila genus. Nature 450: 238–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C. G., Li R., Smith H. E., Woodruff G. C., Oliver B., et al. , 2012. Simplification and desexualization of gene expression in self-fertile nematodes. Curr. Biol. 22: 2167–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C., Pachter L., Salzberg S. L., 2009. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25: 1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C., Roberts A., Goff L., Pertea G., Kim D., et al. , 2012. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 7: 562–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J. M., 2007. Meiotic sex chromosome inactivation. Development 134: 1823–1831. [DOI] [PubMed] [Google Scholar]
- Turner J. M., Mahadevaiah S. K., Fernandez-Capetillo O., Nussenzweig A., Xu X., et al. , 2005. Silencing of unsynapsed meiotic chromosomes in the mouse. Nat. Genet. 37: 41–47. [DOI] [PubMed] [Google Scholar]
- Villeneuve A. M., Meyer B. J., 1987. sdc-1: a link between sex determination and dosage compensation in C. elegans. Cell 48: 25–37. [DOI] [PubMed] [Google Scholar]
- Villeneuve A. M., Meyer B. J., 1990. The regulatory hierarchy controlling sex determination and dosage compensation in Caenorhabditis elegans. Adv. Genet. 27: 117–188. [DOI] [PubMed] [Google Scholar]
- Voutev R., Killian D. J., Ahn J. H., Hubbard E. J., 2006. Alterations in ribosome biogenesis cause specific defects in C. elegans hermaphrodite gonadogenesis. Dev. Biol. 298: 45–58. [DOI] [PubMed] [Google Scholar]
- Wang P. J., Page D. C., McCarrey J. R., 2005. Differential expression of sex-linked and autosomal germ-cell-specific genes during spermatogenesis in the mouse. Hum. Mol. Genet. 14: 2911–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J. R., Zarkower D., 2008. Somatic sexual differentiation in Caenorhabditis elegans. Curr. Top. Dev. Biol. 83: 1–39. [DOI] [PubMed] [Google Scholar]
- Wu H., Luo J., Yu H., Rattner A., Mo A., et al. , 2014. Cellular resolution maps of X chromosome inactivation: implications for neural development, function, and disease. Neuron 81: 103–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Fong Y., Strome S., 2001. The Caenorhabditis elegans maternal-effect sterile proteins, MES-2, MES-3, and MES-6, are associated in a complex in embryos. Proc. Natl. Acad. Sci. USA 98: 5061–5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F., Babak T., Shendure J., Disteche C. M., 2010. Global survey of escape from X inactivation by RNA-sequencing in mouse. Genome Res. 20: 614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Schadt E. E., Wang S., Wang H., Arnold A. P., et al. , 2006. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res. 16: 995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonker S. A., Meyer B. J., 2003. Recruitment of C. elegans dosage compensation proteins for gene-specific versus chromosome-wide repression. Development 130: 6519–6532. [DOI] [PubMed] [Google Scholar]
- Zarkower D., 2006. Somatic sex determination. WormBook 10: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Castillo-Morales A., Jiang M., Zhu Y., Hu L., et al. , 2013. Genes that escape X-inactivation in humans have high intraspecific variability in expression, are associated with mental impairment but are not slow evolving. Mol. Biol. Evol. 30: 2588–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. E., Vibranovski M. D., Landback P., Marais G. A., Long M., 2010. Chromosomal redistribution of male-biased genes in mammalian evolution with two bursts of gene gain on the X chromosome. PLoS Biol. 8: e1000494. [DOI] [PMC free article] [PubMed] [Google Scholar]