Abstract
Objective
During the aging process in men testosterone (T) levels progressively fall and inflammatory biomarkers increase. Although a relationship between these two phenomena has been tested in previous clinical trials, there is inconclusive evidence about the potential anti-inflammatory action of T.
Methods
A total of 108 healthy men >65 years with serum T concentration <475 ng/dL were recruited by direct mailings to alumni of the University of Pennsylvania and Temple University, and randomized to 60-cm2 T or placebo patch for 36-months. Ninety-six subjects completed the trial. Information and stored serum specimens from this trial were used to test the hypothesis of T inhibitory effect on inflammation. 70 men (42 in the T group) who had banked specimens available for assays of T, C-reactive protein (CRP), Tumor necrosis factor (TNF)-alpha, soluble TNF-alpha receptor-1 (TNFR1), interleukin-6 (IL-6) and soluble IL-6 receptors (sIL6r and sgp130) at multiple time points, were evaluated.
Results
The mean age ± SD at baseline was 71.8 ± 4.9 years. Testosterone replacement therapy for 36 months did not induce a significant decrease in inflammatory markers. A trend toward a significant increase was observed in the placebo group for TNF-alpha (p=0.03) and sgp130 (p=0.01). Significant differences, in estimated means of TNFR1 (but not of other inflammatory markers), with lower levels in T group, were observed at 36 month-time point. In T-treated subjects we found an almost significant treatment-time interaction term TNFR1 (p=0.02) independent of total body fat content assessed by DXA. No serious adverse effect was observed.
Conclusions
Transdermal T treatment of older men for 36 months is not associated with significant changes in inflammatory markers.
Keywords: testosterone, inflammatory markers, older men
INTRODUCTION
In men, from the age of 35, there is a progressive decline in serum testosterone (T) levels by 1% per year, due to attrition in testicular Leydig cells and slowing of the hypothalamic GnRH pulse generator (1). The age-related impairment in T levels may coexist with a “low-grade proinflammatory state” characterized by a slight increase in cytokines and acute phase proteins levels (2,3). Epidemiologic studies in older men suggest that lower T and higher levels of inflammatory markers are independently associated with increased risk of sarcopenia, disability and mortality (4,5).
Experimental studies in steers show that exogenous T administration is involved in the development of tolerance to repeated immune challenge (6). In mice and rats T suppresses immune cell differentiation and macrophage activation (7). Moreover, castrated animals show increased levels of interleukin-2 (IL-2) and interferon- γ in peripheral T-cells (8).
Interestingly, hypogonadal men seem more prone to develop autoimmune diseases than eugonadal men (9). Yesilova et al. (10) in idiopathic hypogonadotropic hypogonadal young men found that T deficiency affects both cell-mediated and humoral immunity compared with age-matched healthy controls. The hyperactivation of immune system is normalized after 6-month androgen-stimulating therapy with human menopausal gonadotropin/hCG. This data is also supported by the observation that men with Klinefelter Syndrome have a higher incidence of autoimmune diseases than their age-matched counterparts (9). Thus, T could play a role in the inflammatory process because of its anti-inflammatory and immune-modulator properties.
However, inflammatory cytokines seem also to act as inhibitory factors at pituitary and testicular level, impairing both the secretion of LH and T and T sensitivity to LH (11). Indeed, in models of critical illness characterized by pro-inflammatory status (i.e. post-operative period) there is a steeper decline of T levels (12).
Despite the increasing evidence from observational studies of an association between the progressive decline in T levels and the increase in inflammatory markers (13,14), previous clinical trials have reported inconsistent results without suggesting any potential underlying mechanism. Testosterone replacement therapy has not been always able to lower inflammatory markers including TNF-alpha, IL-6 and CRP, especially in older population (15–18). On the contrary, in middle aged adult subjects a shift of the cytokines balance to a state of reduced inflammation has been reported (19–21). However, several trials were single-blind (20) or with open nonrandomized design (21), and almost all enrolling subjects affected by frank hypogonadism and/or specific clinical conditions such as metabolic syndrome (19) and diabetes (15).
AIM OF THE STUDY
Because of the high discrepancy of data coming from prior clinical trials, the aim of this study is devoted to better understand whether or not transdermal T has an inhibitory effect on inflammatory markers in a cohort of older male subjects with low-normal T serum concentrations. We focused on different components of both IL-6 and TNF-alpha systems.
PATIENTS AND METHODS
Subjects
As described elsewhere, healthy men over 65 years of age were recruited by direct mailings to alumni of the University of Pennsylvania and Temple University and by newspaper and television advertisements (22–23). The study included men who had a serum T concentration at least one SD below the mean for healthy young men (<475 ng/dL; 16.5 nmol/L) (22). A total of 108 men met these criteria and were randomized to receive a T or placebo patch in a double blind fashion for 36 months. Ninety-six subjects completed 36 months of treatment. Other inclusion and exclusion criteria are described elsewhere (22).
The study reported here was performed 10 years later using banked serum specimens from 70 of these men, 42 in the T treatment group and 28 in placebo group, who had sufficient sera remaining for assays. Time points for assays were baseline, 3, 6, 9, 12, 18, 24, 30 and 36 months. The flow diagrams for the original RCT and the post hoc data used in the current analysis of the RCT are reported elsewhere (22). The study protocol was approved by local ethic committee. All participants received a detailed description of the purpose and design of the study and signed informed participation consent.
Study design
Testosterone was administered by scrotal patch (Testoderm; Alza Corp., Palo Alto, CA, USA); placebo patches were identical in appearance to testosterone patches. Each subject was asked to wear a patch at all times except when bathing, change the patch once a day, and shaved the scrotum twice a week. Each subject began by wearing a 60-cm2 testosterone patch, which delivers approximately 6 mg testosterone/24 h, or a 60-cm2 placebo patch. The data manager reviewed serum T concentrations every 3 months and directed a decrease in patch size to 40 cm2 if a man's serum T was above 1000 ng/dL (34.7 nmol/L) or re-education in patch technique if a man in the T-treated group had a value less than 250 ng/dL above baseline. To maintain the blinding in both the above manipulations, the data manager directed that a man in the placebo-treated group be treated similarly.
Storage of the samples
The samples were kept frozen at all times at −80°C. The freeze-thaw cycles that affect some analytes, but not others were very limited.
Hormonal and Cytokine Measurements
Testosterone was measured together with the inflammatory markers in 2008. Total T was assessed by electrochemiluminescence immunoassay with minimum detectable concentration (MDC) of 2 ng/dL and interassay coefficient of variation (CV) <10%. C-Reactive protein (CRP) was assessed by Siemens BNII Nephelometry with MDC of 0.146 mg/L and interassay CV less than 5%. Interleukin 6 (IL-6), soluble IL-6 receptor, (sIL6r), soluble gp130 (sgp130) and tumor necrosis factor receptor-1 (TNFR1) were assessed by ELISA kits provided by R&D systems. The MDC of IL-6 was 0.156 pg/ml and the interassay CV was less than 12 %. The MDC of sIL6r was 3120 pg/ml and interassay CV less than 14%. The MDC of sgp130 was 25 ng/ml and interassay CV less than 9%. The MDC of TNFR1 was 78 pg /ml and interassay CV less than 6%. TNF-alpha was assayed by Bio-Rad Luminex Flow Cytometry provided by Millipore Panel B. The MDC was 0.64 pg/ml and interassay CV less than 10%.
Body composition
Body composition was determined by Dual energy X-ray Absorptiometry (DXA) using a DPX scanner (Lunar Corp., Madison, WI) with acquisition software versions 3.1–3.61 and body composition software version 1.3.
Statistical Analysis
Variables were reported as means ± SD or medians and interquartile range, as appropriate. For measures not normally distributed, log-transformed values were used in the analyses. Differences in baseline characteristics across experimental groups were analyzed using independent sample t tests. Changes within groups from baseline to treatment end were evaluated with paired t tests.
The association between treatment and change in inflammatory markers over time was examined using random-effect regression analyses, modeling an unstructured covariance matrix with intercept and slope as random effects.
Random-effects models were valuable in this context allowing the detection of variation between subjects and autocorrelation between repeated measurements of the same participants over time, giving greater flexibility to model time effects, and handling missing data. The different inflammatory marker measures were entered as dependent variables in separate analyses.
The effect of the interaction between treatment and time has been evaluated by introducing a treatment time interaction term in the mixed models (already including the main terms of treatment and time) in order to test the change over time in inflammatory markers according to treatment. Finally, in order to adjust the results for multiple testing, the significance of the treatment*time interaction terms was additionally evaluated using False Discovery Rate (FDR) q (24).
All analyses were performed using SAS (v. 9.1; SAS Institute, Inc., Cary, NC, USA). Significance level was set at p < 0.05.
RESULTS
The characteristics of the subjects according to treatment group are listed in Table 1. The two groups were comparable in terms of age, body fat mass and lean body mass. Body mass index (BMI) was higher in the T treatment group, but the difference between the two groups did not reach statistical significance. The two groups were also highly similar at baseline with respect to all measured inflammatory markers (Table 1 and Table 3).
Table 1.
Characteristics of study participants (N=70) at baseline according to treatment group.
| Treatment (N=42) | Placebo (N=28) | P# | |
|---|---|---|---|
| Age (years)a | 71.9 ± 4.7 | 71.5 ± 5.3 | 0.75 |
| BMI (Kg/m2)a | 27.0 ± 3.2 | 25.7 ± 2.3 | 0.07 |
| Testosterone (ng/dL)b | 402.2 (245.6) | 372.1 (79.1) | 0.06 |
| Body Fat Mass (Kg)a | 25.1±6.1 | 23.4±5.2 | 0.23 |
| Lean Body Mass (Kg)a | 54.9±5.2 | 54.5±5.4 | 0.74 |
| IL-6 (pg/mL)b | 2.6 (1.9) | 2.6 (1.9) | 0.85 |
| CRP (μg/mL)b | 1.5 (3.0) | 1.58 (3.3) | 0.40 |
| sIL6r (pg/mL)b | 40359 (13934) | 43464 (12447) | 0.48 |
| TNF α (pg/mL)b | 5.3 (3.1) | 5.5 (2.9) | 0.68 |
| TNF-R1 (pg/mL)b | 2088 (687) | 2148 (823) | 0.33 |
| sgp130 (ng/mL)b | 376 (62) | 364 (74) | 0.54 |
Means ± SD.
Medians ± Interquartile range.
Based on t-test.
Table 3.
Concentrations of inflammatory markers (mean ± SD) and change from baseline (delta) by treatment group.
| Baseline | 36 months | Delta | Pc | ||
|---|---|---|---|---|---|
| CRP (μg/mL) a | |||||
| T | 2.8 ± 3.5 | 2.8 ± 4.1 | −0.06 ± 2.9 | 0.40 | |
| Placebo | 4.2 ± 8.0 | 9.2 ± 25.2 | 5.5 ±20.1 | 0.95 | |
| Pb | 0.40 | 0.23 | |||
|
| |||||
| IL-6 (pg/mL) a | |||||
| T | 3.1 ± 1.8 | 3.5± 2.4 | 0.6± 2.8 | 0.71 | |
| Placebo | 3.2 ± 1.9 | 3.3 ± 1.9 | 0.1± 2.9 | 0.41 | |
| Pb | 0.85 | 0.69 | |||
|
| |||||
| sIL6r (pg/mL) a | |||||
| T | 40280±11432 | 43686±10178 | 976± 80016 | 0.30 | |
| Placebo | 44436±11530 | 46971±13099 | 2498±8009 | 0.12 | |
| Pb | 0.48 | 0.27 | |||
|
| |||||
| sgp_130 (ng/mL) a | |||||
| T | 377±49 | 380±302 | 3± 39 | 0.66 | |
| Placebo | 371±49 | 395±49 | 23± 44 | 0.01 | |
| Pb | 0.54 | 0.20 | |||
|
| |||||
| TNF-α (pg/mL) a | |||||
| T | 5.3 ± 2.3 | 6.2 ± 3.6 | 0.4± 2.2 | 0.84 | |
| Placebo | 6.3 ± 3.5 | 5.9 ± 2.6 | −0.4± 2.6 | 0.84 | |
| Pb | 0.68 | 0.99 | |||
|
| |||||
| TNF_R1 (pg/mL) a | |||||
| T | 2094±427 | 2191 ± 448 | 88 ± 240 | 0.07 | |
| Placebo | 2243±589 | 2487±780 | 222 ± 544 | 0.03 | |
| Pb | 0.33 | 0.10 | |||
NOTE: In order to obtain SD, delta was calculated only for participants with available measures at both time points
Means ± SD.
The p values compare the mean change from 0–36 months between the two treatment groups.
The p values compare the mean change from 0–36 months within the two treatment groups
As expected, 36 months of T treatment was associated with an increase in T levels from 490 ± 233 to 682 ± 308 ng/dL. The delta change in T levels was 192.41 ±282.41 and 6.86 ± 83.34 ng/dL in T and placebo group, respectively (Table 2).
Table 2.
Serum testosterone concentrations (means ± SD and delta) by treatment group at baseline and 36 months.
| Baselinea | 36 months | delta | Pb | Pc | |
|---|---|---|---|---|---|
| T (N=42) | 490 ± 233 | 682 ± 308 | 192 ±282 | 0.0006 | 0.06 |
| Placebo (N=28) | 396 ± 102 | 404 ± 102 | 7 ± 83 | 0.95 | <.0001 |
The baseline values were the means of three different measurements.
The p values compare the mean change from 0 to 36 months within the two treatment groups.
The p values compare the mean change from 0 to 36 months between the two treatment groups.
NOTE:
At 36 months, 3 subjects had missing testosterone values. Therefore delta has been calculated only for participants (67) with available measures at both time points.
A trend toward a significant increase was observed in the placebo group, but the trend was significant only for TNFR1 (p=0.03) and sgp130 (p=0.01) and did not reach statistical significance for sIL6r (p=0.12) (Table 3). There was no significant change in inflammatory markers from baseline in the treatment group. The difference in inflammatory markers between the two groups approached statistical significance only for TNFR1 (p=0.10) (Table 3).
The treatment-time interaction term was found almost significant only for CRP (p=0.03) and TNFR1 (p=0.02). No significant difference was found for TNF alpha, IL-6, sIL6r or sgp130. After adjusting for multiple testing using FDR statistical method, treatment*time interaction term was not significant for all the inflammatory markers considered in the Study (Table 4). After adjusting for body fat, the results did not substantially change (data not shown).
Table 4.
Effects of testosterone treatment on inflammatory markers.
| Treatment by time |
||||
|---|---|---|---|---|
| β ± SE | F | P^ | FDR q# | |
| CRP (μg/mL) | −0.12 ± 0.06 | 4.48 | 0.03 | 0.09 |
| IL-6 (pg/mL) | −0.01 ± 0.01 | 0.65 | 0.42 | 0.50 |
| sIL6r (pg/mL) | 15.15 ± 52.92 | 0.08 | 0.77 | 0.77 |
| TNF alpha (pg/mL) | 0.03 ± 0.03 | 1.16 | 0.28 | 0.42 |
| TNF R1 (pg/mL) | −4.88 ± 2.02 | 5.82 | 0.02 | 0.09 |
| sgp130 (ng/mL) | −0.42 ± 0.23 | 3.48 | 0.06 | 0.12 |
treatment*time interaction term by mixed model including the main terms of treatment and time.
multiple testing treatment*time interaction terms evaluated by False Discovery Rate (FDR).
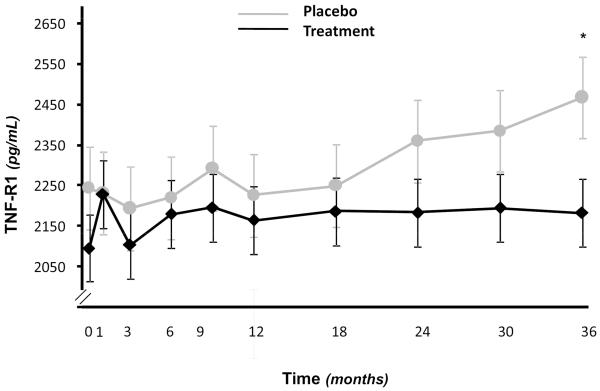
Figure 1 shows the adjusted means of TNFR1 at each time-point obtained from mixed models with random intercept in which time-point was dummy-coded as a categorical variable and appropriate treatment*time-point interaction terms were also included. Significant difference (p<0.05), in estimated means of TNF-R1 (but not of other inflammatory markers), with lower levels in T group, were observed at 36 month-time point. After 36 months, we also found significant differences in estimated means of CRP. However, at 36 months two participants in the placebo group had values of CRP > 4 SD. After the removal of these outlier values the difference in estimated means of CRP at 36 months across treatment groups was no longer significant (data not shown).
Figure 1.
Adjusted means of TNFR1 at each time-point obtained from mixed models with random intercept in which time-point was dummy-coded as a categorical variable and including appropriate treatment*time-point interaction terms. Significant difference, in estimated means of TNF-R1 (but not of other inflammatory markers), with lower levels in T group, was observed at 36 month-time point (p<0.05).
DISCUSSION
In older men with low-normal serum T concentrations we found no significant change in inflammatory markers after 36 month T transdermal treatment. This is a further attempt to understand the role of T in inflammatory pathways, giving the high inconsistency of findings produced by prior RCTs.
To our knowledge this is the first RCT addressing this issue by focusing on different components of both IL-6 and TNF-alpha systems, in a cohort of older men with detailed and complete information on serum concentrations of CRP, TNF-alpha and TNFR1, IL-6 and soluble IL-6 receptors, at baseline and multiple time points. Our data are consistent with other RCTs performed in older subjects showing neutral effects of T on inflammation. Indeed, in hypogonadal, diabetic older men, short-term T administration did not seem to adversely affect IL-6, CRP and TNF-alpha levels (15). Consistently, Basaria and colleagues (17) in 209 older hypogonadal men with mobility limitation from the Testosterone in Older Men with Mobility Limitations Trial did not show significant effects of Transdermal T administration (10 g T gel daily for 6 months) on CRP and IL-6 serum concentration. Short-term treatment with an aromatase inhibitor in elderly hypogonadal men, despite increasing T levels, did not change CRP levels (16). Nakhai-Pour and colleagues (18) in older men with moderately low T levels similarly did not find any significant variation in high-sensitivity CRP levels after 26 week oral T undecanoate supplementation (TU 160 mg daily).
On the contrary, the majority of RCTs including middle-aged and adult subjects have documented a shift of the cytokines balance to a state of reduced inflammation after T administration (19–20; 25–27). In a recent randomized, placebo-controlled, double-blinded, phase III trial of 184 men (aged 35–70), suffering from both the metabolic syndrome and hypogonadism, T administration for 30 weeks (n = 113; TU; 1000 mg IM) resulted in lower levels of CRP, IL-1β, IL-6, and TNF-alpha (19). Similarly, Malkin and colleagues (20) in a single-blind RCT of 27 hypogonadal men (age, 62+/− 9 yr), the majority of whom had coronary artery disease, found, after 1 month intramuscular T, a reduction in IL-1 β, and TNF-alpha. In type 2 diabetic men with partial androgen deficiency was shown a reduction ex vivo of IL-1β, IL-6 and TNF-alpha by antigen-presenting cells after 12 month T treatment (25). Similarly, 3-week T treatment before intracoronary stenting resulted in a significant suppression in high sensitive CRP (hs-CRP) and interleukin-6 (IL-6) levels after the stent implantation (26).
Interestingly, studies evaluating the effects of pharmacologically induced hypogonadism (by GnRH agonist and aromatase inhibitor or Androgen Deprivation Therapy in prostate cancer) on circulating inflammatory cytokines and CRP, have produced inconsistent findings (28–31).
However, we found a significant trend toward an increase for TNFR1 in the placebo group and significant differences in estimated means of TNFR1 only at 36 months time point. Interestingly, a treatment-time interaction term was almost significant for TNFR1.
This data is of importance because the soluble receptors of the main pro-inflammatory cytokines were not measured in the previous RCTs. This information represents one of the main strength points of our study. In fact, TNFR1 is more sensitive than its respective cytokine TNF-alpha (32). Moreover, the population here evaluated, in contrast to the mentioned RCTs, had mean baseline serum T concentration at the low-end of the normal reference range. This factor can be accounted for the different and stronger anti-inflammatory effects reported in moderately or severely hypogonadal men.
Limitations and Strengths
The present study has some limitations. The serum samples had been frozen for more than 10 years, and there are no data directly addressing the stability of inflammatory markers in the freezer. However, the samples were stored at −80°C at all times, and freezing and thawing was kept to a minimum. In addition, there are several reasons to think that inflammatory markers are stable for this length of time. The company that provided the inflammatory marker kits assessed several proteins for stability and has not found evidence of degradation in the freezer. In addition, many proteins have been studied, even in this study population, in samples frozen for considerably longer than 10 years (23). Measurement of CRP in the Honolulu Heart Program showed similar results in samples stored for up to 26 years as those collected more recently (33). Despite these considerations, we cannot exclude the possibility that CRP and other inflammatory markers may have degraded over time and that may have affected the findings.
We should acknowledge that the original study design was primarily aimed at evaluating T effects on bone density and body composition. Thus, we cannot exclude that the present study was underpowered to detect significant changes in some inflammatory markers.
Moreover, only 70 subjects of those 96 who completed 36 months of treatment had sera available for laboratory measurements. This data together with the inclusion of individuals at the low-end of the normal reference range could have led to potential selection biases.
Testosterone was not measured by liquid chromatography tandem mass spectrometry (LC-MS/MS) which is the gold standard method to measure sex hormones in men (34). Unfortunately, we did not have this methodical available for the hormonal assays. Undoubtedly, the evaporation of T over time could be uneven between the storage tubes, limiting the accuracy of the results in an uneven way between the samples. However, by reanalyzing T concentrations, which was assessed in the original study, was demonstrated linearity between the results which is an objective parameter of “freezer”-related accuracy. These limitations are offset by important strengths.
First, this was a randomized, placebo-controlled trial. Second, participants had complete data on serum concentrations of soluble receptors of TNF-alpha and IL-6, which are not easily found in previous investigations. All the inflammatory markers were measured at baseline and at multiple time points (3, 6, 9, 12, 18, 24, 30 and 36 months) allowing statistical analysis by the random effects model which accounts for all data points and missing data. The length of duration of treatment (36 months) is not easily found in other RCTs. The methods for the assays of cytokines in body fluids are considered quite accurate. Third, the study population here evaluated is composed of healthy subjects without mobility limitation or clinical conditions including diabetes, metabolic syndrome negatively influencing both T and inflammatory markers. All these enumerated points have justified the decision to test this research question in a trial when inflammation was not a primary outcome.
In conclusion, in older men with low-normal serum T concentrations, 36 month-T treatment did not produce significant effects on inflammatory markers. Significant differences, in estimated means of TNFR1, with lower levels in T group, were observed at 36 month-time point. The substantially negative findings of the present study require future investigations to address the current inconsistence between RCTs in adult and older populations.
ACKNOWLEDGEMENTS
The Study was supported as a `targeted project' by the US National Institute on Aging and by the Intramural Research Program of the US National Institute on Aging. None of the sponsoring institutions interfered with the collection, analysis, presentation, or interpretation of the data reported here. None of the authors have declared a conflict of interest. We thank all participants in the Study. Authors' responsibilities were as follows: Marcello Maggio, Luigi Ferrucci and Peter Snyder conceived this specific hypothesis of the study; Luigi Ferrucci applied for the funding; Yuri Milaneschi: conducted final statistical analyses; Marcello Maggio and Francesca De Vita wrote the first draft of the manuscript; Gian Paolo Ceda, Fulvio Lauretani, Michele Luci, Chiara Cattabiani, Helen Peachey, Giorgio Valenti, Anne R Cappola and Dan L. Longo contributed to subsequent drafts of the manuscript, and approved the final version of the manuscript. The corresponding author (Marcello Maggio) had full access to all data and had final responsibility for the decision to submit the manuscript for publication.
We thank Fabrizio Ablondi, Maurizio Conca and Pietro Schianchi for their technical supports and all the participants in the Study.
Abbreviations
- BMI
Body mass index
- CRP
C-reactive protein
- CV
coefficient of variation
- DXA
Dual energy X-ray Absorptiometry
- FDR
False Discovery Rate
- GnRH
Gonadotropin-releasing hormone
- hs-CRP
high sensitive CRP
- IL-6
interleukin-6
- LC-MS/MS
liquid chromatography tandem mass spectrometry
- LH
Luteinizing hormone
- sIL6r and sgp130
soluble IL-6 receptors
- T
testosterone
- TNF
Tumor necrosis factor -alpha
- TNFR1
soluble TNF-alpha receptor-1
- TU
T undecanoate.
Footnotes
Competing interests/financial disclosure: None of the sponsoring institutions interfered with the collection, analysis, presentation, or interpretation of the data reported here. None of the authors have declared a conflict of interest.
REFERENCES
- 1.Basaria S. Reproductive aging in men. Endocrinol Metab Clin North Am. 2013;42:255–270. doi: 10.1016/j.ecl.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 2.Brunnsgard H, Pederson M, Pederson BK. Aging and proinflammatory cytokines. Curr Opin Hematol. 2001;8:131–136. doi: 10.1097/00062752-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Franceschi C, Bonafè M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann NY Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 4.Cesari M, Penninx BW, Pahor M, et al. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59:242–248. doi: 10.1093/gerona/59.3.m242. [DOI] [PubMed] [Google Scholar]
- 5.Maggio M, Guralnik JM, Longo DL, Ferrucci L. Interleukin-6 in aging and chronic disease: a magnificent pathway. J Gerontol A Biol Sci Med Sci. 2006;61:575–584. doi: 10.1093/gerona/61.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kahl S, Elsasser TH. Exogenous testosterone modulates tumor necrosis factor-alpha and acute phase protein responses to repeated endotoxin challenge in steers. Domest Anim Endocrinol. 2006;31:301–311. doi: 10.1016/j.domaniend.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Olsen NJ, Kovacs WJ. Gonadal steroids and immunity. Endocr Rev. 1996;17:369–384. doi: 10.1210/edrv-17-4-369. [DOI] [PubMed] [Google Scholar]
- 8.Viselli SM, Stanziale S, Shults K, Kovacs WJ, Olsen NJ. Castration alters peripheral immune function in normal male mice. Immunology. 1995;84:337–342. [PMC free article] [PubMed] [Google Scholar]
- 9.Bizzarro A, Valentini G, Di Martino G, DaPonte A, De Bellis A, Iacono G. Influence of testosterone therapy on clinical and immunological features of autoimmune diseases associated with Klinefelter's syndrome. J Clin Endocrinol Metab. 1987;64:32–36. doi: 10.1210/jcem-64-1-32. [DOI] [PubMed] [Google Scholar]
- 10.Yesilova Z, Ozata M, Kocar IH, et al. The effects of gonadotropin treatment on the immunological features of male patients with idiopathic hypogonadotrpic hypogonadism. J Clin Endocrinol Metab. 2000;85:66–70. doi: 10.1210/jcem.85.1.6226. [DOI] [PubMed] [Google Scholar]
- 11.Spratt DI, Cox P, Orav J, Moloney J, Bigos T. Reproductive axis suppression in acute illness is related to disease severity. J Clin Endocrinol Metab. 1993;76:1548–1554. doi: 10.1210/jcem.76.6.8501163. [DOI] [PubMed] [Google Scholar]
- 12.Maggio M, Ceda GP, De Cicco G, et al. Acute changes in circulating hormones in older patients with impaired ventricular function undergoing on-pump coronary artery bypass grafting. J Endocrinol Invest. 2005;28:711–719. doi: 10.1007/BF03347554. [DOI] [PubMed] [Google Scholar]
- 13.Maggio M, Basaria S, Ble A, et al. Correlation between testosterone and the inflammatory marker soluble interleukin-6 receptor in older men. J Clin Endocrinol Metab. 2006;91:345–347. doi: 10.1210/jc.2005-1097. [DOI] [PubMed] [Google Scholar]
- 14.Kupelian V, Chiu GR, Araujo AB, Williams RE, Clark RV, McKinlay JB. Association of sex hormones and C-reactive protein levels in men. Clin Endocrinol. 2010;72:527–533. doi: 10.1111/j.1365-2265.2009.03713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kapoor D, Clarke S, Stanworth R, Channer KS, Jones TH. The effect of testosterone replacement therapy on adipocytokines and C-reactive protein in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2007;156:595–602. doi: 10.1530/EJE-06-0737. [DOI] [PubMed] [Google Scholar]
- 16.Dougherty RH, Rohrer JL, Hayden D, Rubin SD, Leder BZ. Effect of aromatase inhibition on lipids and inflammatory markers of cardiovascular disease in elderly men with low testosterone levels. Clin Endocrinol (Oxf) 2005;62:228–235. doi: 10.1111/j.1365-2265.2005.02205.x. [DOI] [PubMed] [Google Scholar]
- 17.Basaria S, Davda MN, Travison TG, Ulloor J, Singh R, Bhasin S. Risk factors associated with cardiovascular events during testosterone administration in older men with mobility limitation. J Gerontol A Biol Sci Med Sci. 2013;68:153–160. doi: 10.1093/gerona/gls138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakhai-Pour HR, Grobbee DE, Emmelot-Vonk MH, Bots ML, Verhaar HJ, van der Schouw YT. Oral testosterone supplementation and chronic low-grade inflammation in elderly men: a 26-week randomized, placebo-controlled trial. Am Heart J. 2007;154:1228.e1–7. doi: 10.1016/j.ahj.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Kalinchenko SY, Tishova YA, Mskhalaya GJ, Gooren LJ, Giltay EJ, Saad F. Effects of testosterone supplementation on markers of the metabolic syndrome and inflammation in hypogonadal men with the metabolic syndrome: the double-blinded placebo-controlled Moscow study. Clin Endocrinol (Oxf) 2010;73:602–612. doi: 10.1111/j.1365-2265.2010.03845.x. [DOI] [PubMed] [Google Scholar]
- 20.Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004;89:3313–3318. doi: 10.1210/jc.2003-031069. [DOI] [PubMed] [Google Scholar]
- 21.Giltay EJ, Haider A, Saad F, Gooren LJ. C-reactive protein levels and ageing male symptoms in hypogonadal men treated with testosterone supplementation. Andrologia. 2008;40:398–400. doi: 10.1111/j.1439-0272.2008.00873.x. [DOI] [PubMed] [Google Scholar]
- 22.Snyder PJ, Peachey H, Hannoush P, et al. Effect of testosterone treatment on bone mineral density in men over 65 years of age. J Clin Endocrinol Metab. 1999;84:1966–1972. doi: 10.1210/jcem.84.6.5741. [DOI] [PubMed] [Google Scholar]
- 23.Maggio M, Snyder PJ, Ceda GP, et al. Is the haematopoietic effect of testosterone mediated by erythropoietin? The results of a clinical trial in older men. Andrology. 2013;1:24–28. doi: 10.1111/j.2047-2927.2012.00009.x. [DOI] [PubMed] [Google Scholar]
- 24.Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Annals of Statistics. 2001;29:1165–1188. [Google Scholar]
- 25.Corrales JJ, Almeida M, Burgo R, Mories MT, Miralles JM, Orfao A. Androgen-replacement therapy depresses the ex vivo production of inflammatory cytokines by circulating antigen-presenting cells in aging type-2 diabetic men with partial androgen deficiency. J Endocrinol. 2006;189:595–604. doi: 10.1677/joe.1.06779. [DOI] [PubMed] [Google Scholar]
- 26.Guler N, Batyraliev T, Dulger H, et al. The effects of short term (3 weeks) testosterone treatment on serum inflammatory markers in men undergoing coronary artery stenting. Int J Cardiol. 2006;109:339–343. doi: 10.1016/j.ijcard.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 27.Singh AB, Hsia S, Alaupovic P, et al. The effects of varying doses of T on insulin sensitivity, plasma lipids, apolipoproteins, and C-reactive protein in healthy young men. J Clin Endocrinol Metab. 2002;87:136–143. doi: 10.1210/jcem.87.1.8172. [DOI] [PubMed] [Google Scholar]
- 28.Maggio M, Blackford A, Taub D, et al. Circulating inflammatory cytokine expression in men with prostate cancer undergoing androgen deprivation therapy. J Androl. 2006;27:725–728. doi: 10.2164/jandrol.106.000141. [DOI] [PubMed] [Google Scholar]
- 29.Khosla S, Atkinson EJ, Dunstan CR, O'Fallon WM. Effect of estrogen versus testosterone on circulating osteoprotegerin and other cytokine levels in normal elderly men. J Clin Endocrinol Metab. 2002;87:1550–1554. doi: 10.1210/jcem.87.4.8397. [DOI] [PubMed] [Google Scholar]
- 30.Saylor PJ, Kozak KR, Smith MR, et al. Changes in biomarkers of inflammation and angiogenesis during androgen deprivation therapy for prostate cancer. Oncologist. 2012;17:212–219. doi: 10.1634/theoncologist.2011-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith MR, Lee H, Fallon MA, Nathan DM. Adipocytokines, obesity, and insulin resistance during combined androgen blockade for prostate cancer. Urology. 2008;71:318–322. doi: 10.1016/j.urology.2007.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dibbs Z, Thornby J, White BG, Mann DL. Natural variability of circulating levels of cytokines and cytokine receptors in patients with heart failure: implications for clinical trials. J Am Coll Cardiol. 1999;33:1935–1942. doi: 10.1016/s0735-1097(99)00130-8. [DOI] [PubMed] [Google Scholar]
- 33.Sakkinen P, Abbott RD, Curb JD, Rodriguez BL, Yano K, Tracy RP. C-reactive protein and myocardial infarction. J Clin Epidemiol. 2002;55:445–451. doi: 10.1016/s0895-4356(01)00502-9. [DOI] [PubMed] [Google Scholar]
- 34.Hsing AW, Stanczyk FZ, Bélanger A, et al. Reproducibility of serum sex steroid assays in men by RIA and mass spectrometry. Cancer Epidemiol Biomarkers Prev. 2007;16:1004–1008. doi: 10.1158/1055-9965.EPI-06-0792. [DOI] [PubMed] [Google Scholar]