Abstract
Background
Splenic marginal zone lymphoma (MZL) is a form of indolent B-cell lymphoma that is not well characterized in dogs.
Hypothesis/Objectives
The purpose of this study was to describe clinical characteristics and outcome in dogs with splenic MZL confirmed by histopathology, immunophenotyping, and molecular clonality assessment. We hypothesized that affected dogs would have prolonged survival time with splenectomy alone.
Animals
Thirty-four dogs were included. Twenty-nine dogs were diagnosed after splenectomy, and 5 dogs were diagnosed at necropsy.
Methods
Pathology records were searched for dogs with histologically confirmed splenic MZL. Clinical and outcome data were retrospectively collected by medical record review, and prognostic factors were evaluated. Histopathology was reviewed by a board-certified pathologist, and tissue sections were subjected to immunophenotyping and molecular clonality assessment by PCR.
Results
Immunohistochemistry confirmed a B-cell phenotype for all dogs. Molecular clonality assessment was performed in 33 of 34 dogs, of which 24 had clonal rearrangement of immunoglobulin (Ig) loci, 3 had pseudoclonal rearrangement, and 6 had polyclonal rearrangement. The overall median survival time (MST) for the 29 dogs that underwent splenectomy was 383 days. The MST for 14 of 29 asymptomatic dogs that underwent splenectomy for MZL was 1,153 days as compared to 309 days for 15/29 dogs with clinical signs referable to splenic MZL (P = .018). Lymph node involvement, hemoabdomen, anemia, chemotherapy, and concurrent malignancy did not affect survival outcome.
Conclusions and Clinical Importance
Dogs diagnosed with splenic MZL can have prolonged survival with splenectomy alone, without the use of adjuvant chemotherapy. Asymptomatic dogs may have a better survival outcome.
Keywords: Canine, Indolent lymphoma, Neoplasia
Indolent lymphoma is a subgroup of lymphomas with a low mitotic rate and a slow clinical course of progression.1 Marginal zone lymphoma (MZL) is a form of indolent B-cell lymphoma in humans with 3 recognized subtypes, including splenic, nodal, and mucosal-associated lymphoid tissue forms.2–6 Marginal zone lymphoma originates from the marginal zone of lymphoid follicles and is characterized by a proliferating cuff outside of the mantle cell layer.1,2,7
The incidence and prognosis of MZL in dogs is largely unknown because only 2 reports of a limited number of cases are available.1,8 Additionally, the diagnosis of MZL can be challenging for pathologists because it begins with marginal zone hyperplasia (MZH), and confirmation of the diagnosis ultimately requires immunophenotyping and molecular clonality assessment.1,7 Valli et al reported 66 dogs with indolent lymphoma, of which 46 had MZL.1 In this study, 33 dogs had nodal MZL and 13 dogs had splenic MZL.1 The majority of splenic MZL cases were incidentally identified on routine abdominal ultrasound examination, and all dogs had multifocal areas of neoplastic proliferation located within a solitary splenic lesion.1 Molecular clonality assessment and immunophenotyping were performed on the majority of cases in this study. All cases of splenic MZL were found to be CD79a positive and CD3 negative, and most had clonal rearrangement of immunoglobulin (Ig) heavy chain loci, consistent with B-cell lymphoma.1
Currently, little is known about clinical outcome in dogs with splenic MZL. Only 3 dogs with splenic MZL in Valli’s study had follow-up data available, and none of these dogs died from MZL in the follow-up period (7–19 months).1 In a recent study by Stefanello et al, outcome was described in 5 dogs with splenic MZL, of which 4 of 5 received adjuvant single-agent chemotherapy with doxorubicin.8 Results of that study suggested a possible survival benefit with the addition of adjuvant chemotherapy, but additional studies are warranted to support this conclusion because of the limited number of dogs evaluated in this report.
The purpose of this study was to describe clinical information and outcome in a larger cohort of dogs with a definitive diagnosis of splenic MZL confirmed with histopathology, immunophenotyping, and molecular clonality assessment. An additional aim was to examine possible prognostic factors that may predict outcome in this population and to further explore the potential role of adjuvant chemotherapy.
Materials and Methods
The veterinary histopathology database at the University of California, Davis Veterinary Medical Teaching Hospital (VMTH) was searched for cases of canine splenic MZL between January 2000 and June 2009 that were also seen at the VMTH. The veterinary histopathology database at VDx Veterinary Diagnostics (Davis, CA) was searched for cases of canine splenic MZL between January 2008 and June 2009. Diagnoses could be made from surgical biopsy (splenectomy) or necropsy samples. All medical records were reviewed, and data collected included signalment, body weight, clinical signs associated with the tumor, staging diagnostics, treatment, and date of death. Follow-up information was obtained by contact with referring veterinarians, clients, or both. To meet selection criteria, dogs needed adequate follow-up information available to assess outcome.
All slides were reviewed by a single board-certified pathologist (PFM), and histopathological criteria for diagnosis were based on those previously published for canine splenic MZL.1,7 Immunophenotyping and molecular clonality were performed in all cases. Immunophenotyping was performed by immunohistochemistry with CD79aa and CD20b antibodies to determine a B-cell phenotype and CD3c antibody to determine T-cell phenotype. Molecular clonality assessment was performed with PCR as described previously.1,9,10
The Kaplan–Meier product limit method was used to estimate survival after splenectomy, and the log-rank test was used to assess the effect of various factors on survival. Survival times were censored if dogs were alive at the study’s end, lost to follow-up, or dead from causes other than MZL or treatment for MZL. Death was assumed to be related to MZL if the cause of death could not be definitively determined. Factors assessed for effect on prognosis included regional lymph node involvement, presence of hemoabdomen at diagnosis, anemia (Hct <40%) at diagnosis, use of postoperative adjuvant chemotherapy, presence of a concurrent malignancy at diagnosis, and whether or not splenic MZL was diagnosed as an incidental finding. The diagnosis of splenic MZL was considered an incidental finding if the patient presented for a comorbidity and a splenic mass was found during abdominal ultrasound examination. The diagnosis was not considered incidental if clinical signs were present (eg, anorexia or lethargy) or if the patient presented with a hemoabdomen.
Results
Thirty-four dogs with splenic MZL met selection criteria. Twenty-seven dogs were from the University California, Davis VMTH histopathology database, and thus also patients seen at the VMTH, and 7 dogs were from the histopathology database of VDx Veterinary Diagnostics. Twenty-nine dogs were diagnosed after splenectomy, and 5 dogs were diagnosed at necropsy. There were 8 mixed breed dogs, 5 Rottweilers, 2 each of Bouvier des Flandres, Labrador Retriever, Norwegian Elkhound, Miniature Poodle, and Standard Poodle, and 1 each of 11 other pure breeds. Sex characteristics included 16 castrated males, 1 intact male, and 17 spayed females. Mean age was 10 years (range, 3–15 years), and mean body weight was 24.5 kg (range, 2.6–88.6 kg).
Clinical signs attributable to splenic MZL were present in 15 of 29 dogs that underwent splenectomy. These clinical signs included hemoabdomen, distended abdomen, and nonspecific clinical signs such as lethargy, anorexia, vomiting, and weight loss.
All dogs were confirmed to have splenic MZL on pathology review by PFM. As expected, all lymphomas were CD79a positive and CD3 negative, confirming a B-cell phenotype. Evaluation of CD20 expression was performed in 27 of 29 dogs and all were CD20 positive. DNA was available for 33 of 34 dogs, and 24 dogs (73%) had clonal rearrangement of Ig loci, 3 dogs (9%) had pseudoclonal rearrangement of Ig loci, and 6 dogs (18%) had polyclonal rearrangement of Ig loci. All of the dogs that were identified at necropsy had clonal rearrangement of Ig loci. Survival analysis based on clonality results was evaluated for 28 of 29 dogs that underwent splenectomy. The MST for 19 dogs with clonal rearrangement of Ig loci, 3 dogs with pseudoclonal rearrangement of Ig loci, and 6 dogs with polyclonal rearrangement of Ig loci was 347 days, 309 days, and not reached, respectively. The MST for the dogs with pseudoclonal and clonal rearrangement of Ig loci combined was 322 days as compared to MST that was not reached for the dogs with polyclonal rearrangement of Ig loci. Statistical analysis was not performed because of small sample size (low power) and risk of statistical errors.
Of the 5 dogs identified at necropsy, splenic MZL was considered to be an incidental finding by the pathologist conducting the necropsy in 4 of 5 dogs. Cause of death in these 4 dogs was fungal pneumonia with suspected septicemia, anaplastic carcinoma of the esophagus with metastasis to the pulmonary parenchyma and spinal cord (C5–C6), end-stage renal disease with thrombosis of arterioles, and cardiac hemangiosarcoma with pulmonary metastasis. The only necropsy case in which splenic MZL was not considered an incidental finding had disseminated MZL involving the spleen, jejunum, ileo-colic junction, liver, mesenteric lymph nodes, and peritoneum. The antemortem diagnosis of disseminated neoplasia was the cause for euthanasia, and this was the only dog in the study with dissemination of MZL.
Twenty-nine dogs were diagnosed with splenic MZL after splenectomy. Fine-needle aspiration and cytology were performed presurgically on a clinically detected splenic mass in 13 of 29 dogs. A cytological diagnosis of lymphoma was made in 3 of 13 dogs by immunocytochemistry in 2 of 3 and molecular clonality assessment in the remaining dog to confirm the diagnosis. A cytological diagnosis of lymphoma was not made in the remaining 10 dogs. Instead, the cytological diagnoses were extramedullary hematopoiesis (EMH, n = 2), reactive lymphoid hyperplasia and EMH (n = 2), no clinically relevant abnormalities (n = 2), inconclusive (n = 1), reactive lymphoid hyperplasia (n = 1), atypical lymphoid hyperplasia (n = 1), and plasmacytosis and histiocytosis (n = 1).
Abdominal ultrasonography was performed in all 29 dogs before splenectomy. A solitary splenic mass was identified on ultrasound examination in 19 dogs, and multiple splenic masses and nodules were present in 7 dogs. Splenomegaly with a diffusely mottled echotexture was identified in 2 dogs. The echotexture of the solitary splenic mass was described in 13 of 19 dogs and was reported as mixed echogenicity (n = 8), hypoechoic (n = 4), and hyperechoic (n = 1). The size of the solitary splenic mass was recorded in 17 of 19 dogs with mean size of 5.4 cm (range, 2.5–11 cm), and the splenic capsule was distorted in 4 of 25 dogs. In 1 dog, abdominal ultrasound examination failed to identify a 4-cm splenic mass that was subsequently found during an exploratory laparotomy for septic peritonitis. Thoracic radiographs were performed in 28 of 29 dogs that underwent splenectomy, and no detectable lesions suggestive of neoplasia were identified.
None of the dogs underwent bone marrow evaluation. A CBC was available for review at the time of diagnosis in 25 of 29 dogs that underwent splenectomy, and anemia (Hct <40%) was present in 16 of these dogs (mean Hct, 27.7%; range, 13.9–39.7%). The anemia was characterized as nonregenerative (reticulocyte count <60,000/μL) in 7 of 16 dogs and regenerative (reticulocyte count >60,000/μL) in 9 of 16 dogs. Hemoabdomen was present in 3 of 9 dogs with regenerative anemia. Thrombocytopenia (<150,000/μL) was present in 4 dogs (mean, 55,750/μL; range, 22,000–95,000/μL), whereas thrombocytosis (>400,000/μL) was present in 6 dogs (mean, 709,000/μL; range, 423,000–947,000/μL). Leukocytosis (WBC >13,000/μL) was present in 11 of 25 dogs. The leukocytosis was characterized by mature neutrophilia in 7 of 11 dogs and neutrophilia and monocytosis in 3 of 11 dogs. In 1 dog, leukocytosis (17,410/μL) was characterized by lymphocytosis of 8,705/μL (reference range, 1,000–4,000/μL). On blood smear review, the lymphocytes consisted of a mixed population of cells that were predominantly small mature lymphocytes. Molecular clonality assessment was performed on the blood, and the cells in the blood sample had a clonal gene rearrangement identical to that present in the spleen. These findings were consistent with a leukemic phase of MZL, which resolved after splenectomy without administration of adjuvant chemotherapy or corticosteroids. This dog was euthanized 7 months after splenectomy owing to the progression of chronic renal failure and dynamic airway collapse, but no evidence of lymphoma was found on necropsy. This was the only dog in this study that had molecular clonality assessment identified in peripheral blood.
Twelve concurrent malignancies were diagnosed at the same time as MZL in 10 of 29 dogs that underwent splenectomy. These tumors included cutaneous mast cell tumor (n = 1), digital squamous cell carcinoma (n = 1), hepatocellular carcinoma (n = 1), pheochromocytoma (n = 1), splenic sarcoma (n = 2), soft tissue sarcoma (n = 3), and splenic hemangiosarcoma (n = 3). A splenic hematoma was present in addition to splenic MZL in 5 dogs. Other nonmalignant concurrent disease processes in 14/29 dogs included diabetes mellitus, chronic renal failure, septic abdomen, hepatopathy, lymphangiectasia, neurological disease, cardiac disease, laryngeal paralysis, and dynamic collapsing airway disease.
Adjuvant chemotherapy was administered to 8 dogs. Six dogs received chemotherapy intended to treat MZL, which included chlorambucil and prednisone (n = 1) and the University of Wisconsin–Madison protocol (n = 5). Chemotherapy intended for neoplasia other than MZL included single-agent doxorubicin in 1 dog (splenic hemangiosarcoma) and alternating vinblastine and CCNU with prednisone in 1 dog (cutaneous mast cell tumor).
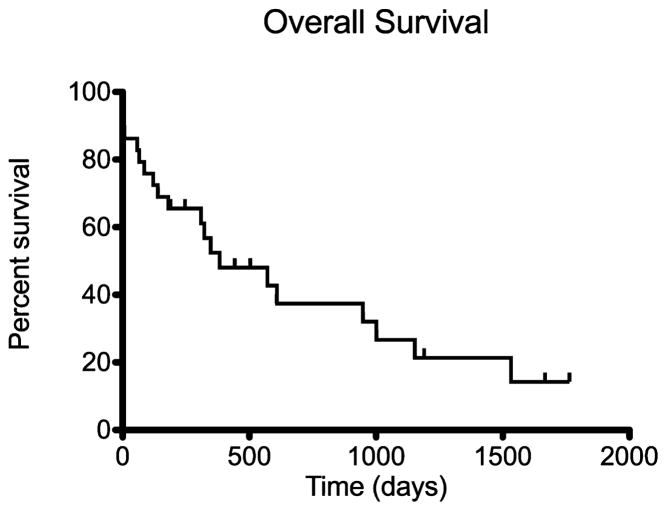
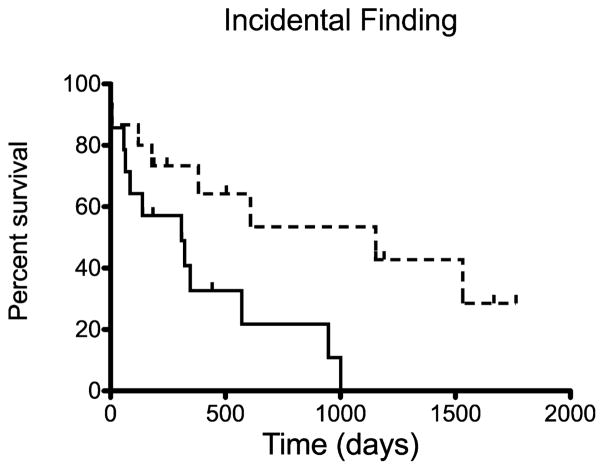
All 29 dogs that underwent splenectomy were included in survival analysis. The overall MST after splenectomy was 383 days (Fig 1). The MST for 25 dogs that survived the immediate postoperative period of >1 week was 571 days. Two dogs that died in the immediate post-operative period had concurrent septic abdomen (1 with pyometra [pseudoclonal rearrangement of Ig loci] and 1 with dehiscence of a cholecystoduodenostomy site [clonal rearrangement of Ig loci]). One dog died at home after discharge (polyclonal rearrangement of Ig loci), and 1 dog had concurrent lymphangiectasia and was euthanized because of the lack of improvement in the postoperative period (clonal rearrangement of Ig loci). The MST for 5 of 29 dogs without a concurrent disease process was 1,001 days, with 3 of 5 dogs still alive. Splenic MZL was considered to be an incidental finding in 14 of 29 dogs undergoing splenectomy, and this group had a MST of 1,153 days as compared to 309 days for 15 of 29 dogs with clinical signs referable to splenic MZL (P = .018, Fig 2 and Table 1). Lymph node involvement, hemoabdomen, anemia, chemotherapy, and concurrent malignancy were not found to influence survival (Table 1).
Fig 1.
Kaplan–Meier survival curve estimating overall survival of the 29 dogs that underwent splenectomy. The overall median survival time of these dogs was 383 days.
Fig 2.
Kaplan–Meier survival curve estimating overall survival of the 29 dogs that underwent splenectomy for marginal zone lymphoma (MZL) when the dogs were symptomatic (solid line) or asymptomatic (dashed line). Splenic MZL was an incidental finding in 14 of 29 dogs with a median survival time of 1,153 days versus 309 days for 15 of 29 dogs with symptoms referable to splenic MZL (P = .018).
Table 1.
Prognostic factors assessed for effect on survival
| n | MST (days) | P Value | |
|---|---|---|---|
| Incidental finding | |||
| Yes | 14 | 1,153 | .018 |
| No | 15 | 309 | |
| Lymph node | |||
| Positive | 6 | 570 | .49 |
| Negative | 23 | 383 | |
| Anemia | |||
| Hct ≥40% | 7 | 609 | .80 |
| Hct <40% | 17 | 139 | |
| Hemoabdomen | |||
| Yes | 5 | 948 | .63 |
| No | 24 | 383 | |
| Adjuvant chemotherapy | |||
| Yes | 8 | 347 | .80 |
| No | 21 | 571 | |
| Concurrent malignancy | |||
| Yes | 10 | 335 | .11 |
| No | 19 | 1,001 | |
Twenty-one dogs that underwent splenectomy died. Cause of death was assessed by necropsy in 2 of 21 dogs. One of these dogs was receiving CHOP-based chemotherapy for splenic MZL, and the protocol was started 61 days before death. This dog received L-asparaginased chemotherapy to avoid a chemotherapy treatment delay for gastrointestinal signs after doxorubicin administration and presented dead on arrival to the hospital 2 days later. The cause of death was unknown, but suspected to be related to clotting factor abnormalities associated with L-asparaginased chemotherapy because of the presence of severe intestinal mucosal congestion and hemorrhage and extensive venous thrombosis of mural and mesenteric blood vessels. In the 2nd dog, necropsy showed the cause of death to be chronic renal failure. There was no evidence of lymphoma in examined tissues in either of these dogs. The cause of death could not be definitively determined in the remaining dogs that underwent splenectomy. Two dogs were lost to follow-up, and 6 dogs were still alive at manuscript preparation. One dog that underwent splenectomy for MZL developed high-grade multicentric lymphoma at a later date. This dog initially received prednisone and vinblastine alternating with CCNU chemotherapy for a cutaneous mast cell tumor, and this chemotherapy protocol started 15 days postsplenectomy. The dog was found to have generalized peripheral lymphadenopathy 184 days after starting chemotherapy for the mast cell tumor (22 days after completion of the chemotherapy protocol). Cytology of a peripheral lymph node confirmed high-grade lymphoma, but immunophenotyping and molecular clonality assessment were not performed. This dog then was started on the University Wisconsin–Madison protocol, did not achieve clinical remission, and was euthanized 122 days later owing to resistant lymphoma.
Discussion
In humans, splenic MZL is considered rare, representing approximately 1% of the cases of non-Hodgkin’s lymphoma.2,3 This lymphoma also is thought to be rare in dogs, but MZL is being recognized more because of increased awareness by veterinary pathologists and with the use of immunophenotyping and molecular clonality assessment. Currently, there are only 2 published reports of splenic MZL in the veterinary literature, totaling 18 dogs, and with follow-up data available for 8 of these dogs.1,8 The goals of this study were to better characterize the clinical aspects of canine splenic MZL in a larger population of dogs in which the diagnosis was confirmed by immunophenotyping and molecular clonality assessment.
By immunohistochemistry, we confirmed B-cell morphology for all cases of splenic MZL included in this study. Clonal rearrangement of Ig was confirmed in 24 dogs (73%) diagnosed with splenic MZL, supporting the diagnosis of B-cell neoplasia when used in conjunction with pathology review and immunophenotyping. Lack of demonstration of a clonal population in 9 of 33 dogs (27%) in our study is consistent with the previously published sensitivity of this PCR-based assay and with the findings of Valli et al.1,11,12 Because there was immunohistochemical confirmation of B-cell linage for all dogs in this study, the pseudoclonal results in 3 dogs are consistent with a diagnosis of MZL and could be because of unknown mutations of the variable (V) and joining (J) segments or lack of primer coverage of unknown V and J segments. The polyclonal results in 6 dogs could be attributable to a mutation in V or J segments of Ig. Based on careful histological review of morphology and immunophenotyping results, the 6 polyclonal cases in this study could be interpreted as false-negative results. Alternatively, this finding could suggest that the polyclonal cases actually were cases of MZH, and this is why survival times based on clonality results also were reported.
Our results demonstrate a good outcome in dogs with splenic MZL after splenectomy, with an overall MST of 383 days and MST of 571 days with exclusion of peri-operative deaths. Lymph node involvement, hemoabdomen, anemia, adjuvant chemotherapy, and concurrent malignancy were not found to predict prognosis. Only whether or not MZL was an incidental finding was found to influence survival. Previous studies suggest a longer survival time than reported here; however, follow-up data were available in a limited number of dogs in these 2 previous studies.1,8 Also, in the study by Stefenello et al, only 3 of 5 dogs had molecular clonality assessment performed, which could have resulted in the inclusion of 2 dogs with MZH considering the difficulty in making an accurate diagnosis of this lymphoma type.8 The relatively short MST despite low incidence of documented metastasis also may have been affected by our conservative method of censoring death. Interestingly, the majority of splenectomized dogs had a concurrent disease process. Only 5 of 29 of these dogs did not have any concurrent disease, and this group had a MST of 1,001 days with 3 dogs still alive. Ten of 29 dogs had 12 total concurrent malignancies and 7 dogs had severe concurrent non-neoplastic conditions that could have contributed to cause of death and thus to a shorter MST. These data suggest that concurrent disease is common in affected dogs, and ultimately, MZL may not be the limiting factor affecting survival. Also, MZL-specific survival times could have been longer if necropsies had been performed, and thus, more information on the cause of death was available.
Results suggest that adjuvant chemotherapy may not prolong survival time in dogs with MZL, indicating that splenectomy may be the treatment of choice, which is similar to the human counterpart of this lymphoma type. The finding of lack of survival benefit with the use of adjuvant chemotherapy in this study is in contrast to a recent publication by Stefanello et al.8 In this report, 4 of 5 dogs received adjuvant doxorubicin chemotherapy after splenectomy with 3 of 4 dogs dead of causes unrelated to the tumor.8 The 1 dog that did not receive adjuvant chemotherapy died of tumor recurrence after 180 days.8 However, because of the small sample size in that study, and lack of definitive confirmation of MZL with molecular clonality assessment, it is difficult to draw conclusions regarding the benefit of adjuvant chemotherapy for these cases. The majority of the dogs in the present study that received chemotherapy were treated with cytotoxic injectable chemotherapy, whereas indolent lymphomas generally are more responsive to continuous chemotherapy (ie, chlorambucil and prednisone). The lack of survival benefit observed may have been related to the type of adjuvant chemotherapy used. In humans, splenectomy is the standard of care for MZL for patients with symptomatic splenomegaly and progressive hematological abnormalities such as lymphocytosis and cytopenias.4,5,13 Splenectomy is not curative in humans, but yields excellent control of the disease with a median time to progression of 4–5 years.4,5,14 The role of chemotherapy in humans remains largely unknown, but chemotherapy is used when splenectomy is contraindicated.2–5,13 A prospective study evaluating the use of adjuvant chemotherapy as compared to no chemotherapy for splenectomized dogs with MZL would be necessary to further evaluate the effect of chemotherapy on survival.
Concurrent lymph node involvement was not found to be prognostic in our study. Splenic MZL is considered an indolent lymphoma, indicating a slow disease progression, and this may explain why lymph node involvement did not affect prognosis.2,5,7 The dogs with lymph node involvement (n = 6) survived longer than those without lymph node involvement (n = 23), but this may be due to small sample size. Additional studies would be required to determine whether prognosis is affected by concurrent lymph node involvement and whether adjuvant chemotherapy is beneficial in this subset of patients.
The definitive diagnosis of MZL requires architectural assessment and hence histopathology. As such, MZL cannot be diagnosed by cytology. Thirteen of the 29 dogs that underwent splenectomy had fine-needle aspiration cytology performed on a splenic mass. A diagnosis of lymphoma was made in only 3 of these dogs. On cytology, MZL is described as having an immature cell morphology with rare mitoses observed.7 Cytological diagnosis may be confused with high-grade lymphoma or confounded by concurrent disease or concurrent reactive hyperplasia. Lack of awareness of this lymphoma type also may confound cytological diagnosis. Our findings suggest that, as with other indolent lymphomas, diagnosis is challenging using cytology alone, and histopathology ultimately is required for a definitive diagnosis.
Lymphocytosis was present in 1 dog, suggesting that, in contrast to humans, a leukemic phase is rare in dogs, although this was the only dog that had molecular clonality assessment of peripheral blood, and no dogs in this study underwent bone marrow evaluation. The lymphocytosis in this dog resolved after splenectomy, and although this dog was euthanized 7 month postsplenectomy owing to progression of chronic renal failure and dynamic airway collapse, the lymphocytosis did not recur and there was no evidence of lymphoma at necropsy. In contrast, human splenic MZL is characterized by splenomegaly and lymphocytosis in up to 75% of patients, with bone marrow infiltration in most patients.2–4,13,15
Later development of high-grade multicentric lymphoma was observed in only 1 dog. This dog’s high-grade lymphoma was resistant to various chemotherapeutics, and the dog was euthanized 122 days later. This dog also had been treated previously with prednisone, vinblastine, and CCNU for a mast cell tumor, which may have contributed to chemoresistance. It is unknown whether this dog developed de novo lymphoma or transformation of MZL because of lack of confirmation with matched molecular clonality assessment. Transformation of MZL to large B-cell lymphoma, mostly in peripheral lymph nodes, has been reported in humans, and although the true incidence is unknown, it is thought to be low.16,17
Limitations of this study include its retrospective design. Survival times are likely underestimated because dogs were considered to be dead of MZL if necropsy was not performed to determine the cause of death. Only 2 of 20 dogs that had splenectomy and subsequently died were necropsied. Additionally, because of the retrospective design, 2 cases were lost to follow-up.
In conclusion, the results of this study suggest that splenic MZL carries a favorable prognosis in dogs treated with splenectomy alone. Many dogs are asymptomatic, which may predict a better prognosis. The diagnosis of splenic MZL can be challenging and ultimately requires histopathology with immunohistochemistry and molecular clonality assessment for definitive confirmation.
Acknowledgments
Funded by the Center for Companion Animal Health, University of California-Davis, Davis, CA.
Abbreviations
- Ig
immunoglobulin
- MST
median survival time
- MZH
marginal zone hyperplasia
- MZL
marginal zone lymphoma
- VMTH
Veterinary Medical Teaching Hospital
Footnotes
HM57, Dako, Carpinteria, CA
RB-9013, Lab Vision Corp, Kalamazoo, MI
CD3-12, Serotec, Oxford, UK
L-asparaginase, Lundbeck, Deerfield, IL
Conflict of Interest: Authors disclose no conflict of interest.
References
- 1.Valli VE, Vernau W, de Lorimier LP, et al. Canine indolent nodular lymphoma. Vet Pathol. 2006;43:241–256. doi: 10.1354/vp.43-3-241. [DOI] [PubMed] [Google Scholar]
- 2.Kahl B, Yang D. Marginal zone lymphomas: Management of nodal, splenic, and MALT NHL. Hematology Am Soc Hematol Educ Program. 2008:359–364. doi: 10.1182/asheducation-2008.1.359. [DOI] [PubMed] [Google Scholar]
- 3.Thieblemont C. Non-MALT marginal zone lymphomas. Ann Oncol. 2008;19(Suppl 4):iv70–73. doi: 10.1093/annonc/mdn202. [DOI] [PubMed] [Google Scholar]
- 4.Thieblemont C, Felman P, Callet-Bauchu E, et al. Splenic marginal-zone lymphoma: A distinct clinical and pathological entity. Lancet Oncol. 2003;4:95–103. doi: 10.1016/s1470-2045(03)00981-1. [DOI] [PubMed] [Google Scholar]
- 5.Oscier D, Owen R, Johnson S. Splenic marginal zone lymphoma. Blood Rev. 2005;19:39–51. doi: 10.1016/j.blre.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Morse HC, 3rd, Kearney JF, Isaacson PG, et al. Cells of the marginal zone–origins, function and neoplasia. Leuk Res. 2001;25:169–178. doi: 10.1016/s0145-2126(00)00107-7. [DOI] [PubMed] [Google Scholar]
- 7.Valli VE ebrary Inc. Veterinary Comparative Hematopathology. 1. Ames, IA: Blackwell Pub; 2007. p. xii. [Google Scholar]
- 8.Stefanello D, Valenti P, Zini E, et al. Splenic marginal zone lymphoma in 5 dogs (2001–2008) J Vet Intern Med. 2010;25:90–93. doi: 10.1111/j.1939-1676.2010.0639.x. [DOI] [PubMed] [Google Scholar]
- 9.Vernau W, Moore PF. An immunophenotypic study of canine leukemias and preliminary assessment of clonality by polymerase chain reaction. Vet Immunol Immunopathol. 1999;69:145–164. doi: 10.1016/s0165-2427(99)00051-3. [DOI] [PubMed] [Google Scholar]
- 10.van Dongen JJ, Langerak AW, Bruggemann M, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: Report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia. 2003;17:2257–2317. doi: 10.1038/sj.leu.2403202. [DOI] [PubMed] [Google Scholar]
- 11.Burnett RC, Vernau W, Modiano JF, et al. Diagnosis of canine lymphoid neoplasia using clonal rearrangements of antigen receptor genes. Vet Pathol. 2003;40:32–41. doi: 10.1354/vp.40-1-32. [DOI] [PubMed] [Google Scholar]
- 12.Avery A. Molecular diagnostics of hematologic malignancies. Top Companion Anim Med. 2009;24:144–150. doi: 10.1053/j.tcam.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Matutes E. Clinical and biological diversity of splenic marginal zone lymphoma. Expert Rev Anticancer Ther. 2009;9:1185–1189. doi: 10.1586/era.09.91. [DOI] [PubMed] [Google Scholar]
- 14.Berger F, Felman P, Thieblemont C, et al. Non-MALT marginal zone B-cell lymphomas: A description of clinical presentation and outcome in 124 patients. Blood. 2000;95:1950–1956. [PubMed] [Google Scholar]
- 15.Thieblemont C. Clinical presentation and management of marginal zone lymphomas. Hematology Am Soc Hematol Educ Program. 2005:307–313. doi: 10.1182/asheducation-2005.1.307. [DOI] [PubMed] [Google Scholar]
- 16.Camacho FI, Mollejo M, Mateo MS, et al. Progression to large B-cell lymphoma in splenic marginal zone lymphoma: A description of a series of 12 cases. Am J Surg Pathol. 2001;25:1268–1276. doi: 10.1097/00000478-200110000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Dungarwalla M, Appiah-Cubi S, Kulkarni S, et al. High-grade transformation in splenic marginal zone lymphoma with circulating villous lymphocytes: The site of transformation influences response to therapy and prognosis. Br J Haematol. 2008;143:71–74. doi: 10.1111/j.1365-2141.2008.07301.x. [DOI] [PubMed] [Google Scholar]