Abstract
This retrospective case series evaluates the outcome of 21 dogs with grade II stage 2 mast cell tumour (MCT) treated with adequate local therapy and adjuvant systemic chemotherapy (prednisone, vinblastine and CCNU). The median survival for all dogs was 1359 days (range, 188–2340). Median disease-free interval was 2120 days (149–2325 days). Dogs treated with surgery and chemotherapy had shorter survival (median, 1103 days; 188–2010 days) than those that underwent surgery, radiation therapy and chemotherapy as part of their treatment (median, 2056 days; 300–2340 days). Two patients had local recurrence in the radiation field and four patients had de novo MCT. Distant metastasis was not observed in any dogs. The results of this study suggest that, in the presence of loco-regional lymph node metastasis in grade II MCT, the use of prednisone, vinblastine and CCNU after adequate local-regional therapy can provide a median survival in excess of 40 months.
Keywords: canine, chemotherapy, mast cell tumour, radiation therapy, surgery
Introduction
Mast cell tumour (MCT) is the most common canine cutaneous malignancy encountered in general practice and grade II (according to the Patnaik classification) MCT accounts for up to 77% of the returned histopathological diagnoses.1–5 Surgery remains the mainstay of treatment for this dermal neoplasm and removal of grade II MCT with 2 cm (or less) lateral margins and one deep fascial plane often provides adequate margins and an excellent local control.6–8 After resection of grade II MCT, local regrowth rates from 0% to up to 27% have been reported for both completely and incompletely excised tumours.4,6,7,9–12 However, when grade II MCTs are incompletely excised, adjuvant therapy is recommended in order to provide adequate local control (ALC) and to decrease the chances of tumour-related death.13,14 Radiation therapy is an effective adjuvant therapy to achieve ALC of incompletely excised grade II MCT (microscopic setting).15–20
Once ALC is achieved, the reported survival of patients with a diagnosis of grade II MCT varies widely.5,21 In an effort to better predict survival in this common clinically relevant disease, numerous investigations have evaluated risk factors such as breed, local recurrence, anatomic location, tumour-mediated clinical signs, grade, mitotic index, density of microvessels, patterns of immunohistochemical staining for various proliferation markers, status of c-kit or stage.3–5,13,20–40
In dogs with locoregional lymph node metastasis (stage 2), a poor prognostic variable, adjuvant chemotherapy and radiation have been recommended and implemented in a small number of patients with grade II stage 2 MCT; these dogs have been reported to enjoy prolonged survival.16,20,39–41
To date, there is no formal study evaluating therapy for only grade II stage 2 MCT. Previous studies have focused on the therapeutic strategies themselves and have comingled patients with divergent stages or tumour burden and then analysed these patients as a whole for survival. Consequently the power to detect clinically relevant differences after stratification of patients is often lacking. Ultimately then, these characteristics of the reported data make application of the outcome to a patient harbouring a specific grade and stage, challenging.
The purpose of this study was to evaluate outcome in dogs with a single grade of MCT (grade II) with lymph node metastasis (stage 2) that received ALC and a single adjuvant chemotherapeutic protocol (prednisone and CCNU (1-[2-chloroethyl]3-cyclohexyl-1-nitrosurea) alternated with vinblastine).
Materials and methods
Electronic medical records of client-owned dogs presented to the University of California at Davis William R. Pritchard Veterinary Medical Teaching Hospital for treatment of MCTs from 1999 to 2012 were reviewed.
To be included in this retrospective study, patients had to have a confirmed histopathological diagnosis of a primary grade II cutaneous stage 2 MCT, ALC of the tumour and adjuvant treatment with alternating vinblastine/CCNU and prednisone combination chemotherapy. Stage 2 was documented via cytology and/or histopathology.39 Patients with suspected or confirmed metastasis to the liver or spleen were excluded. Initial treatment intent included alternating CCNU and vinblastine for six to seven cycles.
Dogs were excluded if they presented with a recurrent MCT, received prior treatment with chemotherapy (including corticosteroids) for MCT, or if the presenting MCT was muco-cutaneous. Data collected from the medical records included signalment, body weight, tumour size, presence of clinical signs, histopathology of the MCT, treatment protocol, disease free and overall survival data. The longest dimension of the tumour was reported (longest diameter). Results of initial staging which included physical examination, complete blood count (CBC), serum biochemistry profile, urinalysis, thoracic radiographs, abdominal ultrasonography and cytology and/or histopathology of the regional lymph node, cytology of liver and spleen were collected.
The stage of disease immediately before surgery was categorized based on the World Health Organization (WHO) criteria (Table 1), with de novo MCT classified independently.42
Table 1.
World Health Organization modified TNM
| Staging system for canine cutaneous MCTs | |
|---|---|
|
| |
| Stage | Description |
| I | One tumour confined to the dermis, without regional lymph node involvement |
| II | One tumour confined to the dermis, with regional lymph node involvement |
| III | Large infiltrating or multiple dermal tumours, with or without regional lymph node involvement |
| IV | Any tumour with distant metastasis or recurrence with metastasis |
The grade of the MCT was determined by review of histopathology reports from UC Davis (n=10) or commercial histopathology laboratories (n=11) using the previously described Patnaik classification system3. When available (n=10), the histopathological grade was confirmed and the mitotic index (number of mitotic figures identified per 10 high power field (HPF) – MF/10 (×400 HPF)) was also scored by one pathologist (C. R.).
ALC was defined as resection of the primary MCT (with adequate histological margins), lymph node excision or incomplete excision of the primary MCT followed by radiation therapy delivered to the tumour site plus surgical excision of the lymph node and/or radiation to the lymph node. For the purpose of this study, histopathological tumour-free margins were defined as the absence of tumour cells within 5 mm of the submitted lateral margins and one deep fascial plane.8,43 When surgical margins were deemed to contain tumour cells based on the pathology report, a second surgery or definitive radiation therapy (total dose of 48 Grays delivered in daily 3 Gray treatments given over 16 consecutive days on a Monday through Friday basis) was prescribed. The radiation therapy field included the draining lymph node bed in all cases and was delivered using a 6-MV linear accelerator (Clinac 4/80, Varian Medical Systems, Palo Alto, CA, USA). Non-graphic (manual) planning or computer-based planning was used, depending on the complexity of the tumour location. When appropriate, blocks were used to spare surrounding tissues. Treatment field margins were set at 3 cm from the scar when possible. For all cases receiving radiation, the regional lymph node bed received the same radiation protocol as the primary site and was included in the same field as the primary site if it was within 6 cm of the surgical scar. When the regional node was more distant, it was treated as a separate field.
The chemotherapy protocol consisted of prednisone (40 mg m−2 PO q24h for 2 weeks, 20 mg m−2 PO q24h for 2 weeks and then 20 mg m−2 PO q48h until the end of the protocol), vinblastine (2 mg m−2 administered by a rapid intravenous bolus) alternated with lomustine (CCNU, 1-[2-chloroethyl]3-cyclohexyl-1-nitrosurea) (60–80 mg m−2 PO delivered to the nearest 5 mg) every 2 weeks, with a plan of treating for six to seven cycles (chemotherapy protocol described in Table 2). The number of cycles intended to be delivered was modified from seven to six cycles after 2008. Chemotherapy was initiated during or after the radiation therapy protocol or as soon as possible following surgery. Prophylactic H1/H2 antagonists (diphenhydramine 2 mg mg−1 PO q12h and famotidine 0.5 mg kg−1 PO q12–24h) were prescribed during the protocol according to the clinician's preference. A combination of S-adenosylmethionine (SAMe) and silybin (Denamarin, Nutramax laboratories, Inc, Edgewood, MD, USA) (dose per dog size, PO, q24h on an empty stomach) was administered as a hepatoprotectant during the chemotherapy protocol according to the clinician's preference. Prophylactic antibiotics were administered for 7–10 days starting 3 days following CCNU administration (enrofloxacin or amoxillin/clavulamate or trimethoprim and sulfamethoxazole, TMS) in order to prevent any morbidity associated with the anticipated CCNU-induced neutropenia.44,45 Toxicity of the protocol was determined on the basis of patient history, clinical pathology data and physical examination findings. Neutropenia, thrombocytopenia, increases in alanine aminotransferase (ALT) activity, and gastrointestinal toxicosis were graded in accordance with the guidelines produced by the Veterinary Cooperative Oncology Group (VCOG) – Common Terminology Criteria for Adverse Events v1.0.46
Table 2.
Chemotherapy protocol (CCNU, vinblastine and prednisone) used for the treatment of stage 2 grade II MCTs
| Week | Drug | Dosage and route | Diagnostic tests |
|---|---|---|---|
| 1 | Vinblastine | 2 mg m−2 IV | CBC, chemistry panel, abdominal US, UA, chest X-rays |
| Prednisone | 40 mg m−2 PO q24h | ||
| 3 | CCNU | 60–80 mg m−2 PO | CBC, Liver panel |
| Prednisone | 20 mg m−2 PO q24h | ||
| 5 | Vinblastine | 2 mg m−2 IV | CBC |
| Prednisone | 20 mg m−2 PO q48h | ||
| 7 | CCNU | 60–80 mg m−2 PO | CBC, Liver panel |
| Prednisone | 20 mg m−2 PO q48h | ||
| 9 | Vinblastine | 2 mg m−2 IV | CBC |
| Prednisone | 20 mg m−2 PO q48h | ||
| Repeat cycle …. | |||
| 27 | CCNU | 60–80 mg m−2 PO | CBC, chemistry panel, abdominal US, UA, chest X-rays |
| Prednisone | 20 mg m−2 PO q48h | ||
| Tapper over 4 weeks | |||
If CCNU was discontinued, chemotherapy was continued with single agent vinblastine given at 2 mg m−2 IV every other week, for the number of intended cycles.
Complete physical examinations were performed at each chemotherapy treatment. An abdominal ultrasound, chest radiographs and bloodwork were recommended at the end of the chemotherapy protocol and every 3 months for 18 months after completion of the treatment and then every 6 months thereafter. Follow-up information was obtained primarily from medical records and phone calls to the referring veterinarians. Owners were contacted by telephone when adequate information was not available from these sources.
For all subjects, efficacy was assessed using disease-free interval (DFI). DFI was defined as the time interval between the time of initiation of treatment (surgery) and the development of de novo MCT, local recurrence or metastasis, whichever came first. Data were censored for dogs alive without evidence of de novo MCT, local relapse or metastasis and for dogs that had died without evidence ofde novo MCT, local relapse or metastasis. Dogs lost to follow-up were included in analyses until the last day follow-up information was collected and were then censored. The occurrence of de novo MCT (i.e., cutaneous location at distant, unrelated site from the original tumour) was also reported. All relapses were confirmed by cytology, histopathology or both whenever possible. Overall survival time was defined as the time interval between the initiation of treatment and death. Cause of death was classified as related to MCT, unrelated or relationship unknown. Dogs were considered dead of disease if the cause was not known, as in most cases this aetiology could not be excluded; patients were censored if they died from unrelated causes based on necropsy.
Descriptive statistics were calculated. DFI was calculated using the Kaplan–Meier product limit method. Median survival time (MST) was determined by means of the Kaplan–Meier product limit method, and the log-rank test was used to determine whether survival times differed significantly among treatment groups. For analyses of survival time, dogs were censored if they were alive at the time of the study or were lost to follow-up. Differences in MST due to mitotic index (less or more than 5/10 HPF), tumour size (less than 3 cm, or equal or more than 3 cm), lymph node assessment (normal or abnormal based on subjective assessment of size and consistency), treatment with surgery alone or surgery followed by radiation therapy, timing of initiation of chemotherapy after surgery (less or more than 30 days) and number of chemotherapy cycles (less or more than six cycles) were compared using the log-rank test. All analyses were performed with standard statistical software (GraphPad Prism, GraphPad La Jolla, CA, USA). For all analyses, a value of P<0.05 was considered significant.
Results
Twenty-one dogs were identified meeting the inclusion criteria. The characteristics of the 21 dogs enrolled in the study are summarized in Table 3. Age and sex did not have any impact on survival.
Table 3.
Patient characteristics
| Age (years) median (range) | 7.6 | (2.2–12.1) |
| Weight (kg) median (range) | 28.6 | (2.2–49.8) |
| Sex | ||
| Spayed female | 10 | |
| Castrated male | 8 | |
| Intact female | 1 | |
| Intact male | 2 | |
| Breed | ||
| Mixed breed | 7 | |
| Labrador retriever | 4 | |
| Other pure breeds | 10 | |
| Longest measurement (cm) median (range) | 1.5 | (0.6–5) |
| Clinical signs | ||
| Mass alone | 19 | |
| Ulceration | 2 |
Results of CBC determination, urine and serum biochemical analysis were available for all patients. All but two patients had three view thoracic radiographs performed prior to treatment; all the available radiographs were assessed as normal.
Five patients were confirmed to have metastasis of their loco regional lymph node based on histopathology, without any prior cytological evaluation. Sixteen dogs had their regional lymph node aspirated, 12 of which were positive for metastasis (i.e., presence of atypical looking mast cells, high number of mast cells, or clusters of mast cells identified on cytological examination).39 One patient had a haemodiluted, non-diagnostic sample and the three remaining patients were diagnosed with a reactive lymph node on cytology. These four patients had their lymph node excised and were histopathologically confirmed to contain metastatic disease.
All patients had an abdominal ultrasound performed prior to initiation of treatment and no evidence of metastasis was seen.
At presentation, tumours were located on an extremity (n=15), thorax (n=1), caudal abdomen (n=3, two of three were noted to be preputial), or head/neck (n=2, one of two reported to be on the rostral muzzle). Two tumours were described as being ulcerated. The remainder of the patients did not exhibit any clinical signs and the main reason for presenting to the veterinarian was the presence of a cutaneous mass. Measurement of the primary tumour was reported in the record in 19/21 cases. The unidimensional tumour measurement ranged from 0.6 to 5 cm (mean, 2 cm; median, 1.5 cm; 5/19 tumours ≥3 cm and 14/19 <3 cm). There was no association found between tumour size and survival time.
The size and consistency of the draining lymph node was available in all patients. Thirteen patients were deemed to have an abnormal draining lymph node on palpation (being either enlarged and/or firm, based on subjective assessment). The size of all lymph nodes ranged from 1 to 4.6 cm (mean, 1.7 cm; median, 1.5 cm). The size of the abnormal draining lymph nodes ranged from 1 to 4.6 cm (mean, 2.1 cm; median, 2 cm). There was no association found between assessment (normal or abnormal) and overall survival time.
The diagnosis of primary grade II cutaneous MCT was confirmed histologically in all 21 dogs. Seventeen patients had lymph node metastasis confirmed histologically and the remaining four were confirmed with only cytology. The mitotic index of the primary tumour was available in 18 of 21 cases and was less than 5 of 10 HPF in 17 of 18 of these cases. The remaining case had a reported mitotic index of 10–20 mitoses per 10 HPF. We were not able to assess differences in outcome based on mitotic index, given that only one patient had a tumour with high mitotic index. All dogs had undergone surgical excision of their primary MCT before commencing treatment with chemotherapy. Nine dogs had one surgery performed that yielded complete surgical margins of the primary tumour. All these dogs had their affected draining loco-regional lymph node removed. Four dogs required a second surgery to achieve complete excision of their primary tumour; these dogs had their affected draining loco-regional lymph node removed as well.
Eight dogs had incomplete excision of their tumour following surgery, and received definitive radiation therapy to the primary site and the affected lymph node bed; of these eight patients, four had their metastatic lymph node resected prior to radiation therapy, the remaining four patients received radiation therapy to the lymph node. These latter patients were all noted to have normal draining lymph node at their scheduled rechecks following completion of their chemotherapy; only three of the four patients had their affected draining lymph node re-sampled following completion of treatment. Repeated cytology for all re-sampled lymph nodes yield blood and fat consistent with aspirating an irradiated lymph node. Resection of the draining lymph node did not have any impact on survival. One patient received radiation therapy and chemotherapy concurrently, whereas chemotherapy was initiated within a median of 18 days (range, 0–36 days) following completion of the definitive radiation in the remainder seven patients. Overall median time to initiate chemotherapy was 22 days (range, 12–78 days) following surgical treatment.
The radiotherapy treatment was overall well-tolerated; according to the Veterinary Radiation Therapy Oncology Group (VRTOG) acute radiation morbidity scoring scheme, three of the eight (37.5%) dogs were noted to have self-limiting grade I toxicities (mild erythema, epilation and dry desquamation), four of the eight (50%) had grade II toxicities (moderate erythema, patchy moist desquamation), one of the eight dog (12.5%) developed a grade III toxicity (severe mucositis). These side effects resolved within 2–3 weeks following completion of radiation therapy. Late toxicity effects reported included alopecia or leukotrichia and hyperpigmentation of the skin within the radiation field.
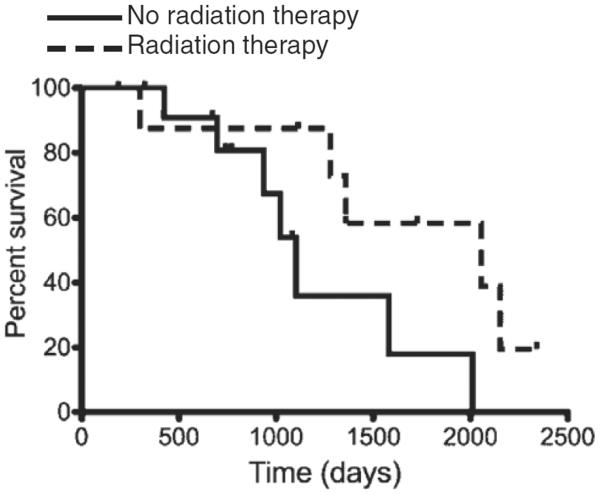
Adjunctive definitive radiation therapy was found to provide a statistical survival advantage (Hazard ratio, 0.54, 95% confidence interval, 0.22 to 0.85, P=0.0496); patients that received radiation therapy had a median survival of 2056 days (range, 300–2340 days) and patients that did not receive radiation therapy had a MST of 1103 days (range, 188–2010 days), as shown in Fig. 1.
Figure 1.
Kaplan–Meier curve depicting survival outcome of the 21 canine MCTs treated with ALC (surgery versus surgery/radiation therapy) and combination chemotherapy with CCNU and vinblastine. Curve compares patients receiving (dash line) or not receiving (solid line) radiation therapy for ALC.
The chemotherapy protocol was well-tolerated; no hospitalizations were required. Data on the haematological and serum chemistry adverse events for the dogs in the study is described in Table 4.41,44,47–51 Main toxicities recorded during the chemotherapy protocol were bone marrow suppression (with neutropenia being the predominant finding) and hepatotoxicity. Myelosuppression and gastrointestinal toxicity were attributed to the most recent drug administered. The highest event of toxicity was recorded for each patient for the ALT value. Bone marrow suppression was assessed based on the nadir CBC available for review. Owing to the retrospective nature of this study, some of the bloodwork done at the local referring clinics was not available for review. Following administration of CCNU, 6.3% dogs had a grade II toxicity, 12.5% had a grade III toxicity, 31.3% had a grade IV toxicity. Administration of vinblastine triggered 12.5% of grade I neutropenia and 4.7% of grade II gastrointestinal toxicity. Most of the patients developed elevation of their ALT, with 15% of grade I, 35% of grade II, 25% of grade III and 20% of grade IV elevation of ALT. None of the patients were reported to have clinical signs attributable to liver disease.
Table 4.
Recorded toxicity for the patients receiving alternating CCNU and vinblastine chemotherapy for treatment of grade II stage 2 MCT
| Grade I (%) | Grade II (%) | Grade III (%) | Grade IV (%) |
|---|---|---|---|
| Neutropenia 7 days post CCNU (% of animals) | |||
| 0 | 6.3 | 12.5 | 31.3 |
| Neutropenia 7 days post Vinblastine (% of animals) | |||
| 12.5 | 0 | 0 | 0 |
| Gastrointestinal toxicity following Vinblastine (% of animals) | |||
| 0 | 4.7 | 0 | 0 |
| Elevation of ALT documented during chemotherapy (% of animals) | |||
| 15 | 35 | 25 | 20 |
The 21 dogs in the study received a median of six CCNU treatments (range, 2–7) and a median of seven vinblastine treatments (range, 2–9). The number of CCNU and vinblastine treatments recommended varied over the study period; therefore patients were classified on an intent-to-treat basis. At the time of data accrual, all patients had completed their treatment protocols. The intended number of chemotherapy cycles (<6 or >6) and the time to start chemotherapy (>30 or <30 following surgery) were not found to have a significant impact on overall survival.
A total of 109 doses of CCNU were administered. Distribution of those doses was as follow: two doses (n=3); three doses (n=2); five doses (n=5), six doses (n=5) and seven doses (n=6). The median number of treatments was 6 and the range was 2–7. Because of rounding of the CCNU doses, the starting dose ranged from 61.8 to 70.7 mg m−2 (median, 64.5 mg m−2; mean, 64.9 mg m−2).
Eleven dogs discontinued CCNU chemotherapy before completing the prescribed protocol. In three dogs, only two CCNU treatments were administered because of local recurrence of disease and euthanasia (n=1) and due to elevated liver values (ALT 721, 19–67 IUL−1; n=1 and ALT 528, 21–72 IUL−1, n=1). In two dogs, only three CCNU treatments were administered because of sudden rise in the liver values (ALT>900, 19–67 IUL−1; n=2). In five dogs, only five CCNU treatments were administered because of reasons unrelated to recurrence of disease (owner preference, n=2, undetermined n=1), or sudden elevation in liver values (ALT 616, 19–67 IUL−1 and ALT 198, 19–67 IUL−1; n=2). In one dog, only six CCNU treatments were administered because of an elevation of ALT (96, 19–67 IUL−1; n=1).
A total of 138 doses of vinblastine were administered to the 21 patients. Distribution of those doses was as follow: 2 doses (n=1); 5 doses, (n=3); 6 doses (n=5); 7 doses (n=8) and 8 doses (n=2), 9 doses (n=1), 10 doses (n=1). The median number of treatment was 7 and the range was 2–10. The administered dose ranged from 1.8 to 2.05 mg m−2 (median, 1.92 mg m−2; mean, 1.92 mg m−2).
Subsequent dose reduction was performed in only one dog due to emesis and diarrhoea of 24-h duration the day following administration of the first dose of vinblastine at 2 mg m−2.
Four dogs discontinued vinblastine chemotherapy before completing the prescribed protocol. In one patient, the vinblastine use was discontinued because of progressive disease and euthanasia after only two cycles of chemotherapy. In three dogs, only five vinblastine treatments were administered because of reasons unrelated to recurrence of disease (undetermined n=1 and owner preference n=2). Two patients received eight total doses, two patients were administered nine and ten doses of vinblastine respectively due the fact that CCNU was discontinued (due to elevated liver values) during their protocol.
Seven patients were still alive at the time of the data analysis. Two patients were lost to follow-up 771 days and 1083 days following their diagnosis, respectively. Four patients (19%) did not follow the intended recheck schedule (two were lost of follow-up at day 771 and 1083; two did not come every 3 months for their follow up but were rechecked later on). Twelve of the 21 dogs died or were euthanized either because of causes suspected to be unrelated to MCT (n=8), or because of cause unknown (n=2) or because of causes related to their tumour (n=2). Local relapse occurred in 2 of 21 patients in the area of previous MCT surgical resection and irradiation. One dog developed local recurrence as well as de novo MCT 149 days following initiation of treatment, while receiving his chemotherapy treatment. This patient had a grade II MCT with a mitotic index of 10–20/HPF. The chemotherapy was discontinued after only two cycles due to recurrence of his disease in the radiation field and euthanasia. The other dog developed local recurrence in his radiation field 2120 days following initiation of treatment. None of the patients developed evidence of distant abdominal spread during the follow-up period. Clinical events were too few for meaningful analysis of the impact of the prognostic variables (mitotic index, tumour size, lymph node assessment, treatment with surgery alone or surgery followed by radiation therapy, timing of initiation of chemotherapy after surgery, and number of chemotherapy cycles) on DFI.
Four patients were diagnosed with de novo cutaneous MCT outside the surgical site during the follow-up period, the new MCT occurred 147, 149, 937 and 1420 days (median, 543 days) after their initial diagnosis.
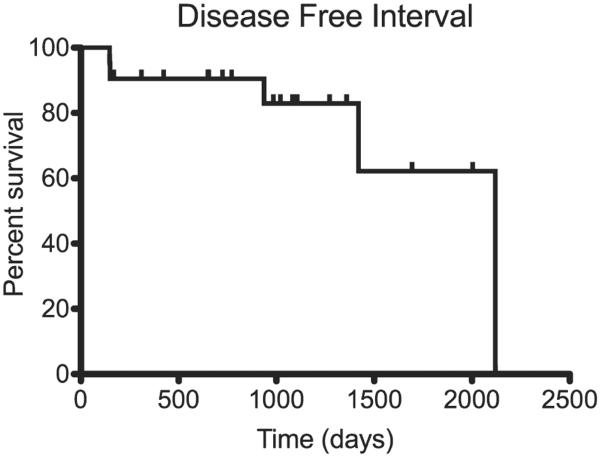
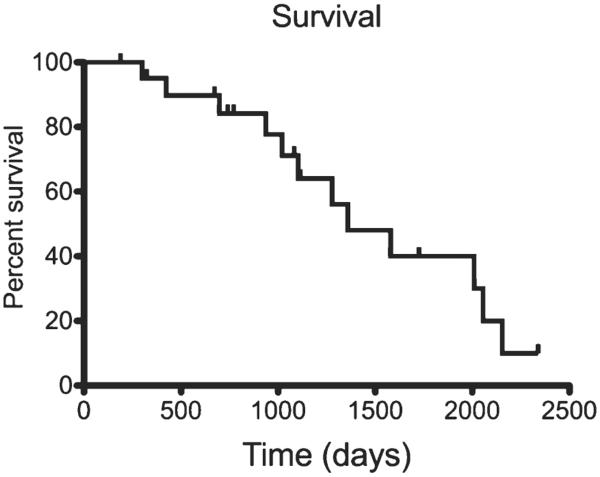
Median time to follow-up in the censored patients was 1083 days (range, 169–2325 days). Analysis of censored DFI curves indicated that the median DFI was 2120 days (range, 149–2325 days) as shown in Fig. 2. The overall MST was 1359 days (range, 188–2340 days), as displayed in Fig. 3. DFI was longer than overall MST as a result of our approach to censoring data in the outcome analysis; for DFI, we censored dogs thought to be disease-free at death; for overall survival, patients were designated dead of disease when the cause of death was not known.
Figure 2.
Kaplan–Meier curve illustrating the DFI of the 21 canine MCTs treated with ALC and combination chemotherapy with CCNU and vinblastine.
Figure 3.
Kaplan–Meier curve depicting overall survival of the 21 canine MCTs treated with ALC and combination chemotherapy with CCNU and vinblastine.
Discussion
The results of this study suggest that dogs with grade II, stage 2 MCT can achieve long-term median survival and extended DFI when treated with ALC and chemotherapy with prednisone, CCNU alternated with vinblastine.
The population characteristics in this study are similar to what has been previously reported.6,9–11,16,19,20,39,40 Fifteen MCTs were located on the extremities. Two tumours were preputial and one was on the rostral muzzle. While these latter locations have been associated with a poorer prognosis, additional studies have supported the notion that the true predictor of behaviour remains the grade and not the anatomical location.26–28,52,53 In this study, there were too few cases in these controversial locations to draw any conclusion as far as a possible correlation between location and outcome.
Multiple chemotherapy protocols have been studied for the treatment of gross MCT. These protocols have evaluated vincristine, cyclophosphamide, hydroxyurea, steroids, tyrosine kinase inhibitors, chlorambucil and steroids, taxanes, vinblastine +/− steroids, CCNU +/− steroids, combination of CCNU and vinblastine at variable doses.20,25,41,47,50,51,54–67 In our institution, it is common use to prescribe an alternating protocol with CCNU, vinblastine and prednisone. All these agents have been studied and have proven efficacy against MCT in the gross disease setting.25,49,50,61,62
The survival outcome and DFI found in this study are concordant with what is described in a previously published report on high-risk MCTs, where an unspecified number of grade II stage 2 MCTs enjoyed long term tumour control when treated with multi modal therapy including surgery, radiation therapy and single agent adjuvant vinblastine chemotherapy.20
In another case series, four dogs with cutaneous grade II, stage 2 MCTs treated with CCNU, vinblastine and prednisone after locoregional radiotherapy had a similar DFI (median, 1159 days; range, 954–1851 days).41
In addition, these results are analogous to those reported from Chaffin et al.16 In this latter study, a subgroup of dogs (16/19) was diagnosed with grade II stage 2 MCT. Post-operative treatment with radiation therapy and oral prednisone provided an extended DFI. While the outcome described in this previous study appears encouraging, it needs to be extended to a larger cohort of patients.
Taken together with the results of our study, these data support the notion that dogs diagnosed with grade II, stage 2 MCT can experience long-term survival with aggressive local therapy. Despite the data suggesting that ALC alone could provide, as a sole therapy, excellent outcome, it is a common practice in our hospital to recommend chemotherapy, because multimodality therapy typically is the optimal choice for disseminated spread.16,68,69 Adjuvant chemotherapy given shortly after ALC, when tumour burden is low, seems reasonable, however, the benefit of adjuvant chemotherapy could only be evaluated in a prospective and randomized clinical trial.
It is compelling to note that the addition of adjuvant radiation therapy to the treatment regimen provided a survival advantage to our study population compared to the patients treated with surgery only. Given the small number of patients included in this study, we could iterate that this result may not be representative of the full patient population and that this data results from a type I error. It is difficult to draw conclusions as far as the plausible explanation for this observed difference, as the only two patients that developed recurrence of disease had received definitive intent radiation therapy and none of the patients developed metastatic mast cell neoplasia following treatment. However, a more aggressive approach utilizing combination therapy may have been necessary in animals that had a worse disease. This may have introduced a potential source of bias into the study and may have affected survival times.
The findings of our study and Dr. Chaffin's study raise the question of what degree of aggressive therapy (local control alone, mono, bi or tri agent chemotherapy) is needed for the patients with grade II stage 2 MCTs and it is possible that the dogs in this study were over-treated.16 Ideally a prospective randomized study should be performed to define the role of each adjuvant component.
In addition, while lymph node metastasis has been reported to be a negative prognostic variable, and while we suspect that the prolonged survival documented here is the result of successful treatment, the results of this study (in association with results of other previous studies) raises the question of the prognostic significance of lymph node metastasis in this specific subset of dogs with grade II, low mitotic index, MCT.16,20,39,40,68,70
It is interesting to note that the majority of these metastatic grade II MCTs had a low mitotic index. This finding demonstrates the considerable variation in biological behaviour that can be seen in the Patnaik grade II MCTs. Although the number of mitotic figures has been documented as a single prognostic parameter for survival, this finding highlights the need for identification of additional prognostic markers (in addition to the markers of proliferation) or a refinement of our grading system.5,21,71 Moreover, it is possible that the only patient that had a high mitotic index had a grade III stage 2 MCT, as histological grading, despite well-described grading schemes, can carry significant interobserver variation.71,72
Only 13 of the 21 patients presented here were deemed to have an abnormal draining lymph node on palpation. This suggests that lymph node size or consistency alone may not be sufficient for accurate staging of cutaneous MCT in dogs and that cytological or histological examination of regional lymph nodes should routinely be performed, regardless of the size of those nodes. This is routinely performed for the staging of other canine malignancies.73
In this case series, a decision was made to excise the draining lymph node either because of the suspected metastasis or because the clinician wanted clarification of the lymph node status to complete the staging. Most of the cases in this study had a histopathological diagnosis of lymph node metastasis. In only four cases, lymph node metastasis was diagnosed on cytology based on evidence of high number of mast cells, and/or clusters of atypical mast cells.39 In 13 of 17 lymph nodes, both cytology and histopathology diagnoses were in agreement. This result is in concordance with the previously reported sensitivity and specificity of cytology for regional lymph node metastasis of MCTs.39,74 However, because mast cells may normally reside in lymphoid tissue, an aspirate sample may not be sufficient for a clinical pathologist to determine if a node contains metastatic disease.75
Interpretation of lymph node histology may be difficult because normal canine lymphoid tissue can contain mast cells. In addition, there are unfortunately no published veterinary pathology criteria describing a standard, repeatable method that could be used to define what constitutes metastatic mast cell disease within a lymph node. At our institution, such a determination is guided by the presence of multi-focal clusters, aggregates of atypical mast cells (variably differentiated, poorly granulated, presence of anisocytosis, anisokaryosis), effacement of the normal architecture of the lymph node by mast cells. Although none of the lymph node samples assessed upon histopathology were described as 'probable' metastasis and all of them contain the features described above, it is still possible that some of them assessed to have metastatic disease may have been in fact, non-metastatic. This lack of histopathological consensus and the subsequent subjective assessment should be seen as a limitation of this retrospective study; until a consensus is formed this limitation may limit prospective clinical trials.
A number of variables, including sex, size of tumour, number and timing of the chemotherapy cycles were evaluated to determine whether they had an impact on overall survival time. None of these factors were found to be significantly associated with survival outcome, although small numbers of dogs were included in this study and this may have led to an insufficient power to detect differences between subgroups.
This study should be interpreted with its limitations in mind. Limitations include the retrospective design of the study and the small sample size. Some of the data was not available for review and not all patients had their spleen and liver cytologically assessed. Not all patients with elevation of their liver values had their liver assessed through cytology or histopathology; it is possible that these liver enzyme elevations could have been related to progressive mast cell disease or prednisone therapy instead of CCNU therapy. Owing to the retrospective setting of this study, not all pathological samples were available for review by the single pathologist.
A small sample size alone can make finding a significant difference between two groups challenging and a larger number of patients could increase the power of our statistical analysis.
In summary, in clinical cases of grade II stage 2 MCT, alternating CCNU and vinblastine, and prednisone following ALC provided a median survival and DFI in excess of 3 years.
The role of chemotherapy in stage 2 grade II MCT still is undefined. Our study does not allow us to draw conclusions with respect to the advantage of this protocol compared with other chemotherapy regimens (prednisone alone, other chemotherapy agents) or treatment modalities (radiation therapy only). Therefore, further prospective, randomized studies are needed to evaluate treatment modalities best suited to stage 2, grade II MCT.
Footnotes
Presented in abstract form at the Veterinary Cancer Society Annual Conference, Albuquerque, New Mexico, November 2011.
References
- 1.London CA, Seguin B. Mast cell tumors in the dog. The Veterinary Clinics of North America. Small Animal Practice. 2003;33:473–489. doi: 10.1016/s0195-5616(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 2.Villamil JA, Henry CJ, Bryan JN, Ellersieck M, Schultz L, Tyler JW, Hahn AW. Identification of the most common cutaneous neoplasms in dogs and evaluation of breed and age distributions for selected neoplasms. Journal of the American Veterinary Medical Association. 2011;239:960–965. doi: 10.2460/javma.239.7.960. [DOI] [PubMed] [Google Scholar]
- 3.Patnaik AK, Ehler WJ, MacEwen EG. Canine cutaneous mast cell tumor: morphologic grading and survival time in 83 dogs. Veterinary Pathology. 1984;21:469–474. doi: 10.1177/030098588402100503. [DOI] [PubMed] [Google Scholar]
- 4.Bostock DE. The prognosis following surgical removal of mastocytomas in dogs. The Journal of Small Animal Practice. 1973;14:27–41. doi: 10.1111/j.1748-5827.1973.tb06891.x. [DOI] [PubMed] [Google Scholar]
- 5.Romansik EM, Reilly CM, Kass PH, Moore PF, London CA. Mitotic index is predictive for survival for canine cutaneous mast cell tumors. Veterinary Pathology. 2007;44:335–341. doi: 10.1354/vp.44-3-335. [DOI] [PubMed] [Google Scholar]
- 6.Fulcher RP, Ludwig LL, Bergman PJ, Newman SJ, Simpson AM, Patnaik AK. Evaluation of a two-centimeter lateral surgical margin for excision of grade I and grade II cutaneous mast cell tumors in dogs. Journal of the American Veterinary Medical Association. 2006;228:210–215. doi: 10.2460/javma.228.2.210. [DOI] [PubMed] [Google Scholar]
- 7.Simpson AM, Ludwig LL, Newman SJ, Bergman PJ, Hottinger HA, Patnaik AK. Evaluation of surgical margins required for complete excision of cutaneous mast cell tumors in dogs. Journal of the American Veterinary Medical Association. 2004;224:236–240. doi: 10.2460/javma.2004.224.236. [DOI] [PubMed] [Google Scholar]
- 8.Scarpa F, Sabattini S, Marconato L, Capitani O, Morini M, Bettini G. Use of histologic margin evaluation to predict recurrence of cutaneous malignant tumors in dogs and cats after surgical excision. Journal of the American Veterinary Medical Association. 2012;240:1181–1187. doi: 10.2460/javma.240.10.1181. [DOI] [PubMed] [Google Scholar]
- 9.Seguin B, Leibman NF, Bregazzi VS, Ogilvie GK, Powers BE, Dernell WS, Fettman MJ, Withrow SJ. Clinical outcome of dogs with grade-II mast cell tumors treated with surgery alone: 55 cases (1996–1999) Journal of the American Veterinary Medical Association. 2001;218:1120–1123. doi: 10.2460/javma.2001.218.1120. [DOI] [PubMed] [Google Scholar]
- 10.Murphy S, Sparkes AH, Smith KC, Blunden AS, Brearley MJ. Relationships between the histological grade of cutaneous mast cell tumours in dogs, their survival and the efficacy of surgical resection. Veterinary Record. 2004;154:743–746. doi: 10.1136/vr.154.24.743. [DOI] [PubMed] [Google Scholar]
- 11.Weisse C, Shofer FS, Sorenmo K. Recurrence rates and sites for grade II canine cutaneous mast cell tumors following complete surgical excision. Journal of the American Animal Hospital Association. 2002;38:71–73. doi: 10.5326/0380071. [DOI] [PubMed] [Google Scholar]
- 12.Seguin B, Besancon MF, McCallan JL, Dewe LL, Tenwolde MC, Wong EK, Kent MS. Recurrence rate, clinical outcome, and cellular proliferation indices as prognostic indicators after incomplete surgical excision of cutaneous grade II mast cell tumors: 28 dogs (1994–2002) Journal of Veterinary Internal Medicine. 2006;20:933–940. doi: 10.1892/0891-6640(2006)20[933:rrcoac]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 13.Abadie JJ, Amardeilh MA, Delverdier ME. Immunohistochemical detection of proliferating cell nuclear antigen and Ki-67 in mast cell tumors from dogs. Journal of the American Veterinary Medical Association. 1999;215:1629–1634. [PubMed] [Google Scholar]
- 14.Michels GM, Knapp DW, DeNicola DB, Glickman N, Bonney P. Prognosis following surgical excision of canine cutaneous mast cell tumors with histopathologically tumor-free versus nontumor-free margins: a retrospective study of 31 cases. Journal of the American Animal Hospital Association. 2002;38:458–466. doi: 10.5326/0380458. [DOI] [PubMed] [Google Scholar]
- 15.Al-Sarraf R, Mauldin GN, Patnaik AK, Meleo KA. A prospective study of radiation therapy for the treatment of grade 2 mast cell tumors in 32 dogs. Journal of Veterinary Internal Medicine. 1996;10:376–378. doi: 10.1111/j.1939-1676.1996.tb02084.x. [DOI] [PubMed] [Google Scholar]
- 16.Chaffin K, Thrall DE. Results of radiation therapy in 19 dogs with cutaneous mast cell tumor and regional lymph node metastasis. Veterinary Radiology & Ultrasound. 2002;43:392–395. doi: 10.1111/j.1740-8261.2002.tb01023.x. [DOI] [PubMed] [Google Scholar]
- 17.Frimberger AE, Moore AS, LaRue SM, Gliatto JM, Bengtson AE. Radiotherapy of incompletely resected, moderately differentiated mast cell tumors in the dog: 37 cases (1989–1993) Journal of the American Animal Hospital Association. 1997;33:320–324. doi: 10.5326/15473317-33-4-320. [DOI] [PubMed] [Google Scholar]
- 18.LaDue T, Price GS, Dodge R, Page RL, Thrall DE. Radiation therapy for incompletely resected canine mast cell tumors. Veterinary Radiology & Ultrasound. 1998;39:57–62. doi: 10.1111/j.1740-8261.1998.tb00326.x. [DOI] [PubMed] [Google Scholar]
- 19.Poirier VJ, Adams WM, Forrest LJ, Green EM, Dubielzig RR, Vail DM. Radiation therapy for incompletely excised grade II canine mast cell tumors. Journal of the American Animal Hospital Association. 2006;42:430–434. doi: 10.5326/0420430. [DOI] [PubMed] [Google Scholar]
- 20.Thamm DH, Turek MM, Vail DM. Outcome and prognostic factors following adjuvant prednisone/vinblastine chemotherapy for high-risk canine mast cell tumour: 61 cases. Journal of Veterinary Medical Science. 2006;68:581–587. doi: 10.1292/jvms.68.581. [DOI] [PubMed] [Google Scholar]
- 21.Elston L, Sueiro FA, Cavalcanti J, Metze K. The importance of the mitotic index as a prognostic factor for canine cutaneous mast cell tumors – a validation study. Veterinary Pathology. 2009;46:362–364. doi: 10.1354/vp.46-2-362. [DOI] [PubMed] [Google Scholar]
- 22.McNiel EA, Prink AL, O'Brien TD. Evaluation of risk and clinical outcome of mast cell tumours in pug dogs. Veterinary and Comparative Oncology. 2006;4:2–8. doi: 10.1111/j.1476-5810.2006.00085.x. [DOI] [PubMed] [Google Scholar]
- 23.Peters JA. Canine mastocytoma: excess risk as related to ancestry. Journal of the National Cancer Institute. 1969;42:435–443. [PubMed] [Google Scholar]
- 24.White CR, Hohenhaus AE, Kelsey J, Procter-Gray E. Cutaneous MCTs: associations with Spay/Neuter Status, Breed, Body Size, and Phylogenetic Cluster. Journal of the American Animal Hospital Association. 2011;47:210–216. doi: 10.5326/JAAHA-MS-5621. [DOI] [PubMed] [Google Scholar]
- 25.Thamm DH, Mauldin EA, Vail DM. Prednisone and vinblastine chemotherapy for canine mast cell tumor–41 cases (1992–1997) Journal of Veterinary Internal Medicine. 1999;13:491–497. doi: 10.1892/0891-6640(1999)013<0491:pavcfc>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 26.Gieger TL, Theon AP, Werner JA, McEntee MC, Rassnick KM, DeCock HE. Biologic behavior and prognostic factors for mast cell tumors of the canine muzzle: 24 cases (1990–2001) Journal of Veterinary Internal Medicine. 2003;17:687–692. doi: 10.1111/j.1939-1676.2003.tb02501.x. [DOI] [PubMed] [Google Scholar]
- 27.Sfiligoi G, et al. Rassnick KM, Scarlett JM, Northrup NC, Gieger TL. Outcome of dogs with mast cell tumors in the inguinal or perineal region versus other cutaneous locations: 124 cases (1990–2001) Journal of the American Veterinary Medical Association. 2005;226:1368–1374. doi: 10.2460/javma.2005.226.1368. [DOI] [PubMed] [Google Scholar]
- 28.Turrel JM, Kitchell BE, Miller LM, Theon A. Prognostic factors for radiation treatment of mast cell tumor in 85 dogs. Journal of the American Veterinary Medical Association. 1988;193:936–940. [PubMed] [Google Scholar]
- 29.Mullins MN, Dernell WS, Withrow SJ, Ehrhart EJ, Thamm DH, Lana SE. Evaluation of prognostic factors associated with outcome in dogs with multiple cutaneous mast cell tumors treated with surgery with and without adjuvant treatment: 54 cases (1998–2004) Journal of the American Veterinary Medical Association. 2006;228:91–95. doi: 10.2460/javma.228.1.91. [DOI] [PubMed] [Google Scholar]
- 30.Preziosi R, Sarli G, Paltrinieri M. Multivariate survival analysis of histological parameters and clinical presentation in canine cutaneous mast cell tumours. Veterinary Research Communications. 2007;31:287–296. doi: 10.1007/s11259-006-3427-9. [DOI] [PubMed] [Google Scholar]
- 31.Kiupel M, Webster JD, Miller RA, Kaneene JB. Impact of tumour depth, tumour location and multiple synchronous masses on the prognosis of canine cutaneous mast cell tumours. Journal of Veterinary Medicine. A, Physiology, Pathology, Clinical Medicine. 2005;52:280–286. doi: 10.1111/j.1439-0442.2005.00726.x. [DOI] [PubMed] [Google Scholar]
- 32.Maglennon GA, Murphy S, Adams V, Miller J, Smith K, Blunden A, Scase TJ. Association of Ki67 index with prognosis for intermediate-grade canine cutaneous mast cell tumours. Veterinary and Comparative Oncology. 2008;6:268–274. doi: 10.1111/j.1476-5829.2008.00168.x. [DOI] [PubMed] [Google Scholar]
- 33.Scase TJ, Edwards D, Miller J, Henley W, Smith K, Blunden A, Murphy S. Canine mast cell tumors: correlation of apoptosis and proliferation markers with prognosis. Journal of Veterinary Internal Medicine. 2006;20:151–158. doi: 10.1892/0891-6640(2006)20[151:cmctco]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 34.Simoes JP, Schoning P. Canine mast cell tumors: a comparison of staining techniques. Journal of Veterinary Diagnostic Investigation. 1994;6:458–465. doi: 10.1177/104063879400600410. [DOI] [PubMed] [Google Scholar]
- 35.Simoes JP, Schoning P, Butine M. Prognosis of canine mast cell tumors: a comparison of three methods. Veterinary Pathology. 1994;31:637–647. doi: 10.1177/030098589403100602. [DOI] [PubMed] [Google Scholar]
- 36.Webster JD, Kiupel M, Kaneene JB, Miller R, Yuzbasiyan-Gurkan V. The use of KIT and tryptase expression patterns as prognostic tools for canine cutaneous mast cell tumors. Veterinary Pathology. 2004;41:371–377. doi: 10.1354/vp.41-4-371. [DOI] [PubMed] [Google Scholar]
- 37.Jones CL, Grahn RA, Chien MB, Lyons LA, London CA. Detection of c-kit mutations in canine mast cell tumors using fluorescent polyacrylamide gel electrophoresis. Journal of Veterinary Diagnostic Investigation. 2004;16:95–100. doi: 10.1177/104063870401600201. [DOI] [PubMed] [Google Scholar]
- 38.London CA, Galli SJ, Yuuki T, Hu ZQ, Helfand SC, Geissler EN. Spontaneous canine mast cell tumors express tandem duplications in the proto-oncogene c-kit. Experimental Hematology. 1999;27:689–697. doi: 10.1016/s0301-472x(98)00075-7. [DOI] [PubMed] [Google Scholar]
- 39.Krick EL, Billings AP, Shofer FS, Watanabe S, Sorenmo KU. Cytological lymph node evaluation in dogs with mast cell tumours: association with grade and survival. Veterinary and Comparative Oncology. 2009;7:130–138. doi: 10.1111/j.1476-5829.2009.00185.x. [DOI] [PubMed] [Google Scholar]
- 40.Murphy S, Sparkes AH, Blunden AS, Brearley MJ, Smith KC. Effects of stage and number of tumours on prognosis of dogs with cutaneous mast cell tumours. Veterinary Record. 2006;158:287–291. doi: 10.1136/vr.158.9.287. [DOI] [PubMed] [Google Scholar]
- 41.Rassnick KM, Bailey DB, Russell DS, Flory AB, Kiselow MA, Intile JL, Malone EK, Balkman CE, Barnard SM. A phase II study to evaluate the toxicity and efficacy of alternating CCNU and high-dose vinblastine and prednisone (CVP) for treatment of dogs with high-grade, metastatic or nonresectable mast cell tumours. Veterinary and Comparative Oncology. 2010;8:138–152. doi: 10.1111/j.1476-5829.2010.00217.x. [DOI] [PubMed] [Google Scholar]
- 42.Owen L. TNM Classification of Tumors in Domestic Animals. 1 edn. World Health Organization; Geneva: 1980. pp. 7–15. [Google Scholar]
- 43.Monteiro B, Boston S, Monteith G. Factors influencing complete tumor excision of mast cell tumors and soft tissue sarcomas: a retrospective study in 100 dogs. Canadian Veterinary Journal. 2011;52:1209–1214. [PMC free article] [PubMed] [Google Scholar]
- 44.Skorupski KA, Hammond GM, Irish AM, Kent MS, Guerrero TA, Rodriguez CO, Griffin DW. Prospective randomized clinical trial assessing the efficacy of denamarin for prevention of CCNU-induced hepatopathy in tumor-bearing dogs. Journal of Veterinary Internal Medicine. 2011;25:838–845. doi: 10.1111/j.1939-1676.2011.0743.x. [DOI] [PubMed] [Google Scholar]
- 45.Chretin JD, Rassnick KM, Shaw NA, Hahn KA, Ogilvie GK, Kristal O, Northrup NC, Moore AS. Prophylactic trimethoprim-sulfadiazine during chemotherapy in dogs with lymphoma and osteosarcoma: a double-blind, placebo-controlled study. Journal of Veterinary Internal Medicine. 2007;21:141–148. doi: 10.1892/0891-6640(2007)21[141:ptdcid]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 46.Veterinary Co-operative Oncology Group Common terminology criteria for adverse events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.0. Veterinary and Comparative Oncology. 2004;2:195–213. doi: 10.1111/j.1476-5810.2004.0053b.x. [DOI] [PubMed] [Google Scholar]
- 47.Cooper M, Tsai X, Bennett P. Combination CCNU and vinblastine chemotherapy for canine mast cell tumours: 57 cases. Veterinary and Comparative Oncology. 2009;7:196–206. doi: 10.1111/j.1476-5829.2009.00190.x. [DOI] [PubMed] [Google Scholar]
- 48.Kristal O, Rassnick KM, Gliatto JM, Northrup NC, Chretin JD, Morrison-Collister K, Cotter SM, Moore AS. Hepatotoxicity associated with CCNU (Lomustine) chemotherapy in dogs. Journal of Veterinary Internal Medicine. 2004;18:75–80. doi: 10.1892/0891-6640(2004)18<75:hawclc>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 49.Rassnick KM, Bailey DB, Flory AB, Balkman CE, Kiselow MA, Intile JL, Autio K. Efficacy of vinblastine for treatment of canine mast cell tumors. Journal of Veterinary Internal Medicine. 2008;22:1390–1396. doi: 10.1111/j.1939-1676.2008.0195.x. [DOI] [PubMed] [Google Scholar]
- 50.Rassnick KM, Moore AS, Williams LE, London CA, Kintzer PP, Engler SJ, Cotter SM. Treatment of canine mast cell tumors with CCNU (lomustine) Journal of Veterinary Internal Medicine. 1999;13:601–605. doi: 10.1892/0891-6640(1999)013<0601:tocmct>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 51.Vickery KR, Wilson H, Vail DM, Thamm DH. Dose-escalating vinblastine for the treatment of canine mast cell tumour. Veterinary and Comparative Oncology. 2008;6:111–119. doi: 10.1111/j.1476-5829.2007.00147.x. [DOI] [PubMed] [Google Scholar]
- 52.Cahalane AK, Payne S, Barber LG, Duda LE, Henry CJ, Mauldin GE, Frimberger AE, Cotter SM, Moore AS. Prognostic factors for survival of dogs with inguinal and perineal mast cell tumors treated surgically with or without adjunctive treatment: 68 cases (1994–2002) Journal of the American Veterinary Medical Association. 2004;225:401–408. doi: 10.2460/javma.2004.225.401. [DOI] [PubMed] [Google Scholar]
- 53.Hillman LA, Garrett LD, de Lorimier LP, Charney SC, Borst LB, Fan TM. Biological behavior of oral and perioral mast cell tumors in dogs: 44 cases (1996–2006) Journal of the American Veterinary Medical Association. 2010;237:936–942. doi: 10.2460/javma.237.8.936. [DOI] [PubMed] [Google Scholar]
- 54.Gerritsen RJ, Teske E, Kraus JS, Rutteman GR. Multi-agent chemotherapy for mast cell tumours in the dog. Veterinary Quarterly. 1998;20:28–31. doi: 10.1080/01652176.1998.9694832. [DOI] [PubMed] [Google Scholar]
- 55.Rassnick KM, Al-Sarraf R, Bailey DB, Chretin JD, Phillips B, Zwhalen CH. Phase II open-label study of single-agent hydroxyurea for treatment of mast cell tumours in dogs. Veterinary and Comparative Oncology. 2010;8:103–111. doi: 10.1111/j.1476-5829.2010.00211.x. [DOI] [PubMed] [Google Scholar]
- 56.Davies DR, Wyatt KM, Jardine JE, Robertson ID, Irwin PJ. Vinblastine and prednisolone as adjunctive therapy for canine cutaneous mast cell tumors. Journal of the American Animal Hospital Association. 2004;40:124–130. doi: 10.5326/0400124. [DOI] [PubMed] [Google Scholar]
- 57.McCaw DL, Miller MA, Bergman PJ, Withrow SJ, Moore AS, Knapp DW, Fowler D, Johnson JC. Vincristine therapy for mast cell tumors in dogs. Journal of Veterinary Internal Medicine. 1997;11:375–378. doi: 10.1111/j.1939-1676.1997.tb00484.x. [DOI] [PubMed] [Google Scholar]
- 58.Rivera P, Akerlund-Denneberg N, Bergvall K, Kessler M, Rowe A, Willmann M, Persson G, Kastengren Froöberg G, Westberg S, von Euler H. Clinical efficacy and safety of a water-soluble micellar paclitaxel (Paccal Vet) in canine mastocytomas. The Journal of Small Animal Practice. 2013;54:20–27. doi: 10.1111/j.1748-5827.2012.01304.x. [DOI] [PubMed] [Google Scholar]
- 59.Vail DM, von Euler H, Rusk AW, Barber L, Clifford C, Elmslie R, Fulton L, Hirschberger J, Klein M, London C, Martano M, McNiel EA, Morris JS, Northrup N, Phillips B, Polton G, Post G, Rosenberg M, Ruslander D, Sahora A, Siegel S, Thamm D, Westberg S, Winter J, Khanna C. A randomized trial investigating the efficacy and safety of water soluble micellar paclitaxel (Paccal Vet) for treatment of nonresectable grade 2 or 3 mast cell tumors in dogs. Journal of Veterinary Internal Medicine. 2012;26:598–607. doi: 10.1111/j.1939-1676.2012.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Camps-Palau MA, Leibman NF, Elmslie R, Lana SE, Plaza S, McKnight JA, Risbon R, Bergman PJ. Treatment of canine mast cell tumours with vinblastine, cyclophosphamide and prednisone: 35 cases (1997–2004) Veterinary and Comparative Oncology. 2007;5:156–167. doi: 10.1111/j.1476-5829.2006.00125.x. [DOI] [PubMed] [Google Scholar]
- 61.McCaw DL, Miller MA, Ogilvie GK, Withrow SJ, Brewer WG, Jr, Klein MK, Bell FW, Anderson SK. Response of canine mast cell tumors to treatment with oral prednisone. Journal of Veterinary Internal Medicine. 1994;8:406–408. doi: 10.1111/j.1939-1676.1994.tb03259.x. [DOI] [PubMed] [Google Scholar]
- 62.Stanclift RM, Gilson SD. Evaluation of neoadjuvant prednisone administration and surgical excision in treatment of cutaneous mast cell tumors in dogs. Journal of the American Veterinary Medical Association. 2008;232:53–62. doi: 10.2460/javma.232.1.53. [DOI] [PubMed] [Google Scholar]
- 63.Hahn KA, Ogilvie G, Rusk T, Devauchelle P, Leblanc A, Legendre A, Powers B, Leventhal PS, Kinet JP, Palmerini F, Dubreuil P, Moussy A, Hermine O. Masitinib is safe and effective for the treatment of canine mast cell tumors. Journal of Veterinary Internal Medicine. 2008;22:1301–1309. doi: 10.1111/j.1939-1676.2008.0190.x. [DOI] [PubMed] [Google Scholar]
- 64.London CA, Hannah AL, Zadovoskaya R, Chien MB, Kollias-Baker C, Rosenberg M, Downing S, Post G, Boucher J, Shenoy N, Mendel DB, McMahon G, Cherrington JM. Phase I dose-escalating study of SU11654, a small molecule receptor tyrosine kinase inhibitor, in dogs with spontaneous malignancies. Clinical Cancer Research. 2003;9:2755–2768. [PubMed] [Google Scholar]
- 65.London CA, Malpas PB, Wood-Follis SL, Boucher JF, Rusk AW, Rosenberg MP, Henry CJ, Mitchener KL, Klein MK, Hintermeister JG, Bergman PJ, Couto GC, Mauldin GN, Michels GM. Multi-center, placebo-controlled, double-blind, randomized study of oral toceranib phosphate (SU11654), a receptor tyrosine kinase inhibitor, for the treatment of dogs with recurrent (either local or distant) mast cell tumor following surgical excision. Clinical Cancer Research. 2009;15:3856–3865. doi: 10.1158/1078-0432.CCR-08-1860. [DOI] [PubMed] [Google Scholar]
- 66.Taylor F, Gear R, Hoather T, Dobson J. Chlorambucil and prednisolone chemotherapy for dogs with inoperable mast cell tumours: 21 cases. The Journal of Small Animal Practice. 2009;50:284–289. doi: 10.1111/j.1748-5827.2009.00732.x. [DOI] [PubMed] [Google Scholar]
- 67.Hosoya K, Kisseberth WC, Alvarez FJ, Lara-Garcia A, Beamer G, Stromberg PC, Couto CG. Adjuvant CCNU (lomustine) and prednisone chemotherapy for dogs with incompletely excised grade 2 mast cell tumors. Journal of the American Animal Hospital Association. 2009;45:14–18. doi: 10.5326/0450014. [DOI] [PubMed] [Google Scholar]
- 68.Dobson J, Cohen S, Gould S. Treatment of canine mast cell tumours with prednisolone and radiotherapy. Veterinary and Comparative Oncology. 2004;2:132–141. doi: 10.1111/j.1476-5810.2004.00048.x. [DOI] [PubMed] [Google Scholar]
- 69.Chabner B, Longo DL. Cancer Chemotherapy and Biotherapy: Principles and Practice. 4 edn. Lippincott Willians & Wilkins; Philadelphia: 2005. Clinical strategies for cancer treatment: the role of drugs. [Google Scholar]
- 70.Warland J, Amores-Fuster I, Newbury W, Brearley M, Dobson J. The utility of staging in canine mast cell tumours. Veterinary and Comparative Oncology. 2012 doi: 10.1111/vco.12012. [DOI] [PubMed] [Google Scholar]
- 71.Kiupel M, Webster JD, Bailey KL, Best S, DeLay J, Detrisac CJ, Fitzgerald SD, Gamble D, Ginn PE, Goldschmidt MH, Hendrick MJ, Howerth EW, Janovitz EB, Langohr I, Lenz SD, Lipscomb TP, Miller MA, Misdorp W, Moroff S, Mullaney TP, Neyens I, O'Toole D, Ramos-Vara J, Scase TJ, Schulman FY, Sledge D, Smedley RC, Smith K, W Snyder P, Southorn E, Stedman NL, Steficek BA, Stromberg PC, Valli VE, Weisbrode SE, Yager J, Heller J, Miller R. Proposal of a 2-tier histologic grading system for canine cutaneous mast cell tumors to more accurately predict biological behavior. Veterinary Pathology. 2011;48:147–155. doi: 10.1177/0300985810386469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Northrup NC, Howerth EW, Harmon BG, Brown CA, Carmicheal KP, Garcia AP, Latimer KS, Munday JS, Rakich PM, Richey LJ, Stedman NL, Gieger TL. Variation among pathologists in the histologic grading of canine cutaneous mast cell tumors with uniform use of a single grading reference. Journal of Veterinary Diagnostic Investigation. 2005;17:561–564. doi: 10.1177/104063870501700606. [DOI] [PubMed] [Google Scholar]
- 73.Williams LE, Packer RA. Association between lymph node size and metastasis in dogs with oral malignant melanoma: 100 cases (1987–2001) Journal of the American Veterinary Medical Association. 2003;222:1234–1236. doi: 10.2460/javma.2003.222.1234. [DOI] [PubMed] [Google Scholar]
- 74.Langenbach A, McManus PM, Hendrick MJ, Shofer FS, Sorenmo KU. Sensitivity and specificity of methods of assessing the regional lymph nodes for evidence of metastasis in dogs and cats with solid tumors. Journal of the American Veterinary Medical Association. 2001;218:1424–1428. doi: 10.2460/javma.2001.218.1424. [DOI] [PubMed] [Google Scholar]
- 75.Bookbinder PF, Butt MT, Harvey HJ. Determination of the number of mast cells in lymph node, bone marrow, and buffy coat cytologic specimens from dogs. Journal of the American Veterinary Medical Association. 1992;200:1648–1650. [PubMed] [Google Scholar]