Abstract
Despite numerous published studies describing adjuvant chemotherapy for canine appendicular osteosarcoma, there is no consensus as to the optimal chemotherapy protocol. The purpose of this study was to determine whether either of two protocols would be associated with longer disease-free interval (DFI) in dogs with appendicular osteosarcoma following amputation. Dogs with histologically confirmed appendicular osteosarcoma that were free of gross metastases and underwent amputation were eligible for enrollment. Dogs were randomized to receive either six doses of carboplatin or three doses each of carboplatin and doxorubicin on an alternating schedule. Fifty dogs were included. Dogs receiving carboplatin alone had a significantly longer DFI (425 versus 135 days) than dogs receiving alternating carboplatin and doxorubicin (P = 0.04). Toxicity was similar between groups. These results suggest that six doses of carboplatin may be associated superior DFI when compared to six total doses of carboplatin and doxorubicin.
Keywords: amputation, bone tumour, canine, chemotherapy, clinical trial
Numerous chemotherapeutic protocols have been described for the adjuvant treatment of appendicular osteosarcoma (OSA) in dogs.1–9 Despite this, there is no consensus among veterinarians as to the optimal protocol. The most commonly studied drugs have been cisplatin, carboplatin, and doxorubicin either as single agents or in combination.1–8 Cisplatin is associated with significant renal and gastrointestinal toxicity compared to carboplatin and the cost of carboplatin has decreased significantly over the past decade, causing carboplatin use to gain favour over cisplatin.9
Outcome in dogs receiving single agent carboplatin chemotherapy has been previously reported. Bergman et al.3 reported on 48 dogs undergoing amputation and receiving four doses of adjuvant carboplatin. The median disease-free interval (DFI) in this study was 257 days and the median survival time (MST) was 321 days. Phillips et al.4 published outcome in 155 dogs undergoing amputation and three to four doses of carboplatin in the adjuvant and/or neoadjuvant setting. The median DFI in this population was similar at 256 days and the MST was 307 days. The effect of administering six instead of four total doses of carboplatin to dogs with OSA has not been fully explored. Recently, Saam et al.5 reported outcome in 65 dogs with appendicular OSA prescribed four to six doses of adjuvant carboplatin. The overall DFI and MST were 137 and 277 days, respectively, in this study. While a survival difference between dogs receiving 4 or ≥6 doses of carboplatin was not found in this study, 2 limitations should be noted. First, only dogs that lived more than 120 days were included in this analysis and, second, dogs prescribed six or more doses of carboplatin received the drug at a low starting dose of 250 mg m−2.
Studies have also described outcome in dogs with OSA receiving six total alternating doses of doxorubicin and carboplatin chemotherapy. Kent et al.7 reported outcome in 32 dogs receiving this protocol and found a median DFI of 227 days and MST of 320 days. Similarly, Bacon et al.8 reported outcome in 50 dogs treated with the same protocol and reported a median DFI of 202 days and an MST of 258 days. These medians are similar to those reported in dogs receiving up to four doses of carboplatin, but a direct prospective comparison of two chemotherapeutic protocols for canine OSA has never been conducted. Therefore, the purpose of this study was to determine whether either of two protocols with the same total number of doses would be associated with longer DFI in dogs with appendicular OSA following amputation. The null hypothesis was that there would be no significant difference between DFI in dogs treated with either protocol.
Methods
Dogs with histologically confirmed appendicular OSA were prospectively enrolled into this randomized, open-label trial. For enrollment, dogs had to have undergone amputation with complete excision of their tumour, no gross metastasis on thoracic radiographs, and were eligible to begin chemotherapy with in 1 month of amputation. Informed owner consent was required and all treatments were administered at the Veterinary Medical Teaching Hospital (VMTH) at the University of California, Davis.
Dogs were randomly assigned to one of two treatment groups of equal size in a sequential manner using a simple randomization technique consisting of a shuffled sealed envelope system. Dogs were randomized to receive either six doses of carboplatin or three doses each of carboplatin and doxorubicin on an alternating schedule. Both drugs were given intravenously and, in both groups, the starting dose of carboplatin was 300 mg m−2 and the starting dose for doxorubicin was 30 mg m−2. All doses were prescribed at 21-day intervals. Dogs receiving the alternating protocol received carboplatin first. Dose adjustments and delays due to gastrointestinal toxicity or myelosuppression were allowed at the discretion of the treating clinician. Concurrent chemotherapy, radiation therapy, and tyrosine kinase inhibitor therapy were not allowed, but no other specific medications, including NSAIDS, were disallowed. Routine monitoring for pulmonary metastasis with thoracic radiographs occurred during therapy at the time of the third chemotherapy dose, at the time of the sixth dose of chemotherapy, and every 3 months thereafter. Dogs presenting between routine recheck appointments had additional thoracic and/or bone radiographs performed if clinical signs suspicious for metastasis were present. All radiographs were taken at the VMTH and were reviewed by a board-certified radiologist. Rescue therapy of any kind was allowed once metastasis was documented.
Data collected for each dog included signallment, weight, tumour location, histologic subtype, mitoses per 3 high power fields (hpf), pre-surgical serum alkaline phosphatase (ALP), time between amputation and the first dose of chemotherapy, treatment group, chemotherapy toxicity, dose reductions, treatment delays, outcome, rescue therapy, cause of death, and necropsy information if available. Breeds representing ≤ 2 study cases were classified as other purebreds in statistical evaluation. Mitoses per 3 hpf were classified as greater than 5 versus less than or equal to 5 based on the publication by Saam et al.5 Tumour location was based on radiographic and/or histopathology reports and, for classification purposes, tumours were categorized as located in the distal radius, proximal humerus, distal femur, proximal tibia, distal tibia, or other location. Pre-surgical ALP was recorded only if run less than 1 month prior to amputation and results were classified as normal or elevated based on the reference range for the lab running the test. Adverse events were graded using the Veterinary Cooperative Oncology Group common terminology criteria for adverse events (VCOG-CTCAE) v 1.0.10
An interim analysis was planned after enrollment of 35 dogs. If a significant difference in DFI was detected at this time, study closure and data maturation was planned after enrollment of a total of 50 dogs. The t-test was used to compare continuous variables between treatment groups. The Fisher’s exact test or Chi Square test were used to compare categorical variables between treatment groups depending on number of dogs in each category. Factors compared between groups included age, sex, breed, weight, time between amputation and the first dose of chemotherapy, tumour location, histologic subtype, mitoses per 3 hpf, and pre-surgical ALP.
Because rescue therapy was allowed, the primary endpoint of the study was DFI. DFI was defined as time from amputation to documented or suspected metastasis. DFI was censored for dogs alive without evidence of metastasis, dogs without evidence of metastasis when lost to follow-up, and dogs known to be disease free at death. If cause of death was not known, metastasis was assumed to be present at the date of death. Survival was defined as time from amputation to death. Survival was censored for dogs alive at study’s end, dogs lost to follow-up, and dogs with a cause of death unrelated to OSA or therapy for OSA. If cause of death was not known, death was attributed to OSA. The Kaplan–Meier method was used to estimate DFI and survival and the log-rank test was used to compare DFI and survival times between groups. Statistical analyses were performed using commercial software (GraphPad Prism version 5.0c) and a P-value of < 0.05 was considered significant.
Results
Interim analysis conducted after 36 dogs enrolled showed significant differences in DFI and MST between groups, therefore, study closure occurred after accrual of 50 dogs. Enrollment occurred between January 2007 and March 2011. Patient and treatment characteristics of dogs in each group are described in Table 1. Characteristics were similar between groups in all variables apart from breed. Purebred Golden Retrievers, Rottweilers and Labrador Retrievers were more likely to receive carboplatin alone and mixed breed dogs and other purebred dogs were more likely to receive carboplatin and doxorubicin (P = 0.009). The median time from amputation to chemotherapy initiation was 15 days (range, 10–41 days) in the carboplatin alone group and 15 days (range, 10–26 days) in the carboplatin and doxorubicin group (P = 0.41). Thirty-two dogs (16 in each treatment group) completed their prescribed six-dose treatment protocol. The remaining 18 dogs stopped treatment early due to progressive disease after five doses (6 dogs), four doses (2 dogs), three doses (1 dog), two doses (7 dogs) or one dose (2 dogs).
Table 1.
Comparison of patient and tumour characteristics between groups
| Carboplatin alone |
Carboplatin and doxorubicin |
P value | |
|---|---|---|---|
| Mean Age (years) | 8.8 | 8.0 | 0.67 |
| Sex | 1.00 | ||
| Female | 12 | 12 | |
| Male | 13 | 13 | |
| Breed | 0.009 | ||
| Golden Retriever | 6 | 3 | |
| Rottweiler | 5 | 2 | |
| Labrador Retriever | 6 | 0 | |
| Mixed Breed | 2 | 6 | |
| Other Purebred | 6 | 14 | |
| Mean Weight (kg) | 38.7 | 38.6 | 0.96 |
| Tumor location | 0.78 | ||
| Distal Radius | 8 | 9 | |
| Proximal Humerus | 6 | 3 | |
| Distal Femur | 4 | 3 | |
| Proximal Tibia | 3 | 3 | |
| Distal Tibia | 2 | 5 | |
| Other | 2 | 2 | |
| Histologic Subtype | 0.56 | ||
| Osteoblastic | 15 | 17 | |
| Chondroblastic | 4 | 3 | |
| Mixed | 2 | 1 | |
| Telangiectatic | 2 | 0 | |
| Fibroblastic | 1 | 2 | |
| Giant Cell | 0 | 1 | |
| Not available | 1 | 1 | |
| Mitotic Index | 1.00 | ||
| ≤5 mitoses/3hpf | 12 | 11 | |
| >5 mitoses/3hpf | 12 | 12 | |
| Not available | 2 | 1 | |
| Presurgical ALP | 0.52 | ||
| Normal | 19 | 16 | |
| Elevated | 5 | 8 | |
| Not available | 1 | 1 |
Ten dogs (40%) in the carboplatin alone group and 13 dogs (52%) in the carboplatin and doxorubicin group experienced toxicity of any grade. Chemotherapy toxicity stratified by treatment group and drug is summarized in Table 2. Carboplatin dose was reduced to 270 mg m−2 due to toxicity in three dogs. Two of these dogs were in the carboplatin and doxorubicin group and one was in the carboplatin alone group. Treatment delay to a 28-day cycle after carboplatin was required in 18 dogs due to a late neutrophil nadir. Twelve of these dogs were in the carboplatin alone group and six dogs were in the carboplatin and doxorubicin group. Doxorubicin dose was reduced to 25 mg m−2 due to toxicity in one dog. There were no treatment delays required after doxorubicin administration.
Table 2.
Summary of chemotherapy-related toxicities in each group
| Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
|
|---|---|---|---|---|
| Carboplatin Alone | ||||
| Carboplatin Toxicity | ||||
| Neutropenia | 1 | 6 | 0 | 1 |
| Thrombocytopenia | 0 | 1 | 0 | 0 |
| Vomiting | 1 | 0 | 0 | 0 |
| Diarrhoea | 0 | 0 | 0 | 0 |
| Anorexia | 0 | 0 | 0 | 0 |
| Carboplatin and Doxorubicin | ||||
| Carboplatin Toxicity | ||||
| Neutropenia | 3 | 3 | 1 | 0 |
| Thrombocytopenia | 0 | 0 | 1 | 0 |
| Vomiting | 0 | 0 | 1 | 0 |
| Diarrhoea | 0 | 1 | 1 | 0 |
| Anorexia | 0 | 2 | 0 | 0 |
| Doxorubicin Toxicity | ||||
| Neutropenia | 0 | 2 | 0 | 0 |
| Thrombocytopenia | 0 | 1 | 0 | 0 |
| Vomiting | 2 | 0 | 1 | 0 |
| Diarrhoea | 0 | 0 | 0 | 0 |
| Anorexia | 0 | 0 | 1 | 0 |
| Hypersensitivity | 0 | 1 | 0 | 0 |
Thirteen dogs (seven in the carboplatin alone group and six in the carboplatin and doxorubicin group) did not complete the recommended follow-up schedule after completion of chemotherapy. These dogs were evaluated at the VMTH for a median of 300 days (range, 140–1125 days) and data collection beyond this point occurred through communication with referring veterinarians and owners. Metastasis was documented or suspected in 35 dogs (70%) including 15 dogs (60%) receiving carboplatin alone and 20 dogs (80%) receiving carboplatin and doxorubicin. Metastases were located in lung only (6 dogs receiving carboplatin and 10 dogs receiving both drugs), bone only (1 dog receiving carboplatin and 2 dogs receiving both drugs), lung and bone (5 dogs receiving carboplatin and 2 dogs receiving both drugs), lung and other organs (3 dogs receiving carboplatin and 5 dogs receiving both drugs), or bone and other organs (1 dog receiving both drugs). Eleven dogs received one of four different investigational cytotoxic therapies after metastasis was documented. In this group, there were four dogs that had received carboplatin and seven dogs that had received carboplatin and doxorubicin. One dog from the carboplatin alone group underwent pulmonary metastasectomy 949 days after amputation followed by investigational cytotoxic therapy and is alive at 1743 days. Overall, the 11 dogs receiving rescue therapy survived a median of 147 days (range, 51–794 days) following detection of metastasis.
At study’s end, 43 dogs had died, 21 from the carboplatin alone group and 22 from the carboplatin and doxorubicin group. One dog from the carboplatin alone group was lost to follow-up at 148 days and six dogs were alive at 599, 1119, 1599, 1699, 1743 and 1766 days. Nine of the 43 dogs that had died underwent necropsy and metastatic OSA was found in all dogs. DFI was censored for nine dogs including five dogs that were alive without metastasis, three dogs that were disease free at death, and one dog that was lost to follow-up. Survival was censored for nine dogs including six dogs that were alive, two dogs that died of causes unrelated to OSA and one dog that was lost to follow-up. The two dogs that died of causes unrelated to OSA were extensively staged prior to euthanasia and cause of death was determined to be metastatic haemangiosarcoma in one dog 647 days after amputation and chronic renal failure in the other dog 1275 days after amputation.
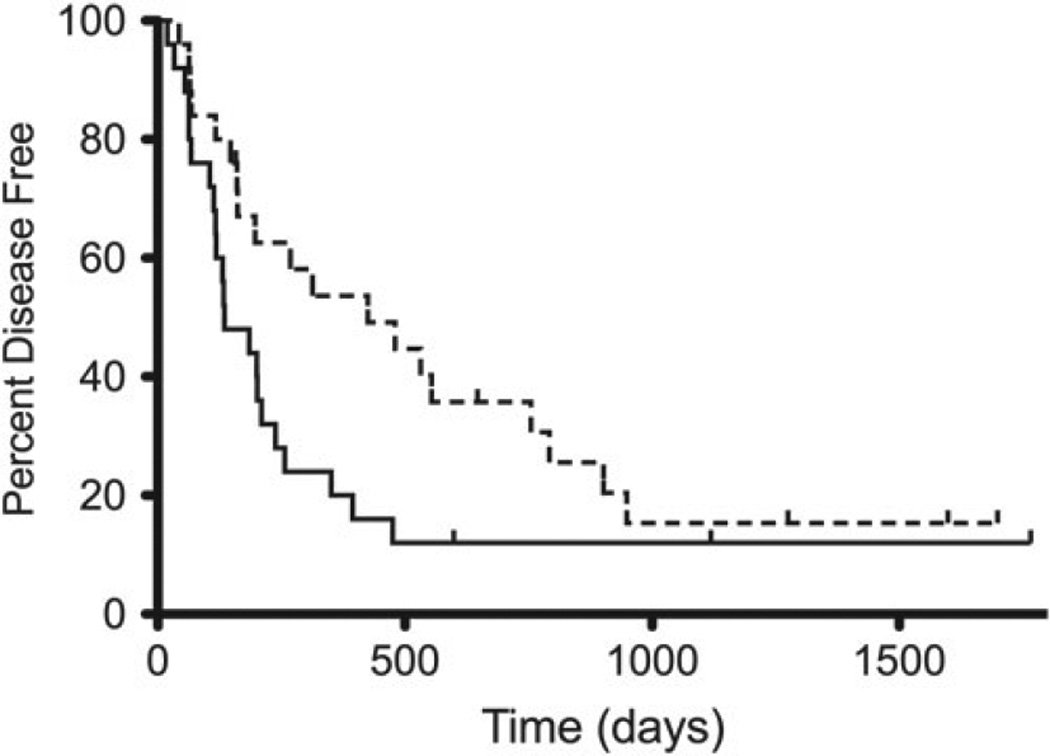
Dogs receiving carboplatin alone had median DFI of 425 days compared to 135 days in dogs receiving carboplatin and doxorubicin (Fig. 1).This difference was statistically significant (P = 0.04). The 1-, 2-, and 3-year disease-free rates were 52, 35, and 15%, respectively, for dogs receiving carboplatin alone and 20, 12, and 12%, respectively, for dogs receiving carboplatin and doxorubicin. This difference was statistically significant (P < 0.001). Dogs receiving carboplatin alone had an MST of 479 days compared to 287 days in dogs receiving carboplatin and doxorubicin. This difference was not statistically significant (P = 0.19).
Figure 1.
Kaplan-Meier curves plotting disease free interval in dogs with OSA receiving carboplatin alone with a dotted line and carboplatin and doxorubicin with a solid line (p = 0.04).
Discussion
This study is the first prospective, randomized, phase III trial comparing two standard-of-care protocols for the adjuvant treatment of appendicular OSA in dogs. The results of this study suggest that dogs receiving six doses of carboplatin may have a longer DFI than dogs receiving carboplatin and doxorubicin following amputation. Previous publications have suggested similar outcome in dogs receiving four doses of carboplatin and six total doses of alternating carboplatin and doxorubicin, however those studies have been retrospective in nature and assessed only one treatment cohort at a time. The median DFI in the carboplatin and doxorubicin group in this study of 135 days is shorter than the median of 202 and 227 days previously reported by Bacon et al. and Kent et al, respectively.7,8 The reason for these differences in DFI is not clear, however, 94% of dogs in the Bacon et al. study underwent 99mTc-MDP bone scans prior to treatment, potentially allowing earlier detection of occult boney metastases than this study, during which bone scans were not routinely performed. In the Kent study, 7 of the 32 dogs studied underwent limb-sparing surgery instead of amputation. Although outcome in these seven dogs was not specified, a study by Lascelles et al. suggests that dogs undergoing limb-sparing surgery plus adjuvant chemotherapy may have longer times to metastasis (median, 315 days) and this may have increased the DFI in the Kent study.7,11 The median DFI of 425 days for dogs prescribed 6 doses of carboplatin in this study is longer than those reported in other studies utilizing a single chemotherapy agent. It is difficult to compare these results directly, however, because those studies focused on outcome after four doses of carboplatin. The effect of administering six instead of four doses of carboplatin has not been fully explored and there is no published information on DFI and MST in dogs with OSA after six adjuvant doses of full-dose carboplatin.
Survival was not considered a primary endpoint of the study reported here due to concerns that rescue therapy initiated after documentation of metastasis could have affected results of survival analysis. In fact, 11 dogs in the study received rescue cytotoxic therapy as part of several clinical trials open for enrollment at the same time as this phase III trial. The MST from detection of metastasis to death in dogs receiving rescue cytotoxic therapy was 147 days and this additional survival time may have affected results. Furthermore, more dogs receiving rescue therapy were in the carboplatin and doxorubicin treatment group than the carboplatin alone group (7 versus 4), which could have negated a survival difference between groups. Saam et al.5 previously reported on 65 dogs with OSA that received adjuvant carboplatin and found that dogs receiving rescue chemotherapy after detection of metastasis lived significantly longer than dogs that did not receive rescue chemotherapy. This finding suggests that rescue therapy can affect the results of survival analysis in a clinical trial such as the one undertaken here. Further study in to the role of rescue therapy in dogs with OSA is necessary and future phase III trials may want to standardize rescue therapy to allow for unbiased survival analysis and comparison.
Many patient and tumour characteristics have been previously shown to predict outcome in dogs with appendicular OSA. Specifically, dogs with proximal humeral tumour location, telangiectatic subtype, high grade, high mitotic rate, and/or elevated serum ALP levels may have a poorer prognosis after amputation and chemotherapy.3,12–18 By assigning dogs to a treatment group randomly, this study was designed to minimize the effect of these prognostic factors on outcome. To confirm that treatment groups were similar, patient and tumour characteristics were compared between groups to look for differences that could affect the results of this study. Results indicated that there were no differences between groups with regard to age, sex, weight, time to chemotherapy start, tumour location, histologic type, mitoses per 3 hpf, or pre-surgical serum ALP levels. A significant difference in breed distribution was found, however, breed has not been shown to be associated with survival in any previous studies so this is unlikely to have impacted results.
Despite the prospective nature of this study, there were several limitations that may have affected results. First, a histopathologic review of biopsy samples by a single pathologist was not conducted to confirm histologic subtype or assign a tumour grade. Grade was infrequently reported in biopsy reports from dogs in this study, but mitotic index was available in 47 of 50 dogs. Mitotic index was, therefore, used as a surrogate for tumour grade, which has been shown in several previous studies to predict prognosis in dogs with OSA.5,15,16 Although mitotic index was shown to independently predict prognosis in some of those studies, evaluation by a single pathologist and application of a previously published grading scheme would have strengthened the results of the study described here. Another limitation is that 13 dogs did not complete the recommended follow-up schedule and this could have resulted in overestimation of DFI in some cases. To minimize this effect, follow-up data from dogs that missed appointments were collected in real time during the study period through contact with referring veterinarians and pet owners. Nonetheless, one of these dogs was lost to follow-up and attempts to contact the owner failed. In addition, some dogs did not have a complete diagnostic work-up at or around the time of death, and only 9 of the 43 dogs that had died underwent necropsy to definitively determine cause of death. To minimize the effects of unknown cause of death on DFI and MST calculations, metastasis and death due to OSA were assumed if a diagnostic work-up did not occur at or around the time of death. Despite this, it is possible that these data loss may have affected study results.
In veterinary medicine, toxicity risk may play a role when deciding between two apparently equivalent chemotherapy protocols. Although toxicity evaluation was not a primary endpoint of this study, these data were collected and reported. Overall, toxicity was similar between treatment groups with 10 dogs in the carboplatin alone group and 13 dogs in the carboplatin and doxorubicin group experiencing at least one adverse event. The majority of these adverse events were mild and did not require dose reduction, however, treatment delay to a 28-daycycle after carboplatin due to delayed neutrophil nadir was common. Treatment delays may result in decreased chemotherapy efficacy and may affect patient outcome, though it is not clear to what extent. The carboplatin alone group was affected by treatment delay more often than the carboplatin and doxorubicin group in this study. Dogs receiving carboplatin alone were delayed to a 28-day cycle after all future doses where dogs receiving both drugs were only delayed after future carboplatin doses and were treated 21 days after doxorubicin. Furthermore, a delayed nadir after carboplatin was more common in the carboplatin alone group (12 dogs) compared to the carboplatin and doxorubicin group (6 dogs). Overall, differences between groups with regard to toxicity were minimal, but the treatment delays that occurred after carboplatin could have affected outcome in some dogs.
In conclusion, this study demonstrated longer than previously reported median DFI in dogs with OSA undergoing amputation and adjuvant therapy with carboplatin. Further study is required and a direct comparison between outcome after four and six carboplatin doses is in order. This study also suggests that six doses of carboplatin may be associated with a longer DFI than six total doses of carboplatin and doxorubicin. Further study and protocol comparison in a larger cohort of dogs is needed to confirm or deny these results and to determine whether a survival benefit may also exist.
Footnotes
This research was presented, in part, at the 2009 annual meeting of the Veterinary Cancer Society in Austin, TX, USA.
Conflict of interest
None of the authors of this paper have a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of the paper.
References
- 1.Straw RC, Withrow SJ, Richter SL, Powers BE, Klein MK, Postorino NC, LaRue SM, Ogilvie GK, Vail DM, Morrison WB, McGee M, Dickinson K. Amputation and cisplatin for the treatment of canine osteosarcoma. Journal of Veterinary Internal Medicine. 1991;5:205–210. doi: 10.1111/j.1939-1676.1991.tb00950.x. [DOI] [PubMed] [Google Scholar]
- 2.Berg J, Weinstein MJ, Schelling SH, Rand WM. Treatment of dogs with osteosarcoma by administration of cisplatin after amputation or limb-sparing surgery: 22 cases (1987–1990) Journal of the American Veterinary Medical Association. 1992;200:2005–2008. [PubMed] [Google Scholar]
- 3.Bergman PJ, MacEwen EG, Kurzman ID, Henry CJ, Hammer AS, Knapp DW, Hale A, Kruth SA, Klein MK, Klausner J, Norris AM, McCaw D, Straw RC, Withrow SJ. Amputation and carboplatin for treatment of dogs with osteosarcoma: 48 cases (1991 to 1993) Journal of Veterinary Internal Medicine. 1996;10:76–81. doi: 10.1111/j.1939-1676.1996.tb02031.x. [DOI] [PubMed] [Google Scholar]
- 4.Phillips B, Powers BE, Dernell WS, Straw RC, Khanna C, Hogge GS, Vail DM. Use of single-agent carboplatin as adjuvant or neoadjuvant therapy in conjunction with amputation for appendicular osteosarcoma in dogs. Journal of the American Animal Hospital Association. 2009;45:33–38. doi: 10.5326/0450033. [DOI] [PubMed] [Google Scholar]
- 5.Saam DE, Liptak JM, Stalker MJ, Chun R. Predictors of outcome in dogs treated with adjuvant carboplatin for appendicular osteosarcoma: 65 cases (1996–2006) Journal of the American Veterinary Medical Association. 2011;238:195–206. doi: 10.2460/javma.238.2.195. [DOI] [PubMed] [Google Scholar]
- 6.Berg J, Weinstein MJ, Springfield DS, Rand WM. Results of surgery and doxorubicin chemotherapy in dogs with osteosarcoma. Journal of the American Veterinary Medical Association. 1995;206:1555–1560. [PubMed] [Google Scholar]
- 7.Kent MS, Strom A, London CA, Seguin B. Alternating carboplatin and doxorubicin as adjunctive chemotherapy to amputation or limb-sparing surgery in the treatment of appendicular osteosarcoma in dogs. Journal of Veterinary Internal Medicine. 2004;18:540–544. doi: 10.1892/0891-6640(2004)18<540:acadaa>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 8.Bacon NJ, Ehrhart NP, Dernell WS, Lafferty M, Withrow SJ. Use of alternating administration of carboplatin and doxorubicin in dogs with microscopic metastases after amputation for appendicular osteosarcoma: 50 cases (1999–2006) Journal of the American Veterinary Medical Association. 2008;232:1504–1510. doi: 10.2460/javma.232.10.1504. [DOI] [PubMed] [Google Scholar]
- 9.Ehrhart NP, Ryan SD, Fan TM. Tumors of the skeletal system. In: Withrow SJ, Vail DM, Page RL, editors. Withrow & MacEwen’s Small Animal Clinical Oncology. 5. St. Louis: Elsevier; 2013. pp. 463–503. [Google Scholar]
- 10.Veterinary Co-operative Oncology Group. Veterinary co-operative oncology Group – Common terminology criteria for adverse events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.0. Veterinary and Comparative Oncology. 2004;2:195–213. doi: 10.1111/j.1476-5810.2004.0053b.x. [DOI] [PubMed] [Google Scholar]
- 11.Lascelles BDX, Dernell WS, Correa MT, Lafferty M, Devitt CM, Kuntz CA, Straw RC, Withrow SJ. Improved survival associated with postoperative wound infection in dogs treated with limb-salvage surgery for osteosarcoma. Annals of Surgical Oncology. 2005;12:1073–1083. doi: 10.1245/ASO.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Boerman I, Selvarajah GT, Nielen M, Kirpensteijn J. Prognostic factors in canine appendicular osteosarcoma – a meta-analysis. BMC Veterinary Research. 2012;8:56. doi: 10.1186/1746-6148-8-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ehrhart N, Dernell WS, Hoffman WE, Weigel RM, Powers BE, Withrow SJ. Prognostic importance of alkaline phosphatase activity in serum from dogs with appendicular osteosarcoma: 75 cases (1990–1996) Journal of the American Veterinary Medical Association. 1998;213:1002–1006. [PubMed] [Google Scholar]
- 14.Garzotto CK, Berg J, Hoffman WE, Rand WM. Prognostic significance of serum alkaline phosphatase activity in canine appendicular osteosarcoma. Journal of Veterinary Internal Medicine. 2000;14:587–592. doi: 10.1892/0891-6640(2000)014<0587:psosap>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 15.Kirpensteijn J, Kik M, Rutteman GR, Teske E. Prognostic significance of a new histologic grading system for canine osteosarcoma. Veterinary Pathology. 2002;39:240–246. doi: 10.1354/vp.39-2-240. [DOI] [PubMed] [Google Scholar]
- 16.Loukopoulos P, Robinson WF. Clinicopathologic relevance of tumor grading in canine osteosarcoma. Journal of Comparative Pathology. 2007;136:65–73. doi: 10.1016/j.jcpa.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Misdorp W, Hart AA. Some prognostic and epidemiologic factors in canine osteosarcoma. J Nat Cancer Inst. 1979;62:537–545. doi: 10.1093/jnci/62.3.537. [DOI] [PubMed] [Google Scholar]
- 18.Hammer AS, Weeren FR, Weisbrode SE, Padgett SL. Prognostic factors in dogs with osteosarcoma of the flat or irregular bones. Journal of the American Animal Hospital Association. 1995;31:321–326. doi: 10.5326/15473317-31-4-321. [DOI] [PubMed] [Google Scholar]