Abstract
Objective
To evaluate long-term outcomes of active surveillance (AS) applied in low-risk prostate cancer patients, and the impact of re-biopsy results on the prediction of progression.
Material and methods
In our clinic, patients who had undergone AS for low-risk localized prostate cancer between the years 2005–2013 were included in the study. Our AS criteria are Gleason score ≤6, prostate-specific antigen (PSA) level <10 ng/mL, number of positive cores <3, maximum cancer involvement ratio <50% each core. Immediate re-biopsy (within 3 months) was performed to 65 patients who accepted AS. Finally, 43 patients who met re-biopsy criteria were included in the study. Prostate biopsy specimens were harvested from 12 cores under the guidance of transrectal ultrasound (TRUS). Re-biopsy was performed within 3 months (1–12 weeks). In re-biopsy, a total of 20 core biopsies were performed including the far lateral (6 cores) and transition zone (2 cores) in addition to standard 12 core biopsy. Our follow-up protocol is PSA measurement and digital rectal examination (DRE) every 3 months within the first 2 years, than every 6 months. Control biopsies was performed one year later and once upon every 3 years to patients whose PSA levels and DREs were normal at follow-up visits. More than 2 tumor invaded cores or 50% tumor in one core, and Gleason score exceeding 6 points were accepted as indications for definitive treatment. Patients were divided into two groups by re-biopsy results and compared according to the time to progression. We have done multivariate regression analysis to predict prognosis by using data on age, PSA level, and detection of tumor in re-biopsy specimens.
Results
Patients’ median age was 61 years and PSA level was 5 (2.7–9) ng/mL. Tumor was detected in 22 (34%) patients at re-biopsy and they underwent definitive treatment. Additionally tumor was detected in 9 patients, but active surveillance was maintained because their pathologic results met active surveillance criteria. Median follow time was 42 (24–117) months. Definitive treatment was performed in 9 (21%) patients. PSA recurrence was not detected in none of 9 patients during 38 months of follow up. Only the presence of tumor in re-biopsy specimens was found predictor of disease progression in multivariate analysis.
Conclusion
We think that AS is safe method for low-risk localized prostate cancer patients, if it is performed in compliance with certain criteria and regular follow up, and early re-biopsy can be useful either during early period or long term follow-up.
Keywords: Active surveillance, prostate cancer, re-biopsy
Introduction
Prostate cancer is the most common cancer among males, except skin cancer. While the incidence of lifetime risk of prostate cancer is 16.7%, the risk of dying from prostate cancer is 2.5%.[1] Although the death rate of prostate cancer has not changed too much over the years, the average five-year survival rate has increased.[2] The advances in treatment that result in increased survival have increased the treatment cost. About 8.5 billion € is spent every year in all of European countries.[3] Advances in surgical and radiotherapy (RT) techniques improve oncologic and functional success, but the risk of incontinence and impotence are still present.[4] Patients do not want to be a candidate to over-treatment and serious complications. Guidelines for patients are published in the United States on this issue.[5] Therefore, higher cancer specific survival rates indicated in large series have thoroughly popularized active surveillance (AS).[6–9] AS is based on the principle of no making or postponement to definitive treatment. The aim is to take notice of upgrading of tumor before missing the chance of treatment through periodic measurements blood prostate specific antigen (PSA) level, digital rectal examinations (DRE) and prostate biopsies. By this way, side effects that occur with definitive treatment will not happen or be postponed.[6]
In our study, we aimed to evaluate outcomes of AS and impact of early re-biopsy on patients who were candidates of AS.
Material and methods
In our clinic, patients for whom AS was applied for low-risk localized prostate cancer between the years 2005–2013 were included in the study. Prostate biopsies were performed because of an elevated PSA level (values above 4 ng/mL, and 2.5 ng/mL before, and after the year 2010 year, respectively) and/or abnormal DRE results.
Active surveillance, radical prostatectomy (RP) and RT have been proposed as treatment choices to the patients whose blood PSA levels and biopsy results met the AS criteria. These criteria are Gleason score ≤6, PSA level of <10 ng/mL, less than 3 cancer-positive cores, maximum cancer involvement ratio <50% of each core. We’ve noted that patients were at an intellectual level to understand advantages and disadvantages of AS. Re-biopsies were performed in 65 patients who accepted AS. Finally, 43 patients whom re-biopsy results met AS criteria were included in the study. At least once a control biopsy was performed a year later in all study population. Transrectal ultrasound (TRUS) guided 12-core prostate biopsies were performed, and biopsies were repeated within 3 months (1–12 weeks). In re-biopsy, a total of 20 core biopsy specimens were obtained from the far lateral (6 cores) and transition zone (2 cores) in addition to standard 12 core biopsy. Our follow protocol is PSA measurement and DRE every 3 months within the first 2 years, than at every 6 months. Control biopsies are performed one year later and once upon every 3 years in patients whose PSA levels and DRE are normal at follow-up visits. Patients were followed up up to 75 years of age in AS protocol, then watchful-waiting protocol is implemented. We have done again biopsy instead of immediate definitive treatment in cases whom PSA levels exceeded 10 ng/mL. Multiparametric magnetic resonance (MR) imaging of the prostate was done for patients whose PSA levels permanently exceeded 10 ng/mL and tumor was not determined at biopsy, and for those in whom TRUS biopsy could not be achieved. Detection more than 2 tumor invaded cores or 50% tumor in one core, Gleason score exceeding 6 were accepted as definitive treatment indications. We have obtained informed consent from all patients for participation in the study.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Statistical analysis
Patients were divided into two groups by re-biopsy results and they were compared according to time to progression. We have done multivariete linear regressions analyze for to evaluate prediction of upgrading via age, PSA level, and whether tumor present on re-biopsy. We have used Kaplan-Meier and multivariate logistic regression analysis. For statistical analysis Statistical Package for the Social Sciences (SPSS Inc; Chicago, IL, USA) version 15 for Windows was used. The level of statistical significance was determined as p<0.05.
Results
Twenty-two of 65 patients who fulfilled AS criteria on first biopsy underwent immediate definitive treatment based on re-biopsy results. Additionally tumor was determined in 9 patients; but we maintained surveillance, because their pathologic results met AS criteria. Totally 43 patients were included in the study (Figure 1). Patients’ median age was 61 years, and PSA level 5 (2.7–9) ng/mL. Median follow-up period was 50 (24–117) months. Our one patient had undergone RP in another center after one year of AS (Table 1).
Figure 1.
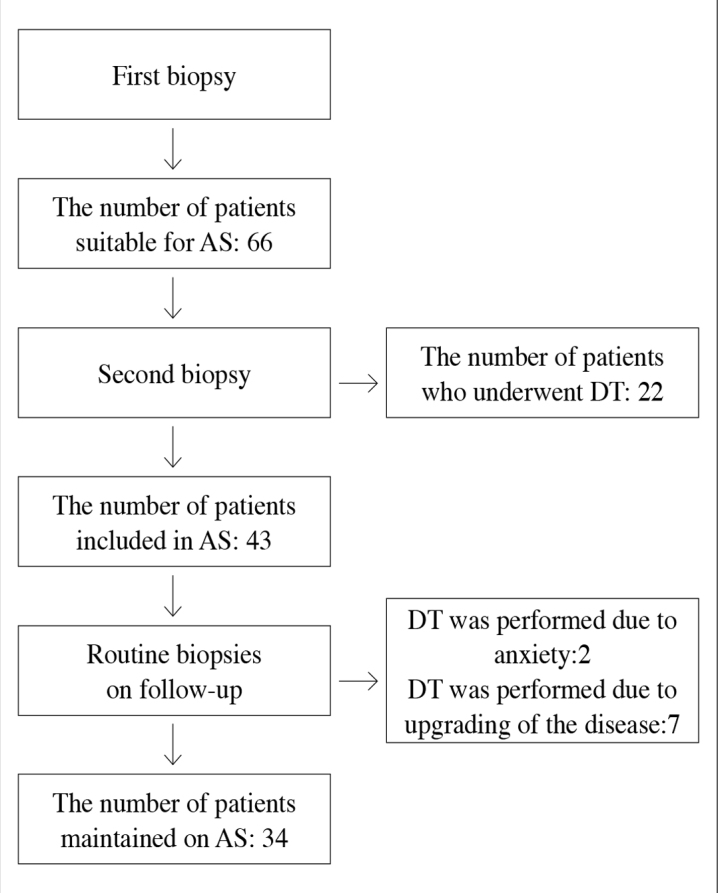
(a) Number of biopsized patients, and those scheduled for definitive treatment (DT), and active surveillance (AS).
Table 1.
Demographic characteristics of the patients
| Patients (n) | 43 |
| Median age (year) | 61 (51–72) |
| Median PSA level (ng/mL) | 5 (2.7–9) |
| Patients requiring definitive treatment as determined by early re-biopsy, n (%) | 22 (34%) |
| Tumor positive patients on active surveillance detected by re-biopsies, n (%) | 9 (21%) |
| Patients on definitive treatment, n, (%) | 9 (21%) |
| Median follow-up period (months) | 50 (24–117) |
| Median follow-up period (months) | 42 (24–117) |
| Patient lost to follow-up n, (%) | 1 (2.3%) |
PSA: prostate-specific antigen
Definitive treatment was performed in 9 (21%) patients. RP was performed in 2 patients on their request. RP was performed in 3 of 7 and RT in 4 of 7 patients whose pathologic results were upgraded (Figure 1). RP was performed via classic retropubic method, and lymph node dissection was performed in all patients. All of three patients’ pathologic results were as follows: Gleason score: 7, extraprostatic extension without seminal vesicular and lymph node involvement. Nevertheless surgical margin was positive at focal area in one patient. Salvage or adjuvant RT or androgen deprivation therapy (ADT) were not performed in RP patients including a surgical margin positive patient. Conformal method was applied as RT treatment. Three of four patients received ADT for 6 months, because their Gleason scores were 7 points. Median follow-up time of 9 patients who had undergone definitive treatment was 38 months (24–93). PSA recurrence was not observed in any patient.
We divided patients into two groups. Group 1, and 2 consisted of patients whose tumors were and were not detected in re-biopsy specimens. We compared two groups in order to evaluate up-grading during follow-up. Pathologic upgrading ratio was significantly higher in Group 1, relative to Group 2 (Figure 2). Only that tumor presenting on re-biopsy was found to have a significant impact factor (Table 2).
Figure 2.
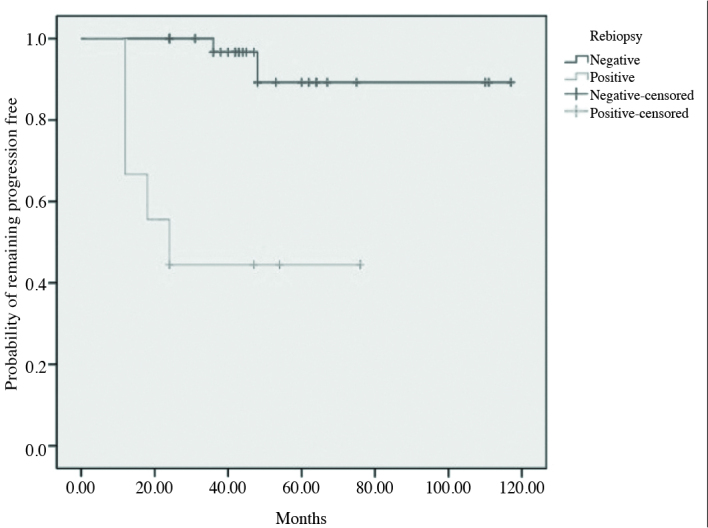
The likelihood of remaining free of disease progression classified by the result of the re-biopsies according to Kaplan-Meier analysis
Table 2.
Multivariate analysis for the prediction of disease progression
| Value | Adjusted odds ratio | p | 95% confidence interval |
|---|---|---|---|
| Age | 0.597 | 0.554 | −0.017–0.030 |
| PSA | −0.379 | 0.706 | −0.074–0.051 |
| Re-biopsy (positive) | 3.537 | 0.001 | 2.02–7.45 |
PSA: prostate-specific antigen
Six patients’ PSA levels exceeded 10 ng/mL during follow-up. Therefore prostate biopsy was done to all six patients before routine biopsy time. Tumor was assessed in none of them. Multiparametric MR imaging of prostate was performed to three of six patients whose PSA levels remained consistently over 10 ng/mL and we maintained follow-up because we didn’t find any significant evidence of tumor.
Discussion
In contrast to safety of AS proven in large series, both physicians and patients do not welcome it. It has been reported that RP has been performed in 94% of the patients whose criteria are suitable for AS in United States of America.[10] Nevertheless, applying definitive treatment to this group has no benefits for survival.[11] We took care that patients intellectual level is satisfactory for understanding AS. Thus, we have learned that our patients have been operated (RP) in another center. Our follow out rate was 2.3% which was in compliance with the current literature (Table 3).
Table 3.
Comperative demographic characteristics
| Researchers | Patients (n) | Median follow-up (Months) | Disease Progression (%) | Survival rates (%) | RP (%) performed upon patient’s request | Patients (n) remaining on active surveillance (PFS) (%) | ||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Biopsy | PSA DT | OS | CSS | |||||
| Klotz et al.[6] | 453 | 82 | 9 | 14 | 78.6 | 97.2 | 3 | 70 |
|
| ||||||||
| Dall’Era et al.[7] | 321 | 47 | 35 | 5 | 97 | 100 | 8 | 54 |
|
| ||||||||
| Van As et al.[8] | 326 | 22 | 13 | 18 | 98 | 100 | 2 | 73 |
|
| ||||||||
| Soloway et al.[9] | 230 | 32 | 10 | - | 100 | 100 | - | 86 |
|
| ||||||||
| Our study | 43 | 42 | 16 | - | 97.6 | 100 | 4.6 | 77 |
OS: overall survival; CSS: cancer-specific survival; PFS: progression-free survival
We have not seen any consensus about AS including its criteria in the literature. Conti et al.[12] reported that 4% of the patients are candidates for AS according to John Hopkins criteria, but in a retrospective study performed in Royal-Marsden hospital which evaluated radical prostatectomy specimens, 82% of the patients had been found to be candidates for AS. We used AS criteria proposed by Epstein criteria, and also Klotz et al.[13]
Very diverse opinions are available in the literature about prostate biopsy. But all of the authors are agree about that the first biopsy must be at least 10 cores and confirmatory biopsy must be performed.[7,14,15] Motamedinia et al.[16] said that the first and the confirmatory biopsies should be obtained from at least 10 cores. Especially, interobserver opinions about pathologic evaluation about low-volume tumor is very variable, so assessments should be performed in experienced centers so as to reduce confusion rates. We have an experienced uropathologist in our hospital, so we think our biopsy results are reliable. Two different studies have revealed that 33–52% of their patients whose histopathology results were appropriate for AS needed definitive treatment, when transperineal prostate biopsies were done. About half of the patients’ tumors were localized in the anterior lob.[17,18] MR image assisted transperineal biopsy is not superior to ultrasound assisted biopsy in patients whose tumors are low grade and volume similar to AS patients.[19] But MR image assisted and 3D mapping method has shown that 25% of AS patients have GS ≥7 tumor and 61% of them are bilateral.[20] Klotz advises that confirmatory biopsy should be performed, and include anterolateral area.[21] We have not done transperinal biopsy for anterolateral sampling because of technical deficiency. Therefore we have done far lateral and transitional zone sampling during re-biopsy.
Re-biopsy or confirmatory biopsy should be done within 1 year.[13,14] Even earlier biopsy (within 3 months) can be more helpful.[15] Thanks to re-biopsy, we performed definitive treatment for 22 of 65 (34%) of our patients whose pathologic results were upgraded. We think that this rate is somewhat higher. Early re-biopsy can discriminate patients who are really suitable for AS. Additionally, the result of re-biopsy appears to have a strong impact on pathologic upgrading. Two different studies revealed that tumor present on re-biopsy is predictive factor for upgrading.[14,21] We have seen that progression rate was higher and time to progression was shorter in patients whose re-biopsy report indicated presence of a tumor.
Immediate, and deferred RP yielded similar pathologic outcomes among AS patients.[11] PSA recurrence was not observed in any of our patients who had undergone RP for tumor upgrading. Urinary incontinence rates were similar between immediate and deferred RP groups.[22]
Active surveillance has yielded the best survival result on low-risk prostate cancer. This result is directly related to tumor volume and grade. Survival rates and overall study results for AS obtained from literature and our study are seen on Table 3. On an average about 21% of our patients needed definitive treatment. Overall survival rates are very high both in our study (97.6%), and in many studies cited in the literature (97–100%).[7–9] But Klotz et al.[6] have reported lower average survival rate of 78.6%. The authors explained this result by their extremely prolonged follow-up period. One of our patients has died secondary to a cardiac event.
Cancer-specific survival is about 100% in all study.[7–9] None of our patients has died because of cancer in our study and definitive treatment has not failed in none of them. Forty-two months may seem to be a long time for follow-up, but it is short time for low-risk prostate cancer.
Physicians may worry about patients lost to follow-up. But current literature and our study have shown that most of patients continue to follow-up.[6–9] Only one patient of our series was lost to follow-up.
Follow-up protocol of AS is not clear yet.[23] We used protocol which was described by Klotz.[13] Routine PSA monitorization and kinetics are not predictive factor for upgrading.[24] But PSA density is an important factor for the prediction of disease progression.[25] We have not decide definitive treatment based on only PSA kinetics, but we used PSA kinetics to decide non-routine biopsy procedures.
In a study, in patients suitable for AS whose Gleason scores were not abnormal, increased number of tumor invaded cores were detected in MR image fusion biopsy specimens.[26] It was shown that predictive value of multiparametric MR image is 2.5-fold higher without concomitant biopsy.[27] We have performed multiparametric MR image examination in 3 patients whose PSA level remained consistently over 10 ng/mL despite negative biopsy results. We have continued follow-up for three patients because tumor has not been seem on multiparametric image of the prostate.
This is second study from Turkey about AS after publication of Soydan et al.[28] in 2013. Although higher number of our patients were followed up for alonger time when compared with their study, still they are far below than those cited in the literature.[6–9] We think that multicenter collaboration must be realized for better results.
In conclusion, we think that AS would be a safe method for low-risk localized prostate cancer patients, if certain criteria are met, and regular follow ups are performed. Immediate (within 3 months) re-biopsy is extremely important. A significant proportion of the patients can be treated at the beginning of AS by immediate re-biopsy, and positive re-biopsy can predict early and high cancer-progression rate.
Footnotes
Ethics Committee Approval: Authors declared that the research was conducted according to the principles of the World Medical Association Declaration of Helsinki “Ethical Principles for Medical Research Involving Human Subjects”, (amended in October 2013).
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – O.T., G.B.; Design –G.B., O.T., H.A.; Supervision - O.T., S.A.; Resources – H.A., A.D.; Materials – G.B.; Data Collection and/or Processing – A.D., H.A.; Analysis and/or Interpretation – K.H., A.D.; Literature Search – G.B., A.D.; Writing Manuscript – G.B., K.H., S.A.; Critical Review – S.A., K.H.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.American Cancer Society. Cancer facts and figures. 2008. Available at http://www.cancer.org/acs/groups/content/@nho/documents/document/2008cafffinalsecuredpdf.pdf.
- 2.De Angelis R, Sant M, Coleman MP, Francisci S, Baili P, Pierannunzio D, et al. EUROCARE-5 Working Group. Cancer survival in Europe 1999–2007 by country and age: results of EUROCARE--5-a population-based study. Lancet Oncol. 2014;15:23–34. doi: 10.1016/S1470-2045(13)70546-1. http://dx.doi.org/10.1016/S1470-2045(13)70546-1. [DOI] [PubMed] [Google Scholar]
- 3.Luengo-Fernandez R, Leal J, Gray A, Sullivan R. Economic burden of cancer across the European Union: a population-based cost analysis. Lancet Oncol. 2013;14:1165–74. doi: 10.1016/S1470-2045(13)70442-X. http://dx.doi.org/10.1016/S1470-2045(13)70442-X. [DOI] [PubMed] [Google Scholar]
- 4.Huang GJ, Sadetsky N, Penson DF. Health related quality of life for men treated for localized prostate cancer with long-term follow up. J Urol. 2010;183:2206–12. doi: 10.1016/j.juro.2010.02.013. http://dx.doi.org/10.1016/j.juro.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network. Prostate Cancer Guidelines For Patients. 2014. Available at: http://www.nccn.org/patients/guidelines/prostate/
- 6.Klotz L, Zhang L, Lam A, Nam R, Mamedov A, Loblaw A. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010;28:126–31. doi: 10.1200/JCO.2009.24.2180. http://dx.doi.org/10.1200/JCO.2009.24.2180. [DOI] [PubMed] [Google Scholar]
- 7.Dall’Era MA, Konety BR, Cowan JE, Shinohara K, Stauf F, Cooperberg MR, et al. Active surveillance for the management of prostate cancer in a contemporary cohort. Cancer. 2008;112:2664–70. doi: 10.1002/cncr.23502. http://dx.doi.org/10.1002/cncr.23502. [DOI] [PubMed] [Google Scholar]
- 8.van As NJ, Norman AR, Thomas K, Khoo VS, Thompson A, Huddart RA, et al. Predicting the probability of deferred radical treatment for localised prostate cancer managed by active surveillance. Eur Urol. 2008;54:1297–305. doi: 10.1016/j.eururo.2008.02.039. http://dx.doi.org/10.1016/j.eururo.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 9.Soloway MS, Soloway CT, Williams S, Ayyathurai R, Kava B, Manoharan M. Active surveillance; a reasonable management alternative for patients with prostate cancer: the Miami experience. BJU Int. 2008;101:165–9. doi: 10.1111/j.1464-410X.2007.07190.x. [DOI] [PubMed] [Google Scholar]
- 10.Cooperberg M, Lubeck DP, Penson D, Mehta SS, Carroll PR, Kane CJ. Sociodemographic and clinical risk characteristics of patients with prostate cancer within the Veterans Affairs health care system: data from CaPSURE. J Urol. 2003;170:905–8. doi: 10.1097/01.ju.0000081200.63275.0b. http://dx.doi.org/10.1097/01.ju.0000081200.63275.0b. [DOI] [PubMed] [Google Scholar]
- 11.Parker C, Muston D, Melia J, Moss S, Dearnaley D. A model of the natural history of screen-detected prostate cancer, and the effect of radical treatment on overall survival. Br J Cancer. 2006;94:1361–8. doi: 10.1038/sj.bjc.6603105. http://dx.doi.org/10.1038/sj.bjc.6603105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conti SL, Dall’era M, Fradet V, Cowan JE, Simko J, Carroll PR. Pathological outcomes of candidates for active surveillance of prostate cancer. J Urol. 2009;181:1628–34. doi: 10.1016/j.juro.2008.11.107. http://dx.doi.org/10.1016/j.juro.2008.11.107. [DOI] [PubMed] [Google Scholar]
- 13.Klotz L. Active surveillance for prostatecancer: for whom? J Clin Oncol. 2005;23:8165–9. doi: 10.1200/JCO.2005.03.3134. [DOI] [PubMed] [Google Scholar]
- 14.Al Otaibi M, Ross P, Fahmy N, Jeyaganth S, Trottier H, Sircar K, et al. Role of repeated biopsy of the prostate in predicting disease progression in patients with prostate cancer on active surveillance. Cancer. 2008;113:286–92. doi: 10.1002/cncr.23575. http://dx.doi.org/10.1002/cncr.23575. [DOI] [PubMed] [Google Scholar]
- 15.Berglund RK, Masterson TA, Vora KC, Eggener SE, Eastham JA, Guillonneau BD. Pathological upgrading and up staging with immediate repeat biopsy in patients eligible for AS. J Urol. 2008;180:1964–8. doi: 10.1016/j.juro.2008.07.051. http://dx.doi.org/10.1016/j.juro.2008.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Motamedinia P, Richard JL, McKiernan JM, DeCastro GJ, Benson MC. Role of immediate confirmatory prostate biopsy to ensure accurate eligibility for active surveillance. Urology. 2012;80:1070–4. doi: 10.1016/j.urology.2012.07.049. http://dx.doi.org/10.1016/j.urology.2012.07.049. [DOI] [PubMed] [Google Scholar]
- 17.Ayres BE, Montgomery BS, Barber NJ, Pereira N, Langley SE, Denham P, et al. The role of transperineal template prostate biopsies in restaging men with prostate cancer managed by active surveillance. BJU Int. 2012;109:1170–6. doi: 10.1111/j.1464-410X.2011.10480.x. http://dx.doi.org/10.1111/j.1464-410X.2011.10480.x. [DOI] [PubMed] [Google Scholar]
- 18.Barzell WE, Melamed MR. Appropriate patient selection in the focal treatment of prostate cancer: the role of transperineal 3-dimensional pathologic mapping of the prostate--a 4-year experience. Urology. 2007;70(Suppl 6):27–35. doi: 10.1016/j.urology.2007.06.1126. [DOI] [PubMed] [Google Scholar]
- 19.Kasivisvanathan V, Dufour R, Moore CM, Ahmed HU, Abd-Alazeez M, Charman SC, et al. Transperineal magnetic resonance image targeted prostate biopsy versus transperineal template prostate biopsy in the detection of clinically significant prostate cancer. J Urol. 2013;189:860–6. doi: 10.1016/j.juro.2012.10.009. http://dx.doi.org/10.1016/j.juro.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Onik G, Miessau M, Bostwick DG. Three-dimensional prostate mapping biopsy has a potentially significant impact on prostate cancer management. J Clin Oncol. 2009;27:4321–6. doi: 10.1200/JCO.2008.20.3497. http://dx.doi.org/10.1200/JCO.2008.20.3497. [DOI] [PubMed] [Google Scholar]
- 21.Patel MI, DeConcini DT, Lopez-Corona E, Ohori M, Wheeler T, Scardino PT. An analysis of men with clinically localized prostate cancer who deferred definitive therapy. J Urol. 2004;171:1520–4. doi: 10.1097/01.ju.0000118224.54949.78. http://dx.doi.org/10.1097/01.ju.0000118224.54949.78. [DOI] [PubMed] [Google Scholar]
- 22.Warlick C, Trock BJ, Landis P, Epstein JI, Carter HB. Delayed versus immediate surgical intervention and prostate cancer outcome. J Natl Cancer Inst. 2006;98:355–7. doi: 10.1093/jnci/djj072. http://dx.doi.org/10.1093/jnci/djj072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roobol MJ, Kranse R, Bangma CH, van Leenders AG, Blijenberg BG, van Schaik RH, et al. Screening for prostate cancer: results of the Rotterdam section of the European randomized study of screening for prostate cancer. Eur Urol. 2013;64:530–9. doi: 10.1016/j.eururo.2013.05.030. http://dx.doi.org/10.1016/j.eururo.2013.05.030. [DOI] [PubMed] [Google Scholar]
- 24.Resnick MJ, Koyama T, Fan KH, Albertsen PC, Goodman M, Hamilton AS, et al. Long-term functional outcomes after treatment for localized prostate cancer. N Engl J Med. 2013;368:436–45. doi: 10.1056/NEJMoa1209978. http://dx.doi.org/10.1056/NEJMoa1209978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross AE, Loeb S, Landis P, Partin AW, Epstein JI, Kettermann A, et al. Prostate-specific antigen kinetics during follow-up are an unreliable trigger for intervention in a prostate cancer surveillance program. J Clin Oncol. 2010;28:2810–6. doi: 10.1200/JCO.2009.25.7311. http://dx.doi.org/10.1200/JCO.2009.25.7311. [DOI] [PubMed] [Google Scholar]
- 26.Lin DW, Newcomb LF, Brown EC, Brooks JD, Carroll PR, Feng Z, et al. Urinary TMPRSS2:ERG and PCA3 in an active surveillance cohort: results from a baseline analysis in the Canary Prostate Active Surveillance Study. Clin Cancer Res. 2013;19:2442–50. doi: 10.1158/1078-0432.CCR-12-3283. http://dx.doi.org/10.1158/1078-0432.CCR-12-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sonn GA, Filson CP, Chang E, Natarajan S, Margolis DJ, Macairan M, et al. Initial experience with electronic tracking of specific tumor sites in men undergoing active surveillance of prostate cancer. Urol Oncol. 2014;32:952–7. doi: 10.1016/j.urolonc.2014.04.003. http://dx.doi.org/10.1016/j.urolonc.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soydan H, Dursun F, Yılmaz Ö, Okçelik S, Ateş F, Karademir K. Our results of active surveillance for localized prostate cancer patients. Turk J Urol. 2013;39:1–5. doi: 10.5152/tud.2013.001. http://dx.doi.org/10.5152/tud.2013.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


