Abstract
Intravascular papillary endothelial hyperplasia (IPEH, Masson’s Tumor) of the kidney is an unusual, benign vascular lesion. A rare case of recurrent IPEH in the kidney was presented in this article. A 50-year-old male with a diagnosis of a suspicious mass in the left kidney was referred to our center for robotic partial nephrectomy. Robotic zero ischemia partial nephrectomy was performed due to the suspicion of a renal malignancy. On the basis of the histopathological results, the patient was diagnosed as IPEH. A urinary ultrasound was performed on the 3rd postoperative month and a hyper echoic solid lesion, which was in the same localization, was detected. Due to the previous atypical pathological result, computed tomography (CT) guided fine-needle aspiration biopsy from the left renal mass was performed but malignant cytology was not confirmed with this biopsy. On follow-up CT done 6 months later, a persistent suspicious left renal mass, measuring 40 × 30 cm in size was detected with no change in its dimensions and appearance. Additionally, magnetic resonance imaging (MRI) scan revealed a bone lesion of 15 × 10 mm in the left hip, which was not present on previous MRI/CT scans. In view of the solid masses in the left kidney, and left hip on CT and MRI scan suspicious for a probably metastatic renal neoplasm, left radical nephrectomy via a left subcostal transperitoneal incision was performed. The ultimate pathological report of the patient was also supported the diagnosis of Masson’s tumor and any renal malignancy was not encountered The patient was discharged on the 4th postoperative day and has been followed up for 4 months without any problems. In this case, we discuss the clinical features, histopathological characteristics, and the management of Masson’s tumor of the kidney in the light of the current literature.
Keywords: Kidney, Masson’s tumor, renal mass
Introduction
Intravascular papillary endothelial hyperplasia (IPEH) or Masson’s tumor that was first described in 1923 by Pierre Masson[1] as a neoplastic lesion, is an unusual, benign, intravascular lesion characterized histologically by papillary structures lined by proliferating endothelium. On the other hand, it was delineated by Henschen[2] in 1932 as a reactive process rather than a neoplasm. Today, it is considered to be a reactive vascular proliferation related to the traumatic vascular stasis and also it is closely associated with thrombus formation in many cases.[3] The term IPEH, which was first used in 1976 by Clearkin and Enzinger[4], is the most descriptive, least confusing and the most frequently used term in the English literature.[5]
Although IPEH may occur in any blood vessel throughout the body, the most common sites of these lesions are head, neck, fingers, and trunk.[5–7] Less frequently, it has been reported in the upper respiratory and gastrointestinal tracts, skeletal muscle, hepatic system, and rarely in the urogenital tract.[6,7] IPEH of the kidney is extremely rare, with only 8 cases previously reported in the English literature.[7–9] In this article, we present a novel case of recurrent IPEH in the kidney. Additionally, the clinical features, histopathological characteristics, and the management of the Masson’s tumor of the kidney are discussed in the light of the current literature.
Case presentation
A 50-year-old male with a detection of a suspicious mass in the left kidney was referred to our center for robotic partial nephrectomy. The patient had no remarkable past medical or family history and his overall health condition was normal in addition to his physical examination. Initial laboratory workup including urinalysis, hemogram, liver and kidney function test results were found to be within normal limits. A computed tomography (CT) scan of the abdomen showed a solid renal mass on the left, measuring 40 × 30 mm, extending into the overlying left renal hilum from the surface of the lower pole (Figure 1a, b). There were some calcifications in the mass on the non-contrast enhanced CT scan (Figure 1a). Nevertheless the mass had an internal homogeneous enhancement in the delayed phase of the contrast CT (Figure 1b). The other abdominal organs including the right kidney, adrenal glands, liver and spleen were all normal in appearance.
Figure 1.
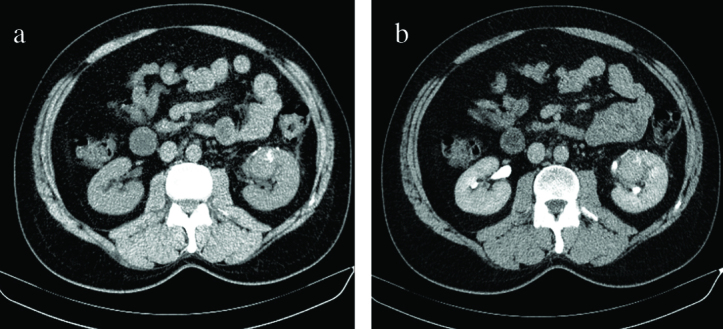
a,b. (a) A non-contrast CT scan of the abdomen showing a solid renal mass on the left side, measuring 4 × 3 cm in size. (b) A 4 × 3 cm mass is seen arising from the lower pole of the left kidney which had an internal homogeneous enhancement in the delayed phase of the contrast CT
CT: computed tomography
Because of the suspicion of a renal malignancy, robotic zero ischemia partial nephrectomy was performed. Estimated bleeding was 150 cc. The patient recovered uneventfully and was discharged on the 3rd postoperative day. On gross examination, partial nephrectomy specimen was 10.5 grams in weight and 35 × 30 × 20 mm in size. Cut sections revealed a purple-red, hemorrhagic mass that measured 20 × 12 × 8 mm close to the surgical margin. Histopathological examination of the mass showed hyalinized papillary and anastomosing channel-like structures that were lined by flat to plump endothelial cells within a large venous vessel out of the parenchyma of the kidney (Figure 2a). The lesion contained extensive fibrin, with no atypia or mitotic activity. Immunohistochemical analysis revealed diffuse strong positivity for CD34 and CD31 (Figure 2b), and cytokeratin and HMB45 negativity. On the basis of the morphologic features and immunohistochemical staining results, the patient was diagnosed as IPEH.
Figure 2.
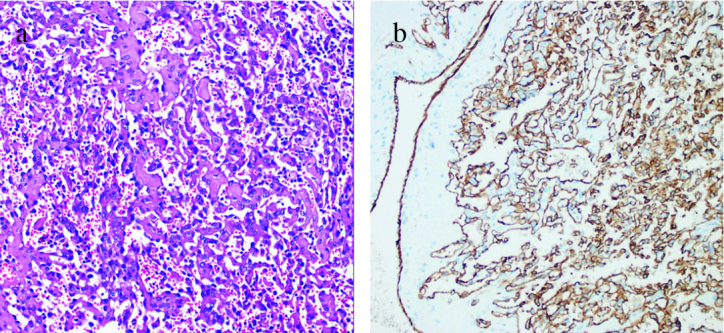
a,b. (a) Tumoural lesion composed of papillary formations and anastomosing channel-like structures (H&E, ×100). (b) Immunohistochemical staining showing diffuse and strong positivity of tumor cells for CD31 (IHC, ×100)
A urinary ultrasound (US) was performed on the 3rd postoperative month and a hyper echoic solid lesion, which was in the same localization as the mass, was detected. This lesion was confirmed by magnetic resonance imaging (MRI) scan. T1-weighted MRI displayed a hypointense solid mass compared to the renal parenchyma and T2 weighted MRI displayed a hyperintense mass which measured 40 × 30 mm in size (Figure 3a, b). Based on the results of the MRI scan, the diagnosis of a renal neoplasm was made. Due to the previous atypical pathological result, CT guided fine-needle aspiration biopsy from the left renal mass was performed. Although the aspiration was clinically significant for fibrin, blood and vascular tissues, malignant cytology was not confirmed. Therefore we decided to follow up the patient for twice a year. On follow-up CT done 6 months later, a persistent suspicious left renal mass, measuring 40 × 30 mm in size was detected with no change in its dimensions and appearance. Additionally, MRI scan revealed a bone lesion of 15 × 10 mm in the left hip, which was not present on previous MRI/CT scans. In view of the solid masses in the left kidney, left hip on CT and MRI scan suspicious for a probably metastatic renal neoplasm, a left radical nephrectomy via a left subcostal transperitoneal incision was performed. Radical nephrectomy material was 640 gr with dimensions of 20 × 12 × 7 cm including perirenal adipose tissue, while it measured 11.5 × 6.5 × 5 cm without it. Nephrectomy material also contained an ureteral segment measuring 6.5 cm in length and 0.5 in diameter. On cut sections, a well-demarcated hemorrhagic lesion measuring 3.5 cm in diameter was detected. Histologic findings of the lesion were similar to the previous diagnosis. Adrenal gland showed hyperplasia without tumoural invasion was detected. Written informed consent was obtained from patient who participated in this case. The patient was discharged on the 4rd postoperative day and followed up for 4 months without any problems.
Figure 3.
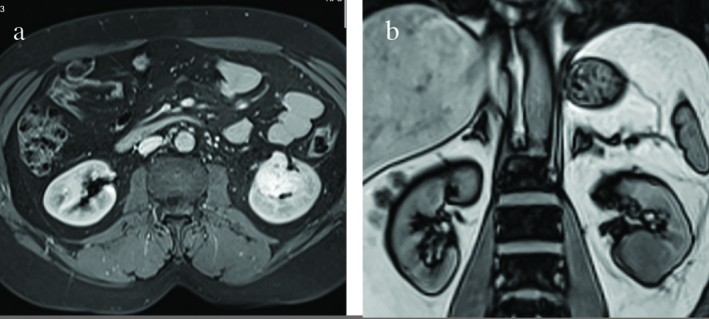
a,b. (a) On axial image of the abdomen, T2-weighted MRI displayed a hyperintense renal mass on the left, which measured 4 × 3 cm in size. (b) On a coronal T1-weighted MR image, the recurrent mass originating from the lower pole of the left kidney appears homogeneous and it is hypointense relative to the renal cortex.
MR: magnetic resonance
Discussion
Masson’s tumor of the kidney is an extremely rare lesion. The first IPEH case in the kidney was reported by Garber et al.[10] in 1990. To date, only 8 cases of renal Masson’s tumors have been reported.[7–9] including 5 (63%) male, and 3 (37%) female patients with a mean age of 47 years (range 7–63). Although the clinical presentation of patients with renal IPEH is mostly asymptomatic, macroscopic hematuria and flank pain are the most presented symptoms.[7–10] The patient had no urinary symptoms and the renal mass was discovered incidentally in our case.
There is no consensus about the radiological findings of IPEH of the kidney since renal IPEH is extremely rare. Radiological examination shows a heterogenic solid lesion with contrast enhancement. Radiologically the lesion resembles a true malignancy with degenerative changes including necrosis and thrombosis. Differential diagnosis of renal IPEH includes renal cell carcinoma, oncocytoma, hemangioma, and angiosarcoma.[7,8] Neither CT nor MRI findings can exactly differentiate renal IPEH from other benign or malignant renal masses.[7,8] Therefore surgical resection is curative for renal IPEH. While radical nephrectomy was performed in 7 of 8 patients with renal IPEH due to the suspect renal neoplasm, partial nephrectomy was done in one patient with renal IPEH who had a renal mass with a diameter of 3 cm.[7] Preoperative diagnosis of the last mentioned patient was a hemangioma.[7] There was a 4 cm mass in the left kidney in our patient. Since we consider that preoperative diagnosis of the patient was renal cell carcinoma, partial nephrectomy was performed. We wanted to check our patient with a follow-up CT scan 3 months after the operation if renal artery had been affected and/or injured at the time of partial nephrectomy, for a possible scarring around it and renal shrinkage, despite a negative tissue diagnosis of malignancy. However, his radiographic examination revealed another solid renal mass, so subsequent follow up examinations were scheduled as described above.
The precise pathogenesis of IPEH is debatable. It is believed that primary endothelial cell proliferation takes place in response to inflammation and stasis in a dilated vascular lumen.[7] Then a pseudotumoural lesion caused by endothelial proliferation with papillary formation proceeded by an accumulation of thrombotic material, which serves to make easy development of the lesion.[5,11] Although IPEH is generally found in the lumen of dilated veins, it is rarely detected in hemangiomas, hematomas, and lymphagiomas.[5] It is the intravascular endothelial proliferation that in many respects mimics an angiosarcoma. Angiosarcoma is the main neoplasm that is confused with the IPEH, because of the presence of papillary formations, anastomosing vascular channels and plump endothelial cells. A helpful point in the differential diagnosis is its intravascular location because angiosarcomas are almost never confined to a vascular lumen.[12] Apart from the usual intravascular location; the lack of necrosis, bizarre cells and high mitotic rate; fibrinous and/or hyaline appearance of the papillary stalks, and the frequent finding of residual organizing thrombi are helpful in the diagnosis of IPEH.
Prognosis is excellent for renal IPEH. Surgical resection of this lesion is satisfactory. Malignant behavior or metastasis has never been reported with renal IPEH.[7–9] Although recurrence rates in various skin cases have been documented in a range of 7–10%, any case with recurrent renal IPEH has not been reported in the English literature.[6–8] It has been considered that there is generally a concomitant vascular tumour in the recurrent cases.[13] In our case, we surprisingly detected a recurrent mass in the left kidney at the same localization with US, and MRI at the postoperative 3rd months, which was not present on preoperative radiograms. To our knowledge, we are presenting the first recurrent renal IPEH in the English literature.
In conclusion, IPEH of the kidney is extremely rare, with only 8 cases previously reported in the English literature. Radiological findings of this lesion are not clear. Therefore it is often misdiagnosed as renal cell carcinoma. Surgical resection including partial or radical nephrectomy for renal IPEH is sufficient which is associated with excellent prognosis. This is the first recurrent renal IPEH reported in the English literature. Follow-up examination should be performed after partial nephrectomy for every benign lesion to detect renal periarterial scarring and renal shrinkage.
Footnotes
Informed Consent: Written informed consent was obtained from patient who participated in this case.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – M.D.B.; Design – E.A., Y.S., A.O.O.; Supervision – M.D.B., Y.S.; Resources – E.A., Y.S., A.O.O.; Materials – E.A., Y.S.; Data Collection and/or Processing – E.A., Y.S., A.O.O.; Analysis and/or Interpretation – E.A., Y.S., M.D.B.; Literature Search – Y.S., A.O.O.; Writing Manuscript – E.A., Y.S.; Critical Review – Y.S., M.D.B.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Masson P. Hemangioendotheliomevegetantintravasculaire. Bull Soc Anat Paris. 1923;93:517–3. [Google Scholar]
- 2.Henschen P. L’ endovasculiteproliferantethrombopoietiquedans la lesion vasculaire locale. Ann Anat Pathol. 1932;9:113–1. [Google Scholar]
- 3.Liu YY, Chiang PH. Adrenal Intravascular Papillary Endothelial Hyperplasia: A Case Report and Literature Review. Urological Science. 2013;24:129–30. http://dx.doi.org/10.1016/j.urols.2013.05.002. [Google Scholar]
- 4.Clearkin KP, Enzinger FM. Intravascular papillary endothelial hyperplasia. Arch Pathol Lab Med. 1976;100:441–4. [PubMed] [Google Scholar]
- 5.Makos CP, Nikolaidou AJ. Intravascular papillary endothelial hyperplasia (Masson’s tumor) of the oral mucosa. Presentation of two cases and review. Oral Oncol Extra. 2004;40:59–62. http://dx.doi.org/10.1016/j.ooe.2003.11.001. [Google Scholar]
- 6.Meadows MC, Sun X, Dardik M, Tarantino DR, Chamberlain RS. Intraabdominal Intravascular Papillary Endothelial Hyperplasia (Masson’s Tumor): A Rare and Novel Cause of Gastrointestinal Bleeding. Case Rep Gastroenterol. 2010;4:124–32. doi: 10.1159/000294148. http://dx.doi.org/10.1159/000294148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johraku A, Miyanaga N, Sekido N, Ikeda H, Michishita N, Saida Y, et al. A case of intravascular papillary endothelial hyperplasia (Masson’s tumor) arising from renal sinus. Jpn J Clin Oncol. 1997;27:433–6. doi: 10.1093/jjco/27.6.434. http://dx.doi.org/10.1093/jjco/27.6.434. [DOI] [PubMed] [Google Scholar]
- 8.Akhtar M, Aslam M, Al-Mana H, Bamefleh H, Al-Khateeb SS, Lindstedt E. Intravascular papillary endothelial hyperplasia of renal vein: report of 2 cases. Arch Pathol Lab Med. 2005;129:516–9. doi: 10.5858/2005-129-516-IPEHOR. [DOI] [PubMed] [Google Scholar]
- 9.Pelosi G, Sonzogni A, Viale G. Intravascular papillary endothelial hyperplasia of the renal vein. Int J Surg Pathol. 2011;19:518–20. doi: 10.1177/1066896909341800. http://dx.doi.org/10.1177/1066896909341800. [DOI] [PubMed] [Google Scholar]
- 10.Garber BB, Prestipino AJ, Pollack HM, Levine SR, Whitmore KE. Masson’s tumor of the kidney: a new renal lesion. J Urol. 1990;143:344–6. doi: 10.1016/s0022-5347(17)39956-1. [DOI] [PubMed] [Google Scholar]
- 11.Bologna-Molina R, Amezcua-Rosas G, Guardado-Luevanos I, Mendoza-Roaf PL, González-Montemayor T, Molina-Frechero N. Intravascular Papillary Endothelial Hyperplasia (Masson’s Tumor) of the Mouth - A Case Report. Case Rep Dermatol. 2010;2:22–6. doi: 10.1159/000279656. http://dx.doi.org/10.1159/000279656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldblum JR, Folpe AL, Weiss S, editors. Enzinger & Weiss’s Soft Tissue Tumors. 6th ed. Vol. 671 Philadelphia: Elsevier Saunders; 2014. Benign vascular tumors and malformations. [Google Scholar]
- 13.Kawashima A, Johsen T, Murayama S, Russel WJ. Intravascular papillary endothelial hyperplasia of the adrenal gland. Br J Radiol. 1986;59:610–3. doi: 10.1259/0007-1285-59-702-610. http://dx.doi.org/10.1259/0007-1285-59-702-610. [DOI] [PubMed] [Google Scholar]


