Abstract
Chronic intestinal pseudo-obstruction (CIPO) is a rare but life-threatening disease characterized by severe intestinal dysmotility. Histopathologic studies in CIPO patients have identified several different mechanisms that appear to be involved in the dysmotility, including defects in neurons, smooth muscle, or interstitial cells of Cajal. Currently there are few mouse models of the various forms of CIPO. We generated a mouse with a point mutation in the RNA recognition motif of the Nup35 gene, which encodes a component of the nuclear pore complex. Nup35 mutants developed a severe megacolon and exhibited a reduced lifespan. Histopathologic examination revealed a degenerative myopathy that developed after birth and specifically affected smooth muscle in the colon; smooth muscle in the small bowel and the bladder were not affected. Furthermore, no defects were found in enteric neurons or interstitial cells of Cajal. Nup35 mice are likely to be a valuable model for the subtype of CIPO characterized by degenerative myopathy. Our study also raises the possibility that Nup35 polymorphisms could contribute to some cases of CIPO.
Chronic intestinal pseudo-obstruction (CIPO) is a rare and debilitating condition in which deficiencies in intestinal peristalsis mimic a mechanical obstruction.1 CIPO can be caused by defects in the enteric nervous system (neuropathy), interstitial cells of Cajal (ICCs), and/or intestinal smooth muscle (myopathy).1, 2, 3, 4, 5 Although CIPO can occur secondary to other clinical complications, or downstream of environmental factors, certain forms of CIPO have a heritable genetic component.1, 6 For example, mutations in genes such as SOX10, FLNA, L1CAM, RAD21, TYMP, ACTG2, and POLG have all been linked to CIPO pathology.3, 7, 8, 9, 10, 11, 12 Nonetheless, the genetic cause of a large portion of heritable CIPO cases remains unknown.3
Mouse models have previously been used to investigate the role of genetic pathways in CIPO pathology.13 Although data from mouse models have implicated the disruption of a number of genetic pathways in intestinal neuropathy, there is a paucity of models that mimic clinical intestinal smooth muscle myopathy. We report a novel mouse model of CIPO associated with a mutation in the RNA recognition motif (RRM) of nucleoporin (NUP)-35. NUP35 (also called NUP53) is part of the multiprotein nuclear pore complex (NPC), which is composed of multiple copies of approximately 30 different NUP proteins forming a complex >40 MDa in size.14 The NPC plays a crucial role in allowing diffusion of small molecules and controlled movement of larger factors in and out of the nucleus. Proper NPC formation is also important for normal nuclear morphology. NUP35 is indispensable for vertebrate NPC formation and nuclear integrity,15, 16, 17, 18, 19 and NUP35 dimerization via its RRM is crucial for its function.19, 20
Here we characterize the colonic changes associated with the Nup35-mutant CIPO mouse model, and show that the mice have colon-specific smooth muscle myopathy without detectable deficits in enteric neurons and with persistence of ICCs. These data suggest that the Nup35-mutant mouse represents a novel model of CIPO associated with degenerative myopathy.
Materials and Methods
Mice
Nup35F192L/F192L mice were isolated from the Australian Phenomics Facility Phenome Bank at the Australian National University (Canberra, ACT, Australia). All animals used in this study were cared for and used in accordance with protocols approved by the Australian National University Animal Experimentation Ethics Committee and the current guidelines from the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes.
NUP35 Sequence Alignment and Protein Modeling
NUP35 sequences were isolated from UniProt (http://www.uniprot.org, last accessed April 1, 2016) and aligned using ClustalW software package version 2.1 (Conway Institute UCD Dublin, Dublin, Ireland; http://www.clustal.org/clustal2).21 RRM sequences and accession numbers are provided in Supplemental Table S1. Protein structure visualization was performed with the UCSF Chimera software package version 1.8 (Resource for Biocomputing, Visualization, and Informatics, University of California, San Francisco, CA).22
IHC Analysis and Histopathology
Mice were sacrificed by cervical dislocation, and the bladder, colon, and small intestine were removed and fixed overnight in formalin. Tissue for hematoxylin and eosin and Masson's trichrome staining was processed as described previously.23 Tissue from three randomly chosen sections of ileum and distal colon were graded by a blinded observer (J.B.F.) using a ×40 objective lens and 8-point graded scales (Table 1).
Table 1.
Colon and Ileum Histologic Examination Scoring
| Region | Layer | Criteria | Score |
|---|---|---|---|
| Colon | Longitudinal muscle | Layered muscle cells with few collagen strands between, <10% collagen | 0 |
| Loss of muscle cells, with prominent collagen between, 10%–50% collagen | 1 | ||
| Substantial loss of muscle cells, some areas may have no muscle cells. Prominent collagen in muscle layer, >50% collagen | 2 | ||
| Circular muscle | Muscle cells plump, eight or more layers, <10% collagen | 0 | |
| Muscle cells smaller and fewer, only four to seven layers, 10%–50% collagen | 1 | ||
| Muscle cells smaller and substantially fewer, less than four layers, >50% collagen | 2 | ||
| Submucosa | Thin with fibroblasts, no or extremely rare macrophages | 0 | |
| Thickened with some macrophages | 1 | ||
| Thickened more than twofold normal with numerous macrophages | 2 | ||
| Mucosa | Goblet cells small at bases of glands and in gland walls, rare at surface | 0 | |
| Goblet cells swollen and commonly observed at surface as well as at bases and in walls of glands | 1 | ||
| Ileum | Longitudinal muscle | Layered muscle cells with few collagen strands between, <10% collagen | 0 |
| Loss of muscle cells, with prominent collagen between, 10%–50% collagen | 1 | ||
| Substantial loss of muscle cells, some areas may have no muscle cells. Prominent collagen in muscle layer, >50% collagen | 2 | ||
| Circular muscle | Muscle cells plump, five or more layers, <10% collagen | 0 | |
| Muscle cells smaller and fewer, only two to four layers, 10% to 50% collagen | 1 | ||
| Muscle cells smaller and substantially fewer, less than layers, >50% collagen | 2 | ||
| Submucosa | Thin with fibroblasts, no or extremely rare macrophages | 0 | |
| Thickened with some macrophages | 1 | ||
| Thickened more than twofold normal with numerous macrophages | 2 | ||
| Mucosa | Mucosa intact | 0 | |
| Disruptions to the mucosa | 1 |
Scoring system designed to be objective, 8-point scale for each region [0 (normal); 7 (severely affected)].
Tissue for whole-mount immunohistochemistry analysis was opened along the mesenteric border and processed for immunohistochemistry analysis, as described previously,24 using the following primary antisera: rabbit anti-Kit (1:100; Merck Millipore, Bayswater, Australia), mouse anti–neuronal class III β-tubulin (1:2000; Covance Princeton, NJ), sheep anti–neuronal nitric oxide synthase (1:200025), and rat anti-F4/80 (1:5026). All primary antisera have been characterized previously.25, 27, 28, 29 Secondary antisera (all from Jackson ImmunoResearch Laboratories, West Grove, PA) were: donkey anti-rat Alexa488 (1:100), donkey anti-rabbit Alexa647 (1:400), donkey anti-mouse Alexa594 (1:200), and donkey anti-sheep Alexa594 (1:100). No staining was observed in preparations in which the primary antisera were omitted. Whole-mount preparations were imaged on Pascal or LSM 800 confocal microscopes (Carl Zeiss, Oberkochen, Germany).
Data Analysis
Graphing and data analysis were conducted using Prism software package version 7 (GraphPad Software, San Diego, CA).
Results
Nup35F192L/F192L-Mutant Mice Exhibit Mortality Associated with Megacolon
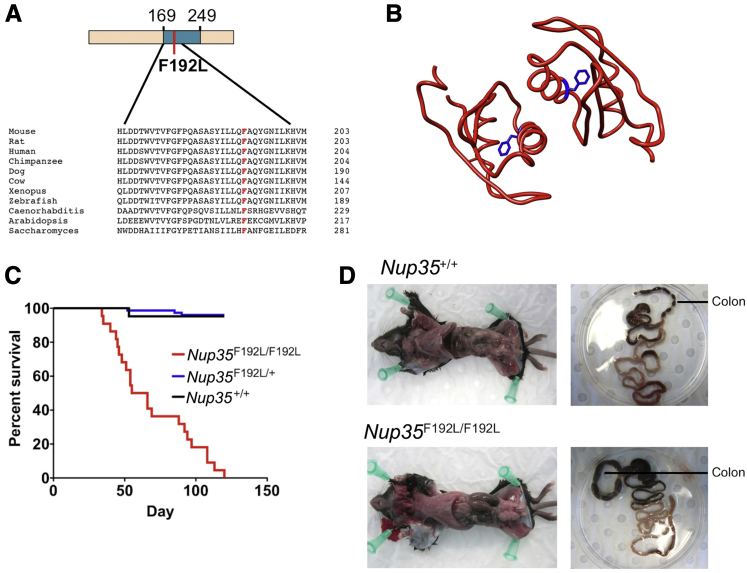
As part of a larger ethylnitrosourea mutagenesis screen for mutant mice with immune phenotypes, mice with an F192L mutation in Nup35 were identified and isolated from the Australian Phenomics Facility Phenome Bank, and the mutation was bred to homozygosity. The crystal structure for the NUP35 RRM dimer has been solved,20 and F192, which is highly conserved (Figure 1A), lies on the α helix of the dimer interaction interface (Figure 1B). A substitution of phenylalanine for leucine, as occurs in the F192L mutation, will delete an aromatic ring that is buried within the hydrophobic core of the RRM (Figure 1B) and is predicted to be highly damaging [1 (PolyPhen); 0 (SIFT [sorting intolerant from tolerant])]. This mutation will thus likely disrupt the RRM interaction interface and thereby interfere with NUP35 dimerization and function.
Figure 1.
Conservation and structural positioning of the nucleoporin (NUP)-35 F192 residue, and mortality and megacolon in Nup35-mutant mice. A: Sequence alignment showing NUP35 RNA recognition motif (RRM) amino acid conservation between species, including the F192 residue (red). Schematic of mouse NUP35 protein showing the RRM (blue, amino acids 169 to 249), and the F192L-mutated residue (red). B: NUP35 RRM dimer crystal structure with F192 (blue). C: Survival in Nup35F192L/F192L (n = 26), Nup35F192L/+ (n = 76), and Nup35+/+ (n = 21), mice. D: Intestines of a Nup35F192L/F192L mouse (bottom) and wild-type littermate (top) within the body cavity (left) and after isolation (right).
Given the essential role of NUP35 in NPC formation and nuclear integrity,15, 16, 17, 18, 19 damaging mutations in the gene encoding this protein could be anticipated to cause embryonic lethality. However, although homozygous mutant mice were born below the expected 25% ratio, viable homozygous Nup35F192L/F192L-mutant mice were still obtained in appreciable numbers [Nup35F192L/F192L, 46 mice (15%); Nup35+/+, 83 mice (28%); and Nup35F192L/+, 171 mice (57%)]. Homozygous mutant mice failed to display a detectable steady-state immune phenotype; however, mortality was observed within this strain, with 50% of mice failing to survive beyond 55 days of age and no mice surviving beyond 120 days of age (Figure 1C). These high mortality rates were not observed in heterozygous Nup35F192L/±mutant mice (Figure 1C). Postmortem examination of mutant mice revealed substantial megacolon (Figure 1D), with no obvious superficial pathology noted in the other organs examined (lung, liver, heart, bladder, spleen, thymus, kidney). The small intestine appeared normal; however, fecal impaction was observed within the colon, without any obvious physical obstruction. Thus, Nup35F192L/F192L-mutant mice develop symptoms consistent with human CIPO that appear to cause mortality.
Ethylnitrosourea randomly introduces point mutations into the genome, and each strain may carry a number of passenger mutations that could be involved in the development of any observed phenotype. Based on exome sequencing data of the pedigree from which the Nup35F192L/F192L-mutant mice were derived, two other exome mutations existed on chromosome 2 within this pedigree that could be loosely linked to the Nup35 locus and may potentially contribute to the observed phenotype. These mutant alleles (in the genes Cst10 and Fbn1) were subsequently found to be present within the Nup35-mutant mouse colony. To confirm that these alleles did not contribute to the observed phenotype, a Nup35-mutant mouse substrain was established that lacked these two mutations, and mortality was still observed, with histopathology comparable to that of the parental mouse strain (see Nup35F192L/F192L-Mutant Mice Display Degenerative Mid- and Distal Colonic Smooth Muscle Loss; data not shown). Thus, the Nup35 F192L mutation appears to be the mutation causative of the observed CIPO phenotype.
Nup35F192L/F192L-Mutant Mice Display Degenerative Mid- and Distal Colonic Smooth Muscle Loss
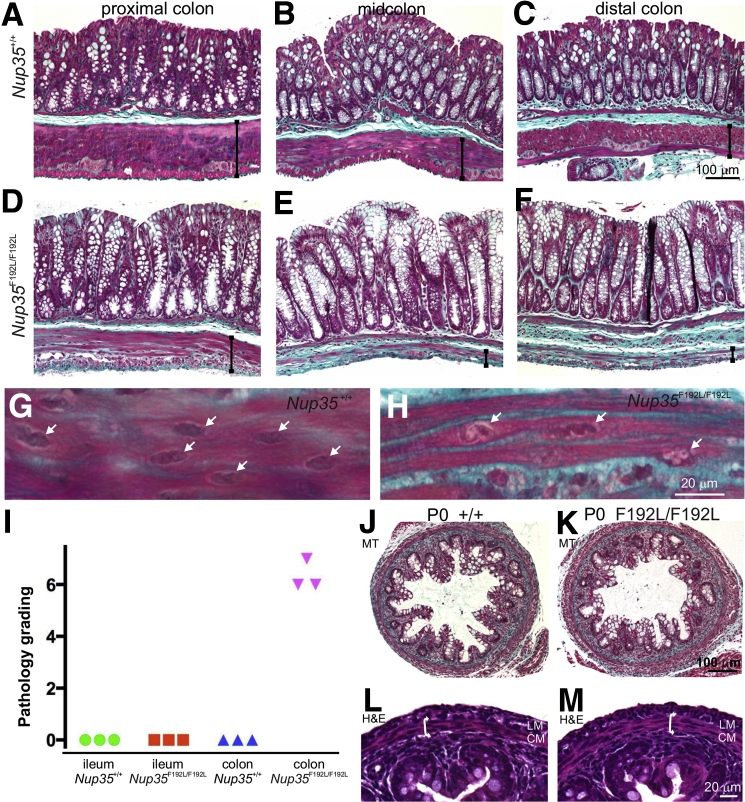
CIPO can be caused by neuropathy, myopathy, and/or defects in ICCs, so we next used histopathology and immunohistochemistry analysis to determine the cause of bowel obstruction in Nup35 mutants. Transverse sections of the ileum, and proximal, mid-, and distal colon of 6- to 8-week-old Nup35F192L/F192L (n = 5), Nup35F192L/+ (n = 2), and Nup35+/+ (n = 6) littermates were stained with hematoxylin and eosin or Masson's trichrome. No differences were observed in the ilea of Nup35F192L/F192L mice compared to those of Nup35F192L/+ or Nup35+/+ mice. However, the distal and midcolon of homozygous Nup35F192L/F192L mice exhibited substantial loss of muscle cells and replacement by connective tissue in the muscularis externa, but not in the muscularis mucosae (Figure 2, A–F). In some areas, no muscle cells were observed in the longitudinal and circular muscle layers, although the layering was still defined by connective tissue. The nuclei of smooth muscle cells in the circular muscle layer of the colon of Nup35F192L/+ and Nup35+/+ mice were very uniform in appearance (Figure 2G). In contrast, the nuclei of the remaining muscle cells in Nup35F192L/F192L mice were very variable; some had chromatin condensations (Figure 2H), whereas others were indistinguishable from those in control mice. There were also patches of thick adherent mucus, and increased numbers of surface goblet cells that appeared to displace surface enterocytes in the mid- and distal colon. There was also thickening of the submucosa of Nup35F192L/F192L mice, with greater numbers of macrophages being present.
Figure 2.
Smooth muscle loss from the external muscle layers of the colon of adult, but not newborn, Nup35-mutant mice. A–F: Masson's trichrome staining of the proximal, mid-, and distal colon from 6- to 8-week-old wild-type (A–C) and Nup35F192L/F192L (D–F) mice. There is a dramatic loss of muscle cells from the circular and longitudinal muscle layers, and replacement by connective tissue (green), in the mid- and distal colon of the mutant mice. There is a less dramatic loss of muscle cells in the proximal colon. The widths of the external muscle layers are indicated by vertical lines. Note greater numbers of surface goblet cells (E and F) and thickened submucosa (F). G and H: High magnification images of circular muscle cells. Although the nuclei (arrows) of WT mice are uniform in appearance (G), many nuclei (arrows) in the remaining smooth muscle cells in the mutant have chromatin condensations (H). I: Pathology grading of distal colon and ileum tissue from three randomly chosen Nup35+/+ and Nup35F192L/F192L mice [0 (normal); 7 (severely affected)], which included assessments of the longitudinal and circular muscle layers, the submucosa and mucosa (Table 1). Although the colons of all mutants were graded as severely affected, the ileum was graded normal. J–M: Masson's trichrome (MT; J and K) and hematoxylin and eosin (H&E) (L and M) staining of transverse sections of distal colon from newborn wild-type (+/+, J and L) and Nup35F192L/F192L (mutant, K and M) mice. There are no detectable differences between the longitudinal muscle layer (LM) and circular muscle layer (CM; double arrow) between mutants and wild-type littermates.
The proximal colon of Nup35F192L/F192L mice displayed only a minor myopathy (Figure 2, A and D), but like more distal regions, there was also a thick, adherent mucus and more surface goblet cells. The histologic characteristics of distal colon and ileum of Nup35+/+ and Nup35F192L/F192L mice were quantified by a blinded observer (J.B.F.) using 8-point scales [0 (normal); 7 (severely affected)] that included appraisals of the longitudinal and circular muscle layers, the submucosa, and mucosa (Table 1). The ilea of Nup35F192L/F192L mice were graded normal, whereas the distal colons of Nup35F192L/F192L mice were graded as severely affected (Figure 2I). The bladder of homozygous Nup35 mutants appeared normal, with no detectable changes in the smooth muscle cells (data not shown). No defects were detected in the gross morphology or histologic appearance of the mutant heart (data not shown). Striated muscle within the pelvic floor also appeared normal (data not shown), suggesting that the myopathy was highly restricted to the external smooth muscle within the colon.
To determine whether the colonic myopathy is congenital or degenerative, sections of colon from newborn Nup35F192L/F192L (n = 5), Nup35F192L/+ (n = 3), and Nup35+/+ (n = 3) littermates were stained with hematoxylin and eosin or Masson's trichrome. The external muscle layers of Nup35F192L/F192L newborn mice were similar in appearance to that of littermates, and no histologic differences were detected between newborn homozygous mutant, heterozygous, and wild-type mice, including the appearance of nuclei in the external muscle layers (Figure 2, J–M).
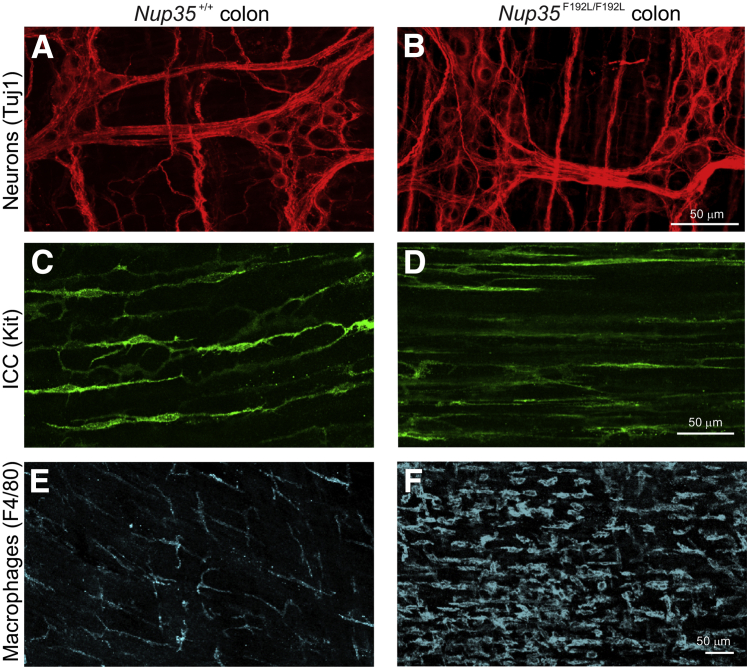
Like smooth muscle cells, ICCs, macrophages in the muscularis layers and enteric neurons, play an essential role in gut motility.30, 31 For example, a congenital absence of enteric neurons from the distal bowel results in bowel obstruction and megacolon.32, 33 Whole-mount preparations of the external muscle layers of the ileum and colon from 6- to 8-week-old mice of Nup35F192L/F192L, Nup35F192L/+, and Nup35+/+ littermates were processed for immunohistochemistry analysis using antisera to the pan-neuronal marker, neuronal class III β-tubulin 1; the enteric neuron subtype marker, neural nitric oxide synthase; the ICC marker, Kit; and the macrophage marker, F4/80.34 Enteric neurons (Figure 3, A and B) and the ICC network (Figure 3, C and D) were present along the entire bowel, although ICCs in the circular muscle layer of the colon of the mutants had fewer fine processes than did those in wild-type mice (Figure 3D). There were many more F4/80+ macrophages in the external muscle and associated with myenteric ganglia, particularly in the mid- and proximal colon of homozygous Nup35 mutants compared to heterozygote and wild-type littermates (Figure 3, E and F). In addition, macrophages were more rounded in shape in Nup35F192L/F192L-mutants compared to those in Nup35F192L/+ and Nup35+/+ littermates (Figure 3, E and F). Collectively, these data show that the megacolon in homozygous Nup35-mutant mice appears to be due to a degenerative myopathy of the colon.
Figure 3.
Neurons and interstitial cells of Cajal (ICCs) are present along the colon of Nup35F192L/F192L-mutant mice. A–F: Whole-mount preparations of external muscle and myenteric plexus from the distal colon (A and B) and midcolon (C–F) of 6- to 8-week-old wild-type (WT; A–C) and Nup35-mutant (D–F) mice after immunostaining for the pan neuron marker, neuronal class III β-tubulin (Tuj)-1 (A and B); the ICC marker, Kit (C and D); and the macrophage marker, F4/80 (E and F). A and D: Tuj1+ myenteric neurons in the distal colon. C and D: Although circular smooth muscle cells have been largely replaced by connective tissue, Kit+ ICCs are present in the circular muscle layer of mutants. However, ICCs in the mutants have simpler morphology and fewer processes than do ICCs in the circular muscle of WT mice. E and F: In WT mice, F4/80+ macrophages are present in the external muscle and are stellate in shape, as described previously.26, 34
Discussion
We describe a novel mouse model of colonic myopathy and CIPO associated with a mutation in Nup35. In several animal models, intestinal distension that is not due to a muscle defect results in secondary muscle hypertrophy.35, 36, 37 Given the absence of any muscle hypertrophy, it is highly likely that the colonic myopathy in the Nup35-mutant mouse is the primary cause of obstruction and distension.
The phenotype associated with the Nup35 mutation is surprising at two levels. First, NUP35 is reported to be indispensable for NPC formation and nuclear integrity,15, 16, 17, 18, 19 and it is essential for Caenorhabditis elegans embryonic development,18 so it is surprising that viable mutant mice were obtained. Second, the phenotype observed is highly selective, with smooth muscle myopathy observed only in the muscularis externa of the colon despite no evidence of tissue-specific NUP35 expression within existing expression databases such as BioGPS (http://biogps.org, last accessed April 1, 2016) and the Human Protein Atlas (http://www.proteinatlas.org, last accessed April 1, 2016). Overall, these data reveal an unexpected link between the NPC and CIPO, and suggest that Nup35 polymorphisms could contribute to disease.
The phenotype within the Nup35-mutant mouse appears distinct from other gene-deficient myopathy models and, to our knowledge, this is the first gene-deficient mouse model demonstrating spontaneous degenerative smooth muscle myopathy and associated fibrosis. Previous models of myopathy associated with CIPO-like disease have involved inducible deletion of the transcriptional regulator, serum response factor, from smooth muscle,38, 39 or deletion of the smooth muscle–restricted factor smoothelin A.40 In both cases, the CIPO phenotype was associated with loss of smooth muscle cell contractility rather than active loss of smooth muscle cells; serum response factor deletion failed to affect smooth muscle cell numbers, whereas smoothelin A loss triggered smooth muscle cell hypertrophy. The large phenotypic differences between the Nup35F192L/F192L mouse and the other models described earlier make it unlikely that Nup35 deficiency causes myopathy by disrupting serum response factor or smoothelin A, implicating a potentially novel pathway in the Nup35F192L/F192L mouse phenotype.
As smooth muscle fibrosis has been reported in a clinical case of hollow visceral myopathy (a form of CIPO associated with degenerative myopathy),41 the Nup35F192L/F192L-mutant mouse represents a potentially valuable model of the disease in humans. Moreover, unlike patients with the congenital neuropathy, Hirschsprung disease,42, 43 CIPO patients with degenerative myopathy are variable in age.3 The colonic myopathy in Nup35-mutant mice was not apparent at birth and so is consistent with the degenerative myopathy seen in a subpopulation of CIPO patients.
The histopathologic characteristics of CIPO vary between patients and include neuropathy, loss of ICCs, as well as degenerative myopathy.3 Mutations linked to CIPO in humans are often associated with neuropathy rather than myopathy (eg, SOX10 and RAD21),3, 12 and so are unlikely to be related to the Nup35-mutant phenotype. The mechanism by which NUP35 deficiency triggers smooth muscle myopathy thus remains unclear. Mutations in FLNA are associated with myopathy in X-linked CIPO8; however, such myopathy is associated with abnormal (additional) layering of the small intestinal muscularis propria rather than smooth muscle cell loss.11 Myopathy associated with fibrosis is observed in patients with mitochondrial neurogastrointestinal encephalomyopathy, a multifactorial disease with CIPO symptoms.9 These patients bear mutations in TYMP, which lead to mitochondrial depletion from smooth muscle that likely causes myopathy.9 There is no reported association between either NUP35 and TYMP, or NUP35 and other mitochondrial components; however, we cannot rule out that mitochondrial abnormalities contribute to myopathy in these mice.
One possible explanation for the phenotype is that nuclear abnormalities associated with NUP35 deficiency cause myopathy. Polymorphisms in LMNA, encoding lamin A, a structural factor that lines the nucleus, have been linked to striated muscle wasting and muscular dystrophy.44, 45 Lamin A is also a relatively widely expressed protein, so the reasons for the tissue-selective effect of LMNA polymorphisms on striated muscle maintenance remain unclear. One theory is that the more fragile nucleus that results from lamin A loss is susceptible to rupture in muscle due to mechanical stress.44, 45 The subsequent loss of nuclear integrity is postulated to cause cell death. The NPC is known to associate with lamins, and interestingly a direct association between NUP35 and lamin B has been reported.17 Furthermore, NUP35 depletion leads to nuclear abnormalities that closely resemble those seen in LMNA-mutant cells.17 It is thus possible that NUP35 deficiencies cause disrupted nuclear morphology that triggers cell death in contractile smooth muscle cells. Consistent with this idea, we did observe some degree of abnormal nuclear morphology within the mutant smooth muscle cells, although these changes could simply be due to ongoing apoptosis. However, some degree of tissue specificity is still required for this mechanism to operate, as altered nuclear morphology should also cause striated muscle loss, which we failed to observe in Nup35-mutant mice. Furthermore, cell loss was observed in only a small subset of smooth muscle cells within the body (colonic smooth muscle cells), suggesting a complex, highly tissue-specific effect.
In some cases, CIPO is associated with inflammation, predominantly of the enteric ganglia, which exhibit inflammatory neuropathy.1 Neither inflammation nor enteric nervous system damage was observed in tissue from the Nup35-mutant mice. However, we did observe increased numbers of macrophages in the affected external muscle and the adjacent submucosa, which is possibly a response to smooth muscle cell degeneration. Changes in the morphology of ICCs may also be secondary to the myopathy and the loss of muscle cells. ICCs form gap junctions with smooth muscle cells,46 and the simpler morphology of ICCs in Nup35 mutants is likely to be due to reduced contacts with smooth muscle cells. The accumulation of gut contents, pressure on the gut wall, and failed propulsion may have induced the increased numbers of surface goblet cells and the adherent mucus that was observed.
As NUP35 does not exhibit clear tissue-specific expression, it is unclear why we observed such a selective phenotype in Nup35-mutant mice. There is emerging evidence of heterogeneity within NPC composition between tissues, which is highlighted by the tissue-specific pathologies associated with loss of other NUPs.14 It is thus possible that the NPC present within colonic smooth muscle is particularly susceptible to NUP35 depletion, leading to selective myopathy within this cell type. Regardless of the mechanism, the Nup35F192L/F192L-mutant mouse phenotype has revealed a novel pathway involved in smooth muscle myopathy that may contribute to CIPO in humans.
Acknowledgments
We thank the Australian Phenomics Facility staff for assistance with animal work, and the Histopathology and Organ Pathology Service of the Australian Phenomics Network.
I.A.P. conceived, designed, funded, and supervised the study, acquired and interpreted data, and wrote the manuscript; L.A.S. acquired, analyzed, and interpreted data and revised the manuscript; A.M.D.L. and S.M.F. acquired, analyzed, and interpreted data; Y.S., L.A.M., and D.R.H. supervised the study; C.C.G. conceived, designed, funded, and supervised the study and revised the manuscript; H.M.Y. conceived, designed, funded, and supervised the study, acquired and interpreted data, and wrote the manuscript; and J.B.F. conceived, designed, and funded the study, acquired, analyzed, and interpreted data, and revised the manuscript.
Footnotes
Supported by a National Health and Medical Research Council (NHMRC) C.J. Martin Fellowship (I.A.P.), NHMRC Program grant 1016953 and NIH grant AI100627 (C.C.G.), NHMRC Senior Research Fellowship APP1002506 (H.M.Y.), and NHMRC Project grants APP1079234 (H.M.Y., L.A.S.) and APP1005811 (J.B.F.). Protein structure visualization was performed with the UCSF Chimera package, which was developed by the Resource for Biocomputing, Visualization and Informatics at the University of California, San Francisco (supported by NIGMSP41-GM103311).
I.A.P. and L.A.S. contributed equally to this work.
I.A.P., C.C.G., H.M.Y., and J.B.F. contributed equally to this work as senior authors.
Disclosures: None declared.
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.ajpath.2016.04.016.
Contributor Information
Ian A. Parish, Email: ian.parish@anu.edu.au.
Heather M. Young, Email: h.young@unimelb.edu.au.
Supplemental Data
References
- 1.De Giorgio R., Cogliandro R.F., Barbara G., Corinaldesi R., Stanghellini V. Chronic intestinal pseudo-obstruction: clinical features, diagnosis, and therapy. Gastroenterol Clin North Am. 2011;40:787–807. doi: 10.1016/j.gtc.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Antonucci A., Fronzoni L., Cogliandro L., Cogliandro R.F., Caputo C., De Giorgio R., Pallotti F., Barbara G., Corinaldesi R., Stanghellini V. Chronic intestinal pseudo-obstruction. World J Gastroenterol. 2008;14:2953–2961. doi: 10.3748/wjg.14.2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonora E., Bianco F., Cordeddu L., Bamshad M., Francescatto L., Dowless D., Stanghellini V., Cogliandro R.F., Lindberg G., Mungan Z., Cefle K., Ozcelik T., Palanduz S., Ozturk S., Gedikbasi A., Gori A., Pippucci T., Graziano C., Volta U., Caio G., Barbara G., D'Amato M., Seri M., Katsanis N., Romeo G., De Giorgio R. Mutations in RAD21 disrupt regulation of APOB in patients with chronic intestinal pseudo-obstruction. Gastroenterology. 2015;148:771–782.e11. doi: 10.1053/j.gastro.2014.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clarke C.M., Plata C., Cole B., Tsuchiya K., La Spada A.R., Kapur R.P. Visceral neuropathy and intestinal pseudo-obstruction in a murine model of a nuclear inclusion disease. Gastroenterology. 2007;133:1971–1978. doi: 10.1053/j.gastro.2007.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knowles C.H., Lindberg G., Panza E., De Giorgio R. New perspectives in the diagnosis and management of enteric neuropathies. Nat Rev Gastroenterol Hepatol. 2013;10:206–218. doi: 10.1038/nrgastro.2013.18. [DOI] [PubMed] [Google Scholar]
- 6.Stanghellini V., Cogliandro R.F., de Giorgio R., Barbara G., Salvioli B., Corinaldesi R. Chronic intestinal pseudo-obstruction: manifestations, natural history and management. Neurogastroenterol Motil. 2007;19:440–452. doi: 10.1111/j.1365-2982.2007.00902.x. [DOI] [PubMed] [Google Scholar]
- 7.Bott L., Boute O., Mention K., Vinchon M., Boman F., Gottrand F. Congenital idiopathic intestinal pseudo-obstruction and hydrocephalus with stenosis of the aqueduct of Sylvius. Am J Med Genet A. 2004;130A:84–87. doi: 10.1002/ajmg.a.30793. [DOI] [PubMed] [Google Scholar]
- 8.Gargiulo A., Auricchio R., Barone M.V., Cotugno G., Reardon W., Milla P.J., Ballabio A., Ciccodicola A., Auricchio A. Filamin A is mutated in X-linked chronic idiopathic intestinal pseudo-obstruction with central nervous system involvement. Am J Hum Genet. 2007;80:751–758. doi: 10.1086/513321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giordano C., Sebastiani M., De Giorgio R., Travaglini C., Tancredi A., Valentino M.L., Bellan M., Cossarizza A., Hirano M., d'Amati G., Carelli V. Gastrointestinal dysmotility in mitochondrial neurogastrointestinal encephalomyopathy is caused by mitochondrial DNA depletion. Am J Pathol. 2008;173:1120–1128. doi: 10.2353/ajpath.2008.080252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holla O.L., Bock G., Busk O.L., Isfoss B.L. Familial visceral myopathy diagnosed by exome sequencing of a patient with chronic intestinal pseudo-obstruction. Endoscopy. 2014;46:533–537. doi: 10.1055/s-0034-1365142. [DOI] [PubMed] [Google Scholar]
- 11.Kapur R.P., Robertson S.P., Hannibal M.C., Finn L.S., Morgan T., van Kogelenberg M., Loren D.J. Diffuse abnormal layering of small intestinal smooth muscle is present in patients with FLNA mutations and X-linked intestinal pseudo-obstruction. Am J Surg Pathol. 2010;34:1528–1543. doi: 10.1097/PAS.0b013e3181f0ae47. [DOI] [PubMed] [Google Scholar]
- 12.Pingault V., Girard M., Bondurand N., Dorkins H., Van Maldergem L., Mowat D., Shimotake T., Verma I., Baumann C., Goossens M. SOX10 mutations in chronic intestinal pseudo-obstruction suggest a complex physiopathological mechanism. Hum Genet. 2002;111:198–206. doi: 10.1007/s00439-002-0765-8. [DOI] [PubMed] [Google Scholar]
- 13.De Giorgio R., Seri M., van Eys G. Deciphering chronic intestinal pseudo-obstruction: do mice help to solve the riddle? Gastroenterology. 2007;133:2052–2055. doi: 10.1053/j.gastro.2007.10.060. [DOI] [PubMed] [Google Scholar]
- 14.Raices M., D'Angelo M.A. Nuclear pore complex composition: a new regulator of tissue-specific and developmental functions. Nat Rev Mol Cell Biol. 2012;13:687–699. doi: 10.1038/nrm3461. [DOI] [PubMed] [Google Scholar]
- 15.Eisenhardt N., Redolfi J., Antonin W. Interaction of Nup53 with Ndc1 and Nup155 is required for nuclear pore complex assembly. J Cell Sci. 2014;127:908–921. doi: 10.1242/jcs.141739. [DOI] [PubMed] [Google Scholar]
- 16.Hawryluk-Gara L.A., Platani M., Santarella R., Wozniak R.W., Mattaj I.W. Nup53 is required for nuclear envelope and nuclear pore complex assembly. Mol Biol Cell. 2008;19:1753–1762. doi: 10.1091/mbc.E07-08-0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawryluk-Gara L.A., Shibuya E.K., Wozniak R.W. Vertebrate Nup53 interacts with the nuclear lamina and is required for the assembly of a Nup93-containing complex. Mol Biol Cell. 2005;16:2382–2394. doi: 10.1091/mbc.E04-10-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodenas E., Klerkx E.P., Ayuso C., Audhya A., Askjaer P. Early embryonic requirement for nucleoporin Nup35/NPP-19 in nuclear assembly. Dev Biol. 2009;327:399–409. doi: 10.1016/j.ydbio.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 19.Vollmer B., Schooley A., Sachdev R., Eisenhardt N., Schneider A.M., Sieverding C., Madlung J., Gerken U., Macek B., Antonin W. Dimerization and direct membrane interaction of Nup53 contribute to nuclear pore complex assembly. EMBO J. 2012;31:4072–4084. doi: 10.1038/emboj.2012.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Handa N., Kukimoto-Niino M., Akasaka R., Kishishita S., Murayama K., Terada T., Inoue M., Kigawa T., Kose S., Imamoto N., Tanaka A., Hayashizaki Y., Shirouzu M., Yokoyama S. The crystal structure of mouse Nup35 reveals atypical RNP motifs and novel homodimerization of the RRM domain. J Mol Biol. 2006;363:114–124. doi: 10.1016/j.jmb.2006.07.089. [DOI] [PubMed] [Google Scholar]
- 21.Thompson J.D., Higgins D.G., Gibson T.J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E. UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 23.Stamp L.A., Obermayr F., Pontell L., Young H.M., Xie D., Croaker D.H., Song Z.M., Furness J.B. Surgical intervention to rescue Hirschsprung disease in a rat model. J Neurogastroenterol Motil. 2015;21:552–559. doi: 10.5056/jnm15079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Obermayr F., Stamp L.A., Anderson C.R., Young H.M. Genetic fate-mapping of tyrosine hydroxylase-expressing cells in the enteric nervous system. Neurogastroenterol Motil. 2013;25:e283–e291. doi: 10.1111/nmo.12105. [DOI] [PubMed] [Google Scholar]
- 25.Norris P.J., Charles I.G., Scorer C.A., Emson P.C. Studies on the localization and expression of nitric oxide synthase using histochemical techniques. Histochem J. 1995;27:745–756. [PubMed] [Google Scholar]
- 26.Young H.M., McConalogue K., Furness J.B., De Vente J. Nitric oxide targets in the guinea-pig intestine identified by induction of cyclic GMP immunoreactivity. Neuroscience. 1993;55:583–596. doi: 10.1016/0306-4522(93)90526-l. [DOI] [PubMed] [Google Scholar]
- 27.Yarden Y., Kuang W.J., Yang-Feng T., Coussens L., Munemitsu S., Dull T.J., Chen E., Schlessinger J., Francke U., Ullrich A. Human proto-oncogene c-kit: a new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J. 1987;6:3341–3351. doi: 10.1002/j.1460-2075.1987.tb02655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glasper E.R., Kozorovitskiy Y., Pavlic A., Gould E. Paternal experience suppresses adult neurogenesis without altering hippocampal function in Peromyscus californicus. J Comp Neurol. 2011;519:2271–2281. doi: 10.1002/cne.22628. [DOI] [PubMed] [Google Scholar]
- 29.Austyn J.M., Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981;11:805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- 30.Huizinga J.D., Lammers W.J. Gut peristalsis is governed by a multitude of cooperating mechanisms. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1–G8. doi: 10.1152/ajpgi.90380.2008. [DOI] [PubMed] [Google Scholar]
- 31.Muller P.A., Koscso B., Rajani G.M., Stevanovic K., Berres M.L., Hashimoto D., Mortha A., Leboeuf M., Li X.M., Mucida D., Stanley E.R., Dahan S., Margolis K.G., Gershon M.D., Merad M., Bogunovic M. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell. 2014;158:300–313. doi: 10.1016/j.cell.2014.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heanue T.A., Pachnis V. Enteric nervous system development and Hirschsprung's disease: advances in genetic and stem cell studies. Nat Rev Neurosci. 2007;8:466–479. doi: 10.1038/nrn2137. [DOI] [PubMed] [Google Scholar]
- 33.Lake J.I., Heuckeroth R.O. Enteric nervous system development: migration, differentiation, and disease. Am J Physiol Gastrointest Liver Physiol. 2013;305:G1–G24. doi: 10.1152/ajpgi.00452.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mikkelsen H.B., Mirsky R., Jessen K.R., Thuneberg L. Macrophage-like cells in muscularis externa of mouse small intestine: immunohistochemical localization of F4/80, M1/70, and Ia-antigen. Cell Tissue Res. 1988;252:301–306. doi: 10.1007/BF00214372. [DOI] [PubMed] [Google Scholar]
- 35.Gabella G. Hypertrophy of intestinal smooth muscle. Cell Tissue Res. 1975;163:199–214. [PubMed] [Google Scholar]
- 36.Won K.J., Torihashi S., Mitsui-Saito M., Hori M., Sato K., Suzuki T., Ozaki H., Karaki H. Increased smooth muscle contractility of intestine in the genetic null of the endothelin ETB receptor: a rat model for long segment Hirschsprung's disease. Gut. 2002;50:355–360. doi: 10.1136/gut.50.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spencer N.J., Bayguinov P., Hennig G.W., Park K.J., Lee H.T., Sanders K.M., Smith T.K. Activation of neural circuitry and Ca2+ waves in longitudinal and circular muscle during CMMCs and the consequences of rectal aganglionosis in mice. Am J Physiol Gastrointest Liver Physiol. 2007;292:G546–G555. doi: 10.1152/ajpgi.00352.2006. [DOI] [PubMed] [Google Scholar]
- 38.Angstenberger M., Wegener J.W., Pichler B.J., Judenhofer M.S., Feil S., Alberti S., Feil R., Nordheim A. Severe intestinal obstruction on induced smooth muscle-specific ablation of the transcription factor SRF in adult mice. Gastroenterology. 2007;133:1948–1959. doi: 10.1053/j.gastro.2007.08.078. [DOI] [PubMed] [Google Scholar]
- 39.Mericskay M., Blanc J., Tritsch E., Moriez R., Aubert P., Neunlist M., Feil R., Li Z. Inducible mouse model of chronic intestinal pseudo-obstruction by smooth muscle-specific inactivation of the SRF gene. Gastroenterology. 2007;133:1960–1970. doi: 10.1053/j.gastro.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 40.Niessen P., Rensen S., van Deursen J., De Man J., De Laet A., Vanderwinden J.M., Wedel T., Baker D., Doevendans P., Hofker M., Gijbels M., van Eys G. Smoothelin-A is essential for functional intestinal smooth muscle contractility in mice. Gastroenterology. 2005;129:1592–1601. doi: 10.1053/j.gastro.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 41.Martin J.E., Benson M., Swash M., Salih V., Gray A. Myofibroblasts in hollow visceral myopathy: the origin of gastrointestinal fibrosis? Gut. 1993;34:999–1001. doi: 10.1136/gut.34.7.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kapur R.P. Practical pathology and genetics of Hirschsprung's disease. Semin Pediatr Surg. 2009;18:212–223. doi: 10.1053/j.sempedsurg.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 43.McKeown S.J., Stamp L., Hao M.M., Young H.M. Hirschsprung disease: a developmental disorder of the enteric nervous system. Wiley Interdiscip Rev Dev Biol. 2013;2:113–129. doi: 10.1002/wdev.57. [DOI] [PubMed] [Google Scholar]
- 44.Azibani F., Muchir A., Vignier N., Bonne G., Bertrand A.T. Striated muscle laminopathies. Semin Cell Dev Biol. 2014;29:107–115. doi: 10.1016/j.semcdb.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 45.Broers J.L., Peeters E.A., Kuijpers H.J., Endert J., Bouten C.V., Oomens C.W., Baaijens F.P., Ramaekers F.C. Decreased mechanical stiffness in LMNA-/- cells is caused by defective nucleo-cytoskeletal integrity: implications for the development of laminopathies. Hum Mol Genet. 2004;13:2567–2580. doi: 10.1093/hmg/ddh295. [DOI] [PubMed] [Google Scholar]
- 46.Ward S.M., Sanders K.M. Involvement of intramuscular interstitial cells of Cajal in neuroeffector transmission in the gastrointestinal tract. J Physiol. 2006;576:675–682. doi: 10.1113/jphysiol.2006.117390. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.