Abstract
Resistin, and its closely related homologs, the resistin-like molecules (RELMs) have been implicated in metabolic dysregulation, inflammation, and cancer. Specifically, RELMβ, expressed predominantly in the goblet cells in the colon, is released both apically and basolaterally, and is hence found in both the intestinal lumen in the mucosal layer as well as in the circulation. RELMβ has been linked to both the pathogenesis of colon cancer and type 2 diabetes. RELMβ plays a complex role in immune system regulation, and the impact of loss of function of RELMβ on colon cancer and metabolic regulation has not been fully elucidated. We therefore tested whether Retnlβ (mouse ortholog of human RETNLβ) null mice have an enhanced or reduced susceptibility for colon cancer as well as metabolic dysfunction. We found that the lack of RELMβ leads to increased colonic expression of T helper cell type-2 cytokines and IL-17, associated with a reduced ability to maintain intestinal homeostasis. This defect leads to an enhanced susceptibility to the development of inflammation, colorectal cancer, and glucose intolerance. In conclusion, the phenotype of the Retnlβ null mice unravels new aspects of inflammation-mediated diseases and strengthens the notion that a proper intestinal barrier function is essential to sustain a healthy phenotype.
Resistin-like molecule β (RETNLβ, alias RELMβ) belongs to a family of small cysteine-rich secreted proteins and is mainly produced by intestinal goblet cells in the colon. Other members of the family include resistin and RELMα.1, 2, 3 RELMβ is secreted both apically as well as basolaterally, and is hence found in both the intestinal lumen in the mucosal layer as well as in the circulation. Colonic RELMβ secretion is induced under a number of different conditions, such as bacterial colonization,4 dextran sodium sulfate (DSS)-induced colonic epithelial injury,5 and in response to high-fat diet.6 Interestingly, the T helper cell type-2 (Th2) cytokine IL-13 also stimulates RELMβ expression and this increase in RELMβ is required for optimal immune defense against parasitic worms.5, 7, 8 Although increased RELMβ levels are associated with Th2-type polarized immune responses, RELMβ itself has been shown to increase tumor necrosis factor-α production in macrophages9, 10 as well as augment CD4(+) Th1 cell responses.11 As one would expect given its main site of expression in the colon, RELMβ also influences the microbiome composition.12 The exact immunological role of RELMβ remains, however, to be determined.
Colorectal cancer is the fourth most common cause of cancer-related mortality in the world.13 More than 8% of all new cancer cases are colon and rectal cancer, and >50,000 individuals are expected to die of the disease in 2015 (Source: National Cancer Institute Surveillance, Epidemiology, and End Results Program). Chronic inflammation of the colon increases the risk of developing a gastrointestinal cancer, but the exact mechanisms are poorly understood. Lack of RELMβ reduces the severity of colitis in the DSS model of colonic injury in C57/Bl6 mice.5, 9 On the other hand, RELMβ is protective in the trinitrobenzensulfonic acid–induced colonic inflammation model. This is probably because of RELMβ's positive effects on maintaining proper barrier function in this context.5, 14 Depending on the challenge, altered intestinal barrier function can lead to a number of different outcomes, such as an increased risk for metabolic disorders and even cancer, although the underlying mechanisms are poorly understood.15, 16, 17, 18
In healthy individuals, there is a positive association of circulating RELMβ with smoking and inverse association with physical activity, both of which are known factors influencing the rate of occurrence of colon cancer.19 Interestingly, a study of 80 colorectal cancer patients shows that RELMβ is expressed at increased levels in most (81.25%) human colorectal cancers. Patients with RELMβ negative cancers display a markedly shorter survival time.20 Circulating levels of RELMβ are increased in murine models of obesity/type 2 diabetes.21 Furthermore, we have previously shown that administration of RELMβ into the circulation induces acute hepatic insulin resistance,22 and transgenic mice overexpressing RELMβ in the liver were shown to exhibit hyperglycemia, hyperlipidemia, and fatty liver.6
Thus, RELMβ has been implicated in both the pathogenesis of colorectal cancer and type 2 diabetes. However, RELMβ appears to play a rather complex role in immune system regulation, and the impact of endogenous RELMβ on colorectal cancer and metabolic regulation has not been fully elucidated. Herein, we have investigated whether Retnlβ (mouse ortholog of human RETNLβ) null mice have an enhanced or reduced susceptibility for colon cancer as well as metabolic dysfunction. We found that the absence of RELMβ promotes a pathological type 2 immune response associated with a reduced ability to maintain intestinal homeostasis, leading to an enhanced susceptibility to the development of colon cancer as well as glucose intolerance.
Materials and Methods
Animals
Retnlβ−/− mice (hereon refered to as RELMβ) were generated in strain 129/SvEvBrd-derived embryonic stem cells (Lexicon Pharmaceuticals, Indianapolis, IN) and can be obtained from the Mutant Mouse Resource Research Center (Mutant Mouse Resource Research Center strain name: B6;129S5-Retnlbtm1Lex/Mmucd; Mutant Mouse Resource Research Center stock number: 032540-UCD). The knockout of RELMβ was confirmed by comparing circulating RELMβ levels in wild-type versus knockout mice (Supplemental Figure S1) through immunoprecipitation of 100 μL using polyclonal rabbit anti-mouse RELMβ antibodies (Covance, Inc., Denver, PA).22 The mice were backcrossed for a minimum of 10 times to either C57/Bl6 or to the FVB background. Mice were maintained on a 12-hour dark/light cycle and housed in groups of four to five with unlimited access to water, chow (No. 5058; Lab-Diet, St. Louis, MO), or high-fat diet (No. D12492, Research Diets Inc., New Brunswick, NJ) as indicated in the individual experiments. The Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center (Dallas, TX) approved all animal experiments.
Induction of Colitis/Colorectal Cancer
Mutagenesis was induced by one i.p. injection of azoxymethane [AOM; 12 mg/kg in phosphate-buffered saline (PBS)] at day-5. This was followed by four rounds (day 0 to 5, 21 to 26, 42 to 47, and 63 to 68) of 2% DSS (molecular weight, 36,000-50,000; MP Biomedicals, Santa Ana, CA) of treatment through drinking water, performed to induce epithelial damage, colitis, and colon cancer (Figure 1A). Animals were analyzed at the indicated time points. Colon cancer was induced by AOM injections alone. Then, weekly AOM injections (10 mg/kg AOM in PBS) were given for 6 weeks and tumor progression was analyzed after 24 weeks.
Figure 1.
Susceptibility for azoxymethane (AOM)/dextran sodium sulfate (DSS)–induced colorectal cancer development increases in RELMβ−/− FVB mice. Body weight (A) during a time course of AOM/DSS treatment and the resultant tumor count (B), tumor size (C), and histological appearance of colons (hematoxylin and eosin stain, D) on day 85 in 11- to 12-week-old female FVB RELMβ−/− mice and littermate controls. Tumor count (E) and size (F) in 5- to 6-month-old FVB RELMβ−/− females and littermate controls after 24 weeks in response to six weekly AOM injections. N = 5 wild type per genotype for all time points (A–D); N = 7 knockout per genotype for all time points (A–D); N = 10 per genotype (E and F). ∗P < 0.05, ∗∗P < 0.01 for the difference between the genotypes (there is also a trend for a difference in body weight between genotypes, P = 0.08). Original magnification, ×5 (D). WT, wild type.
Ablation of Gut Microbiota
Animals were provided 1 g/kg ampicillin (A; Sigma), 250 mg/kg vancomycin (V; Sigma), 1 g/kg neomycin sulfate (N; Sigma), and 1 g/kg metronidazole (M; Sigma) as food admixes in powdered regular chow for the indicated time periods. This antibiotic supplementation was well tolerated and the mice gained weight at the same degree as untreated controls.
Oral Glucose Tolerance Test
The mice were fasted for 3 hours during the light phase and blood samples were drawn from the tail vein before and 15, 30, 60, and 120 minutes after an intragastric load with 2.5 g/kg glucose in PBS. Glucose levels were determined with Sigma Diagnostics Glucose Reagents (Sigma, Sigma Aldrich).
Histological Analysis
Colons were excised, washed with PBS, and cut longitudinally into two pieces. One piece was used for RNA isolation and the other piece was Swiss-rolled and fixed in PBS-buffered 10% formalin for 24 hours. Pieces of adipose tissue and liver were also excised and fixed in PBS-buffered 10% formalin for 24 hours. After paraffin embedding, the tissue sections were deparaffinized and stained with hematoxylin and eosin.
Quantitative Real-Time RT-PCR
Tissues were collected in RNAlater (Ambion, Foster City, CA) and stored at −80°C. Colon pieces were picked from the proximal region about 10 mm from cecum and when necessary tumor tissue was avoided. Lymph nodes were removed from mesenteric adipose tissue. Trizol reagent (Invitrogen, Carlsbad, CA) extraction followed by RNA purification using RNeasy Mini Kit and RNase-Free DNase (Qiagen, Hilden, Germany). RNA was reverse transcribed to cDNA by iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) and IQ SYBR Green Supermix (Bio-Rad) was used for the quantitative PCRs. The relative expression level was calculated by the comparative Ct method using HPRT as endogenous control. Primer sequences can be found in Table 1.
Table 1.
List of Primers Used in This Study
| Primer name | Forward primer | Reverse primer |
|---|---|---|
| HPRT | 5′-AGCAGTACAGCCCCAAAA-3′ | 5′-TTTGGCTTTTCCAGTTTCA-3′ |
| RELMβ | 5′-GCTCTTCCCTTTCCTTCTCCAA-3′ | 5′-AACACAGTGTAGGCTTCATGCTGTA-3′ |
| IL-4 | 5′-ACAGGAGAAGGGACGCCAT-3′ | 5′-GAAGCCCTACAGACGAGCTCA-3′ |
| IL-13 | 5′-GGAGCTGAGCAACATCACACA-3′ | 5′-GGTCCTGTAGATGGCATTGCA-3′ |
| IFNγ | 5′-TCAAGTGGCATAGATGTGGAAGAA-3′ | 5′-TGGCTCTGCAGGATTTTCATG-3′ |
| SAA3 | 5′-TAAAGTCATCAGCGATGCCAGAG-3′ | 5′-CAACCCAGTAGTTGCTCCTCTTC-3′ |
| F4/80 | 5′-CTTTGGCTATGGGCTTCCAGTC-3′ | 5′-GCAAGGAGGACAGAGTTTATCGTG-3′ |
| TNF-α | 5′-GAGAAAGTCAACCTCCTCTCTG-3′ | 5′-GAAGACTCCTCCCAGGTATATG-3′ |
| IL-5 | 5′-CTCTGTTGACAAGCAATGAGACG-3′ | 5′-TCTTCAGTATGTCTAGCCCCTG-3′ |
| IL-10 | 5′-GCTCTTACTGACTGGCATGAG-3′ | 5′-CGCAGCTCTAGGAGCATGTG-3′ |
| IL-17 | 5′-GCTCCAGAAGGCCCTCAGA-3′ | 5′-AGCTTTCCCTCCGCATTGA-3′ |
| GATA3 | 5′-CTACCGGGTTCGGATGTAAGTC-3′ | 5′-GTTCACACACTCCCTGCCTTCT-3′ |
| Tbet | 5′-CCTGGACCCAACTGTCAACT-3′ | 5′-AACTGTGTTCCCGAGGTGTC-3′ |
GATA3, GATA binding protein 3; HPRT, hypoxanthine-guanine phosphoribosyltransferase; IFN, interferon; RELMβ, resistin-like molecule β; SAA, serum amyloid A; Tbet, T-box transcription factor; TNF-α, tumor necrosis factor-α.
Serum Cytokine and Acute Phase Reactant Measurements
Serum IL-13, IL-12p40 [<5% cross-reactivity with the IL-12p30-p40 (IL-12p70) and the IL12p19-p40 (IL-23) heterodimers], IL-12p70, IL-6, and interferon (IFN)γ levels were measured with a multiplex assay (Merck Millipore, Billerica, MA). Serum levels of serum amyloid A and α-1-acid glycoprotein were measured with commercial enzyme-linked immunosorbent assays (Merck Millipore).
Flow Cytometry
Tissues were minced and digested by Collagenase D [Roche (Sigma), St. Louis, MO] and filtered at 100 and 40 μm. Cells were resuspended in cold 0.01% formaldehyde followed by 0.1% Triton. Nonspecific binding was inhibited by incubating cells with an anti-mouse CD16/CD32 antibody (eBioscience, San Diego, CA). After PBS rinsing and 40 μm filtration, cells were incubated with fluorescent-conjugated antibodies against CD3e, CD4, GR1/Ly6G, IL-13 (all eBioscience), and IFNγ (BD Bioscience). Cells were again 40 μm filtered and populations of 100k were analyzed on a Dako CyAn ADP flow cytometer with DAPI as a dead cell marker. The flow cytometry data were analyzed using FlowJo software version 7.6.2 (FlowJo, LLC, Ashland, OR).
Statistical Analysis
Data are in generally expressed as means ± SEM. The Student's t-test and repeated measures analysis of variance were used for comparisons of respectively mean values and time course data between the genotypes. Two-way analysis of variance was used to compare gene expression measurements between genotype in separate cohorts during different stages of colon cancer progression. Ln-transformation was performed as necessary to obtain normal distribution, and a P < 0.05 was considered as significant.
Results
Susceptibility to DSS/AOM-Induced Colorectal Cancer Development Increases in RELMβ−/− Mice in the FVB Background
RELMβ−/− mice on a C57/Bl6 background display reduced susceptibility to colitis in response to high doses (4%) of DSS in the drinking water.9 Colitis is a well-established risk factor for the development of colon cancer. We therefore hypothesized that RELMβ−/− mice are protected against colitis-associated colon cancer. To study this phenomenon in the context of colitis-associated colon cancer, we induced mutagenesis with one AOM injection followed by intermittent bouts with DSS (2%) by administration through the drinking water, an experimental protocol previously shown to be effective for studying the inflammatory components of tumor progression in the colon.23, 24 The C57/Bl6 strain is, however, protected against tumor growth, possibly through its enhanced Th1- and reduced Th2-polarized immune response.25 A predominant Th2-type immune response has been shown to promote colon cancer, whereas Th1-polarized immunity may lead to a more aggressive cell-mediated immune response triggering tumor rejection.26, 27, 28 In fact, the C57/Bl6 mice in our colony were highly sensitive to the acute toxic effects of both AOM and DSS. We had to decrease the AOM dose by 50%. However, this lower AOM dose followed by the bouts of DSS treatment was ineffective with respect to tumor induction. Barely any of the mice developed colon tumors, and no significant differences between genotypes could be detected (data not shown). We therefore crossed the RELMβ−/− mice into the more tumor- and Th2-prone29 FVB background (for >10 generations). We found that female RELMβ−/− FVB mice showed a tendency to gain slightly more body weight along with the AOM/DSS treatment regimen. However, to our surprise, both the number and size of colon tumors were increased compared to wild-type littermate controls by day 85 (Figure 1, A–C). In fact, it was hard to find colon tissue with normal morphology at this stage in the RELMβ−/− mice (Figure 1D). Furthermore, RELMβ−/− FVB mice also displayed larger colons than the wild-type mice (Figure 1D). Although the original data obtained were collected in a female cohort, the study was repeated in males with a similar result (Supplemental Figure S2). To investigate whether the differences in tumor progression between genotypes are dependent on an altered response to DSS or whether another mechanism is at play, we probed for the induction of colon cancer by multiple injections of AOM in the absence of DSS. Overall, this protocol led to fewer colon tumors than the combined AOM/DSS protocol, but even in the context of this experimental setting, RELMβ−/− mice developed about twice as many tumors as their wild-type controls (Figure 1, E and F). Thus, in contrast to our initial hypothesis, lack of RELMβ in a rodent FVB background leads to increased colon tumor growth, in a setting with or without colitis-enhanced tumor progression. As such, RELMβ could be considered a tumor suppressor.
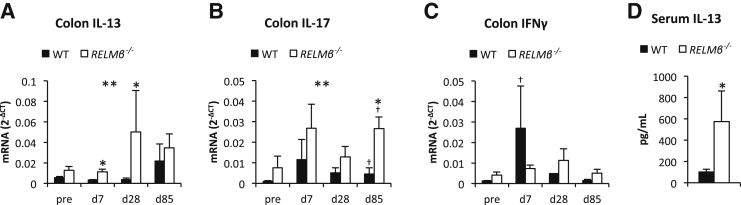
The proposed role for RELMβ in Th1-Th2-immune response polarization11 is expected to enhance host antitumor immunity.26, 27, 28 In the setting here, we therefore hypothesized that the lack of RELMβ skews the immune response toward a Th2 response. This would offer an explanation for the reduction in acute colitis as previously shown6, 9, 11 and the increase in tumor progression in the knockout mice (this study). Consistent with this proposed mechanism, we found that the colonic expression of Th2-type cytokines, such as IL-13, IL-4, and IL-5, is increased in the RELMβ−/− mice (Figure 2A and Supplemental Figure S3, A and B). The expression of IL-4 and IL-5 was only significantly increased at day 28, whereas the IL-13 levels were generally higher in knockout mice regardless of time point (Fgenotype = 11.3, P = 0.002, Ftimepoint = 1.5, P = 0.3). The Th2 differentiation marker GATA3 increased along with colitis and tumor progression in both genotypes, but RELMβ−/− mice displayed generally higher GATA3 level than their littermate controls (Fgenotype = 9.0, P = 0.005, Ftimepoint = 7.9, P < 0.001) (Supplemental Figure S3C). We also found an overall increased colonic IL-17 expression in the RELMβ−/− mice (Fgenotype = 9.5, P = 0.004, Ftimepoint = 1.4, P = 0.3), but the difference between genotypes was most pronounced by day 85 when tumors had developed (Figure 2B). On day 85, the RELMβ−/− mice showed also a higher colonic expression of anti-inflammatory cytokine IL-10 (Supplemental Figure S3D). The colonic expression of the Th1 cytokine IFNγ and the Th1-differentiation transcription factor Tbet did not differ between genotypes (Figure 2C and Supplemental Figure S3E). In wild-type there was, however, a significant increase in colon IFNγ expression at day 7 compared to baseline, indicating that AOM/DSS treatment is associated with a Th1-type immune response in presence of RELMβ. Circulating levels of IFNγ remained, however, unchanged until day 85, when the levels in fact decreased from baseline in both genotypes (27 ± 5 to 16 ± 3 pg/mL, P = 0.03, in wild-type and 30 ± 4 to 11 ± 3 pg/mL, P = 0.008, in RELMβ−/− mice). The expression of tumor necrosis factor-α increased along the AOM/DSS time course to a similar extent in both genotypes, but was higher in RELMβ−/− mice than in wild-type at day 85, probably reflecting the increased tumor burden in knockouts at this stage (Supplemental Figure S3F). Furthermore, the circulating levels of the acute phase reactant serum amyloid A increased in both genotypes in response to AOM/DSS treatment and remained elevated throughout the time course (Supplemental Figure S3G), whereas the circulating levels of α-1-acid glycoprotein and IL-17 remained unchanged (data not shown). Serum IL-6 levels were also unchanged throughout the initial parts of the time course, but were increased by day 85 in wild-type leading to a difference between genotypes (14 ± 5 and 2.1 ± 0.7 pg/mL in respectively, wild-type and RELMβ−/− mice, P = 0.01). The serum levels of IL-12p40, a cytokine that in its homodimeric form is thought to be the natural antagonist of the Th1-associated cytokine IL-12p70 (the active IL-12 heterodimer),30 were significantly increased on days 7 and 85 during the AOM/DSS time course (Supplemental Figure S3H). The multiplex assay used cannot distinguish between IL-12p40 monomer and homodimer. Furthermore, increased IL-12p40 levels may also result from a higher degree of intestinal barrier dysfunction in DSS-treated RELMβ−/− compared to wild-type mice.31 The levels of IL-12p70 remained stable and were similar between genotypes (data not shown). The levels of IL-13 in the circulation were similar in wild-type and RELMβ−/− mice and were also unchanged during the AOM/DSS time course (data not shown). However, when serum IL-13 was measured acutely after 7 days 4% DSS treatment, the levels were significantly higher in the RELMβ−/− mice (Figure 2D).
Figure 2.
Colonic IL-13 and IL-17 expression increases in FVB RELMβ−/− mice. Colon IL-13 (A), IL-17 (B), and interferon (IFN)γ (C) mRNA levels during the azoxymethane/dextran sodium sulfate (DSS) treatment time course (baseline, day 7, 28, and 85) in 11- to 12-week-old female FVB RELMβ−/− mice and littermate controls. D: Serum IL-13 levels in response to 7 days of DSS treatment in 9-month-old male FVB RELMβ−/− mice and littermate controls. N = 4 to 7 per genotype and time point (A–C); N = 3 knockout (D); N = 6 wild type (D). ∗P < 0.05, ∗∗P < 0.01 for the difference between the genotypes (there is also a trend for a difference in IL-13 expression between genotypes at baseline, P = 0.08); †P < 0.05 for the difference from baseline within a genotype (there is also a trend for a difference in IFNγ expression between baseline and day 29 within wild-type mice, P = 0.06). WT, wild type.
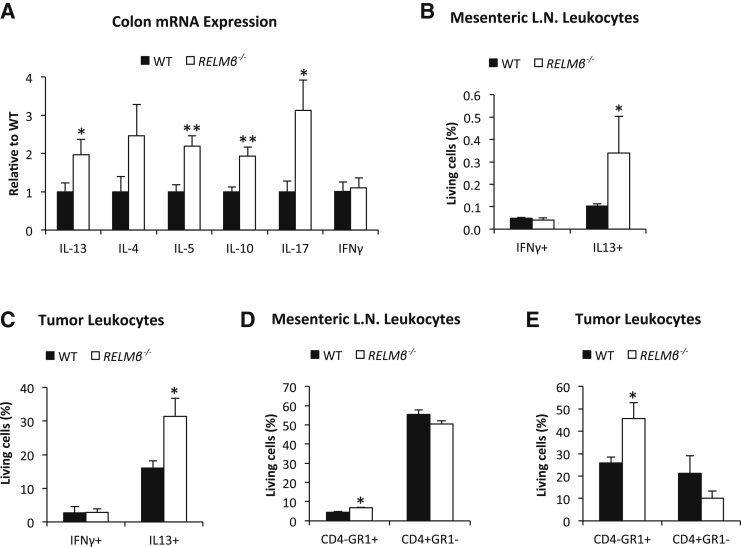
In the cohort of FVB mice that were challenged with multiple injections of AOM in the absence of DSS (Figure 1, E and F), the RELMβ−/− mice displayed about twofold higher colonic expression of IL-13, IL-5, IL-10, and IL-17 than their wild-type controls, whereas IFNγ expression was similar between genotypes (Figure 3A). Fluorescence-activated cell sorter analysis of mesenteric lymph nodes and tumors from this cohort of animals showed that FVB RELMβ−/− mice have a higher number of total IL-13+ and Gr1/Ly6G+ cells in both tissues, whereas the number of IFNγ+ and CD4+ lymphocytes was similar between genotypes (Figure 3, B–E). The number of Gr1/Ly6G+IL-13+ cells was also increased in both mesenteric lymph nodes (0.09 ± 0.01 versus 0.31% ± 0.15% of living cells in respectively, wild-type and RELMβ−/− mice, P = 0.029) and tumors of FVB RELMβ−/− mice (15.0 ± 2.49 versus 30.3% ± 5.23% of living cells in respectively, wild-type and RELMβ−/− mice, P = 0.048). In fact, almost all IL-13+ cells in both tumors and lymph nodes were found within the Gr1/Ly6G+high population and are thus most likely neutrophils32 (Supplemental Figures S4 and S5). Thus, the increased IL-13 expression in FVB RELMβ−/− colon tissue is dependent on an increased number of IL-13–producing neutrophils.
Figure 3.
The number of IL-13+ and Gr1/Ly6G+ leukocytes in mesenteric lymph nodes and tumor tissue increases in azoxymethane-treated RELMβ−/− FVB mice. A: Colon mRNA expression. Percentage of interferon (IFN)γ+, IL13+, CD4-Gr1+, and CD4+Gr1− cells of total living leukocytes in mesenteric lymph node (B–D) and colon tumors (C–E). N = 10 per genotype (A); N = 4 wild type and knockout (B–E). ∗P < 0.05, ∗∗P < 0.01 for the difference between the genotypes. L.N., lymph node; WT, wild type.
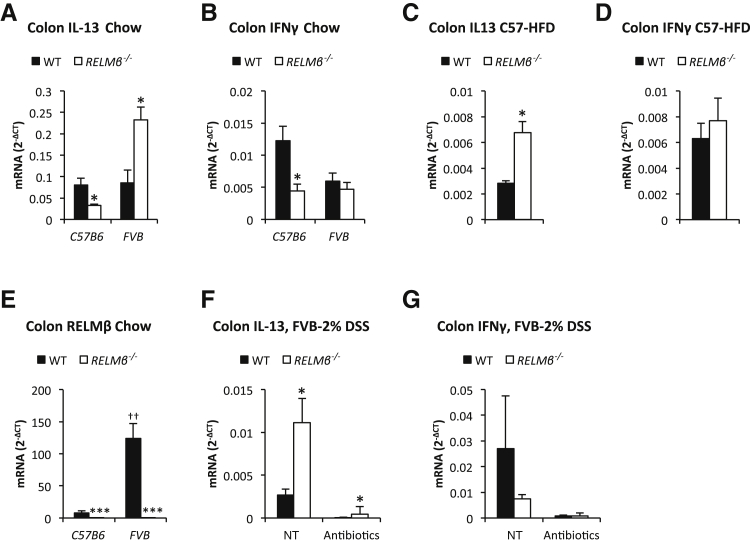
Interestingly, a skewed Th1-Th2 cytokine balance was also seen in absence of proinflammatory/tumorigenic stimuli in the RELMβ−/− mice, although the cytokine expression pattern was, as expected, highly dependent on the genetic background. In our mouse colony, the colonic IL-13 expression is consistently elevated in the RELMβ−/− mice on the FVB background. In contrast, our RELMβ−/− mice on C57/Bl6 background display a reduced expression of both IFNγ and IL-13 under chow-fed conditions, whereas IL-13 is elevated under high-fat diet fed conditions (Figure 4, A–D). The colonic RELMβ expression is substantially higher in FVB wild-type than in C57/Bl6 wild-type, which influences not only the overall phenotypic differences between these mouse strains, but also differentially affects the mechanistic consequences of RELMβ ablation (Figure 4E).
Figure 4.
The T helper cell 1 (Th1)/Th2-type cytokine balance is skewed in RELMβ−/− mice. Colon IL-13 (A) and interferon (IFN)γ (B) mRNA levels in chow-fed male 18-week-old C57/Bl6 and 18-week-old FVB RELMβ−/− mice and their littermate controls. Colon IL-13 (C) and IFNγ (D) mRNA levels in 20-week-old high-fat diet (HFD)-fed male C57/Bl6 RELMβ−/− mice and littermate controls. E: Colon RELMβ mRNA levels in chow-fed 17-week-old male C57/Bl6 and 18-week-old FVB RELMβ−/− mice and their littermate controls. Colon IL-13 (F) and IFNγ (G) mRNA levels in response to five dextran sodium sulfate (DSS) treatments with and without ablation of gut microbiota in 20-week-old female FVB RELMβ−/− mice and littermate controls. N = 4 per group (A, B, and E); N = 6 to 12 per group (C and D); N = 4 to 5 per group (F and G). ∗P < 0.05, ∗∗∗P < 0.001 for the difference between the genotypes; ††P < 0.01 for the difference between the FVB and C57/Bl6 mouse strain. NT, no treatment; WT, wild type.
To further explore the role of RELMβ and gut microbiota for the observed skewed Th1-Th2 cytokine balance (this study), we treated a cohort of FVB mice with antibiotics for 4 weeks and measured the response to DSS treatment with respect to colonic mRNA expression (5 days treatment followed by 2 days without antibiotic exposure). Antibiotic-induced ablation of gut microbiota reduced the absolute levels of IL-13 and IFNg in both wild-type and RELMβ−/− FVB mice, indicating that commensal bacteria potently trigger both baseline and DSS-induced expression of these cytokines. The difference between genotypes remained, however, significant: RELMβ−/− displayed higher levels of IL-13 (with an associated tendency toward lower IFNγ) than controls with or without antibiotics (Figure 4, F and G). Collectively, these observations indicate that the lack of RELMβ has an impact on Th1-Th2 cytokine expression independent of the genetic backgrounds examined herein (FVB versus C57/Bl6), and at least to some extent, independent of potential differences in gut microbiota composition. We cannot formally rule out an effect of the gut microbiota, because antibiotic treatment was initiated in adult mice only (15-week-old), thereby leaving the possibility that gut microbiota may have made developmental contributions.
Glucose Intolerance in C57/Bl6 and FVB RELMβ−/− Mice
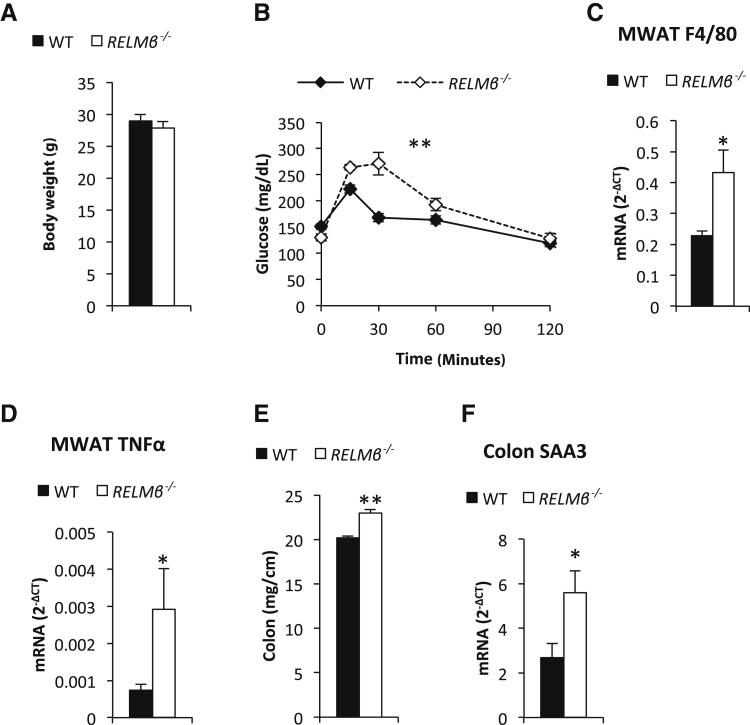
Elevated circulating RELMβ levels are associated with insulin resistance and overexpression or exogenous administration of RELMβ reduces insulin signaling.6, 22 We therefore hypothesized that RELMβ−/− mice would have an improved metabolic profile, at least on challenge with a high-fat diet. To our surprise, rather than being protected against the metabolic perturbations, C57/Bl6 RELMβ−/− mice displayed a reduced glucose tolerance, even in the absence of a dietary challenge and/or obesity (Figure 5, A and B). This was associated with an increased expression of F4/80 and tumor necrosis factor-α mRNA in mesenteric adipose tissue (Figure 5, C and D). However, we did not detect any significant changes in expression of inflammatory markers in the liver (data not shown). Colon hyperplasia and colonic mRNA expression levels of serum amyloid A 3 (an acute phase reactant associated with proinflammatory processes in colon and adipose tissue33, 34) were, however, significantly increased in the C57/Bl6 RELMβ knockouts (Figure 5, E and F). These data indicate that the altered immune response in the colon of these C57/Bl6 RELMβ−/− mice enhances inflammation in this area, which in turn is associated with metabolic dysfunction. Body weight and fat mass differences varied between different C57/Bl6 cohorts, giving inconsistent weight differences (data not shown). Of note is that whenever we found an increase in fat mass in the knockouts, it was mostly an increase in the mesenteric depot (81% heavier, P < 0.05). The gonadal depot was also increased, albeit to a lower degree (48% heavier, P < 0.05), whereas the inguinal depot only trended to be increased compared to control mice (43% heavier, P = 0.1). On average, however, there was no clear effect of RELMβ ablation on measures of obesity in the C57/Bl6 background. It is possible that the overall inflammatory status of the mice, secondary to the lack of RELMβ, varies slightly between cohorts because of subtle environmental alterations affecting energy balance in either direction.
Figure 5.
The susceptibility to the development of metabolic dysregulation increases in RELMβ−/− mice in the C57/Bl6 background. Body weight (A) and glucose tolerance (B) in 16-week-old male C57/Bl6 RELMβ−/− mice and littermate controls. Mesenteric white adipose tissue (MWAT) F4/80 (C) and tumor necrosis factor (TNF)-α mRNA levels (D), colon thickness (E), and serum amyloid A (SAA) 3 mRNA levels (F) in chow-fed 17-week-old male C57/Bl6 RELMβ−/− mice and littermate controls. N = 4 to 6 per group (A–F). ∗P < 0.05, ∗∗P < 0.01 for the difference between the genotypes. WT, wild type.
To explore this further, we embarked on a metabolic characterization of the RELMβ knockout phenotype in the FVB background. As previously mentioned, FVB mice are more prone toward Th2 responses,29 whereas C57/Bl6 mice are characterized by an increased propensity toward Th1-dominated immune responses.25 We have recently shown that an effective proinflammatory response within adipose tissue is important for healthy adipose tissue expansion, as judged by the capacity to safely store excess nutrient in adipose tissue as opposed to ectopic lipid deposition in nonadipose tissues. Consistent with this model, FVB mice are more susceptible to develop glucose intolerance on a high-fat diet, even though they remain comparatively lean.35, 36 According to our observations regarding the balance of Th1-Th2 responses in the colon of RELMβ−/− mice, we argued that the Th2-domination in knockouts might aggravate the metabolic phenotype further. Indeed, we found that the FVB RELMβ−/− mice are severely glucose intolerant and also slightly heavier than wild-type littermate controls, even on a normal chow diet (Figure 6, A and B). This metabolic phenotype was associated with colon hyperplasia, a pronounced expansion of mesenteric adipose tissue (296 ± 56 versus 695 ± 130 mg, P = 0.03), mild hepatomegaly, and an increase in mRNA expression of inflammatory markers in both liver and in mesenteric adipose tissue (Figure 6, C–H). Interestingly, this metabolic phenotype could be prevented by treatment of the mice with antibiotics. In fact, fasting glucose levels were lower in antibiotic-treated RELMβ−/− than in control mice (94.1 ± 1.7 versus 87.0 ± 2.2 mg/dL, P < 0.05), but the overall glucose tolerance as well as markers of inflammation in liver and mesenteric adipose tissue were similar between genotypes (Supplemental Figure S6). The weight of mesenteric adipose tissue was also similar between genotypes (404 ± 36 versus 406 ± 30 mg), but the livers were slightly heavier in the antibiotic-treated RELMβ−/− mice (937 ± 20 versus 1022 ± 31 mg, P = 0.05).
Figure 6.
The susceptibility to the development of metabolic dysregulation increases in RELMβ−/− mice in the FVB background. Body weight (A) and glucose tolerance (B) in chow-fed 17-week-old male FVB RELMβ−/− mice and littermate controls. Colon length, weight, and histological appearance [hematoxylin and eosin (H&E)] (C), liver weight (D), and TNF-α mRNA levels (E), adipose tissue weights related to body weight and mesenteric white adipose tissue (MWAT) appearance (H&E; F), MWAT F4/80 (G), and tumor necrosis factor (TNF)-α (H) mRNA levels in chow-fed 18-week-old male FVB RELMβ−/− mice and littermate controls. N = 4 to 6 per group (A–H). ∗P < 0.05, ∗∗P < 0.01 for the difference between the genotypes. Original magnification: ×5 (C); ×20 (F). GWAT, gonadal white adipose tissue; IWAT, inguinal white adipose tissue; WT, wild type.
The Aggravated Metabolic Profile in RELMβ−/− Mice Is Associated with Colon Hyperplasia
Over the course of our studies that stretched over many years, we encountered RELMβ−/− cohorts with a less pronounced metabolic phenotype, both in the C57/Bl6 and FVB background. In the FVB RELMβ−/− mice, the degree of obesity/weight gain was positively associated with the glucose intolerance (except in the cohorts treated with antibiotics). This was not the case for the C57/Bl6 RELMβ−/− mice. This difference between C57/Bl6 and FVB RELMβ−/− mice is likely related to an overall higher capacity for healthy adipose tissue expansion in the C57 strain than in the FVB background. In addition, this variability in body weight may also take root from a difference in gut microbiota composition between strains and litters, which can affect energy harvest and/or energy balance through altered levels of microbial metabolites.37 The complete absence of glucose intolerance in antibiotic-treated RELMβ−/− mice suggests that the variation in gut microbiota triggers a more or less apparent phenotype in absence of RELMβ. Consistent with this effect of antibiotics, we found that the variability of the metabolic phenotype of RELMβ−/− mice, independent of genetic background, was strongly linked to the degree of colon hyperplasia (a phenomenon typically linked to dysbiosis and inflammatory processes38, 39). In the C57 background, glucose intolerance was most pronounced in RELMβ−/− mice that displayed the thickest colons, regardless of whether the mice were more or less obese than littermate controls (Supplemental Figure S7). In the FVB background, the colons of metabolically compromised RELMβ−/− mice were both longer and heavier, and these knockouts were also consistently heavier than their controls. In antibiotic-treated RELMβ−/− mice (Supplemental Figure S6), the difference in colon weight and length decreased from being 23% and 22%, respectively (P = 0.002 and 0.002 for the differences between genotypes) to 14% and 9% (P = 0.046 and 0.054 for the difference between genotypes) to be larger in RELMβ−/− mice. The remaining differences in colon hyperplasia between genotypes may be because of the fact that the antibiotic treatment started when these mice were 11 weeks old and not at birth. The exact manifestation of the RELMβ phenotype will thus depend on both genetic background and environmental factors, such as subclinical infections.
Discussion
Elevated circulating RELMβ levels are associated with insulin resistance and overexpression or exogenous administration of RELMβ reduces insulin signaling.6, 22 Lack of RELMβ protects against DSS-induced colitis, at least in the acute setting in mice in a C57/Bl6 background.5, 9 Herein, we found that the lack of RELMβ increases the susceptibility for metabolic dysfunction as well as enhances colonic tumor progression rather than being protective.
To date, we do not know how RELMβ exerts its effect and the RELMβ receptor(s) remain elusive. RELMβ has been suggested to play a role for a healthy symbiosis with gut microbiota.5, 14 In line with this notion, RELMβ−/− mice display a modest shift in the microbiome composition.12 It is possible that RELMβ influences the composition of gut microbiota through direct interactions with bacteria in a similar manner as other goblet cell–derived peptides do (eg, the antibacterial lectin RegIIIγ).40 RELMβ has indeed been shown to bind to the chemosensory apparatus of nematodes and thereby inhibit infection by worms.5, 7, 8 Currently, it is however unknown whether this shift in microbiota composition is dependent on a direct bactericidal activity, or indirectly via immune system regulation. We believe that our data both support a direct bactericidal function of RELMβ as well as a model in which RELMβ acts as a bridge between innate and adaptive immunity through fine-tuning the Th1-Th2-Th17 cytokine balance in the colon. From the present study, we cannot elucidate whether the altered colonic expression of Th1-Th2-Th17-type cytokines in the RELMβ−/− mice causes an increased susceptibility to gut microbial interactions, or whether this cytokine expression difference is rather the consequence of an altered gut microbiome. The colonic cytokine profile with elevated Th2-type cytokines and IL-17 associated with enhanced tumor progression in the RELMβ−/− mice is however highly consistent with published results obtained in both preclinical and clinical studies.41 Th1-type immune responses, characterized by an increased IFNγ production, typically promote tumor rejection; however less is known regarding the contribution of Th2 and Th17 responses in tumor progression.42 Although the colonic expression of IFNγ is not significantly different between genotypes in the AOM-DSS colon cancer studies, wild-type mice show a more dynamic IFNγ response with an acute increase at day 7, which may exert a protective effect. The most consistent difference between FVB RELMβ−/− and wild-type mice in our studies is however the elevated expression of Th2 cytokines and IL-17 in the FVB RELMβ−/− mice. Several studies indicate that a predominant Th2 immune response promotes colon cancers in mice,28, 43, 44 and IL-17 is tightly associated with intestinal tumorigenesis in situations of chronic inflammation or infections associated with commensal bacteria as well as with a poor prognosis in patients with colorectal cancer.45, 46, 47
On the basis of our fluorescence-activated cell sorter analysis, at least the Th2 type cytokine IL-13 originates predominantly from infiltrating neutrophils and not from CD4+ T cells. Interestingly, there is emerging evidence of a somewhat surprising link between increased IL-17 levels and Th2 immunity that mechanistically involves neutrophils.48 Typically, IL-17 is involved in proinflammatory immune responses, such as being a major driving force for the recruitment and activation of neutrophils.49 Unexpectedly, Il-17 deficient mice display reduced Th2 cytokines both in the context of Nippostrongylus brasiliensis infection and atopic dermatitis indicating that IL-17 can promote type 2 responses.50, 51 Chen et al52 demonstrate that neutrophils are an important source of Th2 cytokines in the acute response to N. brasiliensis infection, affecting the effector macrophages' capacity to cause parasite damage. Thus, the effect on IL-17 on promoting Th2-type immune responses may be exerted via alternatively activated (N2) neutrophils. These Th2 cytokines are generally thought to exert negative feedback and suppress excessive neutrophilia and tissue damage,53 but Th2 cytokines have also been shown to promote IL-17 production.54 Thus, if the type 2 response fails to suppress (or even augment) the IL-17 response excessive type 2 pathology may be the result. Our data suggest that lack of RELMβ increases the susceptibility to develop such type 2 pathology, leading to an enhanced colon cancer progression. As for the metabolic phenotype of the RELMβ−/− mice, we believe that a similar mechanism is at play. Given the tight positive correlation between colon hyperplasia and glucose intolerance in the RELMβ−/− mice, we propose that lack of RELMβ increases the susceptibility for unfavorable interactions with gut microbiota, leading to inflammatory processes, which in turn negatively affect whole-body metabolic regulation. As a result, the systemic metabolic dysfunction may further enhance colonic tumor growth beyond the impact on local changes in the immune response within the colon microenvironment.
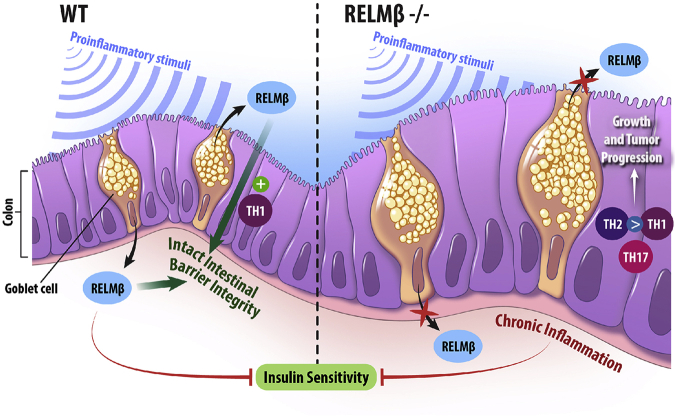
Regardless of the precise mechanism(s) of action, the role of RELMβ in pathogenesis of disease appears to be much context-dependent (ie, the exact outcome depends on interactions between multiple genetic and environmental factors). Both excess and inappropriately low levels of RELMβ increase the susceptibility to disease. An excess of RELMβ enhances proinflammatory responses and may thereby cause tissue damage.55 Disproportionately low levels of RELMβ, on the other hand, lead to a reduced ability for proper intestinal barrier function and thereby increases the susceptibility for developing chronic inflammation with all its metabolic and protumorigenic consequences (Figure 7). In summary, our study adds to the growing body of evidence suggesting the importance a functional intestinal barrier for both tumor prevention and metabolic health.
Figure 7.
Summary and hypothesis. Circulating RELMβ can reduce the insulin sensitivity, whereas local RELMβ in the colon plays a role for the integrity of the intestinal barrier. Thus, lack of RELMβ increases the susceptibility to leaky gut and the development of pathological processes such as chronic inflammation associated with insulin resistance. WT, wild type.
Acknowledgments
We thank the metabolic core facility for Multiplex cytokine analyses, Lora Hooper for advice regarding the antibiotic-supplemented diet, and Jie “Amy” Song for assistance with the mouse genotyping.
I.W.A. and P.E.S. are the guarantors of this work and, as such, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Supported by the Swedish Research Council (2012-1601 and 2013-7107), VINNOVA (2011-01336), NovoNordisk Excellence Project Award, Åke Wiberg Foundation (18431223, M14-0105 and M14-0014), Diabetesfonden (DIA2014-074), Diabetes Wellness Research Foundation (8349/2014SW), and the Magnus Bergvall foundation (2014-00169; I.W.A.), and US NIH grants R01-DK55758, P01-DK088761, and R01-DK099110 (P.E.S.).
Disclosures: None declared.
Current address of J.Y.K.-M., Cardiometabolic Disease, Merck Research Laboratories, Boston, MA.
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.ajpath.2016.04.017.
Contributor Information
Ingrid Wernstedt Asterholm, Email: iwa@neuro.gu.se.
Philipp E. Scherer, Email: philipp.scherer@utsouthwestern.edu.
Supplemental Data
Serum RELMβ levels in wild-type and RELMβ−/− mice. Representative Western blot from >3 independent experiments where immunoprecipitates of 100 μL serum with rabbit polyclonal anti-mouse RELMβ antibodies immobilized to Protein A Sepharose were visualized. Purified RELMβ protein (produced in HEK-293T cells) was loaded as a positive control. IgG light chains in both immunoprecipitate lanes are indicated with arrows. WT, wild type.
Increased susceptibility for dextran sodium sulfate (DSS)/azoxymethane (AOM)–induced colorectal cancer development in RELMβ−/− FVB mice. Tumor count (A) and size (B) and colon appearance on day 85 in 8-month-old male FVB RELMβ−/− and littermate control mice in response to AOM/DSS treatment regimen (Figure 1A). N = 8 knockout (A and B); N = 12 wild type (A and B). ∗P < 0.05, ∗∗P < 0.01 for the difference between the genotypes. WT, wild type.
Skewed T helper cell 1 (Th1)/Th2-type cytokine balance in RELMβ−/− mice. Colon IL-4 (A), IL-5 (B), GATA3 (C), IL-10 (D), Tbet (E), and tumor necrosis factor (TNF)-α (F) mRNA levels, and serum amyloid A (SAA; G) and IL-12-p40 (H) levels during the azoxymethane/dextran sodium sulfate treatment time course (baseline, day 7, 28, and 85) in 11- to 12-week-old female FVB RELMβ−/− mice and littermate controls. N = 4 to 7 per genotype and time point (A–H). ∗P < 0.05, ∗∗P < 0.01 for the difference between the genotypes (there is also a trend for a difference in IL-4 expression at day 7 between genotypes, P = 0.07); †P < 0.05, ††P < 0.01, and †††P < 0.001 for the difference from baseline within a genotype; and ‡‡P < 0.01 for the overall difference between genotype. WT, wild type.
IL-13+ cells are Gr1/Ly6G+high leukocytes. CD4 versus Gr1 fluorescence-activated cell sorter plots of living leukocytes from mesenteric lymph nodes (A) and back gating of the IL-13+ cells (B) in these plots. Tissues were obtained from 5- to 6-month-old FVB RELMβ−/− females and littermate controls after 24 weeks in response to six weekly azoxymethane injections.
IL-13+ cells are Gr1/Ly6G+high leukocytes. CD4 versus Gr1 fluorescence-actived cell sorter plots of living leukocytes from colon tumors (A) and back gating of the IL-13+ cells in these plots (B). Tissues were obtained from 5- to 6-month-old FVB RELMβ−/− females and littermate controls after 24 weeks in response to six weekly azoxymethane injections.
Ablation of gut microbiota protects against metabolic disturbances in the RELMβ−/− FVB mice. Body weight (A) and glucose tolerance (B) in antibiotic-treated chow-fed 11-week-old male FVB RELMβ−/− and littermate control mice. Liver tumor necrosis factor (TNF)-α (C), mesenteric white adipose tissue (MWAT) F4/80 (D), and TNF-α (E) mRNA levels in antibiotic-treated chow-fed 15-week-old male FVB RELMβ−/− and littermate control mice. N = 4 to 5 per group (A–E). ∗∗P < 0.01 for the difference between the genotypes. WT, wild type.
Metabolic disturbances in the RELMβ−/− mice are associated with colon hyperplasia. Body weights (A and D), colon thickness (B and E), and glucose tolerance (C and F) in 4- to 5-month-old high-fat diet (HFD)-fed male C57/Bl6 RELMβ−/− and control mice from two different litters. N = 4 per group (A–F). ∗∗P < 0.01 for the difference between the genotypes. WT, wild type.
References
- 1.Steppan C.M., Bailey S.T., Bhat S., Brown E.J., Banerjee R.R., Wright C.M., Patel H.R., Ahima R.S., Lazar M.A. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 2.Steppan C.M., Brown E.J., Wright C.M., Bhat S., Banerjee R.R., Dai C.Y., Enders G.H., Silberg D.G., Wen X., Wu G.D., Lazar M.A. A family of tissue-specific resistin-like molecules. Proc Natl Acad Sci U S A. 2001;98:502–506. doi: 10.1073/pnas.98.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rajala M.W., Lin Y., Ranalletta M., Yang X.M., Qian H., Gingerich R., Barzilai N., Scherer P.E. Cell type-specific expression and coregulation of murine resistin and resistin-like molecule-alpha in adipose tissue. Mol Endocrinol. 2002;16:1920–1930. doi: 10.1210/me.2002-0048. [DOI] [PubMed] [Google Scholar]
- 4.He W., Wang M.L., Jiang H.Q., Steppan C.M., Shin M.E., Thurnheer M.C., Cebra J.J., Lazar M.A., Wu G.D. Bacterial colonization leads to the colonic secretion of RELMbeta/FIZZ2, a novel goblet cell-specific protein. Gastroenterology. 2003;125:1388–1397. doi: 10.1016/j.gastro.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Hogan S.P., Seidu L., Blanchard C., Groschwitz K., Mishra A., Karow M.L., Ahrens R., Artis D., Murphy A.J., Valenzuela D.M., Yancopoulos G.D., Rothenberg M.E. Resistin-like molecule beta regulates innate colonic function: barrier integrity and inflammation susceptibility. J Allergy Clin Immunol. 2006;118:257–268. doi: 10.1016/j.jaci.2006.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kushiyama A., Shojima N., Ogihara T., Inukai K., Sakoda H., Fujishiro M., Fukushima Y., Anai M., Ono H., Horike N., Viana A.Y., Uchijima Y., Nishiyama K., Shimosawa T., Fujita T., Katagiri H., Oka Y., Kurihara H., Asano T. Resistin-like molecule beta activates MAPKs, suppresses insulin signaling in hepatocytes, and induces diabetes, hyperlipidemia, and fatty liver in transgenic mice on a high fat diet. J Biol Chem. 2005;280:42016–42025. doi: 10.1074/jbc.M503065200. [DOI] [PubMed] [Google Scholar]
- 7.Herbert D.R., Yang J.Q., Hogan S.P., Groschwitz K., Khodoun M., Munitz A., Orekov T., Perkins C., Wang Q., Brombacher F., Urban J.F., Jr., Rothenberg M.E., Finkelman F.D. Intestinal epithelial cell secretion of RELM-beta protects against gastrointestinal worm infection. J Exp Med. 2009;206:2947–2957. doi: 10.1084/jem.20091268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Artis D., Wang M.L., Keilbaugh S.A., He W., Brenes M., Swain G.P., Knight P.A., Donaldson D.D., Lazar M.A., Miller H.R., Schad G.A., Scott P., Wu G.D. RELMbeta/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proc Natl Acad Sci U S A. 2004;101:13596–13600. doi: 10.1073/pnas.0404034101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McVay L.D., Keilbaugh S.A., Wong T.M., Kierstein S., Shin M.E., Lehrke M., Lefterova M.I., Shifflett D.E., Barnes S.L., Cominelli F., Cohn S.M., Hecht G., Lazar M.A., Haczku A., Wu G.D. Absence of bacterially induced RELMbeta reduces injury in the dextran sodium sulfate model of colitis. J Clin Invest. 2006;116:2914–2923. doi: 10.1172/JCI28121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kushiyama A., Sakoda H., Oue N., Okubo M., Nakatsu Y., Ono H., Fukushima T., Kamata H., Nishimura F., Kikuchi T., Fujishiro M., Nishiyama K., Aburatani H., Kushiyama S., Iizuka M., Taki N., Encinas J., Sentani K., Ogonuki N., Ogura A., Kawazu S., Yasui W., Higashi Y., Kurihara H., Katagiri H., Asano T. Resistin-like molecule beta is abundantly expressed in foam cells and is involved in atherosclerosis development. Arterioscler Thromb Vasc Biol. 2013;33:1986–1993. doi: 10.1161/ATVBAHA.113.301546. [DOI] [PubMed] [Google Scholar]
- 11.Nair M.G., Guild K.J., Du Y., Zaph C., Yancopoulos G.D., Valenzuela D.M., Murphy A., Stevens S., Karow M., Artis D. Goblet cell-derived resistin-like molecule beta augments CD4+ T cell production of IFN-gamma and infection-induced intestinal inflammation. J Immunol. 2008;181:4709–4715. doi: 10.4049/jimmunol.181.7.4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hildebrandt M.A., Hoffmann C., Sherrill-Mix S.A., Keilbaugh S.A., Hamady M., Chen Y.Y., Knight R., Ahima R.S., Bushman F., Wu G.D. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology. 2009;137:1716–1724.e1–e2. doi: 10.1053/j.gastro.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parkin D.M., Bray F., Ferlay J., Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 14.Krimi R.B., Kotelevets L., Dubuquoy L., Plaisancie P., Walker F., Lehy T., Desreumaux P., Van Seuningen I., Chastre E., Forgue-Lafitte M.E., Marie J.C. Resistin-like molecule beta regulates intestinal mucous secretion and curtails TNBS-induced colitis in mice. Inflamm Bowel Dis. 2008;14:931–941. doi: 10.1002/ibd.20420. [DOI] [PubMed] [Google Scholar]
- 15.de Kort S., Keszthelyi D., Masclee A.A. Leaky gut and diabetes mellitus: what is the link? Obes Rev. 2011;12:449–458. doi: 10.1111/j.1467-789X.2010.00845.x. [DOI] [PubMed] [Google Scholar]
- 16.Lee K.N., Lee O.Y. Intestinal microbiota in pathophysiology and management of irritable bowel syndrome. World J Gastroenterol. 2014;20:8886–8897. doi: 10.3748/wjg.v20.i27.8886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rogler G. Chronic ulcerative colitis and colorectal cancer. Cancer Lett. 2014;345:235–241. doi: 10.1016/j.canlet.2013.07.032. [DOI] [PubMed] [Google Scholar]
- 18.Teixeira T.F., Collado M.C., Ferreira C.L., Bressan J., Peluzio Mdo C. Potential mechanisms for the emerging link between obesity and increased intestinal permeability. Nutr Res. 2012;32:637–647. doi: 10.1016/j.nutres.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Neilson A.P., Djuric Z., Land S., Kato I. Plasma levels of resistin-like molecule beta in humans. Cancer Epidemiol. 2011;35:485–489. doi: 10.1016/j.canep.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng L.D., Tong Q.S., Weng M.X., He J., Lv Q., Pu J.R., Jiang G.S., Cai J.B., Liu Y., Hou X.H. Enhanced expression of resistin-like molecule beta in human colon cancer and its clinical significance. Dig Dis Sci. 2009;54:274–281. doi: 10.1007/s10620-008-0355-2. [DOI] [PubMed] [Google Scholar]
- 21.Shojima N., Ogihara T., Inukai K., Fujishiro M., Sakoda H., Kushiyama A., Katagiri H., Anai M., Ono H., Fukushima Y., Horike N., Viana A.Y., Uchijima Y., Kurihara H., Asano T. Serum concentrations of resistin-like molecules beta and gamma are elevated in high-fat-fed and obese db/db mice, with increased production in the intestinal tract and bone marrow. Diabetologia. 2005;48:984–992. doi: 10.1007/s00125-005-1735-1. [DOI] [PubMed] [Google Scholar]
- 22.Rajala M.W., Obici S., Scherer P.E., Rossetti L. Adipose-derived resistin and gut-derived resistin-like molecule-beta selectively impair insulin action on glucose production. J Clin Invest. 2003;111:225–230. doi: 10.1172/JCI16521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka T. Development of an inflammation-associated colorectal cancer model and its application for research on carcinogenesis and chemoprevention. Int J Inflam. 2012;2012:658786. doi: 10.1155/2012/658786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Popivanova B.K., Kitamura K., Wu Y., Kondo T., Kagaya T., Kaneko S., Oshima M., Fujii C., Mukaida N. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest. 2008;118:560–570. doi: 10.1172/JCI32453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mills C.D., Kincaid K., Alt J.M., Heilman M.J., Hill A.M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–6173. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- 26.Evans C., Dalgleish A.G., Kumar D. Review article: immune suppression and colorectal cancer. Aliment Pharmacol Ther. 2006;24:1163–1177. doi: 10.1111/j.1365-2036.2006.03075.x. [DOI] [PubMed] [Google Scholar]
- 27.Hou N., Zhang X., Zhao L., Zhao X., Li Z., Song T., Huang C. A novel chronic stress-induced shift in the Th1 to Th2 response promotes colon cancer growth. Biochem Biophys Res Commun. 2013;439:471–476. doi: 10.1016/j.bbrc.2013.08.101. [DOI] [PubMed] [Google Scholar]
- 28.Osawa E., Nakajima A., Fujisawa T., Kawamura Y.I., Toyama-Sorimachi N., Nakagama H., Dohi T. Predominant T helper type 2-inflammatory responses promote murine colon cancers. Int J Cancer. 2006;118:2232–2236. doi: 10.1002/ijc.21639. [DOI] [PubMed] [Google Scholar]
- 29.Kim E.M., Bae Y.M., Choi M.H., Hong S.T. Cyst formation, increased anti-inflammatory cytokines and expression of chemokines support for Clonorchis sinensis infection in FVB mice. Parasitol Int. 2012;61:124–129. doi: 10.1016/j.parint.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Kato K., Shimozato O., Hoshi K., Wakimoto H., Hamada H., Yagita H., Okumura K. Local production of the p40 subunit of interleukin 12 suppresses T-helper 1-mediated immune responses and prevents allogeneic myoblast rejection. Proc Natl Acad Sci U S A. 1996;93:9085–9089. doi: 10.1073/pnas.93.17.9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abdi K., Singh N.J., Spooner E., Kessler B.M., Radaev S., Lantz L., Xiao T.S., Matzinger P., Sun P.D., Ploegh H.L. Free IL-12p40 monomer is a polyfunctional adaptor for generating novel IL-12-like heterodimers extracellularly. J Immunol. 2014;192:6028–6036. doi: 10.4049/jimmunol.1400159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Becher B., Schlitzer A., Chen J., Mair F., Sumatoh H.R., Teng K.W., Low D., Ruedl C., Riccardi-Castagnoli P., Poidinger M., Greter M., Ginhoux F., Newell E.W. High-dimensional analysis of the murine myeloid cell system. Nat Immunol. 2014;15:1181–1189. doi: 10.1038/ni.3006. [DOI] [PubMed] [Google Scholar]
- 33.Asterholm I.W., McDonald J., Blanchard P.G., Sinha M., Xiao Q., Mistry J., Rutkowski J.M., Deshaies Y., Brekken R.A., Scherer P.E. Lack of “immunological fitness” during fasting in metabolically challenged animals. J Lipid Res. 2012;53:1254–1267. doi: 10.1194/jlr.M021725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reigstad C.S., Lunden G.O., Felin J., Backhed F. Regulation of serum amyloid A3 (SAA3) in mouse colonic epithelium and adipose tissue by the intestinal microbiota. PLoS One. 2009;4:e5842. doi: 10.1371/journal.pone.0005842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wernstedt Asterholm I., Tao C., Morley T.S., Wang Q.A., Delgado-Lopez F., Wang Z.V., Scherer P.E. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 2014;20:103–118. doi: 10.1016/j.cmet.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim D.H., Gutierrez-Aguilar R., Kim H.J., Woods S.C., Seeley R.J. Increased adipose tissue hypoxia and capacity for angiogenesis and inflammation in young diet-sensitive C57 mice compared with diet-resistant FVB mice. Int J Obes (Lond) 2013;37:853–860. doi: 10.1038/ijo.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wallace J.G., Gohir W., Sloboda D.M. The impact of early life gut colonization on metabolic and obesogenic outcomes: what have animal models shown us? J Dev Orig Health Dis. 2016;7:15–24. doi: 10.1017/S2040174415001518. [DOI] [PubMed] [Google Scholar]
- 38.Sobhani I., Amiot A., Le Baleur Y., Levy M., Auriault M.L., Van Nhieu J.T., Delchier J.C. Microbial dysbiosis and colon carcinogenesis: could colon cancer be considered a bacteria-related disease? Therap Adv Gastroenterol. 2013;6:215–229. doi: 10.1177/1756283X12473674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Westbrook A.M., Szakmary A., Schiestl R.H. Mechanisms of intestinal inflammation and development of associated cancers: lessons learned from mouse models. Mutat Res. 2010;705:40–59. doi: 10.1016/j.mrrev.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaishnava S., Yamamoto M., Severson K.M., Ruhn K.A., Yu X., Koren O., Ley R., Wakeland E.K., Hooper L.V. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334:255–258. doi: 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monteleone G., Pallone F., Stolfi C. The dual role of inflammation in colon carcinogenesis. Int J Mol Sci. 2012;13:11071–11084. doi: 10.3390/ijms130911071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dunn G.P., Koebel C.M., Schreiber R.D. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 43.Barderas R., Bartolome R.A., Fernandez-Acenero M.J., Torres S., Casal J.I. High expression of IL-13 receptor alpha2 in colorectal cancer is associated with invasion, liver metastasis, and poor prognosis. Cancer Res. 2012;72:2780–2790. doi: 10.1158/0008-5472.CAN-11-4090. [DOI] [PubMed] [Google Scholar]
- 44.Koller F.L., Hwang D.G., Dozier E.A., Fingleton B. Epithelial interleukin-4 receptor expression promotes colon tumor growth. Carcinogenesis. 2010;31:1010–1017. doi: 10.1093/carcin/bgq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hyun Y.S., Han D.S., Lee A.R., Eun C.S., Youn J., Kim H.Y. Role of IL-17A in the development of colitis-associated cancer. Carcinogenesis. 2012;33:931–936. doi: 10.1093/carcin/bgs106. [DOI] [PubMed] [Google Scholar]
- 46.Wu S., Rhee K.J., Albesiano E., Rabizadeh S., Wu X., Yen H.R., Huso D.L., Brancati F.L., Wick E., McAllister F., Housseau F., Pardoll D.M., Sears C.L. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Simone V., Pallone F., Monteleone G., Stolfi C. Role of T17 cytokines in the control of colorectal cancer. Oncoimmunology. 2013;2:e26617. doi: 10.4161/onci.26617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Allen J.E., Sutherland T.E., Ruckerl D. IL-17 and neutrophils: unexpected players in the type 2 immune response. Curr Opin Immunol. 2015;34:99–106. doi: 10.1016/j.coi.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 49.Witowski J., Ksiazek K., Jorres A. Interleukin-17: a mediator of inflammatory responses. Cell Mol Life Sci. 2004;61:567–579. doi: 10.1007/s00018-003-3228-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakajima S., Kitoh A., Egawa G., Natsuaki Y., Nakamizo S., Moniaga C.S., Otsuka A., Honda T., Hanakawa S., Amano W., Iwakura Y., Nakae S., Kubo M., Miyachi Y., Kabashima K. IL-17A as an inducer for Th2 immune responses in murine atopic dermatitis models. J Invest Dermatol. 2014;134:2122–2130. doi: 10.1038/jid.2014.51. [DOI] [PubMed] [Google Scholar]
- 51.Sutherland T.E., Logan N., Ruckerl D., Humbles A.A., Allan S.M., Papayannopoulos V., Stockinger B., Maizels R.M., Allen J.E. Chitinase-like proteins promote IL-17-mediated neutrophilia in a tradeoff between nematode killing and host damage. Nat Immunol. 2014;15:1116–1125. doi: 10.1038/ni.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen F., Wu W., Millman A., Craft J.F., Chen E., Patel N., Boucher J.L., Urban J.F., Jr., Kim C.C., Gause W.C. Neutrophils prime a long-lived effector macrophage phenotype that mediates accelerated helminth expulsion. Nat Immunol. 2014;15:938–946. doi: 10.1038/ni.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen F., Liu Z., Wu W., Rozo C., Bowdridge S., Millman A., Van Rooijen N., Urban J.F., Jr., Wynn T.A., Gause W.C. An essential role for TH2-type responses in limiting acute tissue damage during experimental helminth infection. Nat Med. 2012;18:260–266. doi: 10.1038/nm.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kinyanjui M.W., Shan J., Nakada E.M., Qureshi S.T., Fixman E.D. Dose-dependent effects of IL-17 on IL-13-induced airway inflammatory responses and airway hyperresponsiveness. J Immunol. 2013;190:3859–3868. doi: 10.4049/jimmunol.1200506. [DOI] [PubMed] [Google Scholar]
- 55.Soares M.P., Gozzelino R., Weis S. Tissue damage control in disease tolerance. Trends Immunol. 2014;35:483–494. doi: 10.1016/j.it.2014.08.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Serum RELMβ levels in wild-type and RELMβ−/− mice. Representative Western blot from >3 independent experiments where immunoprecipitates of 100 μL serum with rabbit polyclonal anti-mouse RELMβ antibodies immobilized to Protein A Sepharose were visualized. Purified RELMβ protein (produced in HEK-293T cells) was loaded as a positive control. IgG light chains in both immunoprecipitate lanes are indicated with arrows. WT, wild type.
Increased susceptibility for dextran sodium sulfate (DSS)/azoxymethane (AOM)–induced colorectal cancer development in RELMβ−/− FVB mice. Tumor count (A) and size (B) and colon appearance on day 85 in 8-month-old male FVB RELMβ−/− and littermate control mice in response to AOM/DSS treatment regimen (Figure 1A). N = 8 knockout (A and B); N = 12 wild type (A and B). ∗P < 0.05, ∗∗P < 0.01 for the difference between the genotypes. WT, wild type.
Skewed T helper cell 1 (Th1)/Th2-type cytokine balance in RELMβ−/− mice. Colon IL-4 (A), IL-5 (B), GATA3 (C), IL-10 (D), Tbet (E), and tumor necrosis factor (TNF)-α (F) mRNA levels, and serum amyloid A (SAA; G) and IL-12-p40 (H) levels during the azoxymethane/dextran sodium sulfate treatment time course (baseline, day 7, 28, and 85) in 11- to 12-week-old female FVB RELMβ−/− mice and littermate controls. N = 4 to 7 per genotype and time point (A–H). ∗P < 0.05, ∗∗P < 0.01 for the difference between the genotypes (there is also a trend for a difference in IL-4 expression at day 7 between genotypes, P = 0.07); †P < 0.05, ††P < 0.01, and †††P < 0.001 for the difference from baseline within a genotype; and ‡‡P < 0.01 for the overall difference between genotype. WT, wild type.
IL-13+ cells are Gr1/Ly6G+high leukocytes. CD4 versus Gr1 fluorescence-activated cell sorter plots of living leukocytes from mesenteric lymph nodes (A) and back gating of the IL-13+ cells (B) in these plots. Tissues were obtained from 5- to 6-month-old FVB RELMβ−/− females and littermate controls after 24 weeks in response to six weekly azoxymethane injections.
IL-13+ cells are Gr1/Ly6G+high leukocytes. CD4 versus Gr1 fluorescence-actived cell sorter plots of living leukocytes from colon tumors (A) and back gating of the IL-13+ cells in these plots (B). Tissues were obtained from 5- to 6-month-old FVB RELMβ−/− females and littermate controls after 24 weeks in response to six weekly azoxymethane injections.
Ablation of gut microbiota protects against metabolic disturbances in the RELMβ−/− FVB mice. Body weight (A) and glucose tolerance (B) in antibiotic-treated chow-fed 11-week-old male FVB RELMβ−/− and littermate control mice. Liver tumor necrosis factor (TNF)-α (C), mesenteric white adipose tissue (MWAT) F4/80 (D), and TNF-α (E) mRNA levels in antibiotic-treated chow-fed 15-week-old male FVB RELMβ−/− and littermate control mice. N = 4 to 5 per group (A–E). ∗∗P < 0.01 for the difference between the genotypes. WT, wild type.
Metabolic disturbances in the RELMβ−/− mice are associated with colon hyperplasia. Body weights (A and D), colon thickness (B and E), and glucose tolerance (C and F) in 4- to 5-month-old high-fat diet (HFD)-fed male C57/Bl6 RELMβ−/− and control mice from two different litters. N = 4 per group (A–F). ∗∗P < 0.01 for the difference between the genotypes. WT, wild type.