Abstract
Background and objectives
Awareness of CKD is necessary for patient engagement and adherence to medical regimens. Having an accurate tool to assess awareness is important. Use of the National Health and Nutrition Examination Survey (NHANES) CKD awareness question “Have you ever been told by a doctor or other health professional that you had weak or failing kidneys (excluding kidney stones, bladder infections, or incontinence)?” produces surprisingly low measures of CKD awareness. We sought to compare the sensitivity and specificity of different questions ascertaining awareness of CKD and other health conditions.
Design, setting, participants, & measurements
Between August of 2011 and August of 2014, an in-person questionnaire was administered to 220 adults with CKD, diabetes, hypertension, or hyperlipidemia who received primary care in a public health care delivery system to ascertain awareness of each condition. CKD awareness was measured using the NHANES question, and other questions, asking if patients knew about their “kidney disease”, “protein in the urine”, “kidney problem”, or “kidney damage.” Demographic data were self-reported; health literacy was measured. The sensitivity and specificity of each question was calculated using the medical record as the gold standard.
Results
In this diverse population (9.6% white, 40.6% black, 36.5% Hispanic, 12.3% Asian), the mean age was 58 years, 30% had a non-English language preference, and 45% had low health literacy. Eighty percent of participants had CKD, with a mean eGFR of 47.2 ml/min per 1.73 m2. The sensitivities of each CKD awareness question were: 26.4% for “kidney damage”, 27.7% for “kidney disease”, 33.2% for “weak or failing kidneys”, 39.8% for “protein in the urine”, and 40.1% for “kidney problem.” Specificities ranged from 82.2% to 97.6%. The best two-question combination yielded a sensitivity of 53.1% and a specificity of 83.3%. This was lower than awareness of hypertension (90.1%) or diabetes (91.8%).
Conclusions
CKD awareness is low compared with other chronic diseases regardless of how it is ascertained. Nevertheless, more sensitive questions to ascertain CKD awareness suggest current under-ascertainment.
Keywords: chronic kidney disease; awareness; diabetes mellitus; Health Literacy; Humans; hypertension; kidney; Kidney Calculi; Language; Medical Records; Patient Participation; Renal Insufficiency, Chronic
Introduction
Awareness of chronic diseases is necessary for patient engagement and adherence to medical regimens known to improve health outcomes (1). Increasing awareness of CKD is a priority for many public health organizations, including the National Kidney Disease Education Program and the Centers for Disease Control and Prevention. Consistent with this is the Healthy People 2020 goal of increasing the proportion of persons with CKD who know they have impaired renal function from an age-adjusted prevalence of 9.4%–13.4% (2).
Accurately assessing CKD awareness is key to estimating the effect of national awareness campaigns. Current estimates of CKD awareness, including those that assess progress toward Healthy People 2020 goals, come from the National Health and Nutrition Examination Survey (NHANES), which examines trends in disease prevalence in cross-sectional representative samples of noninstitutionalized United States residents (3). Since 1994, CKD awareness has been ascertained with the question “Have you ever been told by a doctor or other health professional that you had weak or failing kidneys (excluding kidney stones, bladder infections, or incontinence)?” National estimates have been low, ranging from 4% to 9%, including estimates of <50% among individuals with stage 4 CKD (4).
There are reasons to believe that this measure of CKD awareness may lack sensitivity and may be providing flawed estimates. The NHANES awareness question relies on language (“weak or failing kidneys”) that is not often used by clinicians to describe kidney disease. And, unlike individuals aware of other chronic illnesses, such as hypertension, congestive heart failure, or hyperlipidemia, studies have demonstrated that individuals aware of their “weak or failing kidneys” are not more likely to achieve outcomes associated with guideline-concordant care (5).
We sought to compare the sensitivity and specificity of different questions ascertaining CKD awareness, as well as questions measuring awareness of other chronic conditions, in one population of primary care patients. We hypothesized that the NHANES question had low sensitivity but high specificity and that other questions were more sensitive. We also hypothesized that CKD awareness would be lower than awareness of hypertension, diabetes, and hyperlipidemia because of low awareness of, and confidence managing CKD among primary care providers (PCPs) compared with other chronic diseases (6,7).
Materials and Methods
Study Design, Setting, and Participants
This is a cross-sectional study of individual awareness of chronic medical conditions among patients with a routine site for health care. Between August of 2011 and August of 2014, we administered a questionnaire to a convenience sample of 220 English-, Spanish-, and Cantonese-speaking patients who received primary care in the San Francisco Health Network, the integrated public health care delivery system for San Francisco’s uninsured and publically insured residents. After receiving approval from providers to approach their patients, individuals were recruited in person from one clinic (n=83 of a total clinic population of 5655) at dates and times convenient for the research staff or by telephone from two other clinics (n=137 of a total clinic population of 13,421) by language-concordant research assistants. Eligible patients were ≥18 years of age, had seen their PCP at least once in the previous 2 years, and had CKD, hypertension, diabetes, or hyperlipidemia (defined below). Patients who could not read or actively participate in a discussion with health care providers about chronic medical conditions (e.g., very hard of hearing, dementia, active psychosis) were excluded from this study. All study participants provided written consent before participating. A total of 137 patients were concordantly offered the opportunity to participate in a trial examining the effect of self-management support on BP control among patients with CKD (8). The overall study sample was thus enriched with patients with CKD. All study participants received a gift card to a local grocery store and were blinded to the purpose of the research study. This study was approved by the Committee on Human Research at the University of California, San Francisco.
Measurements
The written questionnaire ascertained individual awareness of CKD, diabetes, hypertension, and hyperlipidemia (Supplemental Figure 1). It was developed in English and subsequently forward and backward translated into Spanish and Cantonese by a Committee on Human Research–approved neutral party. CKD awareness was assessed using five different questions, including the question used by NHANES: “Have you ever been told by a doctor or health care provider that you have weak or failing kidneys (excluding kidney stones, bladder infections or incontinence)?” On the basis of use in other studies (9,10) and clinical experience, we also asked if each patient had “ever been told by a doctor or health care professional that” they had: (1) “kidney disease”, (2) “protein in the urine”, (3) “a kidney problem”, or (4) that their “kidneys are damaged.” Awareness of hypertension and diabetes were ascertained with questions similar to those used by NHANES: “Have you ever been told by a doctor or health care provider that you have” (1) “high blood pressure” or (2) “diabetes or problems with high blood sugar?” Hyperlipidemia awareness was assessed by asking about “high cholesterol.” Self-reported demographic information (age, gender, race/ethnicity, educational attainment, health insurance) was also obtained. Health literacy was determined with a validated three-item instrument (11) that included the following questions with Likert-style response scales: (1) “How often do you have someone help you read hospital materials?”; (2) “How confident are you filling out medical forms by yourself?”; and (3) “How often do you have problems learning about your medical conditions because of difficulty understanding written information?” An individual was considered to have limited health literacy if he or she had a score ≥6 of a maximal score of 12 (12).
Definitions
Comorbid conditions and laboratory data were ascertained from each patient’s medical record. CKD was defined by two values of Modification of Diet in Renal Disease (MDRD) eGFR<60 ml/min per 1.73 m2 (the GFR-estimating equation reported in the electronic medical record) separated by a minimum of 3 months or presence of persistent proteinuria, defined by two values of albuminuria >30 mg/g or ≥1+ dipstick proteinuria over a minimum 3-month period. Diabetes was defined by a glycosylated hemoglobin ≥6.5% or use of diabetes medications commonly used in the San Francisco Health Network (insulin, metformin, glyburide, or glipizide). Hyperlipidemia was defined by an LDL cholesterol level >130 mg/dl or prescription of a statin medication; hypertension was defined as the last clinic BP>140/90 mmHg or prescription of the following antihypertensive medications: diuretics, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta blockers, calcium channel blockers, nitrate, hydralazine, clonidine, or minoxidil.
Statistical Analyses
Characteristics of participants were compared by CKD status and by the number of studies for which they were recruited using chi-squared and ANOVA as appropriate. Using the electronic medical record as the gold standard, sensitivity (true-positive rate or the proportion of participants who had CKD who answered yes correctly, otherwise known as CKD awareness) and specificity (true-negative rate or the proportion of participants who did not have CKD who answered no correctly) of each awareness question were calculated for the entire study population by health literacy and CKD stage. Data from individual questions were used to calculate sensitivities and specificities of combinations of questions. Data from individual CKD awareness questions were compared with the sensitivities and specificities of questions ascertaining awareness of diabetes, hypertension, and hyperlipidemia. Sensitivities and specificities of questions ascertaining CKD and hypertension awareness were also ascertained from 2009 to 2012 NHANES participants that were most similar to the study population: adults >18 years of age that reported having at least one health care visit with a provider within the prior year (n=8629 for CKD awareness; n=9041 for hypertension awareness). In NHANES, CKD was defined by single values of MDRD eGFR<60 ml/min per 1.73 m2 or presence of albuminuria >30 mg/g. Hypertension was defined by an average BP of >140/90 mmHg or use of anti-hypertensive medications. Nationally representative estimates of sensitivity and specificity were calculated using appropriate weights using the “SVY” commands in SAS 9.4 (SAS Institute, Inc., Cary, NC).
Results
Study Participants
Overall, 328 participants were approached and 108 declined to participate, giving a 67.1% recruitment rate. The study population was evenly balanced by gender, had a mean age of 58 years, and was racially and ethnically diverse with 9.6% non-Hispanic white, 36.5% Hispanic, 40.6% black, and 12.3% Asian (Table 1). Nearly 30% of the cohort had a non-English language preference and nearly 50% had limited health literacy. Demographic characteristics were similar among those with (n=176) and without CKD (n=44), with statistically significant differences only noted in educational attainment (P=0.004). Characteristics were also similar among study participants recruited for one study (n=83) versus two studies (n=137) (data not shown). Individuals who declined to participate in the study (n=108) were similar to those who agreed to participate, with statistically significant differences only noted in the proportion of individuals with public health insurance compared with no insurance (P=0.004) (data not shown).
Table 1.
Demographic characteristics of study population
| Characteristics | All | No CKD | CKD |
|---|---|---|---|
| n=220 | n=44 | n=176 | |
| Mean age, yrs (SD) | 58.2 (13.2) | 58.9 (11.6) | 47.2 (10.1) |
| Male, % (N) | 50.5 (111) | 61.4 (27) | 47.7 (84) |
| Race/ethnicity, % (N) | |||
| Non-Hispanic white | 9.6 (21) | 29.6 (6) | 8.5 (15) |
| Hispanic | 36.5 (80) | 13.6 (13) | 38.1 (67) |
| Black | 40.6 (89) | 40.9 (18) | 40.3 (70) |
| Asian | 12.3 (27) | 2.3 (1) | 5.7 (10) |
| Other | 1.4 (3) | 13.6 (6) | 7.4 (13) |
| Interview language, % (N) | |||
| English | 70.3 (154) | 90.9 (40) | 65.1 (114) |
| Spanish | 26.0 (57) | 9.1 (4) | 30.3 (53) |
| Cantonese | 3.7 (8) | 0 (0) | 4.6 (8) |
| Educational attainmenta, % (N) | |||
| Did not attend | 15.9 (35) | 11.4 (5) | 17.1 (30) |
| Primary school | 41.4 (91) | 27.3 (12) | 44.9 (79) |
| High school | 25.9 (57) | 22.7 (10) | 26.7 (47) |
| College | 16.8 (37) | 38.6 (17) | 11.4 (20) |
| Low health literacy, % (N) | 45.0 (99) | 38.6 (17) | 46.6 (82) |
| Health insuranceb, % (N) | |||
| None | 30.3 (54) | 33.3 (4) | 30.1 (50) |
| Medicaid | 62.4 (111) | 66.7 (8) | 62.1 (103) |
| Medicare | 22.5 (40) | 0% (0) | 24.1 (40) |
| Hypertension, % (N) | |||
| BP≥140/90 mmHg | 30 (67) | 18 (8) | 34 (59) |
| Use of antihypertensive medications | 84 (184) | 50 (22) | 92 (162) |
| Mean eGFR, ml/min per 1.73 m2 (SD) | 47.2 (10.1) | NA | 47.2 (10.1) |
| CKD stages, % (N) | |||
| Stage 1–2 | 23.6 (52) | NA | 32.3 (52) |
| Stage 3–4 | 49.5 (109) | NA | 61.9 (109) |
| Proteinuria (n=171), % (N) | |||
| Yes | 48.3 (83) | 0 (0) | 47.2 (83) |
| No | 40.0 (88) | 100 (7) | 48.0 (81) |
| Number of primary care clinic visits in the past year, median (IQR) | 4 (3, 6) | 3 (1, 4) | 4 (3, 6) |
NA, not applicable; IQR, interquartile range.
Denotes P<0.05 for comparison among groups with and without CKD.
Sums to >100%, as groups are not mutually exclusive.
Sensitivity and Specificity of CKD Awareness Questions
Sensitivities of CKD awareness questions ranged from 26.4% for “kidney damage” to 40.1% for “kidney problem.” Specificities ranged from 82.2% for “protein in the urine” to 97.6% for “kidney damage.” Sensitivity of the CKD awareness question used by NHANES (“weak or failing kidneys”) was 33.2% in this study population (Table 2). This was higher than the 7.2% noted among comparable 2009–2012 NHANES adult participants with CKD. Specificity of this question was similar in both study populations (92.9% in this study; 98.9% in NHANES).
Table 2.
Sensitivity and specificity of individual CKD awareness questions overall and stratified by language
| Awareness Question | Overall Study Population (n=220) | English (n=114) | Spanish (n=53) | P Value for Difference in Sensitivities by Language | |||
|---|---|---|---|---|---|---|---|
| Sensitivity, % | Specificity, % | Sensitivity, % | Specificity, % | Sensitivity, % | Specificity, % | ||
| “Weak or failing kidneys” (NHANES) | 33.2 | 92.9 | 35.6 | 89.7 | 34.2 | 100.0 | 0.83 |
| “Kidney disease” | 27.7 | 92.9 | 25.7 | 89.7 | 46.0 | 100.0 | 0.04 |
| “Kidney problem” | 40.1 | 90.5 | 40.0 | 86.2 | 42.1 | 100.0 | 0.93 |
| “Kidney damage” | 26.4 | 97.6 | 26.7 | 96.4 | 34.2 | 100.0 | 0.37 |
| “Protein in the urine”a | 39.8 | 82.2 | 31.3 | 82.8 | 53.0 | 62.5 | 0.01 |
NHANES, National Health and Nutrition Examination Survey.
Among those with proteinuria only.
Higher sensitivities of CKD awareness questions were noted among study participants with more advanced CKD (Table 3). Among study participants with CKD stages 1–2, sensitivities ranged from 13.5% (“kidney damage”) to 30.8% (“protein in the urine”). Among those with CKD stages 3–4, sensitivities ranged from 33.0% (“kidney damage”) to 48.2% (“kidney problem”). The question asking about “weak or failing kidneys” was associated with higher sensitivity among study participants with stages 1–2 CKD compared with similar NHANES participants (19.2% versus 3.2% in NHANES), as well as among those with CKD stages 3–4 (43.1% versus 10.5% in NHANES). There were no statistically significant differences in sensitivities when the study population was stratified by health literacy (data not shown), although there were some differences in sensitivities when stratified by language (Table 2).
Table 3.
Sensitivity of individual CKD awareness questions, stratified by CKD stage
| Awareness Question | CKD Stage 1–2 (n=52) | CKD Stage 3–4 (n=109) | P Value |
|---|---|---|---|
| Sensitivity (%) | Sensitivity (%) | ||
| “Weak or failing kidneys” (NHANES) | 19.2 | 43.1 | <0.01 |
| “Kidney disease” | 17.3 | 33.3 | 0.03 |
| “Kidney problem” | 25.0 | 48.2 | 0.01 |
| “Kidney damage” | 13.5 | 33.0 | 0.01 |
| “Protein in the urine”a | 30.8 | 41.7 | 0.36 |
NHANES, National Health and Nutrition Examination Survey.
Among those with proteinuria only.
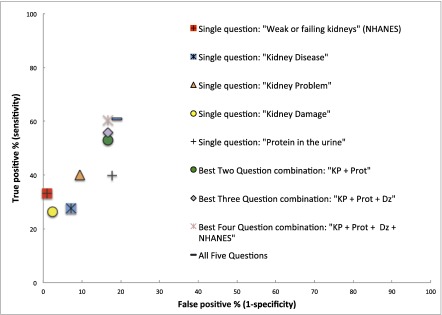
Combinations of questions were associated with much higher sensitivities and somewhat lower specificities compared with single questions of CKD awareness (Figure 1). For example, the two-question combination with the highest sensitivity (“kidney problem” and “protein in the urine”) was associated with a near-doubling of sensitivity compared with the single NHANES question (53.1% versus 33.2%) whereas the specificity changed by a lesser amount (83.3% for the combination versus 92.9% for the NHANES question alone). Combining all five questions yielded a sensitivity of 61.1% and a specificity of 81.0%.
Figure 1.
Awareness of CKD (percentage of true positive responses) differs by how patients are asked. Percentage of true-positive (sensitivity) and false-positive (1-specificity) responses by patients with and without kidney disease answering: “Have you even been told by a doctor or health care provider that you have kidney disease,” using different descriptions of kidney disease. Dz, ”Kidney Disease“; KP, ”Kidney Problem“; NHANES, National Health and Nutrition Examination Survey; Prot, “Protein in the Urine”.
CKD Awareness Compared with Other Chronic Medical Conditions
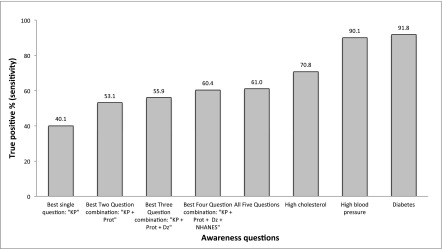
Sensitivities of questions ascertaining CKD awareness were lower than those ascertaining awareness of other chronic medical conditions in this study population (Figure 2) as well as in NHANES. Over 70% of study participants with hyperlipidemia correctly answered that they had “high cholesterol” and over 90% of study participants with hypertension and diabetes correctly answered that they had “high blood pressure” and “diabetes or problems with high blood sugar”, respectively. Specificities of these questions were lower than the CKD awareness questions, ranging from 55.6% for cholesterol, to 60.7% for hypertension, and to 73.1% for diabetes. Among 2009–2012 NHANES participants who reported seeing a health care provider at least once within the past year (n=9041), the sensitivity of the hypertension awareness question was 78.2% and its specificity was 88.2%.
Figure 2.
Percentage of true-positive (sensitivity) responses among patients with chronic health conditions answering whether a provider had ever told them that they had kidney disease, high cholesterol, high BP, or diabetes. The first five bars represent different questions used to ask about kidney disease (see text or Figure 1 for phrasing). Dz, ”Kidney Disease“; KP, ”Kidney Problem“ NHANES, National Health and Nutrition Examination Survey; Prot, ”Protein in the Urine“.
Discussion
This study has two key findings. First, it provides strong circumstantial evidence that current estimates of CKD awareness (4) used to guide important health care policies, such as the goals of the Healthy People 2020 initiative, may be substantially underestimating the true prevalence of CKD awareness by relying on single questions of awareness that may not be understandable by patients and do not ask about proteinuria. Second, regardless of how CKD awareness questions are phrased, awareness of CKD is still lower than awareness of other chronic medical conditions among patients actively engaged in one health care system.
While several studies have confirmed poor knowledge and general understanding about kidney disease (13–15), national estimates of CKD awareness have largely relied on measures of awareness that use language that may not resonate well with individuals with kidney disease (4). NHANES uses the phrase “weak or failing kidneys.” Other geographically diverse studies of CKD awareness have used the phrase “chronic kidney disease” (9,16,17). Estimates of CKD awareness using these phrases have been remarkably similar, around 6%–12%. In our study population, awareness of “weak or failing kidneys” and “kidney disease” were 32.4% and 27.2%, respectively. Higher CKD awareness in our population may be because of more frequent discussions of CKD between providers and patients than in the general community resulting from the regularity of patient visits with their PCPs (median of four visits in the year before this study) and/or the higher risk of kidney disease in our racially/ethnically diverse, poor population.
Despite the higher prevalence of awareness in our study population, we noted that measures of awareness depended on the description of CKD used during ascertainment. For example, the two individual awareness questions with the highest sensitivities in our study used the phrases “protein in the urine” (39.8%) and “kidney problem” (40.1%). When combined, these two questions also yielded the highest sensitivity of CKD awareness among two-question combinations (53.1%), suggesting that they may capture awareness among slightly different patient populations. To our knowledge, neither of these questions has been used to measure CKD awareness in other studies, but they do have face value and may be more understandable to patients than existing awareness questions.
CKD awareness campaigns that target providers (18) and written patient educational materials (19) do not use the phrase “weak or failing kidneys”. Most high-quality educational materials use the phrase “chronic kidney disease” and “protein in the urine” or “albumin in the urine” to introduce to patients the two different laboratory abnormalities that define CKD. The Kidney Disease Improving Global Outcomes guidelines also emphasize albuminuria as a risk factor for CKD progression, development of ESRD, cardiovascular disease, and mortality (20). Including proteinuria or albuminuria in a measure of CKD awareness is thus important. How to ask patients about low eGFR remains unclear. In our study, CKD awareness was higher when asking patients whether they had a “kidney problem” compared with “kidney disease.” While speculative, this may reflect provider word choice when discussing CKD with patients. This is consistent with prior studies that have depicted provider anxiety about disclosure of a kidney disease diagnosis (21), leading to to deficiencies in patient–physician discussions around CKD in primary care (22–24).
In the absence of a perfect measure of CKD awareness, we must consider the trade-offs between sensitivity and specificity and the reason for measuring awareness. Arguably, the reason for measuring awareness is to evaluate the success of public health campaigns about CKD for the overall population and among specific groups. In this context, sensitivity of awareness questions could be considered a measure of campaign success, i.e., percentage of the population with kidney disease that has been told about kidney disease and believes that it has CKD. Specificity can be considered a measure of message accuracy, as low specificity equates to a high percentage of false-positive answers from individuals who inaccurately think that they have kidney disease. For public health officials who must determine how best to spend limited resources on public health campaigns, using a measure with high sensitivity may be important in determining the content and audiences of future campaigns (25).
The NHANES question related to kidney disease awareness has evolved over the years. We suggest performing a pilot study in NHANES with a new CKD awareness question to potentially obtain a more accurate nationally representative measure. If results of the pilot study are similar to those from this study, a more permanent change could be adopted. On the basis of this study’s results, we propose using the following combined awareness question: “Have you ever been told by a doctor or other health provider that you had a kidney problem or protein in the urine?” It is important to note that sensitivity was higher in our study with combinations of three or four questions compared to combinations of two questions, as we propose here. However, long combination questions may score less well with readability instruments due to their length and may be more difficult to answer among individuals with limited health literacy (26), which is common among patients with CKD (27–29). It is also important to note that compound questions have a tendency to yield less consistent answers than single questions, as they may cause misunderstanding among responders (30,31). However, the specific combination question that we propose captures the two essential elements that define CKD and has few syllables and short words. Also, sensitivity and specificity of each question in the proposed combination did not differ among patients with adequate and limited health literacy.
The results of this study should be considered within the context of its limitations. For example, our study population is not generalizable, as participants were from a single health care system that is focused on providing care to a low-income, urban population. Thus, we cannot provide a national estimate of CKD awareness. However, we can be confident in our within-population differences in sensitivities and specificities of awareness questions. Study participants were considered to have hypertension if their last clinic visit BP ≥140/90 mmHg or if they were prescribed antihypertensive medications. While few study participants were given the diagnosis of hypertension solely on the basis of measurement, reliance on clinical BP measures at one time point can lead to misclassification. Also, sensitivity and specificity estimates of CKD awareness using NHANES data rely on single measures of eGFR and albuminuria rather than the two repeated measures used in our study, potentially explaining the large discrepancy in CKD awareness using the same question in both populations.
Nevertheless, this study shows that by using a more sensitive combination of questions to ascertain CKD awareness, estimates of awareness may increase by 1.6-fold in certain patient populations (from 33.2% with “weak or failing kidneys” to 53.1% with “kidney problem or protein in the urine”). While we cannot directly apply this factor to current national estimates of awareness, our data suggest that CKD awareness may have exceeded the Healthy People 2020 target of 13.4% (2). However, CKD awareness remains lower than awareness of diabetes and hypertension (32). Similar to CKD, diabetes and hypertension are often asymptomatic yet still require active patient self-management, including daily medication adherence. Higher awareness of these two diseases is likely a result of a more effective public health strategy, including linking specific medications to disease management. Perhaps future kidney disease awareness campaigns in the United States could highlight the importance of medications for kidney health, including angiotensin-converting enzyme inhibitors and angiotensin receptor blockers, and emphasize the link between kidney disease and hypertension and diabetes. Coupling these chronic diseases may remind providers to mention “kidney disease” or “kidney problem” more often during health care visits, thereby increasing awareness among patients with CKD. This strategy has been successful for the Indian Health Service, where kidney disease and diabetes have been intricately linked and have resulted in lower incidence of ESRD secondary to diabetic nephropathy in the Indian population of the southwestern United States (33).
In summary, we have demonstrated that CKD awareness is low among patients with kidney disease in primary care, particularly compared with awareness of diabetes and hypertension, but that a more sensitive measure of awareness suggests that we have likely met the Healthy People 2020 goal related to CKD awareness. These data do not advocate complacency. On the contrary, they suggest that the nephrology community should consider investing resources to further increase its reach, perhaps with social marketing campaigns. Understanding the phrases about kidney disease that are most comprehensible by patients with, and at risk of CKD is one important first step to further improve upon our metrics of measuring awareness. Devising new tools to help PCPs discuss kidney disease is another. These efforts will allow us to better target future awareness messages and also allow us to more accurately evaluate campaign successes.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank participants from the San Francisco Health Network.
This study was funded by grants K23DK094850, R34DK093992, K24DK92291, and R01DK104130, all from the National Institute of Diabetes and Digestive and Kidney diseases.
Data were presented in poster format at the 2015 Kidney Week in San Diego, CA, November 3–8, 2015.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.00490116/-/DCSupplemental.
References
- 1.Bodenheimer T, Lorig K, Holman H, Grumbach K: Patient self-management of chronic disease in primary care. JAMA 288: 2469–2475, 2002 [DOI] [PubMed] [Google Scholar]
- 2.United States Department of Health and Human Services: Healthy People 2020 Topics and Objectives, 2014. Available at: http://www.healthypeople.gov/2020/topics-objectives/topic/chronic-kidney-disease/objectives. Accessed May 20, 2016.
- 3.National Center for Health Statistics: Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–94. Series 1: program and collection procedures. Vital Health Stat 1 32: 1–407, 1994 [PubMed]
- 4.Plantinga LC, Boulware LE, Coresh J, Stevens LA, Miller ER 3rd, Saran R, Messer KL, Levey AS, Powe NR: Patient awareness of chronic kidney disease: trends and predictors. Arch Intern Med 168: 2268–2275, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tuot DS, Plantinga LC, Hsu CY, Powe NR: Is awareness of chronic kidney disease associated with evidence-based guideline-concordant outcomes? Am J Nephrol 35: 191–197, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plantinga LC, Tuot DS, Powe NR: Awareness of chronic kidney disease among patients and providers. Adv Chronic Kidney Dis 17: 225–236, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tahir MA, Dmitrieva O, de Lusignan S, van Vlymen J, Chan T, Golmohamad R, Harris K, Tomson C, Thomas N, Gallagher H: Confidence and quality in managing CKD compared with other cardiovascular diseases and diabetes mellitus: a linked study of questionnaire and routine primary care data. BMC Fam Pract 12: 83, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tuot DS, Velasquez A, McCulloch CE, Banerjee T, Zhu Y, Hsu CY, Handley M, Schillinger D, Powe NR: The Kidney Awareness Registry and Education (KARE) study: protocol of a randomized controlled trial to enhance provider and patient engagement with chronic kidney disease. BMC Nephrol 16: 166, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tuot DS, Plantinga LC, Judd SE, Muntner P, Hsu CY, Warnock DG, Gutiérrez OM, Safford M, Powe NR, McClellan WM; REGARDS Investigators : Healthy behaviors, risk factor control and awareness of chronic kidney disease. Am J Nephrol 37: 135–143, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saab G, Whaley-Connell AT, McCullough PA, Bakris GL: CKD awareness in the United States: the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis 52: 382–383, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Chew LD, Griffin JM, Partin MR, Noorbaloochi S, Grill JP, Snyder A, Bradley KA, Nugent SM, Baines AD, Vanryn M: Validation of screening questions for limited health literacy in a large VA outpatient population. J Gen Intern Med 23: 561–566, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris NS, MacLean CD, Chew LD, Littenberg B: The Single Item Literacy Screener: evaluation of a brief instrument to identify limited reading ability. BMC Fam Pract 7: 21, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright Nunes JA, Wallston KA, Eden SK, Shintani AK, Ikizler TA, Cavanaugh KL: Associations among perceived and objective disease knowledge and satisfaction with physician communication in patients with chronic kidney disease. Kidney Int 80: 1344–1351, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finkelstein FO, Story K, Firanek C, Barre P, Takano T, Soroka S, Mujais S, Rodd K, Mendelssohn D: Perceived knowledge among patients cared for by nephrologists about chronic kidney disease and end-stage renal disease therapies. Kidney Int 74: 1178–1184, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Lopez-Vargas PA, Tong A, Phoon RK, Chadban SJ, Shen Y, Craig JC: Knowledge deficit of patients with stage 1-4 CKD: a focus group study. Nephrology (Carlton) 19: 234–243, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Whaley-Connell A, Sowers JR, McCullough PA, Roberts T, McFarlane SI, Chen SC, Li S, Wang C, Collins AJ, Bakris GL; KEEP Investigators : Diabetes mellitus and CKD awareness: the Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES). Am J Kidney Dis 53[Suppl 4]: S11–S21, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Szczech LA, Stewart RC, Su HL, DeLoskey RJ, Astor BC, Fox CH, McCullough PA, Vassalotti JA: Primary care detection of chronic kidney disease in adults with type-2 diabetes: the ADD-CKD Study (awareness, detection and drug therapy in type 2 diabetes and chronic kidney disease). PLoS One 9: e110535, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Narva AS, Briggs M: The National Kidney Disease Education Program: improving understanding, detection, and management of CKD. Am J Kidney Dis 53[Suppl 3]: S115–S120, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Tuot DS, Davis E, Velasquez A, Banerjee T, Powe NR: Assessment of printed patient-educational materials for chronic kidney disease. Am J Nephrol 38: 184–194, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group: KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Inter [Suppl] 3: 1–150, 2016 [Google Scholar]
- 21.Glassock R, Delanaye P, El Nahas M: An Age-Calibrated Classification of Chronic Kidney Disease. JAMA 314: 559–560, 2015 [DOI] [PubMed] [Google Scholar]
- 22.Abdel-Kader K, Greer RC, Boulware LE, Unruh ML: Primary care physicians’ familiarity, beliefs, and perceived barriers to practice guidelines in non-diabetic CKD: a survey study. BMC Nephrol 15: 64, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greer RC, Cooper LA, Crews DC, Powe NR, Boulware LE: Quality of patient-physician discussions about CKD in primary care: a cross-sectional study. Am J Kidney Dis 57: 583–591, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blakeman T, Protheroe J, Chew-Graham C, Rogers A, Kennedy A: Understanding the management of early-stage chronic kidney disease in primary care: a qualitative study. Br J Gen Pract 62: e233–e242, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abroms LC, Maibach EW: The effectiveness of mass communication to change public behavior. Annu Rev Public Health 29: 219–234, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Tuot DS, Cavanaugh KL: Evaluating the Merits of CKD Patient Educational Materials: Readability Is Necessary But Not Sufficient. Am J Kidney Dis 65: 814–816, 2015 [DOI] [PubMed] [Google Scholar]
- 27.Green JA, Mor MK, Shields AM, Sevick MA, Palevsky PM, Fine MJ, Arnold RM, Weisbord SD: Prevalence and demographic and clinical associations of health literacy in patients on maintenance hemodialysis. Clin J Am Soc Nephrol 6: 1354–1360, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cavanaugh KL, Wingard RL, Hakim RM, Eden S, Shintani A, Wallston KA, Huizinga MM, Elasy TA, Rothman RL, Ikizler TA: Low health literacy associates with increased mortality in ESRD. J Am Soc Nephrol 21: 1979–1985, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Devraj R, Gordon EJ: Health literacy and kidney disease: toward a new line of research. Am J Kidney Dis 53: 884–889, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Just MA, Carpenter PA: A capacity theory of comprehension: individual differences in working memory. Psychol Rev 99: 122–149, 1992 [DOI] [PubMed] [Google Scholar]
- 31.Mackillop L, Parker-Swift J, Crossley J: Getting the questions right: non-compound questions are more reliable than compound questions on matched multi-source feedback instruments. Med Educ 45: 843–848, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Verhave JC, Troyanov S, Mongeau F, Fradette L, Bouchard J, Awadalla P, Madore F: Prevalence, awareness, and management of CKD and cardiovascular risk factors in publicly funded health care. Clin J Am Soc Nephrol 9: 713–719, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Narva AS: Reducing the burden of chronic kidney disease among American Indians. Adv Chronic Kidney Dis 15: 168–173, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.