Abstract
Alport syndrome is an inherited disease characterized by progressive renal failure, hearing loss, and ocular abnormalities. Inheritance is X-linked (85%) or autosomal recessive (15%). Many renal physicians think of Alport syndrome as primarily affecting men. However, twice as many women are affected by the X-linked diseases. Affected women are commonly undiagnosed, but 15%–30% develop renal failure by 60 years and often hearing loss by middle age. Half of their sons and daughters are also affected. Autosomal recessive Alport syndrome is less common, but is often mistaken for X-linked disease. Recessive inheritance is suspected where women develop early-onset renal failure or lenticonus. Their family may be consanguineous. The prognosis for other family members is very different from X-linked disease. Other generations, including parents and offspring, are not affected, and on average only one in four of their siblings inherit the disease. All women with Alport syndrome should have their diagnosis confirmed with genetic testing, even if their renal function is normal, because of their own risk of renal failure and the risk to their offspring. Their mutations indicate the mode of inheritance and the likelihood of disease transmission to their children, and the mutation type suggests the renal prognosis for both X-linked and recessive disease. Women with X-linked Alport syndrome should be tested at least annually for albuminuria and hypertension. The “Expert guidelines for the diagnosis and management of Alport syndrome” recommend treating those with albuminuria with renin-angiotensin-aldosterone system (RAAS) blockade (and adequate birth control because of the teratogenic risks of angiotensin converting enzyme inhibitors), believing that this will delay renal failure. Current recommendations are that women with autosomal recessive Alport syndrome should be treated with RAAS blockade from the time of diagnosis. In addition, women should be offered genetic counseling, informed of their reproductive options, and monitored closely during pregnancy for the development of hypertension.
Keywords: Alport syndrome, genetic renal disease, ACE inhibitors, albuminuria, Deafness, Female, Genetic Testing, Hearing Loss, Humans, Kidney Failure, Chronic, Mutation, Nephritis, Hereditary
Introduction
Alport syndrome is an inherited disease characterized by progressive renal failure, hearing loss, and ocular abnormalities, including corneal scarring, lenticonus, retinal thinning, and fleck retinopathy (1,2). Inheritance is X-linked (85%) or autosomal recessive (15%) (3). X-linked disease is suspected clinically when men in the family are affected more severely, and disease appears to “skip” a generation. Autosomal recessive inheritance should be considered where disease affects only one generation, there is consanguinity, or a young woman develops ESRD and hearing loss, or lenticonus.
Alport syndrome is considered rare because it affects fewer than one in 2000 individuals (www.orpha.net), but most estimates of its prevalence are from hospital-based series of mainly men, and many at-risk women have never been tested.
X-linked Alport syndrome is caused by mutations in the COL4A5 gene which encodes the collagen IV α5 chain (4). Autosomal recessive disease is caused by two mutations in trans (on different chromosomes) in the COL4A3 or COL4A4 genes which code for the collagen IV α3 and α4 chains, respectively (5). The collagen IV α3, α4, and α5 chains form a heterotrimer that is the predominant network of the basement membranes of the glomerular filter, the cochlea, cornea, lens capsule, and retina (6) – a tissue distribution that explains the clinical features. The collagen IV heterotrimer consists of a long series of Gly-Xaa-Yaa repeats, where Gly is present at each third residue, and X and Y are often proline and hydroxyproline.
In general, mutations in Alport syndrome are different in each family, and are missense (40%) or nonsense (direct or downstream, 40%), most often where a Gly is replaced by another amino acid, or a stop codon, respectively (7). More than 2000 COL4A5 mutations have been described in X-linked Alport syndrome and more than 1000 in COL4A3 and COL4A4 in recessive disease (http://www.lovd.nl).
The clinical features of Alport syndrome are identical in males with X-linked inheritance, and in males and females with recessive disease (Table 1). Features include hematuria, proteinuria, ESRD, lenticonus, retinal thinning (8), and retinopathy. Rare manifestations include leiomyomatosis (soft tissue tumors of the esophagus, bronchus), aortic aneurysms (9), and giant retinal holes. The “severe” phenotype (with renal failure before the age of 30, hearing loss, and often lenticonus and retinopathy) is more common with gene rearrangements, indels (insertions/deletions), and nonsense and splicing mutations (10,11). Gly substitutions with Glu, Arg, or Asp, also produce this phenotype (12). Other Gly and non-Gly substitutions are commonly associated with renal failure after the age of 30, hearing loss, and peripheral retinopathy only.
Table 1.
Features that help distinguish X-linked and autosomal recessive inheritance in females
| Features | X-Linked Alport Syndrome in Females | Autosomal Recessive Alport Syndrome in Females |
| Frequency | 1 in 5000; M/F = 1:2 | 1 in 40,000, M/F = 1:1 |
| Family history | Women are affected twice as often as men but usually have less severe disease | Men and women are affected equally often, and equally severely |
| Inheritance pattern | Disease occurs in several generations of the same family, appears to skip a generation where a woman is undiagnosed | Single generation only |
| Gene mutations | Heterozygous mutation in COL4A5 | Two mutations in COL4A3 or COL4A4 in trans |
| Hematuria | At least 95% | Probably all |
| Proteinuria | Common from early adulthood | Common from adolescence |
| Renal impairment | Prevalence and age not known | Probably all |
| ESRD | 15%–30% by the age of 60 yr | Probably the majority by middle age |
| Hearing loss | Common from middle age | Common earlier than middle age |
| Corneal opacities | Often undetected, occurs even with normal renal function | Not known |
| Lenticonus | Very uncommon | Common |
| Central fleck retinopathy | 30% | 85% |
| Retinal thinning (on OCT) | 50% of hospital-based females | Not known, but probably more common than in women with X-linked disease |
| Macular hole | Uncommon but occurs | Uncommon, maybe 5% |
| Peripheral retinopathy | 50% | Nearly all |
| Leiomyomatosis | Not common but all women with COL4A5-COL4A6 deletions | Not reported |
Please refer to manuscript for references. M/F, Male/Female; OCT, optical coherence tomography.
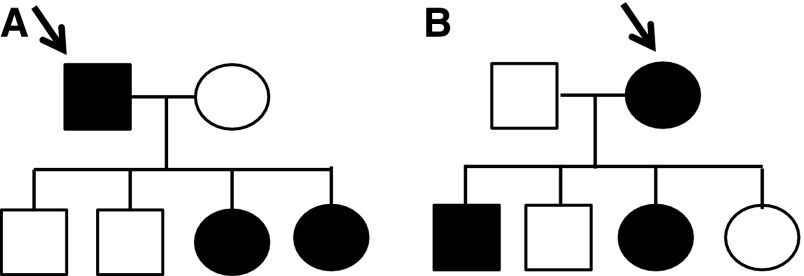
Women with X-linked Alport syndrome are often undiagnosed. However, on average twice as many women are affected as men (Figure 1). The prevalence of Alport syndrome is probably more than reported (one in 10,000), and closer to 1 in 5000. Thus, a renal genetics clinic responsible for a population of 5 million potentially cares for 1000 patients, two-thirds of whom are female. Furthermore, up to one third of these women will develop renal failure (15%–30% with X-linked disease, and all those with the rarer autosomal recessive disease) (10,13). Despite the prevalence and renal risk, Alport syndrome in women and girls has been little studied (14–17).
Figure 1.
More women are affected than men in X-linked Alport syndrome. Families of offspring of a male (A) and a female (B) with X-linked Alport syndrome demonstrating more affected females than males in their combined offspring (three times as many, 3:1 in one generation; and overall twice as many, 4:2 in the two generations). Males are shown as squares and females as circles. Affected individuals are in black.
X-Linked Alport Syndrome
All women with X-linked Alport syndrome are heterozygous for a pathogenic COL4A5 mutation. Whether these women should be described as “affected” or “carriers” has been controversial. Some of us prefer to use “carriers” unless women have clinically significant disease. Others believe that “affected” more accurately reflects their risk and is consistent with the need for a careful, even aggressive, approach to diagnosis and management. We have chosen to use the term affected here.
X-linked Alport syndrome is underdiagnosed in women. The generation skipping observed in X-linked families reflects the presence of undiagnosed women. This occurs because female relatives of affected men are not systematically screened in adult nephrology practice. This contrasts with pediatrics, where the mother and siblings of a child with hematuria are tested routinely.
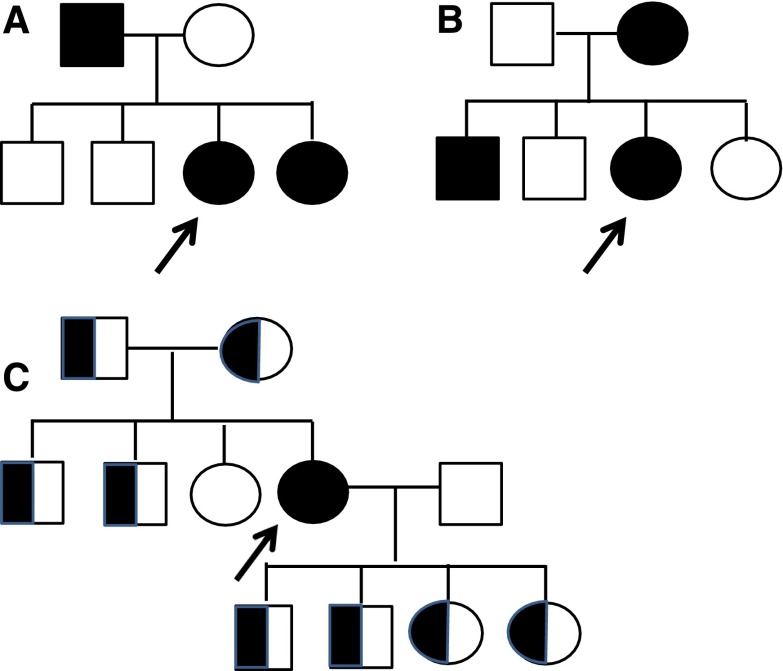
A male with X-linked disease has inherited the disease from his mother in 85% of cases (15% are due to de novo mutations) (Figure 2). On average half the affected male’s sisters and brothers, all his daughters, but none of his sons are also affected.
Figure 2.
The risk of siblings being affected depends on the mode of inheritance and the gender of the affected parent (in X-linked disease). Examples of families of females (arrows) who have inherited X-linked Alport syndrome from their father (A) or mother (B), indicating other family members who are likely to be affected. All the daughters of the affected father are affected, and half the sons and daughters of the affected mother. (C) Indicates the family of a woman with autosomal recessive disease. With autosomal recessive disease, males and females (with two COL4A3 or COL4A4 mutations) are affected equally often and equally severely, and disease occurs in only one generation. Other family members with only one mutation (half-shaded symbols) have thin basement membrane nephropathy and are much less likely to develop renal failure than females with X-linked Alport syndrome.
For females with X-linked disease, the situation is more complex since the disease can be inherited from her father or mother (Figure 2). If a woman inherits the disease from her father, then all her sisters are also affected, and if she inherits the disease from her mother, then half her sisters and half her brothers are affected. In addition, half an affected woman’s sons and half her daughters are affected. It is important to remember that “chance has no memory,” and the likelihood of any family member inheriting the mutation is independent of the outcome for any other.
Clinical Features in X-Linked Alport Syndrome (Table 1)
Clinical features in females depend on mutation type, but also on “lyonization” or the random inactivation of one of the two X chromosomes in any cell. Lyonization produces a mosaic distribution of the mutant collagen IV α5 chain and disease in the female kidney (18) and skin. This may result in a normal clinical phenotype, a severe or an intermediate phenotype, and the staining pattern for diagnostic testing may be confusing.
Hematuria.
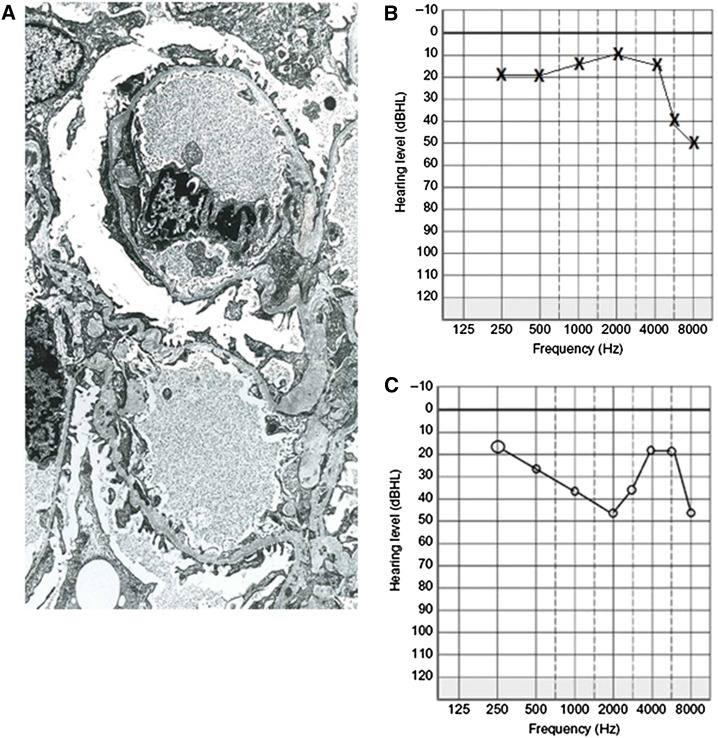
Nearly all females with X-linked Alport syndrome have persistent hematuria from infancy (10,19). Episodes of macroscopic hematuria occur with intercurrent infections, but are also common in thin basement membrane nephropathy and IgA disease. The renal biopsy demonstrates a thinned glomerular basement membrane that is often indistinguishable from that in thin basement membrane nephropathy (Figure 3A). The presence of even short stretches of lamellation suggests Alport syndrome, and lamellation often become more obvious over time.
Figure 3.
Glomerular basement membrane (GBM) appearance and patterns of hearing loss in women with Alport syndrome. (A) Electron micrograph of kidney biopsy from a 40-year-old woman demonstrating a variably thinned glomerular capillary basement membrane, <200 nm in places, but without multilamellation, splitting, or GBM microparticles. The appearance was indistinguishable from thin membrane nephropathy and was misdiagnosed until recent genetic testing confirmed X-linked Alport syndrome; (B) audiometry 30 years later (age 70) from the same woman with X-linked Alport syndrome due to a R373× mutation in COL4A5 demonstrating a high tone sensorineural hearing loss in the left ear (above 6000 Hz); and (C) audiometry from a 50-year-old with X-linked Alport syndrome demonstrating a midtone sensorineural loss in the right ear.
Albuminuria.
Albuminuria is not well-studied in women with Alport syndrome. However, in other diseases it is an early indicator of kidney damage and future progression along the continuum of albuminuria–nephrotic syndrome–ESRD. There is no evidence currently for using renin-angiotensin-aldosterone system (RAAS) blockade in women with X-linked disease prior to the development of overt albuminuria (20,21).
Again there are few data on the prevalence of hypertension in females with Alport syndrome. In our experience, many affected women develop hypertension with increasing age, but this may depend on their renal impairment.
Renal Impairment.
There are no data on how often women with X-linked Alport syndrome develop mild renal impairment, nor on the age at which eGFR starts to decline. These features may vary with the underlying mutations (10).
Hospital-based series suggest that 30% of all women with X-linked Alport syndrome develop ESRD by the age of 60 (10), requiring dialysis or transplantation. However, the risk is probably closer to the 15% seen in community-based affected female relatives of affected men (13).
Some affected women who develop ESRD have had further renal insults such as coincidental renal disease, poorly controlled hypertension, or nephrotoxic medication use.
Women with X-linked disease do not develop post-transplant anti-GBM disease (16) because their normal X chromosome means that they are tolerant of the wildtype collagen IV α5 chain.
Affected women should be strongly advised not to donate a kidney to an affected male relative (15) because of their own risk of ESRD. It is important though to confirm genetically that the mother is actually affected because of the small chance (15%) of a de novo mutation in her son.
Hearing Loss.
Hearing loss is common in women by middle age even if renal function is normal (10). It is typically a bilateral high tone sensorineural hearing deficit that the treating clinician may not associate with her diagnosis of Alport syndrome (Figure 3B). However, the pattern of loss varies and may affect mid-range tones (Figure 3C). Formal audiometric studies are required for confirmation. The hearing loss usually affects the ability to hear noises in the upper register, such as children talking, or where there is background noise. It is often falsely attributed to middle ear infections, age, swimming, and industrial exposure even on the audiometry reports. The defect eventually plateaus, and is helped with hearing aids.
Ocular Abnormalities.
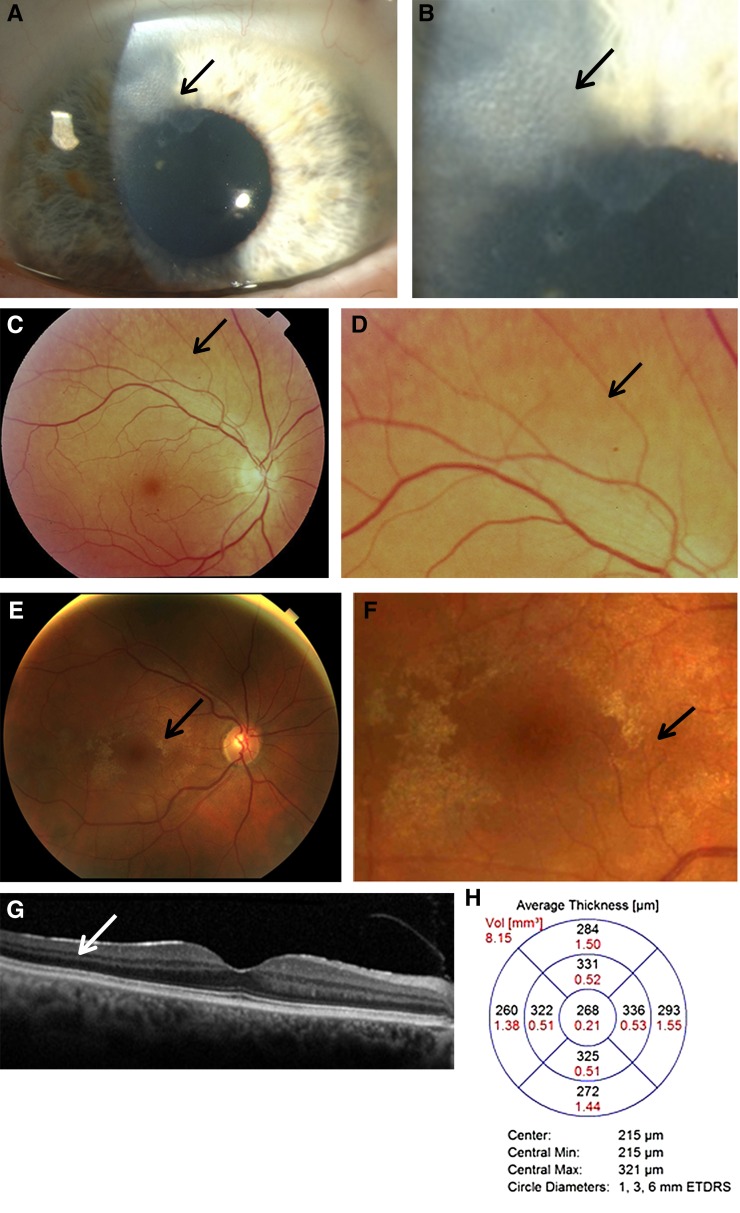
Most ocular abnormalities in Alport syndrome do not affect vision but are useful in identifying the diagnosis, and sometimes suggesting the mode of inheritance (22) (Figure 4, A–H).
Figure 4.
Characteristic ocular features in women with Alport syndrome. (A) Corneal opacity (arrow) in a 60-year-old woman with X-linked Alport syndrome and an in-frame COL4A5 deletion, hearing loss, and normal renal function; and (B) higher powered view of the same abnormality. Her son had a renal transplant and had a similar corneal lesion. (C) Peripheral fleck retinopathy in a 75-year-old woman, with mild renal impairment and hearing loss; and (D) higher power view demonstrating a dappled appearance due to the coalescent fleck retinopathy (arrow). (E) Central fleck retinopathy in a 30-year-old female with a R373× in COL4A5, and normal renal function (arrow). This figure demonstrates how difficult it can be to distinguish the fleck retinopathy from the retinal sheen of youth; and (F) a higher power view of the central fleck retinopathy (arrow). Vision was not affected. (G) Optical coherence tomography demonstrating a sagittal section through the retina and subtle thinning of the temporal versus nasal quadrants, and (H) optical coherence tomography measurements demonstrating different thicknesses in the temporal versus nasal quadrants which was consistent with moderate temporal retinal thinning (7.4%) (24).
Corneal abnormalities are not uncommon in affected females (22), but the association with Alport syndrome may be overlooked (Figure 4, A and B). Individuals have episodes of dry scratchy eyes that last a few days, recur, and affect multiple members of the family at different times. Treatment is with local lubricants. These corneal lesions differ from the posterior polymorphous corneal dystrophy which is rare and has not been reported in women with X-linked disease.
Lenticonus has not been described in women with X-linked disease.
The central fleck retinopathy occurs in fewer women with X-linked disease and is usually less prominent than in men (Figure 4, E and F). Giant macular holes have been reported in women (23).
At least 30% of females with X-linked disease have a peripheral fleck retinopathy (Figure 4, C and D). This is evident on retinal photographs with peripheral and red-free images, and can be confirmed by an ophthalmologist who is familiar with its appearance.
Temporal retinal thinning on optical coherence tomography represents a useful screening test for Alport syndrome, especially where genetic testing is not available (Figure 4, G and H). Severe temporal thinning occurs in 53% of a hospital-based series of females and 90% of males with X-linked Alport syndrome, and the thinning is less prominent in women (24). The test requires dilated pupils, but is widely available, noninvasive, and takes about 10 minutes (24). This technique is more objective than using other ocular features such as peripheral retinopathy, but it is not clear that it distinguishes between X-linked Alport syndrome and thin basement membrane nephropathy.
Other Clinical Features.
Leiomyomatoses are soft tissue tumors of the bronchus and esophagus that occur with a large COL4A5 deletion that extends into intron 2 of the contiguous COL4A6 gene (9). Tumors in females also involve the vagina, and multiple clinical sites may be affected simultaneously. Fewer than 1% of families with X-linked Alport syndrome have the mutations that cause these tumors. However, the tumors occur in affected males and females even with normal renal function. Treatment is surgical.
Aortic aneurysms are rare and there are no reports in women (25).
Cardiac Risk.
People with CKD from any cause, including Alport syndrome, have a two to three times greater risk of cardiac death (26). The most effective method of reducing cardiac risk is to delay the onset of renal failure with RAAS blockade.
Genotype–Phenotype Correlations
Genotype–phenotype correlations are less well-described for women with X-linked disease (10,27). However, rearrangements, insertions/deletions, nonsense, and splicing mutations appear to result in a more severe phenotype, with renal failure more often, and possibly hearing loss. Many missense mutations result in a less severe phenotype (10).
There are rare reports of women with two COL4A5 mutations (28), where the phenotype again depends on the nature of the underlying mutations. We have reported a 20-year-old woman with two mutations but normal renal function, hearing, and ophthalmologic examination.
Autosomal Recessive Alport Syndrome
Autosomal recessive Alport syndrome affects about one in 40,000 individuals (3), and is suspected in young women with renal failure and hearing loss or lenticonus (15). The family may be consanguineous. Typically, the only other affected family member, if any, is a sibling (Figure 2C). The affected woman’s parents, grandparents, and children may have hematuria (and thin basement membrane nephropathy) but do not develop renal failure.
Males and females with autosomal recessive Alport syndrome are affected equally often and severely. Most women with recessive disease develop renal failure by early middle age.
Autosomal recessive Alport syndrome is confirmed when two COL4A3 or COL4A4 mutations are demonstrated in trans. With whole exome sequencing (WES), many individuals with one COL4A3 and one COL4A4 mutation have been described, usually with a less severe phenotype and later age of onset of renal failure than conventional recessive disease (29).
Clinical Features in Autosomal Recessive Disease
The clinical features in women with autosomal recessive Alport syndrome are identical to those in men with X-linked or recessive disease (Table 1). The current understanding is that all females with recessive disease develop ESRD. There is an early peak in childhood (5), and another in adulthood, at about the same age for men with X-linked disease (30). Thus, adult women develop renal failure by about the age of 30 years, as well as hearing loss, lenticonus, and the central and peripheral retinopathy (30,31). Hematuria occurs from infancy, but albuminuria and renal impairment have not been studied.
Anti-GBM disease is even rarer after transplantation in autosomal recessive disease (5). The ocular features that occur in males with X-linked disease (macular hole and posterior polymorphous corneal dystrophy) also occur in females with recessive disease. There are no data on the use of retinal temporal thinning for the diagnosis of recessive disease in women.
Genotype–Phenotype Correlations
There is preliminary evidence that the same types of mutations that cause severe disease in X-linked Alport syndrome, such as nonsense mutations, do so too in autosomal recessive disease (30).
Diagnosis of Alport Syndrome in Women and Girls
The recently-published “Expert guidelines for the diagnosis and management of Alport syndrome” (15) emphasize the use of genetic testing to confirm the diagnosis of Alport syndrome, and to identify the mode of inheritance, help predict the clinical course, enable preimplantation genetic diagnosis, and specify future mutation-specific treatments.
It is critical to distinguish between X-linked and autosomal recessive inheritance in a woman with Alport syndrome (15), both in order to institute therapy to slow the rate of renal function deterioration, and to screen her at-risk family members.
The individual with suspected Alport syndrome should be tested for hematuria, albuminuria, eGFR, BP, audiometry, retinal imaging (including peripheral and red-free views), and retinal thinning with optical coherence tomography.
Renal biopsies are performed for the diagnosis of Alport syndrome less often in girls and women because of the risks of a biopsy and the equivocal results in females. Thus the GBM may demonstrate thinning only and the lack of lamellation does not exclude Alport syndrome.
Again, a renal and skin biopsy may be examined immunohistochemically for collagen IV α5 expression, but the interpretation is hampered by lyonization and patchy staining in X-linked disease. Experts can use staining patterns to distinguish between X-linked and recessive inheritance. Females with X-linked or autosomal recessive Alport syndrome have reduced or absent GBM collagen IV α5 staining, but expression is variable in the epidermis in X-linked disease. It is present with recessive inheritance because the collagen IV α5α5α6 network persists in the skin even when the collagen α3α4α5 network is absent from the GBM with COL4A3 or COL4A4 mutations.
Genetic testing is now performed using WES (32) and possibly further analysis that ensures deletions or duplications are not overlooked (7). WES detects 95% of causative mutations in X-linked and autosomal recessive Alport syndrome, but also mutations in other genes that exacerbate injury.
Management
The management is different for women with X-linked Alport syndrome, where only 15%–30% develop ESRD, and for recessive disease where all affected females do.
In general, if a woman has X-linked disease she should be monitored at least annually for albuminuria and hypertension (15). Her risk factors for renal failure progression include mutation type, a history of early onset renal failure in male relatives (10), proteinuria, and hearing loss (36). She should be treated with RAAS blockade from the onset of albuminuria (21) even if she has a normal BP. Our preference is to use ACE inhibitors for first line treatment, followed by angiotensin receptor blockers. RAAS blockade delays the onset of ESRD by years (21,37), not only through their antihypertensive action, but by minimizing proteinuria (38), directly antagonizing TGFβ, and reducing tubulointerstitial fibrosis. Aldosterone antagonists also reduce proteinuria but further increase the risk of hyperkalemia.
In contrast, the “Expert guidelines” recommend treating women with autosomal recessive Alport syndrome with ACE inhibitors from the time of diagnosis, that is, even before the onset of albuminuria (15). This recommendation is based on the understanding that most females with two COL4A3 or COL4A4 pathogenic mutations develop ESRD by the age of 30, and that RAAS blockade reduces proteinuria and fibrosis and delays renal failure onset. There is currently no evidence for this recommendation but the required studies may never be performed because of the difficulty in recruiting sufficient participants. This recommendation is derived from studies that demonstrated that ACE inhibitors started from the time of diagnosis in boys with X-linked disease delayed the onset of renal failure, regardless of whether albuminuria was present (37).
Other strategies to delay renal failure progression include treating hypertension adequately, preventing incidental renal damage (from infections, hypotension, nephrotoxic drugs, preeclampsia), maintaining a healthy weight and lifestyle, optimizing diabetes control, stopping smoking, and so on. Women should also reduce their risk factors for the cardiac disease associated with renal impairment.
Many women with X-linked or autosomal recessive Alport syndrome develop hearing loss in middle age. All affected women should have a formal ophthalmologic review at least once and be checked regularly if they have renal failure. It is not clear whether RAAS blockade also reduces the risk of the complications such as hearing loss, retinal thinning, and macular hole.
There are many opportunities for women to be involved in their own care to delay ESRD and to reduce the cardiac risk associated with renal impairment.
Pregnancy
Affected women should use contraception to avoid becoming pregnant while being treated with angiotensin converting enzyme (ACE) inhibitors because of their teratogenic risk (33).
It may be difficult for a woman with renal impairment to become pregnant. Renal impairment, hypertension, and albuminuria all predispose to preeclampsia (34). Uncontrolled hypertension during pregnancy may further exacerbate renal impairment.
In addition, all transplant recipients have an increased risk of further renal impairment if they become pregnant.
Some affected women have not had children because of their risk of an affected child. If the familial mutation is known then they can be offered prenatal diagnosis or preimplantation genetic diagnosis, but this requires planning (35) and is expensive.
Psychologic Health
There is little published on the psychologic concerns of women and girls in families with Alport syndrome, but the support groups tell us that these are different at different life stages.
At diagnosis, individuals may need psychologic help to deal with the implications of their diagnosis, the deterioration in their quality of life, and the impact on their children and their relationships. They may need further support at other key stages, such as when their children are diagnosed, or when a family member starts dialysis or undergoes renal transplantation.
Women with Alport syndrome may experience the grief of losing a parent or sibling to renal failure, caring for a relative on dialysis, and of transmitting the disease to their children and grandchildren. They also have to deal with the practical issues of managing frequent clinic visits for family members. The isolation may be compounded by their hearing loss. Their own affected status may be unrecognized or neglected.
Resources
Genetic testing for Alport syndrome is the definitive test for diagnosis, identification of inheritance type, and renal risk assessment. However, many health providers still do not fund mutation testing in spite of its obvious economic advantages. (The cost of the genetic test is about $USD 3500, less for testing at-risk family members, and RAAS blockade delays ESRD and the need for dialysis by up to 13 years, when the cost is potentially $USD 70,000 annually). Where gene testing is not available, the family should be assessed clinically to determine the mode of inheritance as accurately as possible.
Patient support groups are found in many countries (the United States, the United Kingdom, France, Germany, Israel, Australia, The Netherlands, China) (http://alportsyndrome.org/connect/international-groups/). They host websites, and blogs, with access to expert medical advisors who can explain the genetic risks, optimal medical management, and rare clinical complications. Patient Support Groups also coordinate conferences where patients, researchers, and clinicians interact.
The Future
There are many reasons to be hopeful. RAAS blockade means that fewer women with Alport syndrome will develop ESRD. Across the globe, researchers, patient groups, and pharmaceutical companies are partnering to support research into how Alport syndrome causes disease and how to target and test treatments for different mutation types.
Disclosures
None of the authors has a conflict of interest that impacts on the advice presented in this review. J.S. and M.R. conduct research that is funded by Regulus Therapeutics (San Diego, CA). M.R. is also funded by Amgen (Thousand Oaks, CA) and Retrophin (San Diego, CA).
Three of the retinal images (Figure 4, D–F) have been lightened to make the abnormalities more obvious in the color images. They have not been altered in any other way.
Acknowledgments
The need for a document focusing on women with Alport syndrome was recognized at the International Alport meeting in Gottingen in September 2015. The authors are women who include an adult nephrologist, two pediatricians, a geneticist, an ophthalmologist, an anatomical pathologist, and representatives of the US and UK patient support groups. They have all contributed to this review.
We would like to thank the many women whose results are presented here. All of these were studied with approval from the Northern Health or Melbourne Health Human Research Ethics Committee, and all individuals provided written informed consent to the use of photographs.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Gubler M, Levy M, Broyer M, Naizot C, Gonzales G, Perrin D, Habib R: Alport’s syndrome. A report of 58 cases and a review of the literature. Am J Med 70: 493–505, 1981 [DOI] [PubMed] [Google Scholar]
- 2.Habib R, Gubler MC, Hinglais N, Noël LH, Droz D, Levy M, Mahieu P, Foidart JM, Perrin D, Bois E, Grünfeld JP: Alport’s syndrome: experience at Hôpital Necker. Kidney Int Suppl 11: S20–S28, 1982 [PubMed] [Google Scholar]
- 3.Feingold J, Bois E, Chompret A, Broyer M, Gubler MC, Grünfeld JP: Genetic heterogeneity of Alport syndrome. Kidney Int 27: 672–677, 1985 [DOI] [PubMed] [Google Scholar]
- 4.Barker DF, Hostikka SL, Zhou J, Chow LT, Oliphant AR, Gerken SC, Gregory MC, Skolnick MH, Atkin CL, Tryggvason K: Identification of mutations in the COL4A5 collagen gene in Alport syndrome. Science 248: 1224–1227, 1990 [DOI] [PubMed] [Google Scholar]
- 5.Mochizuki T, Lemmink HH, Mariyama M, Antignac C, Gubler MC, Pirson Y, Verellen-Dumoulin C, Chan B, Schröder CH, Smeets HJ, Reeder ST: Identification of mutations in the alpha 3(IV) and alpha 4(IV) collagen genes in autosomal recessive Alport syndrome. Nat Genet 8: 77–81, 1994 [DOI] [PubMed] [Google Scholar]
- 6.Hudson BG, Tryggvason K, Sundaramoorthy M, Neilson EG: Alport’s syndrome, Goodpasture’s syndrome, and type IV collagen. N Engl J Med 348: 2543–2556, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Hertz JM, Thomassen M, Storey H, Flinter F: Clinical utility gene card for: Alport syndrome - update 2014. Eur J Hum Genet 23: 23, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fawzi AA, Lee NG, Eliott D, Song J, Stewart JM: Retinal findings in patients with Alport Syndrome: expanding the clinical spectrum. Br J Ophthalmol 93: 1606–1611, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Antignac C, Zhou J, Sanak M, Cochat P, Roussel B, Deschênes G, Gros F, Knebelmann B, Hors-Cayla MC, Tryggvason K, Gubler MC: Alport syndrome and diffuse leiomyomatosis: deletions in the 5′ end of the COL4A5 collagen gene. Kidney Int 42: 1178–1183, 1992 [DOI] [PubMed] [Google Scholar]
- 10.Jais JP, Knebelmann B, Giatras I, De Marchi M, Rizzoni G, Renieri A, Weber M, Gross O, Netzer KO, Flinter F, Pirson Y, Dahan K, Wieslander J, Persson U, Tryggvason K, Martin P, Hertz JM, Schröder C, Sanak M, Carvalho MF, Saus J, Antignac C, Smeets H, Gubler MC: X-linked Alport syndrome: natural history and genotype-phenotype correlations in girls and women belonging to 195 families: a “European Community Alport Syndrome Concerted Action” study. J Am Soc Nephrol 14: 2603–2610, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Jais JP, Knebelmann B, Giatras I, De Marchi M, Rizzoni G, Renieri A, Weber M, Gross O, Netzer KO, Flinter F, Pirson Y, Verellen C, Wieslander J, Persson U, Tryggvason K, Martin P, Hertz JM, Schröder C, Sanak M, Krejcova S, Carvalho MF, Saus J, Antignac C, Smeets H, Gubler MC: X-linked Alport syndrome: natural history in 195 families and genotype- phenotype correlations in males. J Am Soc Nephrol 11: 649–657, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Persikov AV, Pillitteri RJ, Amin P, Schwarze U, Byers PH, Brodsky B: Stability related bias in residues replacing glycines within the collagen triple helix (Gly-Xaa-Yaa) in inherited connective tissue disorders. Hum Mutat 24: 330–337, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Dagher H, Buzza M, Colville D, Jones C, Powell H, Fassett R, Wilson D, Agar J, Savige J: A comparison of the clinical, histopathologic, and ultrastructural phenotypes in carriers of X-linked and autosomal recessive Alport’s syndrome. Am J Kidney Dis 38: 1217–1228, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Rheault MN: Women and Alport syndrome. Pediatr Nephrol 27: 41–46, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Savige J, Gregory M, Gross O, Kashtan C, Ding J, Flinter F: Expert guidelines for the management of Alport syndrome and thin basement membrane nephropathy. J Am Soc Nephrol 24: 364–375, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Kashtan CE: Alport syndrome and the X chromosome: implications of a diagnosis of Alport syndrome in females. Nephrol Dial Transplant 22: 1499–1505, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Kashtan CE: Women with Alport syndrome: risks and rewards of kidney donation. Nephrol Dial Transplant 24: 1369–1370, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Kashtan CE: Familial hematuria. Pediatr Nephrol 24: 1951–1958, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flinter FA, Cameron JS, Chantler C, Houston I, Bobrow M: Genetics of classic Alport’s syndrome. Lancet 2: 1005–1007, 1988 [DOI] [PubMed] [Google Scholar]
- 20.Gross O, Friede T, Hilgers R, Görlitz A, Gavénis K, Ahmed R, Dürr U: Safety and Efficacy of the ACE-Inhibitor Ramipril in Alport Syndrome: The Double-Blind, Randomized, Placebo-Controlled, Multicenter Phase III EARLY PRO-TECT Alport Trial in Pediatric Patients. ISRN Pediatr 2012: 436046, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Temme J, Peters F, Lange K, Pirson Y, Heidet L, Torra R, Grunfeld JP, Weber M, Licht C, Müller GA, Gross O: Incidence of renal failure and nephroprotection by RAAS inhibition in heterozygous carriers of X-chromosomal and autosomal recessive Alport mutations. Kidney Int 81: 779–783, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Savige J, Sheth S, Leys A, Nicholson A, Mack HG, Colville D: Ocular features in Alport syndrome: pathogenesis and clinical significance. Clin J Am Soc Nephrol 10: 703–709, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rahman W, Banerjee S: Giant macular hole in Alport syndrome. Can J Ophthalmol 42: 314–315, 2007 [PubMed] [Google Scholar]
- 24.Ahmed F, Kamae KK, Jones DJ, Deangelis MM, Hageman GS, Gregory MC, Bernstein PS: Temporal macular thinning associated with X-linked Alport syndrome. JAMA Ophthalmol 131: 777–782, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kashtan CE, Segal Y, Flinter F, Makanjuola D, Gan JS, Watnick T: Aortic abnormalities in males with Alport syndrome. Nephrol Dial Transplant 25: 3554–3560, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH: Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 164: 659–663, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Gross O, Netzer KO, Lambrecht R, Seibold S, Weber M: Meta-analysis of genotype-phenotype correlation in X-linked Alport syndrome: impact on clinical counselling. Nephrol Dial Transplant 17: 1218–1227, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Mohammad M, Nanra R, Colville D, Trevillian P, Wang Y, Storey H, Flinter F, Savige J: A female with X-linked Alport syndrome and compound heterozygous COL4A5 mutations. Pediatr Nephrol 29: 481–485, 2014 [DOI] [PubMed] [Google Scholar]
- 29.Mencarelli MA, Heidet L, Storey H, van Geel M, Knebelmann B, Fallerini C, Miglietti N, Antonucci MF, Cetta F, Sayer JA, van den Wijngaard A, Yau S, Mari F, Bruttini M, Ariani F, Dahan K, Smeets B, Antignac C, Flinter F, Renieri A: Evidence of digenic inheritance in Alport syndrome. J Med Genet 52: 163–174, 2015 [DOI] [PubMed] [Google Scholar]
- 30.Storey H, Savige J, Sivakumar V, Abbs S, Flinter FA: COL4A3/COL4A4 mutations and features in individuals with autosomal recessive Alport syndrome. J Am Soc Nephrol 24: 1945–1954, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colville D, Savige J, Morfis M, Ellis J, Kerr P, Agar J, Fassett R: Ocular manifestations of autosomal recessive Alport syndrome. Ophthalmic Genet 18: 119–128, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Morinière V, Dahan K, Hilbert P, Lison M, Lebbah S, Topa A, Bole-Feysot C, Pruvost S, Nitschke P, Plaisier E, Knebelmann B, Macher MA, Noel LH, Gubler MC, Antignac C, Heidet L: Improving mutation screening in familial hematuric nephropathies through next generation sequencing. J Am Soc Nephrol 25: 2740–2751, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nadeem S, Hashmat S, Defreitas MJ, Westreich KD, Shatat IF, Selewski DT, Onder AM, Chiang M, Weaver DJ Jr, Steinke J, Barcia J, Hernandez J, Hidalgo G, Ingraham SE, Abitbol CL, Pan C, Greenbaum LA: Renin Angiotensin System Blocker Fetopathy: A Midwest Pediatric Nephrology Consortium Report. J Pediatr 167: 881–885, 2015 [DOI] [PubMed] [Google Scholar]
- 34.Alkhunaizi A, Melamed N, Hladunewich MA: Pregnancy in advanced chronic kidney disease and end-stage renal disease. Curr Opin Nephrol Hypertens 24: 252–259, 2015 [DOI] [PubMed] [Google Scholar]
- 35.Braude P, Flinter F: Use and misuse of preimplantation genetic testing. BMJ 335: 752–754, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grünfeld JP, Noël LH, Hafez S, Droz D: Renal prognosis in women with hereditary nephritis. Clin Nephrol 23: 267–271, 1985 [PubMed] [Google Scholar]
- 37.Gross O, Licht C, Anders HJ, Hoppe B, Beck B, Tönshoff B, Höcker B, Wygoda S, Ehrich JH, Pape L, Konrad M, Rascher W, Dötsch J, Müller-Wiefel DE, Hoyer P, Knebelmann B, Pirson Y, Grunfeld JP, Niaudet P, Cochat P, Heidet L, Lebbah S, Torra R, Friede T, Lange K, Müller GA, Weber M; Study Group Members of the Gesellschaft für Pädiatrische Nephrologie: Early angiotensin-converting enzyme inhibition in Alport syndrome delays renal failure and improves life expectancy. Kidney Int 81: 494–501, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Heeg JE, de Jong PE, van der Hem GK, de Zeeuw D: Reduction of proteinuria by angiotensin converting enzyme inhibition. Kidney Int 32: 78–83, 1987 [DOI] [PubMed] [Google Scholar]