Abstract
Background and objectives
A major challenge in early treatment of acute cardiorenal syndrome (CRS) is the lack of predictors for progression of AKI. We aim to investigate the utility of urinary angiotensinogen and other renal injury biomarkers in predicting AKI progression in CRS.
Design, settings, participants, & measurements
In this prospective, multicenter study, we screened 732 adults who admitted for acute decompensated heart failure from September 2011 to December 2014, and evaluated whether renal injury biomarkers measured at time of AKI diagnosis can predict worsening of AKI. In 213 patients who developed Kidney Disease Improving Global Outcomes stage 1 or 2 AKI, six renal injury biomarkers, including urinary angiotensinogen (uAGT), urinary neutrophil gelatinase-associated lipocalin (uNGAL), plasma neutrophil gelatinase-associated lipocalin, urinary IL-18 (uIL-18), urinary kidney injury molecule-1, and urinary albumin-to-creatinine ratio, were measured at time of AKI diagnosis. The primary outcome was AKI progression defined by worsening of AKI stage (50 patients). The secondary outcome was AKI progression with subsequent death (18 patients).
Results
After multivariable adjustment, the highest tertile of three urinary biomarkers remained associated with AKI progression compared with the lowest tertile: uAGT (odds ratio [OR], 10.8; 95% confidence interval [95% CI], 3.4 to 34.7), uNGAL (OR, 4.7; 95% CI, 1.7 to 13.4), and uIL-18 (OR, 3.6; 95% CI, 1.4 to 9.5). uAGT was the best predictor for both primary and secondary outcomes with area under the receiver operating curve of 0.78 and 0.85. These three biomarkers improved risk reclassification compared with the clinical model alone, with uAGT performing the best (category-free net reclassification improvement for primary and secondary outcomes of 0.76 [95% CI, 0.46 to 1.06] and 0.93 [95% CI, 0.50 to 1.36]; P<0.001). Excellent performance of uAGT was further confirmed with bootstrap internal validation.
Conclusions
uAGT, uNGAL, and uIL-18 measured at time of AKI diagnosis improved risk stratification and identified CRS patients at highest risk of adverse outcomes.
Keywords: acute kidney injury, biomarkers, prediction, outcomes, Adult, Angiotensinogen, Area Under Curve, Cardio-Renal Syndrome, creatinine, Humans, Prospective Studies
Introduction
AKI is common in patients with acute decompensated heart failure (ADHF), complicating 25%–51% of hospitalizations (1–3). Coexistence of acute cardiac and renal dysfunction, termed as acute cardiorenal syndrome (CRS) (4), has been shown to correlate with increased mortality and all manner of adverse outcomes (5,6) and may increase the risk of subsequent development of CKD (7).
Although most patients who develop acute CRS experience a mild form of AKI (e.g., Kidney Disease Improving Global Outcomes [KDIGO] or Acute Kidney Injury Network [AKIN] stage 1 or RIFLE R) and do not progress to more severe AKI (KDIGO/AKIN stage 2/3 or RIFLE F) or require dialysis, approximately 29%–48% of these patients progress to a higher stage of AKI (8,9). Recent studies have demonstrated that risk of mortality exponentially increased with increasing stages of AKI in the setting of ADHF (10). Early detection of patients at higher risk for AKI progression would help physicians to plan and initiate the appropriate managements to improve renal safety of therapies, augment surveillance of cardiac and renal dysfunction, and develop renal-preserving treatments (11,12).
Unfortunately, predicting which patients with CRS will suffer progressive AKI or proceed to death is clinically challenging. In patients with ADHF, fluid retention, low protein intake, and decreased creatinine production secondary to muscle atrophy may dissociate creatinine levels from reflecting the true severity of renal dysfunction. Diagnostic mainstays such as the fractional excretion of sodium and urea have been shown to be suboptimal in a variety of clinical settings (13–17). Assessment of biomarkers for renal tubular injury (e.g., neutrophil gelatinase-associated lipocalin [NGAL], kidney injury molecule-1 [KIM-1] and IL-18) at the time of creatinine-based diagnosis of AKI offers prognostic information in multiple clinical settings such as cardiac surgery, ICU, transplantation, and cirrhosis (18–23). However, their utility has not been tested in ADHF.
The intrarenal renin-angiotensin system (RAS) plays a vital role in maintaining hemodynamic balance and cardiorenal interaction, which are often disrupted in acute heart failure (24–26). In animal studies, activation of the intrarenal RAS is an initial response to hypoperfusion and an important contributor to the progression of cardiac and renal injury (26,27). Urinary angiotensinogen (uAGT) has been identified as an indicator of intrarenal RAS activity in both animal and clinical studies (28,29). We have recently shown that uAGT level on admission predicts subsequent development of AKI in patients with ADHF (30). Elevated uAGT is also associated with severe AKI after cardiac surgery (20).
This study aims to investigate the utility of uAGT and other renal injury biomarkers in predicting AKI progression in patients with ADHF. We performed a large prospective, multicenter cohort study in 732 hospitalized adults with ADHF to evaluate the utility of uAGT and other injury biomarkers, measured at the time of stage 1 or 2 AKI diagnoses, for predicting progression of AKI in ADHF.
Materials and Methods
Study Design
We prospectively screened adult (18–80 years) patients with ADHF who were admitted to the six academic teaching hospitals in China from September 2011 to December 2014 according to the protocols previously reported (30). Eligible participants were those with ADHF who developed stage 1 or 2 AKI (determined by three independent nephrologists who were blinded to the urinary measurements) during their hospitalization, and who had at least three measurements of serum creatinine over a 6-month period before admission. Exclusion criteria included exposure to nephrotoxins (i.e., contrast media, aminoglycoside antibiotics, vancomycin, and nonsteroidal anti-inflammatory drugs except aspirin) within 4 weeks before admission or during their hospital stay, pre-existing advanced CKD (preadmission eGFR<30 ml/min per 1.73m2), urinary tract infection or obstruction, cancer, acute coronary syndrome, cardiogenic shock or need for inotropes, cardiac transplantation, or heart failure after cardiac surgery. The patients whose initial AKI diagnoses were stage 3 (n=11; 4.9% of total patients with AKI) were excluded, because they would not progress further (Supplemental Figure 1).
AKI was defined according to the KDIGO Clinical Practice Guidelines for AKI based on serum creatinine criteria (31). Prior CKD was defined as preadmission eGFR<60ml/min per 1.73m2 which was calculated according to at least three measurements of serum creatinine over a six-month period before admission.
The study was approved by the Institutional Review Board of the National Clinical Research Center for Kidney Disease and all of the patients provided written informed consent on admission.
Procedures
All of the study patients received the standard of care for ADHF (32). Spot urine and blood samples were collected every 24 hours for the first 7 days during hospitalization. The remaining urine and blood samples were obtained at the time of routine morning sample collection until discharge (30). Serum creatinine was measured on admission and at least twice a day during the first 3 days and daily thereafter. Death status and date of death were obtained through reviewing the hospital records.
Laboratory Measurements
All of the biomarkers were measured in a central laboratory, and all of the samples were labeled using study identification numbers without personal identifiers or clinical conditions. The urine samples were centrifuged at 3000× g for 10 minutes and the supernatants were stored at −80°C.
Biomarkers measured on the initial day that the serum creatinine first crossed the KDIGO stage 1 or 2 threshold were used for analysis. uAGT, urinary or plasma NGAL (uNGAL or pNGAL), urinary IL-18 (uIL-18), or KIM-1(uKIM-1) levels were quantified using ELISA kits (Immuno-Biological Laboratories Co., Ltd., Fujioka, Japan; Bioporto, Denmark; RayBiotech Inc., GA; R&D Systems, Minneapolis, MN) according to the manufacturers’ instructions. All biomarkers were measured in triplicate. Investigator-calculated intra- and interassay variability ranged 2%–6% and 5%–9% based on blinded replicate samples from study patients. Urinary albumin was measured using an automatic analyzer and reported as the ratio to urinary creatinine (UACR). All of the urinary biomarkers were normalized for urinary creatinine. Urinary and serum creatinine was measured using an automatic biochemical analyzer. The eGFR was estimated by the CKD-Epidemiology Collaboration Equation (33).
Outcome Definitions
The primary outcome was the progression of AKI, defined as worsening of KDIGO stage (from stage 1 to either stage 2 or stage 3, or from stage 2 to stage 3). Patients treated with acute dialysis at any point during hospitalization were defined as stage 3.
The secondary outcome was progression to a higher stage with subsequent death. Patients who died without progression were excluded from the primary analysis because death may have been a competing risk for progression for these patients.
Statistical Analyses
We performed the analyses with SPSS software (version 17.0). To compare continuous variables, we used a two-sample t test or a Mann–Whitney U test. To compare categorical variables, we used the chi-squared or Fisher exact test. All tests were two-tailed and P<0.05 was considered significant.
We categorized each urinary biomarker tertile essentially as a continuous variable and then performed logistic regression on created variables. We determined the adjusted odds ratios (OR) of AKI progression with multiple logistic regression analysis with random intercepts for each center. The selection of covariates for the adjusted model was based on known risk factors of AKI progression in ADHF, including age, gender, hypertension, diabetes, preadmission eGFR, N-terminal pro-B-type natriuretic peptide (NT-proBNP), serum albumin, hemoglobin, diuretic dosage before AKI, use of spironolactone before AKI, use of RAS inhibitors before AKI, and change of serum creatinine from baseline at the time of AKI diagnosis, as well as study site. uAGT, uNGAL, and uIL-18 were also modeled as a continuous variable (log-transformed).
To compare the performance of uAGT and other biomarkers at different cutoff values, a conventional area under the receiver-operating characteristic (ROC) curve (AUC) was generated. To evaluate the utility of the biomarkers on risk classification, we determined the category-free net reclassification improvement (NRI) and the integrated discrimination improvement (IDI), as previously described (34,35).
The performance of uAGT for predicting AKI progression was internally validated by a bootstrap method with 1000 replications (36).
Results
Cohort Characteristics
A total of 732 patients with ADHF enrolled from six hospitals were screened. Among them, 213 (29.1%) developed stage 1 or 2 AKI on admission (n=28) or during hospitalization (n=185).
Among 213 patients who developed stage 1 or 2 AKI, 50 patients (23.5%) progressed to a higher stage of AKI during their hospitalization (19 individuals progressed to stage 2 and 31 progressed to stage 3); 13 of 50 (26.0%) progressors received acute dialysis; 18 of 50 (36.0%) had AKI progression and subsequently died during their hospitalization; 163 patients (76.5%) persisted in stage 1 or 2 AKI; 3 of them died on the day of AKI diagnosis; 22 (13.5% of those with stage 1or 2 AKI) died without progression and were excluded from the outcome analysis.
Table 1 shows the characteristics on admission of 213 patients who developed stage 1 or 2 AKI. Among them, 100 (46.9%) suffered from newly diagnosed ADHF. There was no statistically significant difference in proportion of patients who received RAS inhibitors or diuretics before admission between those with or without AKI progression. Compared with those with AKI that did not progress, patients with progression of AKI had higher levels of serum NT-proBNP and lower levels of serum albumin and hemoglobin.
Table 1.
Characteristics in patients with and without AKI progression
| Demographics | AKI progression | P Value | |
|---|---|---|---|
| Yes (n=50) | No (n=163) | ||
| Age, y | 66.8±17.6 | 70.6±12.2 | 0.09 |
| Male, n (%) | 26 (52.0) | 108 (66.3) | 0.09 |
| Hypertension, n (%) | 24 (48.0) | 105 (64.4) | 0.05 |
| Diabetes, n (%) | 20 (40.0) | 55 (33.7) | 0.49 |
| Prior CKDa, n (%) | 22 (44.0) | 60 (36.8) | 0.41 |
| Prior hospitalization for HF, n (%) | 25 (50.0) | 88 (54.0) | 0.63 |
| Primary diseases of heart failure | |||
| Ischemic heart disease, n (%) | 28 (56.0) | 90 (55.2) | 0.99 |
| Hypertensive heart disease, n (%) | 7 (14.0) | 30 (18.4) | 0.53 |
| Rheumatic heart disease, n (%) | 4 (8.0) | 11 (6.7) | 0.76 |
| Cardiomyopathy, n (%) | 7 (14.0) | 21 (12.9) | 0.81 |
| Other, n (%) | 4 (8.0) | 11 (6.7) | 0.76 |
| Preadmission medication | |||
| ACEI/ARB, n (%) | 15 (30.0) | 52 (32.0) | 0.86 |
| β-blockers, n (%) | 9 (18.0) | 40 (24.5) | 0.44 |
| Spironolactone, n (%) | 10 (20.0) | 44 (27.0) | 0.36 |
| Loop diuretics, n (%) | 14 (28.0) | 53 (32.5) | 0.60 |
| Preadmission (baseline) renal function | |||
| Serum creatinine, mg/dl | 1.45±0.7 | 1.30±0.5 | 0.11 |
| eGFR, ml/min per 1.73m2 b | 59.1±28.3 | 63.4±24.0 | 0.29 |
| Parameters on admission | |||
| LVEF (%) | 42 (40–63) | 41 (36–58) | 0.22 |
| NYHA (class IV), n (%) | 20 (40.0) | 89 (54.6) | 0.08 |
| NT-proBNP, pg/ml | 9000 (3656–24139) | 6647 (3185–9000) | 0.02 |
| Serum creatinine, mg/dl | 1.6±0.8 | 1.5±0.8 | 0.41 |
| Serum albumin, g/dl | 2.9±0.6 | 3.2±0.5 | 0.001 |
| Serum triglyceride, mmol/L | 1.6±0.8 | 1.7±0.7 | 0.39 |
| Serum cholesterol, mmol/L | 3.8±0.9 | 3.8±0.6 | 0.99 |
| Serum sodium, mmol/L | 138.3±5.0 | 138.0±5.0 | 0.71 |
| Serum potassium, mmol/L | 4.2±0.8 | 4.1±0.6 | 0.34 |
| Hemoglobin, g/dl | 10.8±2.4 | 11.8±2.6 | 0.04 |
Continuous variables were expressed as mean±SD or median (25th percentile – 75th percentile, interquartile range). Categorical variables were expressed as a number (%). AKI progression is defined as worsening of AKI stage. HF, heart failure; ACEI, angiotensin converting enzyme inhibitors; ARB, angiotensin II type I receptor blockers; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
Defined as preadmission eGFR<60ml/min per 1.73m2. Preadmission eGFR was calculated by CKD-Epidemiology Collaboration equation according to at least three measurements of serum creatinine over a 6-month period before admission.
Table 2 compares in-hospital characteristics and outcomes between patients with or without progressed AKI. In addition to serum creatinine, the levels of six biomarkers tested at the time of AKI diagnosis were significantly higher in patients with progressed AKI as compared with those without. Clinical outcomes were much worse for patients with progressed AKI who had 2.4-fold mortality compared with those without progression.
Table 2.
Characteristics after admission in patients with and without AKI progression
| Variables | AKI progression | P Value | |
|---|---|---|---|
| Yes (n=50) | No (n=163) | ||
| Timing of AKI | |||
| AKI on admission, n (%) | 6 (12.0) | 22 (13.5) | 0.99 |
| Within 4 d after admission, n (%) | 46 (92.0) | 135(82.8) | 0.17 |
| Use of loop-diureticsa | |||
| Before AKI diagnosis, mg/d | 46.5±22.1 | 40.5±20.6 | 0.08 |
| On the day of AKI diagnosis, mg/d | 49.0±23.5 | 42.1±22.8 | 0.06 |
| Use of spironolactone | |||
| Before AKI diagnosis, n (%) | 20 (40.0) | 78 (47.9) | 0.41 |
| On the day of AKI diagnosis, n (%) | 20 (40.0) | 78 (47.9) | 0.41 |
| Use of RAS inhibitors | |||
| Before AKI diagnosis, n (%) | 19 (38.0) | 73 (44.8) | 0.42 |
| On the day of AKI diagnosis, n (%) | 19 (38.0) | 73 (44.8) | 0.42 |
| Biomarkers on the day of AKI diagnosis | |||
| SCr, mg/dl | 2.1±0.85 | 1.8±0.7 | 0.01 |
| Change in SCr, mg/dlb | 0.7±0.5 | 0.5±0.4 | 0.05 |
| Change in SCrc, % | 48.4±27.7 | 45.9±23.0 | 0.52 |
| uAGT, μg/g Cr | 224.5 (108.8–483.8) | 44.8 (9.3–146.2) | <0.001 |
| pAGT, μg/L | 27.8±5.7 | 26.5±5.5 | 0.15 |
| uNGAL, μg/g Cr | 311.3 (87.5–1883.8) | 58.8 (29.8–197.3) | <0.001 |
| pNGAL, ng/ml | 238.0 (152.9–335.4) | 179.0 (115.2–258.2) | 0.001 |
| uIL-18, ng/g Cr | 748.4 (68.7–1964.9) | 52.0 (16.0–215.2) | <0.001 |
| uKIM-1, μg/g Cr | 4.6 (2.5–8.0) | 2.6 (1.6–4.9) | 0.001 |
| UACR, mg/g Cr | 292.3 (138.2–964.9) | 154.0 (49.8–382.0) | 0.001 |
| In-hospital outcomes | |||
| Hospital-free daysd | 0 (0–18) | 18 (10–20) | 0.001 |
| ICU-free daysd | 13 (0–21) | 22 (15–24) | <0.001 |
| Acute dialysis, n (%) | 13 (26.0) | 0 (0) | <0.001 |
| In-hospital death, n (%) | 18 (36.0) | 25 (15.3) | 0.002 |
Continuous variables were expressed as mean±SD or median (25th percentile – 75th percentile, interquartile range). Categorical variables were expressed as a number (%). SCr, serum creatinine; uAGT, urinary angiotensinogen; pAGT, plasma angiotensinogen; uNGAL, urinary neutrophil gelatinase-associated lipocalin; pNGAL plasma neutrophil gelatinase-associated lipocalin; uIL-18, urinary IL-18; uKIM-1, urinary kidney injury molecule-1; UACR, urinary albumin to creatinine ratio; ICU, Intensive-care unit.
Average daily dose of intravenous furosemide (oral dosage × 0.5, as oral bioavailability is about 50%).
Serum creatinine level on the day of AKI diagnosis minus baseline serum creatinine level.
(SCr level on the day of AKI diagnosis − baseline SCr level)/ baseline SCr level × 100%.
28 minus the number of hospital or ICU days, with a score of zero assigned to patients who died.
The Performance of Traditional and Injury Biomarkers for Predicting Progression of AKI
Medians of the tested injury biomarkers were significantly higher in patients with progressive AKI compared with nonprogressors (Table 2). There were graded responses across the tertiles of these biomarkers (either in urine creatinine-normalized or non-normalized data) in the unadjusted model (P<0.05 in all); however, the OR remained statistically significant only for uAGT (OR, 10.8; 95% confidence interval [95% CI], 3.4 to 34.7), uNGAL (OR, 4.7; 95% CI, 1.7 to 13.4), and uIL-18 (OR, 3.6; 95% CI, 1.4 to 9.5) after adjusting for clinical variables (Supplemental Table 1 and Table 3). When the biomarkers were analyzed as continuous variables, higher levels of these three biomarkers were also associated with the progression of AKI (Supplemental Table 2) in a multivariable model.
Mean level of serum creatinine at time of AKI diagnosis tended to be higher in patients with AKI progression compared with those without. The highest tertile of serum creatinine had a higher risk of AKI progression in the unadjusted model (P=0.04); this association was attenuated and did not reach the statistical significance in the adjusted analysis (Table 3).
Table 3.
Biomarkers for predicting AKI progression: multivariate logistic regression analyses
| Biomarker | Cut Points | N | AKI Progression (%) | Unadjusted OR (95% CI) | P Value | Adjusted ORa (95% CI) | P Value |
|---|---|---|---|---|---|---|---|
| SCr, mg/dl | |||||||
| Low (T1) | 0.8–1.4 | 70 | 14.3 | 1 (referent) | 0.04 | 1 (referent) | 0.21 |
| Medium (T2) | 1.5–2.0 | 72 | 23.6 | 1.9 (0.8 to 4.3) | 2.3 (0.8 to 6.6) | ||
| High (T3) | >2.0 | 71 | 32.4 | 2.9 (1.2 to 6.6) | 2.9 (0.8 to 10.5) | ||
| uAGT, μg/g Cr | |||||||
| Low (T1) | 0.04–27.3 | 71 | 7.0 | 1 (referent) | <0.001 | 1 (referent) | <0.001 |
| Medium (T2) | 27.4–146.4 | 71 | 19.7 | 3.2 (1.1 to 9.5) | 3.7 (1.1 to 12.1) | ||
| High (T3) | >146.4 | 71 | 43.7 | 10.2 (3.7 to 28.4) | 10.8 (3.4 to 34.7) | ||
| uNGAL, μg/g Cr | |||||||
| Low (T1) | 0.2–47.4 | 71 | 9.9 | 1 (referent) | <0.001 | 1 (referent) | 0.01 |
| Medium (T2) | 47.5–185.4 | 71 | 21.1 | 2.4 (0.9 to 6.4) | 2.0 (0.7 to 5.7) | ||
| High (T3) | >185.4 | 71 | 39.4 | 5.9 (2.4 to 14.8) | 4.7 (1.7 to 13.4) | ||
| uIL-18, ng/g Cr | |||||||
| Low (T1) | 1.2–38.5 | 70 | 14.3 | 1 (referent) | <0.001 | 1 (referent) | 0.004 |
| Medium (T2) | 38.6–224.4 | 72 | 11.1 | 0.8 (0.3 to 2.0) | 0.8 (0.3 to 2.3) | ||
| High (T3) | >224.4 | 71 | 45.1 | 4.9 (2.2 to 11.1) | 3.6 (1.4 to 9.5) | ||
| uKIM-1, μg/g Cr | |||||||
| Low (T1) | 0.01–2.2 | 71 | 15.5 | 1 (referent) | 0.002 | 1 (referent) | 0.11 |
| Medium (T2) | 2.3–4.6 | 71 | 16.9 | 1.1 (0.5 to 2.7) | 0.9 (0.3 to 2.5) | ||
| High (T3) | >4.6 | 71 | 38.0 | 3.3 (1.5 to 7.5) | 2.1 (0.8 to 5.3) | ||
| UACR, mg/g Cr | |||||||
| Low (T1) | 1.0–104.7 | 71 | 12.7 | 1 (referent) | 0.02 | 1 (referent) | 0.19 |
| Medium (T2) | 104.8–308.5 | 71 | 23.9 | 2.2 (0.9 to 5.2) | 1.7 (0.6 to 4.4) | ||
| High (T3) | >308.5 | 71 | 33.8 | 3.5 (1.5 to 8.2) | 2.5 (0.9 to 6.9) | ||
| pNGAL, ng/ml | |||||||
| Low (T1) | 8.8–151.3 | 71 | 14.1 | 1 (referent) | 0.04 | 1 (referent) | 0.63 |
| Medium (T2) | 151.4–238.0 | 74 | 24.3 | 2.0 (0.8 to 4.6) | 1.5 (0.6 to 4.0) | ||
| High (T3) | >238.0 | 68 | 32.4 | 2.9 (1.3 to 6.8) | 1.2 (0.4 to 3.2) |
AKI progression is defined as worsening of AKI stage (from stage 1 to either stage 2 or 3 or from stage 2 to 3). Biomarkers are measured at the time of stage 1 or 2 AKI diagnosis. SCr, serum creatinine; T, tertile; uAGT, urinary angiotensinogen; uNGAL, urinary neutrophil gelatinase-associated lipocalin; uIL-18, urinary IL-18; uKIM-1, urinary kidney injury molecule-1; UACR, urinary albumin to creatinine ratio; pNGAL plasma neutrophil gelatinase-associated lipocalin.
Adjusted for age, gender, hypertension, diabetes, preadmission eGFR, NT-proBNP, serum albumin, hemoglobin, diuretic dosage before AKI, use of spironolactone before AKI, use of RAS inhibitors before AKI, and change of serum creatinine from baseline at the time of AKI diagnosis. Site is adjusted as a random effect.
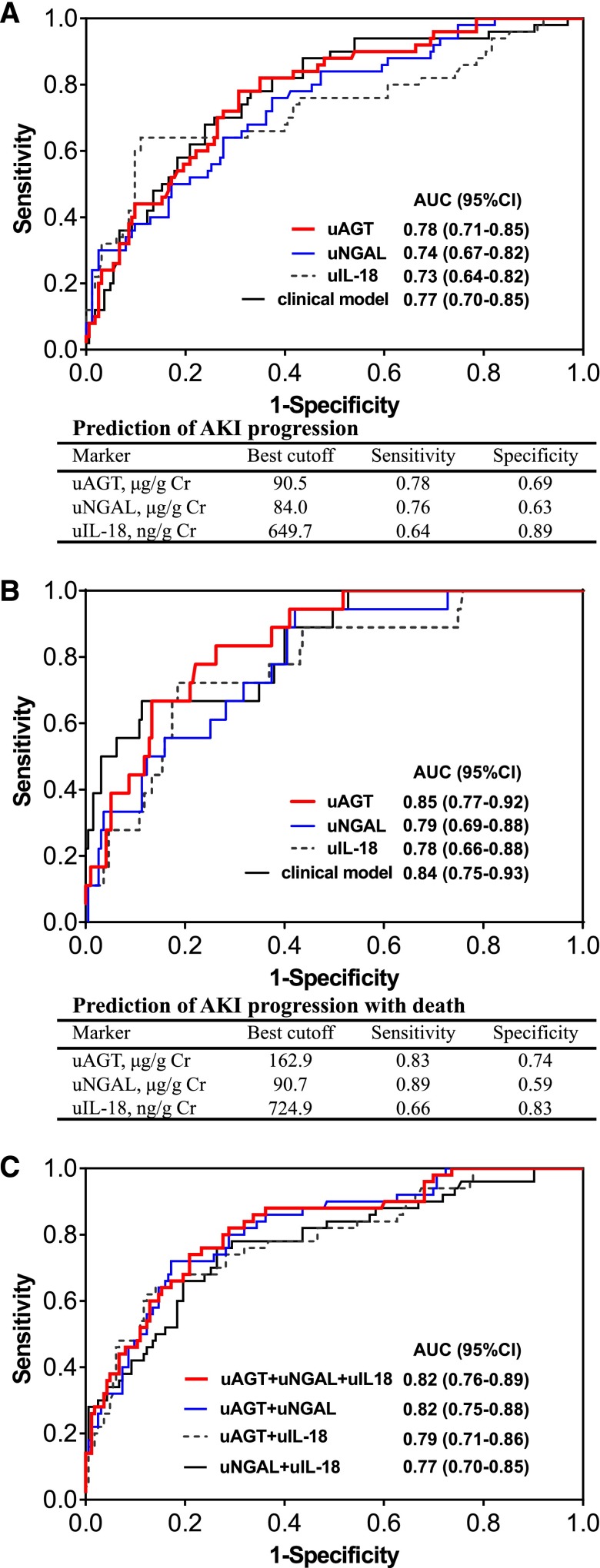
uAGT presented the strongest ability to predict the progression of AKI. In the adjusted model, patients with the highest tertile of uAGT (>146.4 μg/g Cr) had 10.8-fold higher risk of AKI progression compared with those with the lowest tertile of uAGT (0.04–27.3 μg/g Cr) (Table 3). As shown in Figure 1, uAGT provided the largest AUC for predicting both AKI progression (AUC 0.78) and AKI progression with death (AUC 0.85) (Figure 1, A and B). For predicting AKI progression, the performance of uAGT was better in patients with acute on chronic renal injury (AUC 0.83) compared with those without prior CKD (AUC 0.74).
Figure 1.
Receiver-operating characteristics analyses for predicting AKI progression or AKI progression with death. (A) The area under the receiver operating curve (AUCs) of renal injury biomarkers (urinary angiotensinogen [uAGT], urinary neutrophil gelatinase-associated lipocalin [uNGAL], urinary IL-18 [uIL-18]) and clinical model, at the time of AKI diagnosis, for predicting AKI progression. (B) The AUCs of renal injury biomarkers (uAGT, uNGAL, uIL-18) and clinical model, at the time of AKI diagnosis, for predicting AKI progression with subsequent death. (C) The performance of combination of renal injury biomarkers for predicting AKI progression. The clinical risk model includes age, gender, hypertension, diabetes, preadmission eGFR, N-terminal pro-B-type natriuretic peptide (NT-proBNP), serum albumin, hemoglobin, diuretic dosage before AKI, use of spironolactone before AKI, use of RAS inhibitors before AKI, and change of serum creatinine from baseline at the time of AKI diagnosis.
Combination of uAGT with uNGAL or uIL-18 further improved the performance for predicting progression of AKI (Figure 1C). A similar trend was observed in analysis of these biomarkers for predicting AKI progression and/or death (Supplemental Table 3).
The performance of uAGT was further confirmed by the bootstrap internal validation, in which the average AUC for predicting AKI progression (0.78; 95% CI, 0.77 to 0.78) was comparable to that in the test cohort.
The Improvement of the Risk Classification with the Injury Biomarkers to the Clinical Model
Addition of uAGT, uNGAL, and uIL-18 improved risk classification over the clinical model, with uAGT displaying the largest category-free NRI of 0.76 and IDI of 0.14 (Table 4). However, there was no significant improvement in risk classification when pNGAL, uKIM-1, and UACR were added to the clinical model (Table 4).
Table 4.
NRI and IDI of including biomarkers in the clinical model to predict AKI progression or progression with death
| Outcome | Category-Free NRI (95% CI) | P Value | NRI in Progressors (95% CI) | P Value | NRI in Nonprogressors (95% CI) | P Value | IDI (95% CI) | P Value |
|---|---|---|---|---|---|---|---|---|
| Predicting AKI progression | ||||||||
| Clinical risk factorsa | referent | referent | ||||||
| Clinical risk factors plus uAGT | 0.76 (0.46–1.06) | <0.001 | 0.48 (0.22–0.74) | <0.001 | 0.28 (0.13–0.43) | <0.001 | 0.14 (0.10–0.18) | <0.001 |
| Clinical risk factors plus uNGAL | 0.64 (0.32–0.96) | <0.001 | 0.28 (0.01–0.56) | 0.04 | 0.36 (0.21–0.51) | <0.001 | 0.11 (0.07–0.15) | <0.001 |
| Clinical risk factors plus uIL-18 | 0.60 (0.30–0.90) | <0.001 | 0.36 (0.10–0.62) | 0.01 | 0.24 (0.08–0.40) | 0.002 | 0.09 (0.05–0.13) | <0.001 |
| Clinical risk factors plus pNGAL | 0.23 (−0.09–0.55) | 0.15 | −0.08 (-0.36–0.20) | 0.57 | 0.31 (0.17–0.45) | <0.001 | 0.04 (0.00–0.08) | 0.01 |
| Clinical risk factors plus uKIM-1 | 0.17 (−0.15–0.49) | 0.29 | 0.24 (-0.04–0.52) | 0.08 | −0.07 (−0.23–0.09) | 0.39 | 0.01 (0.00–0.03) | 0.30 |
| Clinical risk factors plus UACR | 0.03 (−0.29–0.35) | 0.87 | 0.24 (−0.04–0.52) | 0.09 | −0.21 (−0.37 to −0.05) | 0.006 | 0.01 (0.00–0.03) | 0.36 |
| Predicting AKI progression with death | ||||||||
| Clinical risk factorsa | referent | referent | ||||||
| Clinical risk factors plus uAGT | 0.93 (0.50–1.36) | <0.001 | 0.44 (-0.01–0.90) | 0.06 | 0.49 (0.37–0.61) | <0.001 | 0.19 (0.09–0.29) | <0.001 |
| Clinical risk factors plus uNGAL | 0.86 (0.43–1.30) | <0.001 | 0.44 (-0.01–0.90) | 0.06 | 0.42 (0.30–0.54) | <0.001 | 0.07 (0.01–0.13) | 0.04 |
| Clinical risk factors plus uIL-18 | 0.88 (0.45–1.31) | <0.001 | 0.44 (-0.01–0.90) | 0.06 | 0.44 (0.32–0.56) | <0.001 | 0.08 (0.00–0.16) | 0.03 |
AKI progression is defined as worsening of AKI stage (from stage 1 to either stage 2 or 3 or from stage 2 to 3). NRI, net reclassification improvement; IDI, integrated discrimination improvement.
The clinical risk factors for AKI progression are comprised of age, gender, hypertension, diabetes, preadmission eGFR, NT-proBNP, serum albumin, hemoglobin, diuretic dosage before AKI, use of spironolactone before AKI, use of RAS inhibitors before AKI, and change of serum creatinine from baseline at the time of AKI diagnosis.
To validate the improvement of the risk classification with uAGT to the clinical model, the bootstrap internal validation was conducted and yielded comparable category-free NRI (0.72) and IDI (0.15) with the test set.
Discussion
In this large prospective multicenter cohort of ADHF, we found several renal injury biomarkers at the time of stage 1 or 2 AKI diagnosis which predicted the progression of AKI. Among the renal injury biomarkers, uAGT>146.4 μg/g Cr at the time of AKI diagnosis conveyed a 10.8-fold risk of AKI progression compared with patients in the lowest tertile after controlling for clinical factors; uNGAL>185.4 μg/g Cr denoted 4.7-fold odds of AKI progression compared with uNGAL<47.5 μg/g Cr; and uIL-18 levels of >224.4 ng/g Cr showed a 3.6-fold risk of progression compared with lower levels in the adjusted analysis. All three injury biomarkers, when added to the clinical model, significantly improved the risk classification for the outcomes, i.e., AKI progression and AKI progression with death.
Progression of AKI is associated with increased risk of mortality in patients with CRS (10,30). In our cohort, patients who initially presented with stage 1 or 2 AKI and progressed to higher stages had mortality of 36% versus 15% in those who presented in original stages but not progressed. It is therefore critical to identify patients at highest risk of both AKI progression and death so as to guide prognosis and treatment decisions. Unfortunately, the lack of objective tests to predict progression of acute CRS delays initiation of intervention and hinders clinical trials. Quantitating the severity of injury that the kidney has sustained may allow for earlier prediction of AKI progression. In this study, increase in serum creatinine, the current hallmark for recognizing AKI, was not associated with the risk of AKI progression after adjusting for clinical factors. However, there was a clear correlation between levels of urinary injury biomarkers, such as uAGT, uNGAL, and uIL-18, and the risk of AKI progression. Critically, these injury biomarkers, especially uAGT, showed large effect size (NRI>0.6) to improve risk classification for AKI progression and AKI progression with death beyond the clinical model, suggesting that uAGT, uNGAL, and uIL-18 measurement at the time of AKI diagnosis forecasted the progression of AKI in ADHF.
The goal of most AKI biomarker research has been the discovery of a predictor for developing AKI. Several biomarkers, such as IGF binding protein 7 and tissue inhibitors of metalloproteinases, have been approved by the US Food and Drug Administration as a first-of-a-kind test to help determine if certain hospitalized patients are at risk of developing AKI (37). However, the identification of biomarkers that predict the outcomes of patients with established AKI has not been fully highlighted. Most of these studies are conducted in patients after cardiac surgery (18,19). A recent study tested the ability of 32 biomarkers to predict worsening of renal function in patients with AKIN stage 1 AKI after cardiac surgery (19). They found that uIL-18 was the best predictor of worsening AKI or death. uNGAL and uKIM-1 were also good predictors, but they did not test uAGT, which was the best performer in our study. In a larger study from the Translational Research Investigating Biomarker Endpoints-AKI consortium, uIL-18, UACR, and pNGAL measurement at the time of AKI diagnosis predicted the progression of AKI in adults after cardiac surgery (18). The good performance of uIL-18 and uNGAL for predicting worsening of AKI has also been confirmed in other settings (22,38,39). Consistently, our results confirmed the ability of uIL-18 and uNGAL to predict worsening of AKI in acute CRS. However, we did not find benefit to pNGAL, uKIM-1, and UACR measurements after adjusting for variables known to affect AKI progression, although prior reports show these biomarkers may predict worsening of AKI (18,19). Notably, these prior reports were not specific to ADHF. Increasing data on renal injury biomarkers suggest that their diagnostic and prognostic performance may vary depending on the clinical setting in which they are evaluated (40).
Our study has the following strengths. First, the size of the multicenter cohort was the largest in the literature for this difficult-to-study population and we relied on standardized AKI staging criteria (KDIGO) that are currently employed by the international community. Second, the serum creatinine was measured every day throughout the hospital stay for determination of AKI progression. Serum creatinine before admission was available for the study patients, which allowed us to more precisely determine the baseline renal function and to define AKI. Third, we evaluated multiple biomarkers in acute CRS, in which AKI is physiologically distinct from other settings. Therefore, this work is an opportunity for direct comparisons of these putative biomarkers within this cohort and the assessment of their combinations as predictive models for AKI progression in the setting of ADHF.
The study also had limitations. The number of outcomes was relatively small, although there were sufficient events for the primary endpoint. As is true in the case of most AKI studies, we were not able to use urine output for AKI diagnosis because an indwelling urinary catheter was not present in most of the patients.
In conclusion, uAGT, uNGAL, and uIL-18 measurement at the time of AKI diagnosis predicted AKI progression and worsening of AKI with death in ADHF. uAGT was the best predictor for both outcomes. These renal injury biomarkers, when added to the clinical risk model, may identify a subpopulation that is at the highest risk for the most adverse outcomes. Improvement of risk prediction may improve care of ADHF, guide patient counseling, optimize management, and facilitate clinical trials for acute CRS treatment.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by the Major State Basic Research Development Program of China (2012CB517703 to F.F.H.), the National Natural Science Foundation of China (Key Program) (81430016 to F.F.H.), the National Key Technology Support Program of China (2013BAI09B06 & 2015BAI12B07 to F.F.H.), the National Nature Science Foundation (Innovation team program, 81521003 to Y.H.L), the Major Scientific and Technological Planning Project of Guangzhou (201504010027 to F.F.H.), the Science and Information Technology of Guangzhou Key Project (201400000004-4 to J.N.), and the Science and Technology Planning Project of Guangdong Province (Key Program) (2014B020212023 to C.C.).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Urinary Angiotensinogen: A Promising Biomarker of AKI Progression in Acute Decompensated Heart Failure: What Does It Mean?,” on pages 1515–1517.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.00910116/-/DCSupplemental.
References
- 1.Adams KF Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP; ADHERE Scientific Advisory Committee and Investigators : Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J 149: 209–216, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Cowie MR, Komajda M, Murray-Thomas T, Underwood J, Ticho B; POSH Investigators : Prevalence and impact of worsening renal function in patients hospitalized with decompensated heart failure: results of the prospective outcomes study in heart failure (POSH). Eur Heart J 27: 1216–1222, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Zhou Q, Zhao C, Xie D, Xu D, Bin J, Chen P, Liang M, Zhang X, Hou F: Acute and acute-on-chronic kidney injury of patients with decompensated heart failure: impact on outcomes. BMC Nephrol 13: 51, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ronco C, Haapio M, House AA, Anavekar N, Bellomo R: Cardiorenal syndrome. J Am Coll Cardiol 52: 1527–1539, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Liang KV, Williams AW, Greene EL, Redfield MM: Acute decompensated heart failure and the cardiorenal syndrome. Crit Care Med 36[Suppl]: S75–S88, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Metra M, Nodari S, Parrinello G, Bordonali T, Bugatti S, Danesi R, Fontanella B, Lombardi C, Milani P, Verzura G, Cotter G, Dittrich H, Massie BM, Dei Cas L: Worsening renal function in patients hospitalised for acute heart failure: clinical implications and prognostic significance. Eur J Heart Fail 10: 188–195, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Chawla LS, Eggers PW, Star RA, Kimmel PL: Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med 371: 58–66, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hata N, Yokoyama S, Shinada T, Kobayashi N, Shirakabe A, Tomita K, Kitamura M, Kurihara O, Takahashi Y: Acute kidney injury and outcomes in acute decompensated heart failure: evaluation of the RIFLE criteria in an acutely ill heart failure population. Eur J Heart Fail 12: 32–37, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Shirakabe A, Hata N, Kobayashi N, Shinada T, Tomita K, Tsurumi M, Matsushita M, Okazaki H, Yamamoto Y, Yokoyama S, Asai K, Mizuno K: Prognostic impact of acute kidney injury in patients with acute decompensated heart failure. Circ J 77: 687–696, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Roy AK, Mc Gorrian C, Treacy C, Kavanaugh E, Brennan A, Mahon NG, Murray PT: A Comparison of Traditional and Novel Definitions (RIFLE, AKIN, and KDIGO) of Acute Kidney Injury for the Prediction of Outcomes in Acute Decompensated Heart Failure. Cardiorenal Med 3: 26–37, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haase M, Müller C, Damman K, Murray PT, Kellum JA, Ronco C, McCullough PA: Pathogenesis of cardiorenal syndrome type 1 in acute decompensated heart failure: workgroup statements from the eleventh consensus conference of the Acute Dialysis Quality Initiative (ADQI). Contrib Nephrol 182: 99–116, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Tang WH, Mullens W: Cardiorenal syndrome in decompensated heart failure. Heart 96: 255–260, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Koyner JL, Vaidya VS, Bennett MR, Ma Q, Worcester E, Akhter SA, Raman J, Jeevanandam V, O’Connor MF, Devarajan P, Bonventre JV, Murray PT: Urinary biomarkers in the clinical prognosis and early detection of acute kidney injury. Clin J Am Soc Nephrol 5: 2154–2165, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steiner RW: Interpreting the fractional excretion of sodium. Am J Med 77: 699–702, 1984 [DOI] [PubMed] [Google Scholar]
- 15.Hilberman M, Myers BD, Carrie BJ, Derby G, Jamison RL, Stinson EB: Acute renal failure following cardiac surgery. J Thorac Cardiovasc Surg 77: 880–888, 1979 [PubMed] [Google Scholar]
- 16.Yavuz I, Asgun FH, Bolcal C, Bingol H, Yokusoglu M, Baysan O, Ozgurtas T, Demirkilic U, Tatar H: Importance of urinary measurement of glutathione S-transferase in renal dysfunction patients after on- and off-pump coronary artery bypass surgery. Thorac Cardiovasc Surg 57: 125–129, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Pépin MN, Bouchard J, Legault L, Ethier J: Diagnostic performance of fractional excretion of urea and fractional excretion of sodium in the evaluations of patients with acute kidney injury with or without diuretic treatment. Am J Kidney Dis 50: 566–573, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Koyner JL, Garg AX, Coca SG, Sint K, Thiessen-Philbrook H, Patel UD, Shlipak MG, Parikh CR; TRIBE-AKI Consortium : Biomarkers predict progression of acute kidney injury after cardiac surgery. J Am Soc Nephrol 23: 905–914, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arthur JM, Hill EG, Alge JL, Lewis EC, Neely BA, Janech MG, Tumlin JA, Chawla LS, Shaw AD; SAKInet Investigators : Evaluation of 32 urine biomarkers to predict the progression of acute kidney injury after cardiac surgery. Kidney Int 85: 431–438, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alge JL, Karakala N, Neely BA, Janech MG, Tumlin JA, Chawla LS, Shaw AD, Arthur JM; SAKInet Investigators : Urinary angiotensinogen and risk of severe AKI. Clin J Am Soc Nephrol 8: 184–193, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall IE, Stern EP, Cantley LG, Elias JA, Parikh CR: Urine YKL-40 is associated with progressive acute kidney injury or death in hospitalized patients. BMC Nephrol 15: 133, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall IE, Yarlagadda SG, Coca SG, Wang Z, Doshi M, Devarajan P, Han WK, Marcus RJ, Parikh CR: IL-18 and urinary NGAL predict dialysis and graft recovery after kidney transplantation. J Am Soc Nephrol 21: 189–197, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belcher JM, Garcia-Tsao G, Sanyal AJ, Thiessen-Philbrook H, Peixoto AJ, Perazella MA, Ansari N, Lim J, Coca SG, Parikh CR; TRIBE-AKI Consortium : Urinary biomarkers and progression of AKI in patients with cirrhosis. Clin J Am Soc Nephrol 9: 1857–1867, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ronco C, Cicoira M, McCullough PA: Cardiorenal syndrome type 1: pathophysiological crosstalk leading to combined heart and kidney dysfunction in the setting of acutely decompensated heart failure. J Am Coll Cardiol 60: 1031–1042, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Velez JC: The importance of the intrarenal renin-angiotensin system. Nat Clin Pract Nephrol 5: 89–100, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Rafiq K, Noma T, Fujisawa Y, Ishihara Y, Arai Y, Nabi AH, Suzuki F, Nagai Y, Nakano D, Hitomi H, Kitada K, Urushihara M, Kobori H, Kohno M, Nishiyama A: Renal sympathetic denervation suppresses de novo podocyte injury and albuminuria in rats with aortic regurgitation. Circulation 125: 1402–1413, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graciano ML, Cavaglieri RC, Dellê H, Dominguez WV, Casarini DE, Malheiros DM, Noronha IL: Intrarenal Renin-Angiotensin system is upregulated in experimental model of progressive renal disease induced by chronic inhibition of nitric oxide synthesis. J Am Soc Nephrol 15: 1805–1815, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Kobori H, Harrison-Bernard LM, Navar LG: Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int 61: 579–585, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamamoto T, Nakagawa T, Suzuki H, Ohashi N, Fukasawa H, Fujigaki Y, Kato A, Nakamura Y, Suzuki F, Hishida A: Urinary angiotensinogen as a marker of intrarenal angiotensin II activity associated with deterioration of renal function in patients with chronic kidney disease. J Am Soc Nephrol 18: 1558–1565, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Yang X, Chen C, Tian J, Zha Y, Xiong Y, Sun Z, Chen P, Li J, Yang T, Ma C, Liu H, Wang X, Hou FF: Urinary Angiotensinogen Level Predicts AKI in Acute Decompensated Heart Failure: A Prospective, Two-Stage Study. J Am Soc Nephrol 26: 2032–2041, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group : KDIGO clinical practice guideline for acute kidney injury. Kidney Int [Suppl 2]: 1–138, 2012 [Google Scholar]
- 32.Nieminen MS, Böhm M, Cowie MR, Drexler H, Filippatos GS, Jondeau G, Hasin Y, Lopez-Sendon J, Mebazaa A, Metra M, Rhodes A, Swedberg K, Priori SG, Garcia MA, Blanc JJ, Budaj A, Cowie MR, Dean V, Deckers J, Burgos EF, Lekakis J, Lindahl B, Mazzotta G, Morais J, Oto A, Smiseth OA, Garcia MA, Dickstein K, Albuquerque A, Conthe P, Crespo-Leiro M, Ferrari R, Follath F, Gavazzi A, Janssens U, Komajda M, Morais J, Moreno R, Singer M, Singh S, Tendera M, Thygesen K; ESC Committe for Practice Guideline (CPG) : Executive summary of the guidelines on the diagnosis and treatment of acute heart failure: the Task Force on Acute Heart Failure of the European Society of Cardiology. Eur Heart J 26: 384–416, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cook NR: Statistical evaluation of prognostic versus diagnostic models: beyond the ROC curve. Clin Chem 54: 17–23, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr, Vasan RS: Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 27: 157–172, discussion 207–212, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Efron B, Tibshirani RJ: An introduction to the bootstrap, Boca Raton, Florida, CRC Press, 1994 [Google Scholar]
- 37.Gunnerson KJ, Shaw AD, Chawla LS, Bihorac A, Al-Khafaji A, Kashani K, Lissauer M, Shi J, Walker MG, Kellum JA; Sapphire Topaz investigators : TIMP2•IGFBP7 biomarker panel accurately predicts acute kidney injury in high-risk surgical patients. J Trauma Acute Care Surg 80: 243–249, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siew ED, Ikizler TA, Gebretsadik T, Shintani A, Wickersham N, Bossert F, Peterson JF, Parikh CR, May AK, Ware LB: Elevated urinary IL-18 levels at the time of ICU admission predict adverse clinical outcomes. Clin J Am Soc Nephrol 5: 1497–1505, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hall IE, Coca SG, Perazella MA, Eko UU, Luciano RL, Peter PR, Han WK, Parikh CR: Risk of poor outcomes with novel and traditional biomarkers at clinical AKI diagnosis. Clin J Am Soc Nephrol 6: 2740–2749, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siew ED, Ware LB, Ikizler TA: Biological markers of acute kidney injury. J Am Soc Nephrol 22: 810–820, 2011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.